Effect of Side Chain Substituent Volume on Thermoelectric Properties of IDT-Based Conjugated Polymers
Abstract
1. Introduction
2. Experimental Section
2.1. General
2.2. Synthesis of the Copolymers
2.3. Thin Films Preparation
3. Results and Discussions
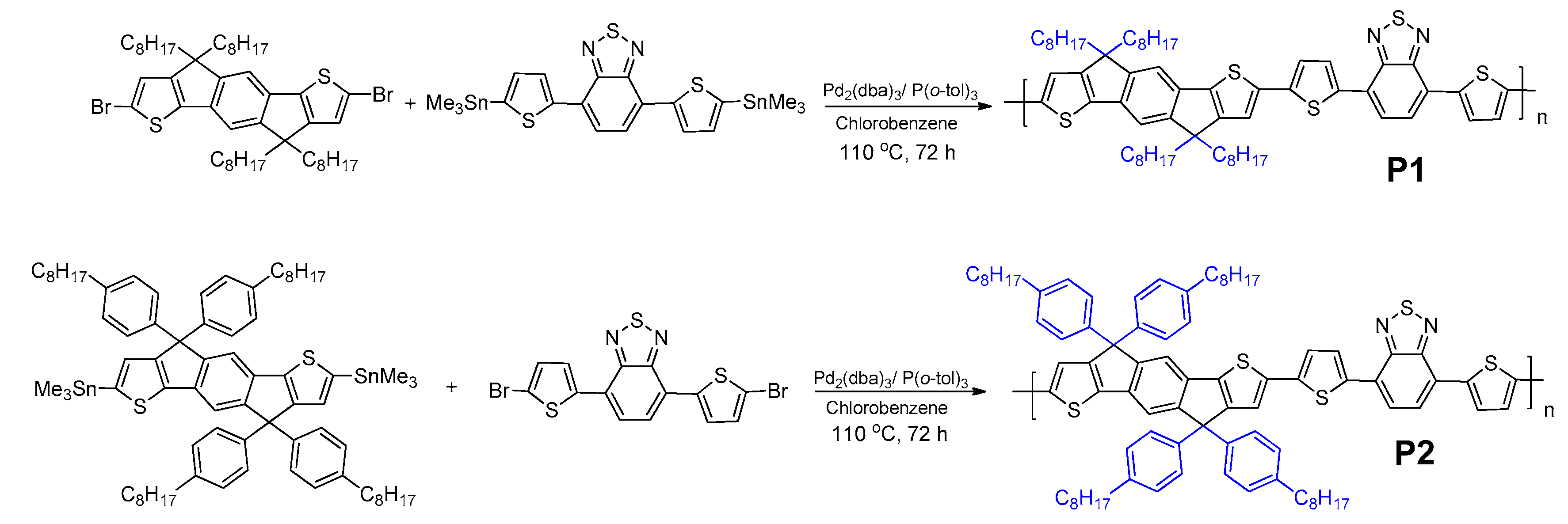
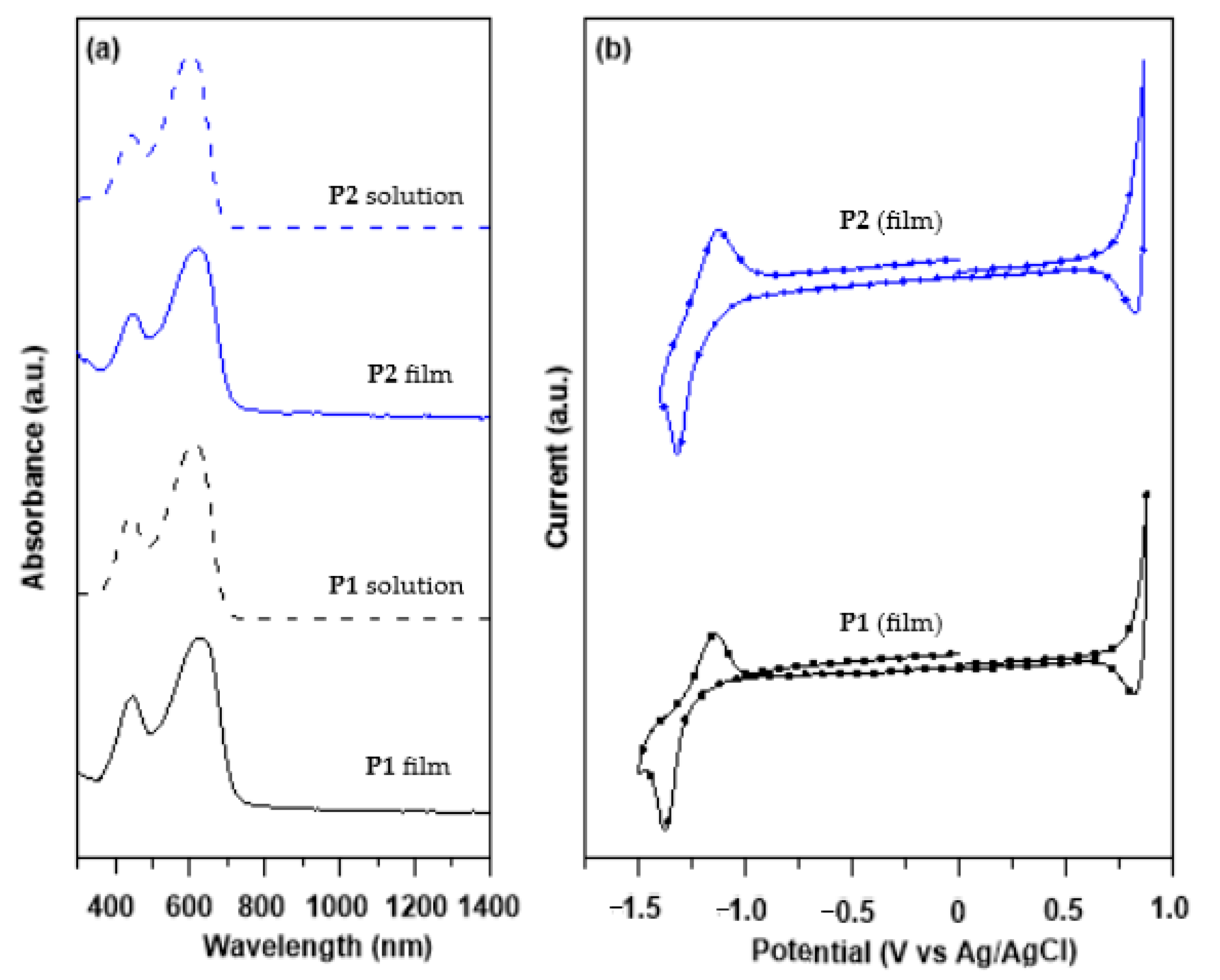
3.1. Polymer Design, Synthesis, and Characterizations
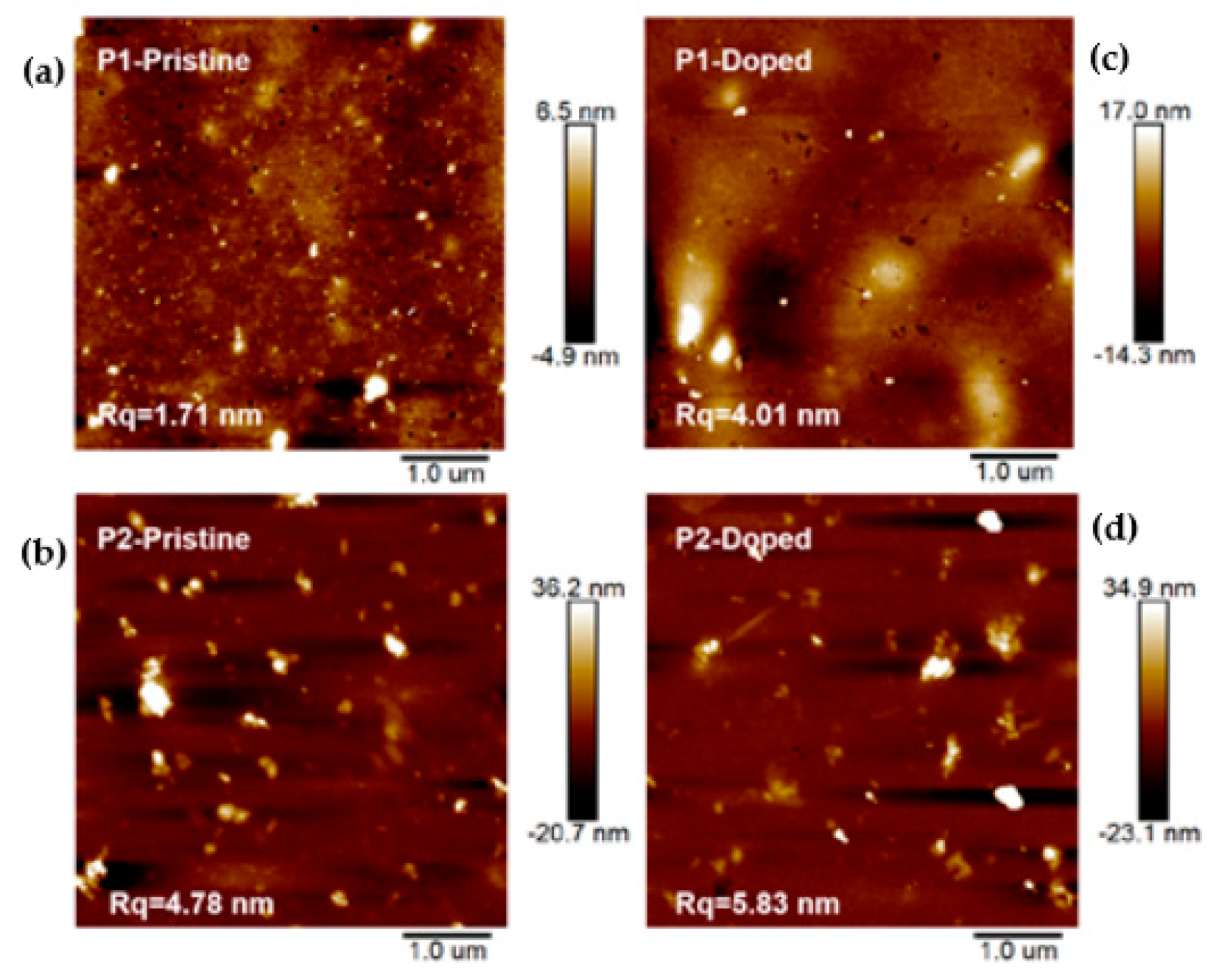
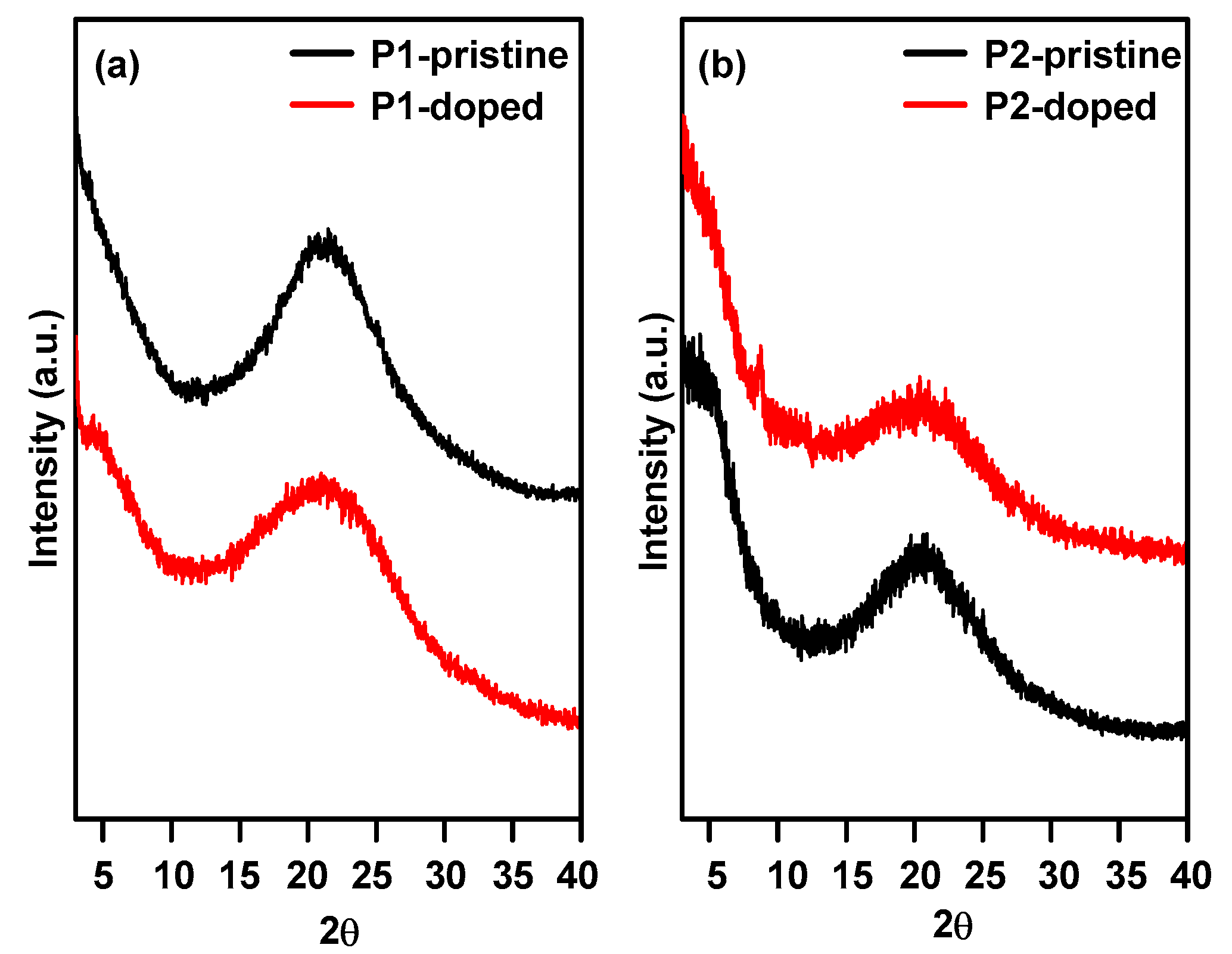
3.2. Morphology Study
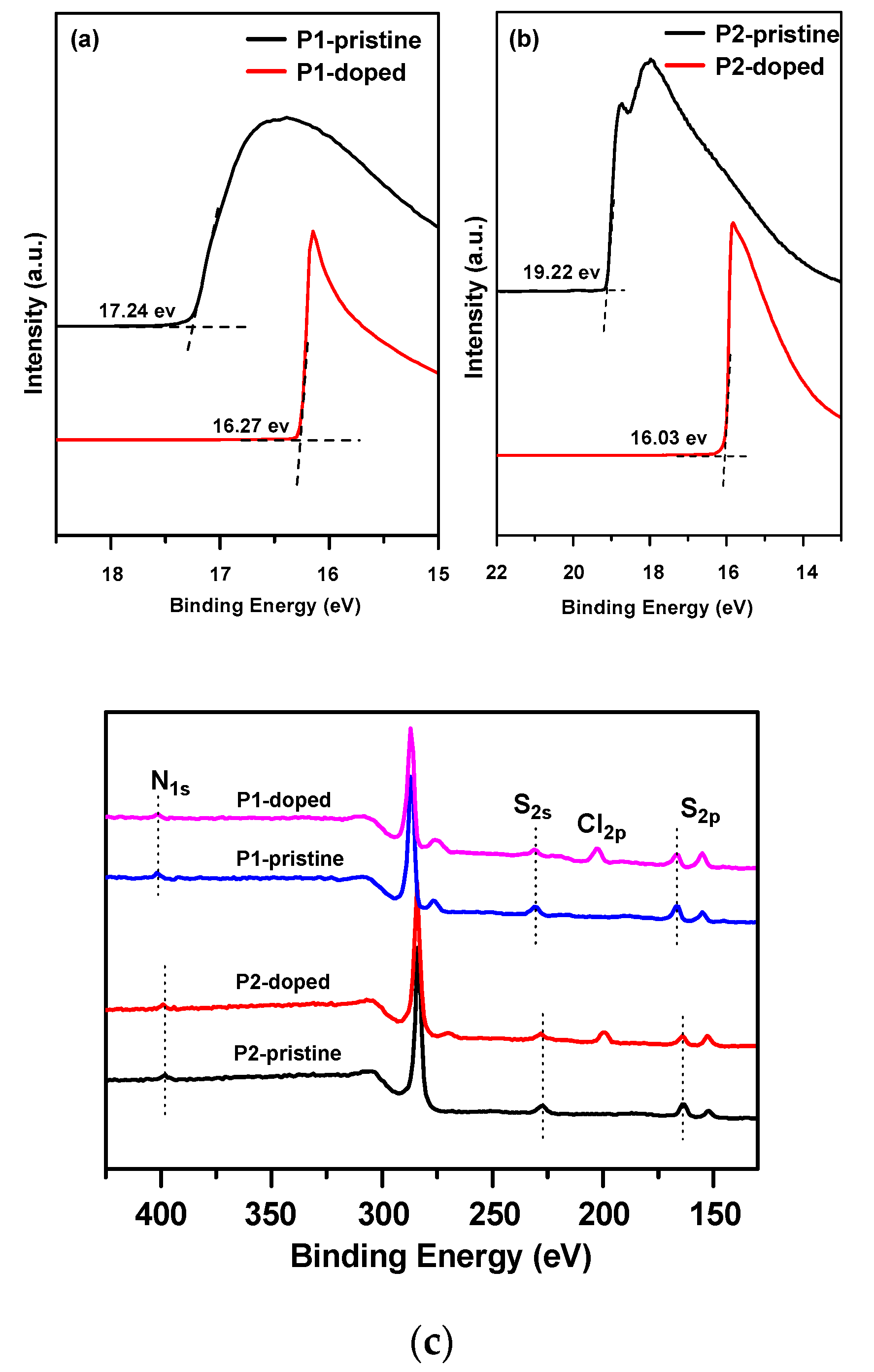
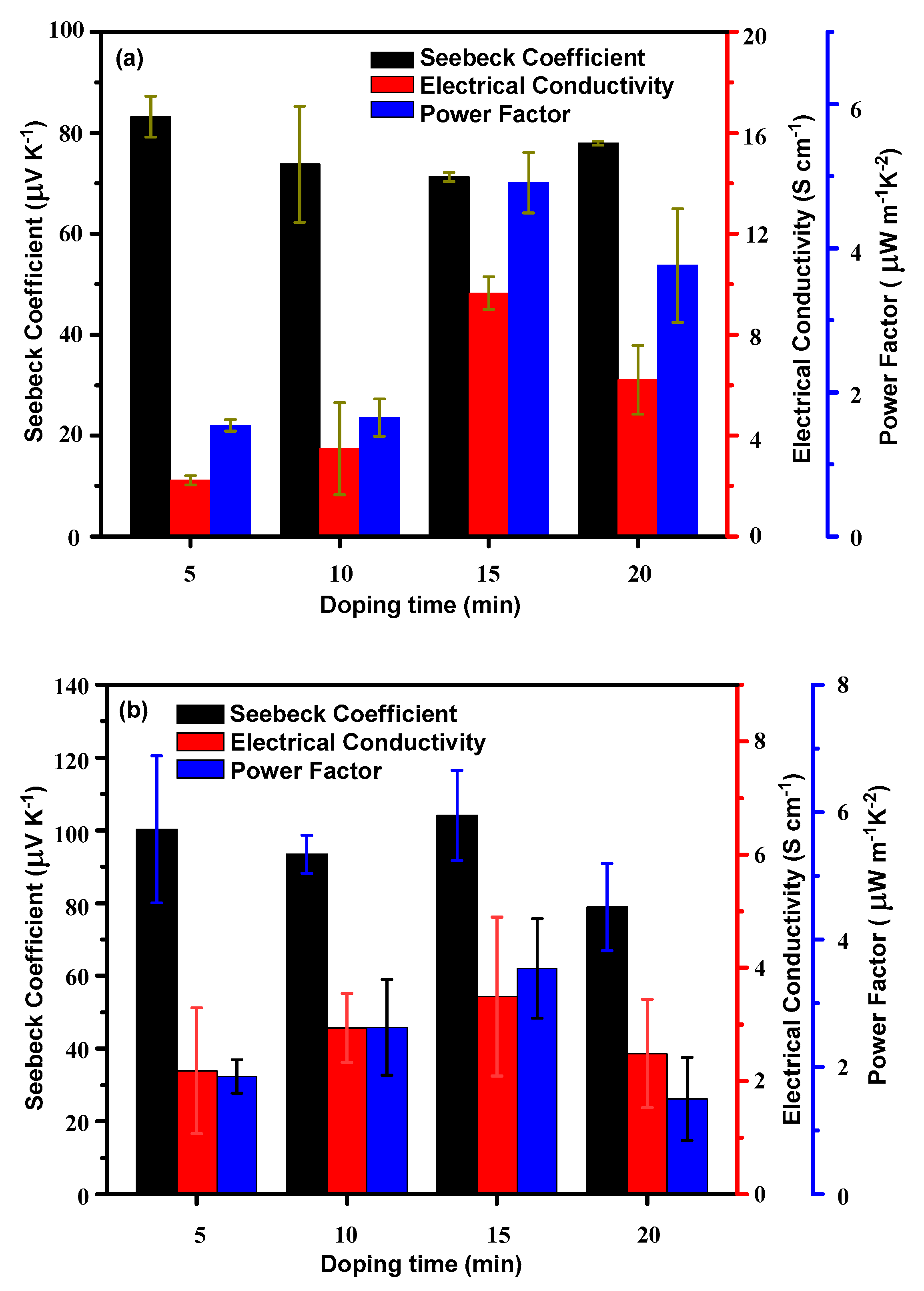
3.3. Characterizations of the Doped Conjugated Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bubnova, O.; Crispin, X. Towards polymer-based organic thermoelectric generators. Energy Environ. Sci. 2012, 5, 9345–9362. [Google Scholar] [CrossRef]
- Russ, B.; Glaudell, A.; Urban, J.J.; Chabinyc, M.L.; Segalman, R.A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 2016, 1, 16050. [Google Scholar] [CrossRef]
- Hong, S.; Gu, Y.; Seo, J.K.; Wang, J.; Liu, P.; Meng, Y.S.; Xu, S.; Chen, R. Wearable thermoelectrics for personalized thermoregulation. Sci. Adv. 2019, 5, eaaw0536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Liang, Z. Solution processed organic thermoelectrics: Towards flexible thermoelectric modules. Energy Environ. Sci. 2015, 8, 401–422. [Google Scholar] [CrossRef]
- Xiao, C.; Li, Z.; Li, K.; Huang, P.; Xie, Y. Decoupling Interrelated Parameters for Designing High Performance Thermoelectric Materials. Acc. Chem. Res. 2014, 47, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Holliday, S.; Donaghey, J.E.; McCulloch, I. Advances in Charge Carrier Mobilities of Semiconducting Polymers Used in Organic Transistors. Chem. Mater. 2013, 26, 647–663. [Google Scholar] [CrossRef]
- Zhou, Q.; Swager, T.M. Method for enhancing the sensitivity of fluorescent chemosensors: Energy migration in conjugated polymers. J. Am. Chem. Soc. 1995, 117, 7017–7018. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef] [PubMed]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef]
- Jung, I.H.; Hong, C.T.; Lee, U.-H.; Kang, Y.H.; Jang, K.-S.; Cho, S.Y. High Thermoelectric Power Factor of a Diketopyrrolopyrrole-Based Low Bandgap Polymer via Finely Tuned Doping Engineering. Sci. Rep. 2017, 7, srep44704. [Google Scholar] [CrossRef]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N.; Chabinyc, M.L. Impact of the Doping Method on Conductivity and Thermopower in Semiconducting Polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. What to Expect from Conducting Polymers on the Playground of Thermoelectricity: Lessons Learned from Four High-Mobility Polymeric Semiconductors. Macromolecules 2014, 47, 609–615. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, F.; Di, C.-A.; Yan, T.-W.; Zou, Y.; Zhou, X.; Zhu, D.; Wang, J.-Y.; Pei, J. Toward High Performance n-Type Thermoelectric Materials by Rational Modification of BDPPV Backbones. J. Am. Chem. Soc. 2015, 137, 6979–6982. [Google Scholar] [CrossRef]
- Kiefer, D.; Giovannitti, A.; Sun, H.; Biskup, T.; Hofmann, A.; Koopmans, M.; Cendra, C.; Weber, S.; Koster, L.J.A.; Olsson, E.; et al. Enhanced n-Doping Efficiency of a Naphthalenediimide-Based Copolymer through Polar Side Chains for Organic Thermoelectrics. ACS Energy Lett. 2018, 3, 278–285. [Google Scholar] [CrossRef]
- Duong, D.T.; Wang, C.; Antono, E.; Toney, M.F.; Salleo, A. The chemical and structural origin of efficient p-type doping in P3HT. Org. Electron. 2013, 14, 1330–1336. [Google Scholar] [CrossRef]
- Lanzi, M.; Salatelli, E.; Giorgini, L.; Marinelli, M.; Pierini, F. Effect of the incorporation of an Ag nanoparticle interlayer on the photovoltaic performance of green bulk heterojunction water-soluble polythiophene solar cells. Polymer 2018, 149, 273–285. [Google Scholar] [CrossRef]
- Fokina, A.; Lee, Y.; Chang, J.H.; Braun, L.; Bae, W.K.; Char, K.; Lee, C.; Zentel, R. Side-chain conjugated polymers for use in the active layers of hybrid semiconducting polymer/quantum dot light emitting diodes. Polym. Chem. 2015, 7, 101–112. [Google Scholar] [CrossRef]
- Tsao, H.N.; Cho, D.M.; Park, I.; Hansen, M.R.; Mavrinskiy, A.; Yoon, D.Y.; Graf, R.; Pisula, W.; Spiess, H.W.; Müllen, K. Ultrahigh Mobility in Polymer Field-Effect Transistors by Design. J. Am. Chem. Soc. 2011, 133, 2605–2612. [Google Scholar] [CrossRef]
- Sun, B.; Hong, W.; Yan, Z.; Aziz, H.; Li, Y. Record High Electron Mobility of 6.3 cm2V−1s−1 Achieved for Polymer Semiconductors Using a New Building Block. Adv. Mater. 2014, 26, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Deng, Y.; Tian, H.; Zhang, J.; Yan, D.; Geng, Y.; Wang, F. Multifluorination toward High-Mobility Ambipolar and Unipolar n-Type Donor-Acceptor Conjugated Polymers Based on Isoindigo. Adv. Mater. 2017, 29, 1606217. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lai, C.; Xiang, X.; Wang, L. Synthesis and characterization of poly-Schiff bases with a donor–acceptor structure containing thiophene units as thermoelectric materials. J. Mater. Chem. C 2015, 3, 2693–2701. [Google Scholar] [CrossRef]
- Wang, L.; Pan, C.; Chen, Z.; Zhou, X.; Gao, C.M.; Wang, L. A study of the thermoelectric properties of benzo[1,2-b:4,5-b′]dithiophene–based donor–acceptor conjugated polymers. Polym. Chem. 2018, 9, 4440–4447. [Google Scholar] [CrossRef]
- Wang, L.; Pan, C.; Liang, A.; Zhou, X.; Zhou, W.; Wan, T.; Wang, L. The effect of the backbone structure on the thermoelectric properties of donor–acceptor conjugated polymers. Polym. Chem. 2017, 8, 4644–4650. [Google Scholar] [CrossRef]
- Pan, C.; Wang, L.; Liu, T.; Zhou, X.; Wan, T.; Wang, S.; Chen, Z.; Gao, C.; Wang, L. Polar Side Chain Effects on the Thermoelectric Properties of Benzo[1,2-b:4,5-b′]Dithiophene-Based Conjugated Polymers. Macromol. Rapid Commun. 2019, 40, e1900082. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Jin, W.-L.; Wang, J.; Ding, Y.-F.; Nong, S.; Shi, K.; Lu, Y.; Dai, Y.-Z.; Zhuang, F.-D.; Lei, T.; et al. Enhancing the n-Type Conductivity and Thermoelectric Performance of Donor-Acceptor Copolymers through Donor Engineering. Adv. Mater. 2018, 30, e1802850. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Z.; Zhao, W.; Jin, W.; Xiang, L.; Wang, Z.; Zeng, Y.; Zou, Y.; Zhang, F.; Yi, Y.; et al. Selenium-Substituted Diketopyrrolopyrrole Polymer for High-Performance p-Type Organic Thermoelectric Materials. Angew. Chem. Int. Ed. 2019, 58, 18994–18999. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xiong, M.; Li, J.-T.; Zhang, S.; Ahmad, Z.; Lu, Y.; Wang, Z.-Y.; Yao, Z.-F.; Wang, J.-Y.; Gu, X.; et al. Pyrazine-Flanked Diketopyrrolopyrrole (DPP): A New Polymer Building Block for High-Performance n-Type Organic Thermoelectrics. J. Am. Chem. Soc. 2019, 141, 20215–20221. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Nguyen, T.L.; Kim, M.; Park, J.; Lee, Y.; Hwang, S.; Kwon, Y.-W.; Kwak, J.; Woo, H.Y. A Planar Cyclopentadithiophene–Benzothiadiazole-Based Copolymer with sp2-Hybridized Bis(alkylsulfanyl)methylene Substituents for Organic Thermoelectric Devices. Macromolecules 2018, 51, 3360–3368. [Google Scholar] [CrossRef]
- Zhou, X.; Pan, C.; Gao, C.; Shinohara, A.; Yin, X.; Wang, L.; Li, Y.; Jiang, Q.; Yang, C.; Wang, L. Thermoelectrics of two-dimensional conjugated benzodithiophene-based polymers: Density-of-states enhancement and semi-metallic behavior. J. Mater. Chem. A 2019, 7, 10422–10430. [Google Scholar] [CrossRef]

| Polymer | Mn (KDa) | Mw (KDa) | PDI | Td (°C) | Tg (°C) |
|---|---|---|---|---|---|
| P1 | 58.54 | 132.7 | 2.27 | 401 | 133 |
| P2 | 87.11 | 166.3 | 1.91 | 392 | 203 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, D.-X.; Liu, T.-C.; Xiao, J.; Fang, J.-K.; Pan, C.-J.; Shao, G. Effect of Side Chain Substituent Volume on Thermoelectric Properties of IDT-Based Conjugated Polymers. Molecules 2021, 26, 963. https://doi.org/10.3390/molecules26040963
Xie D-X, Liu T-C, Xiao J, Fang J-K, Pan C-J, Shao G. Effect of Side Chain Substituent Volume on Thermoelectric Properties of IDT-Based Conjugated Polymers. Molecules. 2021; 26(4):963. https://doi.org/10.3390/molecules26040963
Chicago/Turabian StyleXie, De-Xun, Tong-Chao Liu, Jing Xiao, Jing-Kun Fang, Cheng-Jun Pan, and Guang Shao. 2021. "Effect of Side Chain Substituent Volume on Thermoelectric Properties of IDT-Based Conjugated Polymers" Molecules 26, no. 4: 963. https://doi.org/10.3390/molecules26040963
APA StyleXie, D.-X., Liu, T.-C., Xiao, J., Fang, J.-K., Pan, C.-J., & Shao, G. (2021). Effect of Side Chain Substituent Volume on Thermoelectric Properties of IDT-Based Conjugated Polymers. Molecules, 26(4), 963. https://doi.org/10.3390/molecules26040963







