pH-Sensitive Glycyrrhizin Based Vesicles for Nifedipine Delivery
Abstract
1. Introduction
2. Results
2.1. Optical Spectroscopy
2.2. Nuclear Magnetic Resonance Experiments
2.3. Molecular Dynamics Simulation
3. Materials and Methods
3.1. Materials
3.2. Optical Spectroscopy
3.3. NMR Experiments
3.4. Molecular Dynamics Simulations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| CMC | critical micelle concentration |
| COM | center of mass |
| DDS | drug delivery system |
| GA | glycyrrhizic acid |
| MD | molecular dynamics |
| NF | nifedipine |
| NMR | nuclear magnetic resonance |
References
- Advanced Drug Delivery Systems: Technologies and Global Markets: PHM006H/BCC Research. Available online: https://www.bccresearch.com/market-research/pharmaceuticals/advanced-drug-delivery-systems-phm006h.html (accessed on 15 February 2021).
- Lopalco, A.; Denora, N. Nanoformulations for drug delivery: Safety, toxicity, and efficacy. In Computational Toxicology: Methods and Protocols; Nicolotti, O., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 347–365. ISBN 978-1-4939-7899-1. [Google Scholar]
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular carotenoid complexes of enhanced solubility and stability—The way of bioavailability improvement. Molecules 2019, 24, 3947. [Google Scholar] [CrossRef]
- Jafari, S. Nanoencapsulation Technologies for the Food and Nutraceutical Industries, 1st ed.; Academic Press: Cambridge, MA, USA, 2017; ISBN 978-0-12-809436-5. [Google Scholar]
- Soukoulis, C.; Bohn, T. A Comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutr. 2017, 58, 1–36. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. Available online: https://www.hindawi.com/journals/jnm/2016/1087250/ (accessed on 14 February 2021).
- Zhu, Y.-J.; Chen, F. PH-Responsive drug-delivery systems. Chem. Asian J. 2015, 10, 284–305. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, S.; Fan, P.; Wang, L.; Di, Y.; Lin, K.; Xiao, F.-S. PH-Responsive carrier system based on carboxylic acid modified mesoporous silica and polyelectrolyte for drug delivery. Chem. Mater. 2005, 17, 5999–6003. [Google Scholar] [CrossRef]
- Pompei, R.; Flore, O.; Marccialis, M.A.; Pani, A.; Loddo, B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef]
- Shibata, S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi 2000, 120, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.J.; Yin, A.C.Y. Therapeutic effects of glycyrrhizic acid. Nat. Prod. Commun. 2013, 8, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2020, 153364. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Glycyrrhizin: An alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020, 214, 107618. [Google Scholar] [CrossRef] [PubMed]
- Tolstikova, T.G.; Bryzgalov, M.V.K.; Bryzgalov, A.O. The Complexes of Drugs with Carbohydrate-Containing Plant Metabolites as Pharmacologically Promising Agents. Available online: http://www.eurekaselect.com/85315/article (accessed on 3 October 2019).
- Yang, Y.; Shi, Q.; Liu, Z.; Li, R.; Pan, P.; Hou, Y.; Lu, W.; Bai, G. The synergistic anti-asthmatic effects of glycyrrhizin and salbutamol. Acta Pharmacol. Sin. 2010, 31, 443–449. [Google Scholar] [CrossRef]
- Stakhneva, E.M.; Vavilin, V.A.; Ragino, Y.I.; Safronova, O.G.; Shintyapina, A.B.; Ivanova, M.V. Effects of simvaglyzin and atorvaglyzin on the expression of 3-Hydroxy-3-Methyl-Glutaryl-CoA reductase in rat liver. Bull. Exp. Biol. Med. 2013, 156, 63–65. [Google Scholar] [CrossRef]
- Yang, F.-H.; Zhang, Q.; Liang, Q.-Y.; Wang, S.-Q.; Zhao, B.-X.; Wang, Y.-T.; Cai, Y.; Li, G.-F. Bioavailability enhancement of paclitaxel via a novel oral drug delivery system: Paclitaxel-loaded glycyrrhizic acid micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef]
- Konkina, I.G.; Shitikova, O.V.; Lobov, A.N.; Murinov, Y.I.; Bachurin, S.O. Host-guest complexation in the β-Glycyrrhizic Acid–2,8-Dimethyl-5-[2´-(6″-Methylpyridin-3″-Yl)Ethyl]-2,3,4,5-Tetrahydro-1H-Pyrido[4,3-b]indole system. Russ. Chem. Bull. 2015, 64, 1385–1393. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, B.; Wang, S.; Liang, Q.; Cai, Y.; Yang, F.; Li, G. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Deliv. 2016, 23, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Dushkin, A.V.; Meteleva, E.S.; Tolstikova, T.G.; Khvostov, M.V.; Dolgikh, M.P.; Tolstikov, G.A. Complexing of pharmacons with glycyrrhizic acid as a route to the development of the preparations with enhanced efficiency. Chem. Sustain. Dev. 2010, 4, 437–444. [Google Scholar]
- Lekar’, A.V.; Vetrova, E.V.; Borisenko, N.I.; Yakovishin, L.A.; Grishkovets, V.I. Mass spectrometry of triterpene glycosides molecular complexation with purine bases of nucleic acids. Russ. J. Bioorganic Chem. 2011, 37, 609. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Zaw, A.M.; Sekar, R.; Palak, A.; Allam, A.A.; Ajarem, J.; Chow, B.K.C. glycyrrhizic acid reduces heart rate and blood pressure by a dual mechanism. Molecules 2016, 21, 1291. [Google Scholar] [CrossRef] [PubMed]
- Salvi, M.; Fiore, C.; Armanini, D.; Toninello, A. Glycyrrhetinic acid-induced permeability transition in rat liver mitochondria. Biochem. Pharmacol. 2003, 66, 2375–2379. [Google Scholar] [CrossRef]
- Fiore, C.; Salvi, M.; Palermo, M.; Sinigaglia, G.; Armanini, D.; Toninello, A. On the mechanism of mitochondrial permeability transition induction by glycyrrhetinic acid. Biochim. Biophys. Acta BBA 2004, 1658, 195–201. [Google Scholar] [CrossRef]
- Harada, S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem. J. 2005, 392, 191–199. [Google Scholar] [CrossRef]
- Sapra, B.; Jain, S.; Tiwary, A.K. Transdermal delivery of carvedilol containing glycyrrhizin and chitosan as permeation enhancers: Biochemical, biophysical, microscopic and pharmacodynamic evaluation. Drug Deliv. 2008, 15, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Shelepova, E.A.; Paramonova, E.D.; Kichigina, L.A.; Khalikov, S.S.; Polyakov, N.E. Glycyrrhizin-induced changes in phospholipid dynamics studied by 1H NMR and MD simulation. Arch. Biochem. Biophys. 2020, 686, 108368. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery. Drug Deliv. 2016, 23, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Selyutina, O.Y.; Apanasenko, I.E.; Kim, A.V.; Shelepova, E.A.; Khalikov, S.S.; Polyakov, N.E. Spectroscopic and molecular dynamics characterization of glycyrrhizin membrane-modifying activity. Colloids Surf. B 2016, 147, 459–466. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Polyakov, N.E. Glycyrrhizic acid as a multifunctional drug carrier—From physicochemical properties to biomedical applications: A modern insight on the ancient drug. Int. J. Pharm. 2019, 559, 271–279. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Apanasenko, I.E.; Polyakov, N.E. Membrane-modifying activity of glycyrrhizic acid. Russ. Chem. Bull. 2015, 64, 1555–1559. [Google Scholar] [CrossRef]
- Petrova, S.S.; Schlotgauer, A.A.; Kruppa, A.I.; Leshina, T.V. Self-association of glycyrrhizic acid. NMR Study. Z. Phys. Chem. 2017, 231, 839–855. [Google Scholar] [CrossRef]
- Glazachev, Y.I.; Schlotgauer, A.A.; Timoshnikov, V.A.; Kononova, P.A.; Selyutina, O.Y.; Shelepova, E.A.; Zelikman, M.V.; Khvostov, M.V.; Polyakov, N.E. Effect of glycyrrhizic acid and arabinogalactan on the membrane potential of rat thymocytes studied by potential-sensitive fluorescent probe. J. Membr. Biol. 2020, 253, 343–356. [Google Scholar] [CrossRef]
- Borisenko, S.; Lekar, A.; Milov, A.; Vetrova, E.; Borisenko, N. Mass-spectrometry and quantum-chemical study of self-association processes of glycyrrhizinic acid molecules. Chem. Plant Mater. 2013, 2, 85–92. [Google Scholar] [CrossRef]
- Zeng, C.-X.; Hu, Q. Determination of the Polyacid Dissociation Constants of Glycyrrhizic Acid; CSIR: Pretoria, South Africa, 2008. [Google Scholar]
- Tolstikova, T.G.; Sorokina, I.V.; Brisgalov, A.O.; Dolgich, M.P.; Lifshitz, G.I.; Chvostov, M.V. The Use of a New Approach to Prevention and Therapy of Acute Arterial Hypertension with Complex of Well-Known Drugs With Vegetable Glycosides (Experimental Study). Available online: https://www.rpcardio.com/jour/article/view/1005 (accessed on 21 January 2021).
- Polyakov, N.E.; Khan, V.K.; Taraban, M.B.; Leshina, T.V. Complex of calcium receptor blocker nifedipine with glycyrrhizic acid. J. Phys. Chem. B 2008, 112, 4435–4440. [Google Scholar] [CrossRef]
- Morales-Ríos, M.S.; De la Cerda Medina, A.; Pérez-Alvarez, V.; Joseph-Nathan, P. 1H and 13C NMR study of the antihypertensive drugs nifedipine, nicardipine and related compounds. Magn. Reson. Chem. 2000, 38, 680–683. [Google Scholar] [CrossRef]
- Matsuoka, K.; Miyajima, R.; Ishida, Y.; Karasawa, S.; Yoshimura, T. Aggregate formation of glycyrrhizic acid. Colloids Surf. Physicochem. Eng. Asp. 2016, 500, 112–117. [Google Scholar] [CrossRef]
- Zhang, Q.; Polyakov, N.E.; Chistyachenko, Y.S.; Khvostov, M.V.; Frolova, T.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018, 25, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Meteleva, E.S.; Chistyachenko, Y.S.; Suntsova, L.P.; Khvostov, M.V.; Polyakov, N.E.; Selyutina, O.Y.; Tolstikova, T.G.; Frolova, T.S.; Mordvinov, V.A.; Dushkin, A.V.; et al. Disodium salt of glycyrrhizic acid—A novel supramolecular delivery system for anthelmintic drug praziquantel. J. Drug Deliv. Sci. Technol. 2019, 50, 66–77. [Google Scholar] [CrossRef]
- Kim, A.V.; Shelepova, E.A.; Selyutina, O.Y.; Meteleva, E.S.; Dushkin, A.V.; Medvedev, N.N.; Polyakov, N.E.; Lyakhov, N.Z. Glycyrrhizin-assisted transport of praziquantel anthelmintic drug through the lipid membrane: An experiment and MD simulation. Mol. Pharm. 2019, 16, 3188–3198. [Google Scholar] [CrossRef]
- Stroet, M.; Caron, B.; Visscher, K.M.; Geerke, D.P.; Malde, A.K.; Mark, A.E. Automated topology builder version 3.0: Prediction of solvation free enthalpies in water and hexane. J. Chem. Theory Comput. 2018, 14, 5834–5845. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, M.; Rahman, A. Polymorphic transitions in single crystals: A new molecular dynamics method. J. Appl. Phys. 1981, 52, 7182–7190. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
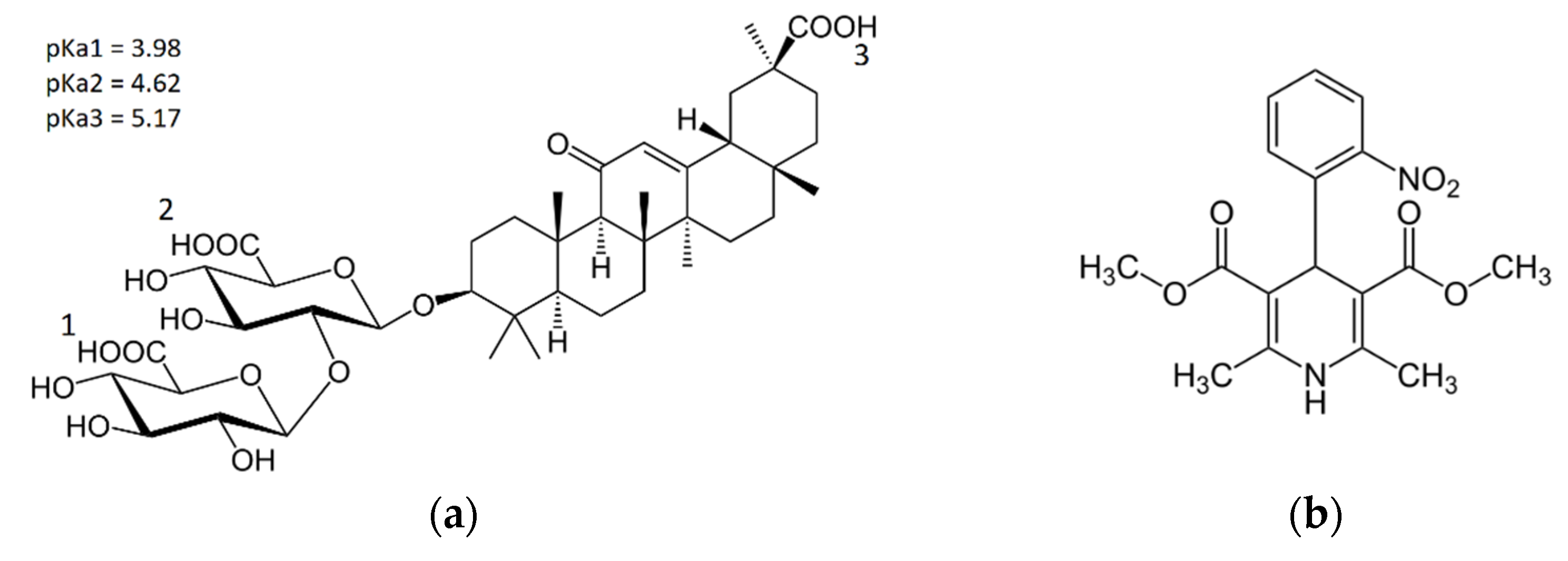






| pH | D, *10−10 m2/s |
|---|---|
| 3.8 | 1.9 ± 0.3 |
| 4.2 | 2.4 ± 0.1 |
| 5 | 2.7 ± 0.1 |
| 6.1 | 2.3 ± 0.1 |
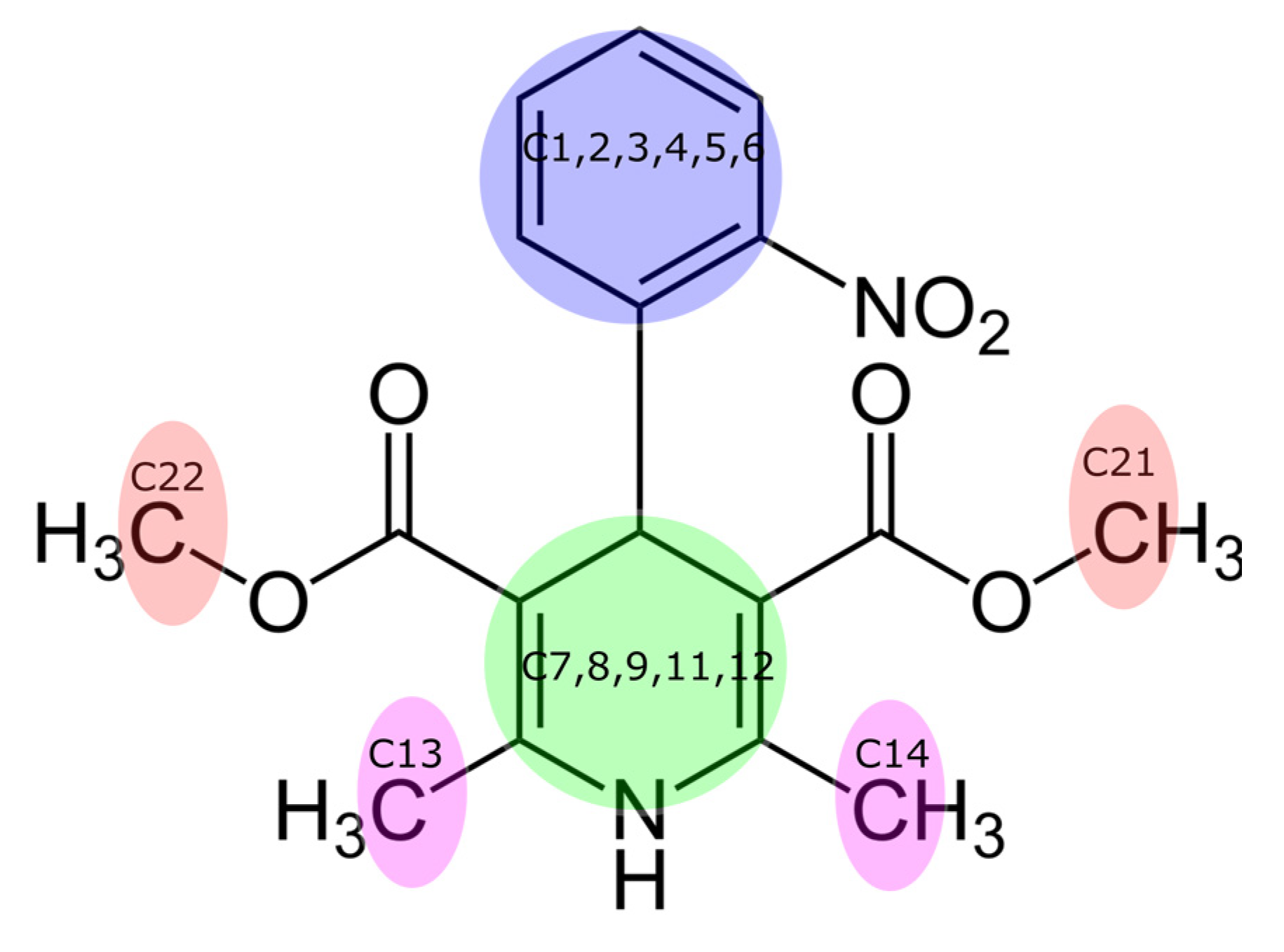
| GA Ionization Form | COM of GA—C13-14 | COM of GA—C21-22 | COM of GA—Benzene Ring | COM of GA—Pyridine Ring | COM of GA—COM of Nifedipine |
|---|---|---|---|---|---|
| GA | 0.99 ± 0.14 | 0.80 ± 0.11 | 0.83 ± 0.15 | 0.86 ± 0.12 | 0.78 ± 0.10 |
| GA1− | 0.95 ± 0.14 | 0.76 ± 0.12 | 0.78 ± 0.18 | 0.82 ± 0.12 | 0.74 ± 0.12 |
| GA2− | 0.79 ± 0.15 | 0.77 ± 0.12 | 0.84 ± 0.16 | 0.73 ± 0.12 | 0.73 ± 0.11 |
| GA3− | 0.88 ± 0.21 | 0.79 ± 0.23 | 0.79 ± 0.19 | 0.84 ± 0.15 | 0.78 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selyutina, O.Y.; Mastova, A.V.; Shelepova, E.A.; Polyakov, N.E. pH-Sensitive Glycyrrhizin Based Vesicles for Nifedipine Delivery. Molecules 2021, 26, 1270. https://doi.org/10.3390/molecules26051270
Selyutina OY, Mastova AV, Shelepova EA, Polyakov NE. pH-Sensitive Glycyrrhizin Based Vesicles for Nifedipine Delivery. Molecules. 2021; 26(5):1270. https://doi.org/10.3390/molecules26051270
Chicago/Turabian StyleSelyutina, Olga Yu., Anna V. Mastova, Ekaterina A. Shelepova, and Nikolay E. Polyakov. 2021. "pH-Sensitive Glycyrrhizin Based Vesicles for Nifedipine Delivery" Molecules 26, no. 5: 1270. https://doi.org/10.3390/molecules26051270
APA StyleSelyutina, O. Y., Mastova, A. V., Shelepova, E. A., & Polyakov, N. E. (2021). pH-Sensitive Glycyrrhizin Based Vesicles for Nifedipine Delivery. Molecules, 26(5), 1270. https://doi.org/10.3390/molecules26051270








