Density Functional Theory Study of Optical and Electronic Properties of (TiO2)n=5,8,68 Clusters for Application in Solar Cells
Abstract
:1. Introduction
2. Results and Discussion
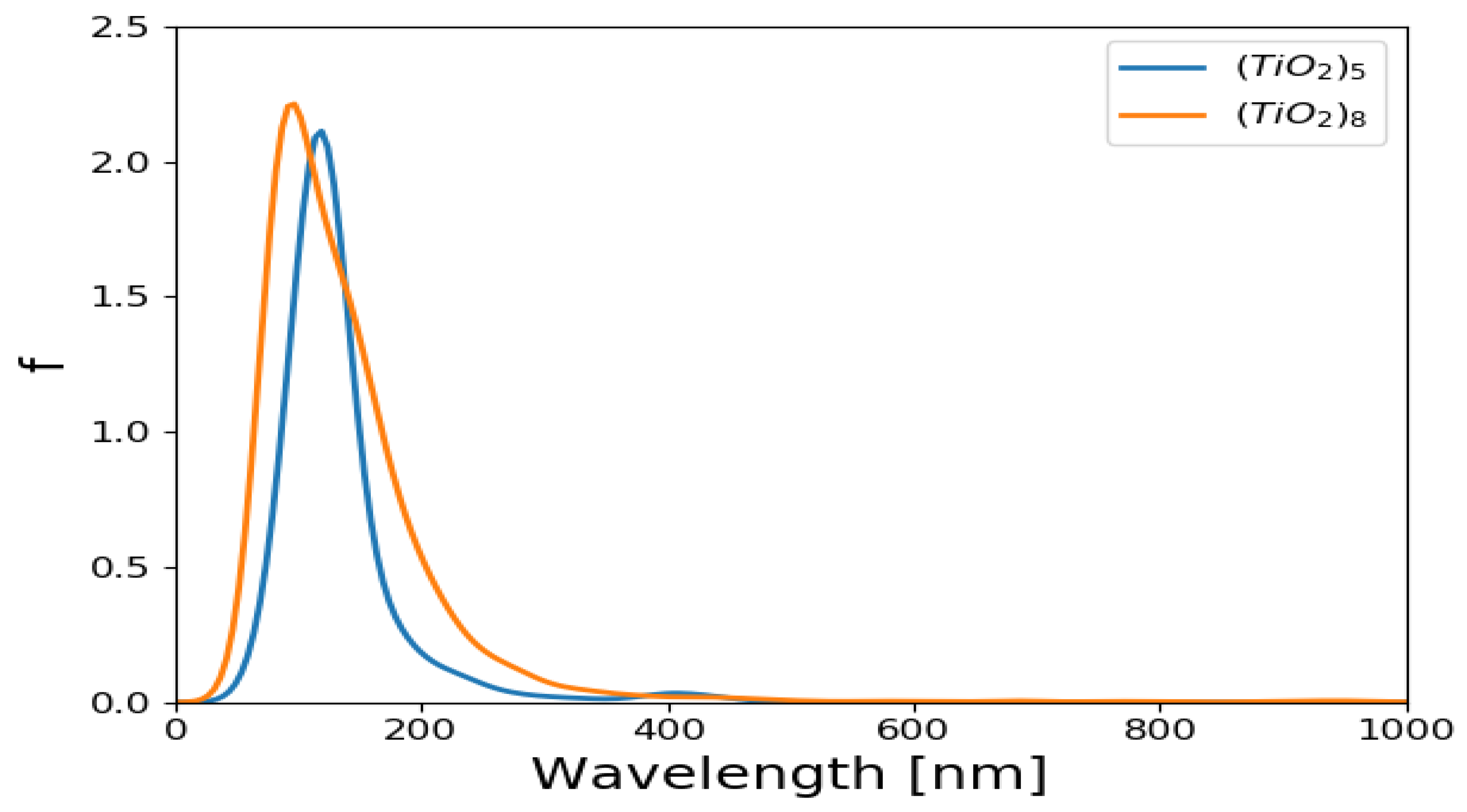
2.1. Optical Properties of (TiO2)5 and (TiO2)8 Brookite Clusters
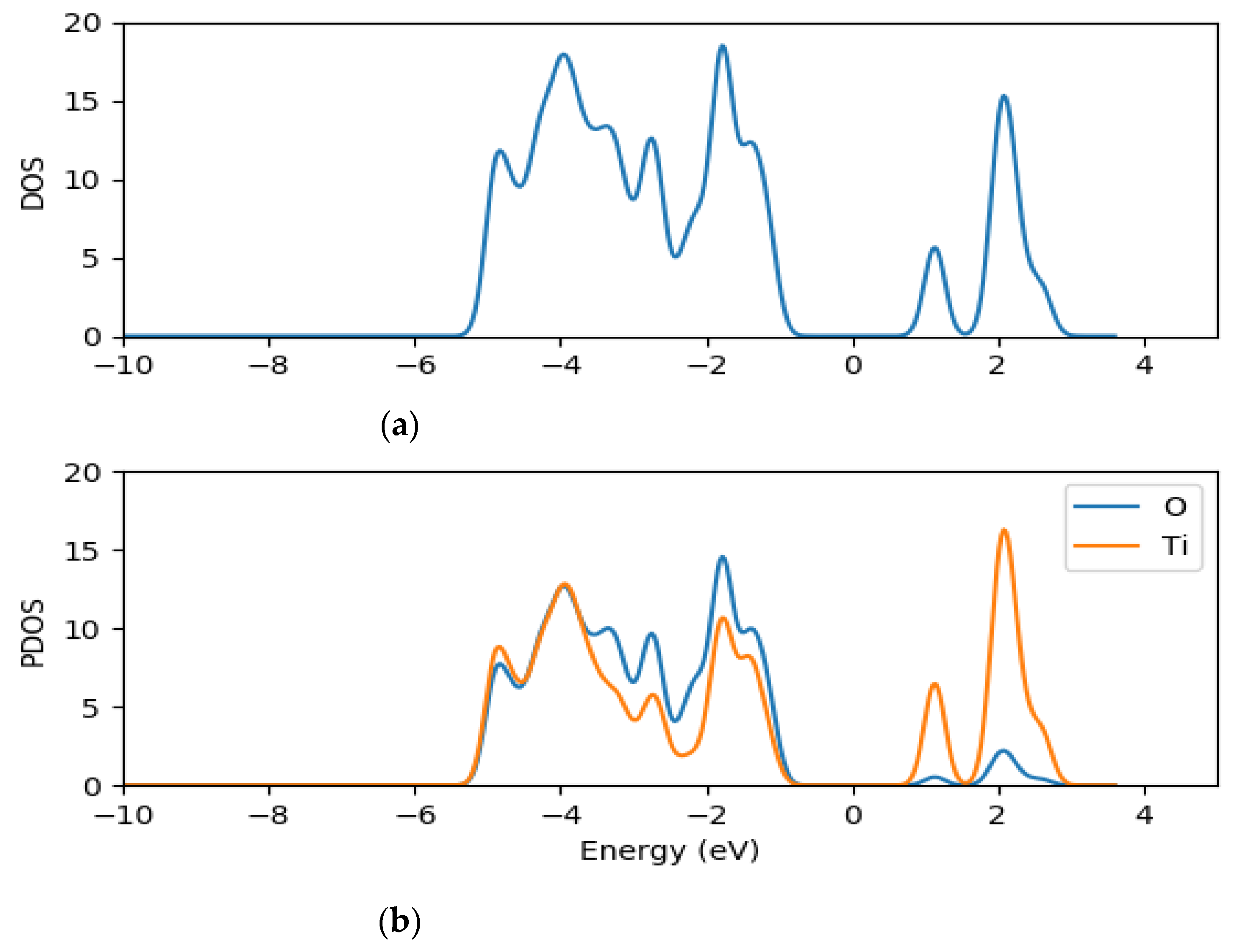
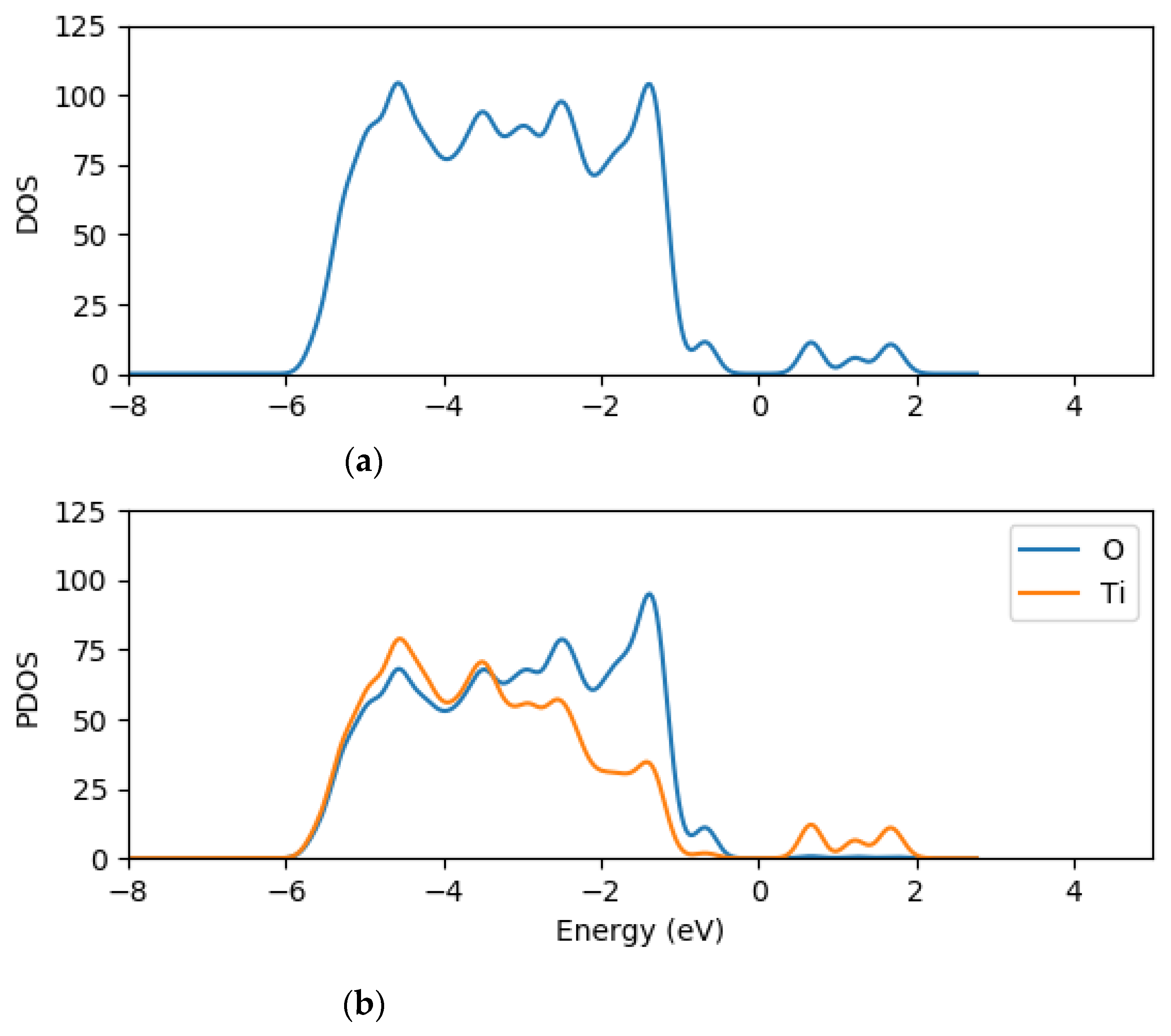
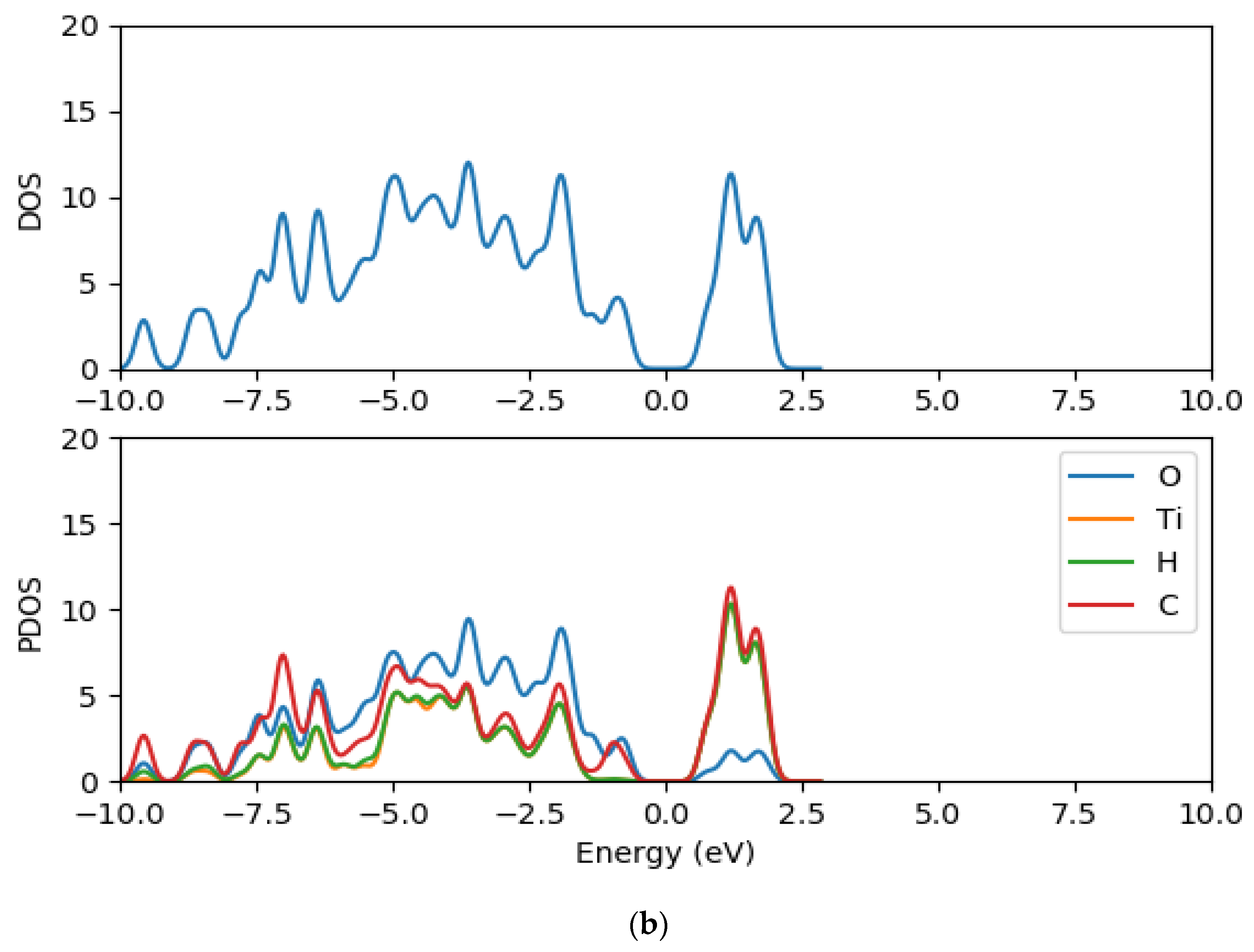
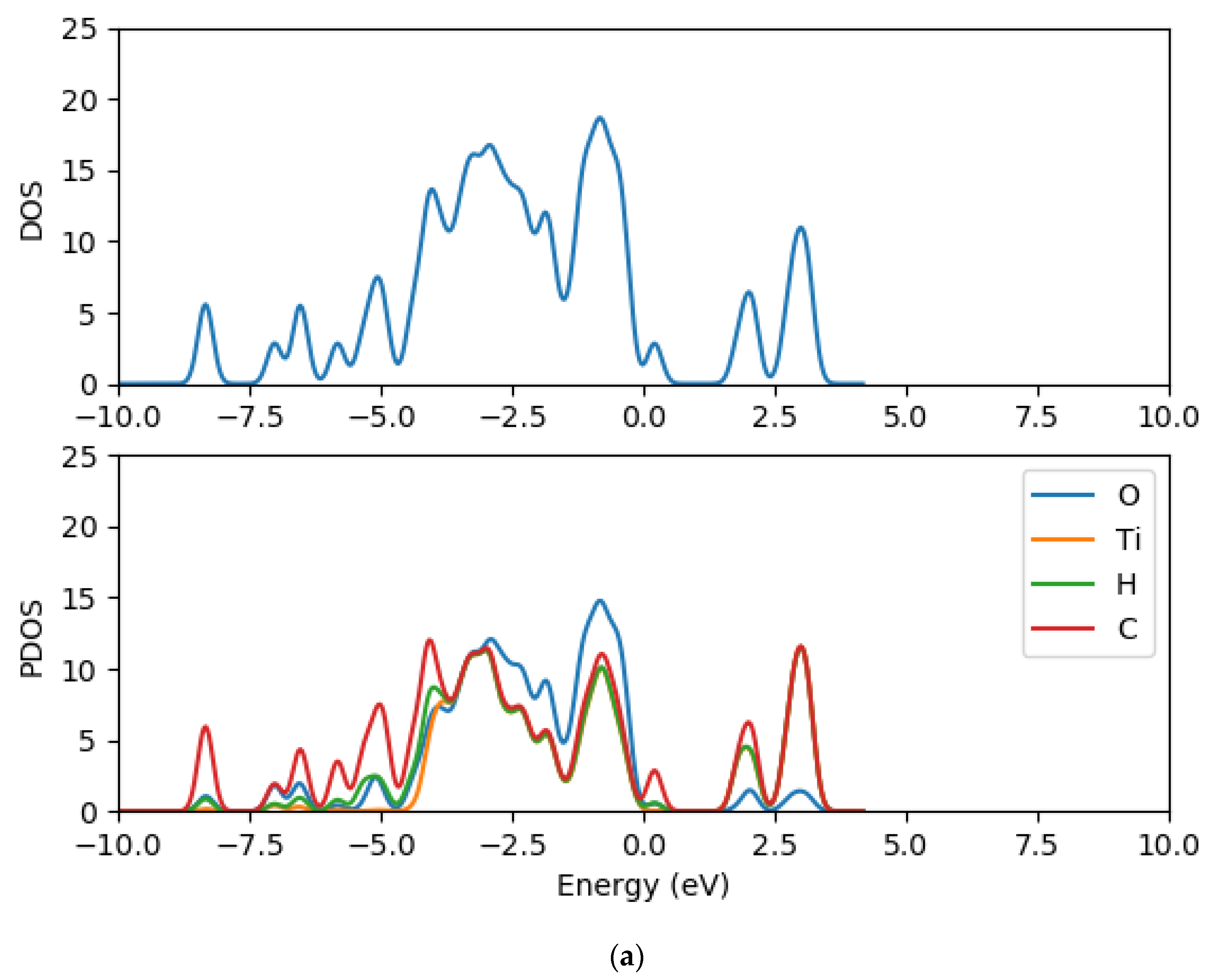
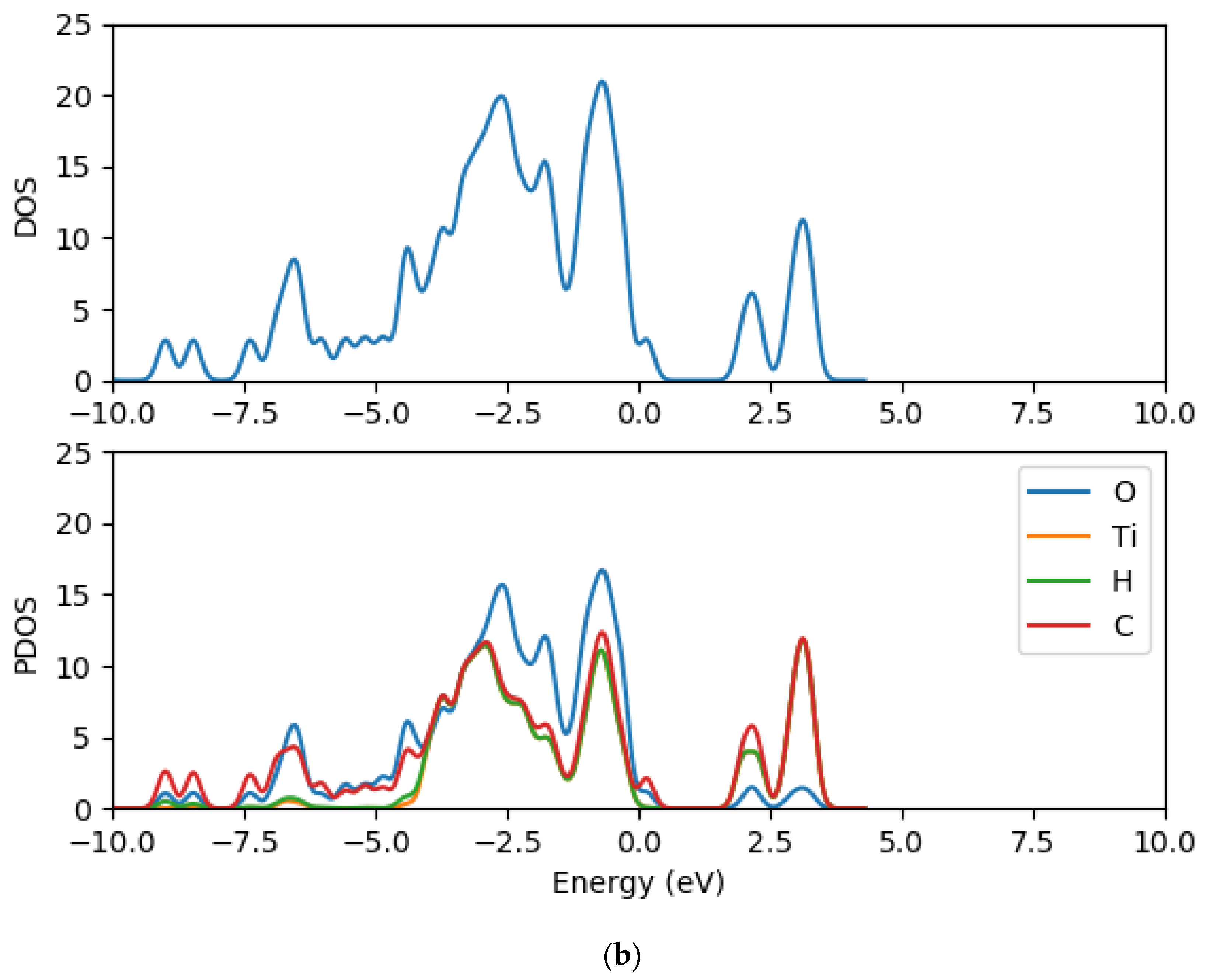
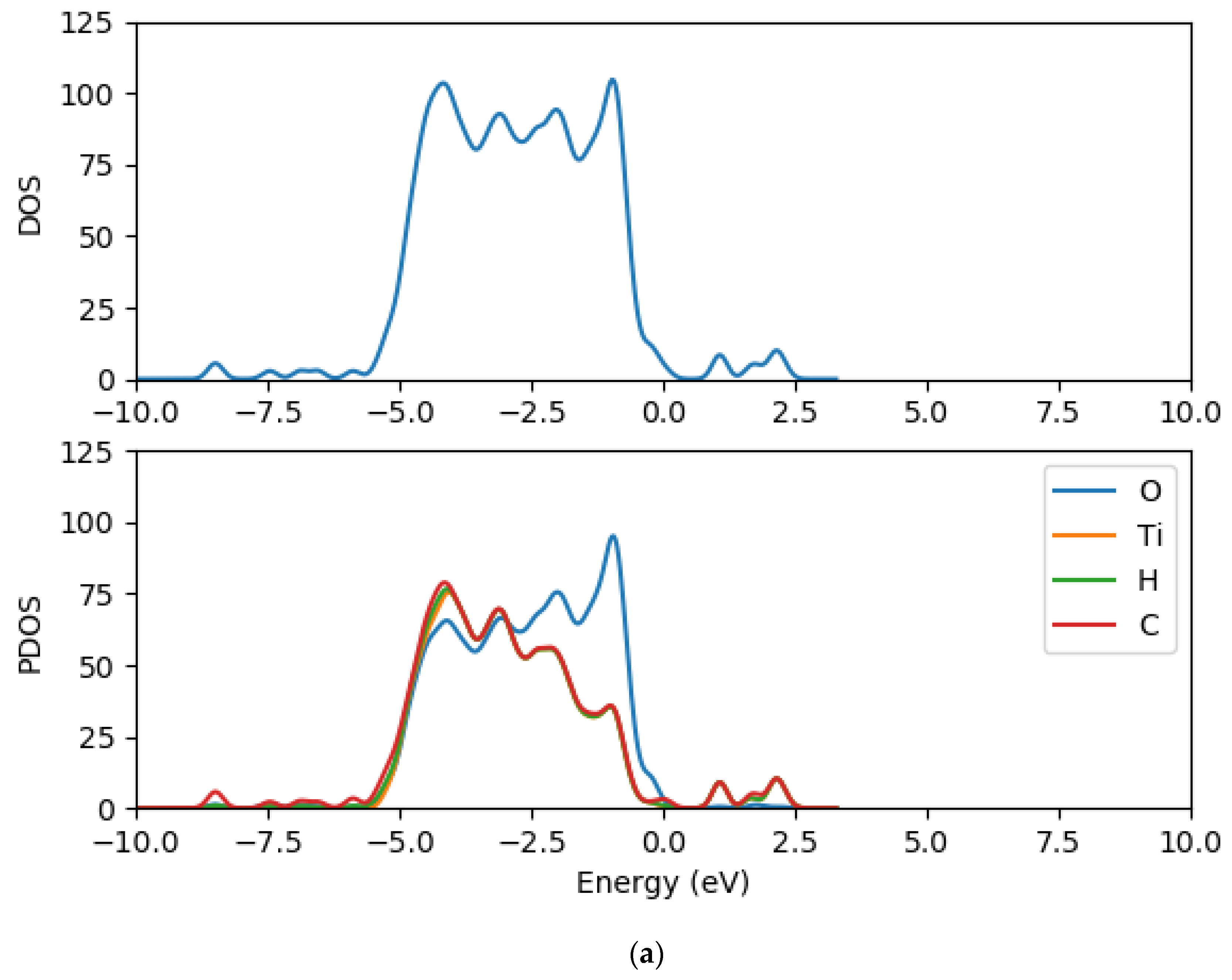
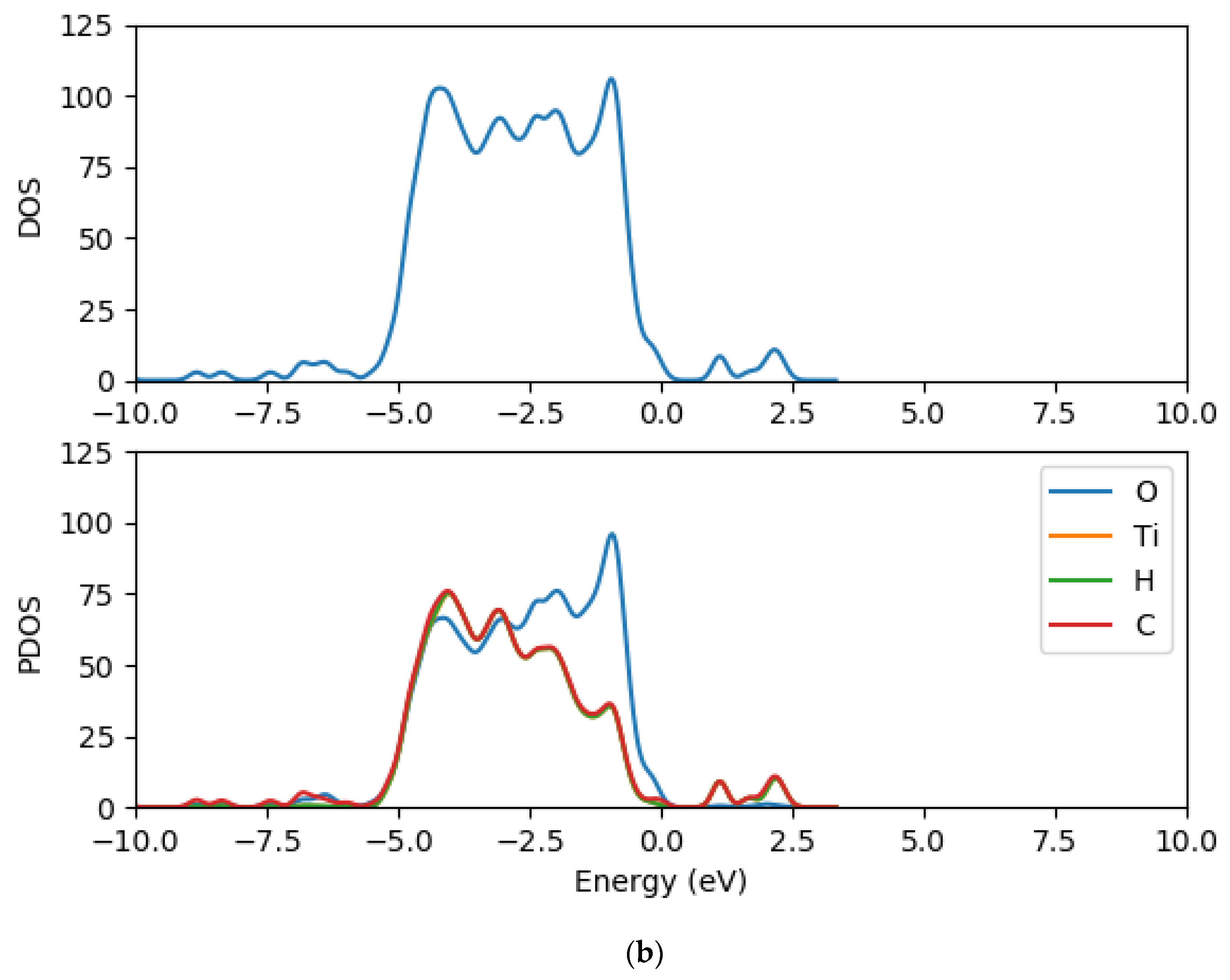
2.2. Electronic Properties of (TiO2)n (n = 5, 8, 68) Brookite Clusters
2.3. Structural Models
2.4. Adsorption of Croconate Dyes on (TiO2)5, (TiO2)8 and (TiO2)68 Clusters
2.5. Adsorption Energy of Croconate Dyes Adsorbed on (TiO2)n n = 5, 8, 68 Brookite Complex
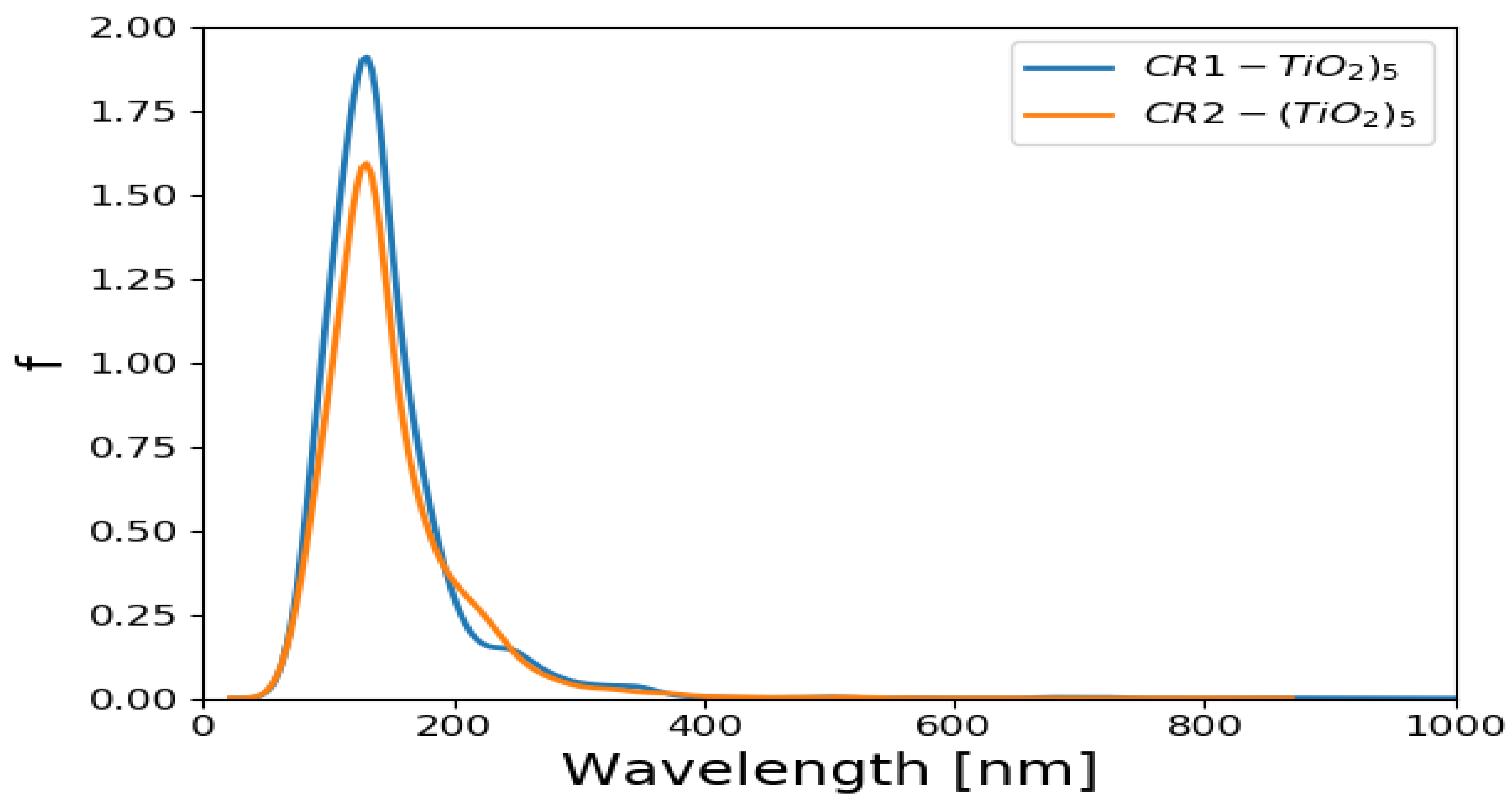
2.6. Absorption Spectrum of CR1 and CR2 Dyes Adsorbed on (TiO2)n n = 5, 8 Brookite Clusters
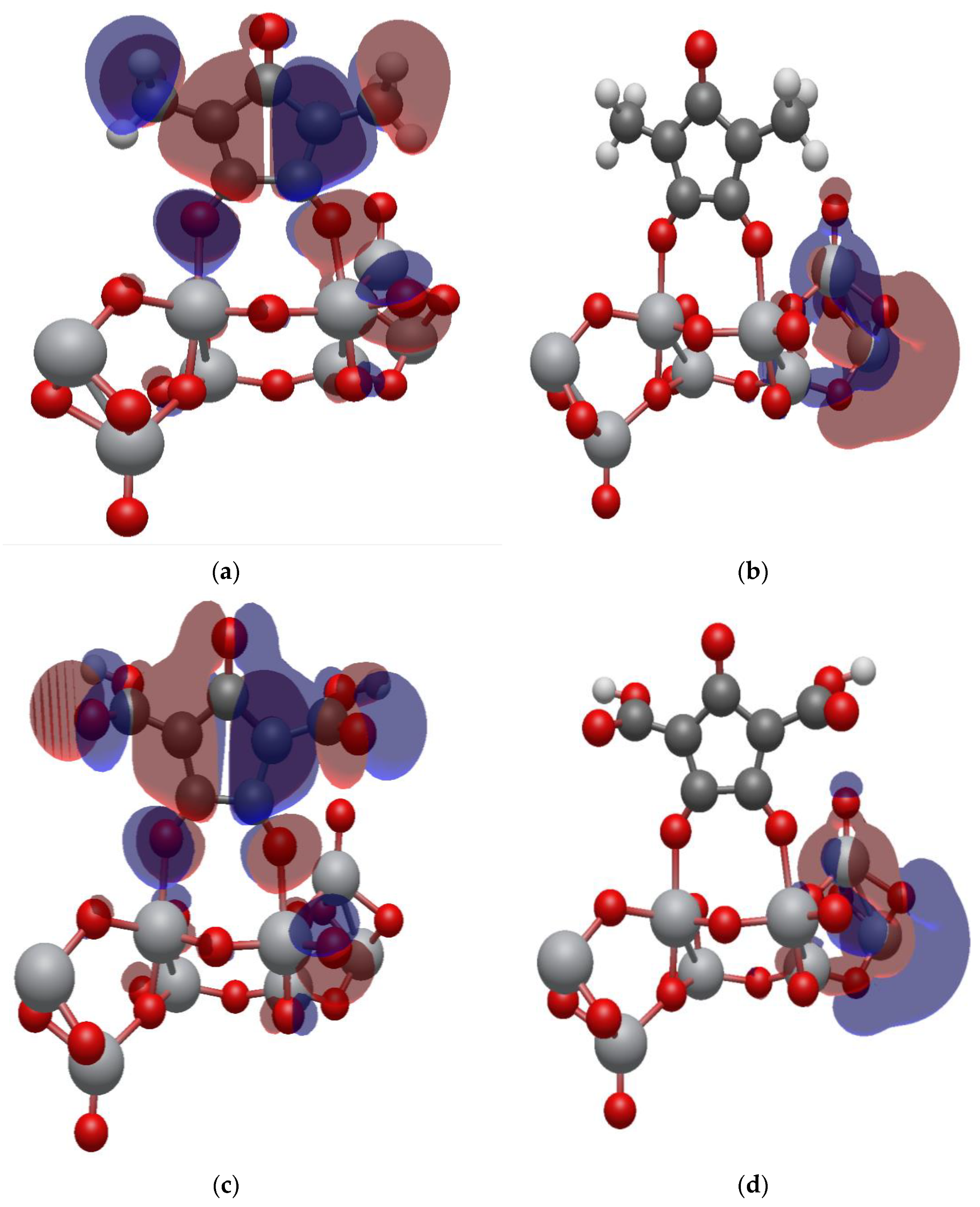
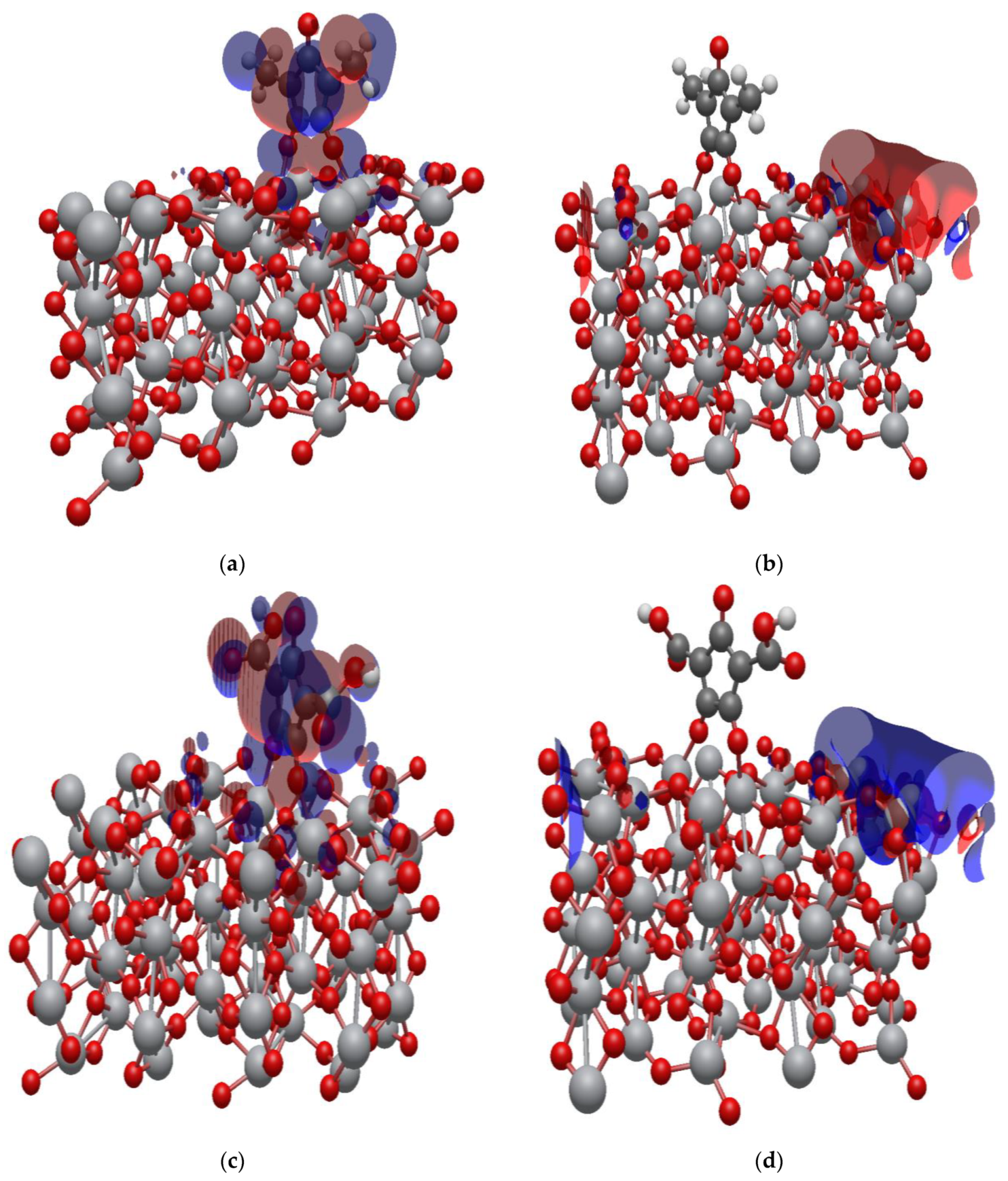
2.7. Isodensity Surfaces of the Croconate Dyes Adsorbed on (TiO2)n, n = 5, 8, 68 Brookite Clusters
2.8. Electronic Properties of CR1 and CR2 dye Molecules Adsorbed on TiO2 Clusters
3. Materials and Methods
Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatib, H. IEA World Energy Outlook 2011—A comment. Energy Policy 2012, 48, 737–743. [Google Scholar] [CrossRef]
- Fotis, P.; Karkalakos, S.; Asteriou, D. The relationship between energy demand and real GDP growth rate: The role of price asymmetries and spatial externalities within 34 countries across the globe. Energy Econ. 2017, 66, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Filippini, M.; Hunt, L.C. US residential energy demand and energy efficiency: A stochastic demand frontier approach. Energy Econ. 2012, 34, 1484–1491. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Zhang, F.; Meng, S. Dye sensitized solar cells principles and new design. In Solar Cells–Dye Sensitized Devices; Kosyachenko, L.A., Ed.; Intechopen: Rijeka, Croatia, 2011; pp. 132–148. [Google Scholar]
- Goodland, R.; Daly, H.E.; El Serafy, S.; Droste, B.V. (Eds.) Environmentally Sustainable Economic Development: Building on Brundtland; UNESCO: Paris, France, 1991. [Google Scholar]
- Anderson, D.; Winne, S. Energy system change and external effects in climate change mitigation. Environ. Dev. Econ. 2007, 12, 359–378. [Google Scholar] [CrossRef]
- Cariou, R.; Benick, J.; Feldmann, F.; Höhn, O.; Hauser, H.; Beutel, P.; Razek, N.; Wimplinger, M.; Bläsi, B.; Lackner, D.; et al. III–V-on-silicon solar cells reaching 33% photoconversion efficiency in two-terminal configuration. Nat. Energy 2018, 3, 326–333. [Google Scholar] [CrossRef]
- Schmidt, J.; Peibst, R.; Brendel, R. Surface passivation of crystalline silicon solar cells: Present and future. Sol. Energy Mater. Sol. Cells 2018, 187, 39–54. [Google Scholar] [CrossRef]
- Ingenito, A.; Nogay, G.; Jeangros, Q.; Rucavado, E.; Allebé, C.; Eswara, S.; Valle, N.; Wirtz, T.; Horzel, J.; Koida, T. A passivating contact for silicon solar cells formed during a single firing thermal annealing. Nat. Energy 2018, 3, 800–808. [Google Scholar] [CrossRef]
- Farooq, W.; Alshahrani, T.; Kazmi, S.A.A.; Iqbal, J.; Khan, H.A.; Khan, M.; Raja, A.A.; Rehman, A. Materials optimization for thin-film copper indium gallium selenide (CIGS) solar cell based on distributed braggs reflector. Optik 2020, 165987, in press. [Google Scholar] [CrossRef]
- Jiang, J.; Giridharagopal, R.; Jedlicka, E.; Sun, K.; Yu, S.; Wu, S.; Gong, Y.; Yan, W.; Ginger, D.S.; Green, M.A.; et al. Highly efficient copper-rich chalcopyrite solar cells from DMF molecular solution. Nano Energy 2020, 69, 104438. [Google Scholar] [CrossRef]
- Ramanujam, J.; Bishop, D.M.; Todorov, T.K.; Gunawan, O.; Rath, J.; Nekovei, R.; Artegiani, E.; Romeo, A. Flexible CIGS, CdTe and a-Si:H based thin film solar cells: A review. Prog. Mater. Sci. 2020, 110, 100619. [Google Scholar] [CrossRef]
- Zhou, B.; Yin, X.; Zhang, J.; Zeng, G.; Li, B.; Zhang, J.; Feng, L. Numerical simulation of an innovative high efficiency solar cell with CdTe/Si composite absorption layer. Opt. Mater. 2020, 110, 110505. [Google Scholar] [CrossRef]
- Ahmmed, S.; Aktar, A.; Rahman, F.; Hossain, J.; Ismail, A.B.M. A numerical simulation of high efficiency CdS/CdTe based solar cell using NiO HTL and ZnO TCO. Optik 2020, 223, 165625. [Google Scholar] [CrossRef]
- Padhy, A.; Vishal, B.; Verma, P.; Dwivedi, G.; Behura, A. Fabrication of parabolic trough hybrid solar PV-T collector using a-Si thin film solar cells in Indian perspective. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Li, G.; Xuan, Q.; Lu, Y.; Pei, G.; Su, Y.; Ji, J. Numerical and lab experiment study of a novel concentrating PV with uniform flux distribution. Sol. Energy Mater. Sol. Cells 2018, 179, 1–9. [Google Scholar] [CrossRef]
- Li, G.; Xuan, Q.; Pei, G.; Su, Y.; Ji, J. Effect of non-uniform illumination and temperature distribution on concentrating solar cell—A review. Energy 2018, 144, 1119–1136. [Google Scholar] [CrossRef]
- Grätzel, M. Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2004, 164, 3–14. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Nozik, A.J.; Beard, M.C.; Luther, J.M.; Law, M.; Ellingson, R.J.; Johnson, J.C. Semiconductor quantum dots and quantum dot arrays and applications of multiple exciton generation to third-generation photovoltaic solar cells. Chem. Rev. 2010, 110, 6873–6890. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Garg, A.; Dixit, A. A review on quantum dot sensitized solar cells: Past, present and future towards carrier multiplication with a possibility for higher efficiency. Sol. Energy 2020, 203, 210–239. [Google Scholar] [CrossRef]
- Polman, A.; Knight, M.; Garnett, E.C.; Ehrler, B.; Sinke, W.C. Photovoltaic materials: Present efficiencies and future challenges. Sciemce 2016, 352, aad4424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Lu, S.; Li, S.; Wang, S.; Lin, X.; Zhang, J.; Kondrotas, R.; Li, K.; Chen, C.; Tang, J. Efficiency improvement of flexible Sb2Se3 solar cells with non-toxic buffer layer via interface engineering. Nano Energy 2020, 71, 104577. [Google Scholar] [CrossRef]
- Maurits, J. Reducing Polysilicon Materials Costs, 13th ed.; Photovoltaics Int.: California, USA, 2011; pp. 4–47. [Google Scholar]
- Stathatos, E. Dye sensitized solar cells as an alternative approach to the conventional photovoltaic technology based on silicon—Recent developments in the field and large scale applications. In Solar Cells–Dye Sensitized Devices; Kosyachenko, L.A., Ed.; Intechopen: Rijeka, Croatia, 2011; pp. 471–492. [Google Scholar]
- Gupta, S.M.; Tripathi, M. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical applications of TiO2 nanostructures: Recent advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.J.; Goslinski, T.; Sobotta, L. Titanium dioxide nanoparticles: Prospects and applications in medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.; Fan, M.; Cooper, A.T.; Yang, H. Photocatalytic applications of micro-and nano-TiO2 in environmental engineering. Crit. Rev. Environ. Sci. Tech. 2008, 38, 197–226. [Google Scholar] [CrossRef]
- Crisan, M.; Braileanu, A.; Răileanu, M.; Zaharescu, M.; Crişan, D.; Drăgan, N.; Anastasescu, M.; Ianculescu, A.; Nitoi, I.; Marinescu, V.E.; et al. Sol–gel S-doped TiO2 materials for environmental protection. J. Non Cryst. Solids 2008, 354, 705–711. [Google Scholar] [CrossRef]
- Garofalo, E.; Cecchini, L.; Bevione, M.; Chiolerio, A. Triboelectric characterization of colloidal TiO2 for energy harvesting applications. Nanomaterials 2020, 10, 1181. [Google Scholar] [CrossRef]
- El Haimeur, A.; Makha, M.; Bakkali, H.; González-Leal, J.M.; Blanco, E.; Dominguez, M.; Voitenko, Z.V. Enhanced performance of planar perovskite solar cells using dip-coated TiO2 as electron transporting layer. Sol. Energy 2020, 195, 475–482. [Google Scholar] [CrossRef]
- Choi, J.; Song, S.; Hörantner, M.T.; Snaith, H.J.; Choi, J. Well-defined nanostructured, single-crystalline TiO2 electron transport layer for efficient planar perovskite solar cells. ACS Nano 2016, 10, 6029–6036. [Google Scholar] [CrossRef]
- Valero, R.; Morales-García, Á.; Illas, F. Theoretical modeling of electronic excitations of gas-phase and solvated TiO2 nanoclusters and nanoparticles of interest in photocatalysis. J. Chem. Theor. Comput. 2018, 14, 4391–4404. [Google Scholar] [CrossRef]
- Mehmood, U.; Rahman, S.U.; Harrabi, K.; Hussein, I.A.; Reddy, B.V.S. Recent advances in dye sensitized solar cells. Adv. Mat. Sci. Eng. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Chen, C.; Zhu, K.; Fan, Z. TiO2 nanotubes infiltrated with nanoparticles for dye sensitized solar cells. Nanotechnology 2011, 22, 235402. [Google Scholar] [CrossRef] [PubMed]
- Maddah, H.A.; Berry, V.; Behura, S.K. Biomolecular photosensitizers for dye-sensitized solar cells: Recent developments and critical insights. Renew. Sustain. Energy Rev. 2020, 121, 109678. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, A.; Gracia, L.; Andrés, J. Density functional theory study of the brookite surfaces and phase transitions between natural titania polymorphs. J. Phys. Chem. B 2006, 110, 23417–23423. [Google Scholar] [CrossRef]
- Zheng, L.; Teng, F.; Ye, X.; Zheng, H.; Fang, X. Photo/electrochemical applications of metal sulfide/TiO2 heterostructures. Adv. Energy Mater. 2020, 10, 1902355. [Google Scholar] [CrossRef]
- Ünlü, B.; Özacar, M. Effect of Cu and Mn amounts doped to TiO2 on the performance of DSSCs. Sol. Energy 2020, 196, 448–456. [Google Scholar] [CrossRef]
- Bandaranayake, K.M.P.; Indika Senevirathna, M.K.; Prasad Weligamuwa, P.M.G.M.; Tennakone, K. Dye-sensitized solar cells made from nanocrystalline TiO2 films coated with outer layers of different oxide materials. Coord. Chem. Rev. 2004, 248, 1277–1281. [Google Scholar] [CrossRef]
- Mattsson, A.; Österlund, L. Adsorption and photoinduced decomposition of acetone and acetic acid on anatase, brookite, and rutile TiO2 nanoparticles. J. Phys. Chem. C 2010, 114, 14121–14132. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, B.; Mosaddeghi, H. Applications of titanium dioxdie nanocoating. In Proceedings of the Nano-Technology in Environments Conference, Isfahan University of Technology, Isfahan, Iran, 21 February 2007; pp. 1–6. [Google Scholar]
- Schaub, R.; Bin, L.; Jin, Z.; Kenneth, J.D.; Jinlong, Y.; Hrvoje, P. Oxygen-mediated diffusion of oxygen vacancies on the TiO2 (110) surface. Science 2003, 299, 377–379. [Google Scholar] [CrossRef]
- Onda, K.; Li, B.; Zhao, J.; Jordan, K.D.; Yang, J.; Petek, H. Wet electrons at the H2O/TiO2 (110) surface. Science 2005, 308, 1154–1158. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Shim, W.-G.; Jung, S.-H.; Yoo, S.-J.; Lee, J.-W. Analysis of adsorption properties of N719 dye molecules on nanoporous TiO2 surface for dye-sensitized solar cell. Appl. Surf. Sci. 2010, 256, 5428–5433. [Google Scholar] [CrossRef]
- Elegbeleye, I.F.; Maluta, N.E.; Maphanga, R.R. Density functional theory studies of ruthenium dye (N3) adsorbed on a TiO2 brookite cluster for application in dye sensitized solar cells. In Advances of Quantum Systems in Chemistry and Physics; Springer: Cham, Switzerland, 2020; pp. 143–155. [Google Scholar] [CrossRef]
- Oprea, C.I.; Panait, P.; Cimpoesu, F.; Ferbinteanu, M.; Gîrţu, M.A. Density Functional Theory (DFT) study of coumarin-based dyes adsorbed on TiO2 nanoclusters—Applications to dye-sensitized solar cells. Materials 2013, 6, 2372–2392. [Google Scholar] [CrossRef] [Green Version]
- Anselmi, C.; Mosconi, E.; Pastore, M.; Ronca, E.; De Angelis, F. Adsorption of organic dyes on TiO2 surfaces in dye-sensitized solar cells: Interplay of theory and experiment. Phys. Chem. Chem. Phys. 2012, 14, 15963–15974. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Khan, S.U.-D.; Rana, U.A.; Tahir, M.H. Red shifting of absorption maxima of phenothiazine based dyes by incorporating electron-deficient thiadiazole derivatives as π-spacer. Arab. J. Chem. 2019, 12, 1447–1453. [Google Scholar] [CrossRef] [Green Version]
- Galappaththi, K.; Lim, A.; Ekanayake, P.; Petra, M.I. Cyanidin-based novel organic sensitizer for efficient dye-sensitized solar cells: DFT/TDDFT study. Int. J. Photoenergy 2017, 2017, 8564293. [Google Scholar] [CrossRef] [Green Version]
- Marcano, E. DFT study of anthocyanidin and anthocyanin pigments for dye sensitized solar cells: Electron injecting from the excited states and adsorption onto TiO2 (anatase) surface. Energy Harvest. Syst. 2018, 5, 29–38. [Google Scholar] [CrossRef]
- Mammino, L.; Ceresoli, D.; Maruani, J.; Brändas, E. Advances in Quantum Systems in Chemistry, Physics, and Biology; Springer: Cham, Switzeland, 2020. [Google Scholar]
- Rodriguez, M.M.; Peng, X.; Liu, L.; Li, Y.; Andino, J.M. Density functional theory and experimental study of CO2 interaction with brookite TiO2. J. Phys. Chem. C 2012, 116, 19755–19764. [Google Scholar] [CrossRef]
- De Lile, J.R.; Kang, S.G.; Son, Y.-A.; Lee, S.W. Investigating polaron formation in anatase and brookite TiO2 by density functional theory with hybrid-functional and DFT + U methods. ACS Omega 2019, 4, 8056–8064. [Google Scholar] [CrossRef] [Green Version]
- Puyad, A.L.; Kumar, C.R.; Bhanuprakash, K. Adsorption of croconate dyes on TiO2 anatase (101) surface: A periodic DFT study to understand the binding of diketo groups. J. Chem. Sci. 2012, 124, 301–310. [Google Scholar] [CrossRef]
- Chitumalla, R.K.; Manho, L.; Xingfa, G.; Joonkyung, J. Substituent effects on the croconate dyes in dye sensitized solar cell applications: A density functional theory study. J. Mol. Mod. 2015, 21, 297. [Google Scholar] [CrossRef]
- Elegbeleye, I.F.; Maluta, N.E.; Maphanga, R.R.; Yaya, A. Density functional theory study of promising polyene-diphenylaniline organic chromophores for dye-sensitized solar cell applications. Cogent Eng. 2018, 5, 1532778. [Google Scholar] [CrossRef]
- Park, J.; Viscardi, G.; Barolo, C.; Barbero, N. Near-infrared Sensitization in dye-sensitized Solar Cells. Chim. Int. J. Chem. 2013, 67, 129–135. [Google Scholar] [CrossRef]
- Walter, M.; Häkkinen, H.; Lehtovaara, L.; Puska, M.; Enkovaara, J.; Rostgaard, C.; Mortensen, J.J. Time-dependent density—Functional theory in the projector augmented-wave method. J. Chem. Phys. 2008, 128, 244101. [Google Scholar] [CrossRef] [Green Version]
- Enkovaara, J.; Rotsgaard, C.; Mortensen, J.J.; Chen, J.; Dułak, M.; Ferrighi, L.; Gavnholt, J.; Glinsvad, C.; Haikola, V.; Hansen, H.A. Electronic structure calculations with GPAW: A real-space implementation of the projector augmented-wave method. J. Phys. Cond. Matt. 2010, 22, 253202. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Holbig, E.; Steinle-Neumann, G. Structural stability of TiO2 at high pressure in density-functional theory based calculations. J. Phys. Cond. Matt. 2010, 22, 295501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Qian, L.; Fu, W.; Xi, J.; Zhenguo, J. Alkaline-earth metal Ca and N codoped TiO2 with exposed {001} facets for enhancing visible light photocatalytic activity. J. Am. Ceram. Soc. 2014, 97, 2615–2622. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Hanwell, M.D.; Donald, C.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]




| Dye | O6–C3 (Å) | C3–C4/ C3–C2 (Å) | C4–R/ C2–R (Å) | C5–O7/C1–O8 C5–O9/C1–O10 (Å) | O6C3C4/ O6C3C2 (°) | C4C3C2 (°) |
|---|---|---|---|---|---|---|
| CR1 | 1.236 | 1.459 | 1.464 | 1.220 | 127.3 | 105.1 |
| CR2 | 1.243 | 1.468 | 1.469 | 1.238 | 128.6 | 102.3 |
| Adsorption Energy of Dyes-(TiO2)n=5,8,68 (eV) | ||
|---|---|---|
| Dyes-(TiO2)5 | CR1-(TiO2)5 | 3.932 |
| CR2-(TiO2)5 | 5.531 | |
| Dyes-(TiO2)8 | CR1-(TiO2)8 | 0.751 |
| CR2-(TiO2)8 | 0.682 | |
| Dyes-(TiO2)68 | D5-(TiO2)68 | 4.743 |
| D7-(TiO2)68 | 4.947 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elegbeleye, I.F.; Maluta, N.E.; Maphanga, R.R. Density Functional Theory Study of Optical and Electronic Properties of (TiO2)n=5,8,68 Clusters for Application in Solar Cells. Molecules 2021, 26, 955. https://doi.org/10.3390/molecules26040955
Elegbeleye IF, Maluta NE, Maphanga RR. Density Functional Theory Study of Optical and Electronic Properties of (TiO2)n=5,8,68 Clusters for Application in Solar Cells. Molecules. 2021; 26(4):955. https://doi.org/10.3390/molecules26040955
Chicago/Turabian StyleElegbeleye, Ife Fortunate, Nnditshedzeni Eric Maluta, and Rapela Regina Maphanga. 2021. "Density Functional Theory Study of Optical and Electronic Properties of (TiO2)n=5,8,68 Clusters for Application in Solar Cells" Molecules 26, no. 4: 955. https://doi.org/10.3390/molecules26040955






