Abstract
Cystic fibrosis (CF) is a genetic disease caused by mutations that impair the function of the CFTR chloride channel. The most frequent mutation, F508del, causes misfolding and premature degradation of CFTR protein. This defect can be overcome with pharmacological agents named “correctors”. So far, at least three different classes of correctors have been identified based on the additive/synergistic effects that are obtained when compounds of different classes are combined together. The development of class 2 correctors has lagged behind that of compounds belonging to the other classes. It was shown that the efficacy of the prototypical class 2 corrector, the bithiazole corr-4a, could be improved by generating conformationally-locked bithiazoles. In the present study, we investigated the effect of tricyclic pyrrolothiazoles as analogues of constrained bithiazoles. Thirty-five compounds were tested using the functional assay based on the halide-sensitive yellow fluorescent protein (HS-YFP) that measured CFTR activity. One compound, having a six atom carbocyle central ring in the tricyclic pyrrolothiazole system and bearing a pivalamide group at the thiazole moiety and a 5-chloro-2-methoxyphenyl carboxamide at the pyrrole ring, significantly increased F508del-CFTR activity. This compound could lead to the synthesis of a novel class of CFTR correctors.
1. Introduction
Cystic fibrosis (CF), one of the most frequent genetic diseases [1], is caused by mutations that impair the expression and function of CFTR chloride channel. CF is a multi-organ pathology, with particularly severe consequences on the lungs, pancreas, and liver [1,2,3]. Pharmacological rescue of mutant CFTR function has become an effective therapeutic strategy to correct the basic defect in a large cohort of CF patients [4,5]. However, the therapy of CF with CFTR pharmacological modulators has to be tailored to the specific mutations carried by each patient. For example, Kalydeco, whose active principle is the “potentiator” VX-770 (Figure 1), is highly effective on patients carrying at least one copy of missense mutations causing CFTR channel gating defects [6,7,8]. Instead, additional types of small molecules, called “correctors”, need to be used to target F508del, the most frequent mutation among CF patients [9]. This mutation causes multiple folding and stability defects in CFTR protein resulting in premature degradation by the ubiquitin-proteasome cell system [10,11]. Highly effective rescue of F508del-CFTR mutant requires combinations of correctors. Correctors have been grouped in three classes according to the additive/synergistic effects that are generated when compounds belonging to separate classes are combined together [11,12]. VX-809 (Figure 1) [13], also known as lumacaftor, the first corrector to be approved for therapeutic use in CF patients, belongs to class 1. It has been postulated that these compounds act by targeting one of the problems caused by F508del, i.e., defective domain-domain interaction [10,11,12]. Other types of class 1 correctors are VX-661 (Figure 1), known as tezacaftor, which is already included in drugs approved for CF patients [14], and ARN23765 (Figure 1) [15]. Class 3 correctors are believed to target NBD1, the domain where F508del is localized [11,12]. VX-445 (elexacaftor) (Figure 1), a highly effective corrector that is now included in triple drug combinations together with VX-770 and VX-661 [16], has been recently classified as a class 3 compound [17]. Class 2 correctors, such as the bithiazole corr-4a (Figure 1) [18], are believed to act with a different mechanism with respect to class 1 and class 3 compounds [11,12]. Although corr-4a was one of the first correctors to be discovered, the development of class 2 correctors into drugs has lagged behind that of other types of correctors. Development of additional type 2 correctors, with the ability to synergize with class 1 and class 3 compounds, would be highly desirable to design novel combinatorial treatments for CF patients.
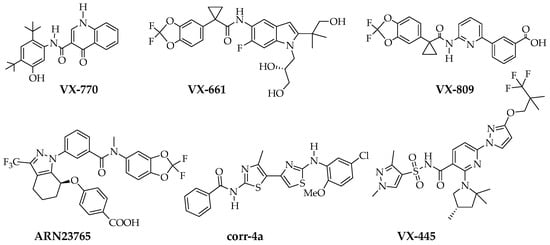
Figure 1.
Structures of VX-770 (potentiator), VX-809 (Class I corrector), VX-661 (Class I corrector), ARN23765 (Class I corrector), corr-4a (Class II corrector) and VX-445 (Class III corrector).
To generate corr-4a derivatives with improved corrector activity, conformationally-locked analogues were previously investigated [19,20]. Thus, by locking the thiazole core into a s-cis conformation, constrained bithiazoles of type 1 (Figure 2) bearing a central ring ranging from 5 to 8 carbon units were obtained [19,20]. In vitro data, on F508del-CFTR cells, suggested that modulating the constraining ring size in these correctors did not significantly enhance their potency (IC50), but significantly affected maximum efficacy. The best compound 1, bearing a cyclohepta ring between the two thiazole moieties, showed a 1.5-fold improved correction compared to free rotable benchmark bithiazole [20]. In particular, structural modifications revealed a correlation between the corrector activity and the presence of the amide and aniline groups into the bithiazole scaffold. The best combination in terms of potency and efficacy was achieved when 5-chloro-2-methoxyphenyl and the pivalamide groups were incorporated: The resulting compound had improved half-effective concentration (2.3 vs. 6 μM) and maximal effect (678 vs. 475 μM/s) with respect to corr-4a. The increase in Vmax is indicative of a higher number of F508del-CFTR proteins trafficking to the plasma membrane. It was postulated that the cyclohepta ring provides the required flexibility in the bithiazole core during protein binding to the active site.
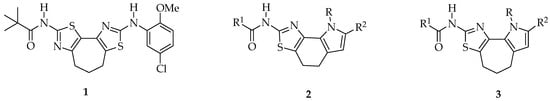
Figure 2.
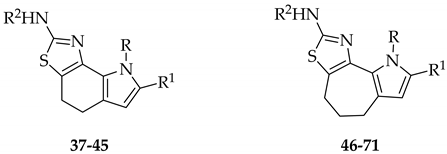
Structure of N-(8-((5-chloro-2-methoxyphenyl)amino)-5,6-dihydro-4Hcyclohepta[1,2-d:3,4-d′] bis(thiazole)-2-yl)pivalamidebithiazole (1), [1,3]thiazolo[5,4-g]indole-2-amines (2) and pyrrolo[3′,2′:6,7]cyclohepta[1,2-d][1,3]thiazol-2-amines (3).
Pyrroles are valuable heterocycles in medicinal chemistry, and offer a high degree of structural diversity as a useful tool for developing new therapeutic agents.
For many years, we have been involved in studies dealing with nitrogen heterocycles and much of our attention has been paid to tricyclic pyrrolo-fused systems [21,22,23,24,25,26,27,28,29,30,31,32]. Based on our past experience, we decided to investigate the effect on corrector activity of the replacement of one thiazole unit with a pyrrole moiety, which could have allowed the incorporation of some common structural features with constrained bithiazoles, aiming at the identification of new correctors. Among all possible pyrrolothiazole derivatives, depending on condensation of the pyrrole unit into the tricyclic scaffold, we chose to maintain the nitrogen position of the parent structure. Thus, we prepared a focused set of pyrrolothiazoles, as analogues of constrained bithiazoles (Figure 2). We decided to start our exploration from new classes of pyrrolothiazoles of type 2 and 3 (Figure 2), incorporating a carbocycle central ring ranging from six to seven carbon atoms and the most relevant structural features such as the 5-chloro-2-methoxyphenyl and the pivalamide groups, that would retain many aspects of the core framework of compounds 1.
2. Results and Discussion
2.1. Chemistry
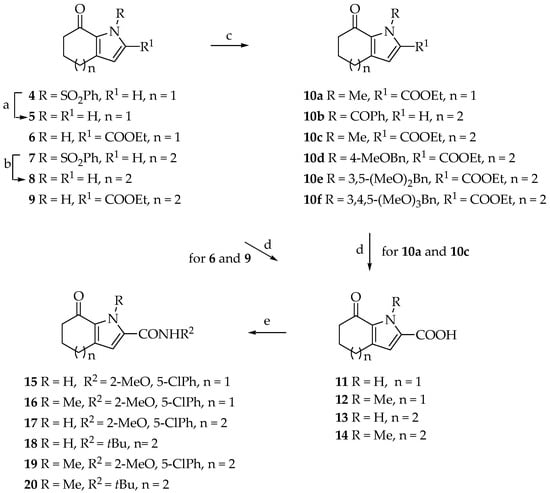
The synthetic strategy used for the preparation of the starting ketones (Scheme 1, Table 1) and of the desired tricyclic ring systems is depicted in Scheme 2 and final compounds reported in Table 2. α-Bromo-ketones (Table 1) were used as key intermediates suitable for the anellation of the thiazole ring upon reaction with thioureas, under typical Hantzsch reaction conditions.
Scheme 1.
Synthesis of tetrahydroindol-7-ones 4–6,10a,11,12,15,16 and tetrahydrocycloheptapyrrol-8-ones 7–9,10b–f,13,14,17–20. Reagents and conditions. (a) KOH, ethanol, reflux, 2 h, 88%; (b) NaOH, ethanol, reflux, 3 h, 80%; (c) NaH, DMF, 0 °C to rt, 1.5 h then alkyl or aralkylhalide, 0 °C to rt, 2–24 h, 80–96%; (d) KOH, ethanol, reflux, 0.5–1.5 h, 71–86%; (e) HOBt, DIPEA, EDC, DMF, rt, 10 min then 1-(5-chloro-2-methoxyphenyl)amine or tert-butylamine, rt or 100 °C, 3–20 h, 60–72%.

Table 1.
Overview of α-bromo-ketones 21–36.
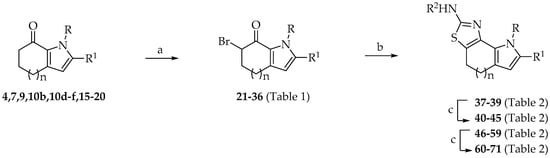
Scheme 2.
[1,3]Thiazolo[5,4-g]indole-2-amines 37–45 and pyrrolo[3′,2′:6,7]cyclohepta[1,2-d][1,3]thiazole-2-amines 46–71. Reagents and conditions. (a) Method A: CuBr2, ethyl acetate, reflux, 2 h, or Method B: pyridine hydrobromide perbromide, THF, rt, 16 h, 60–97%; (b) substituted thiourea, Na2CO3, DMF, rt, 16 h, 53–96%; (c) acetyl chloride, DIPEA, DMF, rt, 24 h, (for derivatives 60–65), 59–73% or acyl chloride, Et3N, 1,4-dioxane, rt, 16 h, (for derivatives 40–45, 66–71), 60–81%.

Table 2.
[1,3]Thiazolo[5,4-g]indole-2-amines 37–45 and pyrrolo[3′,2′:6,7]cyclohepta[1,2-d][1,3]thiazol-2-amines 46–71.
Synthesis of [1,3]Thiazolo[5,4-g]Indole-2-Amines (37–45) and Pyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-2-Amines (46–71)
We started from the synthesis of tetrahydroindol-7-ones 4–6 (n = 1) and tetrahydrocycloheptapyrrol-8-ones 7–9 (n = 2) (Scheme 1), to achieve the target [1,3]thiazoles [33,34,35].
Ketones 5,6,8,9 were functionalized at the pyrrole nitrogen using the suitable alkyl or aryl halide in the presence of sodium hydride to give the corresponding derivatives 10a–f (80–96%) (Scheme 1). In order to mimic the decoration of most active constrained bithiazole derivatives, the ethoxycarbonyl group of ketones 6,9 and 10a,10c was converted into carboxyamide. Thus, hydrolisis with potassium hydroxyde in refluxing ethanol gave carboxy acid derivatives 11–14 in excellent yield (71–86%) (Scheme 1), which were further reacted with the suitable amine in the presence of 1-hydroxy-benzotriazole (HOBt) and N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), as activating agent, allowing the isolation of the corresponding carboxamide derivatives 15–20 (60–72%) (Scheme 1).
α-Bromination of ketones 4,7,9,10b,10d–f,15–20 into α-bromo-derivatives 21–36 was achieved by two different methods (Table 1). Copper(II)bromide was used to obtain α-bromo-ketones 21,23,25–30 (Method A) or pyridine hydrobromide perbromide (Method B) to achieve 24,31–36 (60–97%) (Scheme 2, Table 1).
In the case of ketones bearing an ethoxycarbonyl or a carboxyamide group at the pyrrole ring, the corresponding α-bromo-ketones were achieved in high yields (65–97%) (Scheme 2, Table 1).
However, bromination of 10e, beside the desired α-bromo-ketone 28, led also to 30 as a by-product, bearing a bromine at the aryl moiety (Scheme 2, Table 1). The α-bromo-ketone 22 was obtained in good yield (72%) by deprotection of N-benzoyl derivatives 24 (Scheme 2, Table 1).
α-Bromo-ketones 21–36 were cyclized with thiourea or the suitable substituted thioureas, in presence of sodium carbonate, allowing the isolation of [1,3]thiazoles 37–39 and 46–59 from moderate to excellent yield (53–96%) (Scheme 2, Table 2). Finally, [1,3]thiazoles, bearing an amino group in position 2 of the tricyclic system, were subjected to acylation with the proper acyl chloride (acetyl chloride, trimethylacetyl chloride, isobutyryl chloride) in presence of N,N-diisopropylethylamine (DIPEA) or triethylamine to give compounds 40–45 (60–81%) and 60–71 (59–80%) (Scheme 2, Table 2).
2.2. Biology
All compounds were tested in a CFTR corrector assay that is carried out on CFBE41o-cells expressing F508del-CFTR. Such cells also express the halide-sensitive yellow fluorescent protein (HS-YFP). CFTR-dependent iodide influx causes (HS-YFP) fluorescence quenching. Hence, the quenching rate is proportional to the amount of CFTR channels in the plasma membrane and therefore reflects the efficacy of correctors in rescuing mutant CFTR from intracellular compartments. Cells were incubated for 24 h with compounds at 1 and 10 µM. Treatment was also done in combination with VX-809 (1 µM). After treatment, cells were further stimulated with forskolin and genistein to fully activate CFTR in the plasma membrane and promote anion transport. A representative set of data is shown in Figure 3. As expected, incubation with the control corrector VX-809 elicited a nearly three-fold increase in anion transport that is the result of improved trafficking of mutant CFTR from intracellular compartments to plasma membrane. Among all compounds tested, only compound 44 showed at 10 µM a significant effect, corresponding to 36% of VX-809 activity. Interestingly, combination of 44 with VX-809 elicited a significant additive effect (Figure 3).
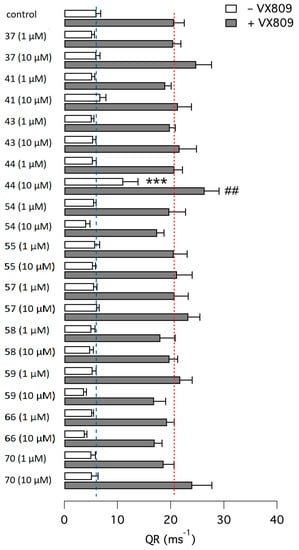
Figure 3.
Functional evaluation of CFTR correctors. The bar graph reports the quenching rate (QR), i.e., the anion transport mediated by F508del-CFTR in the plasma membrane, as determined with the HS-YFP functional assay. Before the assay, CFBE41o-cells were treated for 24 h with indicated compounds at 1 and 10 µM, with and without VX-809 (1 µM). Results were compared to those of cells treated with vehicle (DMSO) or VX-809 (1 µM) alone. Each bar reports the average and SD from six experiments. ***, p < 0.001 vs. control (no VX-809). ##, p < 0.01 vs. VX-809 alone.
From a structure activity point of view a six atom carbocyle ring, a pivalamide group and a 5-chloro-2-methoxyphenyl carboxamide at the thiazole and pyrrole rings respectively are required for corrector activity. In fact, moving to cyclohepta analogues a decrease of activity was observed (compare 44 with 70). Any manipulation of peripheral groups in the pyrrolothiazole structure investigated led either to a decrease of the activity, as in the case of the isopropyl amide analogue 41, or to a loss of activity.
To assess potency and efficacy of compound 44, we treated cells for 24 h at different concentrations in the range 0.1–20 µM. Treatment was done in the absence and in the presence of VX-809 at 1 µM. The resulting dose-response relationships are shown in Figure 4A,B. Interestingly, the apparent affinity of compound 44 appeared to be increased in the presence of VX-809 since the half-effective concentration decreased from 5 to 1.7 µM (Figure 4A,B). We also tested compound 44 in combination with VX-661 and VX-809 at their maximally-effective concentrations (Figure 4C). Compound 44 showed no evidence of additivity with VX-661 and VX-445.
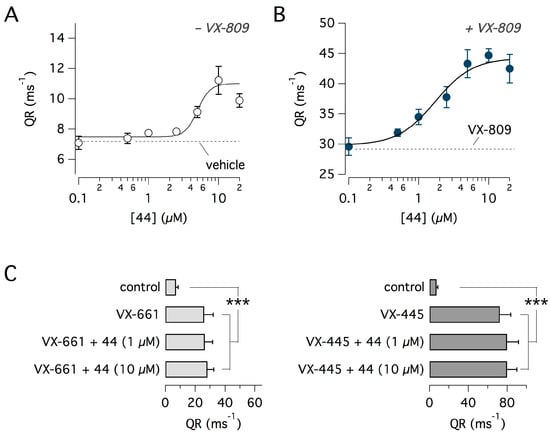
Figure 4.
Characterization of compound 44. (A,B) Dose-response relationships obtained by incubating cells with different concentrations of compound 44 in the absence (A) and presence (B) of VX-809 at 1 µM. After incubation, F508del-CFTR activity in the plasma membrane (i.e., anion transport reported as QR) was determined with the HS-YFP assay. Each symbol shows mean ± SD from six separate experiments. Fitting of data with a Hill equation showed a half-effective concentration of 5 and 1.7 µM in the absence and presence of VX-809, respectively. (C) Effect of combinations of compound 44 (at 1 and 10 µM) with 5 µM VX-661 (left) or 5 µM VX-445 (right). The two types of combinations did not result in additive effects. Each bar is the mean ± SD of eight experiments. ***, p <0.001 vs. control.
3. Materials and Methods
3.1. Chemistry
All melting points were taken on a Büchi melting point M-560 apparatus. IR spectra were determined in bromoform with a Shimadzu FT/IR 8400S spectrophotometer. 1H and 13C NMR spectra were measured at 200 and 50.0 MHz, respectively, in DMSO-d6 or CDCl3 solution using a Bruker Avance II series 200 MHz spectrometer. Column chromatography was performed with Merck silica gel (230−400 mesh ASTM) or a Büchi Sepacor chromatography module (prepacked cartridge system). Elemental analyses (C, H, N) were within ±0.4% of theoretical values and were performed with a VARIO EL III elemental analyzer.
Compounds 4–9, 10a,10c–f were prepared according to our previous published procedures [33,34,35].
3.1.1. Synthesis of [1,3]Thiazolo[5,4-g]Indole-2-Amines and Pyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-2-Amines
General Procedure for the Synthesis of 1-(Benzoyl)-4,5,6,7-Tetrahydrocyclohepta[b]Pyrrol-8(1H)-one (10b)
To a solution of the suitable ketones 8 (6 mmol) in anhydrous DMF (12 mL), NaH (6.6 mmol) was added at 0 °C and the reaction was stirred for 1 h and 30 min at room temperature. Then, benzoyl chloride (9 mmol) was added at 0 °C, and the reaction mixture was stirred at room temperature for 3 h. Then the reaction mixture was poured onto ice and brine (40 mL), and the aqueous solution was extracted with dichloromethane (3 × 40 mL). The organic phase was dried over Na2SO4 and the solvent evaporated under reduced pressure. The crude product was purified by column chromatography (dichloromethane). Colorless oil; yield: 82%; IR: νmax = 1699 (CO) 1645 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.83–2.01 (m, 4H, 2 × CH2), 2.59 (t, J = 6.1 Hz, 2H, CH2), 2.81–2.90 (m, 2H, CH2), 6.13 (d, J = 2.4 Hz, 1H, H-3), 7.17 (d, J = 2.4 Hz, 1H, H-2), 7.40–7.73 (5H, m, Ar); 13C NMR (500 MHz, CDCl3) δ 22.4 (t), 26.0 (t), 27.6 (t), 42.1 (t), 112.4 (d), 128.2 (d), 128.5 (2 × d), 129.6 (2 × d), 130.1 (s), 133.2 (d), 133.8 (s), 137.2 (s), 169.0 (s), 192.1 (s). Anal. Calcd. for C16H15NO2: C, 75.87; H, 5.97; N, 5.53. Found: 76.03; H, 6.12; N, 5.44.
General Procedure for the Synthesis of 7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxylic Acid (11,12) and 8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylic Acid (13,14)
To a solution of suitable ketones 6,9,10a,10c (6.4 mmol) in ethanol (25 mL) a solution of aq. 50% KOH (4.8 mL) was added and the reaction mixture was heated at reflux until the reaction was complete (TLC). After cooling, the solvent was removed under reduced pressure. The residue was added of water and the solution was acidified with HCl 6 M. The solid formed was collected by filtration and dried.
7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxylic Acid (11)
This compound was obtained by reaction of 6 after 35 min. White solid; yield 71%; mp: 280–281 °C; IR: νmax = 3405 (NH), 3176 (OH), 1709 (CO), 1635 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.94–2.06 (m, 2H, CH2), 2.43 (t, J = 5.9 Hz, 2H, CH2), 2.68 (t, J = 5.9 Hz, 2H, CH2), 6.61 (s, 1H, H-3), 12.25 (s, 1H, OH); 13C NMR (50 MHz, DMSO-d6) δ 22.5 (t), 24.5 (t), 38.2 (t), 112.8 (d), 128.4 (s), 130.3 (s), 135.1 (s), 161.7 (s), 188.7 (s). Anal calcd for C9H9NO3: C, 60.33; H, 5.06; N, 7.82. Found: C, 60.47; H, 4.88; N, 7.99.
1-Methyl-7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxylic Acid (12)
This compound was obtained by reaction of 10a after 1 h and 30 min. White solid; yield 86%; mp: 280–281 °C; IR: νmax = 3113 (OH), 1709 (CO), 1686 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.90–2.03 (m, 2H, CH2), 2.46 (t, J = 6.0 Hz, 2H, CH2), 2.68 (t, J = 6.0 Hz, 2H, CH2), 4.13 (s, 3H, CH3), 6.67 (s, 1H, H-3), 13.01 (s, 1H, OH); 13C NMR (50 MHz, DMSO-d6) δ 22.9 (t), 24.1 (t), 34.1 (q), 39.5 (t), 113.9 (d), 128.4 (s), 129.8 (s), 134.6 (s), 162.0 (s), 190.1 (s). Anal calcd for C10H11NO3: C, 62.17; H, 5.74; N, 7.25. Found: C, 62.33; H, 5.59; N, 7.11.
8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylic Acid (13)
This compound was obtained by reaction of 9 after 40 min. White solid; yield 82%; mp: 280–281 °C; IR: νmax = 3296 (NH), 3216 (OH), 1715 (CO), 1686 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.72–1.86 (m, 4H, 2 × CH2), 2.62 (t, J = 5.7 Hz, 2H, CH2), 2.79 (t, J = 5.7 Hz, 2H, CH2), 6.63 (s, 1H, H-3), 11.59 (s, 1H, NH), 12.60 (s, 1H, OH); 13C NMR (50 MHz, DMSO-d6) δ 21.5 (t), 25.4 (t), 26.1 (t), 41.3 (t), 115.6 (d), 127.0 (s), 131.9 (s), 132.1 (s), 161.4 (s), 192.0 (s). Anal calcd for C10H11NO3: C, 62.17; H, 5.74; N, 7.25. Found: C, 61.97; H, 5.88; N, 7.08.
1-Methyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylic Acid (14)
This compound was obtained by reaction of 12 after 45 min. White solid; yield 75%; mp: 280–281 °C; IR: νmax = 3410 (OH), 1721 (CO), 1680 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.63–1.79 (m, 4H, 2 × CH2), 2.63 (t, J = 5.1 Hz, 2H, CH2), 2.75 (t, J = 5.1 Hz, 2H, CH2), 4.06 (s, 3H, CH3), 6.70 (s, 1H, H-3), 12.91 (s, 1H, OH); 13C NMR (50 MHz, DMSO-d6) δ 20.8 (t), 24.5 (t), 24.9 (t), 34.3 (q), 41.4 (t), 116.5 (d), 127.3 (s), 132.8 (s), 133.2 (s), 162.0 (s), 194.1 (s). Anal calcd for C11H13NO3: C, 63.76; H, 6.32; N, 6.76. Found: C, 63.88; H, 6.19; N, 6.59.
General Procedure for the Synthesis of N-(Substituted)-7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxamide (15,16) and N-(Substituted)-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (17–20)
To a suspension of the suitable acid derivatives 11–14 (1.9 mmol) in anhydrous DMF (6 mL) N,N-diisopropylethylamine (1.9 mL, 10.9 mmol), 1-hydroxybenzotriazole hydrate (0.41 g, 3.0 mmol), and EDC (0.53 g, 2.8 mmol) were added. The reaction mixture was stirred at room temperature for 10 min. Then the proper amine (7.6 mmol) was added in one portion, and the resulting suspension was stirred at room temperature or at 100 °C until the reaction was complete (TLC). Then the reaction mixture was poured onto crushed ice and the solid formed was collected by filtration, dried and used in the next step without further purification.
N-(5-Chloro-2-Methoxyphenyl)-7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxamide (15)
This compound was obtained by reaction of 11 with 5-chloro-2-methoxyaniline after 16 h at 100 °C. White solid; yield 60%; mp: 280–281 °C; IR: νmax = 3422 (NH), 3330 (NH), 1675 (CO), 1652 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 2.09–2.21 (m, 2H, CH2), 2.56 (t, J = 6.0 Hz, 2H, CH2), 2.78 (t, J = 6.0 Hz, 2H, CH2), 3.92 (s, 3H, CH3), 6.53 (s, 1H, H-3), 6.80 (d, J = 8.8 Hz, 1H, Ar), 7.01 (dd, J = 8.8, 2.5 Hz, 1H, Ar), 8.33 (s, 1H, NH), 8.48 (d, J = 2.5 Hz, 1H, Ar), 10.08 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 23.1 (t), 24.9 (t), 38.1 (t), 56.2 (q), 108.2 (d), 110.8 (d), 119.8 (d), 123.6 (d), 126.3 (s), 128.0 (s), 130.1 (s), 130.7 (s), 136.3 (s), 146.4 (s), 157.8 (s), 189.5 (s). Anal calcd for C16H15ClN2O3: C, 60.29; H, 4.74; N, 8.79. Found: C, 60.14; H, 4.91; N, 8.94.
N-(5-Chloro-2-Methoxyphenyl)-1-Methyl-7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxamide (16)
This compound was obtained by reaction of 12 with 5-chloro-2- methoxyaniline after 20 h at 100 °C. White solid; yield 60%; mp: 280–281 °C; IR: νmax = 3422 (NH), 1680 (CO), 1658 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 2.03–2.15 (m, 2H, CH2), 2.55 (t, J = 6.1 Hz, 2H, CH2), 2.77 (t, J = 6.1 Hz, 2H, CH2), 3.92 (s, 3H, CH3), 4.26 (s, 3H, CH3), 6.46 (s, 1H, H-3), 6.81 (d, J = 8.7 Hz, 1H, Ar), 7.03 (dd, J = 8.7, 2.5 Hz, 1H, Ar), 8.32 (s, 1H, NH), 8.49 (d, J = 2.5 Hz, 1H, Ar); 13C NMR (50 MHz, CDCl3) δ 23.7 (t), 25.6 (t), 34.8 (q), 40.0 (t), 56.2 (q), 109.4 (d), 110.7 (d), 119.5 (d), 123.4 (d), 126.3 (s), 128.3 (s), 130.2 (s), 131.9 (s), 135.4 (s), 146.4 (s), 159.1 (s), 190.5 (s). Anal calcd for C17H17ClN2O3: C, 61.36; H, 5.15; N, 8.42. Found: C, 61.22; H, 4.97; N, 8.63.
N-(5-Chloro-2-Methoxyphenyl)-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (17)
This compound was obtained by reaction of 13 with 5-chloro-2- methoxyaniline after 3 h at room temperature. White solid; yield 67%; mp: 280–281 °C; IR: νmax = 3588 (NH), 3428 (NH), 1684 (CO), 1672 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.81–1.84 (m, 4H, 2 × CH2), 2.66 (t, J = 5.7 Hz, 2H, CH2), 2.83 (t, J = 5.7 Hz, 2H, CH2), 3.88 (s, 3H, CH3), 6.78 (s, 1H, H-3), 7.11 (d, J = 8.9 Hz, 1H, Ar), 7.21 (dd, J = 8.9, 2.5 Hz, 1H, Ar), 7.91 (dd, J = 2.5 Hz, 1H, Ar), 9.68 (s, 1H, NH), 12.15 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 21.8 (t), 25.6 (t), 26.4 (t), 41.4 (t), 56.1 (q), 112.7 (d), 115.3 (d), 123.1 (d), 123.6 (s), 124.6 (d), 127.5 (s), 129.7 (s), 131.8 (s), 131.9 (s), 149.5 (s), 157.9 (s), 192.3 (s). Anal calcd for C17H17ClN2O3: C, 61.36; H, 5.15; N, 8.42. Found: C, 61.22; H, 5.31; N, 8.55.
N-Tert-Butyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (18)
This compound was obtained by reaction of 13 with tert-butylamine after 16 h at 100 °C. Light brown solid; yield 72%; mp: 280–281 °C; IR: νmax = 3433 (NH), 3324 (NH), 1665 (CO), 1651 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.44 (s, 9H, 3 × CH3), 1.89–1.98 (m, 4H, 2 × CH2), 2.70 (t, J = 5.4 Hz, 2H, CH2), 2.84 (t, J = 5.4 Hz, 2H, CH2), 5.92 (s, 1H, NH), 6.30 (s, 1H, H-3), 9.88 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 22.6 (t), 26.5 (t), 28.0 (t), 28.9 (3 × q), 42.2 (t), 51.8 (s), 110.1 (d), 130.7 (s), 130.8 (s), 132.4 (s), 159.5 (s), 192.7 (s). Anal calcd for C14H20N2O2: C, 67.71; H, 8.12; N, 11.28. Found: C, 67.89; H, 7.98; N, 11.12.
N-(5-Chloro-2-Methoxyphenyl)-1-Methyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (19)
This compound was obtained by reaction of 14 with 5-chloro-2-methoxyaniline after 5 h at room temperature. Light brown solid; yield 67%; mp: 280–281 °C; IR: νmax = 3421 (NH), 1680 (CO), 1646 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.74–1.77 (m, 4H, 2 × CH2), 2.64 (t, J = 5.8 Hz, 2H, CH2), 2.79 (t, J = 5.8 Hz, 2H, CH2), 3.85 (s, 3H, CH3), 4.02 (s, 3H, CH3), 6.79 (s, 1H, H-3), 7.11 (d, J = 8.9 Hz, 1H, Ar), 7.23 (dd, J = 8.9, 2.6 Hz, 1H, Ar), 7.88 (dd, J = 2.6 Hz, 1H, Ar), 9.32 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 20.9 (t), 24.5 (t), 25.1 (t), 34.5 (q), 41.4 (t), 56.1 (q), 112.8 (d), 113.2 (d), 122.7 (d), 123.6 (s), 124.8 (d), 127.7 (s), 130.5 (s), 132.4 (s), 133.0 (s), 149.7 (s), 159.3 (s), 193.7 (s). Anal calcd for C18H19ClN2O3: C, 62.34; H, 5.52; N, 8.08. Found: C, 62.58; H, 5.34; N, 7.88.
N-Tert-Butyl-1-Methyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (20)
This compound was obtained by reaction of 14 with tert-butylamine after 16 h at 50 °C. White solid; yield 67%; mp: 280–281 °C; IR: νmax = 3336 (NH), 1663 (CO), 1635 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.34 (s, 9H, 3 × CH3), 1.63–1.80 (m, 4H, 2 × CH2), 2.51–2.80 (m, 4H, 2 × CH2), 3.93 (s, 3H, CH3), 6.47 (s, 1H, H-3), 7.75 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 20.9 (t), 24.4 (t), 25.1 (t), 28.5 (3 × q), 34.3 (q), 41.3 (t), 50.9 (s), 112.1 (d), 130.9 (s), 132.9 (s), 133.0 (s), 160.9 (s), 193.2 (s). Anal calcd for C15H22N2O2: C, 68.67; H, 8.45; N, 10.68. Found: C, 68.81; H, 8.29; N, 10.53.
General Procedure for the Synthesis of α-Bromo-Ketones (21–36)
Method A. To a suspension of CuBr2 (5.4 mmol) in anhydrous ethyl acetate (30 mL), the suitable ketones (3 mmol) was added and the reaction mixture was heated at reflux for 2 h. After cooling, the reaction mixture was filtered through a celite pad and the filtrate was evaporated under reduced pressure. In the case of compound 23, the residue was purified by column chromatography (dichloromethane).
Method B. To a solution of the suitable ketones (5 mmol) in anhydrous THF (10 mL), a solution of pyridine hydrobromide perbromide (5 mmol) in anhydrous THF (5 mL), was added and the reaction mixture was stirred at room temperature for 16 h. The solid formed was filtered through a celite pad and the filtrate was evaporated under reduced pressure. The residue was dissolved in dichloromethane (10 mL), washed with a solution of 5% aq. NaHCO3 (10 mL), dried over Na2SO4 and evaporated under reduced pressure. In the case of compounds 24,33–36, the residue was purified by chromatography (dichloromethane).
6-Bromo-1-(Phenylsulfonyl)-1,4,5,6-Tetrahydro-7H-Indol-7-One (21)
This compound was obtained by reaction of 4 (method A) and used in the next step without further purification.
7-Bromo-1-(Phenylsulfonyl)-4,5,6,7-Tetrahydrocyclohepta[b]Pyrrol-8(1H)-One (23)
This compound was obtained by reaction of 7 using method A. Colorless oil; yield 82%; IR: νmax = 1622 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.78–2.35 (m, 4H, 2 × CH2), 2.63–3.00 (m, 2H, CH2), 4.64–4.75 (m, 1H, CH), 6.16 (d, J = 2.8 Hz, 1H, H-3), 7.48–7.63 (m, 3H, H-3′, H-4′ and H-5′), 7.70 (d, J = 2.8 Hz, 1H, H-2), 7.94 – 8.04 (m, 2H, H-2′ and H-6′); 13C NMR (50 MHz, CDCl3) δ 22.80 (t), 28.03 (t), 32.24 (t), 55.22 (d), 113.08 (d), 128.04 (2 × d), 128.78 (2 × d), 129.00 (s), 129.67 (d), 133.63 (d), 137.46 (s), 139.39 (s), 184.83 (s). Anal. Calcd. for C15H14BrNO3S: C, 48.92; H, 3.83; N, 3.80. Found: 49.03; H, 3.90; N, 4.00.
1-(Benzoyl)-7-Bromo-4,5,6,7-Tetrahydrocyclohepta[b]Pyrrol-8(1H)-One (24)
This compound was obtained by reaction of 10b using method B. Yellow oil; yield: 60%; IR: νmax = 1711 (CO) 1636 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.93–2.02 (m, 2H, CH2), 2.33–2.42 (m, 2H, CH2), 2.82–3.09 (m, 2H, CH2), 4.63 – 4.70 (m, 1H, CH), 6.12 (d, J = 2.6 Hz, 1H, H-3), 7.24 (d, J = 2.6 Hz, 1H, H-2), 7.41 – 7.76 (m, 5H, Ar); 13C NMR (50 MHz, CDCl3) δ 22.81 (t), 28.53 (t), 32.32 (t), 55.27 (d), 112.46 (d), 128.61 (d), 129.77 (2 × d), 129.92 (2 × d), 130.14 (s), 133.02 (s), 133.38 (d), 136.29 (s), 168.58 (s), 184.62 (s). Anal. Calcd. for C16H14BrNO2: C, 57.85; H, 4.25; N, 4.22. Found: 57.95; H, 4.32; N, 4.01.
Ethyl 7-Bromo-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylate (25)
This compound was obtained by reaction of 9 using method A and used in the next step without further purification.
Ethyl 7-Bromo-1-Methyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylate (26)
This compound was obtained by reaction of 10c (method A) and used in the next step without further purification.
Ethyl 7-Bromo-1-(4-Methoxybenzyl)-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylate (27)
This compound was obtained by reaction of 10d (method A) and used in the next step without further purification.
Ethyl 7-Bromo-1-(3,5-Dimethoxybenzyl)-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylate (28)
This compound was obtained by reaction of 10e (method A) and used in the next step without further purification.
Ethyl 7-Bromo-1-(3,4,5-Trimethoxybenzyl)-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b] Pyrrole-2-Carboxylate (29)
This compound was obtained by reaction of 10f using method A and used in the next step without further purification.
Ethyl 7-Bromo-1-(2-Bromo-3,5-Dimethoxybenzyl)-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxylate (30)
This compound was obtained by reaction of 10e (method A) and used in the next step without further purification.
6-Bromo-N-(5-Chloro-2-Methoxyphenyl)-7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxamide (31)
This compound was obtained by reaction of 15 (method B) and used in the next step without further purification.
6-Bromo-N-(5-Chloro-2-Methoxyphenyl)-1-Methyl-7-Oxo-4,5,6,7-Tetrahydro-1H-Indole-2-Carboxamide (32)
This compound was obtained by reaction of 16 (method B) and used in the next step without further purification.
7-Bromo-N-(5-Chloro-2-Methoxyphenyl)-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (33)
This compound was obtained by reaction of 17 using method B. Brown solid; yield 92%; mp: 280–281 °C; IR: νmax = 3422 (NH), 3273 (NH), 1669 (CO), 1635 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.92–2.45 (m, 4H, 2 × CH2), 2.78–3.00 (m, 2H, CH2), 3.88 (s, 3H, CH3), 5.12–5.17 (m, 1H, CH), 6.79 (s, 1H, H-3), 7.12 (d, J = 8.9 Hz, 1H, Ar), 7.22 (dd, J = 8.9, 2.5 Hz, 1H, Ar), 7.90 (d, J = 2.5 Hz, 1H, Ar), 9.71 (s, 1H, NH), 12.28 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 23.1 (t), 27.1 (t), 32.0 (t), 56.1 (q), 56.7 (d), 112.8 (d), 115.6 (d), 123.2 (d), 123.6 (s), 124.7 (d), 127.4 (s), 128.9 (s), 131.3 (s), 131.6 (s), 149.6 (s), 157.5 (s), 185.4 (s). Anal calcd for C17H16BrClN2O3: C, 49.60; H, 3.92; N, 6.80. Found: C, 49.79; H, 4.05; N, 6.67.
7-Bromo-N-Tert-Butyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (34)
This compound was obtained by reaction of 18 using method B. Light brown solid; yield 97%; mp: 280–281 °C; IR: νmax = 3565 (NH), 3445 (NH), 1658 (CO), 1645 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.44 (s, 9H, 3 × CH3), 1.55–2.05 (m, 2H, CH2), 2.19–2.39 (m, 2H, CH2), 2.77–3.07 (m, 2H, CH2), 4.83–4.87 (m, 1H, CH), 6.20 (s, 1H, NH), 6.39 (s, 1H, H-3), 10.09 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 23.2 (t), 28.2 (t), 28.8 (3 × q), 32.4 (t), 52.0 (s), 54.7 (d), 111.0 (d), 128.1 (s), 132.3 (s), 132.4 (s), 159.3 (s), 185.8 (s). Anal calcd for C14H19BrN2O2: C, 51.39; H, 5.85; N, 8.56. Found: C, 51.23; H, 5.99; N, 8.39.
7-Bromo-N-(5-Chloro-2-Methoxyphenyl)-1-Methyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (35)
This compound was obtained by reaction of 19 using method B. Light brown solid; yield 65%; mp: 280–281 °C; IR: νmax = 3378 (NH), 1661 (CO), 1648 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.76–1.96 (m, 2H, CH2), 2.11–2.46 (m, 2H, CH2), 2.85 (t, J = 4.2 Hz, 2H, CH2), 3.85 (s, 3H, CH3), 3.91 (s, 3H, CH3), 5.11–5.17 (m, 1H, CH), 6.78 (s, 1H, H-3), 7.12 (d, J = 8.8 Hz, 1H, Ar), 7.24 (dd, J = 8.8, 2.5 Hz, 1H, Ar), 7.86 (d, J = 2.5 Hz, 1H, Ar), 9.41 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 26.0 (t), 31.3 (t), 33.2 (t), 34.6 (q), 47.5 (d), 56.1 (q), 111.8 (s), 112.8 (d), 113.3 (d), 123.5 (s), 125.0 (d), 127.2 (d), 130.9 (s), 142.3 (s), 146.3 (s), 159.3 (s), 163.6 (s), 189.6 (s). Anal calcd for C18H18BrClN2O3: C, 50.78; H, 4.26; N, 8.33. Found: C, 50.90; H, 4.14; N, 8.21.
7-Bromo-N-Tert-Butyl-1-Methyl-8-Oxo-1,4,5,6,7,8-Hexahydrocyclohepta[b]Pyrrole-2-Carboxamide (36)
This compound was obtained by reaction of 20 using method B. Brown solid; yield 65%; mp: 280–281 °C; IR: νmax = 3433 (NH), 1669 (CO), 1652 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.35 (s, 9H, 3 × CH3), 1.85–2.49 (m, 4H, 2 × CH2), 2.78–2.84 (m, 2H, CH2), 3.82 (s, 3H, CH3), 5.06–5.12 (m, 1H, CH), 6.46 (s, 1H, H-3), 7.83 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 23.6 (t), 26.4 (t), 28.4 (3 × q), 32.3 (t), 34.3 (q), 41.4 (s), 58.4 (d), 112.3 (d), 129.0 (s), 132.6 (s), 137.0 (s), 166.1 (s), 187.6 (s). Anal calcd for C15H21BrN2O2: C, 52.80; H, 6.20; N, 8.21. Found: C, 52.97; H, 6.03; N, 8.39.
Synthesis of 7-Bromo-4,5,6,7-Tetrahydrocyclohepta[b]Pyrrol-8(1H)-One (22)
To a solution of 24 (5 mmol) in methanol (100 mL), a solution of 5 M aq. NaOH (1.1 mL, 5.5 mmol) was added and the reaction mixture was stirred at room temperature for 4 h. The solution was concentrated, cooled at 0 °C and acidified using HCl 6M. The aqueous phase was extracted with dichloromethane (3 × 20 mL) and the organic phase was washed with a solution of 5% aq. NaHCO3. Then the organic phase was dried over Na2SO4 and evaporated under reduced pressure. The residue was purified by column chromatography (dichloromethane). Pale brown solid; yield: 72 %; mp: 99.9–100.5 °C; IR: νmax = 3425 (NH), 1622 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.94–2.03 (m, 2H, CH2), 2.33–2.39 (m, 2H, CH2), 2.85–3.08 (m, 2H, CH2), 4.83–4.90 (m, 1H, CH), 6.10 (d, J = 2.4 Hz, 1H, H-3), 7.03 (d, J = 2.4 Hz, 1H, H-2), 9.45 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 23.3 (t), 28.5 (t), 32.8 (t), 55.0 (d), 112.2 (d), 126.3 (d), 127.0 (s), 133.0 (s), 185.2 (s). Anal. Calcd. for C9H10BrNO: C, 47.39; H, 4.42; N, 6.14. Found: 47.50; H, 4.27; N, 6.38.
General Procedure for the Synthesis of [1,3]Thiazole Derivatives (37–39)
To a solution of the suitable α-bromo derivative 21,31,32 (0.5 mmol) in anhydrous DMF (5 mL), Na2CO3 (1 mmol) and the proper thiourea (1 mmol) were added and the mixture was stirred at room temperature for 16 h. The reaction was poured onto ice and brine. In the case of formation of a precipitate, the solid was collected by filtration, in the absence of precipitate, the aqueous solution was extracted with dichloromethane (3 × 40 mL). The organic phase was dried over Na2SO4 and the solvent evaporated under reduced pressure. The crude product was purified by column chromatography or crystallization.
8-(Phenylsulfonyl)-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indol-2-Amine (37)
This compound was obtained by reaction of 21 with thiourea and was purified by crystallization from ethanol. Brown solid; yield 90%; mp: 79.2–80.1 °C (EtOH); IR: νmax = 3415–3275 (NH2) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 2.65–2.75 (m, 4H, 2 × CH2), 6.26 (d, J = 3.2 Hz, 1H, H-7), 6.84 (s, 2H, NH2), 7.31 (d, J = 3.2 Hz, 1H, H-8), 7.53–7.75 (m, 3H, H-3′, H-4′ and H-5′), 8.24–8.31 (m, 2H, H-2′ and H-6′); 13C NMR (500 MHz, DMSO-d6) δ 21.52 (t), 22.47 (t), 110.87 (d), 114.78 (s), 120.99 (d), 123.55 (s), 125.39 (s), 128.78 (2 × d), 129.08 (2 × d), 139.97 (d), 137.55 (s), 138.19 (s), 164.89 (s); Anal. Calcd. for C15H13N3O2S2: C, 54.36; H, 3.95; N, 12.68. Found: C, 54.12; H, 4.08; N, 12.79.
2-Amino-N-(5-Chloro-2-Methoxyphenyl)-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indole-7-Carboxamide (38)
This compound was obtained by reaction of 31 with thiourea and was purified by crystallization from methanol. Yellow solid; mp: 214–215 °C; yield 70%; IR: νmax = 3422 (NH), 3330 (NH), 3205–3134 (NH2), 1669 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 2.71–2.90 (m, 4H, 2 × CH2), 3.89 (s, 3H, CH3), 6.77 (s, 1H, H-6), 6.92 (s, 2H, NH2), 7.10–7.19 (m, 2H, Ar), 8.04 (d, J = 2.2 Hz, 1H, Ar), 9.12 (s, 1H, NH), 11.90 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 21.7 (t), 22.3 (t), 56.1 (q), 112.4 (d), 113.4 (d), 116.0 (s), 117.9 (s), 121.7 (s), 121.7 (d), 123.2 (d), 123.7 (s), 128.6 (s), 129.4 (s), 139.1 (s), 148.6 (s), 158.2 (s), 167.1 (s). Anal calcd for C17H15ClN4O2S: C, 54.47; H, 4.03; N, 14.95. Found: C, 54.63; H, 3.85; N, 15.08.
2-Amino-N-(5-Chloro-2-Methoxyphenyl)-8-Methyl-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indole-7-Carboxamide (39)
This compound was obtained by reaction of 32 with thiourea and was purified by column chromatography (dichloromethane). Grey solid; yield 93%; mp: 173–174 °C; IR: νmax = 3426 (NH), 3324–3206 (NH2), 1664 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 2.68–2.82 (m, 4H, 2 × CH2), 3.86 (s, 3H, CH3), 4.23 (s, 3H, CH3), 6.86 (s, 1H, H-6), 6.99–7.22 (m, 4H, Ar and NH2), 8.03 (s, 1H, Ar), 8.74 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 22.0 (t), 22.1 (t), 34.4 (q), 56.2 (q), 112.2 (d), 112.3 (d), 117.0 (s), 117.3 (s), 120.9 (d), 122.8 (s), 123.1 (d), 123.8 (s), 128.7 (s), 131.4 (s), 138.8 (s), 148.5 (s), 159.3 (s), 166.6 (s). Anal calcd for C18H17ClN4O2S: C, 55.59; H, 4.41; N, 14.41. Found: C, 55.72; H, 4.61; N, 14.29.
General Procedure for the Synthesis of N-Substituted[1,3]Thiazole Derivatives (40–45)
To a solution of the suitable 2-amine-thiazole 37–39 (0.46 mmol) in anhydrous 1,4-dioxane (3 mL), Et3N (0.07 mL, 0.51 mmol) and the suitable acyl chloride (0.69 mmol) were added and the reaction mixture was stirred at room temperature for 16 h. Then the reaction mixture was poured onto crushed ice. The precipitate was collected by filtration, dried and purified by column chromatography (dichloromethane).
2-Methyl-N-[8-(Phenylsulfonyl)-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indol-2-yl]Propanamide (40)
This compound was obtained by reaction of 37 with isobutyryl chloride. White solid; mp: 186–187 °C; yield 60%; IR: νmax = 3313 (NH), 1680 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.07 (s, 3H, CH3), 1.10 (s, 3H, CH3), 2.35–2.56 (m, 1H, CH), 2.67–2.89 (m, 4H, 2 × CH2), 6.23 (m, J = 3.3 Hz, 1H, Ar), 7.33–7.53 (m, 4H, Ar), 8.09–8–14 (m, 2H, Ar), 10.10 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 19.2 (2 × q), 22.1 (t), 23.0 (t), 35.3 (d), 111.5 (d), 122.5 (d), 123.0 (s), 125.4 (s), 125.7 (s), 128.1 (2 × d), 128.8 (2 × d), 133.8 (d), 137.1 (s), 138.3 (s), 155.2 (s), 175.1 (s). Anal calcd for C19H19N3O3S2: C, 56.84; H, 4.77; N, 10.47. Found: C, 56.72; H, 4.89; N, 10.63.
N-(5-Chloro-2-Methoxyphenyl)-2-[(2-Methylpropanoyl)Amino]-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indole-7-Carboxamide (41)
This compound was obtained by reaction of 38 with isobutyryl chloride. White solid; mp: 255–256 °C; yield 68%; IR: νmax = 3610 (NH), 3582 (NH), 3561 (NH), 1700 (CO), 1684 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.12 (s, 3H, CH3), 1.15 (s, 3H, CH3), 2.75–2.85 (m, 3H, CH2 and CH), 2.94 (t, J = 7.4 Hz, 2H, CH2), 3.89 (s, 3H, CH3), 6.87 (s, 1H, H-6), 7.07–7.18 (m, 2H, Ar), 8.04 (d, J = 2.2 Hz, 1H, Ar), 9.07 (s, 1H, NH), 11.81 (s, 1H, NH), 11.93 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 19.2 (2 × q), 21.6 (t), 22.0 (t), 33.6 (d), 56.1 (q), 112.5 (d), 112.7 (d), 119.0 (s), 121.7 (d), 122.4 (s), 123.4 (d), 123.7 (s), 124.2 (s), 128.5 (s), 128.8 (s), 138.3 (s), 148.7 (s), 156.0 (s), 158.4 (s), 175.2 (s). Anal calcd for C21H21ClN4O3S: C, 56.69; H, 4.76; N, 12.59. Found: C, 56.55; H, 4.49; N, 12.73.
N-(5-Chloro-2-Methoxyphenyl)-8-Methyl-2-[(2-Methylpropanoyl)Amino]-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indole-7-Carboxamide (42)
This compound was obtained by reaction of 39 with isobutyryl chloride. White solid; mp: 240–241 °C; yield 81%; IR: νmax = 3428 (NH), 3250 (NH), 1664 (CO), 1658 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.31 (s, 3H, CH3), 1.34 (s, 3H, CH3), 2.66–2.99 (m, 5H, 2 × CH2 and CH), 3.91 (s, 3H, CH3), 4.35 (s, 3H, CH3), 6.59 (s, 1H, H-6), 6.79 (d, J = 8.7 Hz, 1H, Ar), 6.98 (dd, J = 8.7, 2.4 Hz, 1H, Ar), 8.23 (s, 1H, NH), 8.49 (d, J = 2.4 Hz, 1H, Ar), 10.34 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 19.1 (2 × q), 22.2 (t), 22.7 (t), 35.0 (q), 35.7 (d), 56.1 (q), 110.6 (d), 110.8 (s), 111.0 (d), 119.2 (d), 119.3 (s), 122.5 (d), 123.9 (s), 125.3 (s), 126.3 (s), 129.0 (s), 129.6 (s), 146.3 (s), 156.5 (s), 159.4 (s), 174.6 (s). Anal calcd for C22H23ClN4O3S: C, 57.57; H, 5.05; N, 12.21. Found: C, 57.72; H, 4.88; N, 12.40.
2,2-Dimethyl-N-[8-(Phenylsulfonyl)-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indol-2-yl]Propanamide (43)
This compound was obtained by reaction of 37 with trimethylacetyl chloride. White solid; mp: 87–88 °C; yield 65%; IR: νmax = 3502 (NH), 1675 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.28 (s, 9H, 3 × CH3), 2.68 – 2.98 (m, 4H, 2 × CH2), 6.20–6.26 (m, 1H, Ar), 7.35–7.68 (m, 4H, Ar), 7.91–8.08 (m, 2H, Ar), 9.94 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 22.0 (t), 22.9 (t), 26.9 (3 × q), 40.5 (s), 110.5 (d), 112.0 (d), 122.7 (s), 123.0 (s), 126.4 (s), 127.6 (2 × d), 128.8 (2 × d), 133.7 (d), 134.0 (s), 138.8 (s), 149.5 (s), 176.2 (s). Anal calcd for C20H21N3O3S2: C, 57.81; H, 5.09; N, 10.11. Found: C, 57.97; H, 4.90; N, 10.23.
N-(5-Chloro-2-Methoxyphenyl)-2-[(2,2-Dimethylpropanoyl)Amino]-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indole-7-Carboxamide (44)
This compound was obtained by reaction of 38 with trimethylacetyl chloride. White solid; yield 65%; mp: 155–156 °C; IR: νmax = 3420 (NH), 3404 (NH), 3259 (NH), 1669 (CO), 1653 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.33 (s, 9H, 3 × CH3), 2.90–3.00 (m, 4H, 2 × CH2), 3.91 (s, 3H, CH3), 6.58 (s, 1H, H-6), 6.79 (d, J = 8.7 Hz, 1H, Ar), 6.97 (dd, J = 8.7, 2.3 Hz, 1H, Ar), 8.18 (s, 1H, NH), 8.52 (d, J = 2.3 Hz, 1H, Ar), 9.17 (s, 1H, NH), 10.24 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 22.2 (t), 22.7 (t), 27.2 (3 × q), 39.2 (s), 56.1 (q), 109.5 (d), 110.6 (d), 119.6 (d), 122.6 (d), 123.9 (s), 124.3 (s), 126.2 (s), 128.8 (s), 129.3 (s), 138.1 (s), 146.3 (s), 150.5 (s), 156.8 (s), 158.8 (s), 176.2 (s). Anal calcd for C22H23ClN4O3S: C, 57.57; H, 5.05; N, 12.21. Found: C, 57.78; H, 5.19; N, 12.08.
N-(5-Chloro-2-Methoxyphenyl)-2-[(2,2-Dimethylpropanoyl)Amino]-8-Methyl-5,8-Dihydro-4H-[1,3]Thiazolo[5,4-g]Indole-7-Carboxamide (45)
This compound was obtained by reaction of 39 with trimethylacetyl chloride. Brown solid; mp: 213–214 °C; yield 78%; IR: νmax = 3430 (NH), 3412 (NH), 1684 (CO), 1675 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.36 (s, 9H, 3 × CH3), 2.78–3.00 (m, 4H, 2 × CH2), 3.90 (s, 3H, CH3), 4.35 (s, 3H, CH3), 6.59 (s, 1H, H-6), 6.78 (d, J = 8.7 Hz, 1H, Ar), 6.96 (dd, J = 8.7, 2.5 Hz, 1H, Ar), 8.23 (s, 1H, NH), 8.51 (d, J = 2.5 Hz, 1H, Ar), 8.96 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 22.4 (t), 22.8 (t), 27.3 (3 × q), 34.9 (q), 39.1 (s), 56.1 (q), 110.6 (d), 111.0 (d), 118.3 (s), 119.1 (d), 122.3 (d), 124.4 (s), 125.0 (s), 126.2 (s), 129.2 (s), 131.4 (s), 138.6 (s), 146.3 (s), 155.3 (s), 159.6 (s), 176.0 (s). Anal calcd for C23H25ClN4O3S: C, 58.40; H, 5.33; N, 11.85. Found: C, 58.56; H, 5.19; N, 11.98.
General Procedure for the Synthesis of [1,3]Thiazole Derivatives (46–59)
To a solution of the suitable bromo derivative 22,23,25–30,33–36 (0.5 mmol) in anhydrous DMF (5 mL), Na2CO3 (1 mmol) and the proper thiourea (1 mmol) were added and the mixture was stirred at room temperature for 16 h. The reaction was poured onto ice and brine. In the case of formation of a precipitate, the solid was collected by filtration, in the absence of precipitate, the aqueous solution was extracted with dichloromethane (3 × 40 mL). The organic phase was dried over Na2SO4 and the solvent evaporated under reduced pressure. The crude product was purified by column chromatography or crystallization.
4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazol-2-Amine (46)
This compound was obtained by reaction of 22 with thiourea and was purified by crystallization from ethanol. Brown solid; yield 64%; mp: 66.4–67.2 °C; IR: νmax = 3455 3416 (NH2), 3287 (NH) cm−1; 1H NMR (200 MHz, CDCl3) δ 2.02–2.09 (m, 2H, CH2), 2.87–2.94 (4H, m, 2 × CH2), 4.97 (s, 2H, NH2), 5.99 (d, J = 2.9 Hz, 1H, H-7), 6.58 (d, J = 2.9 Hz, 1H H-8), 9.27 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 24.31 (t), 27.57 (t), 28.52 (t), 110.28 (d), 116.18 (d), 117.02 (s), 120.53 (s), 124.79 (s), 139.16 (s), 144.61 (s). Anal. Calcd. for C10H11N3S: C, 58.51; H, 5.40; N, 20.47. Found: C, 58.65; H, 5.53; N, 20.30.
9-(Phenylsulfonyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazol-2-Amine (47)
This compound was obtained by reaction of 23 with thiourea and was purified by column chromatography (dichloromethane: ethyl acetate 8:2). Yellow solid; yield 64 %, mp: 173–174 °C; IR: νmax = 3252–3143 (NH2) cm−1; 1H NMR (200 MHz, CDCl3) δ 2.01–2.11 (m, 2H, CH2), 2.41 (t, J = 6.7 Hz, 2H, CH2), 2.45 (t, J = 6.7 Hz, 2H, CH2), 4.68 (s, 2H, NH2), 6.17 (d, J = 3.0 Hz, 1H, H-7), 7.35 (d, J = 3.0 Hz, 1H, H-8), 7.40–7.55 (m, 3H, H-3′, H-4′ and H-5′), 7.86 (d, J = 7.0 Hz, 2H, H-2′ and H-6′); 13C NMR (50 MHz, CDCl3) δ 24.0 (t), 25.0 (t), 31.8 (t), 113.5 (d), 123.2 (d), 125.3 (s), 126.7 (s), 127.2 (2 × d), 128.4 (2 × d), 130.5 (s), 133.0 (d), 138.3 (s), 140.1 (s), 162.7 (s). Anal. Calcd. for C16H15N3O2S2: C, 55.63; H, 4.38; N, 12.16. Found: C, 55.79; H, 4.22; N, 11.89.
Ethyl 2-Amino-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (48)
This compound was obtained by reaction of 25 with thiourea and was purified by column chromatography (dichloromethane: ethyl acetate 8:2). White solid; yield 62%; mp: 190.3–191.1 °C; IR: νmax = 3370 (NH), 3202–3156 (NH2), 1692 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.27 (t, J = 7.1 Hz, 3H, CH3), 1.85–1.99 (m, 2H, CH2), 2.75–2.85 (m, 4H, 2 × CH2), 4.22 (q, J = 7.1 Hz, 2H, CH2), 6.63 (s, 1H, H-7), 6.88 (s, 2H, NH2), 9.64 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 14.33 (q), 23.91 (t), 27.04 (t), 27.64 (t), 59.55 (t), 116.41 (d), 118.95 (s), 119.33 (s), 121.62 (s), 129.87 (s), 137.86 (s), 159.91 (s), 165.52 (s). Anal. Calcd. for C13H15N3O2S: C, 56.30; H, 5.45; N, 15.15. Found: C, 56.16; H, 5.57; N, 15.30.
Ethyl 2-Amino-9-Methyl-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (49)
This compound was obtained by reaction of 26 with thiourea and was purified by column chromatography (dichloromethane: ethyl acetate 8:2). Yellow solid; yield 60%, mp: 113–114 °C; IR: νmax = 3255–3188 (NH2), 1690 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.34 (t, J = 7.1 Hz, 3H, CH3), 2.05–2.09 (m, 2H, CH2), 2.61 (t, J = 6.4 Hz, 2H, CH2), 2.76 (t, J = 6.4 Hz, 2H, CH2), 4.16 (s, 3H, CH3), 4.27 (q, J = 7.1 Hz, 2H, CH2), 4.86 (s, 2H, NH2), 6.79 (s, 1H, H-7); 13C NMR (50 MHz, CDCl3) δ 14.5 (q), 25.7 (t), 25.8 (t), 29.9 (t), 35.2 (q), 59.6 (t), 117.3 (d), 121.8 (s), 124.2 (s), 126.2 (s), 132.7 (s), 139.0 (s), 161.5 (s), 163.3 (s). Anal. Calcd. for C14H17N3O2S: C, 57.71; H, 5.88; N, 14.42. Found: C, 57.93; H, 5.67; N, 14.28.
Ethyl 2-Amino-9-(4-Methoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (50)
This compound was obtained by reaction of 27 with thiourea and was purified by column chromatography (dichloromethane: ethyl acetate 8:2). Yellow solid; yield 69%, mp: 59 °C; IR: νmax = 3253–3175 (NH2), 1715 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.27 (t, J = 7.1 Hz, 3H, CH3), 2.05–2.12 (m, 2H, CH2), 2.63 (t, J = 6.5 Hz, 2H, CH2), 2.73 (t, J = 6.5 Hz, 2H, CH2), 3.73 (s, 3H, CH3), 4.17 (q, J = 7.1 Hz, 2H, CH2), 4.73 (s, 2H, NH2), 6.09 (s, 2H, CH2), 6.73 (d, J = 8.6 Hz, 2H, H-3′ and H-5′), 6.86 (s, 1H, H-7) 6.89 (d, J = 8.6 Hz, 2H, H-2′ and H-6′); 13C NMR (50 MHz, CDCl3) δ 14.4 (q), 25.5 (t), 25.7 (t), 30.2 (t), 48.6 (t), 55.1 (q), 59.6 (t), 113.5 (2 × d), 118.6 (d), 121.4 (s), 124.7 (s), 126.3 (s), 127.5 (2 × d), 132.5 (s), 132.6 (s), 139.1 (s), 158.1 (s), 161.1 (s), 163.4 (s). Anal. Calcd. for C21H23N3O3S: C, 63.45; H, 5.83; N, 10.57. Found: C, 63.31; H, 5.97; N, 10.44.
Ethyl 2-Amino-9-(3,5-Dimethoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (51)
This compound was obtained by reaction of 28 with thiourea and was purified by column chromatography (dichloromethane: ethyl acetate 8:2). Brown solid; yield 58%, mp: 140.6–141.0 °C; IR: νmax = 3377–3253 (NH2), 1696 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.27 (t, J = 7.1 Hz, 3H, CH3), 2.03–2.13 (m, 2H, CH2), 2.63 (t, J = 6.6 Hz, 2H, CH2), 2.73 (t, J = 6.6 Hz, 2H, CH2), 3.66 (s, 6H, 2 × CH3), 4.19 (q, J = 7.1 Hz, 2H, CH2), 4.77 (s, 2H, NH2), 6.08 (s, 2H, CH2), 6.14 (s, 2H, H-2′ and H-6′), 6.23 (s, 1H, H-4′), 6.87 (s, 1H, H-7); 13C NMR (50 MHz, CDCl3) δ 14.4 (q), 25.6 (t), 25.7 (t), 30.2 (t), 49.1 (t), 55.1 (2 × q), 59.6 (t), 98.6 (d), 104.1 (2 × d), 118.6 (d), 121.7 (s), 124.7 (s), 126.1 (s), 132.7 (s), 139.1 (s), 142.9 (s), 160.5 (2 × s), 161.1 (s), 163.5 (s). Anal. Calcd. for C22H25N3O4S: C, 61.81; H, 5.89; N, 9.83. Found: C, 61.68; H, 5.72; N, 9.99.
Ethyl 2-Amino-9-(3,4,5-Trimethoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (52)
This compound was obtained by reaction of 29 with thiourea and was purified by column chromatography (dichloromethane: ethyl acetate 8:2). Yellow solid; yield 92%, mp: 149.5–150.3 °C; IR: νmax = 3408–3357 (NH2), 1701 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.30 (t, J = 7.1 Hz, 3H, CH3), 2.03–2.11 (m, 2H, CH2), 2.63 (t, J = 6.4 Hz, 2H, CH2), 2.73 (t, J = 6.4 Hz, 2H, CH2), 3.70 (s, 6H, 2 × CH3), 3.76 (s, 3H, CH3), 4.22 (q, J = 7.1 Hz, 2H, CH2), 4.78 (s, 2H, NH2), 6.14 (s, 2H, CH2), 6.23 (s, 2H, H-2′ and H-6′), 6.86 (s, 1H, H-7); 13C NMR (50 MHz, CDCl3) δ 14.4 (q), 25.5 (t), 25.6 (t), 30.4 (t), 48.8 (t), 55.7 (2 × q), 59.7 (t), 60.7 (q), 103.6 (2 × d), 118.7 (d), 121.6 (s), 124.9 (s), 126.1 (s), 132.6 (s), 135.9 (s), 136.3 (s), 139.3 (s), 152.8 (2 × s), 161.2 (s), 163.6 (s). Anal. Calcd. for C23H27N3O5S: C, 60.38; H, 5.95; N, 9.18. Found: C, 60.61; H, 6.11; N, 9.02.
Ethyl 2-Amino-9-(2-Bromo-3,5-Dimethoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (53)
This compound was obtained by reaction of 30 with thiourea and was purified by column chromatography (dichloromethane: ethyl acetate 8:2). Brown solid; yield 53%, mp: 189.3–189.9 °C; IR: νmax = 3334–3227 (NH2), 1695 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.25 (t, J = 7.1 Hz, 3H, CH3), 2.00–2.09 (m, 2H, CH2), 2.69–2.84 (m, 4H, 2 × CH2), 3.56 (s, 3H, CH3), 3.84 (s, 3H, CH3), 4.18 (q, J = 7.1 Hz, 2H, CH2), 4.62 (s, 2H, NH2), 5.43 (s, 1H, H-4′), 6.21 (s, 2H, CH2), 6.28 (s, 1H, H-6′), 6.91 (s, 1H, H-7); 13C NMR (50 MHz, CDCl3) δ 14.3 (q), 26.6 (t), 26.7 (t), 28.4 (t), 51.1 (t), 55.1 (q), 56.2 (q), 59.7 (t), 97.2 (d), 101.3 (s), 103.2 (d), 118.7 (d), 121.9 (s), 124.6 (s), 126.0 (s), 132.4 (s), 138.3 (s), 142.4 (s), 156.1 (s), 159.7 (s), 160.9 (s), 162.9 (s). Anal. Calcd. for C22H24BrN3O4S: C, 52.18; H, 4.78; N, 8.30. Found: C, 51.93; H, 4.55; N, 8.57.
2-Amino-N-(5-Chloro-2-Methoxyphenyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (54)
This compound was obtained by reaction of 33 with thiourea and was purified by column chromatography (petroleum ether: ethyl acetate 7:3). Yellow solid; mp: 240–241 °C; yield 96%; IR: νmax = 3422 (NH), 3307 (NH), 3239–3205 (NH2), 1658 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.84–2.01 (m, 2H, CH2), 2.74–2.89 (m, 4H, 2 × CH2), 3.87 (s, 3H, CH3), 6.73–6.82 (m, 3H, H-7 and NH2), 7.06–7.17 (m, 2H, Ar), 7.92 (s, 1H, Ar), 9.29 (s, 1H, NH), 10.43 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 24.0 (t), 27.1 (t), 27.8 (t), 56.1 (q), 112.5 (d), 114.8 (d), 118.7 (s), 121.5 (s), 122.6 (d), 122.7 (s), 123.8 (d), 123.7 (s), 128.3 (s), 129.1 (s), 137.6 (s), 149.2 (s), 158.4 (s), 165.4 (s). Anal calcd for C18H17ClN4O2S: C, 55.59; H, 4.41; N, 14.41. Found: C, 55.44; H, 4.58; N, 14.26.
2-Amino-N-Tert-Butyl-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (55)
This compound was obtained by reaction of 34 with thiourea and was purified by column chromatography (petroleum ether: ethyl acetate 7:3). Light yellow solid; mp: 166–167 °C; yield 74%; IR: νmax = 3433–3378 (NH2), 3312 (NH), 3186 (NH), 1635 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.35 (s, 9H, 3 × CH3), 1.85–1.96 (m, 2H, CH2), 2.72–2.85 (m, 4H, 2 × CH2), 6.54 (s, 1H, H-7), 6.71 (s, 2H, NH2), 7.56 (s, 1H, NH), 10.26 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 24.1 (t), 27.1 (t), 27.9 (t), 28.8 (3 × q), 50.4 (s), 113.4 (d), 117.1 (s), 120.5 (s), 124.6 (s), 127.2 (s), 138.0 (s), 159.8 (s), 165.1 (s). Anal calcd for C15H20N4OS: C, 59.18; H, 6.62; N, 18.41. Found: C, 59.05; H, 6.81; N, 18.28.
2-Amino-N-(5-Chloro-2-Methoxyphenyl)-9-Methyl- 4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (56)
This compound was obtained by reaction of 35 with thiourea and was purified by crystallization from diethyl ether. Yellow solid; yield 93%; mp: 181–182 °C; IR: νmax = 3428 (NH), 3268–3182 (NH2), 1669 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.99–2.10 (m, 2H, CH2), 2.63 (t, J = 6.4 Hz, 2H, CH2), 2.78 (t, J = 6.4 Hz, 2H, CH2), 3.92 (s, 3H, CH3), 4.13 (s, 3H, CH3), 6.89 (s, 1H, H-7), 7.12–7.30 (m, 4H, Ar and NH2), 8.06 (d, J = 1.8 Hz, 1H, Ar), 8.92 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 25.2 (t), 25.5 (t), 29.2 (t), 35.0 (q), 56.2 (q), 112.4 (d), 113.8 (d), 121.2 (d), 122.9 (s), 123.5 (d), 125.0 (s), 126.4 (s), 126.8 (s), 127.3 (s), 128.8 (s), 147.8 (s), 155.4 (s), 156.0 (s), 160.6 (s). Anal calcd for C19H19ClN4O2S: C, 56.64; H, 4.75; N, 13.91. Found: C, 56.42; H, 4.99; N, 14.09.
2-Amino-N-Tert-Butyl-9-Methyl-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (57)
This compound was obtained by reaction of 36 with thiourea and was purified by crystallization from diethyl ether. Light yellow solid; yield 90%; mp: 228–229 °C; IR: νmax = 3416–3302 (NH2), 3188 (NH), 1646 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.34 (s, 9H, 3 × CH3), 1.87–2.00 (m, 2H, CH2), 2.52 (t, J = 6.5 Hz, 2H, CH2), 2.69 (t, J = 6.5 Hz, 2H, CH2), 4.01 (s, 3H, CH3), 6.52 (s, 1H, H-7), 6.83 (s, 2H, NH2), 7.25 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 25.3 (t), 26.0 (t), 28.8 (3 × q), 29.1 (t), 34.6 (q), 50.4 (s), 112.7 (d), 121.0 (s), 121.8 (s), 126.3 (s), 129.8 (s), 138.5 (s), 161.5 (s), 164.4 (s). Anal calcd for C16H22N4OS: C, 60.35; H, 6.96; N, 17.59. Found: C, 60.57; H, 7.11; N, 17.46.
N-Tert-Butyl-2-[(5-Chloro-2-Methoxyphenyl)Amino]-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (58)
This compound was obtained by reaction of 34 with 1-(5-chloro-2-methoxyphenyl)thiourea and was purified by column chromatography (petroleum ether: ethyl acetate 7:3). Orange solid; mp: 134–135 °C; yield 65%; IR: νmax = 3438 (NH), 3405 (NH), 3291 (NH), 1710 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.45 (s, 9H, 3 × CH3), 2.00–2.15 (m, 2H, CH2), 2.83–2.97 (m, 4H, 2 × CH2), 3.89 (s, 3H, CH3), 5.77 (s, 1H, NH), 6.32 (s, 1H, H-7), 6.77 (d, J = 8.6 Hz, 1H, Ar), 6.91 (dd, J = 8.6, 2.0 Hz, 1H, Ar), 7.26 (s, 1H, NH), 7.88 (d, J = 2.0 Hz, 1H, Ar), 9.83 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 24.2 (t), 27.6 (t), 28.5 (t), 29.2 (3 × q), 51.4 (s), 56.0 (q), 110.6 (d), 110.9 (d), 115.9 (d), 120.1 (s), 121.1 (d), 122.1 (s), 124.6 (s), 126.1 (s), 127.7 (s), 130.8 (s), 146.0 (s), 160.0 (s), 160.7 (s), 176.1 (s). Anal calcd for C22H25ClN4O2S: C, 59.38; H, 5.66; N, 12.59. Found: C, 59.53; H, 5.79; N, 12.42.
N-tert-Butyl-2-[(5-Chloro-2-Methoxyphenyl)Amino]-9-Methyl-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (59)
This compound was obtained by reaction of 36 with 1-(5-chloro-2-methoxyphenyl)thiourea and was purified by column chromatography (petroleum ether: ethyl acetate 9:1). Light brown solid; mp: 163–164 °C; yield 85%; IR: νmax = 3588 (NH), 3559 (NH), 1703 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.45 (s, 9H, 3 × CH3), 2.02–2.15 (m, 2H, CH2), 2.66 (t, J = 6.5 Hz, 2H, CH2), 2.85 (t, J = 6.5 Hz, 2H, CH2), 3.90 (s, 3H, CH3), 4.26 (s, 3H, CH3), 5.74 (s, 1H, NH), 6.30 (s, 1H, H-7), 6.77 (d, J = 8.6 Hz, 1H, Ar), 6.89 (dd, J = 8.6, 2.4 Hz, 1H, Ar), 7.48 (s, 1H, NH), 8.39 (d, J = 2.4 Hz, 1H, Ar); 13C NMR (50 MHz, CDCl3) δ 25.8 (t), 26.3 (t), 29.1 (3 × q), 29.4 (t), 35.5 (q), 51.3 (s), 56.0 (q), 110.5 (d), 111.2 (d), 116.5 (d), 120.5 (d), 120.6 (s), 123.5 (s), 124.2 (s), 126.3 (s), 127.1 (s), 130.8 (s), 139.6 (s), 145.4 (s), 158.5 (s), 162.0 (s). Anal calcd for C23H27ClN4O2S: C, 60.18; H, 5.93; N, 12.21. Found: C, 59.95; H, 6.08; N, 12.45.
General Procedure for the Synthesis of N-Acetyl-[1,3]Thiazole Derivatives (60–65)
To a solution of the suitable 2-amine-thiazole 47,49–53 (0.5 mmol) in anhydrous dichloromethane (5 mL), DIPEA (0.1 mL, 0.6 mmol) and acetyl chloride (0,04 mL, 0.55 mmol) were added at 0 °C, and the reaction mixture was stirred at room temperature for 24 h. Water (3 mL) was added and the organic phase was separated and the aqueous phase was extracted with dichloromethane (2 × 5 mL). The organic phase was dried over Na2SO4 and the solvent evaporated under reduced pressure. The residue was purified by column chromatography (dichloromethane: ethyl acetate 95:5).
N-[9-(Phenylsulfonyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazol-2-yl]Acetamide (60)
This compound was obtained by reaction of 47. White solid; yield 73%, mp: 222.6–223.4 °C; IR: νmax = 3408 (NH), 1713 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.83 (s, 3H, CH3), 2.04–2.10 (m, 4H, 2 × CH2), 2.32–2.36 (m, 2H, CH2), 6.27 (s, 1H, H-7), 7.30–7.36 (m, 3H, H-3′, H-4′ and H-5′), 7.44 (s, 1H, H-8), 7.71 (d, J = 6.4 Hz, 2H, H-2′ and H-6′), 11.95 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 21.8 (q), 22.4 (t), 23.6 (t), 34.1 (t), 113.2 (d), 123.1 (d), 125.5 (s), 127.4 (2 × d), 128.7 (2 × d), 130.1 (s), 131.1 (s), 133.6 (d), 136.6 (s), 137.9 (s), 155.8 (s), 168.8 (s). Anal calcd for C18H17N3O3S2: C, 55.80; H, 4.42; N, 10.84. Found: C, 55.99; H, 4.27; N, 10.71.
Ethyl 2-(Acetylamino)-9-Methyl-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (61)
This compound was obtained by reaction of 49. Pale yellow solid; yield: 62%, mp: 193.8–194.2 °C; IR: νmax = 3399 (NH), 1701 (CO), 1597 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.35 (t, J = 7.1 Hz, 3H, CH3), 1.88 (s, 3H, CH3), 2.06–2.14 (m, 2H, CH2), 2.61 (t, J = 6.3 Hz, 2H, CH2), 2.87 (t, J = 6.3 Hz, 2H, CH2), 4.12 (s, 3H, CH3), 4.28 (q, J = 7.1 Hz, 2H, CH2), 6.83 (s, 1H, H-7), 10.5 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 14.4 (q), 22.1 (q), 25.1 (t), 25.5 (t), 30.0 (t), 34.9 (q), 59.8 (t), 117.5 (d), 122.4 (s), 124.5 (s), 130.1 (s), 132.0 (s), 137.4 (s), 155.3 (s), 161.4 (s), 167.8 (s). Anal calcd for C16H19N3O3S: C, 57.64; H, 5.74; N, 12.60. Found: C, 57.48; H, 5.89; N, 12.77.
Ethyl 2-(Acetylamino)-9-(4-Methoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (62)
This compound was obtained by reaction of 50. Yellow solid; yield 65 %, mp: 87.6–88.4 °C; IR: νmax = 3403 (NH), 1696 (CO), 1557 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.28 (t, J = 7.0 Hz, 3H, CH3), 1.92 (s, 3H, CH3), 2.11–2.19 (m, 2H, CH2), 2.61 (t, J = 6.3 Hz, 2H, CH2), 2.82 (t, J = 6.3 Hz, 2H, CH2), 3.69 (s, 3H, CH3), 4.20 (q, J = 7.0 Hz, 2H, CH2), 6.00 (s, 2H, CH2), 6.63 (d, J = 8.3 Hz, 2H, H-3′ and H-5′), 6.77 (d, J = 8.3 Hz, 2H H-2′ and H-6′), 6.91 (s, 1H, H-7), 10.11 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 14.3 (q), 22.5 (q), 24.7 (t), 25.2 (t), 30.8 (t), 48.5 (t), 55.1 (q), 59.8 (t), 113.5 (2 × d), 118.6 (d), 122.0 (s), 125.0 (s), 127.2 (2 × d), 130.2 (s), 131.7 (s), 132.0 (s), 137.6 (s), 155.2 (s), 158.2 (s), 161.0 (s), 167.8 (s). Anal calcd for C23H25N3O4S: C, 62.85; H, 5.73; N, 9.56. Found: C, 62.65; H, 5.58; N, 9.72.
Ethyl 2-(Acetylamino)-9-(3,5-Dimethoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (63)
This compound was obtained by reaction of 51. Brown solid; Yield: 72%, mp: 70.2–71.0 °C; IR: νmax = 3264 (NH), 1695 (CO), 1597 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.27 (t, J = 7.1 Hz, 3H, CH3), 1.94 (s, 3H, CH3), 2.10–2.25 (m, 2H, CH2), 2.62 (t, J = 6.3 Hz, 2H, CH2), 2.83 (t, J = 6.3 Hz, 2H, CH2), 3.57 (s, 6H, 2 × CH3), 4.18 (q, J = 7.1 Hz, 2H, CH2), 5.98 (s, 2H, CH2), 6.04 (s, 2H, H-2′ and H-6′), 6.17 (s, 1H, H-4′), 6.93 (s, 1H, H-7), 9.96 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 14.3 (q), 22.6 (q), 24.8 (t), 25.3 (t), 30.7 (t), 49.1 (t), 55.0 (2 × q), 59.8 (t), 98.1 (d), 103.9 (2 × d), 118.8 (d), 122.2 (s), 124.9 (s), 130.1 (s), 132.0 (s), 137.8 (s), 142.4 (s), 154.9 (s), 160.6 (2 × s), 161.0 (s), 167.6 (s). Anal calcd for C24H27N3O5S: C, 61.39; H, 5.80; N, 8.95. Found: C, 61.57; H, 5.69; N, 9.11.
Ethyl 2-(Acetylamino)-9-(3,4,5-Trimethoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (64)
This compound was obtained by reaction of 52. Pale brown solid; yield: 59%, mp: 73.1–73.8 °C; IR: νmax = 3405 (NH), 1703 (CO), 1597 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.30 (t, J = 7.1 Hz, 3H, CH3), 2.00 (s, 3H, CH3), 2.13–2.17 (m, 2H, CH2), 2.61–2.65 (m, 2H, CH2), 2.80–2.85 (m, 2H, CH2), 3.61 (s, 6H, 2 × CH3), 3.73 (s, 3H, CH3), 4.23 (q, J = 7.1 Hz, 2H, CH2), 6.08 (s, 2H, CH2), 6.13 (s, 2H, H-2′ and H-6′), 6.91 (s, 1H, H-7), 10.41 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 14.4 (q), 22.4 (q), 24.9 (t), 25.3 (t), 30.6 (t), 48.8 (t), 55.7 (2 × q), 59.9 (t), 60.7 (q), 103.2 (2 × d), 118.9 (d), 122.0 (s), 125.2 (s), 130.1 (s), 132.1 (s), 135.3 (s), 136.4 (s), 137.8 (s), 152.9 (2 × s), 155.3 (s), 161.2 (s), 168.0 (s). Anal calcd for C25H29N3O6S: C, 60.10; H, 5.85; N, 8.41. Found: C, 59.91; H, 5.98; N, 8.27.
Ethyl 2-(Acetylamino)-9-(2-Bromo-3,5-Dimethoxybenzyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxylate (65)
This compound was obtained by reaction of 53. White solid; yield 69%, mp: 192.0–192.8 °C; IR: νmax = 3413 (NH), 1704 (CO), 1593 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.29 (t, J = 7.0 Hz, 3H, CH3), 1.94–2.12 (m, 5H, CH3 and CH2), 2.73–2.77 (m, 2H, CH2), 2.89–2.92 (m, 2H, CH2), 3.56 (s, 3H, CH3), 3.82 (s, 3H, CH3), 4.18 (q, J = 7.0 Hz, 2H, CH2), 5.44 (s, 1H, H-4′), 6.14 (s, 1H, CH2), 6.25 (s, 1H, H-6′), 6.95 (s, 1H, H-7), 9.16 (s, 1H, NH); 13C NMR (200 MHz, CDCl3) δ 14.3 (q), 23.1 (q), 26.2 (t), 26.7 (t), 28.2 (t), 51.0 (t), 55.1 (q), 56.2 (q), 59.8 (t), 97.1 (d), 101.1 (s), 103.4 (d), 118.9 (d), 122.3 (s), 124.8 (s), 129.8 (s), 131.8 (s), 137.2 (s), 142.1 (s), 153.5 (s), 156.1 (s), 159.8 (s), 160.9 (s), 167.5 (s). Anal calcd for C24H26BrN3O5S: C, 52.56; H, 4.78; N, 7.66. Found: C, 52.71; H, 4.59; N, 7.81.
General procedure for the synthesis of N-substituted[1,3]thiazole derivatives (66–71)
To a solution of the suitable 2-amine-thiazole 47,54,56 (0.46 mmol) in anhydrous 1,4-dioxane (3 mL), Et3N (0.07 mL, 0.51 mmol) and the suitable acyl chloride (0.69 mmol) were added and the reaction mixture was stirred at room temperature for 16 h. Then the reaction mixture was poured onto crushed ice. The precipitate was collected by filtration, dried and purified by column chromatography (dichloromethane).
2-Methyl-N-[9-(Phenylsulfonyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazol-2-yl]Propanamide (66)
This compound was obtained by reaction of 47 with isobutyryl chloride. Light yellow solid; mp: 212–213 °C; yield 61%; IR: νmax = 3250 (NH), 1686 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.04 (s, 3H, CH3), 1.08 (s, 3H, CH3), 1.63–2.74 (m, 2H, CH2), 2.05–2.40 (m, 5H, 2 × CH2 and CH), 6.27 (d, J = 3.3 Hz, 1H, Ar), 7.31–7.52 (m, 4H, Ar), 7.70–7.74 (m, 2H, Ar), 10.89 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 19.2 (2 × q), 22.8 (t), 23.9 (t), 33.6 (t), 35.3 (d), 113.4 (d), 123.3 (d), 125.7 (s), 127.3 (2 × d), 128.7 (2 × d), 130.1 (s), 131.1 (s), 133.5 (d), 136.9 (s), 138.5 (s), 155.1 (s), 175.5 (s). Anal calcd for C20H21N3O3S2: C, 57.81; H, 5.09; N, 10.11. Found: C, 57.98; H, 4.91; N, 10.27.
N-(5-Chloro-2-Methoxyphenyl)-2-[(2-Methylpropanoyl)Amino]-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (67)
This compound was obtained by reaction of 54 with isobutyryl chloride. Yellow oil; yield 68%; IR: νmax = 3422 (NH), 3250 (NH), 3193 (NH), 1686 (CO), 1646 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.20 (s, 3H, CH3), 1.24 (s, 3H, CH3), 1.91–2.10 (m, 2H, CH2), 2.51–2.72 (m, 1H, CH), 2.77–2.92 (m, 4H, 2 × CH2), 3.84 (s, 3H, CH3), 6.44 (s, 1H, H-7), 6.72 (d, J = 8.7 Hz, 1H, Ar), 6.91 (dd, J = 8.7, 2.5 Hz, 1H, Ar), 8.15 (s, 1H, NH), 8.43 (d, J = 2.5 Hz, 1H, Ar), 9.29 (s, 1H, NH), 9.71 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 19.3 (2 × q), 23.9 (t), 27.4 (t), 28.4 (t), 35.5 (d), 56.1 (q), 110.7 (d), 112.0 (d), 119.4 (d), 122.4 (s), 122.6 (d), 123.3 (s), 125.5 (s), 126.2 (s), 128.7 (s), 129.4 (s), 137.1 (s), 146.3 (s), 154.6 (s), 158.6 (s), 174.7 (s). Anal calcd for C22H23ClN4O3S: C, 57.57; H, 5.05; N, 12.21. Found: C, 57.71; H, 4.92; N, 12.38.
N-(5-Chloro-2-Methoxyphenyl)-9-Methyl-2-[(2-Methylpropanoyl)Amino]-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (68)
This compound was obtained by reaction of 56 with isobutyryl chloride. Yellow oil; yield 80%; IR: νmax = 3345 (NH), 3254 (NH), 1679 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.22 (s, 3H, CH3), 1.25 (s, 3H, CH3), 2.07–2.19 (m, 2H, CH2), 2.68 (t, J = 6.5 Hz, 2H, CH2), 2.90 (t, J = 6.5 Hz, 2H, CH2), 3.92 (s, 3H, CH3), 4.07–4.25 (m, 4H, CH and CH3), 6.57 (s, 1H, H-7), 6.96–7.02 (m, 2H, Ar), 8.27 (s, 1H, Ar), 9.29 (s, 1H, NH), 13.37 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 19.2 (2 × q), 25.4 (t), 29.6 (t), 34.2 (t), 35.4 (d), 35.5 (q), 56.1 (q), 110.6 (d), 112.7 (d), 119.2 (d), 122.5 (d), 123.3 (s), 124.1 (s), 125.8 (s), 126.3 (d), 129.1 (s), 129.9 (s), 131.7 (s), 137.8 (s), 146.4 (s), 154.2 (s), 159.7 (s), 173.7 (s). Anal calcd for C23H25ClN4O3S: C, 58.40; H, 5.33; N, 11.85. Found: C, 58.57; H, 5.19; N, 11.99.
2,2-Dimethyl-N-[9-(Phenylsulfonyl)-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazol-2-yl]Propanamide (69)
This compound was obtained by reaction of 47 with trimethylacetyl chloride. White solid; mp: 67–68 °C; yield 62%; IR: νmax = 3387 (NH), 1677 (CO) cm−1; 1H NMR (200 MHz, CDCl3) δ 1.32 (s, 9H, 3 × CH3), 1.99–2.12 (m, 2H, CH2), 2.37–2.46 (m, 4H, 2 × CH2), 6.22 (d, J = 3.3 Hz, 1H, Ar), 7.36–7.68 (m, 6H, Ar), 8.99 (s, 1H, NH); 13C NMR (50 MHz, CDCl3) δ 23.5 (t), 24.9 (t), 27.3 (3 × q), 31.9 (t), 39.1 (s), 114.4 (d), 123.9 (d), 126.5 (s), 126.7 (2 × d), 128.7 (2 × d), 129.7 (s), 131.2 (s), 133.2 (d), 137.4 (s), 139.8 (s), 153.5 (s), 176.1 (s). Anal calcd for C21H23N3O3S2: C, 58.72; H, 5.40; N, 9.78. Found: C, 58.90; H, 5.35; N, 9.89.
N-(5-Chloro-2-Methoxyphenyl)-2-[(2,2-Dimethylpropanoyl)Amino]-4,5,6,9-Tetrahydropyrrolo [3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (70)
This compound was obtained by reaction of 54 with trimethylacetyl chloride. Yellow solid; yield 63%; mp: 155–156 °C; IR: νmax = 3423 (NH), 3395 (NH), 3255 (NH), 1666 (CO), 1655 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.25 (s, 9H, 3 × CH3), 1.93–2.06 (m, 2H, CH2), 2.87 (t, J = 5.2 Hz, 2H, CH2), 3.01 (t, J = 5.2 Hz, 2H, CH2), 3.87 (s, 3H, CH3), 6.92 (s, 1H, H-7), 7.08–7.21 (m, 2H, Ar), 7.93 (d, J = 2.4 Hz, 1H, Ar), 9.20 (s, 1H, NH), 10.09 (s, 1H, NH), 11.65 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 23.8 (t), 26.5 (t), 26.6 (3 × q), 27.8 (t), 40.2 (s), 56.1 (q), 112.5 (d), 113.7 (d), 122.0 (s), 122.6 (d), 123.4 (s), 123.7 (s), 124.0 (d), 124.3 (s), 128.0 (s), 128.4 (s), 136.8 (s), 149.2 (s), 155.6 (s), 158.4 (s), 176.4 (s). Anal calcd for C23H25ClN4O3S: C, 58.40; H, 5.33; N, 11.85. Found: C, 58.61; H, 5.57; N, 11.69.
N-(5-Chloro-2-Methoxyphenyl)-2-[(2,2-Dimethylpropanoyl)Amino]-9-Methyl-4,5,6,9-Tetrahydropyrrolo[3′,2′:6,7]Cyclohepta[1,2-d][1,3]Thiazole-8-Carboxamide (71)
This compound was obtained by reaction of 56 with trimethylacetyl chloride. Yellow solid; yield 63%; mp: 280–281 °C; IR: νmax = 3433 (NH), 3250 (NH), 1669 (CO) cm−1; 1H NMR (200 MHz, DMSO-d6) δ 1.26 (s, 9H, 3 × CH3), 1.95–2.09 (m, 2H, CH2), 2.61 (t, J = 6.4 Hz, 2H, CH2), 2.85 (t, J = 6.4 Hz, 2H, CH2), 3.87 (s, 3H, CH3), 4.17 (s, 3H, CH3), 6.87 (s, 1H, H-7), 7.07–7.20 (m, 2H, Ar), 8.03 (s, 1H, Ar), 8.88 (s, 1H, NH), 11.65 (s, 1H, NH); 13C NMR (50 MHz, DMSO-d6) δ 26.6 (3 × q), 27.1 (t), 29.0 (t), 29.2 (t), 48.9 (s), 56.2 (q), 64.6 (q), 112.4 (d), 118.1 (d), 121.3 (d), 123.8 (s), 124.9 (s), 126.8 (d), 131.6 (s), 133.7 (s), 137.4 (s), 143.7 (s), 148.6 (s), 154.7 (s), 159.5 (s), 165.9 (s), 179.3 (s). Anal calcd for C24H27ClN4O3S: C, 59.19; H, 5.59; N, 11.50. Found: C, 59.02; H, 5.73; N, 11.64.
3.2. Biology
The CF bronchial epithelial cells, CFBE41o-, with stable expression of F508del-CFTR [36] was stably transfected with the halide-sensitive yellow fluorescent protein, HS-YFP [37,38]. Cells were cultured with MEM plus 10% fetal calf serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin. For fluorescence-based measurement of CFTR activity, CFBE41o- cells were plated (50,000 cells/well) on clear-bottom 96-well black microplates (CLS3603, Corning). After 24 h after plating, cells were treated with test compounds (or vehicle, DMSO) at 37 °C for 24 h. At the time of assay, cells were washed three times with PBS (in mM: 137 NaCl, 2.7 KCl, 8.1 Na2HPO4, 1.5 KH2PO4, 1 CaCl2, and 0.5 MgCl2) to remove culture medium plus test compounds. Cells were then stimulated for 30 min with forskolin (20 µM) plus genistein (50 µM) in 60 µL PBS. The microplate was subsequently transferred to a microplate reader (FluoStar Galaxy; BMG Labtech) equipped with high-quality excitation (ET500/20X: 500 ± 10 nm) and emission (ET535/30M: 535 ± 15 nm) filters for YFP (Chroma Technology). Assay consisted of a continuous 14-s fluorescence reading in each well, with injection at 2 s of 165 µL of an iodide-rich solution (modified PBS, with Cl− replaced by I−; final I− concentration in the well: 100 mM). Fluorescence was read every 0.2 s, with 20 excitation flashes per time point. Data were normalized to the initial background-subtracted fluorescence. To determine fluorescence quenching rate (QR) reflecting the extent of I− influx, the data points corresponding to the final 11 s of the fluorescence reading for each well were fitted with an exponential function (Igor software, Wavemetrics) to extrapolate initial slope (dF/dt).
4. Conclusions
Thirty-five compounds having a pyrrolothiazole structure were synthesized to assess their ability in the rescue of chloride channel function of F508del −CFTR. Our study led to the identification of one compound with promising activity as a corrector. This compound having a six atom carbocyle ring and bearing a pivalamide group at the thiazole moiety and a 5-chloro-2-methoxyphenyl carboxamide at the pyrrole ring had improved activity compared to other substitutions. Any manipulation of peripheral groups in the pyrrolothiazole structure led to a decrease of the activity, as in the case of the isopropyl amide analogue or of the cyclohepta analogue, or to a loss of activity.
Compared to the parent constrained bithiazoles, replacement of the thiazole ring with the pyrrole one did not lead to an improvement in activity. However, the presence of 5-chloro-2-methoxyphenyl and the pivalamide groups is confirmed as an important structural requirement to obtain activity.
The drug discovery is a long process, requiring subsequent advancements of knowledge and synthetic strategies to select lead scaffolds, to reach good activity and selectivity of final compounds. Although, in this study we found corrector activity only for one compound, the pyrrolothiazole 44, our results may represent the starting point for the development of a new series of CFTR correctors. This preliminary result gives in fact important insight to further investigate this class of compounds in the near future, paving the way to new tricyclic structures containing the pyrrole moiety as valuable correctors.
Intriguingly, compound 44 showed additive effects when combined with VX-809 but not with VX-661. This is a surprising result since these two correctors are structurally similar and are believed to act with a similar mechanism of action.
Our work offers the basis for further structural modifications to explore the SAR and the chemical space around the tricyclic core. Other positional isomers could be investigated, maintaining the decoration at the pyrrole and thiazole rings. Overall, these results provide us a basis for selection and optimization of lead candidates and it could represent a resource for medicinal chemists who are interested in this field, to explore diverse pharmacophore structural space for enhancing the discovery of new compounds.
Author Contributions
V.C., M.B. and V.S. carried out synthesis and characterizations; M.G. and M.R. performed functional studies; L.J.V.G. supervision and draft preparation; A.M. and P.B. supervision and writing—original draft preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Italian Cystic Fibrosis Research Foundation, grant number FFC#4/2018 (adopted by FFC Delegation from Vercelli), and FFC#6/2019 (adopted by FFC Delegation from Alba Cuneo).
Acknowledgments
This work was financially supported by Ministero dell’Istruzione dell’Università e della Ricerca (MIUR).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors upon reasonable request.
References
- Elborn, J.S. Cystic fibrosis. Lancet 2016, 388, 2519–2531. [Google Scholar] [CrossRef]
- Gelfond, D.; Borowitz, D. Gastrointestinal complications of cystic fibrosis. Clin. Gastroenterol. Hepatol. 2013, 11, 333–342. [Google Scholar] [CrossRef]
- Stoltz, D.A.D.; Meyerholz, K.; Welsh, M.J. Origins of Cystic Fibrosis Lung Disease. N. Engl. J. Med. 2015, 372, 351–362. [Google Scholar] [CrossRef]
- Bosch, B.; De Boeck, K. Searching for a cure for cystic fibrosis. A 25-year quest in a nutshell. Eur. J. Pediatr. 2016, 175, 1–8. [Google Scholar] [CrossRef]
- De Boeck, K.; Davies, J.C. Where are we with transformational therapies for patients with cystic fibrosis? Curr. Opin. Pharmacol. 2017, 34, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Cao, D.; Neuberger, T.; Turnbull, A.; Singh, A.; Joubran, J.; Hazlewood, A.; et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci. USA 2009, 106, 18825–18830. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Burton, B.; Huang, C.J.; Worley, J.; Cao, D.; Johnson, J.P.; Urrutia, A.; Joubran, J.; Seepersaud, S.; Sussky, K.; et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J. Cyst. Fibros. 2012, 11, 237–245. [Google Scholar] [CrossRef]
- Spanò, V.; Venturini, A.; Genovese, M.; Barreca, M.; Raimondi, M.V.; Montalbano, A.; Galietta, L.J.V.; Barraja, P. Current development of CFTR potentiators in the last decade. Eur. J. Med. Chem. 2020, 204, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Montalbano, A.; Carbone, A.; Scudieri, P.; Galietta, L.J.V.; Barraja, P. An overview on chemical structures as ΔF508-CFTR correctors. Eur. J. Med. Chem. 2019, 180, 430–448. [Google Scholar] [CrossRef]
- Farinha, C.M.; King-Underwood, J.; Sousa, M.; Correia, A.R.; Henriques, B.J.; Roxo-Rosa, M.; Da Paula, A.C.; Williams, J.; Hirst, S.; Gomes, C.M.; et al. Revertants, low temperature, and correctors reveal the mechanism of F508del-CFTR rescue by VX-809 and suggest multiple agents for full correction. Chem. Biol. 2013, 20, 943–955. [Google Scholar] [CrossRef]
- Okiyoneda, T.; Veit, G.; Dekkers, J.F.; Bagdany, M.; Soya, N.; Xu, H.; Roldan, A.; Verkman, A.S.; Kurth, M.; Simon, A.; et al. Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat. Chem Biol. 2013, 9, 444–454. [Google Scholar] [CrossRef]
- Veit, G.; Xu, H.; Dreano, E.; Avramescu, R.G.; Bagdany, M.; Beitel, L.K.; Roldan, A.; Hancock, M.A.; Lay, C.; Li, W.; et al. Structure-guided combination therapy to potently improve the function of mutant CFTRs. Nat. Med. 2018, 24, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Hadida, S.; Grootenhuis, P.D.J.; Burton, B.; Stack, J.H.; Straley, K.S.; Decker, C.J.; Miller, M.; McCartney, J.; Olson, E.R.; et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci. USA 2011, 108, 18843–18848. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; van der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Pedemonte, N.; Bertozzi, F.; Caci, E.; Sorana, F.; Di Fruscia, P.; Tomati, V.; Ferrera, L.; Rodríguez-Gimeno, A.; Berti, F.; Pesce, E.; et al. Discovery of a picomolar potency pharmacological corrector of the mutant CFTR chloride channel. Sci. Adv. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Keating, D.; Marigowda, G.; Burr, L.; Daines, C.; Mall, M.A.; McKone, E.F.; Ramsey, B.W.; Rowe, S.M.; Sass, L.A.; Tullis, E.; et al. VX-445–Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis and One or Two Phe508del Alleles. N. Engl. J. Med. 2018, 379, 1612–1620. [Google Scholar] [CrossRef]
- Veit, G.; Roldan, A.; Hancock, M.A.; da Fonte, D.F.; Xu, H.; Hussein, M.; Frenkiel, S.; Matouk, E.; Velkov, T.; Lukacs, G.L. Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (Trikafta) combination. JCI Insight 2020, 5, e139983. [Google Scholar] [CrossRef]
- Pedemonte, N.; Lukacs, G.L.; Du, K.; Caci, E.; Zegarra-moran, O.; Galietta, L.J.V.; Verkman, A.S. Small-molecule correctors of defective F508-CFTR cellular processing identified by high-throughput screening. J. Clin. Investig. 2005, 115, 2564–2571. [Google Scholar] [CrossRef]
- Yu, G.J.; Yoo, C.L.; Yang, B.; Lodewyk, M.W.; Meng, L.; El-Idreesy, T.T.; Fettinger, J.C.; Tantillo, D.J.; Verkman, A.S.; Kurth, M.J. Potent s-cis-locked bithiazole correctors of ΔF508 cystic fibrosis transmembrane conductance regulator cellular processing for cystic fibrosis therapy. J. Med. Chem. 2008, 51, 6044–6054. [Google Scholar] [CrossRef][Green Version]
- Coffman, K.C.; Nguyen, H.H.; Phuan, P.W.; Hudson, B.M.; Yu, G.J.; Bagdasarian, A.L.; Montgomery, D.; Lodewyk, M.W.; Yang, B.; Yoo, C.L.; et al. Constrained bithiazoles: Small molecule correctors of defective δf508-CFTR protein trafficking. J. Med. Chem. 2014, 57, 6729–6738. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Rocca, R.; Barreca, M.; Giallombardo, D.; Montalbano, A.; Carbone, A.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Bai, R.; et al. Pyrrolo[2′,3′:3,4]cyclohepta[1,2-d][1,2]oxazoles, a New Class of Antimitotic Agents Active against Multiple Malignant Cell Types. J. Med. Chem. 2020, 63, 12023–12042. [Google Scholar] [CrossRef]
- Montalbano, A.; Parrino, B.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Cirrincione, G. Synthesis of the new oligopeptide pyrrole derivative isonetropsin and its one pyrrole unit analogue. Tetrahedron 2013, 69, 2550–2554. [Google Scholar] [CrossRef]
- Spanò, V.; Attanzio, A.; Cascioferro, S.; Carbone, A.; Montalbano, A.; Barraja, P.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. Synthesis and antitumor activity of new thiazole nortopsentin analogs. Mar. Drugs 2016, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Barraja, P.; Caracausi, L.; Diana, P.; Spanò, V.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis and antiproliferative activity of the ring system [1,2]oxazolo[4,5-g]indole. ChemMedChem 2012, 7, 1901–1904. [Google Scholar] [CrossRef]
- Whatmore, J.L.; Swann, E.; Barraja, P.; Newsome, J.J.; Bunderson, M.; Beall, H.D.; Tooke, J.E.; Moody, C.J. Comparative study of isoflavone, quinoxaline and oxindole families of anti-angiogenic agents. Angiogenesis 2002, 5, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Diana, P.; Barraja, P.; Montalbano, A.; Cirrincione, G.; Dattolo, G.; Almerico, A.M. New tricyclic systems of biological interest. Annelated 1,2,3-triazolo[1,5-a]pyrimidines through domino reaction of 3-azidopyrroles and methylene active nitriles. Tetrahedron 2002, 58, 9723–9727. [Google Scholar] [CrossRef]
- Frasson, I.; Spanò, V.; Di Martino, S.; Nadai, M.; Doria, F.; Parrino, B.; Carbone, A.; Cascioferro, S.M.; Diana, P.; Cirrincione, G.; et al. Synthesis and photocytotoxic activity of [1,2,3]triazolo[4,5-h][1,6] naphthyridines and [1,3]oxazolo[5,4-h][1,6]naphthyridines. Eur. J. Med. Chem. 2019, 162, 176–193. [Google Scholar] [CrossRef]
- Lauria, A.; Patella, C.; Diana, P.; Barraja, P.; Montalbano, A.; Cirrincione, G.; Dattolo, G.; Almerico, A.M. A new tetracyclic ring system of biological interest. indolo[3,2-e][1,2,3]Triazolo[1,5-a]pyrimidines through domino reactions of 2-azidoindole. Heterocycles 2003, 60, 2669–2675. [Google Scholar]
- Barraja, P.; Spanò, V.; Diana, P.; Carbone, A.; Cirrincione, G.; Vedaldi, D.; Salvador, A.; Viola, G.; Dall’Acqua, F. Pyrano[2,3-e]isoindol-2-ones, new angelicin heteroanalogues. Bioorg. Med. Chem. Lett. 2009, 19, 1711–1714. [Google Scholar] [CrossRef]
- Barraja, P.; Spanò, V.; Diana, P.; Carbone, A.; Cirrincione, G. Synthesis of the new ring system 6,8-dihydro-5H-pyrrolo[3,4-h]quinazoline. Tetrahedron Lett. 2009, 50, 5389–5391. [Google Scholar] [CrossRef]
- Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Salvador, A.; Brun, P.; Vedaldi, D.; Diana, P.; Cirrincione, G.; Barraja, P. Pyrazolo[3,4-h]quinolines promising photosensitizing agents in the treatment of cancer. Eur. J. Med. Chem. 2015, 102, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Barreca, M.; Rocca, R.; Bortolozzi, R.; Bai, R.; Carbone, A.; Raimondi, M.V.; Palumbo Piccionello, A.; Montalbano, A.; Alcaro, S.; et al. Insight on [1,3]thiazolo[4,5-e]isoindoles as tubulin polymerization inhibitors. Eur. J. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Barraja, P.; Caracausi, L.; Diana, P.; Carbone, A.; Montalbano, A.; Cirrincione, G.; Brun, P.; Palù, G.; Castagliuolo, I.; Dall’Acqua, F.; et al. Synthesis of pyrrolo[3,2-h]quinolinones with good photochemotherapeutic activity and no DNA damage. Bioorg. Med. Chem. 2010, 18, 4830–4843. [Google Scholar] [CrossRef]
- Spanò, V.; Frasson, I.; Giallombardo, D.; Doria, F.; Parrino, B.; Carbone, A.; Montalbano, A.; Nadai, M.; Diana, P.; Cirrincione, G.; et al. Synthesis and antiproliferative mechanism of action of pyrrolo[3′,2′:6,7]cyclohepta[1,2-d]pyrimidin-2-amines as singlet oxygen photosensitizers. Eur. J. Med. Chem. 2016, 123, 447–461. [Google Scholar] [CrossRef]
- Spanò, V.; Giallombardo, D.; Cilibrasi, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Frasson, I.; Salvador, A.; Richter, S.N.; Doria, F.; et al. Pyrrolo[3′,2′:6,7]cyclohepta[1,2-b]pyridines with potent photo-antiproliferative activity. Eur. J. Med. Chem. 2017, 128, 300–318. [Google Scholar] [CrossRef]
- Bebok, Z.; Collawn, J.F.; Wakefield, J.; Parker, W.; Li, Y.; Varga, K.; Sorscher, E.J.; Clancy, J.P. Failure of cAMP agonists to activate rescued ΔF508 CFTR in CFBE41o- airway epithelial monolayers. J. Physiol. 2005, 569, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.V.; Haggie, P.M.; Verkman, A.S. Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett. 2001, 499, 220–224. [Google Scholar] [CrossRef]
- Sondo, E.; Tomati, V.; Caci, E.; Esposito, A.I.; Pfeffer, U.; Pedemonte, N.; Galietta, L.J.V. Rescue of the mutant CFTR chloride channel by pharmacological correctors and low temperature analyzed by gene expression profiling. Am. J. Physiol.—Cell Physiol. 2011, 301, C872–C885. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).