Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea biebersteinii Afan. Extract
Abstract
1. Introduction
2. Results and Discussion
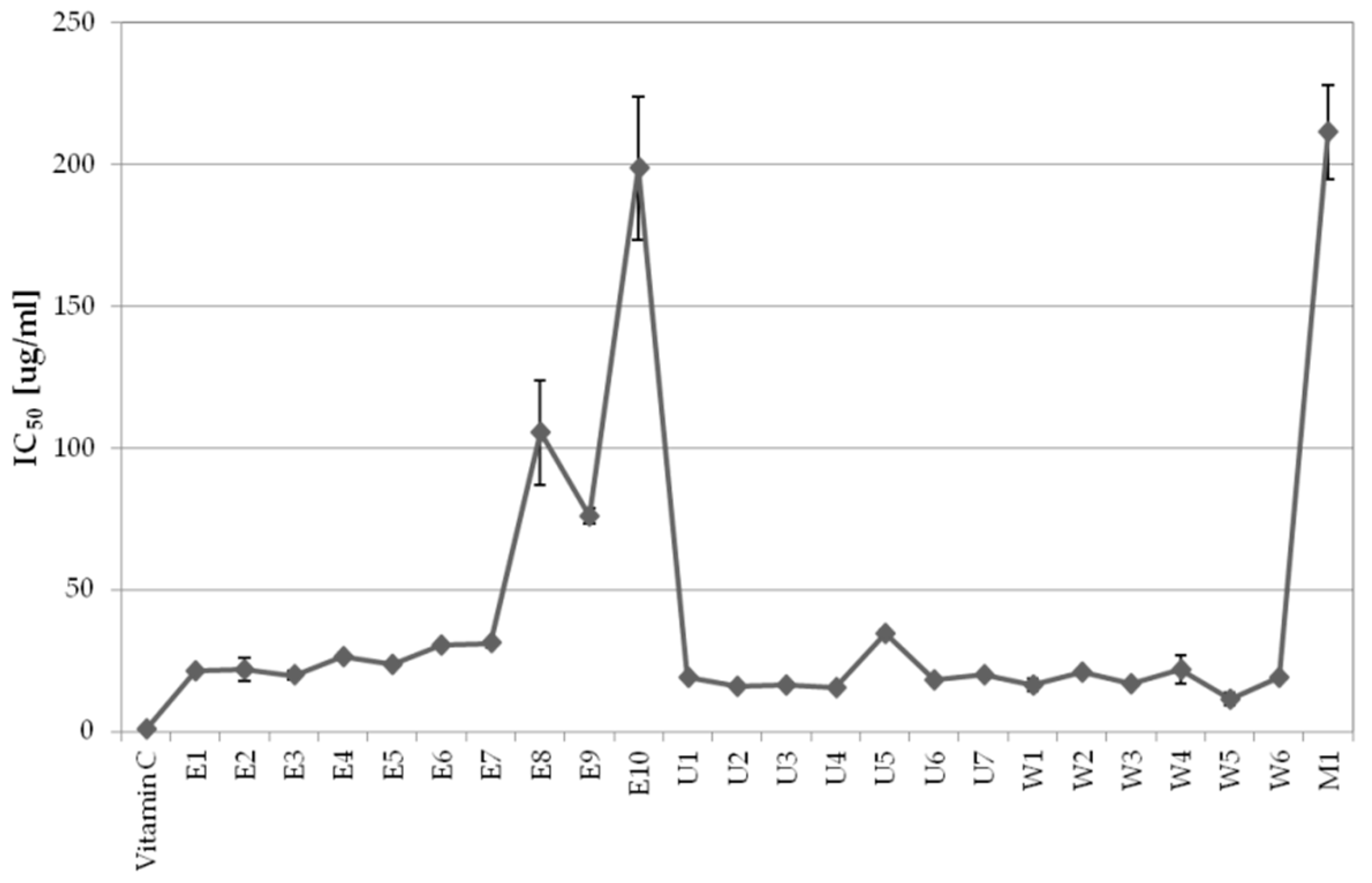
2.1. Activity-Guided Optimization of A. biebersteinii Extraction Conditions
2.1.1. The Influence of Extraction Conditions on Antioxidant Properties
2.1.2. The Influence of Extraction Conditions on Tyrosinase Inhibitory Properties
2.2. The Identification of Active Components in EtOH Extracts from Achillea biebersteinii by HPLC and HPLC–ESI-Q-TOF-MS
2.3. The Fractionation of E6 Extract
2.3.1. Tyrosinase Inhibitory Activity of A. biebersteinii Fractions
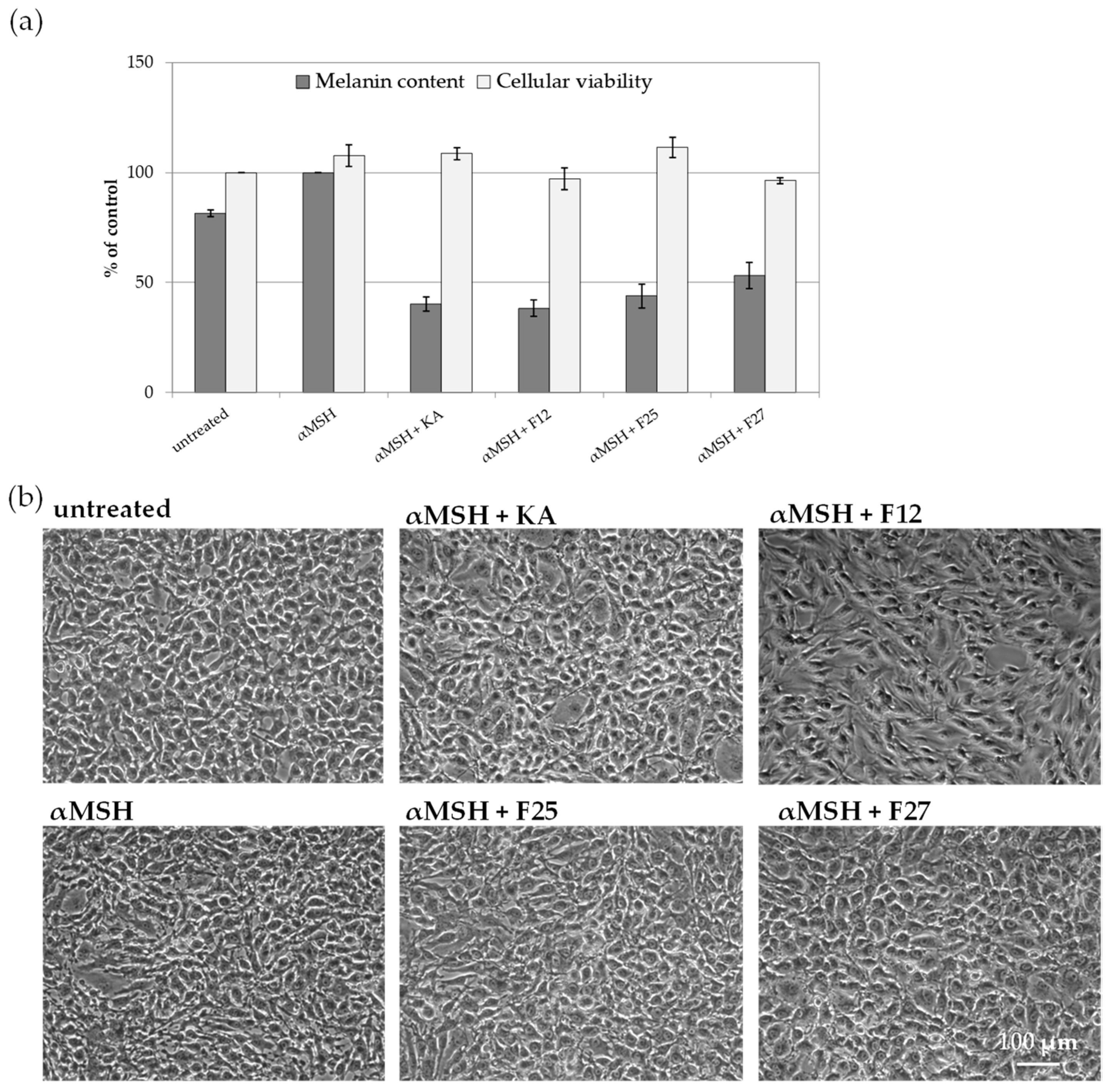
2.3.2. The Influence of A. biebersteinii Fractions of Melanin Content and Viability of B16F10 Cells
2.3.3. HPLC-ESI-Q-TOF-MS/MS—Based Identification of the Antityrosinase Constituents of the Fractions
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. The Optimization of Extraction
3.3.1. Accelerated Solvent Extraction (ASE)
3.3.2. Maceration
3.3.3. Ultrasonic Extraction
3.3.4. Shaking Extraction
3.4. HPLC-ESI-Q-TOF-MS/MS Analysis of the Extracts
3.5. Fractionation of the Extract
3.6. DPPH Scavenging Assay
3.7. Mushroom Tyrosinase Inhibitory Assay
3.8. Murine Tyrosinase Inhibitory Assay
3.9. Melanin Release Assay in B16F10
3.9.1. Melanin Release from B16F10
3.9.2. Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Rhein, L.D.; Fluhr, J.W. Skin aging. Current therapeutic strategies. MedPharmPolska 2013, 328–330. (In Polish) [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Uyama, H. Tyrosinase inhibitors from natural and synthetic sources: Structure, inhibition mechanism and perspective for the future. Cell. Mol. Life Sci. 2005, 62, 1707–1723. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, M.M.; Sun, Y.; Wang, L.F.; Wang, H.; Zhang, Y.J.; Li, H.Y.; Zhuang, P.W.; Yang, Z. Synergistic Promotion on Tyrosinase Inhibition by Antioxidants. Molecules 2018, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Hormozi, A.; Baharvand, P. Achillea biebersteinni Afan may inhibit scar formation: In vitro study. Mol. Genet Genomic Med. 2019, 7, e640. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Koca, U.; Pesin, I.; Yilmazer, D. Evaluation of the Wound Healing Potential of Achillea biebersteinii Afan. (Asteraceae) by In vivo Excision and Incision Models. Evid. Based Complement Alternat. Med. 2011, 2011, 474026. [Google Scholar] [CrossRef] [PubMed]
- Sökmen, A.; Sökmen, M.; Daferera, D.; Polissiou, M.; Candan, F.; Ünlü, M.; Akpulat, H.A. The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan.(Asteraceae). Phytother. Res. 2004, 18, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Jaffal, S.M.; Abbas, M.A. Antinociceptive action of Achillea biebersteinii methanolic flower extract is mediated by interaction with cholinergic receptor in mouse pain models. Inflammopharmacology 2018, 27, 961–968. [Google Scholar] [CrossRef]
- Zengin, G.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Guler, G.O.; Mahomoodally, M.F.; Glamočlija, J.; Ćirić, A.; Soković, M. Shedding the light on biological and chemical fingerprints of three Achillea species (A. biebersteinii, A. millefolium and A. teretifolia). Food Funct. 2017, 22, 1152–1165. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. Hydroglycolic Extracts–Bioactive Ingredients for Cosmetic Use. Molecules 2020, 25, 3368. [Google Scholar] [CrossRef] [PubMed]
- Şabanoğlu, S.; Gökbulut, A.; Altun, M.L. Characterization of phenolic compounds, total phenolic content and antioxidant activity of three Achillea species. J. Res. Pharm. 2019, 23, 567–576. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products; United State Code Title 21, Chapter IX, Subchapter VI; Federal Food, Drug, and Cosmetic Act: Silver Spring, MD, USA.
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahram, A.; Hassan-Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef]
- Yoshimori, A.; Oyama, T.; Takahashi, S.; Abe, H.; Kamiya, T.; Abe, T.; Tanuma, S. Structure-activity relationships of the thujaplicins for inhibition of human tyrosinase. Bioorg. Med. Chem. 2014, 22, 6193–6200. [Google Scholar] [CrossRef]
- Varasteh-Kojourian, M.; Abrishamchi, P.; Matin, M.M.; Asili, J.; Ejtehadi, H.; Khosravitabar, F. Antioxidant, cytotoxic and DNA protective properties of Achillea eriophora DC. And Achillea biebersteinii Afan. Extracts: A comparative study. Avicenna J. Phytomed. 2017, 7, 157–168. [Google Scholar]
- Reyes-Carmona, J.; Yousef, G.G.; Martinez-Peniche, R.A.; Lila, M.A. Antioxidant capacity of fruit extracts of blackberry (Rubus sp.) produced in different climatic regions. J. Food Sci. 2005, 70, 497–503. [Google Scholar] [CrossRef]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Geros, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef]
- Kukuła-Koch, W.; Koch, W.; Angelis, A.; Halabalaki, M.; Aligiannis, N. Application of pH-zone refining hydrostatic countercurrent chromatography (hCCC) for the recovery of antioxidant phenolic and the isolation of alkaloids from Siberian barberry herb. Food Chem. 2016, 203, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef]
- Ylmaz, M.A.; Ertas, A.; Yener, I.; Akdeniz, M.; Cakir, O.; Altun, M.; Demirtas, I.; Boga, M.; Temel, H. A comprehensive LC-MS/MS method validation for the quantitative investigation of 37 fingerprint phytochemicals in Achillea species: A detailed examination of A.coarctata and A.monocephala. J. Pharm. Biomed. Anal. 2018, 154, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Bulut, G.; Mollica, A.; Haznedaroglu, M.Z.; Dogan, A.; Aktumsek, A. Bioactivities of Achillea phrygia and Bupleurum croceum based on the composition of phenolic compounds: In vitro and in silico approaches. Food Chem. Toxicol. 2017, 107, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Sim, M.O.; Woo, K.W.; Jeong, D.; Jung, H.K.; An, B.; Cho, H.W. Antioxidant and anti-melanogenic activities of compound isolated from the aerial parts of Achillea alpina L. Chem. & Biodivers. 2019. [Google Scholar]
- Agar, O.T.; Dikmen, M.; Ozturk, N.l.; Yilmaz, M.A.; Temel, H.; Turkmenoglu, F.P. Comparative Studies on Phenolic Composition, Antioxidant, Wound Healing and Cytotoxic Activities of Selected Achillea, L. Species Growing in Turkey. Molecules 2015, 20, 17976–18000. [Google Scholar] [CrossRef]
- Venditti, A.; Guarcini, L.; Bianco, A.; Rosselli, S.; Bruno, M.; Senatore, F. Phytochemical analysis of Achillea ligustica All. From Lipari Island (Aeolian Islands). Nat. Prod. Res. 2015, 30, 912–919. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M.; Miroliaei, M. Variation in Polyphenolic Profiles, Antioxidant and Antimicrobial Activity of Different Achillea Species as Natural Sources of Antiglycative Compounds. Chem. & Biodivers. 2018, 15, e1800075. [Google Scholar]
- Saeidnia, S.; Gohari, A.; Mokhber-Dezfuli, N.; Kiuchi, F. A review on phytochemistry and medicinal properties of the genus Achillea. DARU 2011, 19, 173–186. [Google Scholar]
- Dorjsembe, B.; Lee, H.J.; Kim, M.; Dulamjav, B.; Jigjid, T.; Nho, C.W. Achillea asiatica extract and its active compounds induce cutaneous wound healing. J. Ethnopharmacol. 2017, 206, 306–314. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef]
- An, S.M.; Jae-Sook, K.; Boo, Y.C. p-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother. Res. 2010, 24, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of melanogenesis by aloesin: A competitive inhibitor of tyrosinase. Pigment Cell Res. 2002, 15, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Kazuya, I.; Kishimoto, N.; Kakino, Y.; Mochida, K.; Fujita, T. In Vitro Antioxidative Effects and Tyrosinase Inhibitory Activities of Seven Hydroxycinnamoyl Derivatives in Green Coffee Beans. J. Agric. Food Chem. 2004, 52, 4893–4898. [Google Scholar]
- Ha, J.H.; Park, S.N. Mechanism underlying inhibitory effect of six dicaffeoylquinic acid isomers on melanogenesis and the computational molecular modeling studies. Bioorganic & Med. Chem. 2018, 26, 4201–4208. [Google Scholar]
- Maruyama, H.; Kawakami, F.; Lwin, T.T.; Imai, M.; Shamsa, F. Biochemical Characterization of Ferulic Acid and Caffeic Acid Which Effectively Inhibit Melanin Synthesis via Different Mechanisms in B16 Melanoma Cells. Biol. Pharm. Bull. 2018, 41, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Long, X.; Liu, Z.; Shao, H.; Liu, L. Analysis of phenolic acids of Jerusalem artichoke (Helianthus tuberosus L.) responding to salt-stress by liquid chromatography/tandem mass spectrometry. Sci. World J. 2014, 2014, 568043. [Google Scholar] [CrossRef]
- Kim, P.S.; Shin, J.H.; Jo, D.S.; Shin, D.W.; Choi, D.H.; Kim, W.J.; Park, K.; Kim, J.K.; Joo, C.G.; Lee, J.S.; et al. Anti-melanogenic activity of schaftoside in Rhizoma Arisaematis by increasing autophagy in B16F1 cells. Biochem. Biophys. Res. Commun. 2018, 503, 309–315. [Google Scholar] [CrossRef]
- Matejic, J.S.; Dzamic, A.M.; Mihajilov-Krstev, T.; Randelovic, V.N.; Krivosej, Z.D.; Marin, P.D. Total phenolic content, flavonoid concentration, antioxidant and antimicrobial activity of methanol extracts from three Seseli, L. Taxa. Cent. Eur. J. Biol. 2012, 7, 1116–1122. [Google Scholar] [CrossRef]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of tyrosinase activity and melanin pigmentation by 2-hydroxytyrosol. Acta. Pharm. Sin. B 2014, 4, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]

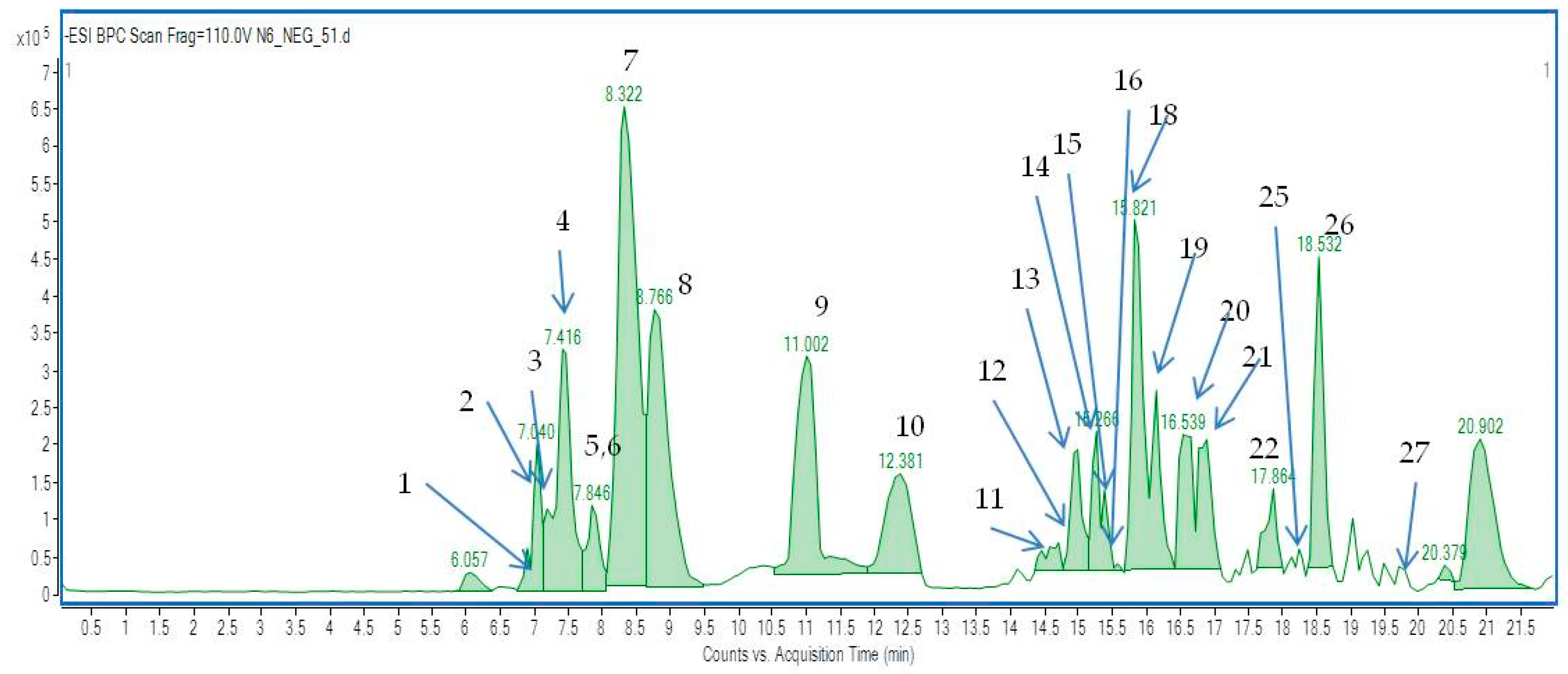



| No. | Ionization Mode | Rt [min] | Molecular Formula | m/z Experimental | m/z Calculated | Delta [ppm] | DBE | Tentative Identification | MS/MS Fragments | Ref. ** | Fraction No. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | [M − H]− | 6.9 | C5H8O4 | 131.035 | 131.0350 | −0.13 | 2 | Glutaric acid | 88,113 | 12 | |
| 2 | [M − H]− | 7.0 | C15H18O3 | 245.0465 | 245.1183 | 1.29 | 7 | Achillin | 211,179,171,101 | [28] | |
| 3 | [M − H]− | 7.1 | C6H12O7 | 195.051 | 195.0510 | 0.13 | 1 | Gluconic acid | 59,71,129 | 12 | |
| 4 | [M − H]− | 7.5 | C7H12O6 | 191.0561 | 191.0561 | 0.006 | 2 | Quinic acid | 85,173,111 | [26,29] | |
| 5 | [M − H]− | 7.8 | C9H8O4 | 179.0343 | 179.0350 | 3.79 | 6 | Caffeic acid * | 136 | comparison with a standard, [13,27,30,31] | |
| 6 | [M − H]− | 8.0 | C16H18O8 | 337.0923 | 337.0929 | 10.33 | 8 | Coumaroyl-quinic acid Isomers | [14] | ||
| 7 | [M − H]− | 8.3 | C4H5O5 | 133.0138 | 133.0142 | 3.33 | 2 | Malic acid | 115, 89, 71 | ||
| 8 | [M − H]− | 8.7 | C6H8O7 | 191.0193 | 191.0197 | 2.22 | Isocitric acid | 173, 129,111,87 | |||
| 9 | [M − H]− | 11.0 | C6H8O7 | 191.0190 | 191.0197 | 3.78 | Citric acid | 129, 111, 87 | |||
| 10 | [M − H]− | 12.4 | C4H6O4 | 117.0193 | 117.0195 | −1.42 | 2 | Succinic acid | 73,99,67 | 12 | |
| 11 | [M − H]− | 14.5 | C7H10O5 | 173.0455 | 173.0451 | 2.57 | 3 | Shikimic acid | 85,111,129 | 12 | |
| 12 | [M − H]− | 14.6 | C16H18O10 | 369.0819 | 369.0827 | 2.22 | 8 | Fraxetin-8-O-glucoside | 173,304,129,111 | 25,27 | |
| 13 | [M − H]− | 15.0 | C13H16O9 | 315.0704 | 315.0722 | 5.55 | 6 | Protocatechuic acid glucoside | 153,161,109 | 12 | |
| 14 | [M − H]− | 15.3 | C16H18O9 | 353.0867 | 353.0878 | 0.58 | 8 | 3-Caffeoylquinic acid * | 191,173 | comparison with a standard [13,14,31] | 12 |
| 15 | [M − H]− | 15.2 | C15H18O9 | 341.0886 | 341.0878 | −2.32 | 7 | Caffeoylglucoside (Isomer I) | 251, 203, 179, 161 | [32] | 27 |
| 16 | [M − H]− | 15.7 | C15H18O9 | 341.0899 | 341.0878 | −6.12 | 7 | Caffeoylglucoside (Isomer II) | 281, 251, 179, 161 | [32] | 27 |
| 17 | [M − H]− | 15.8 | C26H28O14 | 563.1401 | 563.1406 | 0.94 | 13 | Schaftoside or isoschaftoside | 353,191 | [28,33] | 25,27 |
| 18 | [M − H]− | 15.85 | C16H18O9 | 353.0864 | 353.0878 | 0.02 | 8 | 5-Caffeoylquinic acid * | 191,173 | comparison with a standard [13,14,31] | 12 |
| 19 | [M − H]− | 16.2 | C16H18O9 | 353.0868 | 353.0878 | 2.84 | 8 | 4-Caffeoylquinic acid * | 191,173 | comparison with a standard [13,14,31] | 12 |
| 20 | [M − H]− | 16.6 | C21H20O12 | 477.0674 | 477.0675 | 0.13 | 13 | Quercetin-O- glucopyranose | 301,255,178,151 | 25 | |
| 21 | [M − H]− | 16.9 | C25H24O12 | 515.1178 | 515.1195 | 5.23 | 14 | 1,3-Dicaffeoylquinic acid (cynarin) * | 353 | comparison with a standard [13,14,31] | 25,27 |
| 22 | [M − H]− | 17.9 | C17H26O4 | 293.1768 | 293.1758 | −3.29 | 5 | Gmelinin B | 111,193,163 | 25,27 | |
| 23 | [M − H]− | 18.1 | C15H10O6 | 285.0398 | 285.0405 | 2.31 | 11 | Kaempferol | 113,137 | [14,26,27] | |
| 24 | [M − H]− | 18.2 | C14H6O8 | 300.9959 | 300.999 | 10.23 | 12 | Ellagic acid | 129, 179, 151, | ||
| 25 | [M − H]− | 18.3 | C10H10O4 | 193.0536 | 193.0506 | −15.29 | 6 | Ferulic acid | 161, 134 | comparison with a standard [26,27,31] | 25,27 |
| 26 | [M − H]− | 18.5 | C17H14O8 | 345.0609 | 345.0616 | 4.6 | 11 | Axillarin | 315,287,129,81 | [28] | |
| 27 | [M − H]− | 19.7 | C18H16O8 | 359.0772 | 359.0772 | 0.67 | 11 | Jaceidin | 301,258,286,344, 329 | [28] | |
| 28 | [M + H]+ | 15.8 | C21H20O10 | 433.2009 | 433.1129 | 2.58 | 12 | Isovitexin | 428,367 | [28] | |
| 29 | [M + H]+ | 17.9 | C15H10O5 | 271.0601 | 271.0601 | 0 | 11 | Apigenin | [27,29,30,31] |
| Code | Extraction Method | Extrahent | Temperature [ °C] | Time [min] |
|---|---|---|---|---|
| E1 | ASE | 75% EtOH | 60 | 5 |
| E2 | ASE | 75% EtOH | 80 | 5 |
| E3 | ASE | 75% EtOH | 100 | 5 |
| E4 | ASE | 75% EtOH | 120 | 5 |
| E5 | ASE | 75% EtOH | 140 | 5 |
| E6 | ASE | 75% EtOH | 160 | 5 |
| E7 | ASE | 75% EtOH | 180 | 5 |
| E8 | ASE | 75% EtOH | 80 | 2 |
| E9 | ASE | 75% EtOH | 80 | 7 |
| E10 | ASE | 75% EtOH | 80 | 10 |
| U1 | UAE | 75% EtOH | 40 | 5 |
| U2 | UAE | 75% EtOH | 40 | 10 |
| U3 | UAE | 75% EtOH | 40 | 20 |
| U4 | UAE | 75% EtOH | 40 | 30 |
| U5 | UAE | 75% EtOH | 40 | 40 |
| U6 | UAE | 75% EtOH | 60 | 10 |
| U7 | UAE | 75% EtOH | 20 | 10 |
| W1 | SE | 75% EtOH | RT | 5 |
| W2 | SE | 75% EtOH | RT | 10 |
| W3 | SE | 75% EtOH | RT | 15 |
| W4 | SE | 75% EtOH | RT | 20 |
| W5 | SE | 75% EtOH | RT | 25 |
| W6 | SE | 75% EtOH | RT | 30 |
| M1 | MACERATION | 75% EtOH | RT | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea biebersteinii Afan. Extract. Molecules 2021, 26, 964. https://doi.org/10.3390/molecules26040964
Strzępek-Gomółka M, Gaweł-Bęben K, Angelis A, Antosiewicz B, Sakipova Z, Kozhanova K, Głowniak K, Kukula-Koch W. Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea biebersteinii Afan. Extract. Molecules. 2021; 26(4):964. https://doi.org/10.3390/molecules26040964
Chicago/Turabian StyleStrzępek-Gomółka, Marcelina, Katarzyna Gaweł-Bęben, Apostolis Angelis, Beata Antosiewicz, Zuriyadda Sakipova, Kaldanay Kozhanova, Kazimierz Głowniak, and Wirginia Kukula-Koch. 2021. "Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea biebersteinii Afan. Extract" Molecules 26, no. 4: 964. https://doi.org/10.3390/molecules26040964
APA StyleStrzępek-Gomółka, M., Gaweł-Bęben, K., Angelis, A., Antosiewicz, B., Sakipova, Z., Kozhanova, K., Głowniak, K., & Kukula-Koch, W. (2021). Identification of Mushroom and Murine Tyrosinase Inhibitors from Achillea biebersteinii Afan. Extract. Molecules, 26(4), 964. https://doi.org/10.3390/molecules26040964








