Synthesis of Highly Potent Anti-Inflammatory Compounds (ROS Inhibitors) from Isonicotinic Acid
Abstract
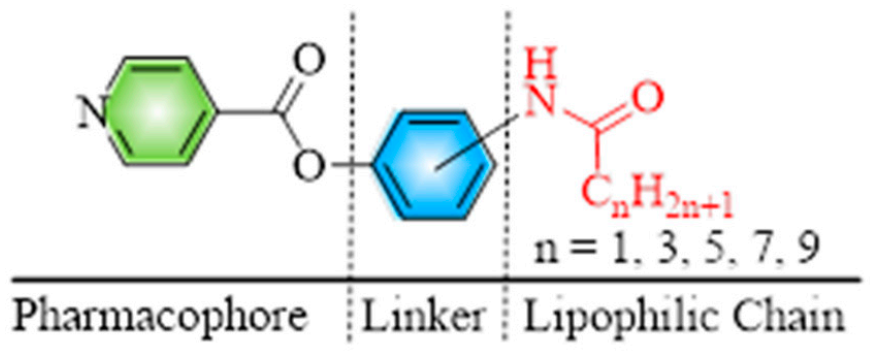
1. Introduction
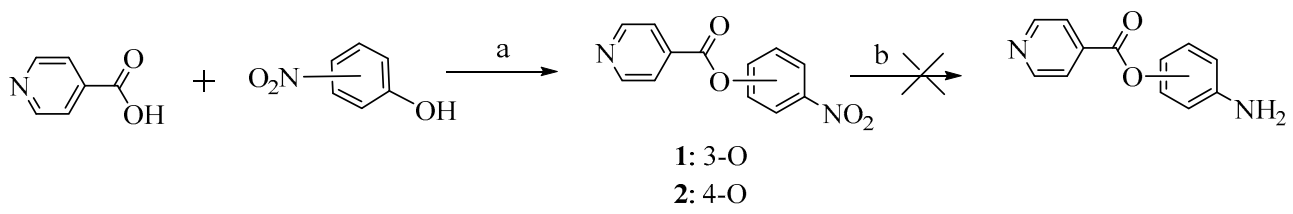
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, L.Z.; Wu, J.; Li, K.; Wu, Q.Q.; Chen, R.; Liu, X.H.; Ruan, B.F. Novel phthalide derivatives: Synthesis and anti-inflammatory activity in vitro and in vivo. Eur. J. Med. Chem. 2020, 206. [Google Scholar] [CrossRef]
- Chelombitko, M.A. Role of Reactive Oxygen Species in Inflammation: A Minireview. Moscow Univ. Biol. Sci. Bull. 2018, 73, 199–202. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxidants Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Shrestha, A.; Jin Oh, H.; Kim, M.J.; Pun, N.T.; Magar, T.B.T.; Bist, G.; Choi, H.; Park, P.H.; Lee, E.S. Design, synthesis, and structure-activity relationship study of halogen containing 2-benzylidene-1-indanone derivatives for inhibition of LPS-stimulated ROS production in RAW 264.7 macrophages. Eur. J. Med. Chem. 2017, 133, 121–138. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.C.; Henriques, R.R.; de Abreu Lopes Junior, M.A.; Farias, A.B.; do Couto Nogueira, T.L.; Quimas, J.V.F.; Romeiro, N.C.; da Silva, L.L.; de Souza, A.L.F. Acylhydrazones as isoniazid derivatives with multi-target profiles for the treatment of Alzheimer’s disease: Radical scavenging, myeloperoxidase/acetylcholinesterase inhibition and biometal chelation. Bioorg. Med. Chem. 2020, 28, 115470. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Li, X.; Chen, G.; Li, Q.-S.; Luo, Y.; Ruan, B.-F.; Chen, X.-W.; Zhu, H.-L. Design, synthesis and biological evaluation of pyridine acyl sulfonamide derivatives as novel COX-2 inhibitors. Bioorg. Med. Chem. 2011, 19, 6827–6832. [Google Scholar] [CrossRef]
- Desogus, A.; Schenone, S.; Brullo, C.; Tintori, C.; Musumeci, F. Bcr-Abl tyrosine kinase inhibitors: A patent review. Expert Opin. Ther. Pat. 2015, 25, 397–412. [Google Scholar] [CrossRef]
- Ghiano, D.G.; Recio-Balsells, A.; Bortolotti, A.; Defelipe, L.A.; Turjanski, A.; Morbidoni, H.R.; Labadie, G.R. New one-pot synthesis of anti-tuberculosis compounds inspired on isoniazid. Eur. J. Med. Chem. 2020, 208, 112699. [Google Scholar] [CrossRef]
- Fu, Y.-D.; Huang, M.-J.; Guo, J.-W.; You, Y.-Z.; Liu, H.-M.; Huang, L.-H.; Yu, B. Targeting histone demethylase KDM5B for cancer treatment. Eur. J. Med. Chem. 2020, 208, 112760. [Google Scholar] [CrossRef]
- Judge, V.; Narasimhan, B.; Ahuja, M.; Sriram, D.; Yogeeswari, P.; De Clercq, E.; Pannecouque, C.; Balzarini, J. Isonicotinic acid hydrazide derivatives: Synthesis, antimicrobial activity, and QSAR studies. Med. Chem. Res. 2012, 21, 1451–1470. [Google Scholar] [CrossRef]
- Scott, F.; Fala, A.M.; Pennicott, L.E.; Reuillon, T.D.; Massirer, K.B.; Elkins, J.M.; Ward, S.E. Development of 2-(4-pyridyl)-benzimidazoles as PKN2 chemical tools to probe cancer. Bioorg. Med. Chem. Lett. 2020, 30, 127040. [Google Scholar] [CrossRef]
- Koltun, D.O.; Parkhill, E.Q.; Kalla, R.; Perry, T.D.; Elzein, E.; Li, X.; Simonovich, S.P.; Ziebenhaus, C.; Hansen, T.R.; Marchand, B.; et al. Discovery of potent and selective inhibitors of calmodulin-dependent kinase II (CaMKII). Bioorg. Med. Chem. Lett. 2018, 28, 541–546. [Google Scholar] [CrossRef]
- Luo, Z.; Yue, X.; Yang, H.; Liu, H.; Klein, R.S.; Tu, Z. Design and synthesis of pyrazolopyridine derivatives as sphingosine 1-phosphate receptor 2 ligands. Bioorg. Med. Chem. Lett. 2018, 28, 488–496. [Google Scholar] [CrossRef]
- Bayrak, H.; Demirbas, A.; Demirbas, N.; Karaoglu, S.A. Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 4362–4366. [Google Scholar] [CrossRef]
- Fiorino, F.; Ciano, A.; Magli, E.; Severino, B.; Corvino, A.; Perissutti, E.; Frecentese, F.; Di Vaio, P.; Izzo, A.A.; Capasso, R.; et al. Synthesis, in vitro and in vivo pharmacological evaluation of serotoninergic ligands containing an isonicotinic nucleus. Eur. J. Med. Chem. 2016, 110, 133–150. [Google Scholar] [CrossRef]
- Yue, X.; Dhavale, D.D.; Li, J.; Luo, Z.; Liu, J.; Yang, H.; Mach, R.H.; Kotzbauer, P.T.; Tu, Z. Design, synthesis, and in vitro evaluation of quinolinyl analogues for α-synuclein aggregation. Bioorg. Med. Chem. Lett. 2018, 28, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Hayat, F.; Azam, A.; Shin, D. Recent progress on the discovery of antiamoebic agents. Bioorg. Med. Chem. Lett. 2016, 26, 5149–5159. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.J.; Khan, S.A.; Alam, O.; Singh, V.; Arora, A. Thiazolidin-4-one, azetidin-2-one and 1,3,4-oxadiazole derivatives of isonicotinic acid hydrazide: Synthesis and their biological evaluation. J. Serbian Chem. Soc. 2011, 76, 1057–1067. [Google Scholar] [CrossRef]
- Al-Omar, M.A.; Amr, A.E.G.E.; Al-Salahi, R.A. Anti-inflammatory, analgesic, anticonvulsant and antiparkinsonian activities of some pyridine derivatives using 2,6-disubstituted isonicotinic acid hydrazides. Arch. Pharm. (Weinheim) 2010, 343, 648–656. [Google Scholar] [CrossRef]
- Helfand, S.L.; Werkmeister, J.; Roder, J.C. Chemiluminescence response of human natural killer cells: I. the relationship between target cell binding, chemiluminescence, and cytolysis. J. Exp. Med. 1982, 156, 492–505. [Google Scholar] [CrossRef] [PubMed]



| Compounds | Anti-Inflammatory Activity | |
|---|---|---|
| % Inhibition * | IC50 (µg/mL) ** | |
| 5 | 95.9 | 1.42 ± 0.1 |
| 6 | 67.3 | 8.6 ± 0.5 |
| 7a | 29.3 | na |
| 7b | 25..8 | na |
| 7c | 15.7 | na |
| 7d | 21.6 | na |
| 7e | 13.2 | na |
| 8a | 66.6 | 19.6 ± 3.4 |
| 8b | 85.4 | 3.7 ± 1.7 |
| 8c | 44.6 | na |
| 8d | -7.7 | na |
| 8e | 5.8 | na |
| Ibuprofen | 73.2 | 11.2 ± 1.9 |
| Compound | Binding Energy (Kcal/mol) | Hydrogen Bonding | Hydrophobic Interaction | ||
|---|---|---|---|---|---|
| Ligand | Residue | Distance (Å) | |||
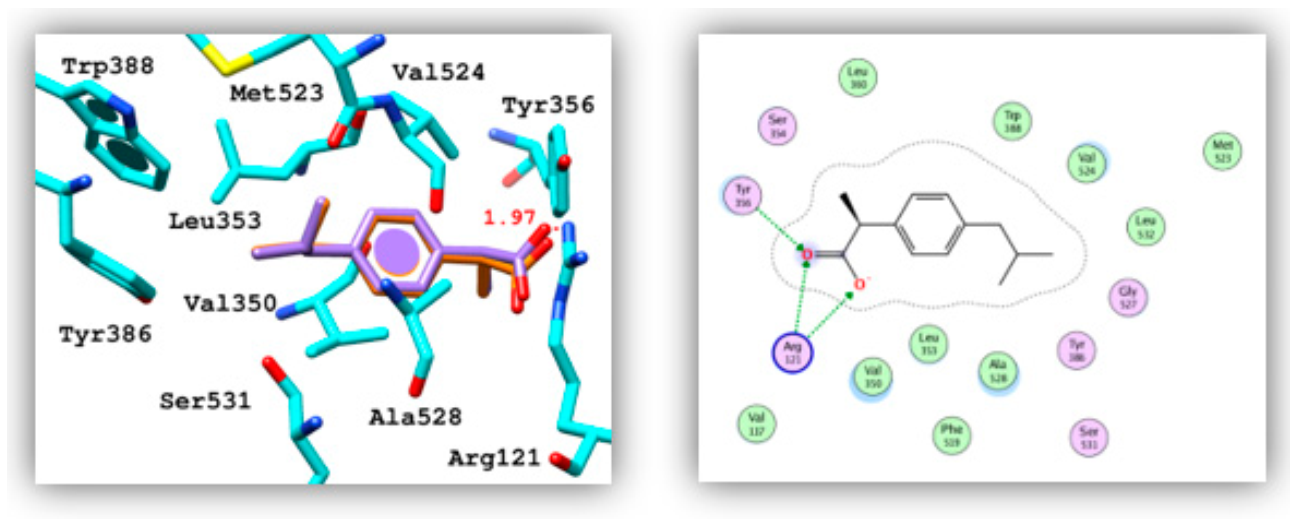
| Ibuprofen | −7.61 | Lig:CO2−Salt bridge | Tyr356:OH Arg121 | 1.97 3.55 | Val350, Leu353, Tyr356, Leu360, Tyr386, Trp388, Val524, Ala528 |
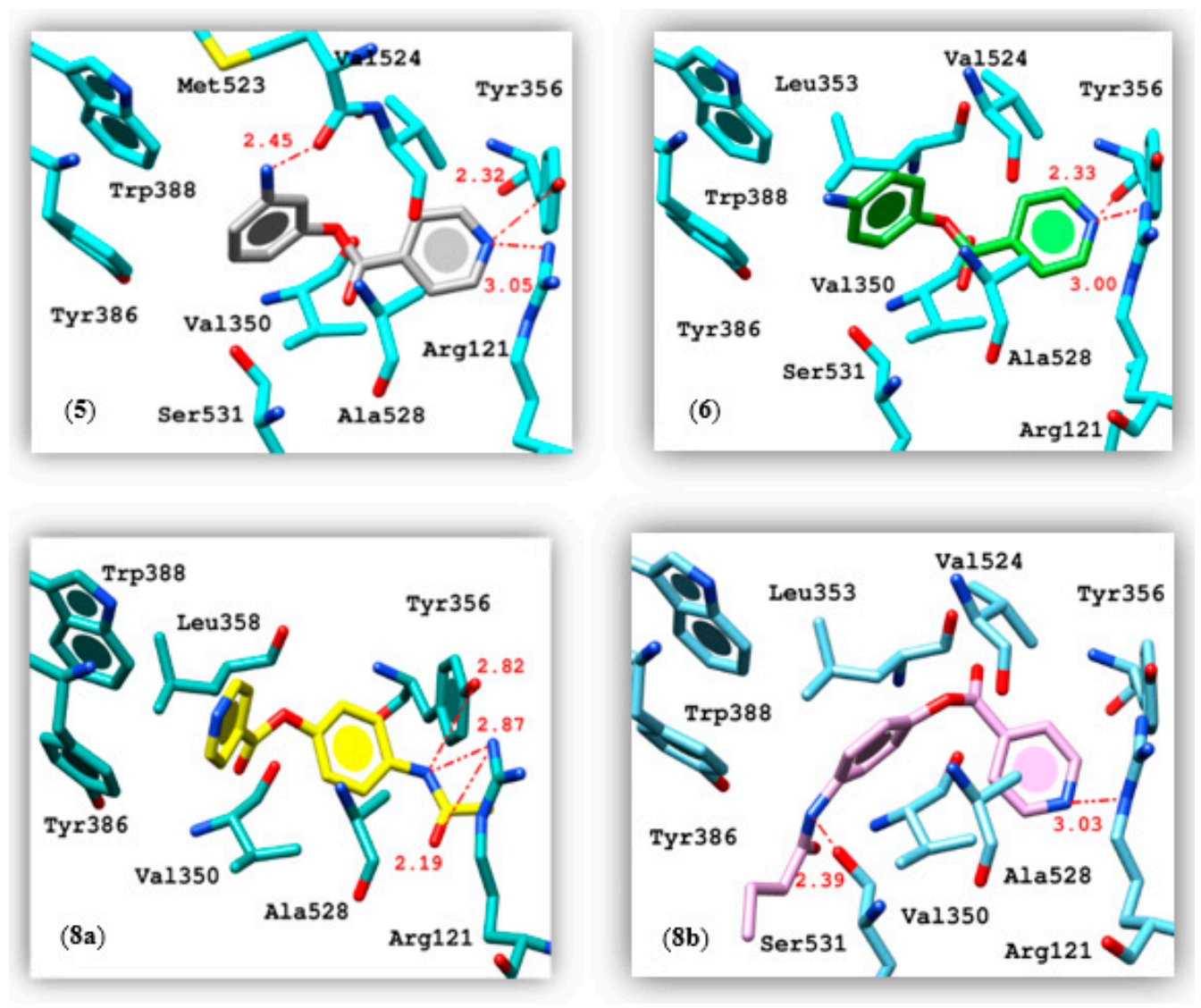
| 5 | −6.66 | Lig:N(1) | Arg121:NH2+ | 3.05 | Val350, Leu353, Tyr386, Val524, Ala528 |
| Lig:N(1) | Tyr356:OH | 2.32 | |||
| Lig:N(3) | Met523:CO | 2.45 | |||
| 6 | −6.56 | Lig:N(1) | Arg121:NH2+ | 3.00 | Val350, Leu353, Tyr386, Val524, Ala528 |
| Lig:N(1) | Tyr356:OH | 2.33 | |||
| 8a | −6.86 | Lig:N(3) Lig:O(3) Lig:N(3) | Arg121:NH2+ Arg121:NH2+ Tyr356:OH | 2.87 2.19 2.82 | Val117, Val350, Leu353, Tyr356, Phe519, Val524, Ala528, Trp388 |
| 8b | −7.26 | Lig:N(1) Lig:N(3) | Arg121:NH Ser531:OH | 3.03 2.39 | Phe206, Phe210, Val345, Val350, Leu353, Phe382, Tyr386, Val524, Ala528 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaqoob, S.; Nasim, N.; Khanam, R.; Wang, Y.; Jabeen, A.; Qureshi, U.; Ul-Haq, Z.; El-Seedi, H.R.; Jiang, Z.-H.; Khan, F.-A. Synthesis of Highly Potent Anti-Inflammatory Compounds (ROS Inhibitors) from Isonicotinic Acid. Molecules 2021, 26, 1272. https://doi.org/10.3390/molecules26051272
Yaqoob S, Nasim N, Khanam R, Wang Y, Jabeen A, Qureshi U, Ul-Haq Z, El-Seedi HR, Jiang Z-H, Khan F-A. Synthesis of Highly Potent Anti-Inflammatory Compounds (ROS Inhibitors) from Isonicotinic Acid. Molecules. 2021; 26(5):1272. https://doi.org/10.3390/molecules26051272
Chicago/Turabian StyleYaqoob, Sana, Nourina Nasim, Rahila Khanam, Yan Wang, Almas Jabeen, Urooj Qureshi, Zaheer Ul-Haq, Hesham R. El-Seedi, Zi-Hua Jiang, and Farooq-Ahmad Khan. 2021. "Synthesis of Highly Potent Anti-Inflammatory Compounds (ROS Inhibitors) from Isonicotinic Acid" Molecules 26, no. 5: 1272. https://doi.org/10.3390/molecules26051272
APA StyleYaqoob, S., Nasim, N., Khanam, R., Wang, Y., Jabeen, A., Qureshi, U., Ul-Haq, Z., El-Seedi, H. R., Jiang, Z.-H., & Khan, F.-A. (2021). Synthesis of Highly Potent Anti-Inflammatory Compounds (ROS Inhibitors) from Isonicotinic Acid. Molecules, 26(5), 1272. https://doi.org/10.3390/molecules26051272










