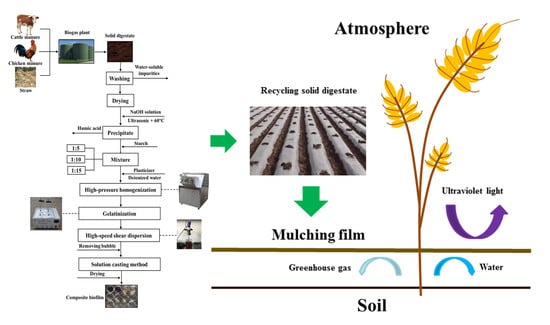
Upgrading Solid Digestate from Anaerobic Digestion of Agricultural Waste as Performance Enhancer for Starch-Based Mulching Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition Analysis
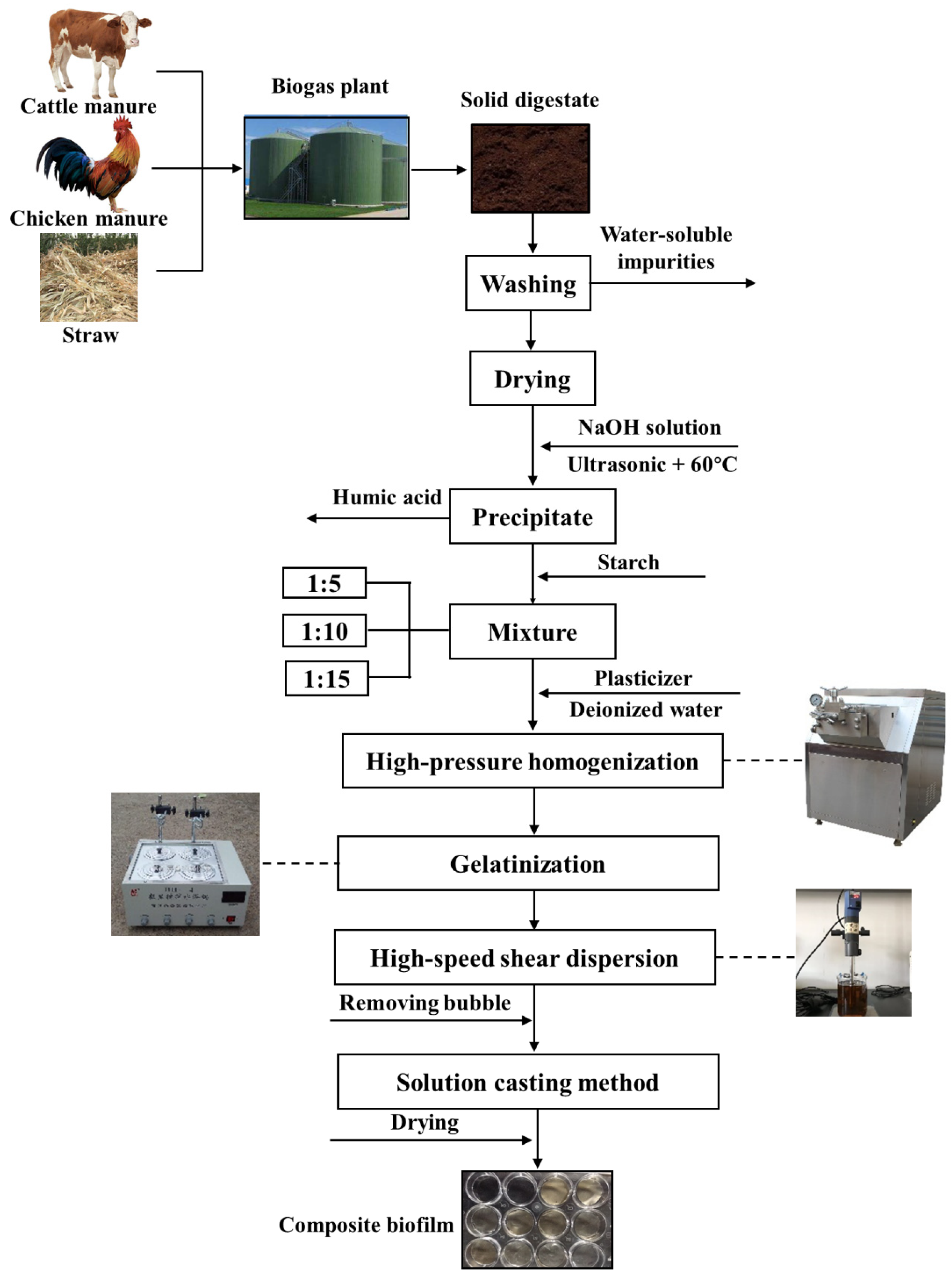
2.3. Pretreatment
2.4. Biofilm Preparation
2.5. Morphology
2.6. Crystallinity
2.7. Fourier-Transform Infrared Spectroscopy (FTIR)
2.8. Mechanical Property
2.9. Thermal Stability
2.10. Transparency
2.11. Water Vapor Permeability
2.12. Gas Transmission Rate
2.13. Statistical Analysis
3. Results and Discussion
3.1. Pretreatment of Solid Digestates
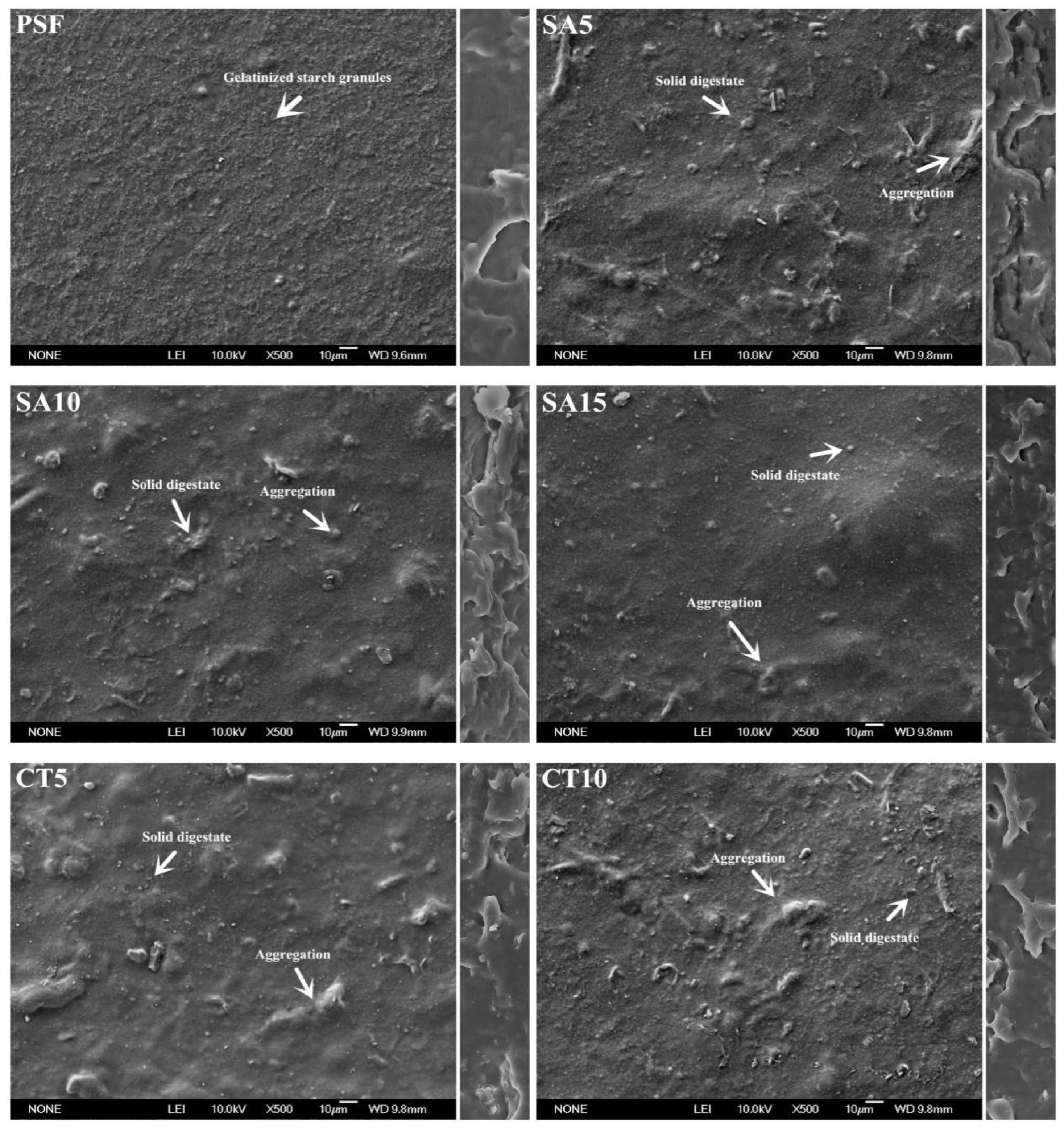
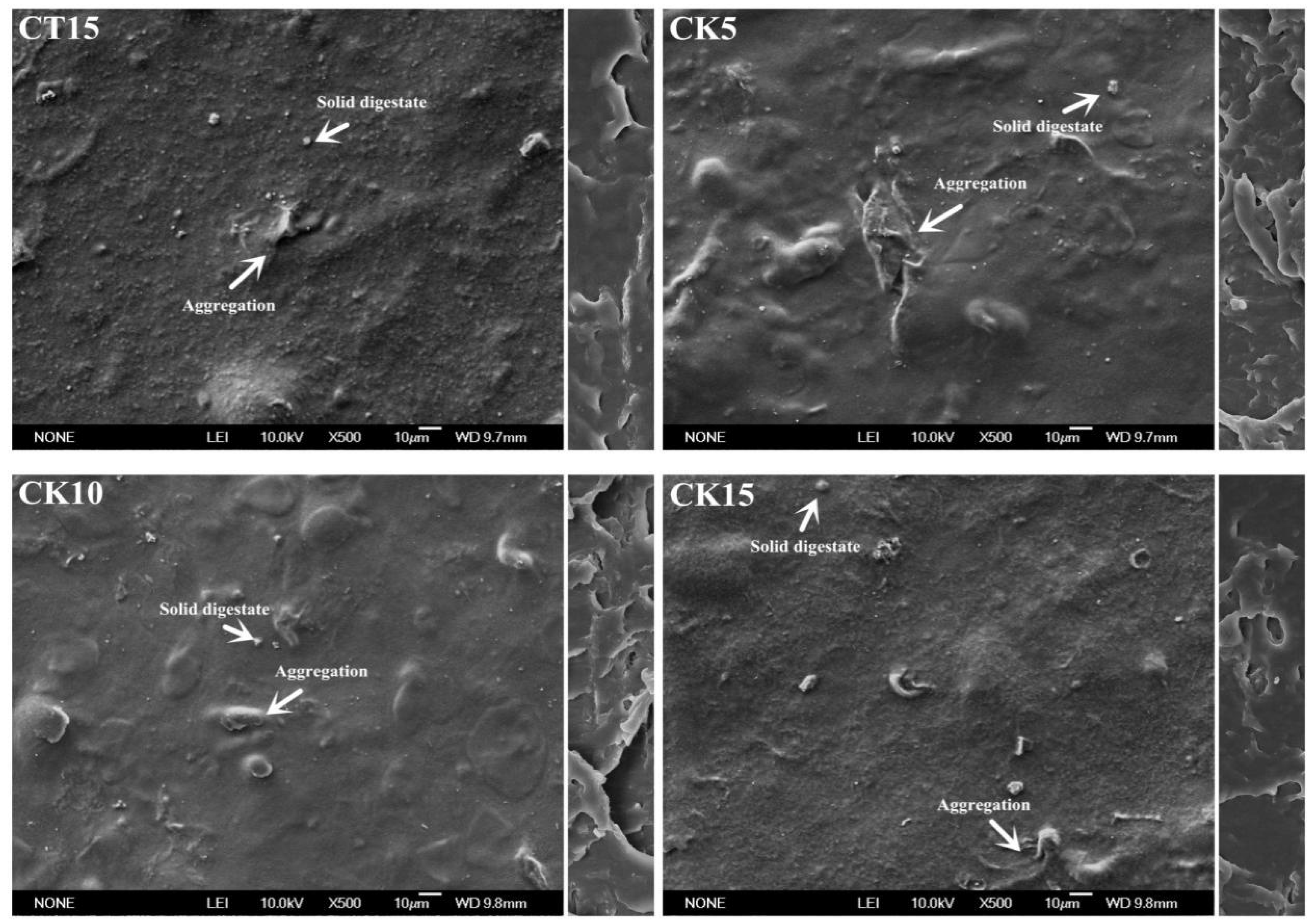
3.2. Morphology
3.3. Crystallinity
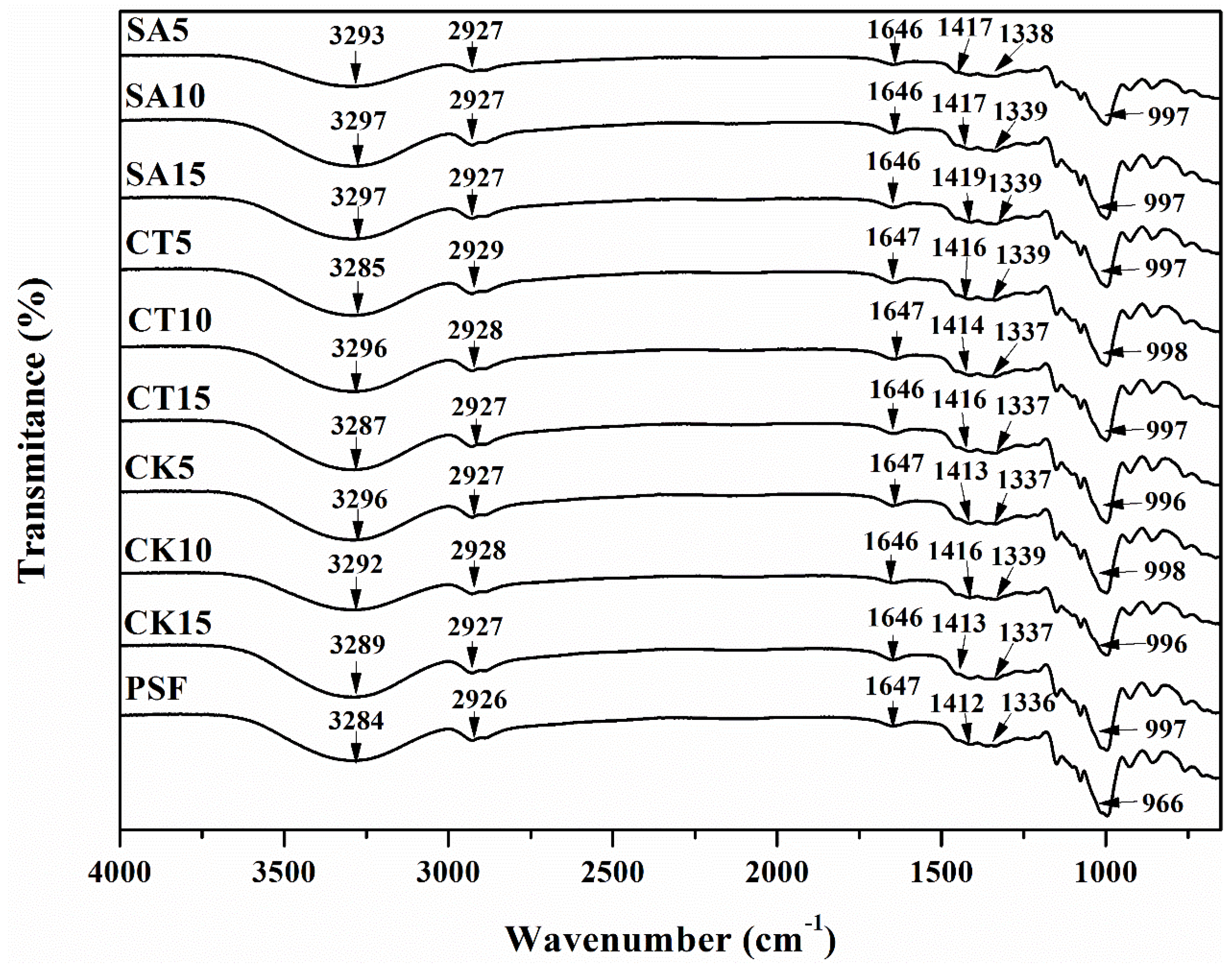
3.4. FTIR Analysis
3.5. Mechanical Properties
3.6. Thermal Stability
3.7. Transparency
3.8. WVP and GTR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Nomenclature
| Tg | Glass transition temperature, °C |
| Tm | Degradation temperature, °C |
| GHC | Greenhouse gas |
| SEM | Scanning electron microscopy |
| XC | Relative crystalline degree, dimensionless |
| IC | Intensity of crystalline region, dimensionless |
| IA | Intensity of amorphous region, dimensionless |
| FTIR | Fourier-transform infrared spectroscopy |
| Ab | Absorbance, dimensionless |
| Ty | Transmittance (%) |
| RH | Relative humidity, % |
| WVTR | Water vapor transmission rate, g/m2h |
| WVP | Water vapor permeability, gm/m2hPa |
| M | Weight gain of beaker, g |
| t | Time when the change is less than 0.01 g, h |
| A | Effective area of film, m2 |
| T | Thickness of film, m |
| P | Saturation vapor pressure of water, Pa |
| R1 | Relative humidity value in beaker |
| R2 | Relative humidity value in desiccator |
| GTR | Gas transmission rate, 10−6 m3/m2hPa |
| N2O | Nitrous oxide |
| CH4 | Methane |
| XRD | X-ray diffraction |
| DSC | Differential scanning calorimeter |
References
- Hernández, J.; Bonachela, S.; Granadosa, M.R.; López, J.C.; Magán, J.J.; Montero, J.I. Microclimate and agronomical effects of internal impermeable screens in an unheated Mediterranean greenhouse. Biosyst. Eng. 2017, 163, 66–77. [Google Scholar] [CrossRef]
- Sun, H.T.; Shao, X.R.; Ma, Z.S. Effect of incorporation nanocrystalline corn straw cellulose and polyethylene glycol on properties of biodegradable films. J. Food. Sci. 2016, 81, 2529–2538. [Google Scholar] [CrossRef]
- O’Loughlin, J.; Finnan, J.; Mcdonnell, K. Accelerating early growth in miscanthus with the application of plastic mulch film. Biomass Bioenery. 2017, 100, 52–61. [Google Scholar] [CrossRef]
- Xu, Y.C.; Shen, Q.R.; Li, M.L.; Dittert, K.; Sattelmacher, B. Effect of soil water status and mulching on N2O and CH4 emission from lowland rice field in China. Biol. Fertil. Soils 2004, 39, 215–217. [Google Scholar] [CrossRef]
- Adhikari, R.; Bristow, K.L.; Casey, P.S.; Freischmidt, G.; Hornbuckle, J.W.; Adhikari, B. Preformed and sprayable polymeric mulch film to improve agricultural water use efficiency. Agric. Water Manag. 2016, 169, 1–13. [Google Scholar] [CrossRef]
- Briassoulis, D.; Babdo, E.; Hiskakis, M.; Scarascia, G.; Picuno, P.; Guarde, D.; Dejean, C. Review, mapping and analysis of the agricultural plastic waste generation and consolidation in Europe. Waste Manag. Res. 2013, 31, 1262–1278. [Google Scholar] [CrossRef]
- Li, R.; Hou, X.Q.; Jia, Z.K.; Han, Q.F.; Yang, B.P. Effects of rainfall harvesting and mulching technologies on soil water, temperature, and maize yield in Loess Plateau region of China. Soil Res. 2012, 50, 105–113. [Google Scholar] [CrossRef]
- Lardjane, N.; Belhaneche-Bensemra, N. Migration of additives in simulated landfills and soil burial degradation of plasticized PVC. J. Appl. Polym. Sci. 2010, 111, 525–531. [Google Scholar] [CrossRef]
- Narayanan, M.; Loganathan, S.; Valapa, R.B.; Thomas, S.; Varghese, T.O. UV Protective Poly (lactic acid)/Rosin Films for Sustainable Packaging. Int. J. Biol. Macromol. 2017, 99, 37–45. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Kumar, A. Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioproc. Biosyst. Eng. 2019, 42, 1893–1901. [Google Scholar] [CrossRef]
- Teodoro, A.P.; Mali, S.; Romero, N.; Carvalho, G.M.D. Cassava starch films containing acetylated starch nanoparticles as reinforcement: Physical and mechanical characterization. Carbohydr. Polym. 2015, 126, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.; Rezaei, M.; Behrooz, R. Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. Int. J. Biol. Macromol. 2014, 70, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Chuayjuljit, S.; Su-uthai, S.; Charuchinda, S. Poly(vinyl chloride) film filled with microcrystalline cellulose prepared from cotton fabric waste: Properties and biodegradability study. Waste Manag. Res. 2009, 28, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Wang, K.Y. Study on technology optimization of lignin removal cellulose extraction from wheat bran by combination of ultrasound and hydrogen peroxide. Biotechnology 2016, 15, 135–140. [Google Scholar]
- Yuan, H.R.; Li, R.P.; Zhang, Y.T.; Li, X.J.; Liu, C.M.; Meng, Y.; Lin, M.N.; Yang, Z.Y. Anaerobic digestion of ammonia-pretreated corn stover. Biosyst. Eng. 2015, 129, 142–148. [Google Scholar] [CrossRef]
- Chen, L.H.; Cong, R.G.; Shu, B.R.; Mi, Z.F. A sustainable biogas model in China: The case study of Beijing Deqingyuan biogas project. Renew. Sust. Energy Rev. 2017, 78, 773–779. [Google Scholar] [CrossRef]
- Xia, A.; Murphy, J.D. Microalgal cultivation in treating liquid digestate from biogas systems. Trends Biotechnol. 2016, 34, 264–275. [Google Scholar] [CrossRef]
- Ribeiro, E.M.; Barros, R.M.; Filho, G.L.T.; dos Santos, I.F.S.; Sampaio, L.C.; dos Santos, T.V.; dGB da Silva, F.; Silva, A.P.M.; de Freitas, V.R. Feasibility of biogas and energy generation from poultry manure in Brazil. Waste Manag. Res. 2018, 36, 221–235. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory Press: Golden, CO, USA, 2008. [Google Scholar]
- AOAC International. Official Methods of Analysis for ash (Acid-Insoluble) of Fertilizers; AOAC 955.03-1955; AOAC International: Washington, DC, USA, 2015. [Google Scholar]
- Head, M.J.; Zhou, W.J. Evaluation of NaOH leaching techniques to extract humic acids from palaeosols. Nucl. Instrum. Methods Phys. Res. Sect. B 2000, 172, 434–439. [Google Scholar] [CrossRef]
- Qi, B.C.; Aldrich, C.; Lorenzen, L. Effect of ultrasonication on the humic acids extracted from lignocellulose substrate decomposed by anaerobic digestion. Chem. Eng. J. 2004, 98, 153–163. [Google Scholar] [CrossRef]
- Veeken, A.; Nierop, K.; Wilde, V.D.; Hamelers, B. Characterisation of NaOH-extracted humic acids during composting of a biowaste. Bioresour. Technol. 2000, 72, 33–41. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Wang, L.J.; Li, D.; Wei, Q.; Adhikari, B. Effects of high-pressure homogenization on the properties of starch-plasticizer dispersions and their films. Carbohydr. Polym. 2011, 86, 202–207. [Google Scholar] [CrossRef]
- Kang, X.Y.; Kuga, S.; Wang, C.; Zhao, Y.; Wu, M.; Huang, Y. Green preparation of cellulose nanocrystal and its application. ACS Sustain. Chem. Eng. 2018, 6, 2954–2960. [Google Scholar] [CrossRef]
- Chen, M.J.; Zhang, X.Q.; Liu, C.F.; Sun, R.C.; Lu, F.H. Approach to renewable lignocellulosic biomass film directly from bagasse. ACS Sustain. Chem. Eng. 2014, 2, 1164–1168. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM D882-12; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Barzegar, B.; Azizi, M.H.; Barzegar, M.; Hamidi-Esfahani, Z. Effect of potassium sorbate on antimicrobial and physical properties of starch-clay nanocomposite films. Carbohydr. Polym. 2014, 110, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z.Y. Moisture and oxygen barrier properties of cellulose nanomaterial- based films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Chen, H.T.; Lin, L.X.; Wang, H.Y.; Liu, L.X. Study on Manufacturing Technology and Performance of Biogas Residue Film; InTech China Press: Shanghai, China, 2012. [Google Scholar]
- Montero, B.; Rico, M.; Rodríguezllamazares, S.; Barral, L.; Bouza, R. Effect of nanocellulose as a filler on biodegradable thermoplastic starch films from tuber, cereal and legume. Carbohydr. Polym. 2016, 157, 1094–1104. [Google Scholar] [CrossRef]
- Pawlak-Krucezk, H.; Niedzwiecki, L.; Sieradzka, M.; Mlonka-Mędrala, A.; Baranowski, M.; Serafin-Tkaczuk, M.; Magdziarz, A. Hydrothermal carbonization of agricultural and municipal solid waste digestates–Structure and energetic properties of the solid products. Fuel 2020, 275, 117837. [Google Scholar] [CrossRef]
- Urbanowska, A.; Polowczyk, I.; Kabsch-Korbutowicz, M.; Seruga, P. Characteristics of changes in particle size and zeta potential of the digestate fraction from the municipal waste biogas plant treated with the use of chemical coagulation/precipitation processes. Energies 2020, 13, 5861. [Google Scholar] [CrossRef]
- Slavutsky, A.M.; Bertuzzi, M.A. Water barrier properties of starch films reinforced with cellulosenanocrystals obtained from sugarcane bagasse. Carbohydr. Polym. 2014, 110, 53–61. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Niklasson, C.; Taherzadeh, M.J. Enhancement of solubilization rate of cellulose in anaerobic digestion and its drawbacks. Process Biochem. 2011, 46, 1509–1514. [Google Scholar] [CrossRef]
- Lopez, O.; García, M.A.; Villar, M.A.; Gentili, A.; Rodriguez, M.S.; Albertengo, L. Thermo-compression of biodegradable thermoplastic corn starch films containing chitin and chitosan. LWT-Food. Sci. Technol. 2014, 57, 106–115. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Kennedy, J.F. Studies on the properties of natural fibers-reinforced themoplastics starch composites. Carbohydr. Polym. 2005, 62, 19–24. [Google Scholar] [CrossRef]
- El, M.N.; Abdelouahdi, K.; Barakat, A.; Zahouily, M.; Fihri, A.; Solhy, A.; Achaby, M.E. Bio-nanocomposite films reinforced with cellulose nanocrystals: Rheology of film-forming solutions, transparency, water vapor barrier and tensile properties of films. Carbohydr. Polym. 2015, 129, 156–167. [Google Scholar]
- Tongdeesoontorni, W.; Mauer, L.J.; Wongruong, S.; Sriburi, P.; Rachtanapun, P. Effect of carboxymethyl cellulose concentration on physical properties of biodegradable cassava starch-based films. Chem. Cent. J. 2011, 5, 6–14. [Google Scholar] [CrossRef]
- Zhang, C.W.; Nair, S.S.; Chen, H.Y.; Yan, N.; Farnood, R.; Li, F.Y. Thermally stable, enhanced water barrier, high strength starch bio-composite reinforced with lignin containing cellulose nanofibrils. Carbohydr. Polym. 2020, 230, 115626. [Google Scholar] [CrossRef]
- Lu, Y.S.; Weng, L.H.; Cao, X.D. Morphological, thermal and mechanical properties of ramie crystallites—reinforced plasticized starch biocomposites. Carbohydr. Polym. 2006, 63, 198–204. [Google Scholar] [CrossRef]
- Li, Y.; Shoemaker, C.F.; Ma, J.G.; Shen, X.R.; Zhong, F. Paste viscosity of rice starches of different amylose content and carboxymethylcellulose formed by dry heating and the physical properties of their films. Food Chem. 2008, 109, 616–623. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers on the crystallinity and mechanical properties of starch-based films at different relative humidity values. Carbohydr. Polym. 2009, 77, 293–299. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch-CMC-nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef]
- Savadekar, N.R.; Karande, V.S.; Vigneshwaran, N.; Bharimalla, A.K.; Mhaske, S.T. Preparation of nano cellulose fibers and its application in kappa-carrageenan based film. Int. J. Biol. Macromol. 2012, 51, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Yu, L.; Liu, H.S.; Khalid, S.; Meng, L.H.; Chen, L. Preparation and characterization of starch-based composite films reinforced by corn and wheat hulls. J. Appl. Polym. Sci. 2017, 134, 45159. [Google Scholar] [CrossRef]
- Shi, A.M.; Wang, L.J.; Li, D.; Adhikari, B. Characterization of starch films containing starch nanoparticles. Part 1: Physical and mechanical properties. Carbohydr. Polym. 2013, 96, 593–601. [Google Scholar] [CrossRef]


| No. | Code | Solid Digestate | Solid Digestate/Starch | Thickness (mm) | Moisture Content (%) |
|---|---|---|---|---|---|
| 1 | SA5 | Straw | 1:5 | 0.053 ± 0.005 a | 13.45 ± 0.15 a |
| 2 | SA10 | Straw | 1:10 | 0.052 ± 0.004 a | 13.59 ± 0.21 a |
| 3 | SA15 | Straw | 1:15 | 0.049 ± 0.007 a | 13.43 ± 0.20 a |
| 4 | CT5 | Cattle manure | 1:5 | 0.049 ± 0.007 a | 13.54 ± 0.19 a |
| 5 | CT10 | Cattle manure | 1:10 | 0.049 ± 0.004 a | 13.44 ± 0.11 a |
| 6 | CT15 | Cattle manure | 1:15 | 0.054 ± 0.005 a | 13.33 ± 0.45 a |
| 7 | CK5 | Chicken manure | 1:5 | 0.056 ± 0.009 a | 13.31 ± 0.11 a |
| 8 | CK10 | Chicken manure | 1:10 | 0.052 ± 0.004 a | 13.53 ± 0.29 a |
| 9 | CK15 | Chicken manure | 1:15 | 0.055 ± 0.005 a | 13.33 ± 0.30 a |
| 10 | PSF | Pure starch film | / | 0.053 ± 0.002 a | 13.74 ± 0.19 a |
| Solid Digestate | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | Humic Acid (%) | |
|---|---|---|---|---|---|---|
| Straw | Raw | 25.63 ± 1.23 | 13.42 ± 1.03 | 16.00 ± 1.69 | 25.46 ± 2.17 | 4.37 ± 0.12 |
| Pretreated | 37.66 ± 1.28 | 11.19 ± 0.93 | 9.09 ± 0.71 | 14.23 ± 1.21 | 2.82 ± 0.10 | |
| Cattle manure | Raw | 16.39 ± 1.20 | 24.22 ± 0.37 | 15.40 ± 1.01 | 8.12 ± 0.23 | 2.26 ± 0.13 |
| Pretreated | 26.86 ± 1.93 | 19.74 ± 0.91 | 11.03 ± 0.09 | 6.23 ± 0.11 | 0.93 ± 0.03 | |
| Chicken manure | Raw | 14.32 ± 0.01 | 19.26 ± 0.93 | 9.07 ± 0.06 | 4.73 ± 0.01 | 0.55 ± 0.001 |
| Pretreated | 15.05 ± 0.02 | 12.20 ± 1.11 | 7.38 ± 0.10 | 3.23 ± 0.01 | 0.53 ± 0.001 | |
| Film | Crystallinity (%) | Mechanical Properties | WVP (10−8 gm/m2hPa) | Thermal Properties (°C) | Transparency (%) | GTR (10−6 m3/m2hPa) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strength (MPa) | Elongation (%) | Elastic Modulus (MPa) | Tg | Tm | UV-B | UV-A | Vis Region | N2O | CH4 | |||
| SA5 | 24.03 ± 0.28 g | 6.66 ± 0.04 d,e | 9.05 ± 0.36 a | 1567.63 ± 59.93 e | 3.49 ± 0.03 b | 67.06 ± 1.97 f,g | 179.57 ± 2.00 d | 0.91 ± 0.07 a | 6.54 ± 0.34 b | 38.89 ± 0.47 c | 2.68 ± 0.02 b | 0.98 ± 0.01 b |
| SA10 | 22.36 ± 0.52 f | 7.49 ± 0.52 f | 12.67 ± 0.17 c,d | 1179.90 ± 9.57 d | 4.09 ± 0.01 f | 60.44 ± 0.50 d | 151.46 ± 2.34 c | 1.12 ± 0.06 a | 7.83 ± 0.47 c | 52.81 ± 0.32 g | 3.20 ± 0.01 f | 1.17 ± 0.01 f |
| SA15 | 17.47 ± 0.19 c | 6.33 ± 0.30 d | 14.06 ± 0.01 d,e | 923.11 ± 15.88 c | 3.43 ± 0.01 b | 55.64 ± 0.86 b | 140.12 ± 1.65 b | 5.80 ± 0.29 c | 19.97 ± 0.71 e | 59.26 ± 0.22 i | 2.85 ± 0.01 b | 1.04 ± 0.01 b |
| CT5 | 22.22 ± 0.55 f | 7.74 ± 0.29 f | 9.91 ± 0.26 a,b | 1562.37 ± 36.25 e | 3.91 ± 0.01 c,d | 68.39 ± 1.36 g | 222.86 ± 0.67 f | 1.09 ± 0.08 a | 5.54 ± 0.28 a | 32.91 ± 0.17 a | 3.25 ± 0.01 c,d | 1.18 ± 0.01 c,d |
| CT10 | 19.37 ± 0.08 d | 5.40 ± 0.14 c | 11.64 ± 0.07 c | 912.23 ± 2.03 c | 3.82 ± 0.01 c | 63.56 ± 0.81 e | 218.71 ± 1.38 e | 3.94 ± 0.25 b | 14.02 ± 0.53 d | 52.08 ± 0.58 f | 3.18 ± 0.01 c | 1.15 ± 0.01 c |
| CT15 | 17.27 ± 0.31 c | 4.43 ± 0.04 b | 14.63 ± 0.74 e | 687.67 ± 31.12 b | 3.19 ± 0.05 a | 57.49 ± 1.97 b,c | 152.81 ± 1.04 c | 17.26 ± 0.51 e | 30.39 ± 0.53 h | 57.07 ± 0.40 h | 2.41 ± 0.04 a | 0.88 ± 0.02 a |
| CK5 | 20.97 ± 0.38 e | 6.97 ± 0.21 e | 9.35 ± 0.13 a | 1314.10 ± 3.63 d | 4.09 ± 0.01 f | 66.09 ± 0.26 f | 232.63 ± 1.39 g | 1.04 ± 0.06 a | 7.51 ± 0.44 c | 33.67 ± 0.15 b | 2.98 ± 0.01 f | 1.08 ± 0.01 f |
| CK10 | 18.13 ± 0.73 c | 4.13 ± 0.23 b | 11.42 ± 0.15 b,c | 926.70 ± 91.74 c | 4.03 ± 0.01 e,f | 60.61 ± 0.38 d | 224.56 ± 0.77 f | 9.53 ± 0.33 d | 23.83 ± 0.61 f | 46.01 ± 0.18 d | 3.16 ± 0.01 e,f | 1.15 ± 0.01 e,f |
| CK15 | 16.31 ± 0.04 b | 6.13 ± 0.19 d | 18.17 ± 1.38 f | 707.08 ± 94.44 b | 3.93 ± 0.01 d,e | 58.70 ± 0.17 c,d | 219.79 ± 1.67 e | 13.76 ± 0.39 e | 28.44 ± 0.61 g | 48.79 ± 0.25 e | 2.91 ± 0.01 d,c | 1.06 ± 0.01 d,c |
| PSF | 15.16 ± 0.10 a | 3.04 ± 0.02 a | 21.34 ± 0.12 e | 326.26 ± 0.01 a | 4.73 ± 0.09 g | 42.99 ± 1.00 a | 101.00 ± 0.01 a | 56.02 ± 0.69 f | 67.49 ± 0.20 i | 77.91 ± 0.24 j | 3.64 ± 0.07 g | 1.32 ± 0.03 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, N.; Mou, H.; Zhou, Y.; Ju, X.; Yang, S.; Liu, S.; Dong, R. Upgrading Solid Digestate from Anaerobic Digestion of Agricultural Waste as Performance Enhancer for Starch-Based Mulching Biofilm. Molecules 2021, 26, 832. https://doi.org/10.3390/molecules26040832
Zhao N, Mou H, Zhou Y, Ju X, Yang S, Liu S, Dong R. Upgrading Solid Digestate from Anaerobic Digestion of Agricultural Waste as Performance Enhancer for Starch-Based Mulching Biofilm. Molecules. 2021; 26(4):832. https://doi.org/10.3390/molecules26040832
Chicago/Turabian StyleZhao, Nan, Huawei Mou, Yuguang Zhou, Xinxin Ju, Shoujun Yang, Shan Liu, and Renjie Dong. 2021. "Upgrading Solid Digestate from Anaerobic Digestion of Agricultural Waste as Performance Enhancer for Starch-Based Mulching Biofilm" Molecules 26, no. 4: 832. https://doi.org/10.3390/molecules26040832
APA StyleZhao, N., Mou, H., Zhou, Y., Ju, X., Yang, S., Liu, S., & Dong, R. (2021). Upgrading Solid Digestate from Anaerobic Digestion of Agricultural Waste as Performance Enhancer for Starch-Based Mulching Biofilm. Molecules, 26(4), 832. https://doi.org/10.3390/molecules26040832