Cytotoxic Flavonoids from the Leaves and Twigs of Murraya tetramera
Abstract
1. Introduction
2. Results and Discussions
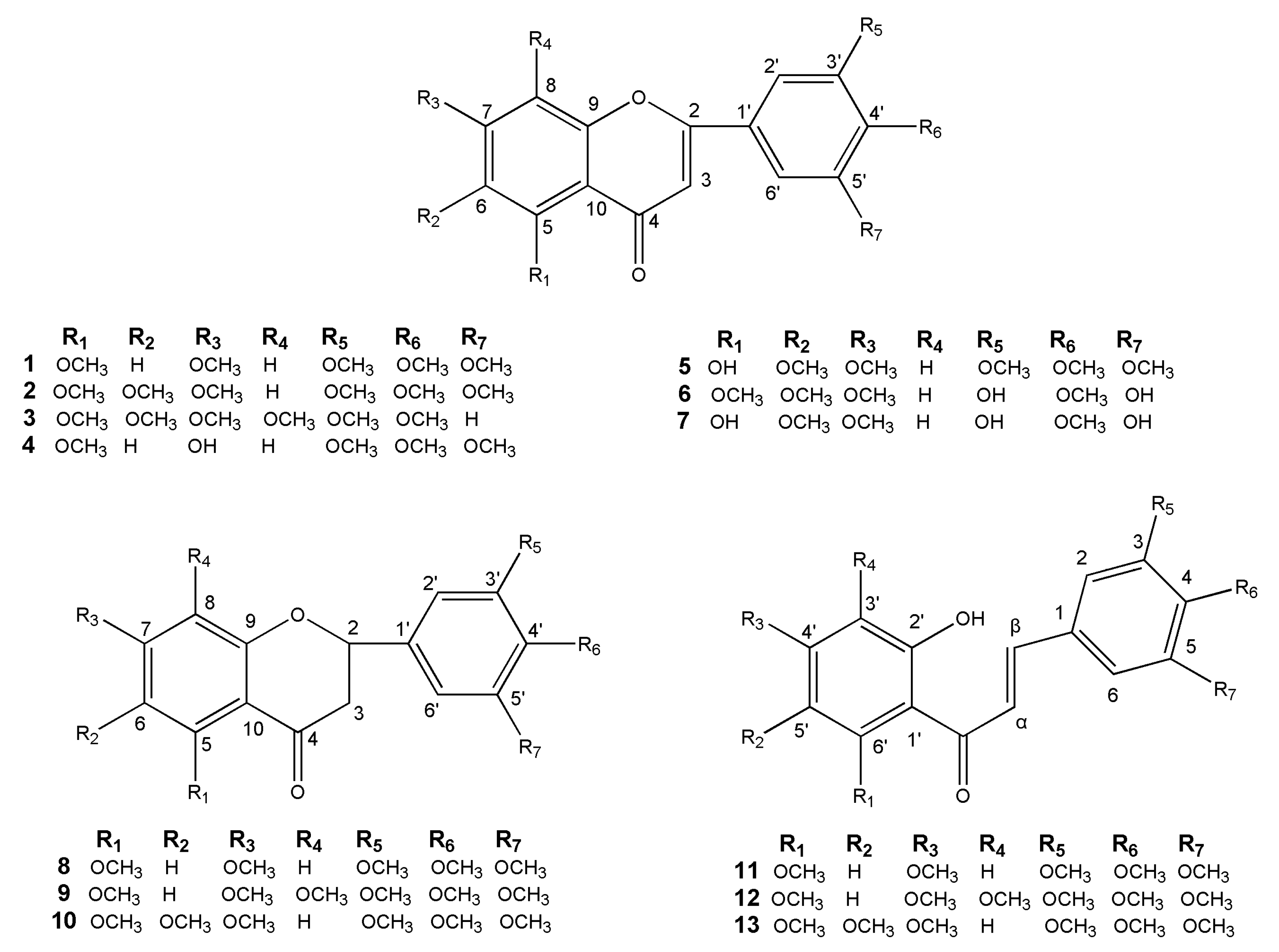
2.1. Flavonoids Isolated from M. tetramera
2.2. Structure Elucidation of the New Flavone
2.3. Cytotoxicities of Isolated Flavonoids
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. Ca-Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.; Zhang, X.L.; Lu, Y.; Zhao, L.Y.; Guo, Y.X.; Guo, S.S.; Kang, Q.Z.; Liu, J.J.; Dai, L.P.; Zhang, L.G.; et al. Protein 4.1R affects photodynamic therapy for B16 melanoma by regulating the transport of 5-aminolevulinic acid. Exp. Cell Res. 2021, 399, 112465. [Google Scholar] [CrossRef]
- Han, B.; Sha, L.J.; Yu, X.M.; Yang, M.; Cao, Y.; Zhao, J. Identification of dual therapeutic targets assisted by in situ automatous DNA assembly for combined therapy in breast cancer. Biosens. Bioelectron. 2021, 176, 112913. [Google Scholar] [CrossRef] [PubMed]
- Vo, P.H.T.; Nguyen, T.D.T.; Tran, H.T.; Nguyen, Y.N.; Doan, M.T.; Nguyen, P.H.; Lien, G.T.K.; To, D.C.; Tran, M.H. Cytotoxic components from the leaves of Erythrophleum fordii induce human acute leukemia cell apoptosis through caspase 3 activation and PARP cleavage. Bioorg. Med. Chem. Lett. 2021, 31, 127673. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Controlled Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Pan, L.; Chai, H.; Kinghorn, A.D. The continuing search for antitumor agents from higher plants. Phytochem. Lett. 2010, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, A.; Tayarani-Najaran, Z. Potent cytotoxic natural flavonoids: The limits of perspective. Curr. Pharm. Des. 2018, 24, 5555–5579. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.R.A.; Ma, T.M.T. In vitro cytotoxic screening of 300 selected Chinese medicinal herbs against human gastric adenocarcinoma SGC-7901 cells. Afr. J. Pharm. Pharmacol. 2012, 6, 592–600. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Passreiter, C.M.; Suckow-Schnitker, A.; Kulawik, A.; Addae-Kyereme, J.; Wright, C.W.; Wätjen, W. Prenylated flavanone derivatives isolated from Erythrina addisoniae are potent inducers of apoptotic cell death. Phytochemistry 2015, 117, 237–244. [Google Scholar] [CrossRef]
- Al-Ashaal, H.A.; El-Sheltawy, S.T. Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm. Biol. 2010, 49, 276–282. [Google Scholar] [CrossRef]
- Liang, H.Z.; Zhao, M.B.; Tu, P.F.; Jiang, Y. Polymethoxylated flavonoids from Murraya paniculata (L.) Jack. Biochem. Syst. Ecol. 2020, 93, 104162. [Google Scholar] [CrossRef]
- Editorial Committee of Flora of China. Flora of China; Science Press: Beijing, China, 1997; p. 145. [Google Scholar]
- Lv, H.N.; Wen, R.; Zhou, Y.; Zeng, K.W.; Li, J.; Guo, X.Y.; Tu, P.F.; Jiang, Y. Nitrogen oxide inhibitory trimeric and dimeric carbazole alkaloids from Murraya tetramera. J. Nat. Prod. 2015, 78, 2432–2439. [Google Scholar] [CrossRef]
- Zhou, Y.; Lv, H.N.; Wang, W.G.; Tu, P.F.; Jiang, Y. Flavonoids and anthraquinones from Murraya tetramera C. C. Huang (Rutaceae). Biochem. Syst. Ecol. 2014, 57, 78–80. [Google Scholar] [CrossRef]
- Lv, H.N.; Zhou, Y.; Wen, R.; Shi, M.L.; Zeng, K.W.; Xia, F.; Tu, P.F.; Jiang, Y. Murradiate and murradiol, two structurally unique heterodimers of carbazole-monoterpene and carbazole-phenylethanol from Murraya tetramera. Phytochem. Lett. 2016, 15, 113–115. [Google Scholar] [CrossRef]
- Lyu, H.N.; Zhou, Y.; Wen, R.; Tu, P.F.; Jiang, Y. Nitric oxide inhibitory carbazole alkaloids from the folk medicine Murraya tetramera C.C. Huang. Chem. Biodivers. 2020, 17, e2000490. [Google Scholar] [CrossRef] [PubMed]
- You, C.X.; Yang, K.; Wang, C.F.; Zhang, W.J.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Cytotoxic compounds isolated from Murraya tetramera Huang. Molecules 2014, 19, 13225–13234. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Wu, M.Z.; Chen, H.P.; Liu, Y.P. Compounds isolated from Murraya tetramera Huang and their cytotoxic activity. Nat. Prod. Res. Dev. 2019, 31, 627–632. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Chen, H.P.; Chen, L.; Liu, Y.P.; Li, Y. Carbazole alkaloids from Murraya tetramera Huang and their cytotoxic activity. Nat. Prod. Res. Dev. 2019, 31, 269–272, 305. [Google Scholar] [CrossRef]
- Mateeva, N.N.; Kode, R.N.; Redda, K.K. Synthesis of novel flavonoid derivatives as potential HIV-integrase inhibitors. J. Heterocycl. Chem. 2002, 39, 1251–1258. [Google Scholar] [CrossRef]
- Kong, C.H.; Liang, W.J.; Hu, F.; Xu, X.H.; Wang, P.; Jiang, Y.; Xing, B.S. Allelochemicals and their transformations in the Ageratum conyzoides intercropped citrus orchard soils. Plant Soil 2004, 264, 149–157. [Google Scholar] [CrossRef]
- Passador, E.A.P.; Da Silva, M.F.D.G.F.; Fo, E.R.; Fernandes, J.B.; Vieira, P.C.; Pirani, J.R. A pyrano chalcone and a flavanone from Neoraputia magnifica. Phytochemistry 1997, 45, 1533–1537. [Google Scholar] [CrossRef]
- Nagase, H.; Omae, N.; Omori, A.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Yamakuni, T.; Ohizumi, Y. Nobiletin and its related flavonoids with CRE-dependent transcription-stimulating and neuritegenic activities. Biochem. Bioph. Res. Co. 2005, 337, 1330–1336. [Google Scholar] [CrossRef]
- Facundo, V.A.; Morais, S.M.; Braz Filho, R. Chemical constituents of Ottionia corcovadensis Miq. from Amazon Forest: 1H and 13C chemical shift assignments. Quim. Nova 2004, 27, 79–83. [Google Scholar] [CrossRef]
- Rwangabo, P.C.; Claeys, M.; Pieters, L.; Corthout, J.; Vandenberghe, D.A.; Vlietinck, A.J. Umuhengerin, a new antimicrobially active flavonoid from Lantana trifolia. J. Nat. Prod. 1988, 51, 966–968. [Google Scholar] [CrossRef]
- Kinoshita, T.; Firman, K. Highly oxygenated flavonoids from Murraya paniculata. Phytochemistry 1996, 42, 1207–1210. [Google Scholar] [CrossRef]
- Da Silva, B.F.; Rodrigues-Fo, E. Production of a benzylated flavonoid from 5,7,3′,4′,5′-pentamethoxyflavanone by Penicillium griseoroseum. J. Mol. Catal. B Enzym. 2010, 67, 184–188. [Google Scholar] [CrossRef]
- Ferracin, R.J.; Da Silva, M.; Fernandes, J.B.; Vieira, P.C. Flavonoids from the fruits of Murraya paniculata. Phytochemistry 1998, 47, 393–396. [Google Scholar] [CrossRef]
- Sherie, E.A.; Gupta, R.K.; Krishnamurti, M. Synthesis of chalcones, flavanones isolated from Popowia cauliflora and their analogues. Agric. Biol. Chem. 1981, 45, 531–533. [Google Scholar] [CrossRef][Green Version]
- Yao, H.; Jin, Y.R.; Shan, J.; Wang, X.Z.; Zhang, M.C.; Li, X.W. Two new natural methoxyflavonoids from leaves of Murraya paniculata(L.) Jack. Chem. Res. Chin. Univ. 2013, 29, 884–887. [Google Scholar] [CrossRef]
- Gupta, R.K.; Krishnamurti, M.; Parthasarathi, J. Synthesis of some recently isolated chalcones, their analogues and corresponding flavanones. Agric. Biol. Chem. 1979, 43, 2603–2605. [Google Scholar] [CrossRef]
- Cao, X.D.; Ding, Z.S.; Jiang, F.S.; Ding, X.H.; Chen, J.Z.; Chen, S.H.; Lv, G.Y. Antitumor constituents from the leaves of Carya cathayensis. Nat. Prod. Res. 2012, 26, 2089–2094. [Google Scholar] [CrossRef]
- You, C.X.; Guo, S.S.; Zhang, W.J.; Geng, Z.F.; Liang, J.Y.; Lei, N.; Du, S.S.; Deng, Z.W. Chemical constituents of Murraya tetramera Huang and their repellent activity against Tribolium castaneum. Molecules 2017, 22, 1379. [Google Scholar] [CrossRef]
- Wu, S.B.; Ji, Y.P.; Zhu, J.J.; Zhao, Y.; Xia, G.; Hu, Y.H.; Hu, J.F. Steroids from the leaves of Chinese Melia azedarach and their cytotoxic effects on human cancer cell lines. Steroids 2009, 74, 761–765. [Google Scholar] [CrossRef]
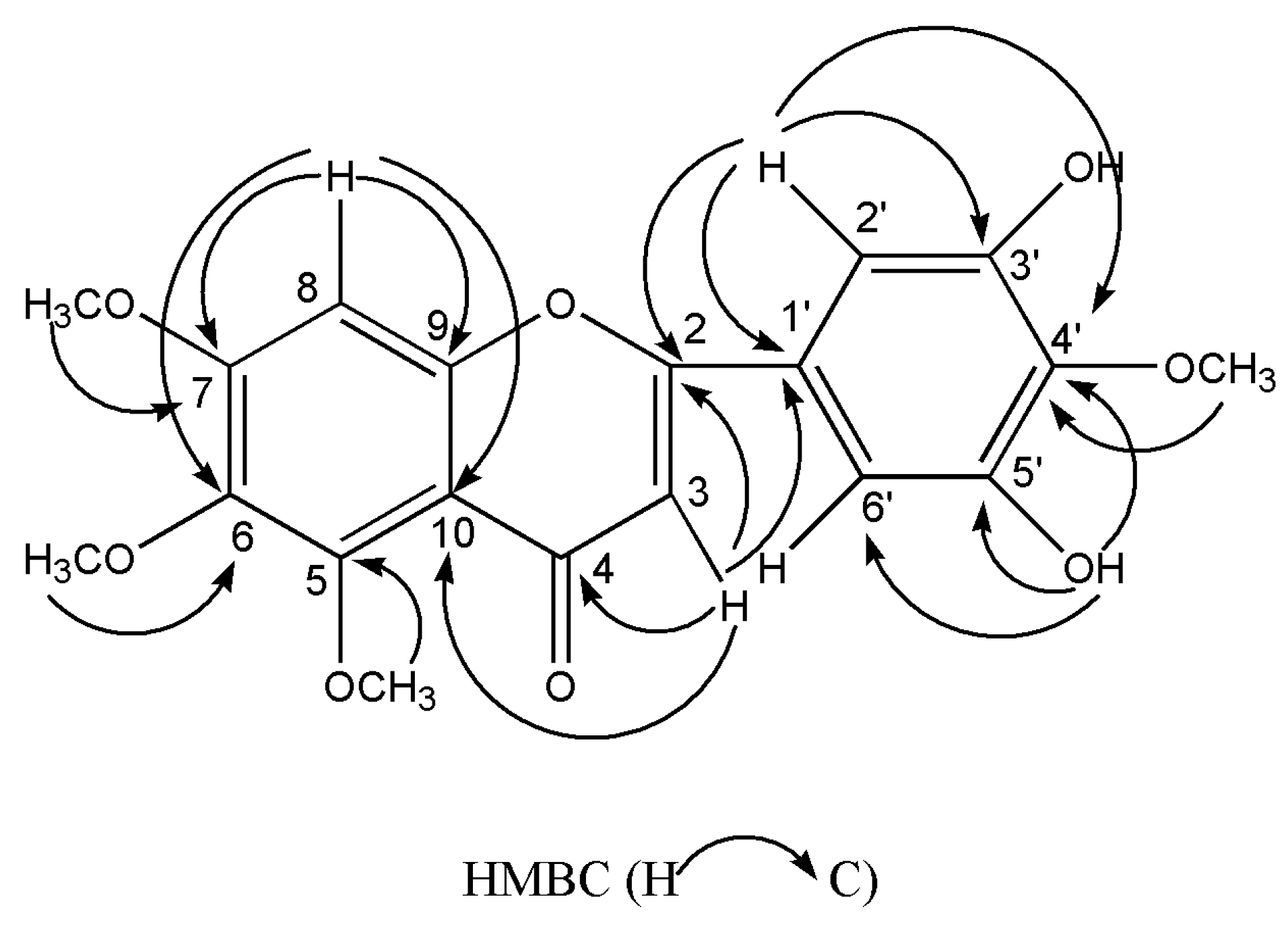
| Position | δH (ppm) | δC (ppm) |
|---|---|---|
| 2 | 161.0 | |
| 3 | 6.45, s | 107.2 |
| 4 | 176.0 | |
| 5 | 152.1 | |
| 6 | 140.3 | |
| 7 | 158.0 | |
| 8 | 7.10, s | 97.6 |
| 9 | 154.4 | |
| 10 | 112.5 | |
| 1′ | 126.4 | |
| 2′ | 7.00, s | 105.9 |
| 3′ | 151.6 | |
| 4′ | 138.9 | |
| 5′ | 151.6 | |
| 6′ | 7.00, s | 105.9 |
| 5-OCH3 | 3.80, s | 62.3 |
| 6-OCH3 | 3.77, s | 61.5 |
| 7-OCH3 | 3.96, s | 56.9 |
| 4′-OCH3 | 3.76, s | 60.3 |
| 3′, 5′-OH | 9.51, s |
| Compound | IC50 ± SD (µg/mL) | |
|---|---|---|
| B16 | MDA-MB-231 | |
| 1 | >100 | >100 |
| 2 | 14.74 ± 3.97 | 34.19 ± 3.38 |
| 3 | 11.18 ± 2.75 | 23.46 ± 2.95 |
| 4 | 14.97 ± 1.96 | >100 |
| 5 | 8.66 ± 1.80 | 3.80 ± 1.49 |
| 6 | >100 | >100 |
| 7 | 3.87 ± 0.68 | 14.93 ± 2.71 |
| 8 | 13.03 ± 1.19 | 26.46 ± 2.53 |
| 9 | 12.76 ± 3.38 | 16.02 ± 1.12 |
| 10 | 23.55 ± 3.51 | 25.29 ± 3.84 |
| 11 | >100 | >100 |
| 12 | 11.53 ± 1.61 | 7.89 ± 1.71 |
| 13 | 7.00 ± 0.64 | 5.95 ± 0.65 |
| DOX 1 | 0.51 ± 0.01 | 2.02 ± 0.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, C.-X.; Zhang, K.; Li, X.; Liu, J.; Zhang, W.-J.; Yu, X.-X. Cytotoxic Flavonoids from the Leaves and Twigs of Murraya tetramera. Molecules 2021, 26, 1284. https://doi.org/10.3390/molecules26051284
You C-X, Zhang K, Li X, Liu J, Zhang W-J, Yu X-X. Cytotoxic Flavonoids from the Leaves and Twigs of Murraya tetramera. Molecules. 2021; 26(5):1284. https://doi.org/10.3390/molecules26051284
Chicago/Turabian StyleYou, Chun-Xue, Kun Zhang, Xin Li, Jing Liu, Wen-Juan Zhang, and Xiao-Xue Yu. 2021. "Cytotoxic Flavonoids from the Leaves and Twigs of Murraya tetramera" Molecules 26, no. 5: 1284. https://doi.org/10.3390/molecules26051284
APA StyleYou, C.-X., Zhang, K., Li, X., Liu, J., Zhang, W.-J., & Yu, X.-X. (2021). Cytotoxic Flavonoids from the Leaves and Twigs of Murraya tetramera. Molecules, 26(5), 1284. https://doi.org/10.3390/molecules26051284






