Multi-Hollow Surface Dielectric Barrier Discharge for Bacterial Biofilm Decontamination
Abstract
1. Introduction
2. Results and Discussion
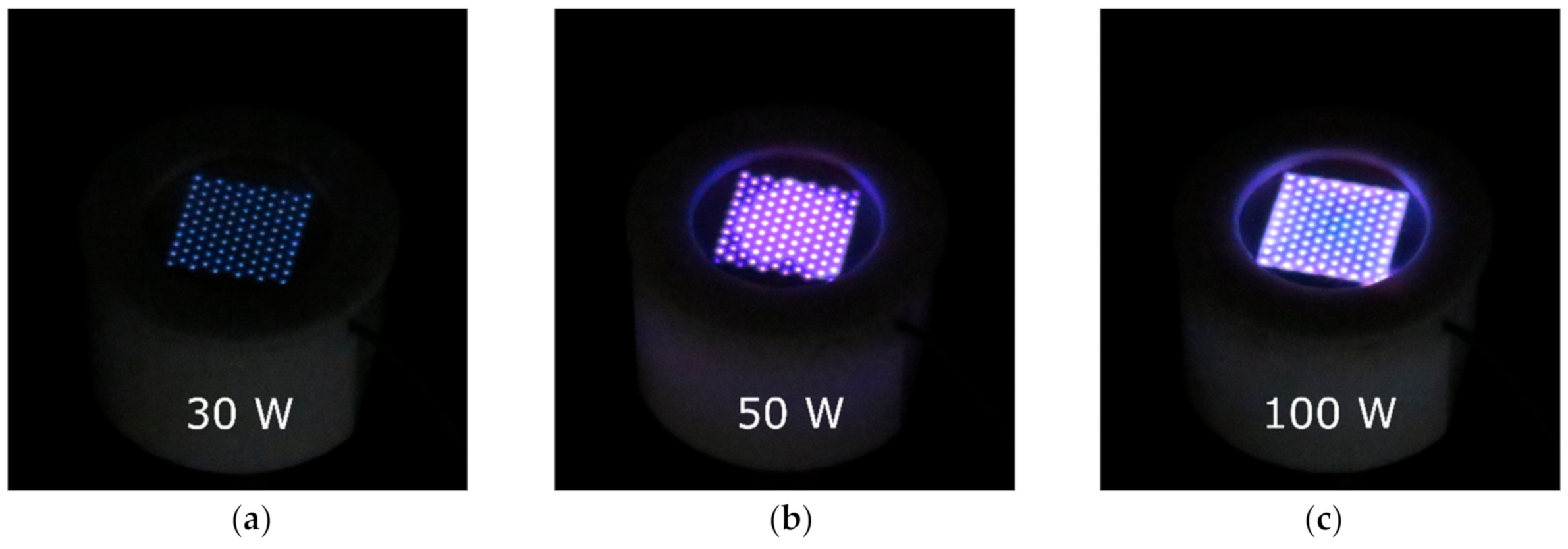
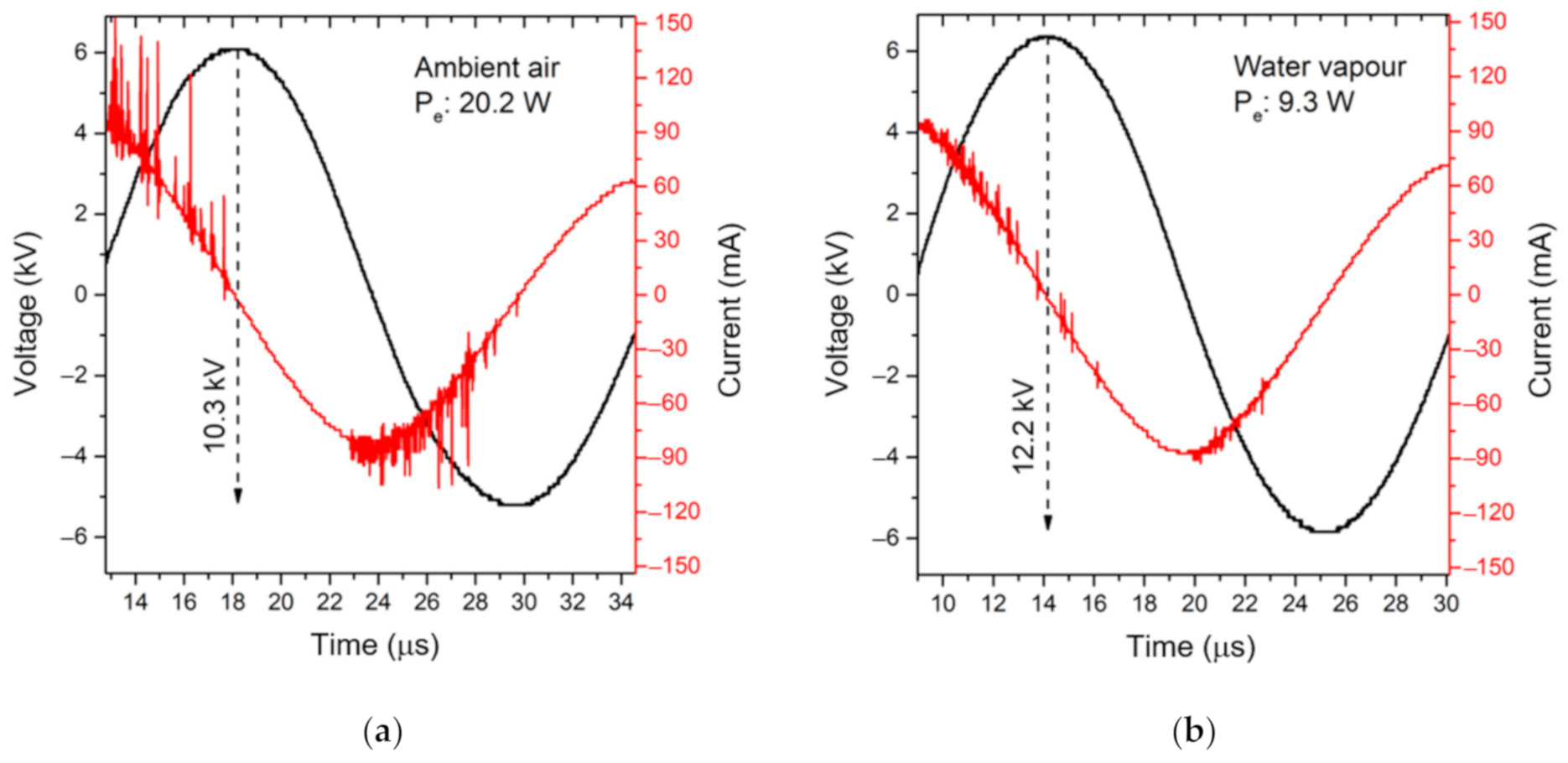
2.1. Characterization of the MSDBD Device for Plasma-Activated Media Generation
2.2. Characterization of Generated Plasma-Activated Media
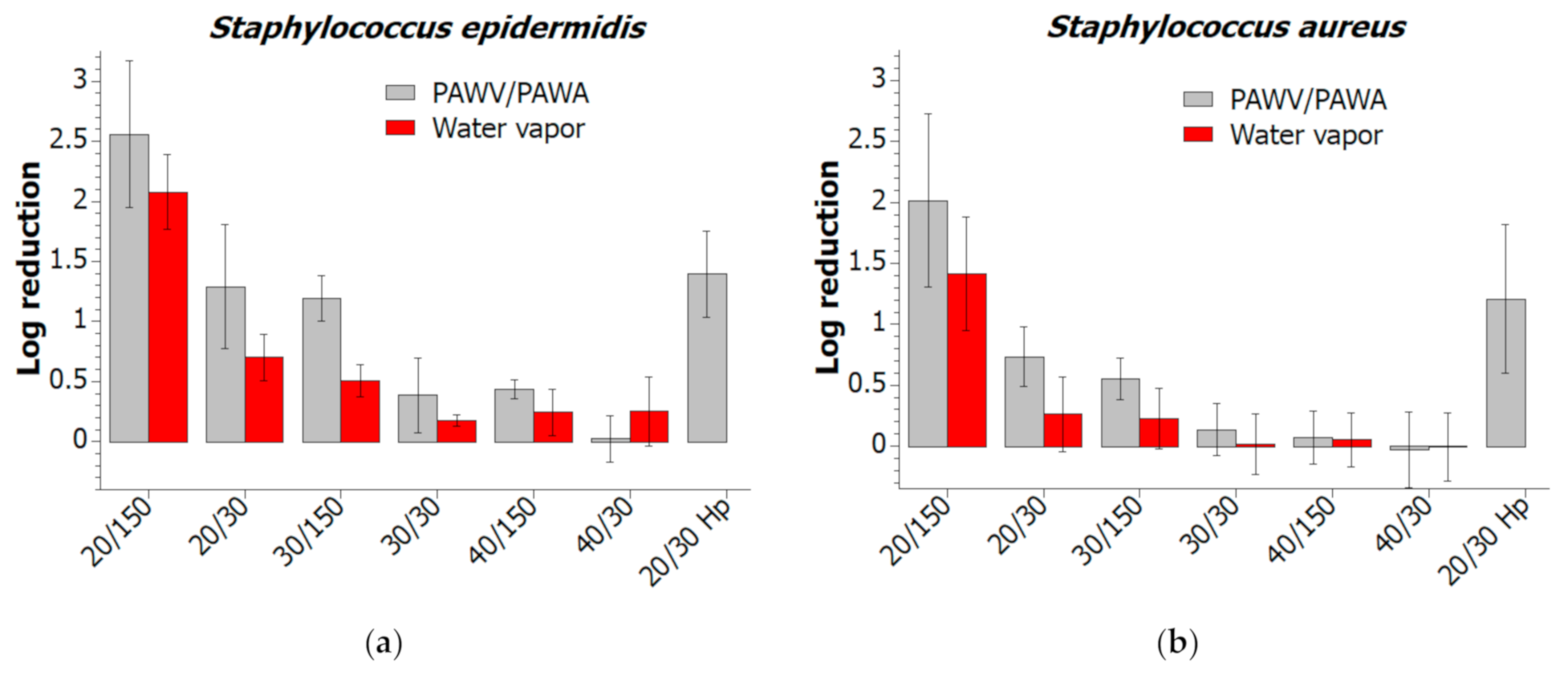
2.3. Bacterial Biofilm Decontamination
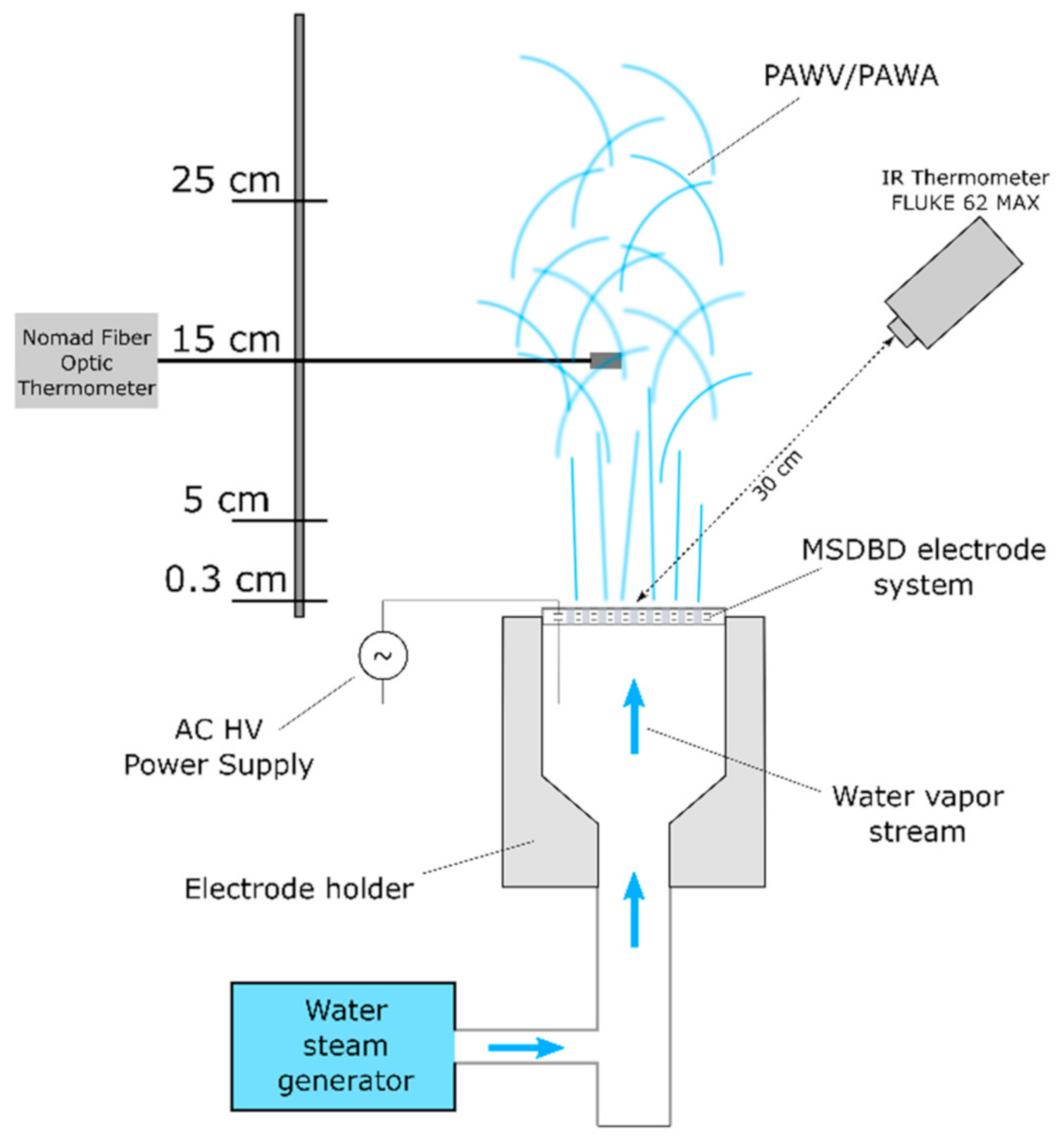
3. Materials and Methods
3.1. Plasma-Activated Media Generation
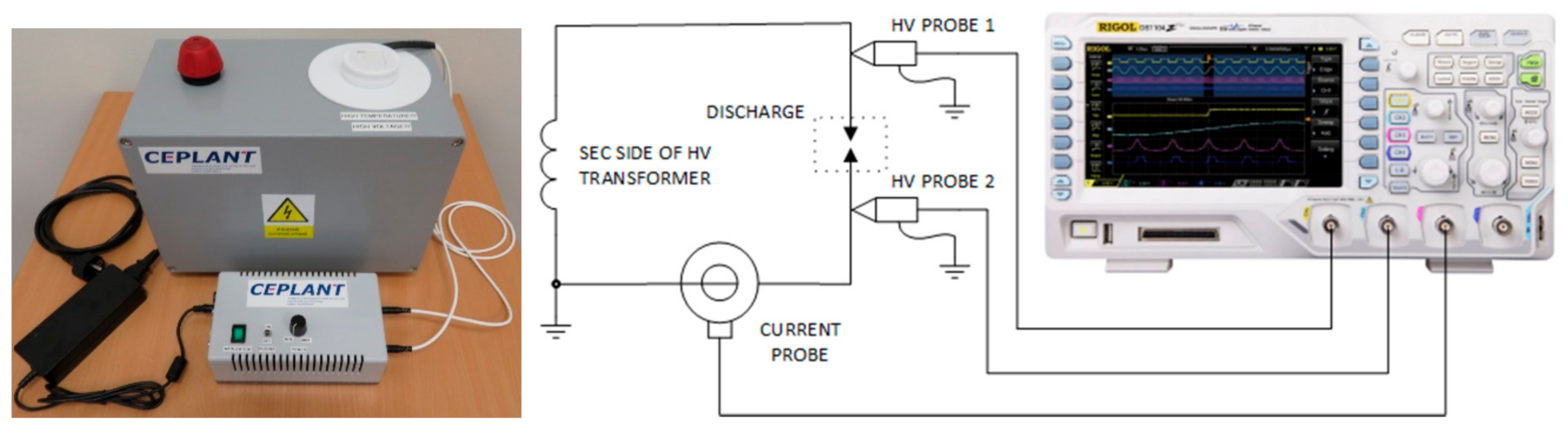
3.1.1. Device for the Generation of Plasma-Activated Media
3.1.2. Electrical Measurements
3.1.3. Temperature Measurements
3.1.4. Characterization of Plasma-Activated Media
3.2. Decontamination of Bacterial Biofilms
3.2.1. Bacterial Biofilm Preparation
3.2.2. Decontamination Effect Evaluation
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Locke, B.R.; Shih, K.-Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sour. Sci. Technol. 2011, 20, 034006. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, T. Characterization of dielectric barrier discharges with water in correlation to productions of OH and H2O2 in gas and liquid phases. Jpn. J. Appl. Phys. 2019, 58, 046001. [Google Scholar] [CrossRef]
- Erben, D.; Hola, V.; Jaros, J.; Rahel, J. Bacterial growth on chitosan-coated polypropylene textile. ISRN Microbiol. 2012, 2012, 749694. [Google Scholar] [CrossRef] [PubMed]
- Rahel, J.; Jonasova, E.; Nesvorna, M.; Klubal, R.; Erban, T.; Hubert, J. The toxic effect of chitosan/metal-impregnated textile to synanthropic mites. Pest. Manag. Sci. 2013, 69, 722–726. [Google Scholar] [CrossRef]
- Ivanova, T.V.; Krumpolec, R.; Homola, T.; Musin, E.; Baier, G.; Landfester, K.; Cameron, D.C.; Černák, M. Ambient air plasma pre-treatment of non-woven fabrics for deposition of antibacterial poly (l-lactide) nanoparticles. Plasma Process. Polym. 2017, 14, 1–9. [Google Scholar] [CrossRef]
- Kováčová, M.; Bodík, M.; Mičušík, M.; Humpolíček, P.; Šiffalovič, P.; Špitálsky, Z. Increasing the effectivity of the antimicrobial surface of carbon quantum dots-based nanocomposite by atmospheric pressure plasma. Clin. Plasma Med. 2020, 19–20, 100111. [Google Scholar] [CrossRef]
- Kováčová, M.; Kleinová, A.; Vajďák, J.; Humpolíček, P.; Kubát, P.; Bodík, M.; Marković, Z.; Špitálský, Z. Photodynamic-active smart biocompatible material for an antibacterial surface coating. J. Photochem. Photobiol. B Biol. 2020, 211, 112012. [Google Scholar] [CrossRef]
- Tudu, B.K.; Sinhamahapatra, A.; Kumar, A. Surface Modification of Cotton Fabric Using TiO2 Nanoparticles for Self-Cleaning, Oil-Water Separation, Antistain, Anti-Water Absorption, and Antibacterial Properties. ACS Omega 2020, 5, 7850–7860. [Google Scholar] [CrossRef]
- Attia, N.F.; Elashery, S.E.A.; Oh, H. Nanomaterials-based antibacterial textiles. In Nanosensors and Nanodevices for Smart Multifunctional Textiles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 135–147. [Google Scholar]
- Guo, J.; Huang, K.; Wang, J. Bactericidal effect of various non-thermal plasma agents and the influence of experimental conditions in microbial inactivation: A review. Food Control. 2015, 50, 482–490. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Cui, D.; Du, M.; Wang, J.; Ma, R.; Jiao, Z. Evaluating the roles of OH radicals, H2O2, ORP and pH in the inactivation of yeast cells on a tissue model by surface micro-discharge plasma. J. Phys. D Appl. Phys. 2019, 52, 395201. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Hayashi, N.; Tsutsui, S.; Tomari, T.; Guan, W. Sterilization of medical equipment using oxygen radicals produced by water vapor RF plasma. IEEE Trans. Plasma Sci. 2008, 36, 1302–1303. [Google Scholar] [CrossRef]
- Fumagalli, F.; Kylián, O.; Amato, L.; Hanuš, J.; Rossi, F. Low-pressure water vapour plasma treatment of surfaces for biomolecules decontamination. J. Phys. D Appl. Phys. 2012, 45, 135203. [Google Scholar] [CrossRef]
- Wang, L.; Deng, L.H.; Li, B.; Fang, B.; Zhao, W.X.; Xu, H.L. Low-pressure OH radicals reactor generated by dielectric barrier discharge from water vapor. Phys. Plasmas 2020, 27. [Google Scholar] [CrossRef]
- Tučeková, Z.; Koval’ová, Z.; Zahoranová, A.; Machala, Z.; Černák, M. Inactivation of Escherichia coli on PTFE surfaces by diffuse coplanar surface barrier discharge. Eur. Phys. J. Appl. Phys. 2016, 75, 24711–24716. [Google Scholar] [CrossRef]
- Procházka, V.; Tučeková, Z.; Dvorák, P.; Kováčik, D.; Slavíček, P.; Zahoranová, A.; Voráč, J. Coplanar surface barrier discharge ignited in water vapor—A selective source of OH radicals proved by (TA)LIF measurement. Plasma Sour. Sci. Technol. 2018, 27, 015001. [Google Scholar] [CrossRef]
- Moldgy, A.; Nayak, G.; Aboubakr, H.A.; Goyal, S.M.; Bruggeman, P.J. Inactivation of virus and bacteria using cold atmospheric pressure air plasmas and the role of reactive nitrogen species. J. Phys. D Appl. Phys. 2020, 53. [Google Scholar] [CrossRef]
- Sarani, A.; Nikiforov, A.Y.; Leys, C. Atmospheric pressure plasma jet in Ar and Ar/ H2 O mixtures: Optical emission spectroscopy and temperature measurements. Phys. Plasmas 2010, 17, 063504. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sour. Sci. Technol. 2017, 26, 53001. [Google Scholar] [CrossRef]
- Falkenstein, Z.; Coogan, J.J. Microdischarge behaviour in the silent discharge of nitrogen—Oxygen and water—Air mixtures. J. Phys. D Appl. Phys. 1997, 30, 817–825. [Google Scholar] [CrossRef]
- Akitsu, T.; Ohkawa, H.; Tsuji, M.; Kimura, H.; Kogoma, M. Plasma sterilization using glow discharge at atmospheric pressure. Surf. Coat. Technol. 2005, 193, 29–34. [Google Scholar] [CrossRef]
- Malik, M.A.; Schoenbach, K.H. New approach for sustaining energetic, efficient and scalable non-equilibrium plasma in water vapours at atmospheric pressure. J. Phys. D Appl. Phys. 2012, 45, 132001. [Google Scholar] [CrossRef]
- Liu, K.; Hu, H.; Lei, J.; Hu, Y.; Zheng, Z. Comparison of pulsating DC and DC power air-water plasma jet: A method to decrease plume temperature and increase ROS. Phys. Plasmas 2016, 23, 123510. [Google Scholar] [CrossRef]
- Krumpolec, R.; Richter, V.; Zemánek, M.; Homola, T. Multi-hollow surface dielectric barrier discharge for plasma treatment of patterned silicon surfaces. Surfaces Interfaces 2019, 16, 181–187. [Google Scholar] [CrossRef]
- Homola, T.; Krumpolec, R.; Zemánek, M.; Kelar, J.; Synek, P.; Hoder, T.; Černák, M. An Array of Micro-hollow Surface Dielectric Barrier Discharges for Large-Area Atmospheric-Pressure Surface Treatments. Plasma Chem. Plasma Process. 2017, 37, 1149–1163. [Google Scholar] [CrossRef]
- Gebremariam, G.; Admassu, S.; Berhanu, T.; Tučeková, Z.; Krumpolec, R.; Černák, M. Optimization and influence of multi-hollow surface dielectric barrier discharge plasma operating conditions on the physical quality of peanut. Eur. Phys. J. D 2019, 73. [Google Scholar] [CrossRef]
- Gebremical, G.G.; Emire, S.A.; Berhanu, T. Effects of Multihollow Surface Dielectric Barrier Discharge Plasma on Chemical and Antioxidant Properties of Peanut. J. Food Qual. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Nayak, G.; Aboubakr, H.A.; Goyal, S.M.; Bruggeman, P.J. Reactive species responsible for the inactivation of felinecalicivirus by a two-dimensional array of integrated coaxialmicrohollow dielectric barrier discharges in air. Plasma Process. Polym. 2018, 15, 1700119. [Google Scholar] [CrossRef]
- Černák, M.; Krumpolec, R.; Tučeková, Z.; Kelar, J.; Zemánek, M.; Kováčik, D. A Method and Device for Generating Low-Temperature Electrical Water-Based Plasma at Near-Atmospheric Pressures and Its Use. EP3,585,136 A1, 25 December 2019. [Google Scholar]
- Homola, T.; Prukner, V.; Hoffer, P.; Šimek, M. Multi-hollow surface dielectric barrier discharge: An ozone generator with flexible performance and supreme efficiency. Plasma Sour. Sci. Technol. 2020, 29. [Google Scholar] [CrossRef]
- Malik, M.A.; Schoenbach, K.H.; Abdel-Fattah, T.M.; Heller, R.; Jiang, C. Low Cost Compact Nanosecond Pulsed Plasma System for Environmental and Biomedical Applications. Plasma Chem. Plasma Process. 2017, 37, 59–76. [Google Scholar] [CrossRef]
- Wei, L.S.; Pongrac, B.; Zhang, Y.F.; Liang, X.; Prukner, V.; Simek, M.S. Influence of Duty Cycle on Ozone Generation and Discharge Using Volume Dielectric Barrier Discharge. Plasma Chem. Plasma Process. 2018, 38, 355–364. [Google Scholar] [CrossRef]
- Russell, A.D. Lethal effects of heat on bacterial physiology and structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Stringer, S.C.; George, S.M.; Peck, M.W. Thermal inactivation of Escherichia coli O157:H7. J. Appl. Microbiol. 2000, 88, 79S–89S. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, A.; Ricker, E.B.; Nuxoll, E. Thermal mitigation of Pseudomonas aeruginosa biofilms. Biofouling 2015, 31, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Julák, J.; Scholtz, V.; Vaňková, E. Medically important biofilms and non-thermal plasma. World J. Microbiol. Biotechnol. 2018, 34, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, D.X.; Liu, Z.C.; Yang, A.J.; Chen, H.L.; Shama, G.; Kong, M.G. A Model of Plasma-Biofilm and Plasma-Tissue Interactions at Ambient Pressure. Plasma Chem. Plasma Process. 2014, 34, 403–441. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Von Keudell, A.; Awakowicz, P.; Benedikt, J.; Raballand, V.; Yanguas-Gil, A.; Opretzka, J.; Fl??tgen, C.; Reuter, R.; Byelykh, L.; Halfmann, H.; et al. Inactivation of bacteria and biomolecules by low-pressure plasma discharges. Plasma Process. Polym. 2010, 7, 327–352. [Google Scholar] [CrossRef]
- Dobrynin, D.; Friedman, G.; Fridman, A.; Starikovskiy, A. Inactivation of bacteria using dc corona discharge: Role of ions and humidity. New J. Phys. 2011, 13, 103033. [Google Scholar] [CrossRef] [PubMed]
- Klämpfl, T.G.; Isbary, G.; Shimizu, T.; Li, Y.F.; Zimmermann, J.L.; Stolz, W.; Schlegel, J.; Morfill, G.E.; Schmidt, H.U. Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl. Environ. Microbiol. 2012, 78, 5077–5082. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, T.; Misra, N.N.; Patil, S.; Cullen, P.J.; Bourke, P.; Keener, K.M.; Mosnier, J.P. Post-discharge gas composition of a large-gap DBD in humid air by UV–Vis absorption spectroscopy. Plasma Sour. Sci. Technol. 2014, 23, 065033. [Google Scholar] [CrossRef]
- Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza sativa L.) Seed Sterilization and Germination Enhancement via Atmospheric Hybrid Nonthermal Discharge Plasma. ACS Appl. Mater. Interfaces 2016, 8, 19268–19275. [Google Scholar] [CrossRef]
- Stephan, K.D.; McLean, R.J.C.; Deleon, G.; Melnikov, V. Effect of feed-gas humidity on nitrogen atmospheric-pressure plasma jet for biological applications. Technol. Health Care 2016, 24, 943–948. [Google Scholar] [CrossRef]
- Yagyu, Y.; Hatayama, Y.; Hayashi, N.; Mishima, T.; Nishioka, T.; Sakudo, A.; Ihara, T.; Ohshima, T.; Kawasaki, H.; Suda, Y. Direct Plasma Disinfection of Green Mold Spore on Citrus by Atmospheric Pressure Dielectric Barrier Discharge for Agricultural Applications. Trans. Mater. Res. Soc. Jpn. 2016, 41, 127–130. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, Y.; Lai, A.C.K.; Huang, G. Inactivation of airborne bacteria by cold plasma in air duct flow. Build. Environ. 2016, 106, 120–130. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control. 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Eto, H.; Ono, Y.; Ogino, A.; Nagatsu, M. Low-temperature sterilization of wrapped materials using flexible sheet-type dielectric barrier discharge. Appl. Phys. Lett. 2008, 93, 221502. [Google Scholar] [CrossRef]
- Kang, M.H.; Pengkit, A.; Choi, K.; Jeon, S.S.; Choi, H.W.; Shin, D.B.; Choi, E.H.; Uhm, H.S.; Park, G. Differential inactivation of fungal spores in water and on seeds by ozone and arc discharge plasma. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef]
- Sasaki, S.; Honda, R.; Hokari, Y.; Takashima, K.; Kanzaki, M.; Kaneko, T. Characterization of plasma-induced cell membrane permeabilization: Focus on OH radical distribution. J. Phys. D Appl. Phys. 2016, 49, 334002. [Google Scholar] [CrossRef]
- Julák, J.; Hujacová, A.; Scholtz, V.; Khun, J.; Holada, K. Contribution to the Chemistry of Plasma-Activated Water. Plasma Phys. Rep. 2018, 44, 125–136. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. npj Biofilms Microbiomes 2021, 7, 11. [Google Scholar] [CrossRef]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hozák, P.; Scholtz, V.; Khun, J.; Mertová, D.; Vaňková, E.; Julák, J. Further Contribution to the Chemistry of Plasma-Activated Water: Influence on Bacteria in Planktonic and Biofilm Forms. Plasma Phys. Rep. 2018, 44, 799–804. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D Appl. Phys. 2019, 52. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sour. Sci. Technol. 2016, 25. [Google Scholar] [CrossRef]
- Hazan, R.; Que, Y.A.; Maura, D.; Rahme, L.G. A method for high throughput determination of viable bacteria cell counts in 96-well plates. BMC Microbiol. 2012, 12, 7. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelar Tučeková, Z.; Vacek, L.; Krumpolec, R.; Kelar, J.; Zemánek, M.; Černák, M.; Růžička, F. Multi-Hollow Surface Dielectric Barrier Discharge for Bacterial Biofilm Decontamination. Molecules 2021, 26, 910. https://doi.org/10.3390/molecules26040910
Kelar Tučeková Z, Vacek L, Krumpolec R, Kelar J, Zemánek M, Černák M, Růžička F. Multi-Hollow Surface Dielectric Barrier Discharge for Bacterial Biofilm Decontamination. Molecules. 2021; 26(4):910. https://doi.org/10.3390/molecules26040910
Chicago/Turabian StyleKelar Tučeková, Zlata, Lukáš Vacek, Richard Krumpolec, Jakub Kelar, Miroslav Zemánek, Mirko Černák, and Filip Růžička. 2021. "Multi-Hollow Surface Dielectric Barrier Discharge for Bacterial Biofilm Decontamination" Molecules 26, no. 4: 910. https://doi.org/10.3390/molecules26040910
APA StyleKelar Tučeková, Z., Vacek, L., Krumpolec, R., Kelar, J., Zemánek, M., Černák, M., & Růžička, F. (2021). Multi-Hollow Surface Dielectric Barrier Discharge for Bacterial Biofilm Decontamination. Molecules, 26(4), 910. https://doi.org/10.3390/molecules26040910





