Abstract
Colorectal cancer is a common cancer worldwide and reduced expression of the DNA repair endonuclease XPF (xeroderma pigmentosum complementation group F) is associated with colorectal cancer. Bacopa monnieri extracts were previously found to exhibit chemical-genetic synthetic lethal effects in a Saccharomyces cerevisiae model of colorectal cancer lacking Rad1p, a structural and functional homologue of human XPF. However, the mechanisms for B. monnieri extracts to limit proliferation and promote an apoptosis-like event in RAD1 deleted yeast was not elucidated. Our current analysis has revealed that B. monnieri extracts have the capacity to promote mutations in rad1∆ cells. In addition, the effects of B. monnieri extracts on rad1∆ yeast is linked to disruption of the vacuole, similar to the mammalian lysosome. The absence of RAD1 in yeast sensitizes cells to the effects of vacuole disruption and the release of proteases. The combined effect of increased DNA mutations and release of vacuolar contents appears to induce an apoptosis-like event that is dependent on the meta-caspase Yca1p. The toxicity of B. monnieri extracts is linked to sterol content, suggesting saponins may be involved in limiting the proliferation of yeast cells. Analysis of major constituents from B. monnieri identified a chemical-genetic interaction between bacopasaponin C and rad1∆ yeast. Bacopasaponin C may have potential as a drug candidate or serve as a model for the development of analogs for the treatment of colorectal cancer.
1. Introduction
The incidence of colorectal cancer is increasing worldwide, due in part to changes in diet and lifestyle [1,2,3]. Surgery, radiotherapy, chemotherapy, or a combination of these strategies are utilized in the treatment of colorectal cancer [4]. While surgery is the most common treatment, advanced disease limits its effectiveness. In many cases, the use of chemotherapy is needed to prevent cancer recurrence [4,5].
Commonly used chemotherapeutic drugs, such as 5-fluorouracil, capecitabine (metabolized to 5-fluorouracil), and oxaliplatin [5,6,7], do not specifically target cancer cells [7,8]. Resulting side effects from generalized toxicity are often difficult for patients to tolerate [5,9,10,11,12]. Targeted therapies that exploit differences between cancer and normal cells are thus desirable [13,14].
A difference between normal cells and a subset of colorectal cancers is reduced expression of ERCC4 (excision repair cross-complementation group 4), also called DNA repair endonuclease XPF [15,16]. XPF functions in nucleotide excision repair (NER) and double-strand break (DSB) repair pathways [15,17]. Previously, we utilized a Saccharomyces cerevisiae model of colorectal cancer lacking Rad1p, a structural and functional homologue of human XPF [15,17,18]. Screening medicinal plant extracts for chemical-genetic interactions in Saccharomyces cerevisiae deleted for RAD1 identified Bacopa monnieri (L.) Wettst as a potential source of drug candidates. Exposure of rad1∆ yeast, but not wild type cells, to B. monnieri extracts resulted in nuclear fragmentation suggesting the promotion of an apoptosis-like event [19].
B. monnieri is a widely utilized medicinal plant from the Indian Ayurvedic tradition [20]. Preparations containing B. monnieri are used to promote cognitive performance and improve memory [21,22]. In addition, B. monnieri extracts have been found to exhibit anti-tumor activity [23,24]. The best characterized bioactive compounds from B. monnieri are saponins, referred to as bacosides, bacopasides, and bacopasaponins [25,26,27]. The saponin fraction from B. monnieri extracts contains bacopaside I, bacoside A3, bacopaside II, bacopasaponin X, and bacopasaponin C [28]. We speculated that one or more of these compounds was responsible for promoting an apoptosis-like event and specifically limiting growth in yeast deleted for RAD1 [19]. These bioactive compounds from B. monnieri may have potential as drug candidates for the treatment of ERCC4 deficient colorectal cancer.
In this study, we investigate the molecular mechanisms that promote the apoptosis-like event following exposure to B. monnieri extract, using the yeast model for ERCC4 deficient colorectal cancer. Our analysis indicates that B. monnieri extracts increase mutation frequency but not chromosomal instability in yeast deleted for RAD1. Loss of integrity of the yeast vacuole, similar to the mammalian lysosome, was observed. The toxicity of B. monnieri extracts was dependent on sterol content, suggesting a role for bacosides in limiting the proliferation of yeast cells. Analysis of major constituents from B. monnieri indicates that bacopasaponin C is the bioactive compound responsible for preferentially limiting the growth of RAD1 deleted yeast. We propose a model in which bacopasaponin C, and perhaps other bioactive compounds from B. monnieri, promotes disruption of the vacuole leading to induction of an apoptosis-like event facilitated by leakage of vacuolar components.
2. Results
2.1. Mutation Frequency But Not Chromosome Instability Is Enhanced Following Exposure to B. monnieri Extracts
The ability of B. monnieri extracts to induce an apoptosis-like effect in yeast deleted for RAD1 [19] as well as apoptosis in some cancer cells lines [29,30,31] suggested an impact on DNA integrity. Using the CAN1 forward mutation assay, a significant increase in the mutation rate was observed in rad1∆ cells exposed to B. monnieri extracts. Similarly, treatment with methyl methanesulfonate (MMS) or oxaliplatin (OxPT), compounds known to induce DNA damage [8,32], resulted in an enhanced mutation frequency in the CAN1 locus, as judged by increased numbers of canavanine resistant colonies (Figure 1A). Exposure to B. monnieri extracts did not promote increased chromosome instability in rad1∆ cells compared to WT. Although treatment with MMS or oxaliplatin produced a significant increase in the appearance of A-like fakers due to recombination at the Mat alpha locus (Figure 1B). These results are consistent with a connection between DNA damage but not chromosome instability and exposure to B. monnieri extracts. Sensitivity of rad1∆ cells to B. monnieri extracts was complemented by episomal expression of RAD1 indicating the effect was not due to a mutation at a second site in the genome (Figure S1).
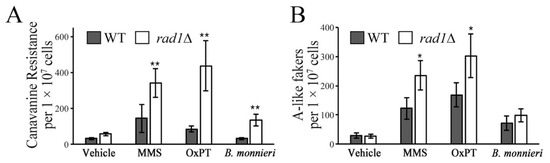
Figure 1.
Extracts from B. monnieri increase mutation frequency in rad1∆ yeast. Strains WT (BY4742) and rad1∆ (12806) were grown in synthetic medium lacking arginine with 2% glucose in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or exposed 325 µg/mL B. monnieri extract, 300 µM MMS, or 2.5 mM oxaliplatin for 16 h. (A) Mutation frequency was monitored by screening for resistance to L-canavanine (50 µg/mL) due to loss-of-function mutations in CAN1 on SC medium lacking arginine. (B) Chromosome instability at the MAT alpha locus was assaying by selecting for A-like fakers using a mating assay. A-like fakers were scored based on the formation of diploids between strains from the BY4742 background (MAT alpha, TRP1, lys2) and EG103 (MAT alpha, trp1, LYS2) by selecting for yeast prototrophic for both tryptophan and lysine. Statistical analysis employed Student’s t-test with ** p < 0.01, * p < 0.05.
2.2. B. monnieri Extracts Promote Vacuole Damage
The vacuole is the hydrolytic compartment of yeast and has a similar function as mammalian lysosomes in the detoxification and recycling of macromolecules [33,34]. Lysosomes can also participate in the regulation of apoptosis in mammalian cells [35,36]. Similarly, the yeast vacuole has been reported to be involved in the promotion of apoptosis-like events [37]. These processes appear to be mediated by the release of lysosomal/vacuolar hydrolases into the cytosol following membrane permeabilization [38]. B. monnieri extracts contain saponins such as bacosides, bacopasides, bacopasaponins [25,27,39] and these compounds have the potential to promote membrane permeabilization [40,41], which has been implicated in lysosomal mediated apoptosis [42,43]. We observed that exposure of yeast cells to B. monnieri extracts was found to increase the appearance in the cytoplasm of the vacuolar Prc1p-GFP fusion (Figure 2A,B), suggesting permeabilization of the vacuole membrane. The vacuolar membrane marker Mam3p-RFP was utilized to examine the structure of vacuoles in cells showing cytoplasmic Prc1p-GFP. It was observed that cells exhibiting diffuse Prc1p-GFP fluorescence had a loss of vacuolar structure. The effect of B. monnieri extracts was not selective to yeast deleted for RAD1 as the WT strain also exhibited an increased number of cells with diffuse Prc1p-GFP. Although the number of cells with diffuse GFP fluorescence was greater in the rad1∆ samples, the difference did not exhibit statistical significance. Analysis of cytoplasmic and vacuolar fractions indicated that exposure to B. monnieri extracts causes an increase in release of GFP from the vacuole (Figure 2C,D). The Prc1p-GFP fusion was not observed in the cytoplasmic fraction, perhaps due to its relatively large size of 87 kDa; however, free GFP derived from this fusion protein was released into the cytoplasm. The rad1∆ strain exhibited a statistically significant increase in cytoplasmic GFP following exposure to B. monnieri extracts, relative to the WT strain.

Figure 2.
Vacuoles exhibit damage following exposure to B. monnieri extracts. (A) Strains WT (BY4742) and rad1∆ (12806) co-transformed with PRC1-GFP (pWC022) and MAM3-RFP (pLJ521) expression plasmids were grown in synthetic medium lacking uracil and leucine with 2% glucose in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or 325 µg/mL B. monnieri extracts for 16 h prior to visualization at a magnification of 60×. (B) Quantitation of cells exhibiting vacuolar disruption was performed using approximately 100 cells per sample. B. monnieri extracts significantly increased vacuolar disruption relative to vehicle control in both WT and rad1∆ yeast. Values are mean ± SD (n = 3). (C) Cytoplasmic and crude vacuolar fractions were prepared from strains WT (BY4742) and rad1∆ (12806) transformed with PRC1-GFP (pWC022) grown with vehicle (−) or 325 µg/mL B. monnieri extract (+) for 16 h. Visualization utilized immunoblots with and anti-GFP anti-body (GFP B-2). A Coomassie stained gel of the same extracts was used as a loading control. (D) Quantitation of GFP levels. Values are relative to WT vacuole fraction grown with vehicle. Statistical analysis employed one-way ANOVA with post-hoc Tukey test with ** p < 0.01, * p < 0.05, NS—not significant.
2.3. Vacuole Permeabilization with Chloroquine Does Not Mimic Effects of B. monnieri Extracts
The effects of B. monnieri extracts on vacuolar structure suggested that increased permeabilization may contribute to toxicity in rad1∆ yeast. Chloroquine exposure promotes lysosomal permeabilization [44] and can be used to evaluate the contribution of vacuolar permeabilation alone to sensitzation of rad1∆ cells. Toxicity from chloroquine was observed in both WT and rad1∆ yeast cells. However, both WT and rad1∆ strains were similarly affected by chloroquine (Figure 3). This suggests that increased vacuolar permeabilzation alone is not sufficient to sensitize rad1∆ cells to the effects of B. monnieri extracts.

Figure 3.
Chloroquine is not specifically toxic to yeast deleted for RAD1. (A,B) Yeast strains WT and rad1∆ (12806) were grown in synthetic medium with 2% glucose in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or chloroquine at the concentrations listed. Values are mean ± SD (n = 3). Growth was monitored by measuring OD at 600 nm with vehicle control (0) set at 100%. Statistical analysis employed Student’s t-test and no significant differences were observed between WT and rad1∆ samples. (B) Serial dilutions of liquid cultures from were spotted onto solid synthetic medium with 2% glucose. The volume of each culture used was based on the OD 600 nm of vehicle samples for each strain background. For vehicle samples 105, 104, and 103 cells were placed onto agar plates and the same culture volume was used for samples treated with B. monnieri extracts. Samples were incubated for 3 days and photographed.
2.4. Deletion of PEP4 Limits Toxicity of B. monnieri Extracts in rad1∆ Yeast
PEP4 encodes a vacuolar aspartyl protease (proteinase A) that is required for maturation of vacuolar proteinases [45]. If release of vacuolar proteases was involved in sensitivity of rad1∆ cells to B. monnieri extracts, then deletion of PEP4 would be expected to have a protective role. Consistent with this idea, yeast deleted for both RAD1 and PEP4 show resistance to B. monnieri extracts to similar levels as the WT strain (Figure 4). It appears that even though vacuolar permeabilization alone (chloroquine exposure) is not sufficient to induce sensitivity of rad1∆, cells release of vacuolar proteases is involved.

Figure 4.
Deletion of PEP4, encoding a vacuolar protease, is protective against toxicity from B. monnieri extracts in rad1∆ cells. (A,B) Yeast strains WT, rad1∆ (12806), pep4∆ (12098), and rad1∆ pep4∆ (LJ467) were grown in synthetic medium with 2% glucose in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or B. monnieri extracts at the concentrations listed. Values are mean ± SD (n = 3). Growth was monitored by measuring OD at 600 nm with vehicle control (0) set at 100%. Statistical analysis employed Student’s t-test with ** p < 0.01, * p < 0.05. (B) Serial dilutions of liquid cultures from were spotted onto solid synthetic medium with 2% glucose as described in Figure 3. Samples were incubated for 3 days and photographed.
2.5. Mitochondrial Structure Is Not Disrupted by Exposure to B. monnieri Extracts
The morphology of mitochondria was evaluated in WT and rad1∆ cells expressing YFP targeted to the mitochondrial matrix (Cox4pMLS-YFP) [46]. Mitochondria exhibited typical tubular morphology in both WT and rad1∆ strains grown with vehicle or B. monnieri extracts (Figure 5A). Analysis of YFP levels in cytoplasmic (post-mitochondrial supernatent, PMS) and mitochondrial fractions did not reveal any indication of disruption to the mitochondrial inner membrane. YFP was primarily localized to the mitochondria with only trace amounts present in the PMS fraction in all samples regardless of exposure to B. monnieri extracts (Figure 5B,C).
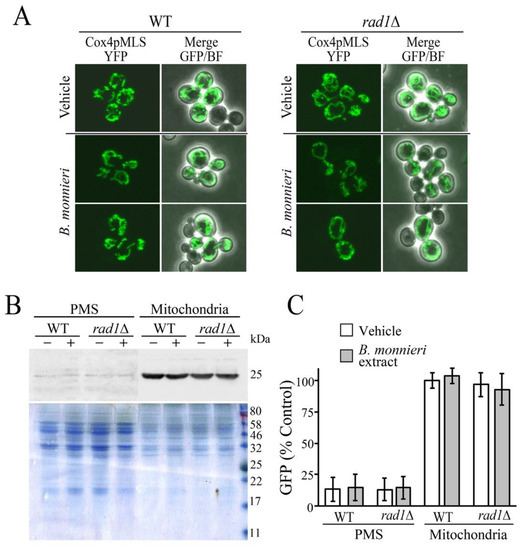
Figure 5.
Mitochondrial morphology and integrity is not altered following exposure to B. monnieri extracts. (A) Strains WT (BY4742) and rad1∆ (12806) transformed with a plasmid expressing a mitochondrial matrix targeted YFP, Cox4pMLS-YFP (pLD207) were grown in synthetic medium lacking uracil with 2% glucose in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or 325 µg/mL B. monnieri extracts for 16 h prior to visualization at a magnification of 60×. (B) Post-mitochondrial (PMS, cytosol) and mitochondrial fractions were prepared from strains as described in (A) treated with vehicle (−) and 325 µg/mL B. monnieri extract (+). Visualization utilized immunoblots with and anti-GFP anti-body (GFP B-2). A Coomassie stained gel of the same extracts was used as a loading control. (C) Quantitation of GFP levels. Values are relative to WT vacuole fraction grown with vehicle. Statistical analysis employed one-way ANOVA with post-hoc Tukey test, no significant differences were found between WT and rad1∆ samples.
2.6. Stabilization of Plasma Membrane Does Not Prevent Toxicity from B. monnieri Extracts
Previously, we observed that rad1∆ cells exposed to B. monnieri extracts had reduced integrity of their plasma membrane [19]. To examine if the reduced plasma membrane integrity was a key element of the toxicity of B. monnieri extracts toward rad1∆ cells, sorbitol was added as an osmotic stabilizer. The growth rate of both WT and rad1∆ cells was reduced in medium supplemented with 0.8 M sorbitol; however, enhanced toxicity of B. monnieri extracts in rad1∆ cells was still observed (Figure 6).

Figure 6.
Osmotic stabilization of plasma membrane does not prevent B. monnieri toxicity. (A) Yeast strains WT and rad1∆ (12806) were grown in synthetic medium with 2% glucose supplemented with 0.8 M sorbitol in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or B. monnieri extracts at the concentrations listed. Values are mean ± SD (n = 4). Growth was monitored by measuring OD at 600 nm with vehicle control (0) set at 100%. Statistical analysis employed Student’s t-test with ** p < 0.01. (B) Serial dilutions of liquid cultures from A were spotted onto solid synthetic medium with 2% glucose as described in Figure 3. Samples were incubated for 3 days and photographed.
2.7. Toxicity of B. monnieri Extracts Is Enhanced in Yeast Competent for Sterol Production
Several mechanisms for the toxicity of saponins, a common component of B. monnieri extracts, have been described and interactions with sterols is a common factor [47,48,49]. Yeast deleted for ERG6 have impaired production of ergosterol, the equivalent of cholesterol in fungi. The contribution of sterols to B. monnieri extract toxicity was evaluated by comparing WT, rad1∆, erg6∆, and erg6∆ rad1∆ strains for sensitivity. Yeast with an intact ERG6 gene were sensitized to B. monnieri extract compared to erg6∆ strains (Figure 7). Deletion of RAD1 increased toxicity of B. monnieri extract regardless of the status of ERG6. It appears that an effect mediated by altered membrane composition or structure may be involved in toxicity from bioactive compounds in extracts from B. monnieri.

Figure 7.
Effect of sterols on the toxicity of B. monnieri extracts in yeast deleted for RAD1. (A,B) Yeast strains WT, rad1∆ (12806), erg6∆ (PJ100), and rad1∆ erg6∆ (PJ101) were grown in synthetic medium with 2% glucose in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or B. monnieri extracts at the concentrations listed. Values are mean ± SD (n = 3). Growth was monitored by measuring OD at 600 nm with vehicle control (0) set at 100%. Statistical analysis employed Student’s t-test with ** p < 0.01, * p < 0.05. (C) Serial dilutions of liquid cultures from A and B were spotted onto solid synthetic medium with 2% glucose as described in Figure 4. Samples were incubated for 3 days and photographed.
2.8. The Yeast Meta-Caspase Yca1p Is Required for Toxicity of B. monnieri Extracts in rad1∆ Cells
The sensitivity of rad1∆ cells appears to be linked to an apoptotic-like response [19]. Blocking the induction of this apoptotic-like event may be protective against toxicity from B. monnieri extracts. Yeast contain a metacaspase, encoded by YCA1, homologous to those found in mammalian cells and is involved in induction of an apoptosis-like pathway following hydrogen peroxide exposure [50]. Disruption of YCA1 provided significant protection to rad1∆ cells against toxicity from B. monnieri extracts (Figure 8). These results confirm that rad1∆ yeast are sensitized to B. monnieri extracts due to activation of an apoptosis-like pathway.

Figure 8.
The meta-caspase Yca1p is required in rad1∆ cells to promote toxicity from B. monnieri extracts. (A,B) Yeast strains WT, rad1∆ (12806), yca1∆ (LJ464), and rad1∆ yca1∆ (LJ465) were grown in synthetic medium with 2% glucose in the presence of 2% DMSO and 0.2% Tween 80 (vehicle control) or B. monnieri extracts at the concentrations listed. Values are mean ± SD (n = 3). Growth was monitored by measuring OD at 600 nm with vehicle control (0) set at 100%. Statistical analysis employed Student’s t-test with ** p < 0.01, * p < 0.05. (B) Serial dilutions of liquid cultures from were spotted onto solid synthetic medium with 2% glucose as described in Figure 3. Samples were incubated for 3 days and photographed.
2.9. Yeast Deleted for RAD52 Exhibit Synthetic Lethal Interactions with B. monnieri Extracts
A synthetic lethal interaction has been observed in rad1∆ yeast with VMA6, encoding a subunit of the vacuolar H+-ATPase (V-ATPase) important for vacuole function [51]. Interestingly, synthetic lethal interactions between VMA6 have been reported with RAD52 and REV3 involved in DNA repair [52,53,54]. Rad1p and Rad52p have primary roles in double-strand break (DSB) repair [55,56] while Rev3p is involved in translesion synthesis and post-replication repair [57]. Yeast deleted for RAD52 exhibited increased sensitivity to B. monnieri extracts compared to rad1∆ cells. In contrast, the sensitivity of rev3∆ cells to B. monnieri extracts was similar to the WT strain (Figure 9). Although a strict correlation between sensitivity to B. monnieri extracts and synthetic lethal interactions with VMA6 was not observed it appears the defects in double-strand break repair may sensitize cells to the effects of compounds present in extracts from B. monnieri. The contribution of VMA6 to the effects of B. monnieri extracts is not clear but does suggest the vacuole as a potential target.

Figure 9.
Yeast deleted for RAD52 are sensitized to B. monnieri extracts. (A) Yeast cultures were grown as described in Figure 3 with the indicated concentration of B. monnieri extracts. Strains utilized include WT (BY4742), rad1∆ (12806), rad52 (10540), and rev3∆ (12085). Values are mean ± SD (n = 3). Statistical analysis employed Student’s t-test with ** p < 0.01 and * p < 0.05. (B) Serial dilutions of liquid cultures from were spotted onto solid synthetic medium with 2% glucose as described in Figure 3.
2.10. Bacopasaponin C Preferentially Limits the Growth of RAD1 Deleted Yeast
Major chemical constituents of B. monnieri have been previously identified including several biologically active compounds [27,58]. The most studied fraction bacoside A is a blend of bacoside A3, bacopasides I, II, X, and bacopasaponin C [25,39]. We examined the purified compounds from B. monnieri, including those found in the bacoside A fraction and bacopaside I, for chemical genetic effects with rad1∆ yeast. Bacoside A3 and bacopaside II did not cause substantial toxicity to either the WT or rad1∆ strain at the concentrations tested (Figure 10). Bacopaside I and bacopaside X exhibited toxicity toward yeast cells but reduced growth of both WT and rad1∆ cells to a similar degree. In contrast, bacopasaponin C was capable of preferentially limiting the growth of yeast deleted for RAD1. The growth inhibition profile of bacopasaponin C was similar to that observed for extracts from B. monnieri, suggesting that this compound may contribute to the synthetic lethal activity toward rad1∆ cells (Figure 10).

Figure 10.
Bacopasaponin C exhibits chemical-genetic interactions with RAD1. Five of the major constituents of B. monnieri extracts were examined for synthetic lethal interactions with yeast deleted for RAD1. Strains utilized were WT (BY4742) and rad1∆ (12806). Strains were grown in synthetic medium with 2% glucose in the presence of vehicle (2% DMSO and 0.2% Tween 80), bacopaside I, bacoside A3, bacopaside X, bacopaside II, or bacopasaponin C at the indicated concentrations. Growth was monitored by measuring OD at 600 nm with vehicle control (0) set at 100%. Values are mean ± SD (n = 3). Statistical analysis employed Student’s t-test ** p < 0.01.
3. Discussion
DNA damage is a major inducer of apoptosis due to either exposure of cells to compounds that form DNA lesions or defects in DNA repair systems [59,60]. However, apoptotic pathways can be activated by several types of cellular insults [61]. Yeast, although a unicellular organism, contains genes homologous to apoptotic regulators [61,62]. This conservation of key components of apoptosis pathways in yeast has allowed its use to facilitate the understanding of basic processes that occur in mammalian cells [61].
Previously we reported that B. monnieri extracts induced apoptosis-like effects in yeast cells lacking the single-stranded DNA endonuclease encoded by RAD1. The cause for the appearance of the apoptosis-like characteristics in yeast cells deleted for RAD1 and exposed to B. monnieri extracts was not identified. In this study, the potential of B. monnieri extracts to induce DNA mutations and chromosomal instability was evaluated using the CAN1 forward mutation assay [63] and appearance of A-like fakers [64]. Our analysis revealed an increase in the mutation frequency following exposure of B. monnieri extracts, although chromosomal instability was not significantly increased in yeast deleted for RAD1 compared to the WT strain. Exposure to MMS or oxaliplatin resulted in increased mutations and chromosomal instability. These findings indicated that DNA damage may be involved in the induction of an apoptosis-like events following exposure to B. monnieri extracts.
Several inducers have been found to promote apoptosis-like events in yeast cells [50,62,65]. In addition to the mitochondrial pathway, the yeast vacuole, similar to mammalian lysosomes, can participate in the appearance of apoptotic characteristics [37,66]. The vacuole/lysosome contains numerous hydrolases capable of degrading cellular macromolecules [34]. The release of vacuolar/lysosomal contents into the cytosol, through a process termed lysosomal membrane permeabilization (LMP), has been implicated in the induction of apoptosis in both yeast and mammalian cells [37,38]. Our analysis indicates that exposure to B. monnieri extracts increases the number of yeast with the diffuse appearance of a GFP fusion with Prc1p, vacuolar carboxypeptidase Y. A marker protein for the vacuolar membrane, Mam3p-RFP, was also observed with a diffuse localization in a fraction of cells exposed to B. monnieri extracts. It appears that treatment with B. monnieri extracts leads to severe vacuolar damage in some cells. This effect was observed in both wild-type and rad1∆ yeast cells, indicating that loss of RAD1 is not enhancing the ability of B. monnieri extracts to promote vacuolar damage. Although release of GFP cleaved from the GFP-Prc1p fusion was released from rad1∆ cells at a higher level than WT cells. B. monnieri extracts do not appear to have a general effect to disrupt all intracellular organelles. Mitochondrial morphology and integrity were not altered in WT or rad1∆ cells following treatment with B. monnieri extracts. We suspect that rad1∆ cells are more sensitive to the effects of vacuolar damage from B. monnieri extracts.
One of the triggers of LMP is exposure to lysosomotropic agents that accumulate within the vacuole/lysosome and cause destabilization of the membrane [67]. Among the best-characterized compounds from Bacopa monnieri are triterpenoid saponins, known as bacosides [27,39]. The mechanisms for the activity of saponins typically involves the interaction with sterols, leading to membrane disruption [47,48,49]. The lipid and protein content of membranes from intracellular organelles is distinct and saponins can display specificity toward particular organelles [40]. If saponins were involved in promoting vacuolar damage, then altering the sterol content should modulate the toxicity of B. monnieri extracts. ERG6, encodes Δ(24)-sterol C-methyltransferase in the ergosterol synthesis pathway, and the erg6∆ strain is similar to wild-type yeast except it exhibits decreased levels of sterols [19]. Interestingly, a comparison of wild type and cells lacking ERG6 revealed that yeast competent for sterol synthesis were sensitized to B. monnieri extracts relative to those with reduced sterol content. The increased sensitivity of rad1∆ yeast to B. monnieri extracts was apparent with or without an intact ERG6 gene. Overall, these results are consistent with saponins, or other sterol interacting compounds, from B. monnieri promoting the synthetic lethal effects seen in the absence of RAD1. Chloroquine is a lysosomotropic agent and would be expected to mimic the effects of B. monnieri extracts if vacuolar leakage was the key event in toxicity. However, while toxic to yeast, rad1∆ cells are not sensitized to chloroquine relative to the wild type strain. It appears that more than vacuolar leakage is required to limit growth of cells lacking RAD1. Our analysis did not indicate limited permeabilization of the vacuole membrane, instead a complete loss of the vacuole structure was observed. It is possible that extensive damage to the vacuole structure is required to sensitize rad1∆ cells to compounds present in B. monnieri extracts.
Release of vacuolar components may contribute to B. monnieri toxicity in rad1∆ yeast. A major function of the yeast vacuole is to provide a secure location for digestive enzymes [34]. Release of proteases from the vacuole would be expected to lead to cell damage. PEP4 encodes a protease important for activation of many digestive enzymes in the vacuole [45] and deletion of this gene limits toxicity of B. monnieri extracts on rad1∆ cells. The cellular insult from release of vacuolar proteases into the cytoplasm may be sufficient to induce and apoptosis-like event in yeast cells. Limiting induction of apoptosis through deletion of the gene for the yeast meta-caspase (YCA1) would then be expected to also be protective against toxicity of B. monnieri extracts. We observed a strong protective effect in rad1∆ cells in which YCA1 was absent. Thus it appears that release of vacuolar proteases and induction of an apoptosis-like event are important to mediate toxicity from B. monnieri extracts.
To better understand why yeast deleted for RAD1 display increased sensitivity to B. monnieri extracts we searched the BioGRID database [68] for synthetic lethal interactions between RAD1 and gene deletions not involved in DNA repair. Deletion of VMA6, encoding a subunit of the yeast vacuolar H+-ATPase (V-ATPase) has been reported to be required for survival of rad1∆ yeast [69]. Interestingly, yeast deleted for RAD52, involved in DSB repair [53], and REV3, important for translesion synthesis [54], are also reported to require VMA6 for survival [69]. Testing yeast deleted for RAD52 or REV3 revealed that of these strains only rad52∆ cells were sensitized to B. monnieri extract. As Rad1p is also involved in DSB repair, it is possible that deficiency in DSB repair may sensitize cells to components of B. monnieri extracts. Our findings are consistent with the report that yeast with impaired vacuolar function, due to deletion of V-ATPase subunits, are more prone to DNA damage compared to wild-type cells [69]. We propose that exposure to B. monnieri extracts, resulting in vacuolar damage and impaired vacuolar function, causes a synthetic sick/lethal chemical-genetic interaction in cells deficient in DSB repair, such as rad1∆ and rad52∆ yeast.
B. monnieri contains several bioactive natural products that may contribute to reduced proliferation of cells lacking RAD1. Bacosides, bacopasides, and bacosaponins have been isolated from B. monnieri [25,26,27] and these compounds exhibit various biological activities including neuroprotection, inhibition of water channels, and anti-tumor effects [24,70,71,72]. While our analysis of bioactive compounds from B. monnieri was not exhaustive, a chemical-genetic synthetic lethal effect between bacopasaponin C and RAD1 was observed.
The five bacoside compounds examined are dammarane-type triterpenoid saponins that have three sugar chains linked to a nonpolar triterpene aglycone skeleton [73]. Unique aspects of the structures of the bacopasides tested may provide a clue regarding functional groups that are involved in the chemical-genetic interactions between bacopasaponin C and rad1∆ yeast. The aglycone group is distinct between bacoside A3 and bacopaside II, with these compounds containing jujubogenin and pseudojujubogenin groups, respectively. However, bacopaside II and bacoside A3 do not exhibit toxicity toward yeast cells at the doses examined, indicating that the identity of the aglycone group is not sufficient to produce a toxic effect. The sugar moiety, an α-L-arabinofuranosyl-(1→2)-[β-D-glucopyranosyl-(1→3)]-α-L-arabinopyranosyl group, is shared between bacopaside X and bacopasaponin C although enhanced toxicity in rad1∆ yeast is only observed with bacopasaponin C. The aglycone group is different between bacopaside X and bacopasaponin C with these compounds containing jujubogenin and pseudojujubogenin, respectively (Figure 11). These findings indicate that neither the aglycone nor sugar moiety alone is sufficient to confer enhanced toxicity to rad1∆ yeast cells. Although the presence of pseudojujubogenin appears necessary for specificity against yeast lacking RAD1.
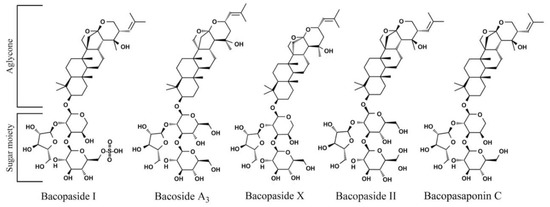
Figure 11.
Structures of major components present in extracts from B. monnieri. Structures were prepared with ChemDraw Ultra, version 12.
The predicted bacoside A content of B. monnieri extracts is approximately 6%. Bacopasaponin C comprises 0.3 to 0.6% of the material in ethanolic B. monnieri extracts [74]. A rough calculation would indicate that the content of bacopasaponin C from B. monnieri extracts utilized in our analysis at approximately 1 to 2 µg/mL. While we did not perform a quantitative analysis of bacoside content this approximate value for bacopasaonin C in the extract is 10-fold lower than that needed to produce toxicity using the purified compound. It is possible that bacopaside X, bacopaside I, or other compounds may contribute to toxicity to rad1∆ cells in the B. monnieri extract.
Overall our analysis has revealed a novel mechanism for the chemical-genetic synthetic lethal interaction between RAD1 and bacopasaponin C present in B. monnieri extracts. The absence of RAD1 in yeast results in increased mutation frequency and appears to sensitize cells to the effects of vacuolar disruption and release of proteases, such as Pep4p, following exposure to B. monnieri extracts. These effects together are likely sufficient to induce an apoptosis-like event that is dependent on the meta-caspase Yca1p. Among the bacoside compounds examined only bacopasaponin C preferentially limited the growth of rad1∆ yeast, indicating this was the active component, although other compounds present in the B. monnieri extract may also contribute to the toxic effect. Bacopasaponin C may have potential as a drug candidate for the treatment of colorectal cancer as well as other cancers that show impaired activity or expression of ERCC4 (XPF), the human homologue of RAD1.
4. Materials and Methods
4.1. Yeast Strains and Plasmids
The majority of S. cerevisiae strains used in this study were derived from BY4742 (Mat α, leu2∆0, lys2∆0, ura3∆0, his3∆1) [75] and KanMX4 containing single deletion strains were obtained from Open Biosystems (Layafette, CO, USA). Strain EG103 (MAT α, leu2–3112, his3∆1, trp1–289, ura3–52) has been described previously [19,76]. Gene deletions in strains LJ464 (BY4742, yca1∆:URA3), LJ465 (BY4742, rad1∆:KanMX4, yca1∆::URA3), and LJ467 (BY4742, pep4∆::KanMX4, rad1∆::URA3) were verified by in vivo PCR with a BioRad MJ Mini thermocycler (Hercules, CA, USA) using flanking primers [77]. Yeast transformations were performed using the lithium acetate procedure [78]. Cells were propagated at 30 °C either in enriched yeast extract, peptone-based medium supplemented with 2% glucose (YPD) or synthetic complete medium with 2% glucose (SC) [79]. The CAN1 forward mutation assays utilized SC medium without arginine (SC-Arg) supplemented with 50 µg/mL canavanine (Sigma-Aldrich, St. Louis, MO, USA).
The PRC1 coding sequence (−16 to +1596) was PCR amplified introducing a SpeI site at the 5′ end and replacing the stop codon with a NotI site. Plasmid pLJ457 containing the PGK1 promoter and a PHO84-GFP fusion (URA3, CEN) [80] was digested with XbaI and NotI liberating the PHO84 sequences. PRC1 was ligated into the cut pLJ457 plasmid resulting in pWC022 (PRC1-GFP). The RAD1 gene was PCR amplified using Pfu polymerase introducing BamHI and SalI restriction sites. The RAD1 PCR product was digested with BamHI and SalI and ligated into pRS315 cut with the same enzymes to generate plasmid pLJ540. The YCA1 disruption plasmid pLJ524, PEP4 disruption plasmid pLJ538, and RAD1 disruption plasmid pLJ539 was generated using standard techniques and utilized pRS306 (URA3) [81]. Transformation of yeast strains with pLJ524, pLJ538, or pLJ539 digested with BamHI resulted in the deletion of YCA1, PEP4, and RAD1 sequences. The sequence integrity of the plasmid was verified by DNA sequencing (Macrogen, Seoul, Korea).
4.2. Growth Tests
Growth analysis utilized cultures in 96 well plates (50 µL each well) with shaking at 200 rpm. Cells were allowed to double at least 5 times prior to measurement of OD 600 nm. Serial dilution analysis also utilized cells grown in 96 well plates. Control cultures containing vehicle only were diluted to OD 600 nm of 1 and the same dilution was used for each treated sample in the respective set. 10-fold serial dilutions were prepared and 10 µL of each sample was spotted onto SC medium and grown for 3 days at 30 °C before being photographed. DMSO was present in all cultures at 2%, a concentration that did not inhibit the growth of yeast strains. Tween-80 was included at a concentration of 0.2% to enhance the solubility of the B. monnieri extract and pure compounds. Purified compounds were tested using the same procedure as for crude extracts.
4.3. Analysis of Mutation Rates and Chromosome Instability
Canavanine resistance and formation of A-like fakers were utilized to monitor mutation frequency and chromosome instability, respectively. Yeast were exposed to vehicle, B. monnieri extract at 325 µg/mL, methyl methanesulfonate (MMS) at 300 µM, and oxaliplatin (OxPT) at 2.5 mM. MMS and OxPT were used as positive controls to induce DNA damage with the selected concentrations showing approximately 50% growth inhibition in the rad1∆ strain. Ten independent cultures for each strain were allowed to double at least five times and the cell number was estimated using OD 600 nm.
Canavanine resistant yeast were selected by plating 500,000 cells onto SC-arginine medium supplemented with 50 mg/liter canavanine. Mutation frequency was determined using the number of canavanine resistant colonies divided by the number of total cells determined using OD 600 nm. The presence of A-like fakers was monitored using a yeast mating assay selecting for cross complementation of auxotrophic markers. The total cell number was estimated using OD 600 nm. 500,000 yeast from erg6∆ and rad1∆ erg6∆ in the BY4742 background were mixed with 2 million cells of the EG103 strain. A-like fakers were identified following mating by selecting for diploids (LYS2/lys2∆, trp1∆/TRP1) capable of growing on medium lacking both lysine and tryptophan. Results are from two independent trials and reported as colonies formed per 1 × 107 cells.
4.4. Fluorescence Imaging
Fluorescence from the Prc1p-GFP, Mam3p-RFP, or Cox4pMLS-YFP fusions were visualized in live cells [82,83] and viewed directly at a magnification of 60× with an Olympus FV1000 or FV10-DOC confocal laser scanning microscope, equipped with universal plan super apochromat phase-contrast oil-immersion objective (Olympus Bioimaging Center, Mahidol University). To evaluate vacuolar and mitochondrial damage, 100 cells were examined for each condition. Yeast were treated with vehicle or 325 µg/mL B. monnieri extract (~IC50 concentration for rad1∆ yeast) for 16 h prior to visualization.
4.5. Cellular Fractionation
Cytosolic and vacuolar fractions were prepared following the procedure of Indge et al. [84]. Vacuolar and cytosolic fractions were separated using 10% SDS-PAGE with 20 µg of protein from each sample applied. Mitochondria and post-mitochondrial supernatents (PMS) were prepared by differential centrifugation following conversion of yeast to spheroplasts by digestion with zymolyase (20T) and lysis with a Dounce homogenizer [85]. Mitochondrial and PMS samples were separated using 15% SDS-PAGE with 15 µg of protein. Samples were transferred to nitrocellulose membranes and probed with an anti-GFP antibody, GFP-B2 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), separate gels were stained with Coomassie blue to serve as loading controls. Visualization of immunoblots used an HRP conjugated secondary antibody and enhanced chemiluminescence (ECL) detection (Merck Millipore, Burlington, MA, USA) with a G:Box Chemi XL1.4 chemiluminescence imaging system (Syngene, Frederick, MD, USA).
4.6. B. monnieri Extracts and Pure Compounds
B. monnieri powder was obtained from Herbal One Co. Ltd. (Nakhon Pathom, Thailand). B. monnieri (20 g) was extracted with 95% ethanol (100 mL) for 3 days. Insoluble material was removed by filtration, and the solvent was evaporated under reduced pressure using an SVC100 SpeedVac vacuum concentrator (Thermo Fisher Inc., Waltham, MA, USA). Dried extracts were weighed and stored at −20 °C prior to use. Samples were resuspended with DMSO at a concentration of 100 mg/mL. Bacoside A3, bacopaside I, bacopaside II, bacopaside X, and bacopasaponin C were purchased from ChromaDex Corp. (Los Angeles, CA, USA). Compounds were dissolved in DMSO at a concentration of 0.1 mg/mL.
4.7. Statistical Analysis
Experimental data are reported as the mean ± the standard deviation (SD). Significant differences between or among groups are indicated with, * p < 0.05 and ** p < 0.01. Data were analyzed with one-way ANOVA with post-hoc Tukey test or Student’s t-test as appropriate.
Supplementary Materials
The following are available online, Figure S1: Sensitivity of rad1∆ cells to B. monnieri extracts was complemented by episomal expression of RAD1.
Author Contributions
Conceptualization, L.T.J., C.H., S.S., and K.I.; Methodology, L.T.J., H.M.A., C.H., S.S., and K.I.; Formal Analysis, L.T.J., H.M.A., and C.H.; Investigation, L.T.J., H.M.A., C.H., T.T., and M.D.; Writing—Original Draft Preparation, L.T.J. and C.H.; Writing—Review & Editing, L.T.J., A.N.J., S.S., and K.I.; Visualization, L.T.J., C.H., T.T., and M.D.; Supervision, L.T.J.; Project Administration, L.T.J.; Funding Acquisition, L.T.J., A.N.J., and K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Thailand Science Research and Innovation RSA6180082 (L.T.J.), RSA6280058 (A.N.J.), DBG6080005 (K.I.), and IRN61W0005 (K.I.); The Center of Excellence for Innovation in Chemistry (PERCH-CIC); The Ministry of Higher Education, Science, Research and Innovation. (K.I.); and The Central Instrument Facility, Faculty of Science, Mahidol University (A.N.J.).
Acknowledgments
We thank W. Chindaudomsate and C. Outten for plasmids pWC022 and pLD207 as well as the Olympus Bioimaging Center, Mahidol University for their assistance with imaging studies.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Sample Availability
Yeast strains and plasmids are available from the authors.
References
- Watson, A.J.; Collins, P.D. Colon cancer: A civilization disorder. Dig. Dis. 2011, 29, 222–228. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer Incidence and Mortality Worldwide; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Watanabe, T.; Itabashi, M.; Shimada, Y.; Tanaka, S.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hyodo, I.; Igarashi, M.; Ishida, H.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2012, 17, 1–29. [Google Scholar] [CrossRef] [PubMed]
- de Gramont, A.; Schmoll, H.J.; Cervantes, A.; Tournigand, C. The evolving role of oxaliplatin in the management of colorectal cancer. Colorectal Dis. 2003, 5, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Fan, J.; Ma, Y.; Ma, Y.; Wang, H. Capecitabine-based chemotherapy for metastatic colorectal cancer. J. Cancer Res. Clin. Oncol. 2011, 137, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Raymond, E.; Faivre, S.; Woynarowski, J.M.; Chaney, S.G. Oxaliplatin: Mechanism of action and antineoplastic activity. Semin. Oncol. 1998, 25, 4–12. [Google Scholar]
- Teixeira, L.; Barry, S.; Debourdeau, P.; Cohen, A.; Tournigand, C. Cardiotoxicity of 5-fluorouracil. Bull. Cancer 2004, 91, 154–158. [Google Scholar]
- Ng, M.; Cunningham, D.; Norman, A.R. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur. J. Cancer 2005, 41, 1542–1546. [Google Scholar] [CrossRef]
- Han, R.; Yang, Y.M.; Dietrich, J.; Luebke, A.; Mayer-Proschel, M.; Noble, M. Systemic 5-fluorouracil treatment causes a syndrome of delayed myelin destruction in the central nervous system. J. Biol. 2008, 7, 12. [Google Scholar] [CrossRef]
- McQuade, R.M.; Bornstein, J.C.; Nurgali, K. Anti-Colorectal Cancer Chemotherapy-Induced Diarrhoea: Current Treatments and Side-Effects. Int. J. Clin. Exp. Med. 2014, 5, 393–406. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Brookman, K.W.; Lamerdin, J.E.; Thelen, M.P.; Hwang, M.; Reardon, J.T.; Sancar, A.; Zhou, Z.Q.; Walter, C.A.; Parris, C.N.; Thompson, L.H. ERCC4 (XPF) encodes a human nucleotide excision repair protein with eukaryotic recombination homologs. Mol. Cell. Biol. 1996, 16, 6553–6562. [Google Scholar] [CrossRef] [PubMed]
- Facista, A.; Nguyen, H.; Lewis, C.; Prasad, A.R.; Ramsey, L.; Zaitlin, B.; Nfonsam, V.; Krouse, R.S.; Bernstein, H.; Payne, C.M.; et al. Deficient expression of DNA repair enzymes in early progression to sporadic colon cancer. Genome Integr. 2012. [Google Scholar] [CrossRef]
- Ahmad, A.; Robinson, A.R.; Duensing, A.; van Drunen, E.; Beverloo, H.B.; Weisberg, D.B.; Hasty, P.; Hoeijmakers, J.H.; Niedernhofer, L.J. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol. Cell. Biol. 2008, 28, 5082–5092. [Google Scholar] [CrossRef]
- Bardwell, A.J.; Bardwell, L.; Tomkinson, A.E.; Friedberg, E.C. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science 1994, 265, 2082–2085. [Google Scholar] [CrossRef]
- Aung, H.S.; Huangteerakul, C.; Jensen, A.N.; Sukrong, S.; Jensen, L.T. Interrogation of ethnomedicinal plants for synthetic lethality effects in combination with deficiency in the DNA repair endonuclease RAD1 using a yeast cell-based assay. J. Ethnopharmacol. 2018, 223, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Harwansh, R.K.; Bahadur, S.; Banerjee, S.; Kar, A.; Chanda, J.; Biswas, S.; Ahmmed, S.M.; Katiyar, C.K. Development of Ayurveda—Tradition to trend. J. Ethnopharmacol. 2017, 197, 10–24. [Google Scholar] [CrossRef]
- Pase, M.P.; Kean, J.; Sarris, J.; Neale, C.; Scholey, A.B.; Stough, C. The cognitive-enhancing effects of Bacopa monnieri: A systematic review of randomized, controlled human clinical trials. J. Altern. Complement. Med. 2012, 18, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Limpeanchob, N.; Jaipan, S.; Rattanakaruna, S.; Phrompittayarat, W.; Ingkaninan, K. Neuroprotective effect of Bacopa monnieri on beta-amyloid-induced cell death in primary cortical culture. J. Ethnopharmacol. 2008, 120, 112–117. [Google Scholar] [CrossRef]
- Rohini, G.; Devi, C.S. Bacopa monniera extract induces apoptosis in murine sarcoma cells (S-180). Phytother. Res. 2008, 22, 1595–1598. [Google Scholar] [CrossRef]
- Peng, L.; Zhou, Y.; Kong de, Y.; Zhang, W.D. Antitumor activities of dammarane triterpene saponins from Bacopa monniera. Phytother. Res. 2010, 24, 864–868. [Google Scholar] [CrossRef]
- Garai, S.; Mahato, S.B.; Ohtani, K.; Yamasaki, K. Dammarane-type triterpenoid saponins from Bacopa monniera. Phytochemistry 1996, 42, 815–820. [Google Scholar] [CrossRef]
- Chakravarty, A.K.; Garai, S.; Masuda, K.; Nakane, T.; Kawahara, N. Bacopasides III-V: Three new triterpenoid glycosides from Bacopa monniera. Chem. Pharm. Bull. (Tokyo) 2003, 51, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishna, C.; Rao, C.V.; Trimurtulu, G.; Vanisree, M.; Subbaraju, G.V. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry 2005, 66, 2719–2728. [Google Scholar] [CrossRef]
- Rastogi, S.; Pal, R.; Kulshreshtha, D.K. Bacoside A3—A triterpenoid saponin from Bacopa monniera. Phytochemistry 1994, 36, 133–137. [Google Scholar] [CrossRef]
- Rohini, G.; Sabitha, K.E.; Devi, C.S. Bacopa monniera Linn. extract modulates antioxidant and marker enzyme status in fibrosarcoma bearing rats. Indian J. Exp. Biol. 2004, 42, 776–780. [Google Scholar] [PubMed]
- Janani, P.; Sivakumari, K.; Geetha, A.; Ravisankar, B.; Parthasarathy, C. Chemopreventive effect of bacoside A on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. J. Cancer Res. Clin. Oncol. 2010, 136, 759–770. [Google Scholar] [CrossRef]
- Mallick, M.N.; Akhtar, M.S.; Najm, M.Z.; Tamboli, E.T.; Ahmad, S.; Husain, S.A. Evaluation of anticancer potential of Bacopa monnieri L. against MCF-7 and MDA-MB 231 cell line. J. Pharm. Bioallied. Sci. 2015, 7, 325–328. [Google Scholar] [CrossRef]
- Xiao, W.; Chow, B.L. Synergism between yeast nucleotide and base excision repair pathways in the protection against DNA methylation damage. Curr. Genet. 1998, 33, 92–99. [Google Scholar] [CrossRef]
- Jones, E.W. Three proteolytic systems in the yeast saccharomyces cerevisiae. J. Biol. Chem. 1991, 266, 7963–7966. [Google Scholar] [CrossRef]
- Knop, M.; Schiffer, H.H.; Rupp, S.; Wolf, D.H. Vacuolar/lysosomal proteolysis: Proteases, substrates, mechanisms. Curr. Opin. Cell Biol. 1993, 5, 990–996. [Google Scholar] [CrossRef]
- Yamashima, T.; Kohda, Y.; Tsuchiya, K.; Ueno, T.; Yamashita, J.; Yoshioka, T.; Kominami, E. Inhibition of ischaemic hippocampal neuronal death in primates with cathepsin B inhibitor CA-074: A novel strategy for neuroprotection based on ‘calpain-cathepsin hypothesis’. Eur. J. Neurosci. 1998, 10, 1723–1733. [Google Scholar] [CrossRef]
- Foghsgaard, L.; Wissing, D.; Mauch, D.; Lademann, U.; Bastholm, L.; Boes, M.; Elling, F.; Leist, M.; Jaattela, M. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J. Cell. Biol. 2001, 153, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.M.; Parker, R. The RNase Rny1p cleaves tRNAs and promotes cell death during oxidative stress in Saccharomyces cerevisiae. J. Cell. Biol. 2009, 185, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Kroemer, G. Lysosomal membrane permeabilization in cell death. Oncogene 2008, 27, 6434–6451. [Google Scholar] [CrossRef]
- Deepak, M.; Sangli, G.K.; Arun, P.C.; Amit, A. Quantitative determination of the major saponin mixture bacoside A in Bacopa monnieri by HPLC. Phytochem. Anal. 2005, 16, 24–29. [Google Scholar] [CrossRef]
- Wassler, M.; Jonasson, I.; Persson, R.; Fries, E. Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Release of secretory proteins. Biochem. J. 1987, 247, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.C.; Favre, M.; Bensa, J.C. Membrane cell permeabilization with saponin and multiparametric analysis by flow cytometry. Cytometry 1991, 12, 550–558. [Google Scholar] [CrossRef]
- Roberg, K.; Ollinger, K. Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am. J. Pathol. 1998, 152, 1151–1156. [Google Scholar]
- Liu, N.; Raja, S.M.; Zazzeroni, F.; Metkar, S.S.; Shah, R.; Zhang, M.; Wang, Y.; Bromme, D.; Russin, W.A.; Lee, J.C.; et al. NF-kappaB protects from the lysosomal pathway of cell death. EMBO J. 2003, 22, 5313–5322. [Google Scholar] [CrossRef]
- de Duve, C.; de Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Lysosomotropic agents. Biochem. Pharmacol. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Ammerer, G.; Hunter, C.P.; Rothman, J.H.; Saari, G.C.; Valls, L.A.; Stevens, T.H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol. Cell. Biol. 1986, 6, 2490–2499. [Google Scholar] [CrossRef]
- Hu, J.; Dong, L.; Outten, C.E. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J. Biol. Chem. 2008, 283, 29126–29134. [Google Scholar] [CrossRef]
- Armah, C.N.; Mackie, A.R.; Roy, C.; Price, K.; Osbourn, A.E.; Bowyer, P.; Ladha, S. The membrane-permeabilizing effect of avenacin A-1 involves the reorganization of bilayer cholesterol. Biophys. J. 1999, 76, 281–290. [Google Scholar] [CrossRef]
- Lorent, J.; Le Duff, C.S.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. Induction of highly curved structures in relation to membrane permeabilization and budding by the triterpenoid saponins, alpha- and delta-Hederin. J. Biol. Chem. 2013, 288, 14000–14017. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. The amphiphilic nature of saponins and their effects on artificial and biological membranes and potential consequences for red blood and cancer cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef]
- Madeo, F.; Herker, E.; Maldener, C.; Wissing, S.; Lachelt, S.; Herlan, M.; Fehr, M.; Lauber, K.; Sigrist, S.J.; Wesselborg, S.; et al. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 2002, 9, 911–917. [Google Scholar] [CrossRef]
- Bauerle, C.; Ho, M.N.; Lindorfer, M.A.; Stevens, T.H. The Saccharomyces cerevisiae VMA6 gene encodes the 36-kDa subunit of the vacuolar H(+)-ATPase membrane sector. J. Biol. Chem. 1993, 268, 12749–12757. [Google Scholar] [CrossRef]
- Muris, D.F.; Bezzubova, O.; Buerstedde, J.M.; Vreeken, K.; Balajee, A.S.; Osgood, C.J.; Troelstra, C.; Hoeijmakers, J.H.; Ostermann, K.; Schmidt, H.; et al. Cloning of human and mouse genes homologous to RAD52, a yeast gene involved in DNA repair and recombination. Mutat. Res. 1994, 315, 295–305. [Google Scholar] [CrossRef][Green Version]
- Symington, L.S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol Mol. Biol. Rev. 2002, 66, 630–670. [Google Scholar] [CrossRef] [PubMed]
- Gan, G.N.; Wittschieben, J.P.; Wittschieben, B.O.; Wood, R.D. DNA polymerase zeta (pol zeta) in higher eukaryotes. Cell Res. 2008, 18, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Paques, F.; Haber, J.E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 1999, 63, 349–404. [Google Scholar] [CrossRef] [PubMed]
- Schiestl, R.H.; Prakash, S. RAD1, an excision repair gene of Saccharomyces cerevisiae, is also involved in recombination. Mol. Cell. Biol. 1988, 8, 3619–3626. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Washington, M.T.; Haracska, L.; Prakash, S.; Prakash, L. Eukaryotic polymerases iota and zeta act sequentially to bypass DNA lesions. Nature 2000, 406, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Ganjewala, D.; Srivastava, A.K. Recent Progress on Chemical Composition and Bioactivities of Bacopa monnieri (Linn.) a Plant of Ayurveda. Med. Aromat. Plant. Sci. Biotechnol. 2011, 5, 102–108. [Google Scholar]
- De Zio, D.; Cianfanelli, V.; Cecconi, F. New insights into the link between DNA damage and apoptosis. Antioxid. Redox. Signal. 2013, 19, 559–571. [Google Scholar] [CrossRef]
- Wang, J.Y. DNA damage and apoptosis. Cell Death Differ. 2001, 8, 1047–1048. [Google Scholar] [CrossRef]
- Falcone, C.; Mazzoni, C. External and internal triggers of cell death in yeast. Cell Mol. Life Sci. 2016, 73, 2237–2250. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Eisenberg, T.; Buttner, S.; Meisinger, C.; Kroemer, G.; Madeo, F. Apoptosis in yeast: Triggers, pathways, subroutines. Cell Death Differ. 2010, 17, 763–773. [Google Scholar] [CrossRef]
- Lang, G.I.; Murray, A.W. Estimating the per-base-pair mutation rate in the yeast Saccharomyces cerevisiae. Genetics 2008, 178, 67–82. [Google Scholar] [CrossRef]
- Novoa, C.A.; Ang, J.S.; Stirling, P.C. The A-Like Faker Assay for Measuring Yeast Chromosome III Stability. Methods Mol. Biol 2018, 1672, 1–9. [Google Scholar] [CrossRef]
- Ludovico, P.; Sousa, M.J.; Silva, M.T.; Leao, C.L.; Corte-Real, M. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology (Reading) 2001, 147, 2409–2415. [Google Scholar] [CrossRef]
- Mason, D.A.; Shulga, N.; Undavai, S.; Ferrando-May, E.; Rexach, M.F.; Goldfarb, D.S. Increased nuclear envelope permeability and Pep4p-dependent degradation of nucleoporins during hydrogen peroxide-induced cell death. FEMS Yeast Res. 2005, 5, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Boya, P.; Andreau, K.; Poncet, D.; Zamzami, N.; Perfettini, J.L.; Metivier, D.; Ojcius, D.M.; Jaattela, M.; Kroemer, G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J. Exp. Med. 2003, 197, 1323–1334. [Google Scholar] [CrossRef]
- Stark, C.; Breitkreutz, B.J.; Chatr-Aryamontri, A.; Boucher, L.; Oughtred, R.; Livstone, M.S.; Nixon, J.; Van Auken, K.; Wang, X.; Shi, X.; et al. The BioGRID Interaction Database: 2011 update. Nucleic Acids Res. 2011, 39, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Hu, B.; Arno, M.J.; Panaretou, B. Genomic screening in vivo reveals the role played by vacuolar H+ ATPase and cytosolic acidification in sensitivity to DNA-damaging agents such as cisplatin. Mol. Pharmacol 2007, 71, 416–425. [Google Scholar] [CrossRef]
- Dhanasekaran, M.; Tharakan, B.; Holcomb, L.A.; Hitt, A.R.; Young, K.A.; Manyam, B.V. Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother Res. 2007, 21, 965–969. [Google Scholar] [CrossRef]
- Liu, X.; Yue, R.; Zhang, J.; Shan, L.; Wang, R.; Zhang, W. Neuroprotective effects of bacopaside I in ischemic brain injury. Restor. Neurol. Neurosci. 2013, 31, 109–123. [Google Scholar] [CrossRef]
- Pei, J.V.; Kourghi, M.; De Ieso, M.L.; Campbell, E.M.; Dorward, H.S.; Hardingham, J.E.; Yool, A.J. Differential Inhibition of Water and Ion Channel Activities of Mammalian Aquaporin-1 by Two Structurally Related Bacopaside Compounds Derived from the Medicinal Plant Bacopa monnieri. Mol. Pharmacol. 2016, 90, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Chin, S.P.; Sukumaran, S.D.; Buckle, M.J.; Kiew, L.V.; Chung, L.Y. In Silico and In Vitro Analysis of Bacoside A Aglycones and Its Derivatives as the Constituents Responsible for the Cognitive Effects of Bacopa monnieri. PLoS ONE 2015, 10, e0126565. [Google Scholar] [CrossRef] [PubMed]
- Phrompittayarat, W.; Putalun, W.; Tanaka, H.; Jetiyanon, K.; Wittaya-areekul, S.; Ingkaninan, K. Comparison of Various Extraction Methods of Bacopa monnieri. Naresuan Univ. J. 2007, 15, 29–34. [Google Scholar]
- Brachmann, C.B.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived from Saccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Liu, X.F.; Elashvili, I.; Gralla, E.B.; Valentine, J.S.; Lapinskas, P.; Culotta, V.C. Yeast lacking superoxide dismutase: Isolation of genetic suppressors. J. Biol. Chem. 1992, 267, 18298–18302. [Google Scholar] [CrossRef]
- Longtine, M.S.; McKenzie, A., 3rd; Demarini, D.J.; Shah, N.G.; Wach, A.; Brachat, A.; Philippsen, P.; Pringle, J.R. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 1998, 14, 953–961. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast 1991, 7, 253–263. [Google Scholar] [CrossRef]
- Sherman, F.; Fink, G.R.; Lawrence, C.W. Methods in Yeast Genetics; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1978; pp. 178–179. [Google Scholar]
- Jensen, L.T.; Ajua-Alemanji, M.; Culotta, V.C. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J. Biol. Chem. 2003, 278, 42036–42040. [Google Scholar] [CrossRef] [PubMed]
- Sikorski, R.S.; Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 1989, 122, 19–27. [Google Scholar] [CrossRef]
- Sundin, B.A.; Chiu, C.H.; Riffle, M.; Davis, T.N.; Muller, E.G. Localization of proteins that are coordinately expressed with Cln2 during the cell cycle. Yeast 2004, 21, 793–800. [Google Scholar] [CrossRef]
- Jensen, L.T.; Carroll, M.C.; Hall, M.D.; Harvey, C.J.; Beese, S.E.; Culotta, V.C. Down-regulation of a manganese transporter in the face of metal toxicity. Mol. Biol. Cell 2009, 20, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Indge, K.J. The isolation and properties of the yeast cell vacuole. J. Gen. Microbiol. 1968, 51, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.T.; Culotta, V.C. Role of Saccharomyces cerevisiae ISA1 and ISA2 in iron homeostasis. Mol. Cell Biol. 2000, 20, 3918–3927. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).