Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars
Abstract
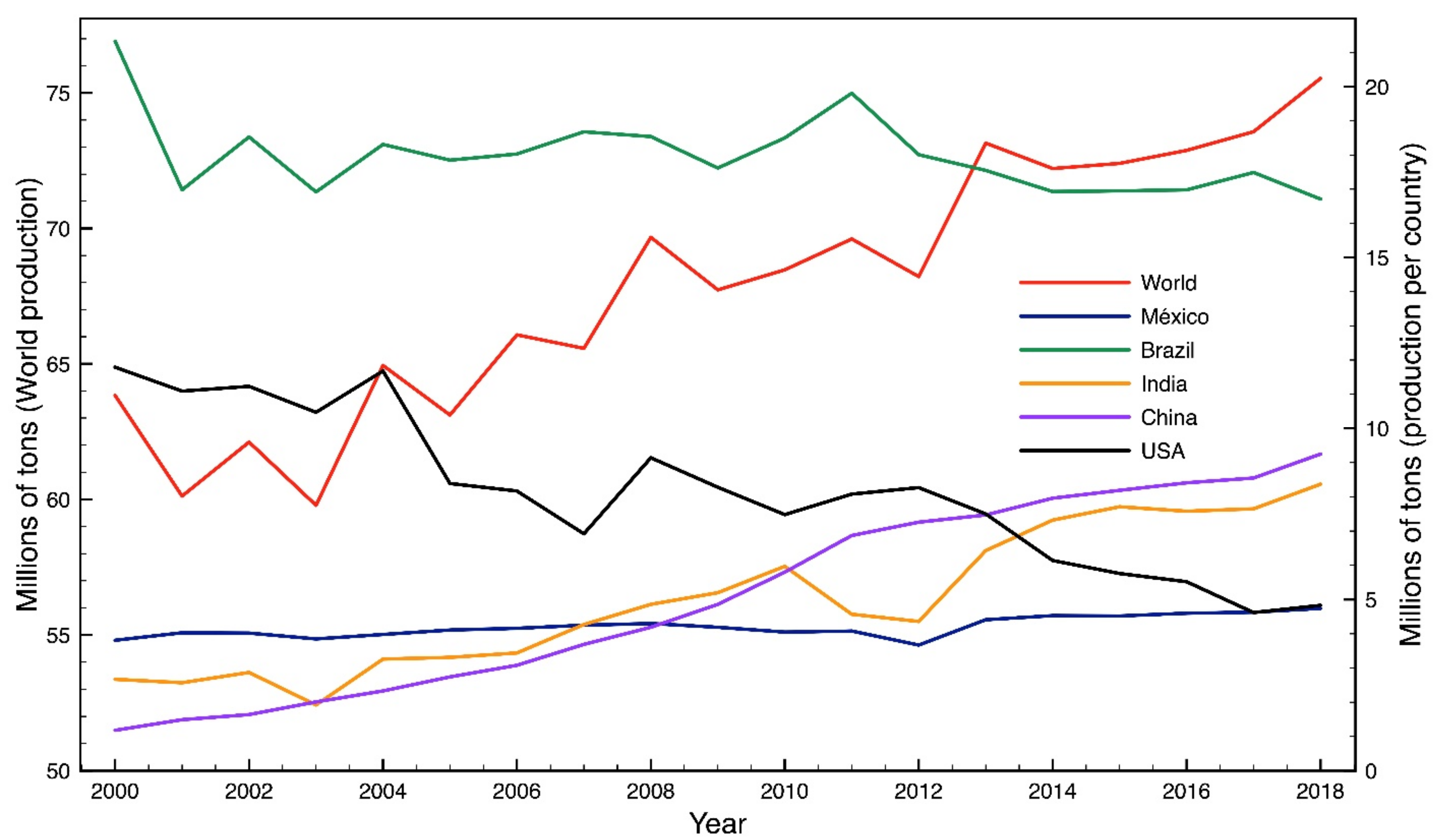
1. Introduction
2. Results
2.1. Results for the Proximate and Chemical Analysis of Orange Peel
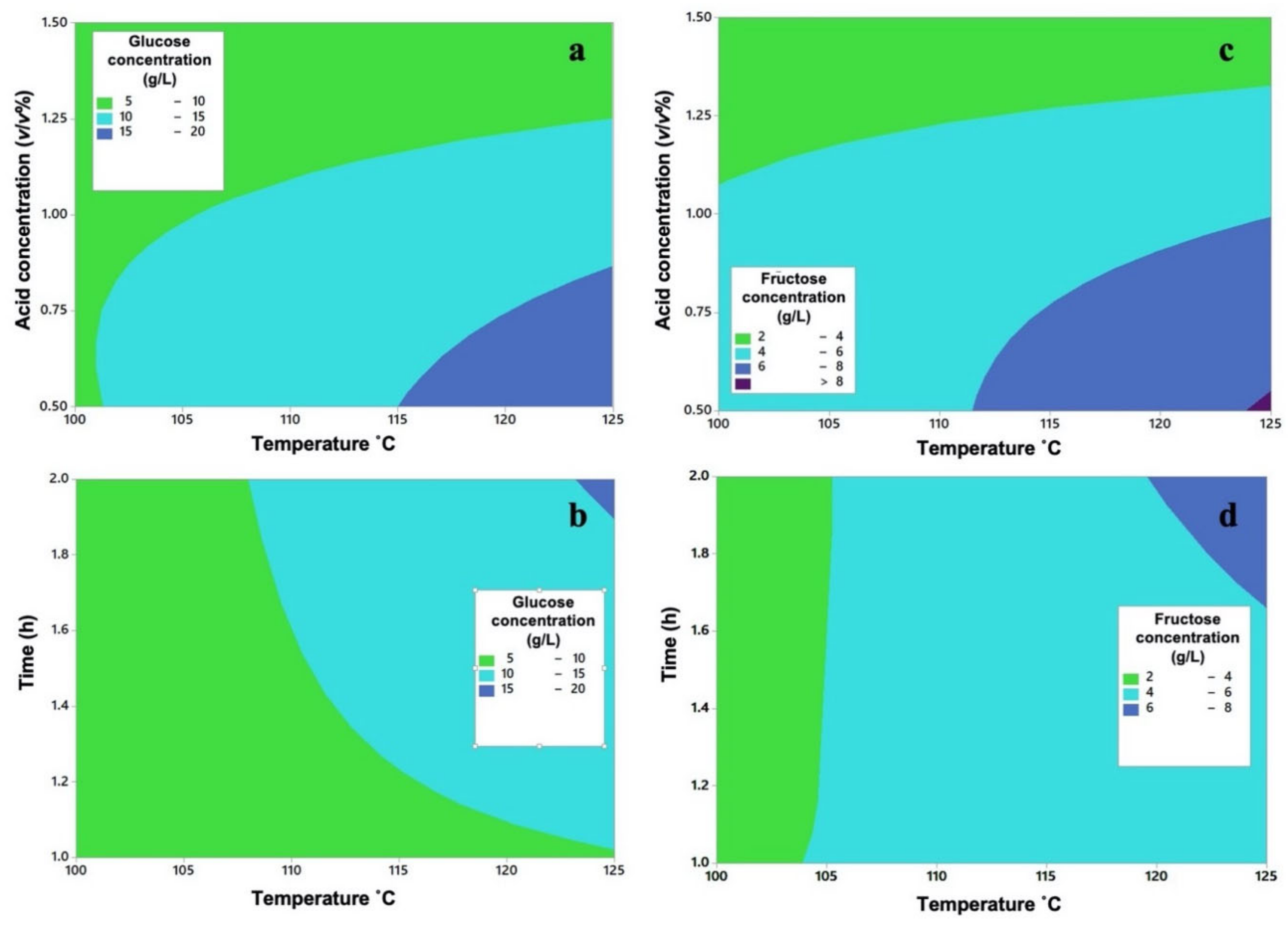
2.2. Design of Experiments of the Dilute Acid Hydrolysis
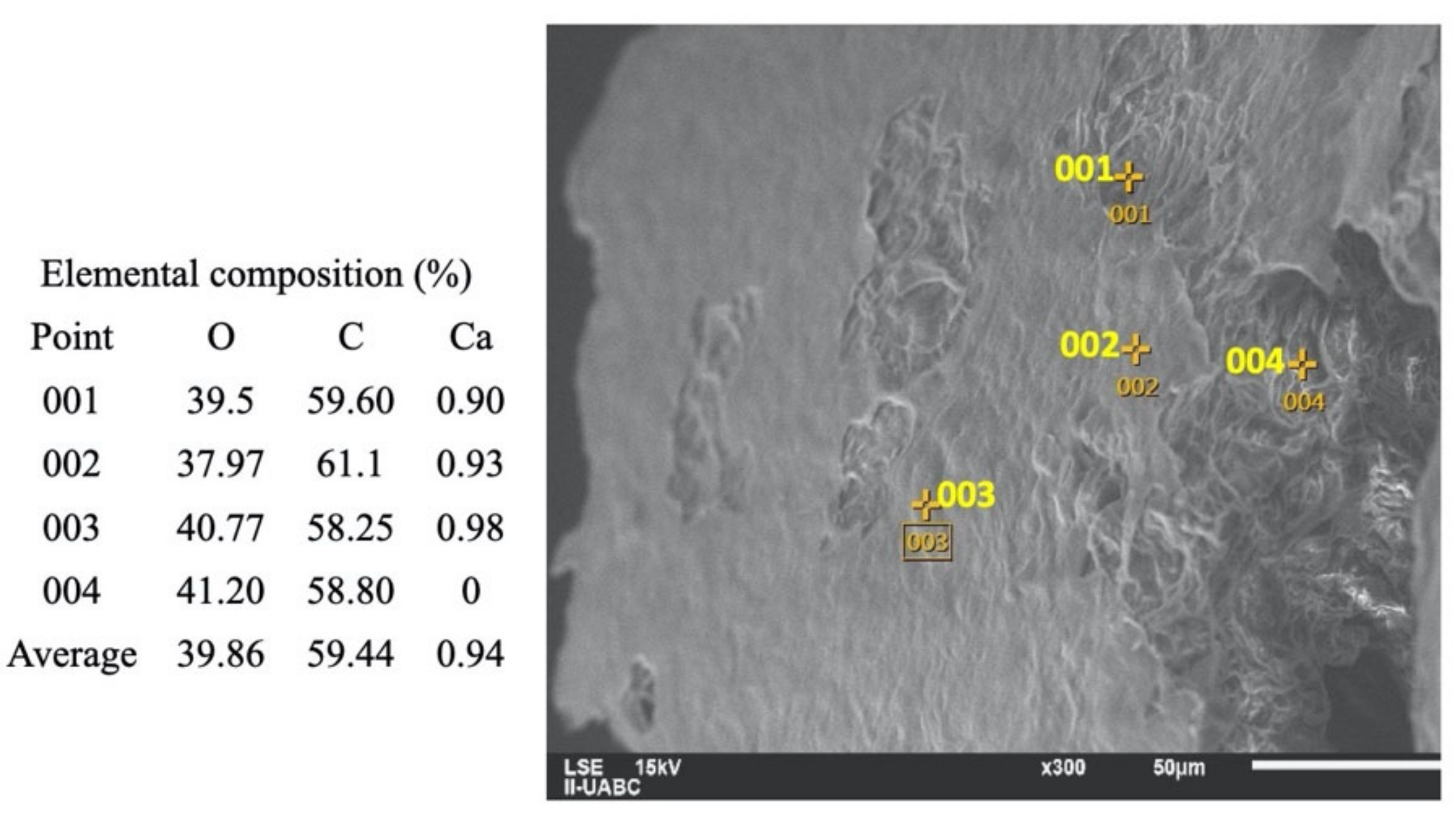
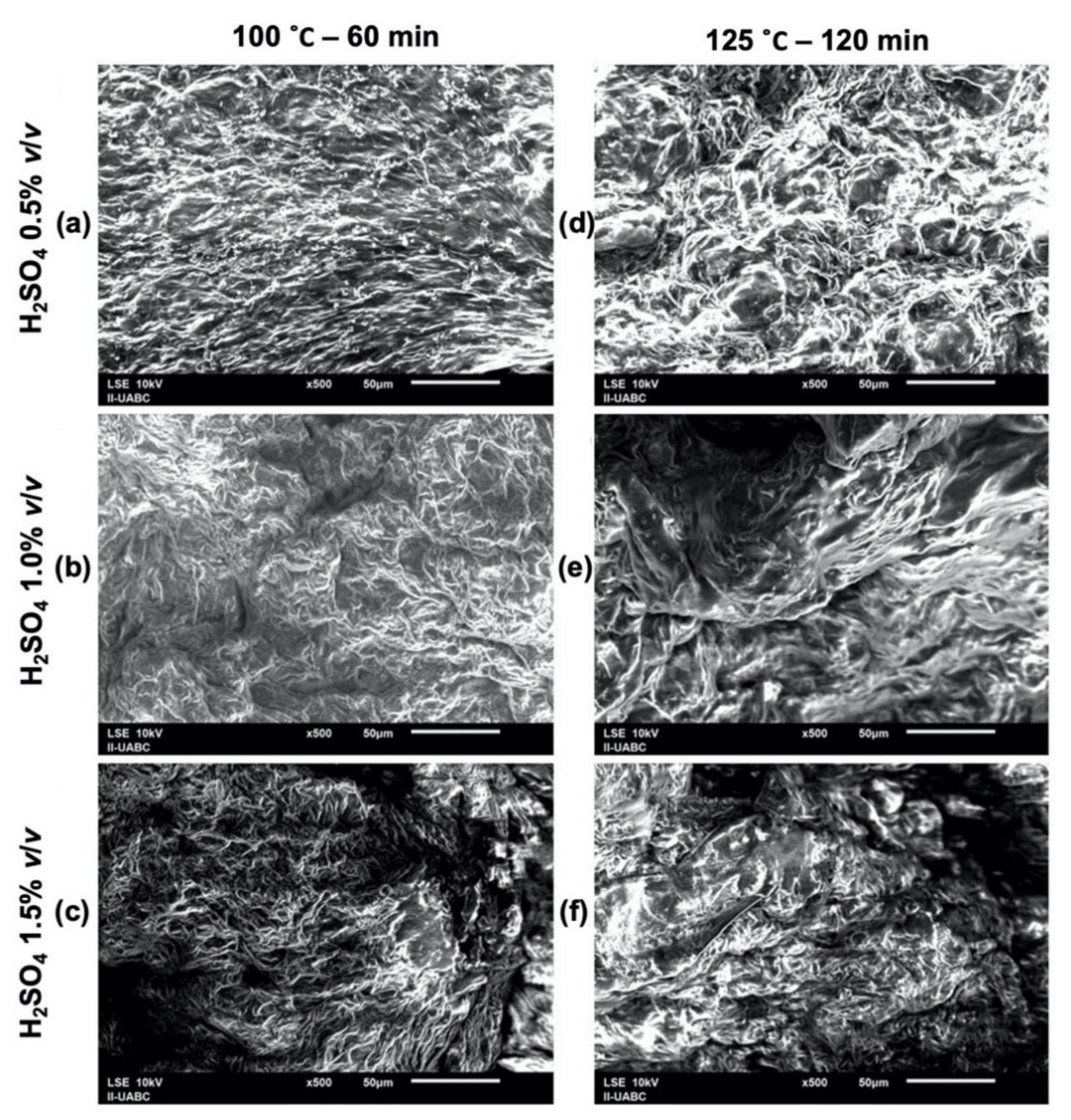
2.3. SEM-EDS Analyses
3. Discussion
4. Materials and Methods
4.1. Proximate and Chemical Analysis of Orange Peel
4.2. Design of Experiments for the Orange Peel Diluted Acid Hydrolysis
4.3. SEM-EDS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Food and Agriculture Organization FAOSTAT. Food Production. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 24 June 2020).
- Sistema de Información Agroalimentaria y Pesquera (SIAP). Cierre de la Producción por Estado. 2020. Available online: http://infosiap.siap.gob.mx:8080/agricola_siap_gobmx/AvanceNacionalCultivo.do (accessed on 24 June 2020).
- Gaind, S. Exploitation of orange peel for fungal solubilization of rock phosphate by solid state fermentation. Waste Biomass Valor. 2016, 8, 1351–1360. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A. Techno-economic and environmental assessment of p-Cymene and pectin production from orange peel. Waste Biomass Valor. 2015, 6, 253–261. [Google Scholar] [CrossRef]
- Siles, J.A.; Li, Q.; Thompson, I.P. Biorefinery of waste orange peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef]
- Aboagye, D.; Banadda, N.; Kiggundu, N.; Kabenge, I. Assessment of orange peel waste availability in Ghana and potential bio-oil yield using fast pyrolysis. Renew. Sust. Energ. Rev. 2017, 70, 814–821. [Google Scholar] [CrossRef]
- Maschke, R.W.; Geipel, K.; Bley, T. Modeling of plant in vitro cultures: Overview and estimation of biotechnological processes. Biotechno. Bioeng. 2015, 112, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Santi, G.; Crognale, S.; Annibale, A.D.; Petruccioli, M.; Ruzzi, M.; Valentini, R.; Moresi, M. Orange peel pretreatment in a novel lab-scale direct steam-injection apparatus for ethanol production. Biomass Bioenerg. 2014, 61, 146–156. [Google Scholar] [CrossRef]
- Molina-Calle, M.; Priego-Capote, F.; Luque de Castro, M.D. Development and application of a quantitative method for determination of flavonoids in orange peel: Influence of sample pretreatment on composition. Talanta 2015, 144, 349–355. [Google Scholar] [CrossRef]
- Voros, V.; Drioli, E.; Fonte, C.; Szekely, G.; Fonte, C.; Szekely, G. Process Intensification via Continuous and Simultaneous Isolation of Antioxidants: An Upcycling Approach for Olive Leaf Waste. ACS Sustain. Chem. Eng. 2019, 7, 18444–18452. [Google Scholar] [CrossRef]
- Sarkar, O.; Katakojwala, R.; Mohan, S.V. Low Carbon Hydrogen production from waste based Biorefinery System and Environmental Sustainability Assessment. Green Chem. 2021, 23, 561–574. [Google Scholar] [CrossRef]
- Deng, J.-J.; Zhang, M.-S.; Li, Z.-W.; Lu, D.L.; Mao, H.-H.; Zhu, M.-J.; Li, J.-Z.; Luo, X.-C. One-step processing of shrimp shell waste with a chitinase fused to a carbohydrate-binding module. Green Chem. 2020, 22, 6862–6873. [Google Scholar] [CrossRef]
- Ayala, J.R.; Montero, G.; Campbell, H.E.; García, C.; Coronado, M.A.; León, J.A.; Sagaste, C.A.; Pérez, L.J. Extraction and Characterization of Orange Peel Essential Oil from Mexico and United States of America. J. Essent. Oil. Bear. Plants 2017, 20, 897–914. [Google Scholar] [CrossRef]
- Chen, W.-H.; Jang, M.-F.; Jheng, S.-L.; Lo, C.-J.; Wang, W. Cellulosic sugars from biomass: Effect of acid presoaking on pretreatment efficiency and operating cost estimation for sugar production. Bioresour. Technol. Rep. 2019, 7, 100259. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Wu, Z.-Y.; Sriariyanun, M. Evaluation of Macaranga tanarius as a biomass feedstock for fermentable sugars production. Bioresour. Technol. 2019, 294, 122195. [Google Scholar] [CrossRef] [PubMed]
- Kassaye, S.; Pant, K.K.; Jain, S. Hydrolysis of cellulosic bamboo biomass into reducing sugars via a combined alkaline solution and ionic liquid pretreatment steps. Renew. Energ. 2017, 104, 177–184. [Google Scholar] [CrossRef]
- Khamis, N.A.; Shamsudin, S.; Rahman, N.S.A.; Kasim, K.F. Effects of autohydrolysis on rice biomass for reducing sugars production. Mater. Today Proc. 2019, 16, 2078–2087. [Google Scholar] [CrossRef]
- Láinez, M.; Héctor, A.; Ruiz, H.A.; Castro-Luna, A.A.; Martínez-Hernández, S. Release of simple sugars from lignocellulosic biomass of Agave salmiana leaves subject to sequential pretreatment and enzymatic saccharification. Biomass Bioenerg. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Martínez, C.M.; Adamovic, T.; Cantero, D.A.; Cocero, M.J. Scaling up the production of sugars from agricultural biomass by ultrafast hydrolysis in supercritical water. J. Supercrit. Fluid. 2019, 143, 242–250. [Google Scholar] [CrossRef]
- Timung, R.; Goud, V.V. Subcritical water hydrolysis of spent Java Citronella biomass for production of reducing sugar. Mater. Today Proc. 2018, 5, 23128–23135. [Google Scholar] [CrossRef]
- Elliston, A.; Collins, S.R.A.; Faulds, C.B.; Roberts, I.N.; Waldron, K.W. Biorefining of waste paper biomass: Increasing the concentration of glucose by optimizing enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2014, 172, 3621–3634. [Google Scholar] [CrossRef]
- Modenbach, A.A.; Nokes, S.E. The use of high-solids loadings in biomass pretreatment-a review. Biotechnol. Bioeng. 2012, 6, 1430–1442. [Google Scholar] [CrossRef]
- Murthy, G.S.; Johnston, D.B.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Design and Evaluation of an Optimal Controller for Simultaneous Saccharification and Fermentation Process. Appl. Biochem. Biotechnol. 2012, 166, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, M.; Joshi, M.; Adivarekar, R. Bioscouring of cotton using lipase from marine bacteria bacillus sonorensis. Appl. Biochem. Biotechnol. 2015, 175, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Buyukkileci, A.O.; Fernandez, M.M.L.; Tari, C. Utilization of orange peel, a food industrial waste, in the production of exo-polygalacturonase by pellet forming Aspergillus sojae. Bioprocess. Biosyst. Eng. 2015, 38, 749–760. [Google Scholar] [CrossRef]
- Tai, C.; Keshwani, D. Impact of pretreatment with dilute sulfuric acid under moderate temperature on hydrolysis of corn stover with two enzyme systems. Appl. Biochem. Biotechnol. 2014, 172, 2628–2639. [Google Scholar] [CrossRef]
- Chaiprapat, S.; Wongchana, S.; Loykulnant, S.; Kongkaew, C.; Charnnok, B. Evaluating sulfuric acid reduction, substitution, and recovery to improve environmental performance and biogas productivity in rubber latex industry. Process. Saf. Environ. 2015, 94, 420–429. [Google Scholar] [CrossRef]
- Lindgren, M.; Siljander, S.; Suihkonen, R.; Pohjanne, P.; Vuorinen, J. Erosion-corrosion resistance of various stainless steel grades in high-temperature sulfuric acid solution. Wear 2016, 364–365, 10–21. [Google Scholar] [CrossRef]
- Jin, M.; Sarks, C.; Bals, B.D.; Posawatz, N.; Gunawan, C.; Dale, B.E.; Balan, V. Toward high solids loading process for lignocellulosic biofuel production at a low cost. Biotechnol. Bioeng. 2016, 5, 980–988. [Google Scholar] [CrossRef]
- Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples. Available online: https://www.nrel.gov/docs/gen/fy08/42621.pdf (accessed on 20 August 2018).
- ASTM International. ASTM E872-82–Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM International. ASTM E830-87–Standard Test Method for Ash in the Analysis Sample of Refuse Derived Fuel; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- TAPPI. Preparation of Wood for Chemical Analysis Test Method T264 cm-07 (TAPPI); TAPPI: Peachtree Corners, GA, USA, 2007. [Google Scholar]
- TAPPI. Water Solubility of Wood and Pulp Test Method T207 cm-99 (TAPPI); TAPPI: Peachtree Corners, GA, USA, 1999. [Google Scholar]
- ASTM International. ASTM D1106-96–Standard Test Method for Acid-Insoluble Lignin in Wood; ASTM International: West Conshohocken, PA, USA, 2001. [Google Scholar]
- ASTM International. ASTM D1104-56–Method of Test for Hollocelulose in Wood; ASTM International: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Rowell, R.M.; Pettersen, R.; Tshabalala, M.A. Handbook of Wood Chemistry and Wood Composites, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2013; pp. 33–74. [Google Scholar]
- Chong, Y.; Yan, A.; Yang, X.; Cai, Y.; Chen, J. An optimum fermentation model established by genetic algorithm for biotransformation from crude polydatin to resveratrol. Appl. Biochem. Biotechnol. 2012, 166, 446–457. [Google Scholar] [CrossRef]
- Dai, J.-Y.; Zhao, P.; Cheng, X.-L.; Xiu, Z.-L. Enhanced production of 2,3-Butanediol from sugarcane molasses. Appl. Biochem. Biotechnol. 2015, 175, 3014–3024. [Google Scholar] [CrossRef]
- Bustamante, J.; Stempvoort, S.; García-Gallarreta, M.; Houghton, J.A.; Briers, H.K.; Budarin, V.L.; Matharu, A.S.; Clark, J.H. Microwave assisted hydro-distillation of essential oils from wet citrus peel waste. J. Clean. Prod. 2016, 137, 598–605. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis). Flavour Fragr. J. 2004, 20, 80–85. [Google Scholar] [CrossRef]
- Ji, W.; Shen, Z.; Wen, Y. Hydrolysis of wheat straw by dilute sulfuric acid in a continuous mode. Chem. Eng. J. 2015, 260, 20–27. [Google Scholar] [CrossRef]
- García, J.F.M.; Sánchez, S.; Cuevas, M. Evaluation of the effect of the dilute acid hydrolysis on sugars release from olive prunings. Renew. Energ. 2013, 51, 382–387. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Julio-Altamiranda, Y.T.; Mercado-Pacheco, J.D.; Sánchez-Tuirán, E.L.; González-Delgado, A.D. Evaluation of mechanical-green solvent pretreatment of oil palm wastes for reducing sugars production in North-Colombia. Sustain. Chem. Pharm. 2020, 16, 100256. [Google Scholar] [CrossRef]
- Manmai, N.; Unpaprom, Y.; Ponnusamy, V.K.; Ramaraj, R. Bioethanol production from the comparison between optimization of sorghum stalk and sugarcane leaf for sugar production by chemical pretreatment and enzymatic degradation. Fuel 2020, 278, 118262. [Google Scholar] [CrossRef]
- Martins, E.H.; Ratuchne, A.; Machado, G.O.; Knob, A. Canola meal as a promising source of fermentable sugars: Potential of the Penicillium glabrum crude extract for biomass hydrolysis. Biocatal. Agric. Biotechnol. 2020, 27, 101713. [Google Scholar] [CrossRef]
- Wang, H.; Srinivasan, R.; Yu, F.; Steele, P.; Li, Q.; Mitchell, B.; Samala, A. Effect of acid, steam explosion, and size reduction pretreatments on Bio-oil production from sweetgum, switchgrass, and corn stover. Appl. Biochem. Biotechnol. 2012, 167, 285–287. [Google Scholar] [CrossRef] [PubMed]
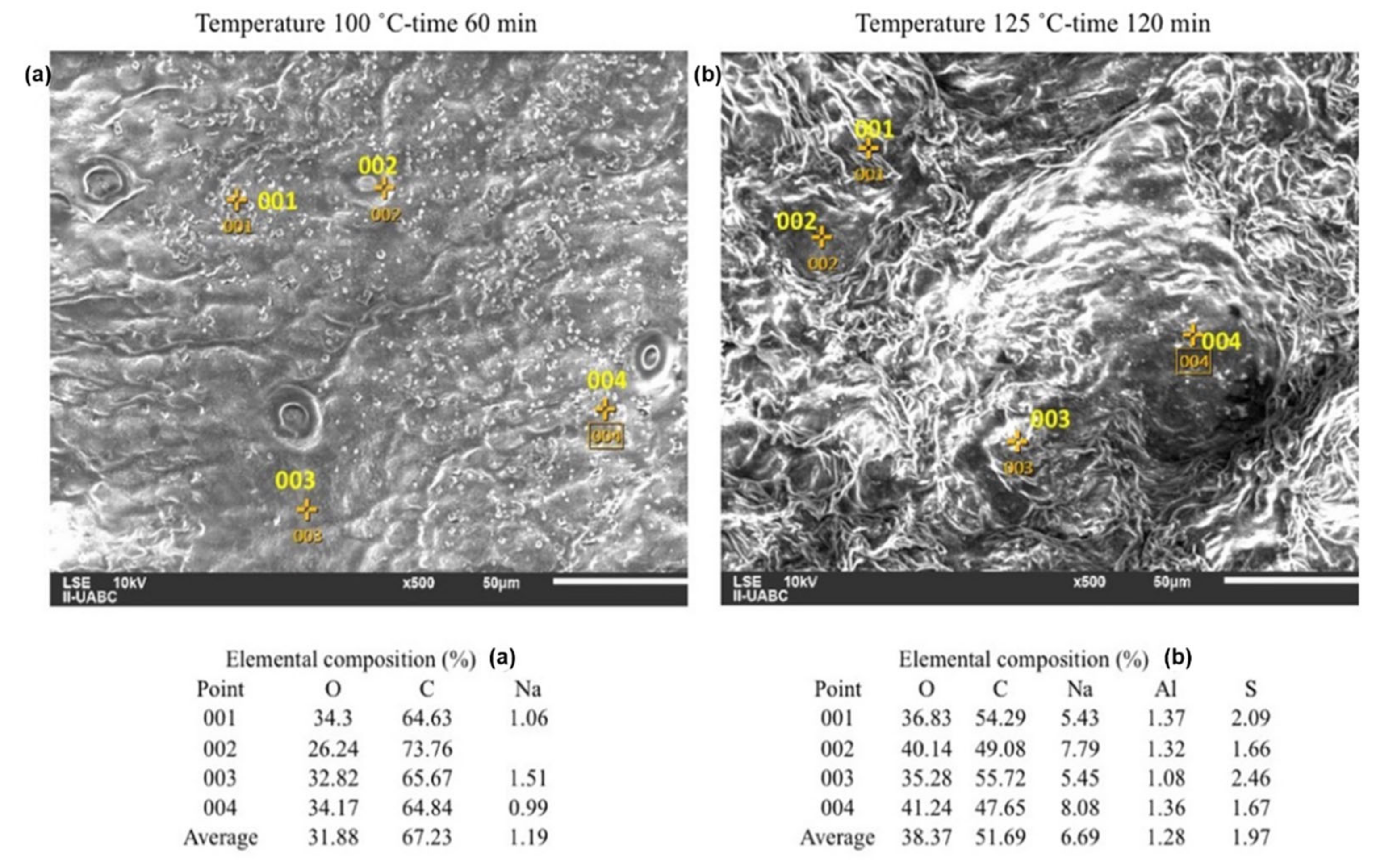
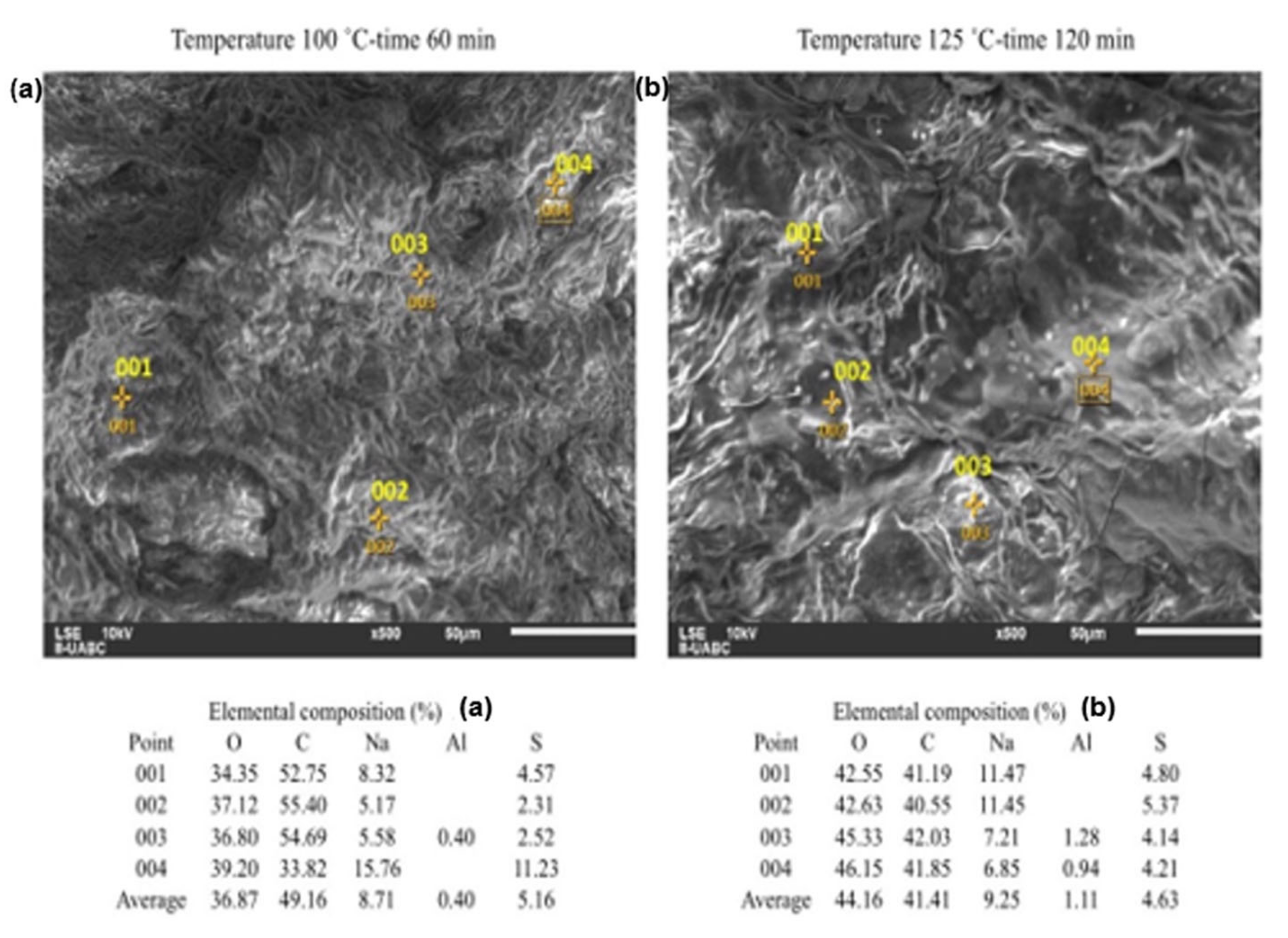
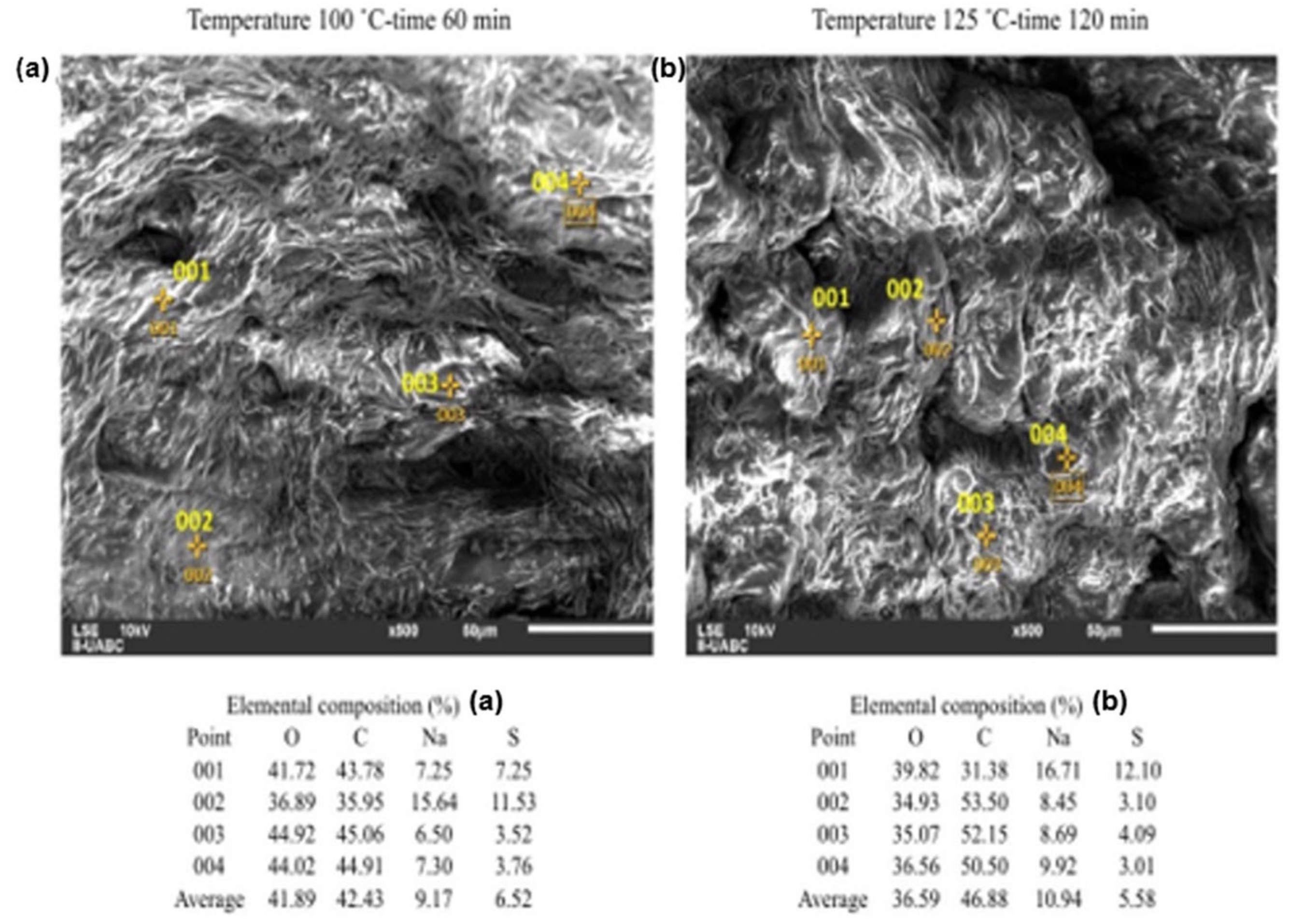
| Analysis | Composition (% in Weight) | Standard Deviation | |
|---|---|---|---|
| Proximate | Moisture | 73.530% | 0.477% |
| Volatiles | 99.261% | 0.074% | |
| Ash | 0.052% | 0.004% | |
| Fixed carbon | 0.687% | 0.078% | |
| Chemical | Acetone extractables | 6.821% | 0.604% |
| Hot water extractables | 40.399% | 2.595% | |
| Lignin determination | 19.801% | 3.595% | |
| Holocellulose determination | 78.110% | 4.404% | |
| Cellulose determination | 69.096% | 9.015% | |
| Hemicellulose determination | 5.433% | 5.433% | |
| Run | H2SO4 Concentration (%v/v) (A) | Temperature (°C) (B) | Time (h) (C) | Glucose (g/L) | Average Glucose (g/L) | Standard Deviation (Glucose) | Fructose (g/L) | Average Fructose (g/L) | Standard Deviation (Fructose) |
|---|---|---|---|---|---|---|---|---|---|
| 1,2 | 0.5 | 100 | 1 | 11.302 10.016 | 10.659 | 0.909 | 4.651 5.092 | 4.871 | 0.312 |
| 3,4 | 0.5 | 100 | 2 | 7.940 8.821 | 8.380 | 0.623 | 3.367 3.499 | 3.433 | 0.093 |
| 5,6 | 0.5 | 125 | 1 | 13.843 17.009 | 15.426 | 2.239 | 5.242 8.936 | 7.089 | 2.612 |
| 7,8 | 0.5 | 125 | 2 | 24.585 19.189 | 21.887 | 3.816 | 9.709 8.862 | 9.285 | 0.599 |
| 9,10 | 1 | 100 | 1 | 8.212 7.998 | 8.105 | 0.151 | 4.231 3.184 | 3.707 | 0.740 |
| 11,12 | 1 | 100 | 2 | 9.500 10.309 | 9.904 | 0.572 | 3.580 5.600 | 4.59 | 1.428 |
| 13,14 | 1 | 125 | 1 | 9.686 11.373 | 10.529 | 1.193 | 4.049 5.090 | 4.569 | 0.736 |
| 15,16 | 1 | 125 | 2 | 16.727 15.826 | 16.276 | 0.637 | 6.803 7.908 | 7.355 | 0.781 |
| 17,18 | 1.5 | 100 | 1 | 7.080 6.039 | 6.559 | 0.736 | 3.880 2.377 | 3.128 | 1.063 |
| 19,20 | 1.5 | 100 | 2 | 3.397 4.192 | 3.794 | 0.562 | 1.768 1.742 | 1.755 | 0.018 |
| 21,22 | 1.5 | 125 | 1 | 3.064 4.274 | 3.669 | 0.856 | 1.829 2.015 | 1.922 | 0.132 |
| 23,24 | 1.5 | 125 | 2 | 8.604 8.709 | 8.656 | 0.074 | 3.600 3.704 | 3.652 | 0.074 |
| Variation Source | Glucose | Fructose | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Square Sum | Freedom Degrees | Mean Square | F0 | F Critic | Square Sum | Freedom Degrees | Mean Square | F0 | F | |
| Critic | ||||||||||
| Acid concentration (A) | 292.829 | 2 | 146.415 | 71.54 | 3.89 | 52.911 | 2 | 26.456 | 26.06 | 3.89 |
| Temperature (B) | 140.568 | 1 | 140.568 | 68.68 | 4.75 | 25.911 | 1 | 25.577 | 25.20 | 4.75 |
| Time (C) | 32.441 | 1 | 32.441 | 15.85 | 4.75 | 3.813 | 1 | 3.813 | 3.76 | 4.75 |
| AB | 67.025 | 2 | 33.512 | 16.37 | 3.89 | 13.803 | 2 | 6.902 | 6.80 | 3.89 |
| AC | 7.251 | 2 | 3.625 | 1.77 | 3.89 | 3.267 | 2 | 1.634 | 1.61 | 3.89 |
| BC | 69.629 | 1 | 69.629 | 34.02 | 4.75 | 12.447 | 1 | 12.448 | 12.26 | 4.75 |
| ABC | 6.403 | 2 | 3.201 | 1.56 | 3.89 | 0.787 | 2 | 0.393 | 0.39 | 3.89 |
| Error | 24.560 | 12 | 2.047 | 12.181 | 12 | 1.015 | ||||
| Total | 640.705 | 23 | 124.786 | 23 | ||||||
| Sample | Stage | Analysis | Conditions and Equipment | Reference |
|---|---|---|---|---|
| Orange peel | Proximate analysis | Moisture | 45 °C for 48 h inside a muffle | [30] |
| Volatiles | 950 °C for 7 min without air | [31] | ||
| Ash | 580 °C for 4 h inside a muffle | [32] | ||
| Fixed carbon | The difference in % of the sample, moisture, volatiles and ash analysis | [32] | ||
| Chemical analysis | Acetone extractable | 8 h Soxhlet extraction with acetone | [33] | |
| Water extractable | 3 h boiling water with condenser reflux | [34] | ||
| Lignin % | 4 h, 15 mL of H2SO4, stirring and 560 mL of distilled water | [35] | ||
| Holocellulose % | 150 mL water, 0.2 mL of acetic acid and 1 g of sodium chlorite per hour for 4 h. | [36] | ||
| Cellulose % | 25 mL of NaOH at 17.5 %, 100 mL of NaOH at 8.3%, 10 mL of acetic acid and water for 105 min. | [37] | ||
| Essential oil extraction | Hydrodistillation | 65 g of orange peel, 90 min, orange peel grinding of 1 min, 500 mL of water | [13] | |
| Hydrolysis | Diluted acid hydrolysis | H2SO4 concentration, time and temperature according to the factorial design | [38,39] | |
| pH stabilization | NaOH at 0.5 N until a pH of 4.8–5.2 was reached | [42] | ||
| Orange peel drying | 65 °C for 24 h | |||
| SEM-EDS | SEM micrography | JEOL JSM-6010LA SEM, working distance 11 mm, 10 kV, 50 Pa, 300x, 500x | [48] | |
| EDS analysis | ||||
| Hydrosylate | Reducing sugars determination | 3,5 DNS reagent | 2.5 g of 3,5-Dinitric salicylic acid, 7.5 g of mixed potassium sodium tartrate, 4 g of NaOH in 250 mL of water | [44,45,47] |
| Calibration curve | Glucose and fructose calibration curves prepared from 0.2 g/L to 2 g/L with water | [43,44] | ||
| Sample preparation | 1:10 dilution of the hydrosylate in distillated water and 1:1 diluted hydrosylate with DNS reagent | |||
| Reducing sugars quantification | Perkin-Elmer Lambda 25 UV-Vis Spectrometer at 575 nm measured in quartz cell | [43,44,47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala, J.R.; Montero, G.; Coronado, M.A.; García, C.; Curiel-Alvarez, M.A.; León, J.A.; Sagaste, C.A.; Montes, D.G. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules 2021, 26, 1348. https://doi.org/10.3390/molecules26051348
Ayala JR, Montero G, Coronado MA, García C, Curiel-Alvarez MA, León JA, Sagaste CA, Montes DG. Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules. 2021; 26(5):1348. https://doi.org/10.3390/molecules26051348
Chicago/Turabian StyleAyala, José R., Gisela Montero, Marcos A. Coronado, Conrado García, Mario A. Curiel-Alvarez, José A. León, Carlos A. Sagaste, and Daniela G. Montes. 2021. "Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars" Molecules 26, no. 5: 1348. https://doi.org/10.3390/molecules26051348
APA StyleAyala, J. R., Montero, G., Coronado, M. A., García, C., Curiel-Alvarez, M. A., León, J. A., Sagaste, C. A., & Montes, D. G. (2021). Characterization of Orange Peel Waste and Valorization to Obtain Reducing Sugars. Molecules, 26(5), 1348. https://doi.org/10.3390/molecules26051348







