Determination of Polybrominated Diphenyl Ethers in Water Samples Using Effervescent-Assisted Dispersive Liquid-Liquid Icroextraction with Solidification of the Aqueous Phase
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of EA-DLLME-SAP Parameters
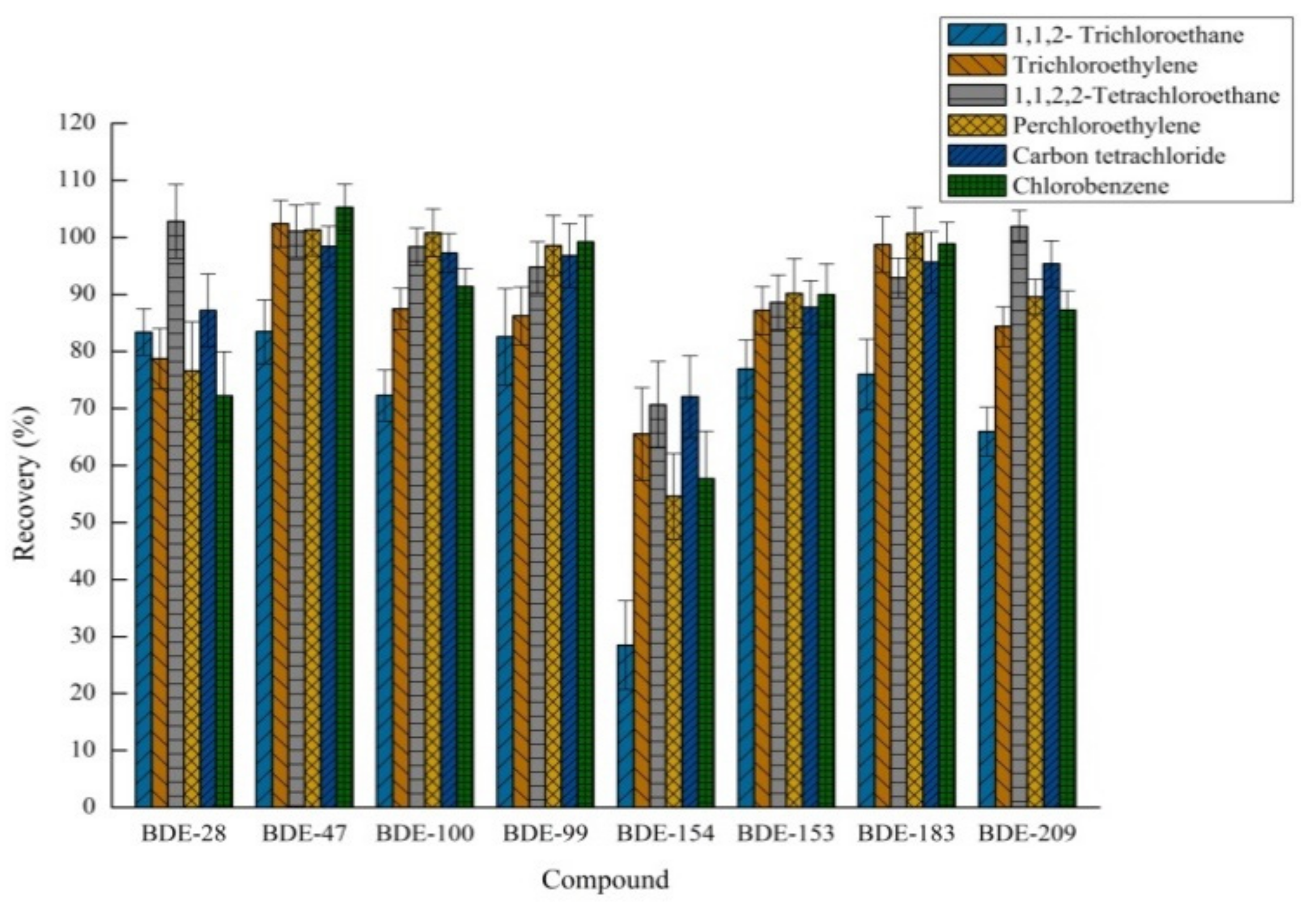
2.1.1. The Effect of Different Extraction Solvents
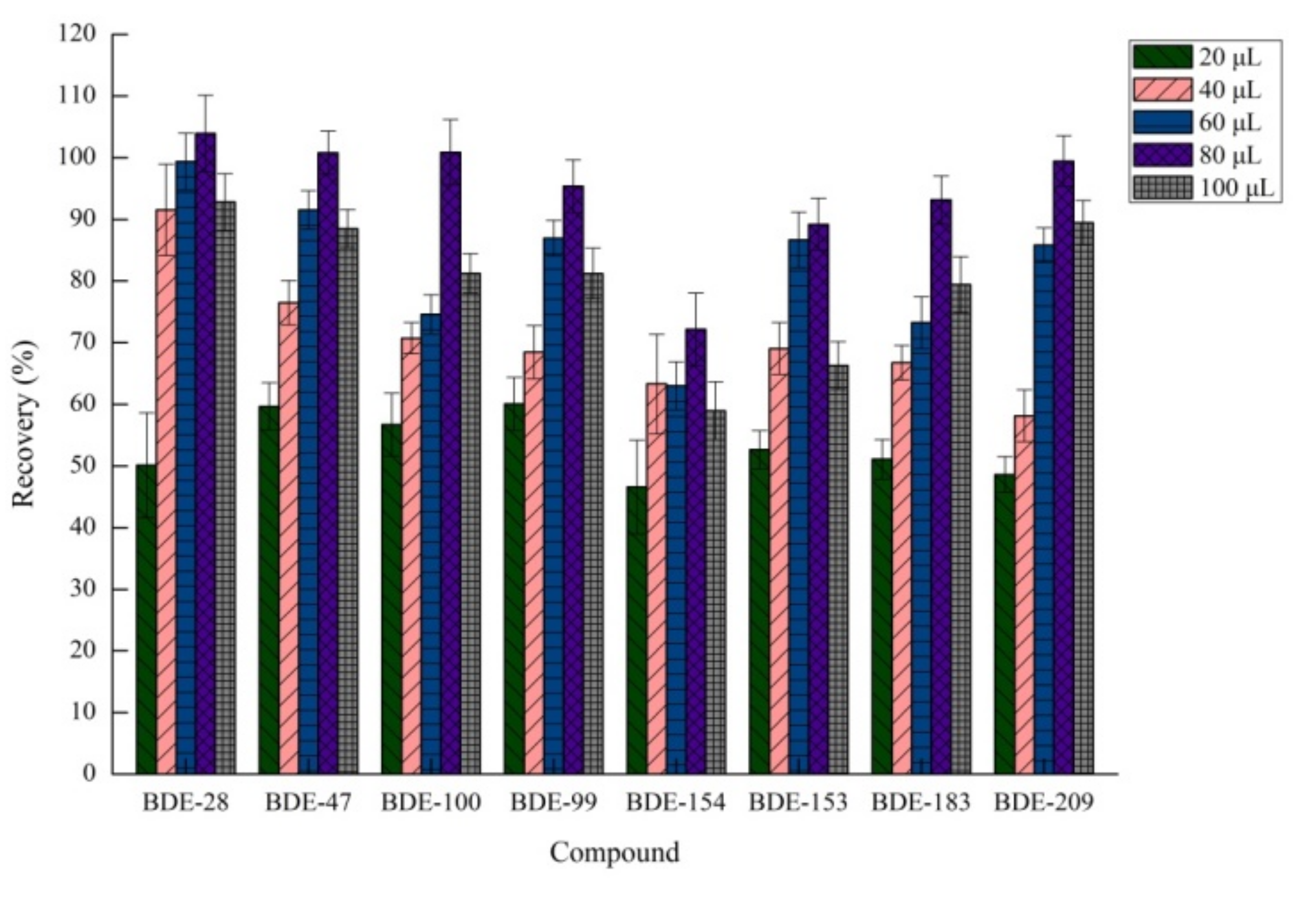
2.1.2. Effects of Extraction Solvent Volume
2.1.3. Effects of Sodium Bicarbonate
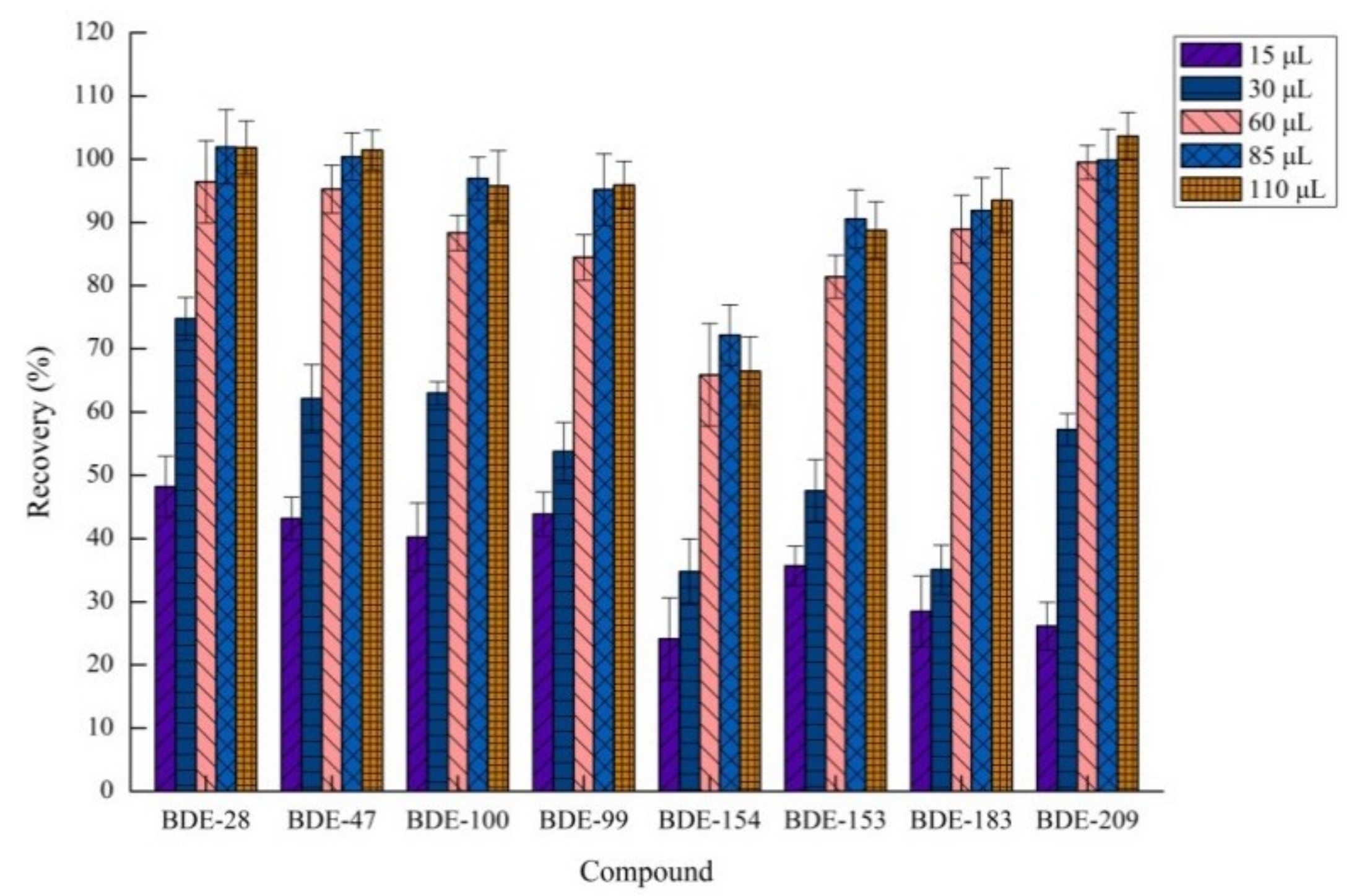
2.1.4. Effects of Volume of Acetic Acid
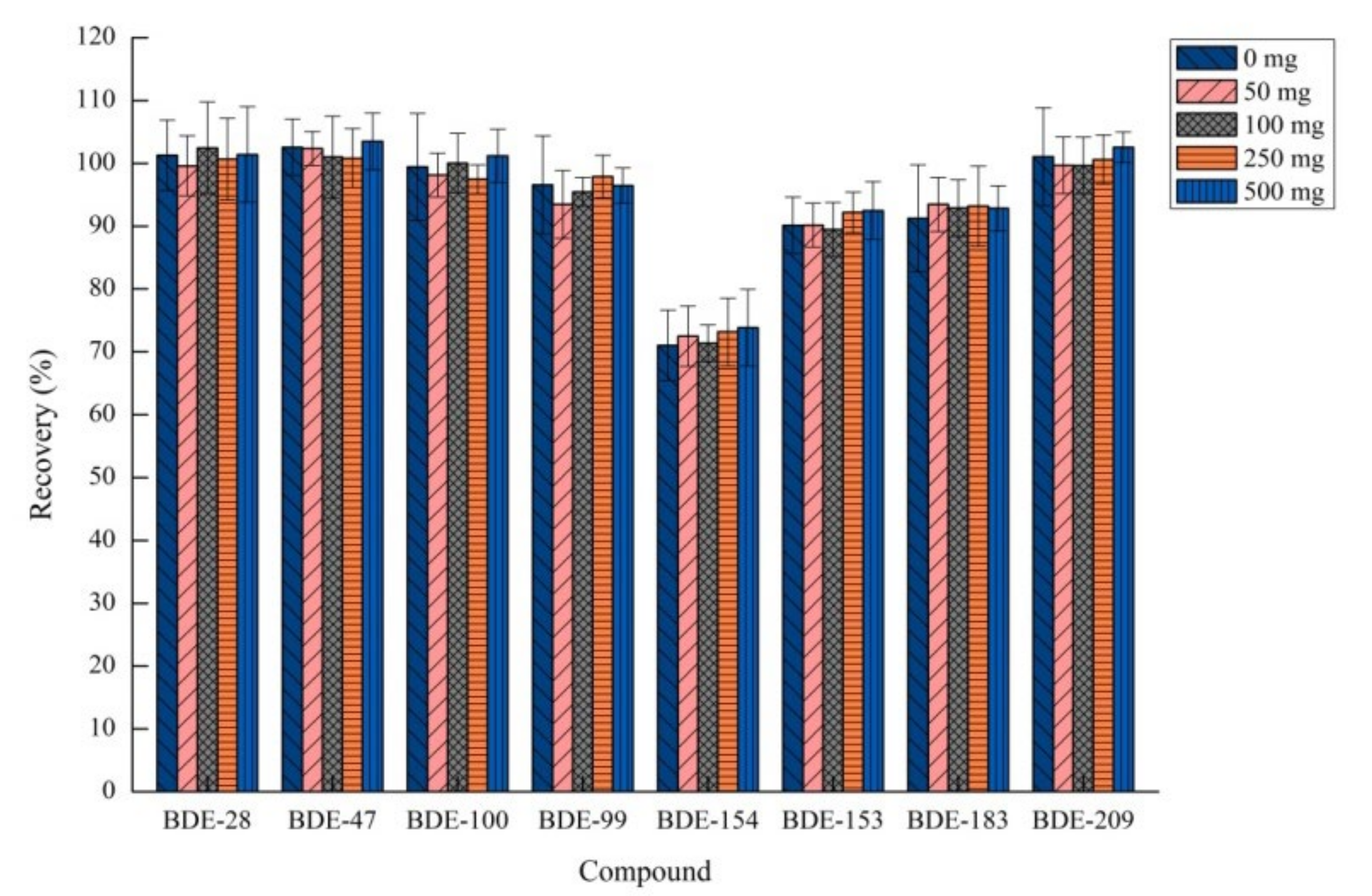
2.1.5. Effects of Sodium Chloride Amount
2.2. Method Validation
2.3. Application to Water Sample Analysis
2.4. Comparison of EA-DLLME-SAP with Other Extraction Techniques
3. Materials and Methods
3.1. Chemicals
3.2. Instrument
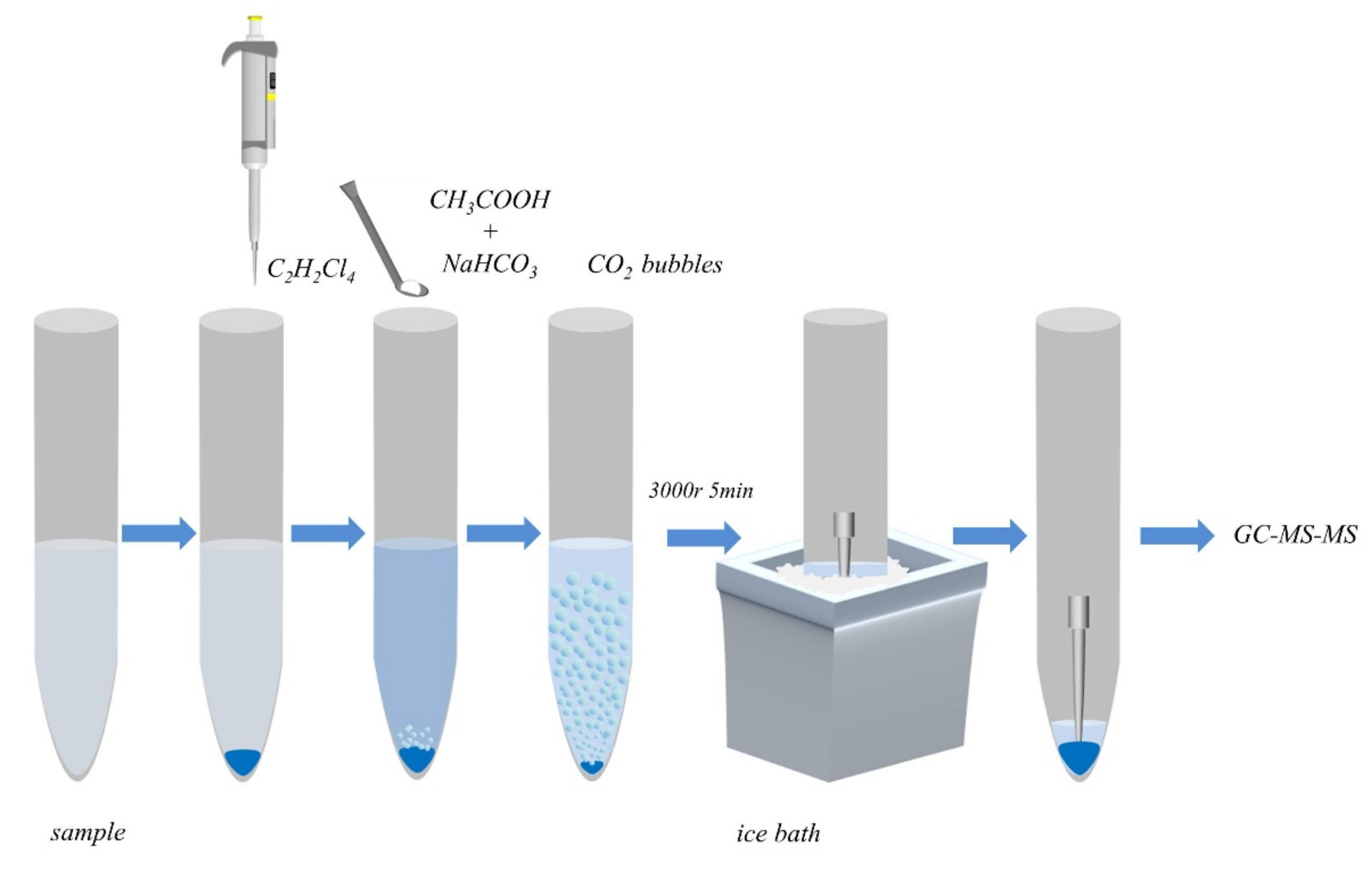
3.3. EA-DLLME-SAP Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBDEs | Polybrominated diphenyl ethers |
| EA-DLLME-SAP | Effervescent-assisted dispersive liquid-liquid microextraction with solidification of the aqueous phase |
| GC-MS-MS | Gas Chromatography-Tandem Mass Spectrometry |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| LLE | Liquid-liquid extraction |
| SPE | Solid-phase extraction |
| LPME | Liquid phase microextraction |
| SPME | Solid phase microextraction |
| SBSE | Stir bar adsorption extraction |
| DLLME | Dispersive liquid-liquid microextraction |
| EA-DLLME | Effervescent-assisted dispersive liquid-liquid microextraction |
| DLLME-SFO | Dispersive liquid-liquid microextraction based on the solidification of floating organic drop |
| DLLME-SAP | Dispersive liquid-liquid microextraction with solidification of the aqueous phase |
| LOQ | Limit of quantification |
| AEI | Advanced Electron Ionization |
References
- Sun, R.; Pan, C.; Li, Q.X.; Peng, F.; Mai, B. Occurrence and congener profiles of polybrominated diphenyl ethers in green mussels (Perna viridis) collected from northern South China Sea and the associated potential health risk. Sci. Total. Environ. 2020, 698, 134276. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, M.; Harrad, S.; Abdallah, M.A.-E.; Drage, D.S.; Berresheim, H. Phasing-out of legacy brominated flame retardants: The UNEP Stockholm Convention and other legislative action worldwide. Environ. Int. 2020, 144, 106041. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.Q.; Tomioka, K.; Tue, N.M.; Tri, T.M.; Minh, T.B.; Takahashi, S. PBDEs and novel brominated flame retardants in road dust from northern Vietnam: Levels, congener profiles, emission sources and implications for human exposure. Chemosphere 2018, 197, 389–398. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Tang, C.-X.; Dong, Y.; Wu, C.-C.; Bao, L.-J.; Zeng, E.Y. Effects of cooking on oral bioaccessibility of PBDEs, MeO-PBDEs, and OH-PBDEs in fish (tilapia) and chicken egg. Sci. Total. Environ. 2020, 748, 142310. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Song, Q.; Yuan, W.; Ruan, J.; Duan, H.; Li, Y.; Li, J. Human exposure to PBDEs in e-waste areas: A review. Environ. Pollut. 2020, 267, 115634. [Google Scholar] [CrossRef] [PubMed]
- Lana, N.B.; Berton, P.; Covaci, A.; Atencio, A.G.; Ciocco, N.F.; Altamirano, J.C. Ultrasound leaching–dispersive liquid–liquid microextraction based on solidification of floating organic droplet for determination of polybrominated diphenyl ethers in sediment samples by gas chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1285, 15–21. [Google Scholar] [CrossRef]
- Guo, W.; Holden, A.; Smith, S.C.; Gephart, R.; Petreas, M.; Park, J.-S. PBDE levels in breast milk are decreasing in California. Chemosphere 2016, 150, 505–513. [Google Scholar] [CrossRef]
- Karakas, F.; Aksoy, A.; Imamoglu, I. Development of a fate and transport model for biodegradation of PBDE congeners in sediments. Environ. Pollut. 2020, 266, 115116. [Google Scholar] [CrossRef]
- Trinh, M.M.; Tsai, C.L.; Chang, M.B. Characterization of polybrominated diphenyl ethers (PBDEs) in various aqueous samples in Taiwan. Sci. Total. Environ. 2019, 649, 388–395. [Google Scholar] [CrossRef]
- Wu, Z.; Lin, T.; Guo, T.; Li, Y.; Li, Z.; Guo, Z. Occurrence, air-sea exchange, and gas-particle partitioning of atmospheric polybrominated diphenyl ethers from East Asia to the Northwest Pacific Ocean. Chemosphere 2020, 240, 124933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yuan, L.; Lin, Q.; Ma, S.; Yu, Y. Polybrominated diphenyl ethers in the environment and human external and internal exposure in China: A review. Sci. Total. Environ. 2019, 696, 133902. [Google Scholar] [CrossRef]
- Chokwe, T.B.; Okonkwo, J.O.; Sibali, L.L.; Ncube, E.J. Alkylphenol ethoxylates and brominated flame retardants in water, fish (carp) and sediment samples from the Vaal River, South Africa. Environ. Sci. Pollut. Res. 2015, 22, 11922–11929. [Google Scholar] [CrossRef]
- Clarke, B.O.; Porter, N.A.; Symons, R.K.; Marriott, P.J.; Stevenson, G.J.; Blackbeard, J.R. Investigating the distribution of polybrominated diphenyl ethers through an Australian wastewater treatment plant. Sci. Total. Environ. 2010, 408, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Oros, D.R.; Hoover, D.; Rodigari, F.; Crane, D.; Sericano, J. Levels and Distribution of Polybrominated Diphenyl Ethers in Water, Surface Sediments, and Bivalves from the San Francisco Estuary. Environ. Sci. Technol. 2005, 39, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Yao, H.; Wang, H.; Li, H.; Lu, S.; Zhang, X.; Xiang, X. Polybrominated diphenyl ethers (PBDEs) in water, surface sediment, and suspended particulate matter from the Yellow River, China: Levels, spatial and seasonal distribution, and source contribution. Mar. Pollut. Bull. 2018, 129, 106–113. [Google Scholar] [CrossRef]
- Sacks, V.P.; Lohmann, R. Freely dissolved PBDEs in water and porewater of an urban estuary. Environ. Pollut. 2012, 162, 287–293. [Google Scholar] [CrossRef]
- Roy, G.; Vuillemin, R.; Guyomarch, J. On-site determination of polynuclear aromatic hydrocarbons in seawater by stir bar sorptive extraction (SBSE) and thermal desorption GC–MS. Talanta 2005, 66, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Kokosa, J.M. Selecting an extraction solvent for a greener liquid phase microextraction (LPME) mode-based analytical method. TrAC Trends Anal. Chem. 2019, 118, 238–247. [Google Scholar] [CrossRef]
- Santos, M.S.F.; Moreira, J.L.; Madeira, L.M.; Alves, A. Determination of polybrominated diphenyl ethers in water at ng/L level by a simple DLLME–GC–(EI) MS method. J. Anal. Chem. 2015, 70, 1390–1400. [Google Scholar] [CrossRef]
- Ali, J.; Tuzen, M.; Feng, X.; Kazi, T.G. Determination of trace levels of selenium in natural water, agriculture soil and food samples by vortex assisted liquid-liquid microextraction method: Multivariate techniques. Food Chem. 2021, 344, 128706. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Sorouraddin, S.M.; Mogaddam, M.R.A. Liquid phase microextraction of pesticides: A review on current methods. Microchim. Acta 2014, 181, 829–851. [Google Scholar] [CrossRef]
- Zhong, Z.; Li, G.; Luo, Z.; Zhu, B. Microwave-assisted dispersive liquid-liquid microextraction coupling to solidification of floating organic droplet for colorants analysis in selected cosmetics by liquid chromatography. Talanta 2019, 194, 46–54. [Google Scholar] [CrossRef]
- Zhou, G.-S.; Yuan, Y.-C.; Yin, Y.; Tang, Y.-P.; Xu, R.-J.; Liu, Y.; Chen, P.-D.; Yin, L.; Duan, J.-A. Hydrophilic interaction chromatography combined with ultrasound-assisted ionic liquid dispersive liquid–liquid microextraction for determination of underivatized neurotransmitters in dementia patients’ urine samples. Anal. Chim. Acta 2020, 1107, 74–84. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Hosseini, M.-R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, J.; Liu, W.; Jin, L.; Zhou, P.; Zhang, Y.; Zhang, B.; Dahlgren, R.A.; Wang, X.; Zhou, Y. Magnetic effervescent tablet-assisted ionic liquid-based dispersive liquid-liquid microextraction of polybrominated diphenyl ethers in liquid matrix samples. Talanta 2019, 195, 785–795. [Google Scholar] [CrossRef]
- Yang, M.; Wu, X.; Jia, Y.; Xi, X.; Yang, X.; Lu, R.; Zhang, S.; Gao, H.; Zhou, W. Use of magnetic effervescent tablet-assisted ionic liquid dispersive liquid-liquid microextraction to extract fungicides from environmental waters with the aid of ex-perimental design methodology. Anal. Chim. Acta 2016, 906, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Karadaş, C.; Kara, D. Dispersive liquid–liquid microextraction based on solidification of floating organic drop for pre-concentration and determination of trace amounts of copper by flame atomic absorption spectrometry. Food Chem. 2017, 220, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Afshar Mogaddam, M.R.; Farajzadeh, M.A.; Azadmard Damirchi, S.; Nemati, M. Dispersive solid phase extraction com-bined with solidification of floating organic drop–liquid–liquid microextraction using in situ formation of deep eutectic solvent for extraction of phytosterols from edible oil samples. J. Chromatogr. A 2020, 1630, 461523. [Google Scholar] [CrossRef] [PubMed]
- El-Deen, A.K.; Shimizu, K. A green air assisted-dispersive liquid-liquid microextraction based on solidification of a novel low viscous ternary deep eutectic solvent for the enrichment of endocrine disrupting compounds from water. J. Chromatogr. A 2020, 1629, 461498. [Google Scholar] [CrossRef] [PubMed]
- Caleb, J.; Alshana, U.; Ertaş, N. Smartphone digital image colorimetry combined with solidification of floating organic drop-dispersive liquid-liquid microextraction for the determination of iodate in table salt. Food Chem. 2021, 336, 127708. [Google Scholar] [CrossRef]
- March, J.G.; Cerdà, V. A novel procedure for phase separation in dispersive liquid-liquid microextraction based on solidi-fication of the aqueous phase. Talanta 2016, 156–157, 204–208. [Google Scholar] [CrossRef]
- Werner, J. Ionic liquid ultrasound-assisted dispersive liquid-liquid microextraction based on solidification of the aqueous phase for preconcentration of heavy metals ions prior to determination by LC-UV. Talanta 2018, 182, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, X.; Ling, Y.; Huang, W.; Wang, J.; Zheng, Z.; Wang, X.; Wang, H. Ultrasonic-enhanced preconcentration of trace Pb(II) using hydrophobic, lighter-than-water ionic liquid microextraction combined with solidification of the aqueous solution prior to detection by graphite furnace atomic absorption spectrometry in human fluids. Spectrochim. Acta Part B At. Spectrosc. 2019, 157, 27–36. [Google Scholar] [CrossRef]
- Berton, P.; Lana, N.B.; Ríos, J.M.; García-Reyes, J.F.; Altamirano, J.C. State of the art of environmentally friendly sample preparation approaches for determination of PBDEs and metabolites in environmental and biological samples: A critical review. Anal. Chim. Acta 2016, 905, 24–41. [Google Scholar] [CrossRef]
- Lu, W.; Chen, L. Recent Advances in Dispersive Liquid-Liquid Microextraction for Organic Compounds Analysis in Environmental Water: A Review. Curr. Anal. Chem. 2012, 8, 78–90. [Google Scholar] [CrossRef]
- Mansour, F.R.; Khairy, M.A. Pharmaceutical and biomedical applications of dispersive liquid–liquid microextraction. J. Chromatogr. B 2017, 1061–1062, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Piao, H.; Jiang, Y.; Qin, Z.; Tao, S.; Ma, P.; Sun, Y.; Wang, X.; Song, D. Development of a novel acidic task-specific ionic liquid-based effervescence-assisted microextraction method for determination of triazine herbicides in tea beverage. Talanta 2020, 208, 120414. [Google Scholar] [CrossRef]
- Moghadam, A.G.; Rajabi, M.; Hemmati, M.; Asghari, A. Development of effervescence-assisted liquid phase microextraction based on fatty acid for determination of silver and cobalt ions using micro-sampling flame atomic absorp-tion spectrometry. J. Mol. Liq. 2017, 242, 1176–1183. [Google Scholar] [CrossRef]
- Wu, X.; Yang, M.; Zeng, H.; Xi, X.; Zhang, S.; Lu, R.; Gao, H.; Zhou, W. Effervescence-assisted dispersive solid-phase ex-traction using ionic-liquid-modified magnetic β-cyclodextrin/attapulgite coupled with high-performance liquid chroma-tography for fungicide detection in honey and juice. J. Sep. Sci. 2016, 39, 4422–4428. [Google Scholar] [CrossRef] [PubMed]
- Lasarte-Aragonés, G.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Effervescence assisted dispersive liquid–liquid microextrac-tion with extractant removal by magnetic nanoparticles. Anal. Chim. Acta 2014, 807, 61–66. [Google Scholar] [CrossRef]
- Xu, L.; Miao, X.; Yang, Z.; Li, H.; Qiu, B. Solid-Phase Extraction Combined with Dispersive Liquid-Liquid Microextraction Based on Solidification of Floating Organic Droplet for Simultaneous Determination of Organochlorine Pesticides and Polychlorinated Biphenyls in Fish. Food Anal. Methods 2019, 12, 1871–1885. [Google Scholar] [CrossRef]
- L’Homme, B.; Scholl, G.; Eppe, G.; Focant, J.-F. Validation of a gas chromatography–triple quadrupole mass spectrometry method for confirmatory analysis of dioxins and dioxin-like polychlorobiphenyls in feed following new EU Regulation 709/2014. J. Chromatogr. A 2015, 1376, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, G.; Hu, J.; Liu, X.; Zhao, X.; Wang, X. Dispersive liquid–liquid microextraction followed by reversed phase-high performance liquid chromatography for the determination of polybrominated diphenyl ethers at trace levels in landfill leachate and environmental water samples. Anal. Chim. Acta 2008, 615, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Zhao, Z.; Zhang, W.; Lin, K.; Huang, C.; Wang, X. Solid-phase extraction combined with dispersive liquid–liquid microextraction for the determination for polybrominated diphenyl ethers in different environmental matrices. J. Chromatogr. A 2009, 1216, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liang, T.; Guan, L. Ultrasound-assisted dispersive liquid–liquid microextraction combined with gas chroma-tography-mass spectrometry in negative chemical ionization mode for the determination of polybrominated diphenyl ethers in water. J. Sep. Sci. 2013, 36, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, B.; Huo, S.; Deng, L.; Pan, H.; Xia, X.; Zhang, J.; Ren, Y.; Liu, H. Polybrominated diphenyl ethers occurrence in major inflowing rivers of Lake Chaohu (China): Characteristics, potential sources and inputs to lake. Chemosphere 2013, 93, 1624–1631. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Wang, X.; Zou, Y.; Wang, W.; Ma, M.; Li, Y.; Wang, H. Extraction and determination of polybrominated diphenyl ethers in water and urine samples using solidified floating organic drop microextraction along with high per-formance liquid chromatography. Microchim. Acta 2012, 176, 303–309. [Google Scholar] [CrossRef]

| Analyte | Sample | Linear Equation (ng·L−1) | R2 | LOQ (ng·L−1) | Intra-Day RSD (%) (n = 6) | Inter-Day RSD (%) (n = 6) | EF |
|---|---|---|---|---|---|---|---|
| BDE-28 | Tap water | y = (0.0321 ± 0.0011)x − 0.0014 | 0.9991 | 1.0 | 5.1 | 4.5 | 101.5 |
| Lake water | y = (0.0367 ± 0.0015)x − (0.0062 ± 0.0001) | 0.9991 | 1.0 | 4.2 | 2.9 | 101.2 | |
| River water | y = (0.0336 ± 0.0012)x − (0.0044 ± 0.0001) | 0.9991 | 1.0 | 4.6 | 4.1 | 102.3 | |
| Reservoir water | y = (0.0283 ± 0.0013)x − (0.0039 ± 0.0001) | 0.9997 | 1.0 | 6.5 | 3.3 | 100.8 | |
| BDE-47 | Tap water | y = (0.0193 ± 0.0007)x + 0.0019 | 0.9998 | 1.0 | 2.2 | 3.8 | 100.6 |
| Lake water | y = (0.0204 ± 0.0012)x − 0.0006 | 0.9999 | 1.0 | 3.8 | 4.2 | 102.5 | |
| River water | y = (0.0193 ± 0.0009)x + 0.0009 | 0.9999 | 1.0 | 3.1 | 6.5 | 101.9 | |
| Reservoir water | y = (0.0176 ± 0.0010)x + 0.0005 | 0.9999 | 1.0 | 5.2 | 5.1 | 101.7 | |
| BDE-100 | Tap water | y = (0.0205 ± 0.0009)x + 0.0016 | 0.9999 | 1.0 | 4.9 | 5.6 | 98.6 |
| Lake water | y = (0.0214 ± 0.0014)x + 0.0007 | 0.9999 | 1.0 | 5.6 | 4.3 | 99.1 | |
| River water | y = (0.0210 ± 0.0007)x + 0.0004 | 0.9999 | 1.0 | 4.6 | 6.9 | 98.4 | |
| Reservoir water | y = (0.0192 ± 0.0009)x − 0.0007 | 0.9999 | 1.0 | 3.9 | 3.1 | 99.2 | |
| BDE-99 | Tap water | y = (0.0182 ± 0.0008)x + 0.0009 | 0.9999 | 1.0 | 3.2 | 5.2 | 95.2 |
| Lake water | y = (0.0193 ± 0.0006)x + 0.0015 | 0.9998 | 1.0 | 2.5 | 4.9 | 94.6 | |
| River water | y = (0.0190 ± 0.0008)x + 0.001 | 0.9999 | 1.0 | 3.8 | 3.2 | 95.7 | |
| Reservoir water | y = (0.0170 ± 0.0009)x + 0.0006 | 0.9999 | 1.0 | 2.4 | 4.6 | 94.1 | |
| BDE-154 | Tap water | y = (0.0147 ± 0.0011)x + (0.0015 ± 0.0001) | 0.9999 | 2.0 | 6.1 | 5.9 | 70.6 |
| Lake water | y = (0.0177 ± 0.0008)x − (0.0033 ± 0.0001) | 0.9999 | 2.0 | 5.8 | 7.6 | 68.5 | |
| River water | y = (0.0160 ± 0.0007)x − 0.0008 | 0.9992 | 2.0 | 7.6 | 4.2 | 71.2 | |
| Reservoir water | y = (0.0146 ± 0.0004)x + 0.0015 | 0.9999 | 2.0 | 2.3 | 3.4 | 70.5 | |
| BDE-153 | Tap water | y = (0.0137 ± 0.0010)x + 0.0004 | 0.9997 | 2.0 | 3.4 | 2.8 | 88.6 |
| Lake water | y = (0.0150 ± 0.0006)x − 0.0001 | 0.9998 | 2.0 | 1.9 | 8.2 | 87.9 | |
| River water | y = (0.0132 ± 0.0008)x + (0.0032 ± 0.0001) | 0.9998 | 2.0 | 3.4 | 4.8 | 89.2 | |
| Reservoir water | y = (0.0134 ± 0.0003)x − 0.0014 | 0.9999 | 2.0 | 2.8 | 6.7 | 88.1 | |
| BDE-183 | Tap water | y = (0.0097 ± 0.0006)x − 0.0002 | 0.9999 | 2.0 | 2.3 | 2.3 | 91.8 |
| Lake water | y = (0.0101 ± 0.0004)x − 0.0022 | 0.9999 | 2.0 | 4.5 | 7.5 | 93.5 | |
| River water | y = (0.0089 ± 0.0004)x − 0.0012 | 0.9999 | 2.0 | 6.6 | 3.4 | 92.6 | |
| Reservoir water | y = (0.0090 ± 0.0005)x − 0.0010 | 0.9999 | 2.0 | 4.8 | 3.4 | 93.1 | |
| BDE-209 | Tap water | y = (0.0045 ± 0.0001)x − 0.0004 | 0.9999 | 5.0 | 3.5 | 5.9 | 100.3 |
| Lake water | y = (0.0042 ± 0.0001)x + (0.0062 ± 0.0001) | 0.9999 | 5.0 | 2.8 | 2.6 | 101.6 | |
| River water | y = (0.0045 ± 0.0002)x − (0.0024 ± 0.0001) | 0.9999 | 5.0 | 5.1 | 1.8 | 102.5 | |
| Reservoir water | y = (0.0037 ± 0.0002)x + 0.0007 | 0.9999 | 5.0 | 3.8 | 3.2 | 101.4 |
| Tap Water | Lake Water | River Water | Reservoir Water | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Analyte | Concentration (ng·L−1) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | Recovery (%) | RSD (%) |
| BDE-28 | 1 | 101.1 | 5.8 | 100.5 | 4.6 | 99.8 | 4.7 | 100.3 | 3.8 |
| 10 | 99.8 | 3.6 | 102.1 | 6.5 | 100.4 | 4.6 | 102.6 | 5.1 | |
| 100 | 100.6 | 2.5 | 101.4 | 4.5 | 99.5 | 4.8 | 102.4 | 4.3 | |
| BDE-47 | 1 | 101.3 | 4.3 | 99.5 | 7.6 | 100.6 | 4.8 | 101.5 | 1.9 |
| 10 | 100.1 | 6.7 | 99.1 | 4.8 | 99.8 | 7.1 | 100.7 | 5.6 | |
| 100 | 100.8 | 4.2 | 100.3 | 3.5 | 101.1 | 4.5 | 100.9 | 4.2 | |
| BDE-100 | 1 | 98.4 | 3.9 | 95.9 | 4.5 | 97.2 | 3.5 | 96.5 | 5.1 |
| 10 | 96.5 | 4.5 | 98.7 | 4.1 | 96.8 | 5.4 | 99.2 | 2.1 | |
| 100 | 99.1 | 4.2 | 97.2 | 4.8 | 98.9 | 4.8 | 98.3 | 5.6 | |
| BDE-99 | 1 | 95.5 | 2.8 | 93.7 | 2.2 | 95.3 | 3.5 | 95.2 | 4.6 |
| 10 | 97.2 | 3.1 | 94.2 | 6.2 | 93.1 | 4.3 | 94.5 | 3.8 | |
| 100 | 96.8 | 3.2 | 94.5 | 4.3 | 94.8 | 4.5 | 93.8 | 3.4 | |
| BDE-154 | 2 | 71.2 | 4.1 | 68.2 | 8.5 | 67.2 | 5.9 | 70.1 | 5.7 |
| 20 | 69.4 | 7.6 | 70.3 | 7.8 | 72.1 | 6.8 | 68.5 | 6.4 | |
| 200 | 70.6 | 3.9 | 70.8 | 5.1 | 71.6 | 8.4 | 69.2 | 4.6 | |
| BDE-153 | 2 | 90.1 | 4.5 | 87.6 | 3.1 | 88.1 | 3.5 | 89.2 | 5.2 |
| 20 | 87.7 | 3.6 | 88.9 | 4.8 | 90.2 | 4.5 | 87.6 | 4.5 | |
| 200 | 88.6 | 3.4 | 88.6 | 3.6 | 89.4 | 4.2 | 89.5 | 8.5 | |
| BDE-183 | 2 | 91.9 | 5.4 | 91.3 | 6.4 | 93.5 | 2.8 | 91.8 | 7.8 |
| 20 | 92.9 | 3.5 | 94.3 | 4.8 | 91.6 | 6.1 | 93.2 | 5.6 | |
| 200 | 93.2 | 6.5 | 92.6 | 5.3 | 93.1 | 4.6 | 92.8 | 4.5 | |
| BDE-209 | 5 | 99.5 | 4.9 | 98.6 | 5.2 | 98.4 | 4.3 | 99.2 | 5.8 |
| 50 | 101.8 | 3.1 | 101.3 | 3.1 | 100.4 | 3.6 | 100.6 | 3.6 | |
| 500 | 100.6 | 5.6 | 99.8 | 3.2 | 99.5 | 2.4 | 101.4 | 4.3 | |
| Methods | Dispersive Solvent | Sample Volume (mL) | Extraction Time (min) | Centrifuge Time (min) | Solidification Time (min) | Injection Time (min) | Linearity Range (ng·L−1) | Recovery(%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| UA-DLLME a-GC-NCI-MS | Acetone (1000 μL) | 5 | 2 | 5 | / | 17.5 | 1.0–200 (BDEs 28, 47, 99, and 100); 5.0–200 (BDEs 153, 154, and 183); 5.0–500 (BDE 209) | 70.6–105.1 | [45] |
| SFOME b-HPLC-VWD | / | 40 | 25 | / | 10 | 26.3 | 500–75,000 (BDEs 28, 47, 99, 154, and 183); 5000–500,000 (BDE 209) | 92.0–118.0 | [47] |
| DLLME c-HPLC-VWD | Acetonitrile (1000 μL) | 5 | <0.5 (equilibrium 1 h) | 5 | / | 32.0 | 50–50,000 (BDEs 28 and 99); 100–100,000 (BDEs 47 and 209) | 87.0–119.1 | [43] |
| DLLME c-GC-EI-MS | Acetonitrile (1000 μL) | 25 | <0.5 | 5 | / | 27.0 | 5.0–10,000 (BDE 100) | 91.0–107.0 | [19] |
| EA-DLLME-SAP d-GC-MS-MS | / | 5 | <0.5 | 5 | 10 | 13.0 | 1.0–100 (BDEs 28, 47, 99 and 100); 2.0–200 (BDEs 153, 154, and 183); 5.0–500 (BDE 209) | 67.2–102.6 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Q.; Chen, S.; Cheng, L.; Jing, X.; Wang, X.; Guan, S.; Song, W.; Rao, Q. Determination of Polybrominated Diphenyl Ethers in Water Samples Using Effervescent-Assisted Dispersive Liquid-Liquid Icroextraction with Solidification of the Aqueous Phase. Molecules 2021, 26, 1376. https://doi.org/10.3390/molecules26051376
Wang Y, Zhang Q, Chen S, Cheng L, Jing X, Wang X, Guan S, Song W, Rao Q. Determination of Polybrominated Diphenyl Ethers in Water Samples Using Effervescent-Assisted Dispersive Liquid-Liquid Icroextraction with Solidification of the Aqueous Phase. Molecules. 2021; 26(5):1376. https://doi.org/10.3390/molecules26051376
Chicago/Turabian StyleWang, Yue, Qicai Zhang, Shanshan Chen, Lin Cheng, Xu Jing, Xianli Wang, Shuhui Guan, Weiguo Song, and Qinxiong Rao. 2021. "Determination of Polybrominated Diphenyl Ethers in Water Samples Using Effervescent-Assisted Dispersive Liquid-Liquid Icroextraction with Solidification of the Aqueous Phase" Molecules 26, no. 5: 1376. https://doi.org/10.3390/molecules26051376
APA StyleWang, Y., Zhang, Q., Chen, S., Cheng, L., Jing, X., Wang, X., Guan, S., Song, W., & Rao, Q. (2021). Determination of Polybrominated Diphenyl Ethers in Water Samples Using Effervescent-Assisted Dispersive Liquid-Liquid Icroextraction with Solidification of the Aqueous Phase. Molecules, 26(5), 1376. https://doi.org/10.3390/molecules26051376







