Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction
Abstract
1. Introduction
2. Results and Discussion
2.1. General Observations
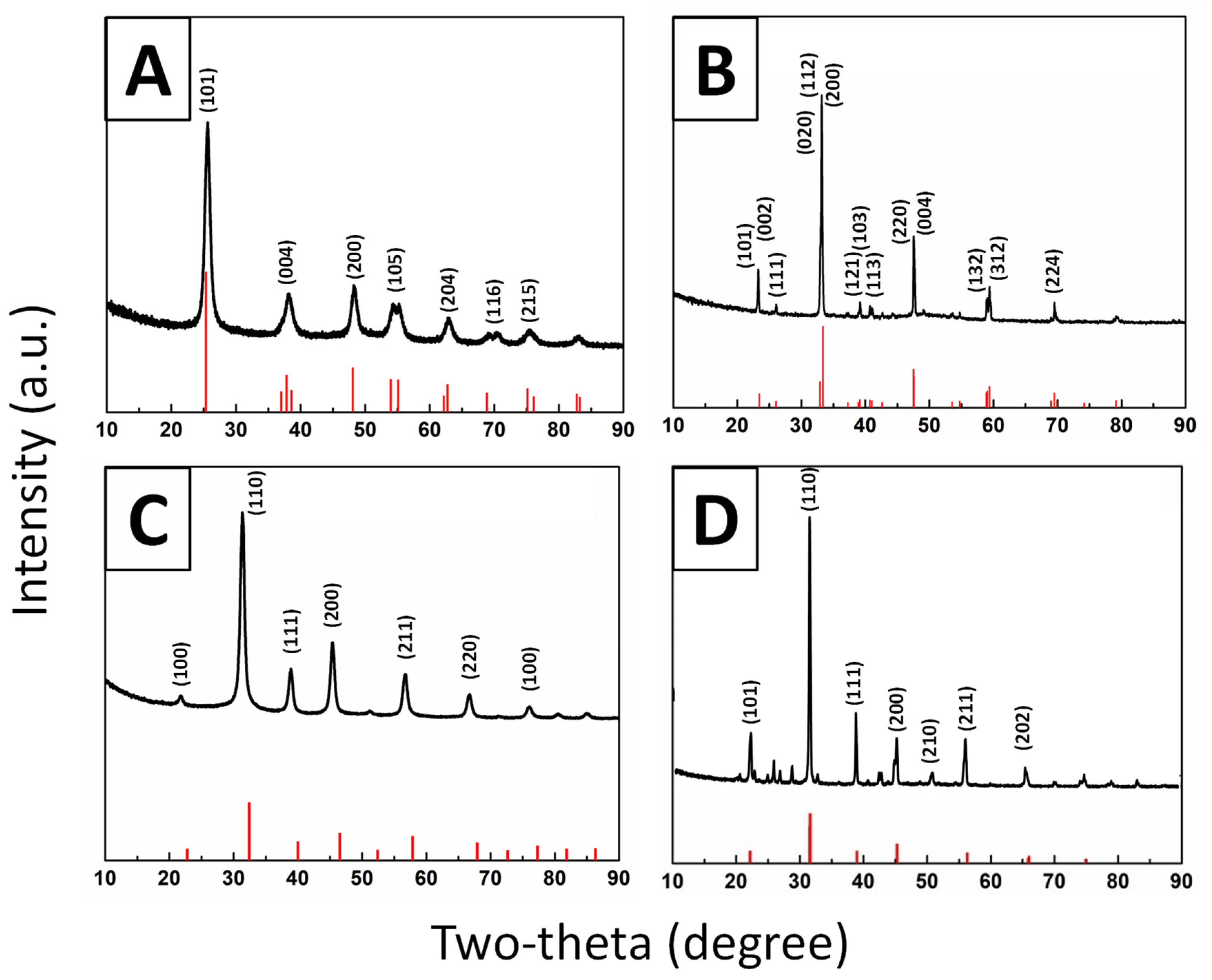
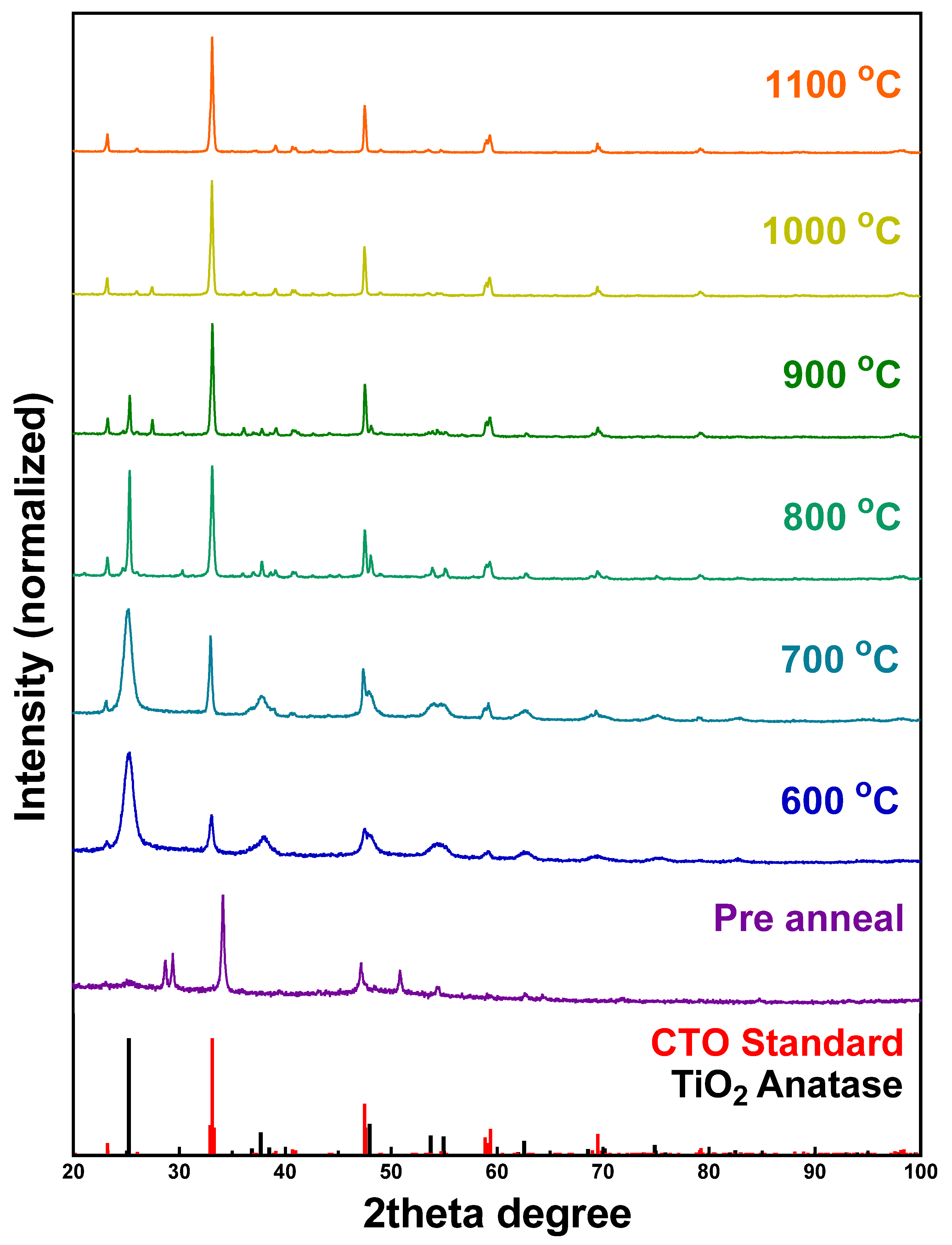
2.2. CaTiO3
2.3. BaTiO3
2.4. SrTiO3
2.5. Mechanistic Insights into Structure
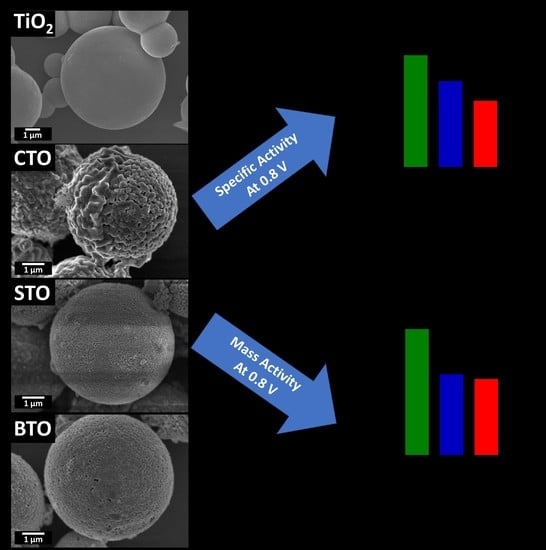
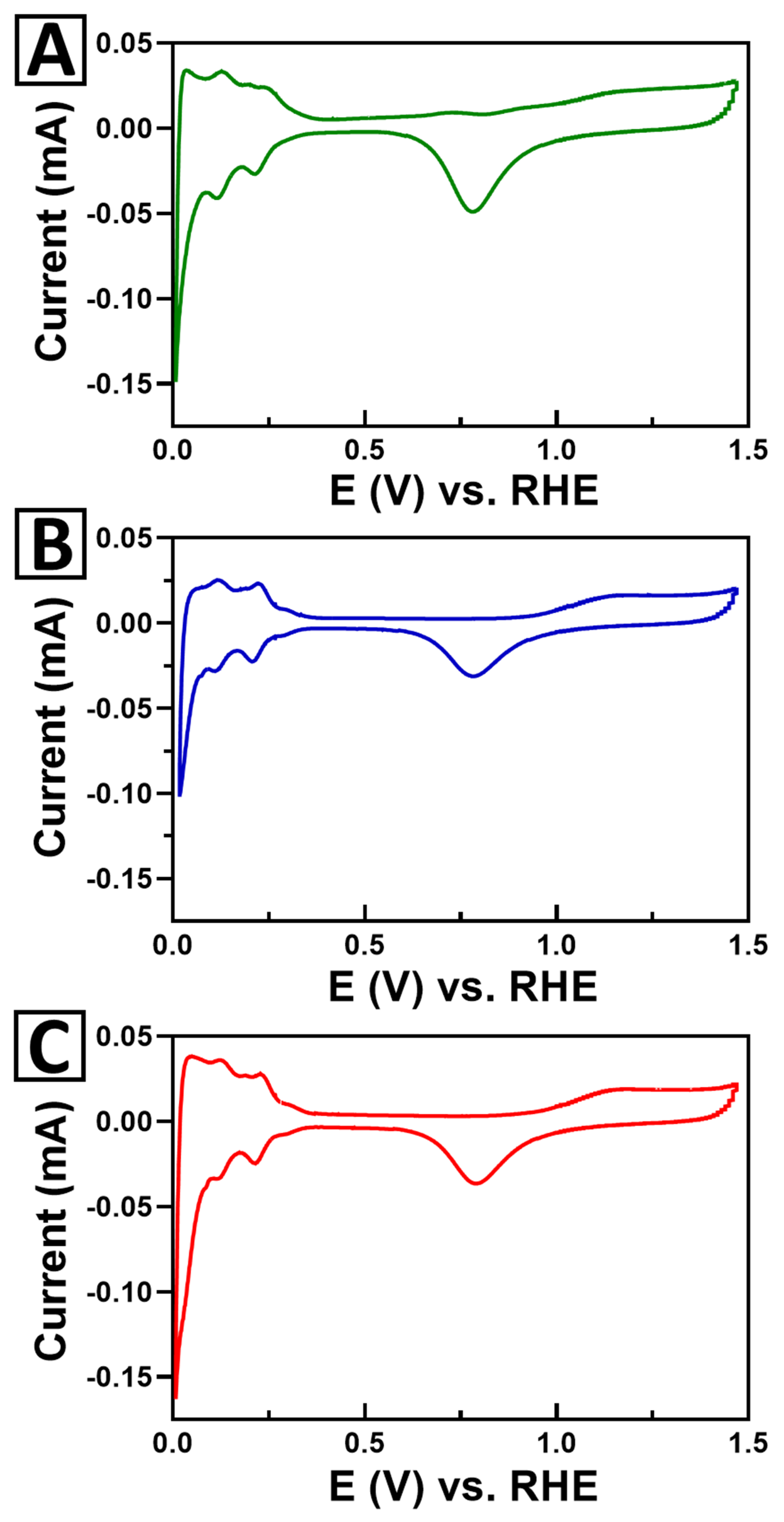
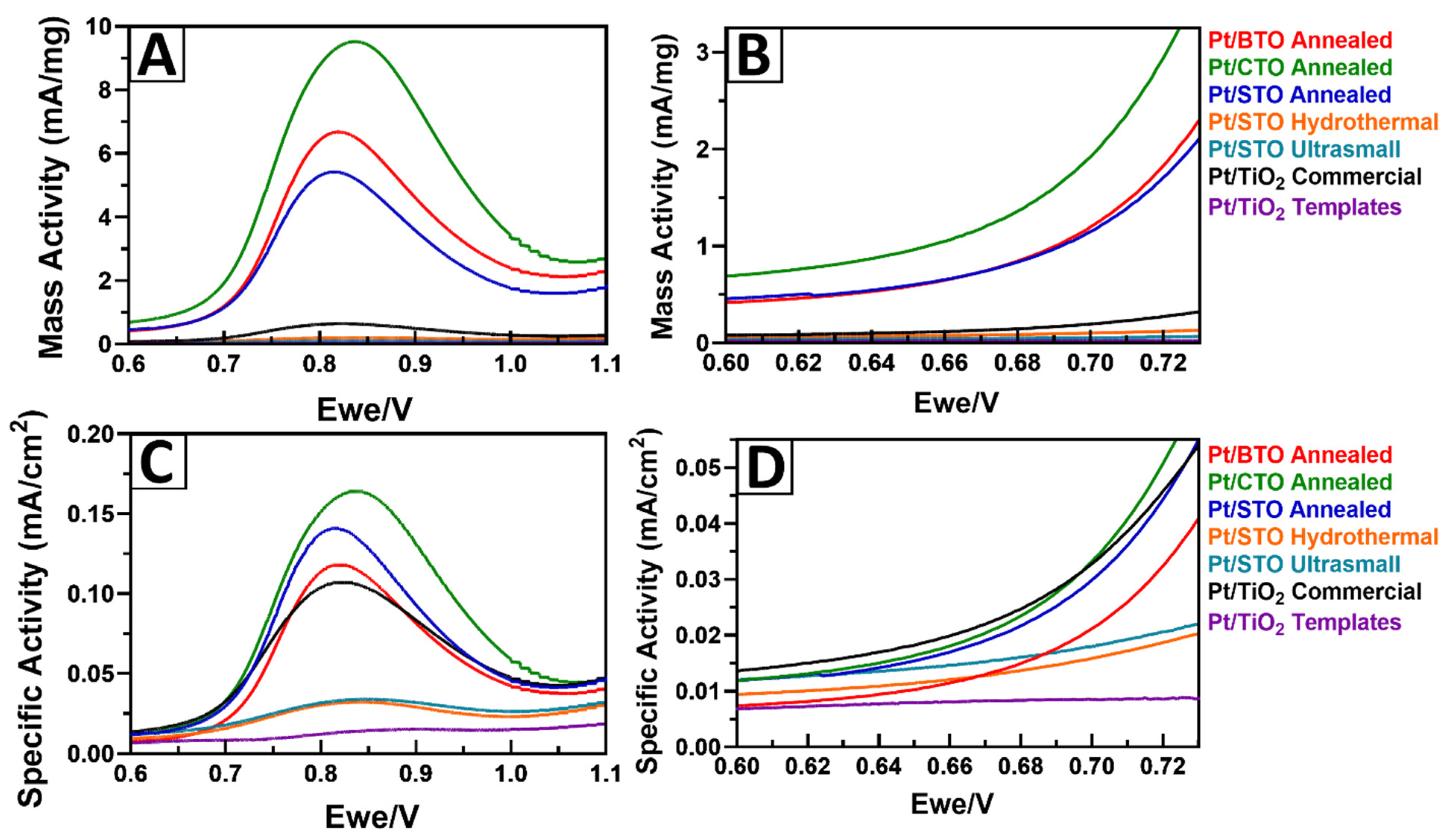
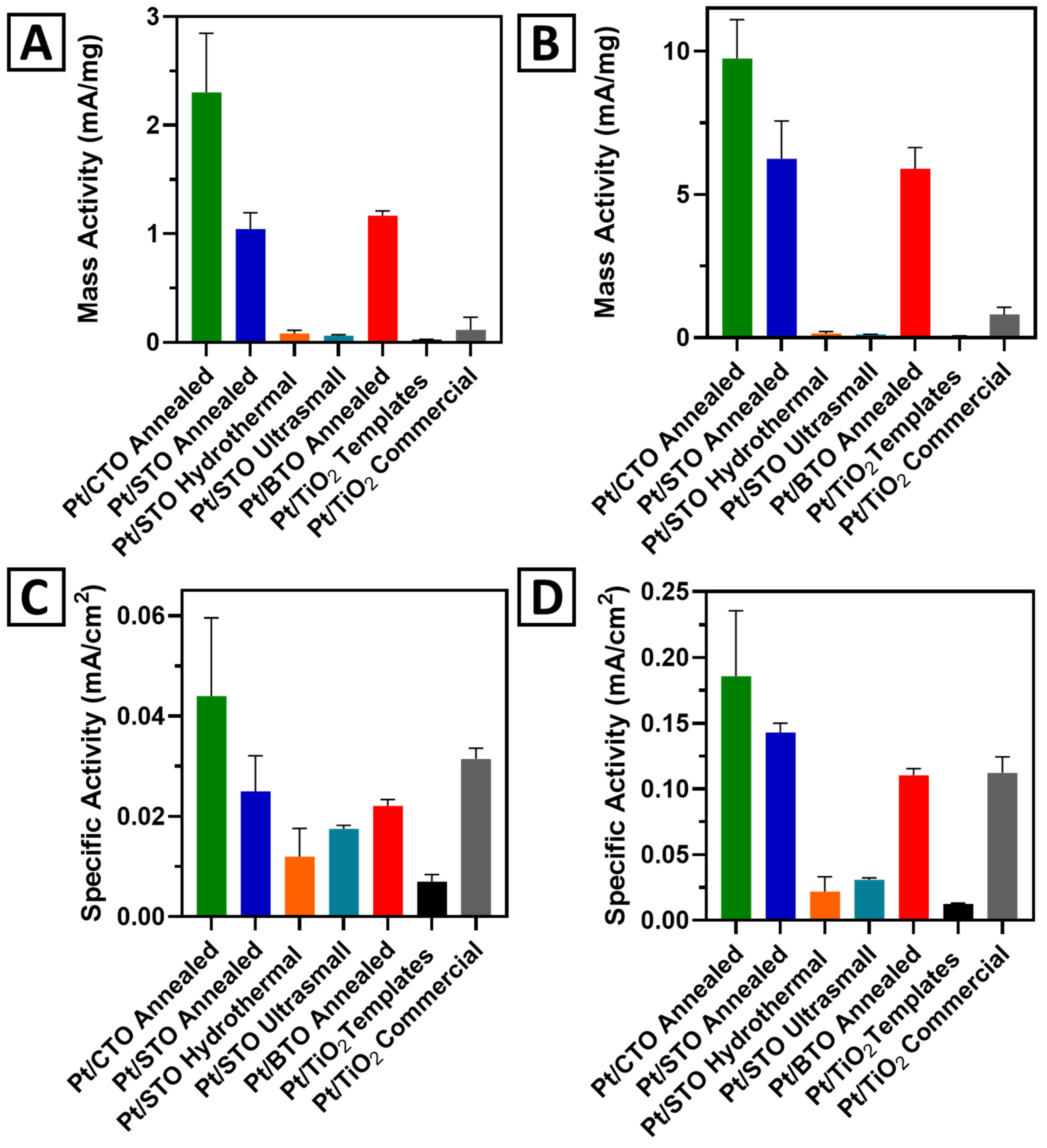
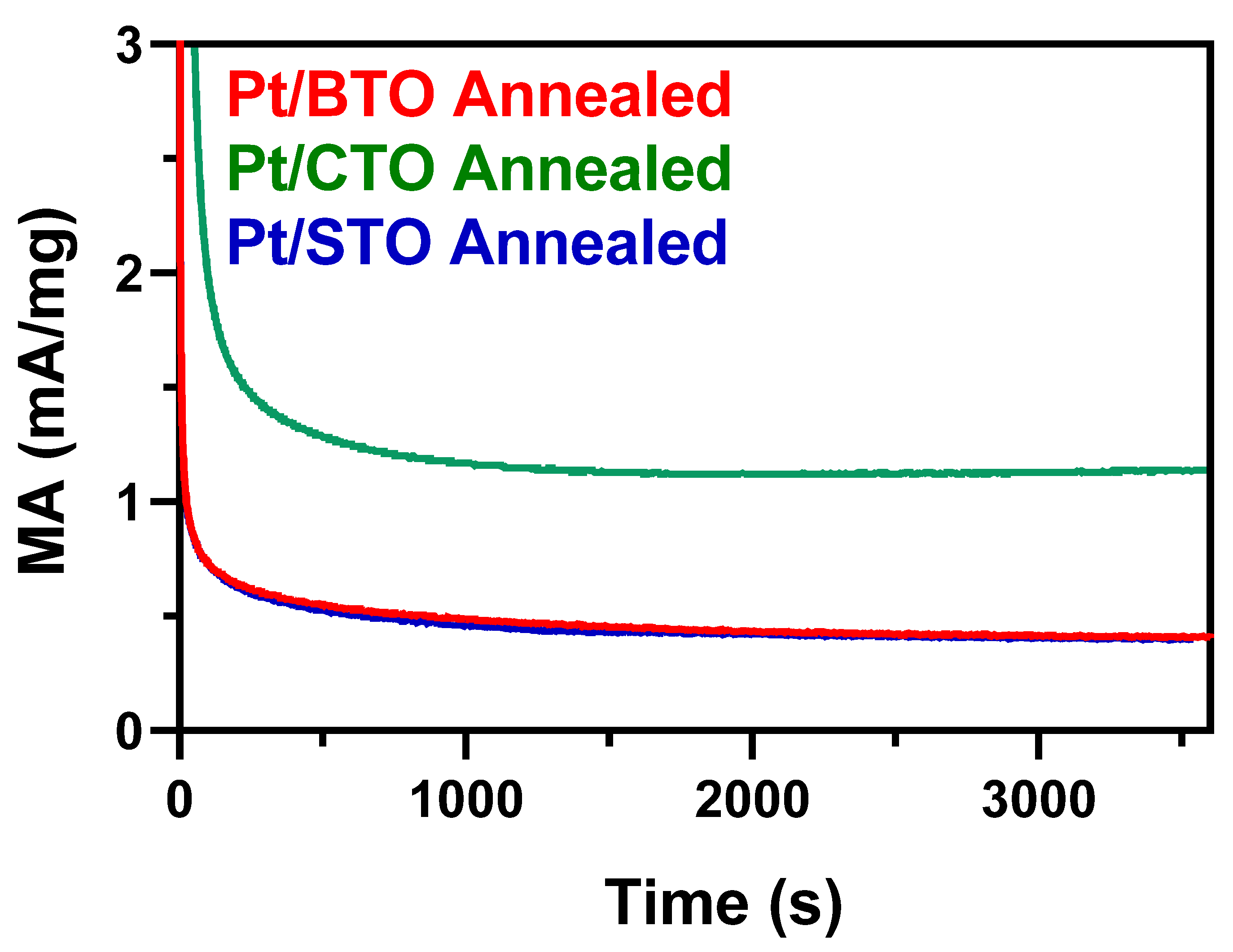
2.6. Probing the Effect of Chemical Composition on MOR
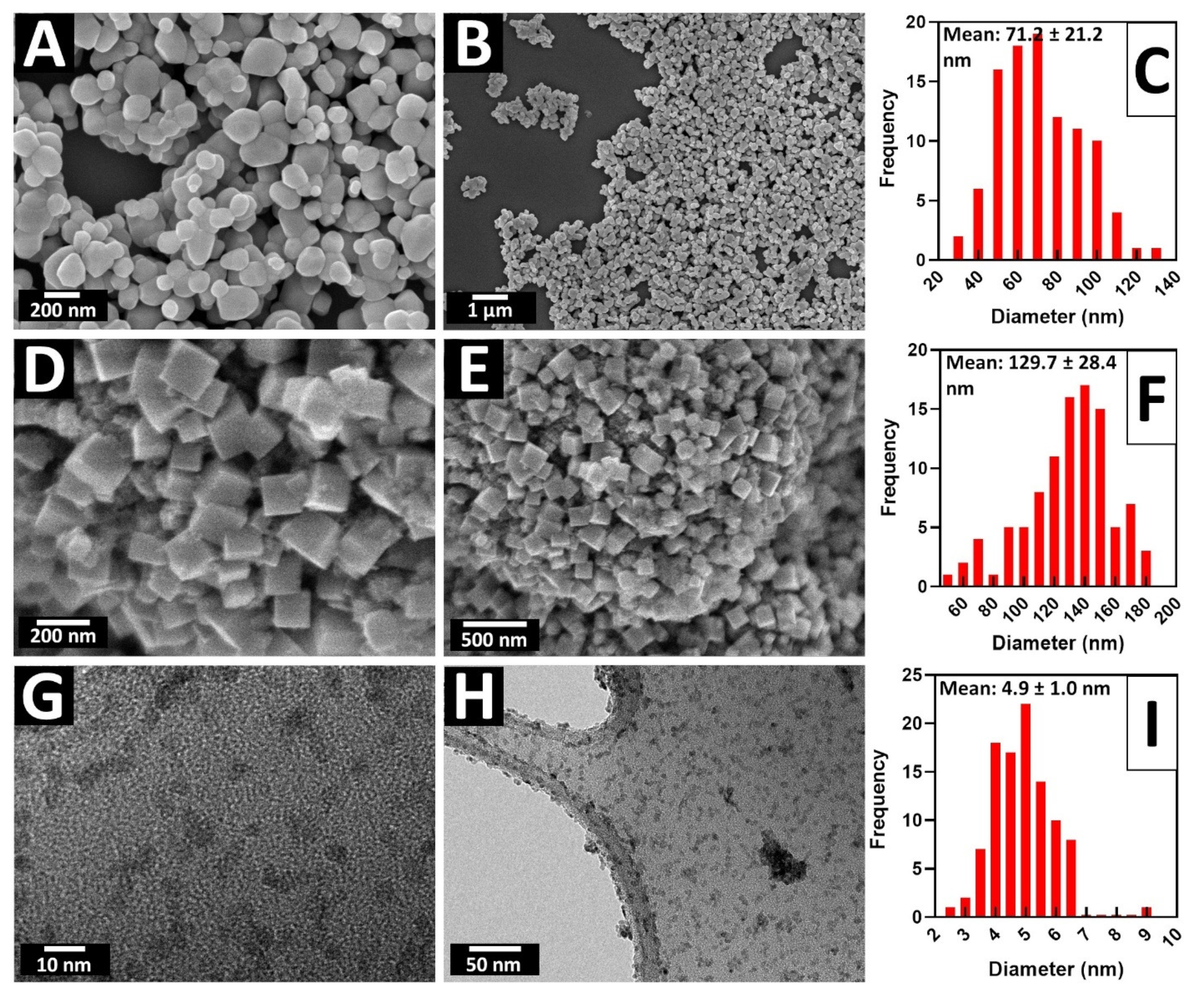
2.7. Probing the Effect of Size and Morphology on MOR: STO Series
3. Materials and Methods
3.1. Materials
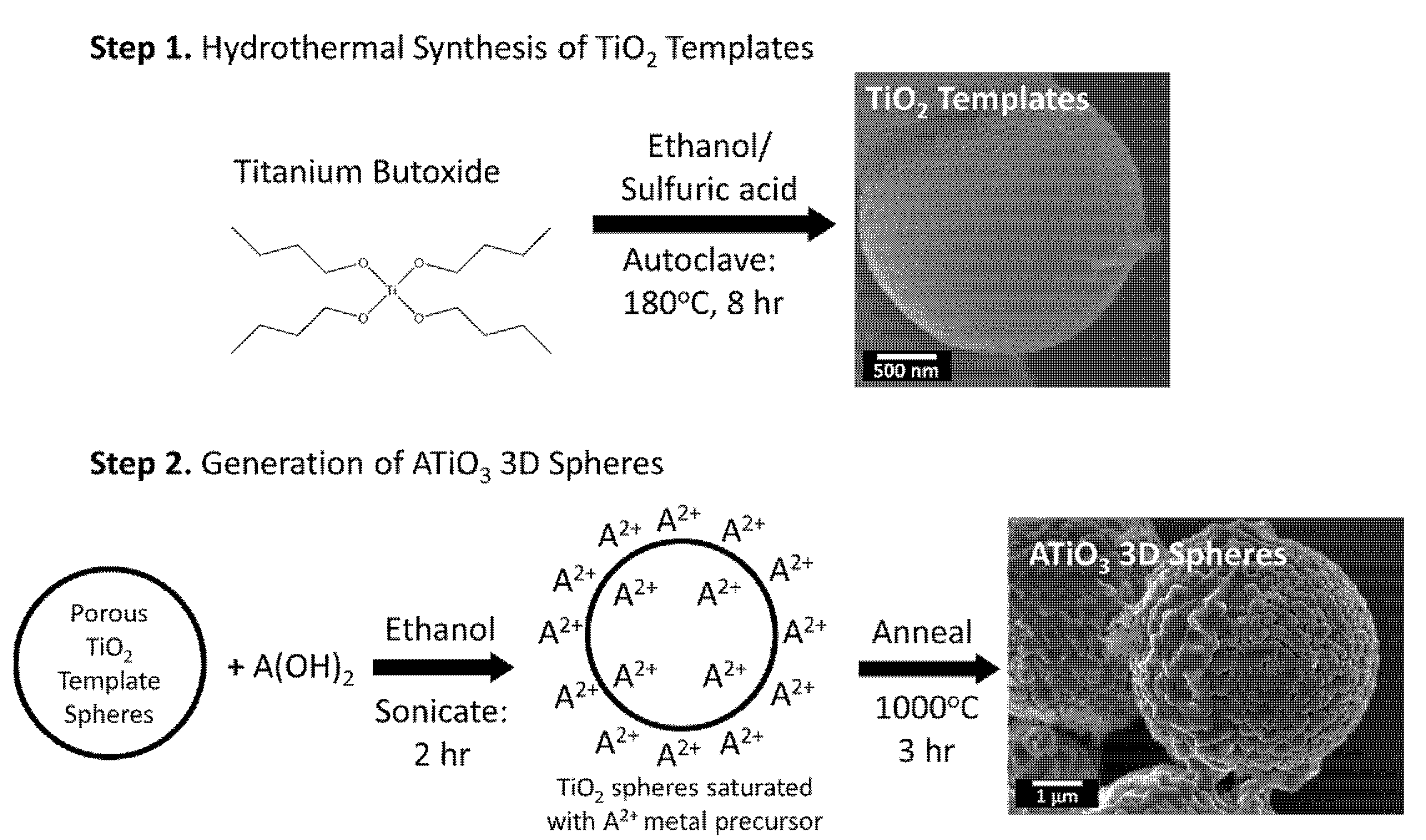
3.2. Synthesis
3.2.1. Synthesis of TiO2 Templates
3.2.2. Synthesis of CaTiO3, BaTiO3, and SrTiO3
3.2.3. Hydrothermal Synthesis of SrTiO3
3.2.4. Ultra-Small SrTiO3
3.2.5. Depositing Pt Nanoparticles onto ATiO3 Micron-Scale Spheres
3.3. Structural and Morphological Characterization
3.3.1. X-Ray Diffraction (XRD)
3.3.2. Electron Microscopy
3.3.3. BET Surface Area
3.3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cohen, R.E. Origin of Ferroelectricity in Perovskite Oxides. Nat. Cell Biol. 1992, 358, 136–138. [Google Scholar] [CrossRef]
- Phelan, D.; Stock, C.; Rodriguez-Rivera, J.A.; Chi, S.; Leão, J.; Long, X.; Xie, Y.; Bokov, A.A.; Ye, Z.-G.; Ganesh, P.; et al. Role of Random Electric Fields in Relaxors. Proc. Natl. Acad. Sci. USA 2014, 111, 1754–1759. [Google Scholar] [CrossRef]
- Jankowska-Sumara, I.; Szot, K.; Majchrowski, A.; Roleder, K. Thermal Hysteresis of Local Instabilities in Paraelectric Phase of PbZr0.96Sn0.04 O3 Single Crystals. J. Appl. Phys. 2013, 113, 187209. [Google Scholar] [CrossRef]
- Talanov, M.V.; Bokov, A.A.; Marakhovsky, M.A. Effects of Crystal Chemistry and Local Random Fields on Relaxor and Piezoelectric Behavior of Lead-Oxide Perovskites. Acta Mater. 2020, 193, 40–50. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, K.; Wang, J. Perovskites for Photovoltaics: A Combined Review of Organic–Inorganic Halide Perovskites and Ferroelectric Oxide Perovskites. J. Mater. Chem. A 2015, 3, 18809–18828. [Google Scholar] [CrossRef]
- Jiang, Y.; Ning, H.; Yu, J. Optical Bandgap Tuning of Ferroelectric Semiconducting BiFeO3-Based Oxide Perovskites via Chemical Substitution for Photo-Voltaics. AIP Adv. 2018, 8, 125334. [Google Scholar] [CrossRef]
- Jones, R.E., Jr.; Zurcher, P.; Chu, P.; Taylor, D.; Lii, Y.; Jiang, B.; Maniar, P.; Gillespie, S.J. Memory Applications Based on Ferroelectric and High-Permittivity Dielectric Thin Films. Microelectron. Eng. 1995, 29, 3–10. [Google Scholar] [CrossRef]
- Dubey, A.K.; Ravikumar, K.; Basu, B. Perovskite Ceramics as New-Generation Materials for Orthopedic Applications. Trans. Indian Inst. Met. 2019, 72, 1999–2010. [Google Scholar] [CrossRef]
- Wang, R.; Ni, S.; Liu, G.; Xu, X. Hollow CaTiO3 Cubes Modified by La/Cr Co-doping for Efficient Photocatalytic Hydrogen Production. Appl. Catal. B Environ. 2018, 225, 139–147. [Google Scholar] [CrossRef]
- Lu, L.; Ni, S.; Liu, G.; Xu, X. Structural Dependence of Photocatalytic Hydrogen Production over La/Cr Co-doped Perovskite Compound ATiO 3 (A = Ca, Sr and Ba). Int. J. Hydrogen Energy 2017, 42, 23539–23547. [Google Scholar] [CrossRef]
- Luo, B.; Wang, X.; Tian, E.; Song, H.; Wang, H.; Li, L. Enhanced Energy-Storage Density and High Efficiency of Lead-Free CaTiO3–BiScO3 Linear Dielectric Ceramics. ACS Appl. Mater. Interfaces 2017, 9, 19963–19972. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Wang, Q.; Zeng, Y.; Liao, K.; Zhang, S.; Zhong, Q. Novel 3D Hierarchical Bifunctional NiTiO3 Nanoflower for Superior Visible Light Photoreduction Performance of CO2 to CH4 and High Lithium Storage Performance. Energy 2019, 169, 580–586. [Google Scholar] [CrossRef]
- Scofield, M.E.; Koenigsmann, C.; Bobb-Semple, D.; Tao, J.; Tong, X.; Wang, L.; Lewis, C.S.; Vukmirovic, M.B.; Zhu, Y.; Adzic, R.R.; et al. Correlating the Chemical Composition and Size of Various Metal Oxide Substrates with the Catalytic Activity and Stability of as-Deposited PT Nanoparticles for the Methanol Oxidation Reaction. Catal. Sci. Technol. 2015, 6, 2435–2450. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Fang, S.; Tang, Y. Synthesis of Sawtooth-like Li4Ti5O12 Nanosheets as Anode Materials for Li-Ion Batteries. Electrochim. Acta 2010, 55, 6596–6600. [Google Scholar] [CrossRef]
- Xia, H.; Luo, Z.; Xie, J. Nanostructured Lithium Titanate and Lithium Titanate/Carbon Nanocomposite as Anode Materials for Advanced Lithium-Ion Bat-Teries. Nanotechnol. Rev. 2014, 3, 161–175. [Google Scholar] [CrossRef]
- Lan, A.; Mukasyan, A.S. Perovskite-Based Catalysts for Direct Methanol Fuel Cells. J. Phys. Chem. C 2007, 111, 9573–9582. [Google Scholar] [CrossRef]
- Lan, A.; Mukasyan, A.S. Complex SrRuO3−Pt and LaRuO3−Pt Catalysts for Direct Alcohol Fuel Cells. Ind. Eng. Chem. Res. 2008, 47, 8989–8994. [Google Scholar] [CrossRef]
- White, J.H.; Sammells, A.F.; Shiratsuchi, R.; Aikoh, Y.; Nogami, G. Perovskite Anode Electrocatalysis for Direct Methanol Fuel Cells. J. Electrochem. Soc. 1993, 140, 2167–2177. [Google Scholar] [CrossRef]
- Tiano, A.L.; Santulli, A.C.; Koenigsmann, C.; Feygenson, M.; Aronson, M.; Harrington, R.; Parise, J.B.; Wong, S.S. Toward a Reliable Synthesis of Strontium Ruthenate: Parameter Control and Property Investigation of Submicrometer-Sized Structures. Chem. Mater. 2011, 23, 3277–3288. [Google Scholar] [CrossRef]
- Abdullah, M.; Nelson, R.J.; Kittilstved, K.R. On the Formation of Superoxide Radicals on Colloidal ATiO3 (A = Sr and Ba) Nanocrystal Surfaces. Nanoscale Adv. 2020, 2, 1949–1955. [Google Scholar] [CrossRef]
- Niederberger, M.; Garnweitner, G.; Pinna, N.; Antonietti, M. Nonaqueous and Halide-Free Route to Crystalline BaTiO3, SrTiO3, and (Ba,Sr)TiO3Nanoparticles via a Mechanism Involving C−C Bond Formation. J. Am. Chem. Soc. 2004, 126, 9120–9126. [Google Scholar] [CrossRef]
- Anjana, P.S.; Sebastian, M.T. Synthesis, Characterization, and Microwave Dielectric Properties of ATiO3 (A=Co, Mn, Ni) Ceramics. J. Am. Ceram. Soc. 2006, 89, 2114–2117. [Google Scholar] [CrossRef]
- Wang, R.; Xie, R.-J.; Hanada, K.; Matsusaki, K.; Kawanaka, H.; Bando, H.; Sekiya, T.; Itoh, M. Enhanced Piezoelectricity around the Tetragonal/Orthorhombic Morphotropic Phase Boundary in (Na,K)NbO3–ATiO3 Solid Solutions. J. Electroceramics 2007, 21, 263–266. [Google Scholar] [CrossRef]
- Moghtada, A.; Ashiri, R. Enhancing the Formation of Tetragonal Phase in Perovskite Nanocrystals Using an Ultrasound Assisted Wet Chemical Method. Ultrason. Sonochem. 2016, 33, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Sunstrom, J.E.; Kauzlarich, S.M.; Antonio, M.R. Synthesis, Structure and Characterization of Ce1-xAxTiO3 (0.0. ltoreq. x. ltoreq. 0.8; A = strontium, barium). Chem. Mater. 1993, 5, 182–191. [Google Scholar] [CrossRef]
- Demirörs, A.F.; Imhof, A. BaTiO3, SrTiO3, CaTiO3, and BaxSr1−xTiO3Particles: A General Approach for Monodisperse Colloidal Perovskites. Chem. Mater. 2009, 21, 3002–3007. [Google Scholar] [CrossRef]
- Xu, L.; Cui, Q.; Zhang, H.; Jiao, A.; Tian, Y.; Li, S.; Li, H.; Chen, M.; Chen, F. Ultra-Clean PtPd Nanoflowers Loaded on Go Supports with Enhanced Low-Temperature Electrocatalytic Activity for Fuel Cells in Harsh Environ-Ment. Appl. Surf. Sci. 2020, 511, 145603. [Google Scholar] [CrossRef]
- Cargnello, M.; Chen, C.; Diroll, B.T.; Doan-Nguyen, V.V.; Gorte, R.J.; Murray, C.B. Efficient removal of organic ligands from supported nanocrystals by fast thermal annealing enables catalytic studies on well-defined active phases. J. Am. Chem. Soc. 2015, 137, 6906–6911. [Google Scholar] [CrossRef]
- Li, D.; Wang, C.; Tripkovic, D.; Sun, S.; Markovic, N.M.; Stamenkovic, V.R. Surfactant Removal for Colloidal Nanoparticles from Solution Synthesis: The Effect on Catalytic Performance. ACS Catal. 2012, 2, 1358–1362. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Lin, X.-X.; Zhang, X.-F.; Wang, A.-J.; Zhu, X.-Y.; Fenga, J.-J. Bimetallic PtPd Alloyed Core-Shell Nanodendrites Supported on Reduced Graphene Oxide: One-Pot Green Synthesis and Efficient Electrocatalytic Performances for Glycerol Oxidation and Hydrogen Evolution. J. Alloy. Compd. 2018, 735, 2123–2132. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Li, Y.; Yang, C. H2SO4 induced mesoporous TiO2 nano-photocatalyst synthesized free of template under microwave. Powder Technol. 2018, 335, 54–61. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y. Strategic synthesis of hierarchical TiO2 microspheres with enhanced photocatalytic activity. Chemistry 2010, 16, 11266–11270. [Google Scholar] [CrossRef]
- Cai, S.; Yu, S.; Wan, W.; Wen, W.; Zhou, Y. Self-Template Synthesis of ATiO3 (A = Ba, Pb and Sr) Perovskites for Photocatalytic Removal of NO. RSC Adv. 2017, 7, 27397–27404. [Google Scholar] [CrossRef]
- Hamnett, A. Mechanism of Methanol Oxidation. In Interfacial Electrochemistry: Theory, Experiment and Applications; Wieckowski, A., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 843–879. [Google Scholar]
- Hartnig, C.; Jorissen, L.; Lehnert, W.; Scholta, J. Direct Methanol Fuel Cells. In Materials for Fuel Cells; Gasik, M., Ed.; Woodhead Publishing: Oxford, UK, 2008; pp. 185–208. [Google Scholar]
- Tang, H.; Su, Y.; Zhang, B.; Lee, A.F.; Isaacs, M.A.; Wilson, K.; Hailian, T.; Karen, W.; Huang, J.; Haruta, M.; et al. Classical Strong Metal–Support Interactions between Gold Nanoparticles and Titanium Dioxide. Sci. Adv. 2017, 3, e1700231. [Google Scholar] [CrossRef] [PubMed]
- Sztaberek, L.; Mabey, H.; Beatrez, W.; Lore, C.; Santulli, A.C.; Koenigsmann, C. Sol–Gel Synthesis of Ruthenium Oxide Nanowires To Enhance Methanol Oxidation in Supported Platinum Nanoparticle Catalysts. ACS Omega 2019, 4, 14226–14233. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J.; Marshall, C.L.; Elam, J.W.; Miller, J.T.; Liu, H.; Enterkin, J.A.; Kennedy, R.M.; Stair, P.C.; Poeppelmeier, K.R.; et al. In situ XANES Study of Methanol Decomposition and Partial Oxidation to Syn-Gas over Supported Pt Catalyst on SrTiO3 Nanocubes. Catal. Today 2014, 237, 71–79. [Google Scholar] [CrossRef]
- Hasa, B.; Kalamaras, E.; Papaioannou, E.; Sygellou, L.; Katsaounis, A. Electrochemical Oxidation of Alcohols on Pt–TiO2 Binary Electrodes. Int. J. Hydrogen Energy 2013, 38, 15395–15404. [Google Scholar] [CrossRef]
- Koenigsmann, C.; Scofield, M.E.; Liu, H.; Wong, S.S. Designing Enhanced One-Dimensional Electrocatalysts for the Oxygen Reduction Reaction: Probing Size-and Composition-Dependent Electrocatalytic Behavior in Noble Metal Nanowires. J. Phys. Chem. Lett. 2012, 3, 3385–3398. [Google Scholar] [CrossRef]
- Polo-Garzon, F.; Wu, Z. Acid–Base Catalysis over Perovskites: A Review. J. Mater. Chem. A 2018, 6, 2877–2894. [Google Scholar] [CrossRef]
- Polo-Garzon, F.; Yang, S.-Z.; Fung, V.; Foo, G.S.; Bickel, E.E.; Chisholm, M.F.; Jiang, D.-E.; Wu, Z. Controlling Reaction Selectivity through the Surface Termination of Perovskite Catalysts. Angew. Chem. Int. Ed. 2017, 56, 9820–9824. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, R.; Wang, H.; Linkov, V.; Ji, S. Evolution of Nanoscale Amorphous, Crystalline and Phase-Segregated Ptnip Nanoparticles and Their Electrocatalytic Effect on Methanol Oxidation Reaction. Phys. Chem. Chem. Phys. 2014, 16, 3593. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, H.; Li, H.; Key, J.; Ji, S.; Wang, R. Synthesis of Ultrafine Amorphous PtP Nanoparticles and the Effect of PtP Crystallinity on Methanol Oxidation. RSC Adv. 2014, 4, 20722–20728. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, R.; Wang, H.; Liao, S.; Key, J.V.L.; Ji, S. The Effect of PtRuIr Nanoparticle Crystallinity in Electrocatalytic Methanol Oxidation. Materials 2013, 6, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.K.; Maram, P.S.; Navrotsky, A. Thermodynamics of Nanoscale Calcium and Strontium Titanate Perovskites. J. Am. Ceram. Soc. 2013, 96, 3670–3676. [Google Scholar] [CrossRef]
- Eglitis, R.I. Comparative First-Principles Calculations of SrTiO3, BaTiO3, PbTiO3 and CaTiO3 (001), (011) and (111) Surfaces. Ferroelectrics 2015, 483, 53–67. [Google Scholar] [CrossRef]
- Wang, Y.X.; Arai, M.; Sasaki, T.; Wang, C.L. First-Principles Study of the (001) Surface of CubicCaTiO3. Phys. Rev. B 2006, 73, 035411. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, T.; Prabhuram, J.; Chen, R.; Wong, C. Preparation and Characterization of a PtRu/C Nanocatalyst for Direct Methanol Fuel Cells. Electrochim. Acta 2005, 51, 754–763. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y. Facile Synthesis of SrTiO3 Hollow Microspheres Built as Assembly of Nanocubes and Their Associated Photocatalytic Activity. J. Colloid Interface Sci. 2011, 358, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-D.; Wang, G.-C.; Liang, R.-P.; Xia, X.-H.; Yu, H.-W. Controllable Deposition of Platinum Nanoparticles on Graphene As an Electrocatalyst for Direct Methanol Fuel Cells. J. Phys. Chem. C 2011, 115, 15639–15645. [Google Scholar] [CrossRef]
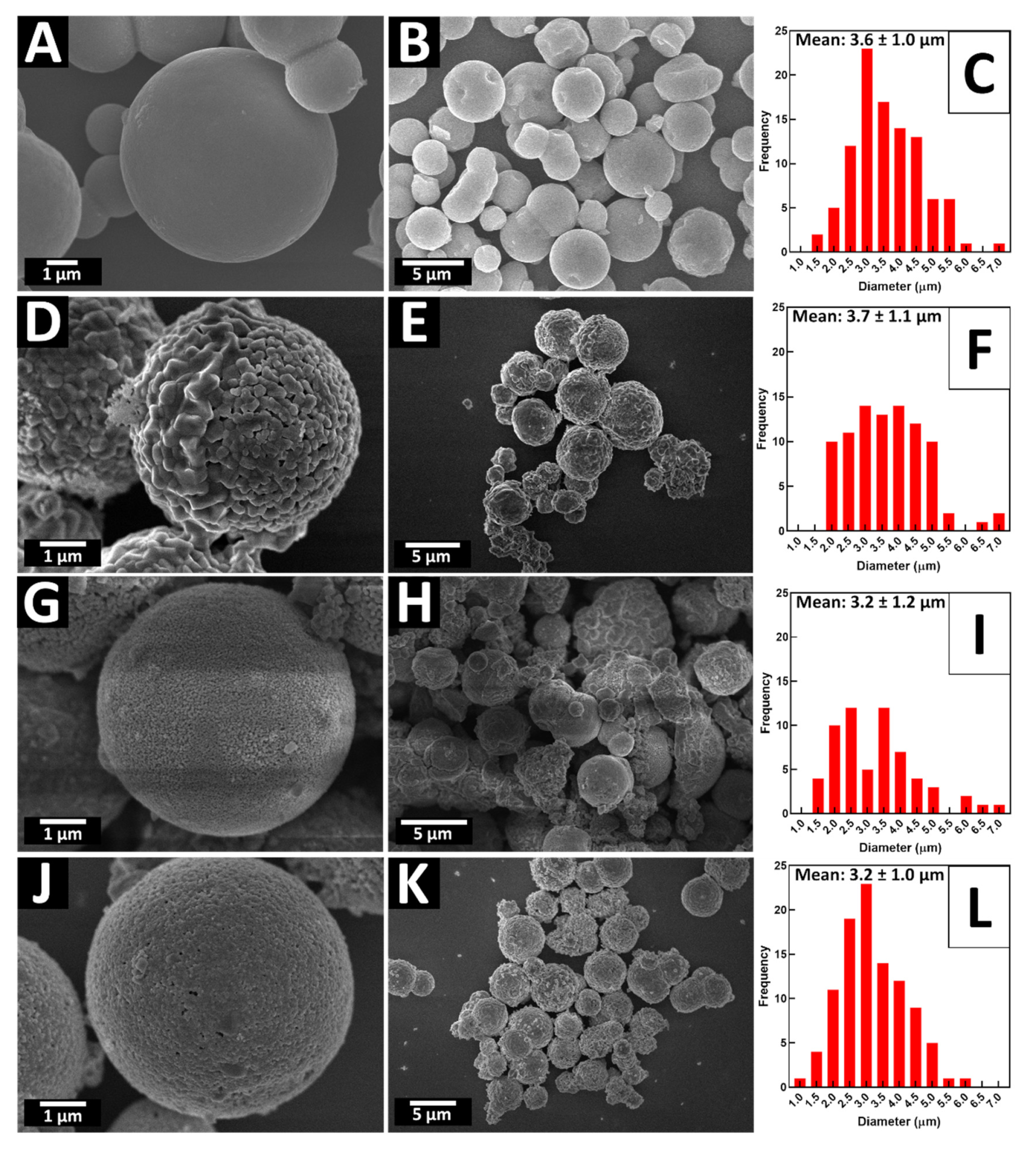


| Sample | BET Surface Area (m2/g) | Particle Size (Derived from Either SEM or TEM Measurements) | Crystallite Size from Debye–Scherrer Equation (Calculated from XRD) |
|---|---|---|---|
| TiO2 templates | 179.22 | 3.6 ± 1.0 μm | 5.7 ± 0.7 nm |
| STO— ultra-small | 81.53 | 4.9 ± 1.0 nm | 4.3 ± 0.4 nm |
| STO— Hydro- thermal | 74.36 | 130 ± 28 nm | 31.1 ± 3.4 nm |
| BTO— annealed | 36.07 | 3.2 ± 1.0 μm | 34.4 ± 8.1 nm |
| TiO2— commercial | 9.93 | 71.1 ± 21.2 nm | 39.9 ± 6.3 nm |
| STO— annealed | 5.74 | 3.2 ± 1.2 μm | 32.1 ± 2.6 nm |
| CTO— annealed | 2.59 | 3.7 ± 1.1 μm | 36.0 ± 8.0 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurley, N.; Li, L.; Koenigsmann, C.; Wong, S.S. Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction. Molecules 2021, 26, 909. https://doi.org/10.3390/molecules26040909
Hurley N, Li L, Koenigsmann C, Wong SS. Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction. Molecules. 2021; 26(4):909. https://doi.org/10.3390/molecules26040909
Chicago/Turabian StyleHurley, Nathaniel, Luyao Li, Christopher Koenigsmann, and Stanislaus S. Wong. 2021. "Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction" Molecules 26, no. 4: 909. https://doi.org/10.3390/molecules26040909
APA StyleHurley, N., Li, L., Koenigsmann, C., & Wong, S. S. (2021). Surfactant-Free Synthesis of Three-Dimensional Perovskite Titania-Based Micron-Scale Motifs Used as Catalytic Supports for the Methanol Oxidation Reaction. Molecules, 26(4), 909. https://doi.org/10.3390/molecules26040909