Prospects and Challenges of Microwave-Combined Technology for Biodiesel and Biolubricant Production through a Transesterification: A Review
Abstract
1. Introduction
2. Recent Stand-Alone Microwave Applications
3. Limitations of Stand-Alone Microwave Technology
4. Intensification of Microwave-Combined Reactor Technology
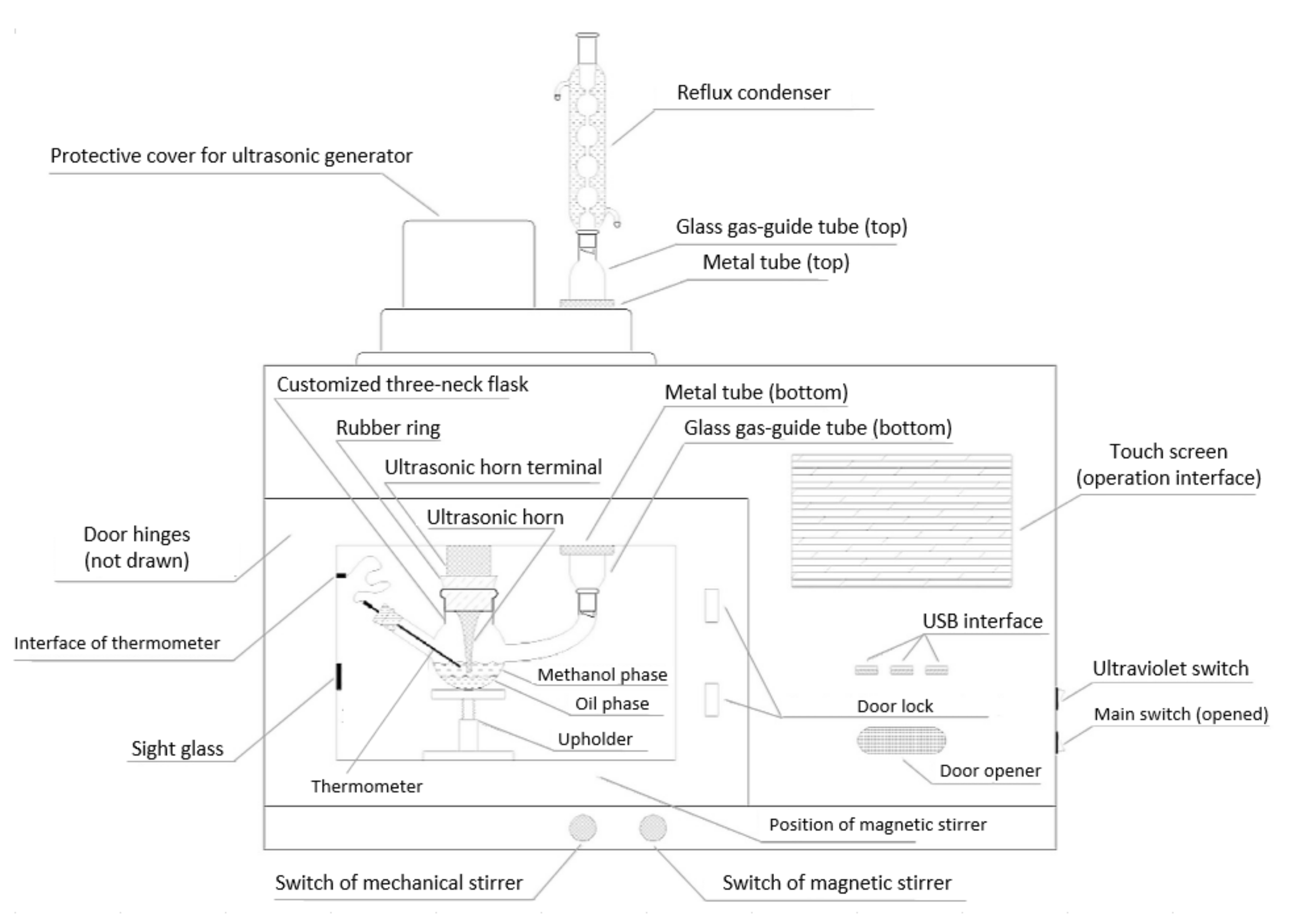
4.1. Simultaneous Microwave and Acoustic Cavitation Technology
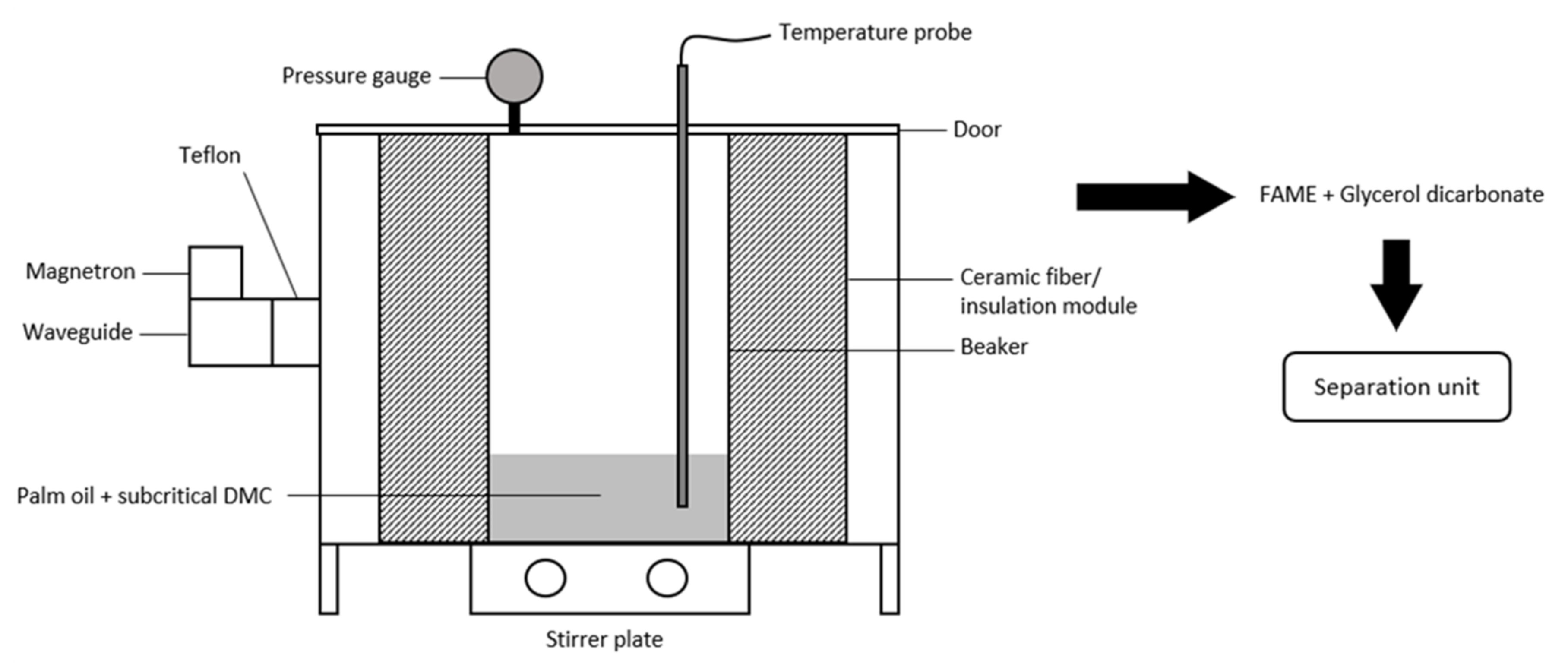
4.2. Simultaneous Microwave and Supercritical/ Subcritical Approach
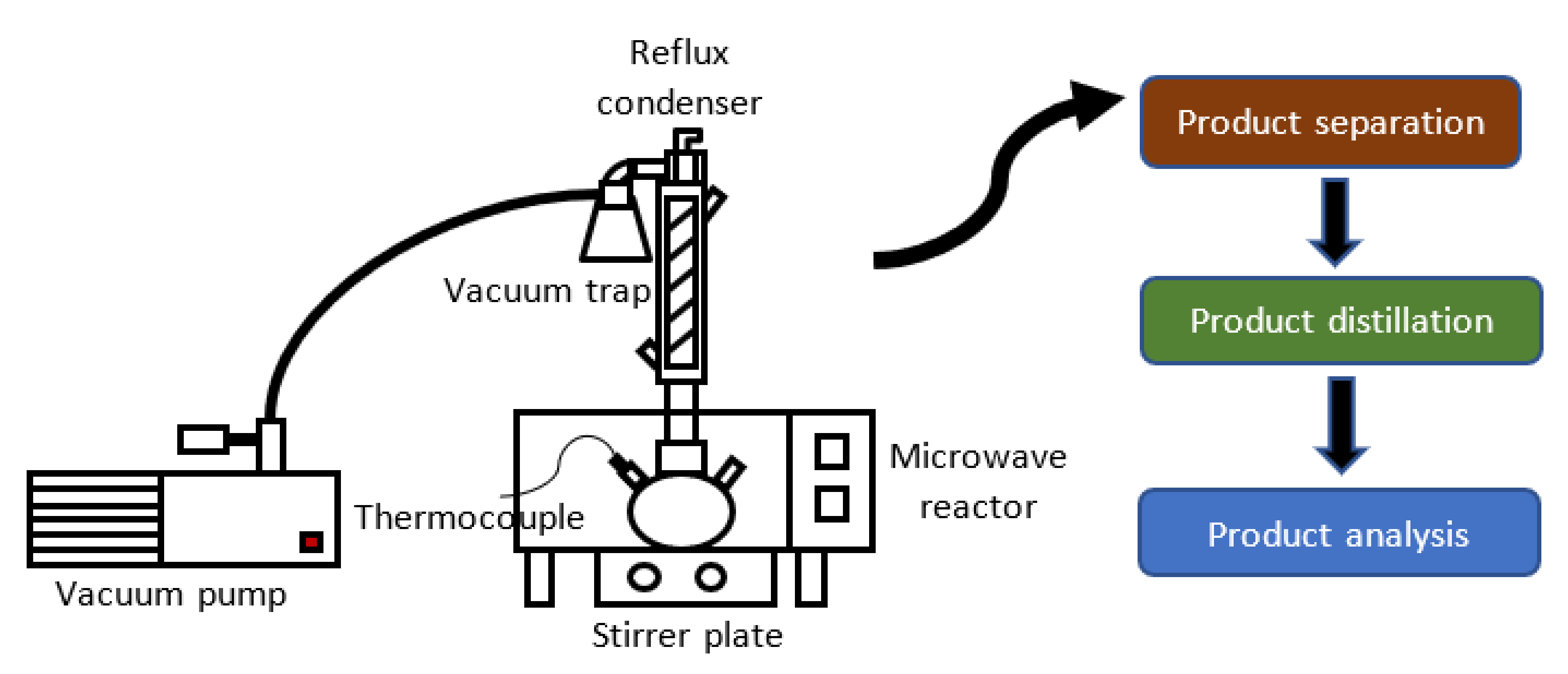
4.3. Vacuum-Microwave Combined Technology
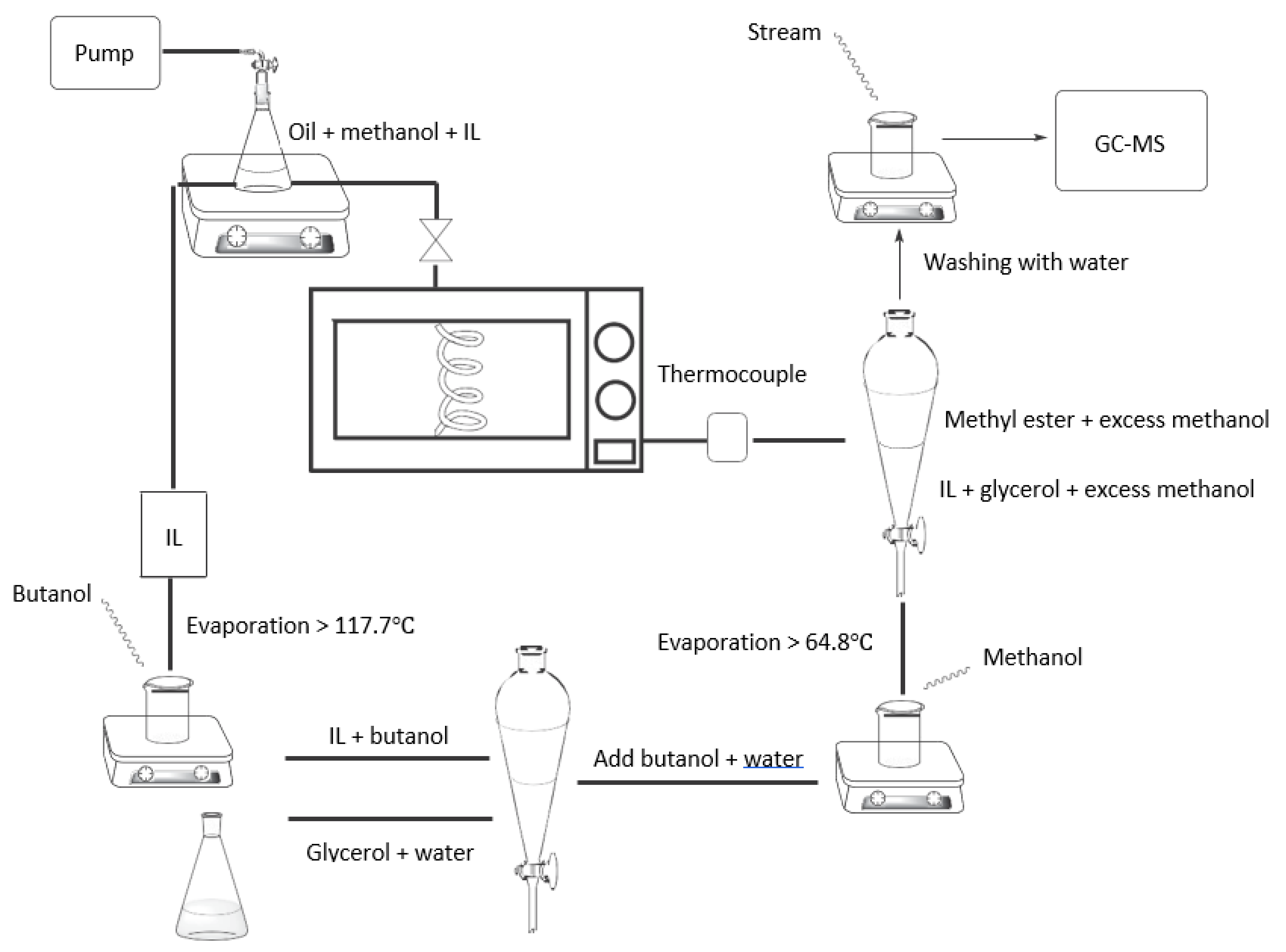
4.4. Simultaneous Microwave and Ionic Solvent Approach
5. Prospects and Challenges of Microwave-Combined Technology
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; An, Y.; Borrion, A.; He, W.; Wang, N.; Chen, Y.; Li, G. Process characteristics for microwave assisted hydrothermal carbonization of cellulose. Bioresour. Technol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; De Bruyn, M.; Budarin, V.L.; Gronnow, M.J.; Shuttleworth, P.S.; Breeden, S.; Macquarrie, D.J.; Clark, J.H. Direct microwave-assisted hydrothermal depolymerization of cellulose. J. Am. Chem. Soc. 2013. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz, A.; Díaz-Ortiz, À.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.A.; Romero-Sierra, C.; Davie, S.J. Non-thermal effects of microwave radiation on birds. Nature 1967, 216, 1139. [Google Scholar] [CrossRef]
- Kesari, V.; Basu, B.N. Epilogue. In High Power Microwave Tubes: Basics and Trends. Morgan & Claypool Publishers: San Rafael, CA, USA, 2018; Volume 2, pp. 10–17. ISBN 978-1-6817-4561-9. [Google Scholar]
- Dudley, G.B.; Richert, R.; Stiegman, A.E. On the existence of and mechanism for microwave-specific reaction rate enhancement. Chem. Sci. 2015, 6, 2144–2152. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave material processing-A review. AIChE J. 2012, 58, 330–363. [Google Scholar] [CrossRef]
- Tangy, A.; Pulidindi, I.N.; Perkas, N.; Gedanken, A. Continuous flow through a microwave oven for the large-scale production of biodiesel from waste cooking oil. Bioresour. Technol. 2017, 224, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Fernández, Y.; Arenillas, A.; Bermúdez, J.M.; Menéndez, J.A. Comparative study of conventional and microwave-assisted pyrolysis, steam and dry reforming of glycerol for syngas production, using a carbonaceous catalyst. J. Anal. Appl. Pyrolysis 2010, 88, 155–159. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Microwave pyrolysis, a novel process for recycling waste automotive engine oil. Energy 2010, 35, 2985–2991. [Google Scholar] [CrossRef]
- Omar, R.; Robinson, J.P. Conventional and microwave-assisted pyrolysis of rapeseed oil for bio-fuel production. J. Anal. Appl. Pyrolysis 2014, 105, 131–142. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Vadivambal, R.; Jayas, D.S. Non-uniform temperature distribution during microwave heating of food materials-A review. Food Bioprocess Technol. 2010, 3, 161–171. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, D.; Jain, V.; Sharma, A.K. Microwave processing of materials and applications in manufacturing industries: A review. Mater. Manuf. Process. 2015, 30, 1–29. [Google Scholar] [CrossRef]
- Abdelmoez, W.; Ashour, E.; Naguib, S.M. A Review on green trend for oil extraction using subcritical water technology and biodiesel production. J. Oleo Sci. 2015, 64, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Martinez-guerra, E.; Gude, V.G.; Mondala, A.; Holmes, W.; Hernandez, R. Microwave and ultrasound enhanced extractive-transesterification of algal lipids. Appl. Energy. 2014, 129, 354–363. [Google Scholar] [CrossRef]
- Debowski, M.; Zielinski, M.; Kisielewska, M.; Kazimierowicz, J. Evaluation of anaerobic digestion of dairy wastewater in an innovative multi-section horizontal flow reactor. Energies 2020, 13, 2392. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Rusanowska, P. Influence of microwave heating on biogas production from Sida hermaphrodita silage. Bioresour. Technol. 2017. [Google Scholar] [CrossRef]
- Zieliński, M.; Kisielewska, M.; Dudek, M.; Rusanowska, P.; Nowicka, A.; Krzemieniewski, M.; Kazimierowicz, J.; Dębowski, M. Comparison of microwave thermohydrolysis and liquid hot water pretreatment of energy crop Sida hermaphrodita for enhanced methane production. Biomass Bioenergy 2019. [Google Scholar] [CrossRef]
- Nayak, S.N.; Bhasin, C.P.; Nayak, M.G. A review on microwave-assisted transesterification processes using various catalytic and non-catalytic systems. Renew. Energy 2019. [Google Scholar] [CrossRef]
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907. [Google Scholar] [CrossRef]
- Arniza, M.Z.; Hoong, S.S.; Idris, Z.; Yeong, S.K.; Hassan, H.A.; Din, A.K.; Choo, Y.M. Synthesis of transesterified palm olein-based Polyol and rigid polyurethanes from this polyol. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Mazo, P.; Rios, L.; Estenoz, D.; Sponton, M. Self-esterification of partially maleated castor oil using conventional and microwave heating. Chem. Eng. J. 2012, 185–186, 347–351. [Google Scholar] [CrossRef]
- Campos, D.C.; Dall’Oglio, E.L.; de Sousa, P.T., Jr.; Vasconcelos, L.G.; Kuhnen, C.A. Investigation of dielectric properties of the reaction mixture during the acid-catalyzed transesterification of Brazil nut oil for biodiesel production. Fuel 2014, 117, 957–965. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. 14-Lubricant use and disposal. In Woodhead Publishing Series in Energy; Bart, J.C.J., Gucciardi, E., Cavallaro, S.B.T.-B., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 755–823. ISBN 978-0-85709-263-2. [Google Scholar]
- Guan, L.; Feng, X.L.; Xiong, G.; Xie, J. Application of dielectric spectroscopy for engine lubricating oil degradation monitoring. Sens. Actuators A Phys. Sens. 2011, 168, 22–29. [Google Scholar] [CrossRef]
- Kappe, C.O. Microwave dielectric heating in synthetic organic chemistry. Chem. Soc. Rev. 2008, 37, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Lidstrom, P.; Tierney, J.P.; Wathey, B.; Westman, J. Microwave assisted organic synthesis-A review. Tetrahedron 2005, 57, 9225–9283. [Google Scholar] [CrossRef]
- Man, A.K.; Shahidan, R. Microwave-assisted chemical reactions. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 651–657. [Google Scholar] [CrossRef]
- Peixoto, C.; Silva, J.; Maria, L.; Dutra, U.; Pacheco, A.; Queiroz, J.; Murilo, F.; Luna, T.; Silva, P. Chemical modification of Tilapia oil for biolubricant applications. J. Clean. Prod. 2018, 191, 158–166. [Google Scholar] [CrossRef]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H. Microwave-assisted methyl ester production from palm fatty acid distillate over a heterogeneous carbon-based solid acid catalyst. Chem. Eng. Technol. 2015, 38, 1837–1844. [Google Scholar] [CrossRef]
- Sajjadi, B.; Abdul Aziz, A.R.; Ibrahim, S. Investigation, modelling and reviewing the effective parameters in microwave-assisted transesterification. Renew. Sustain. Energy Rev. 2014, 37, 762–777. [Google Scholar] [CrossRef]
- Chuah, L.F.; Yusup, S.; Abd Aziz, A.R.; Bokhari, A.; Klemeš, J.J.; Abdullah, M.Z. Intensification of biodiesel synthesis from waste cooking oil (palm olein) in a hydrodynamic cavitation reactor: Effect of operating parameters on methyl ester conversion. Chem. Eng. Process. Process Intensif. 2015, 95, 235–240. [Google Scholar] [CrossRef]
- Yunus, R.; Fakhru’I-Razi, A.; Ooi, T.L.; Biak, D.R.A.; Iyuke, S.E. Kinetics of transesterification of palm-based methyl esters with trimethylolpropane. J. Am. Oil Chem. Soc. 2004, 81, 497–503. [Google Scholar] [CrossRef]
- Esipovich, A.L.; Rogozhin, A.E.; Belousov, A.S.; Kanakov, E.A.; Danov, S.M. A comparative study of the separation stage of rapeseed oil transesteri fi cation products obtained using various catalysts. Fuel Process. Technol. 2018, 173, 153–164. [Google Scholar] [CrossRef]
- Kouzu, M.; Fujimori, A.; Fukakusa, R.; Satomi, N.; Yahagi, S. Continuous production of biodiesel by the CaO-catalyzed transesterification operated with continuously stirred tank reactor. Fuel Process. Technol. 2018, 181, 311–317. [Google Scholar] [CrossRef]
- Pisarello, L.M.; Maquirriain, M.; Olalla, P.S.; Rossi, V.; Querini, C.A. Biodiesel production by transesteri fi cation in two steps: Kinetic effect or shift in the equilibrium conversion? Fuel Process. Technol. 2018, 181, 244–251. [Google Scholar] [CrossRef]
- Wu, L.; Wei, T.; Tong, Z.; Zou, Y.; Lin, Z.; Sun, J. Bentonite-enhanced biodiesel production by NaOH-catalyzed transesteri fi cation of soybean oil with methanol. Fuel Process. Technol. 2016, 144, 334–340. [Google Scholar] [CrossRef]
- Kamil, R.N.M.; Yusup, S.; Rashid, U. Optimization of polyol ester production by transesterification of Jatropha-based methyl ester with trimethylolpropane using Taguchi design of experiment. Fuel 2011, 90, 2343–2345. [Google Scholar] [CrossRef]
- Padmaja, K.V.; Rao, B.V.S.K.; Reddy, R.K.; Bhaskar, P.S.; Singh, A.K.; Prasad, R.B.N. 10-Undecenoic acid-based polyol esters as potential lubricant base stocks. Ind. Crops Prod. 2012, 35, 237–240. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, R.; Li, T.; Zhou, J.; Cen, K. Biodiesel from wet microalgae: Extraction with hexane after the microwave-assisted transesterification of lipids. Bioresour. Technol. 2014, 170, 69–75. [Google Scholar] [CrossRef]
- Zhang, S.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Zhang, D.Y.; Efferth, T. Rapid microwave-assisted transesterification of yellow horn oil to biodiesel using a heteropolyacid solid catalyst. Bioresour. Technol. 2010, 101, 931–936. [Google Scholar] [CrossRef]
- Happe, M.; Ellert, C.; Aeby, S.; Zahno, S.; Mabillard, E.; Héritier, J.-C.; Fischer, F.; Grand, P.; Corthay, F.; Tièche, F.; et al. Microwave barrel reactor use in trimethylolpropane oleate synthesis by Candida antarctica lipase in a biphasic non-solvent process. Green Chem. 2012, 14, 2337. [Google Scholar] [CrossRef]
- Yadav, G.D.; Hude, M.P.; Talpade, A.D. Microwave assisted process intensification of lipase catalyzed transesterification of 1,2 propanediol with dimethyl carbonate for the green synthesis of propylene carbonate: Novelties of kinetics and mechanism of consecutive reactions. Chem. Eng. J. 2015, 281, 199–208. [Google Scholar] [CrossRef]
- Azcan, N.; Yilmaz, O. Microwave assisted transesterification of waste frying oil and concentrate methyl ester content of biodiesel by molecular distillation. Fuel 2013, 104, 614–619. [Google Scholar] [CrossRef]
- Guldhe, A.; Singh, B.; Rawat, I.; Bux, F. Synthesis of biodiesel from Scenedesmus sp. by microwave and ultrasound assisted in situ transesterification using tungstated zirconia as a solid acid catalyst. Chem. Eng. Res. Des. 2014, 92, 1503–1511. [Google Scholar] [CrossRef]
- Hincapié, G.M.; Valange, S.; Barrault, J.; Moreno, J.A.; López, D.P. Effect of microwave-assisted system on transesterification of castor oil with ethanol. Univ. Sci. 2014, 19, 193–200. [Google Scholar] [CrossRef]
- Kumar, R.; Ravi Kumar, G.; Chandrashekar, N. Microwave assisted alkali-catalyzed transesterification of Pongamia pinnata seed oil for biodiesel production. Bioresour. Technol. 2011, 102, 6617–6620. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel 2011, 90, 1963–1967. [Google Scholar] [CrossRef]
- Tippayawong, N.; Sittisun, P. Continuous-flow transesterification of crude jatropha oil with microwave irradiation. Sci. Iran. 2012, 19, 1324–1328. [Google Scholar] [CrossRef]
- Mazzocchia, C.; Modica, G.; Kaddouri, A.; Nannicini, R. Fatty acid methyl esters synthesis from triglycerides over heterogeneous catalysts in the presence of microwaves. Comptes Rendus Chim. 2004, 7, 601–605. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Ansori, A.; Wibowo, S.; Bhuana, D.S.; Mahfud, M. Optimization of transesterification process of biodiesel from nyamplung (Calophyllum inophyllum Linn) using microwave with CaO catalyst optimization of transesterification process of biodiesel from nyamplung (Calophyllum inophyllum Linn) using Microwa. Korean Chem. Eng. Res. 2018, 56, 435–440. [Google Scholar] [CrossRef]
- Porter, M.; Scott, J. Microwave-enhanced process to maximize biodiesel production capacity. U.S. Patent Application No. 11/340,137, 3 August 2006. Available online: https://www.lens.org/lens/patent/012-964-764-949-921 (accessed on 1 February 2021).
- Dall’Oglio, E.; Garofalo, M.; Souza, P. Biodiesel production process using vegetal oils or animal fat and induction through microwaves. U.S. Patent Application No. 11/407,621, 25 October 2007. Available online: https://www.lens.org/lens/patent/060-863-280-373-223 (accessed on 1 February 2021).
- Meikrantz, D.H. Microwave-enhanced biodiesel method and apparatus. U.S. Patent Application No. 11/737,809, 23 October 2008. Available online: https://www.lens.org/lens/patent/062-737-925-079-925 (accessed on 1 February 2021).
- De La Hoz, A.; Alczar, J.; Jos, A.M.; de Muoz, J.M.; Prieto, P.; De, A.; Diaz-Ortiz, A. Reproducibility and Scalability of microwave-assisted reactions. In Microwave Heating; Chandra, A., Ed.; IntechOpen: London, UK, 2011; ISBN 978-953-307-573-0. [Google Scholar]
- Nomanbhay, S.; Ong, M.Y. A Review of microwave-assisted reactions for biodiesel production. Bioengineering 2017, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.A.M.; Yunus, R.; Hamid, H.A.; Ghassan, A.A.K.; Omar, R.; Rashid, U.; Abbas, Z. An acceleration of microwave-assisted transesterification of palm oil-based methyl ester into trimethylolpropane ester. Sci. Rep. 2020, 10, 19652. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G.; Patil, P.; Martinez-Guerra, E.; Deng, S.; Nirmalakhandan, N. Microwave energy potential for biodiesel production. Sustain. Chem. Process. 2013, 1, 5. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Basak, T. On the analysis of microwave power and heating characteristics for food processing: Asymptotes and resonances. Food Res. Int. 2006, 39, 1046–1057. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Chase, H.A. Catalytic microwave pyrolysis of waste engine oil using metallic pyrolysis char. Appl. Catal. B Environ. 2015, 176–177, 601–617. [Google Scholar] [CrossRef]
- Mazo, P.C.; Rios, L.A. Esterification and transesterification assisted by microwaves of crude palm oil. Homogeneous catalysis. Lat. Am. Appl. Res. 2010, 40, 337–342. [Google Scholar]
- Farag, S.; Chaouki, J. A modified microwave thermo-gravimetric-analyzer for kinetic purposes. Appl. Therm. Eng. 2015, 75, 65–72. [Google Scholar] [CrossRef]
- Zhu, H.; He, J.; Hong, T.; Yang, Q.; Wu, Y.; Yang, Y.; Huang, K. A rotary radiation structure for microwave heating uniformity. Appl. Therm. Eng. 2018, 141, 648–658. [Google Scholar] [CrossRef]
- Ameta, S.C.; Punjabi, P.B.; Ameta, R.; Ameta, C. (Eds.) Microwave-assisted organic synthesis, 1st ed.; Apple Academic Press, Inc.: New York, NY, USA, 2014. [Google Scholar]
- Hoogenboom, R.; Wilms, T.F.A.; Erdmenger, T.; Schubert, U.S. Microwave-assisted chemistry: A closer look at heating efficiency. Aust. J. Chem. 2009, 62, 236–243. [Google Scholar] [CrossRef]
- Kremsner, J.M.; Kappe, C.O. Silicon carbide passive heating elements in microwave-assisted organic synthesis. J. Org. Chem. 2006, 71, 4651–4658. [Google Scholar] [CrossRef]
- Dominguez, A.; Fernández, Y.; Fidalgo, B.; Pis, J.J.; Menéndez, J.A. Biogas to Syngas by microwave-assisted reforming in the presence of char. Energy Fuels 2017, 21, 2066–2071. [Google Scholar] [CrossRef]
- Yu, G.-W.; Nie, J.; Lu, L.-G.; Wang, S.-P.; Li, Z.-G.; Lee, M.-R. Transesterification of soybean oil by using the synergistic microwave-ultrasonic irradiation. Ultrason. Sonochem. 2017, 39, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.W.S.; Ng, H.K.; Gan, S. Advances in ultrasound-assisted transesterification for biodiesel production. Appl. Therm. Eng. 2016, 100, 553–563. [Google Scholar] [CrossRef]
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. A review of cleaner intensification technologies in biodiesel production. J. Clean. Prod. 2017, 146, 181–193. [Google Scholar] [CrossRef]
- Cheeke, J.D.N. Fundamentals and Applications of Ultrasonic Waves; CRC Press: Boca Raton, FL, USA, 2017; ISBN ISBN 9781351833196. [Google Scholar]
- Oliveira, P.A. Ultrasound Methods for Biodiesel Production and Analysis; Baesso, R.M., Ed.; IntechOpen: Rijeka, Croatia, 2018; p. 7. ISBN 978-1-78923-347-6. [Google Scholar]
- Yasui, K. Fundamentals of Acoustic Cavitation and Sonochemistry BT-Theoretical and Experimental Sonochemistry Involving Inorganic Systems; Ashokkumar, M., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2011; pp. 1–29. ISBN 978-90-481-3887-6. [Google Scholar]
- Pecha, R.; Gompf, B. Microimplosions: Cavitation collapse and shock wave emission on a nanosecond time scale. Phys. Rev. Lett. 2000, 84, 1328–1330. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of synthesis of biodiesel from non-edible oil using sequential combination of microwave and ultrasound. Fuel Process. Technol. 2013, 106, 62–69. [Google Scholar] [CrossRef]
- Ardebili, S.M.S.; Hashjin, T.T.; Ghobadian, B.; Najafi, G.; Mantegna, S.; Cravotto, G. Optimization of biodiesel synthesis under simultaneous ultrasound-microwave irradiation using response surface methodology (RSM). Green Process. Synth. 2015, 4, 259–267. [Google Scholar] [CrossRef]
- Martinez-guerra, E.; Gude, V.G. Chemical engineering and processing: Process intensification alcohol effect on microwave-ultrasound enhanced transesterification reaction. Chem. Eng. Process. Process Intensif. 2016, 101, 1–7. [Google Scholar] [CrossRef]
- Martinez-Guerra, E.; Gude, V.G. Synergistic effect of simultaneous microwave and ultrasound irradiations on transesterification of waste vegetable oil. Fuel 2014, 137, 100–108. [Google Scholar] [CrossRef]
- Gholami, A.; Hajinezhad, A.; Pourfayaz, F.; Ahmadi, M.H. The effect of hydrodynamic and ultrasonic cavitation on biodiesel production: An exergy analysis approach. Energy 2018. [Google Scholar] [CrossRef]
- Patience, N.A.; Galli, F.; Rigamonti, M.G.; Schieppati, D.; Boffito, D.C. Ultrasonic intensification to produce diester biolubricants. Ind. Eng. Chem. Res. 2019, 58, 7957–7963. [Google Scholar] [CrossRef]
- Bokhari, A.; Chuah, L.F.; Yusup, S.; Klemeš, J.J.; Akbar, M.M.; Kamil, R.N.M. Cleaner production of rubber seed oil methyl ester using a hydrodynamic cavitation: Optimisation and parametric study. J. Clean. Prod. 2016, 136, 31–41. [Google Scholar] [CrossRef]
- Maddikeri, G.L.; Gogate, P.R.; Pandit, A.B. Intensified synthesis of biodiesel using hydrodynamic cavitation reactors based on the interesterification of waste cooking oil. Fuel 2014, 137, 285–292. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Valorisation of high acid value waste cooking oil into biodiesel using supercritical methanolysis: Experimental assessment and statistical optimisation on typical Egyptian feedstock. Energy 2018, 162, 408–420. [Google Scholar] [CrossRef]
- Ong, M.Y.; Chew, K.W.; Show, P.L.; Nomanbhay, S. Optimization and kinetic study of non-catalytic transesterification of palm oil under subcritical condition using microwave technology. Energy Convers. Manag. 2019, 196, 1126–1137. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Tran-Nguyen, P.L.; Ismadji, S.; Gunawan, S.; Ju, Y.H. Biodiesel production under subcritical solvent condition using subcritical water treated whole Jatropha curcas seed kernels and possible use of hydrolysates to grow Yarrowia lipolytica. Fuel 2014, 120, 46–52. [Google Scholar] [CrossRef]
- Saka, S.; Isayama, Y. A new process for catalyst-free production of biodiesel using supercritical methyl acetate. Fuel 2009, 88, 1307–1313. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y. State of the art of biodiesel production under supercritical conditions. Prog. Energy Combust. Sci. 2017, 63, 173–203. [Google Scholar] [CrossRef]
- Saka, S.; Isayama, Y.; Ilham, Z.; Jiayu, X. New process for catalyst-free biodiesel production using subcritical acetic acid and supercritical methanol. Fuel 2010, 89, 1442–1446. [Google Scholar] [CrossRef]
- Wei, C.; Huang, T.; Chen, H. Biodiesel production using supercritical methanol with carbon dioxide and acetic acid. Hindawi 2013, 2013. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Optimization of supercritical dimethyl carbonate method for biodiesel production. Fuel 2012, 97, 670–677. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T.; Mohamed, A.R. Optimization of supercritical dimethyl carbonate (SCDMC) technology for the production of biodiesel and value-added glycerol carbonate. Fuel 2010, 89, 3833–3839. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Patil, P.D.; Reddy, H.; Muppaneni, T.; Schaub, T.; Holguin, F.O.; Cooke, P.; Lammers, P.; Nirmalakhandan, N.; Li, Y.; Lu, X.; et al. In situ ethyl ester production from wet algal biomass under microwave-mediated supercritical ethanol conditions. Bioresour. Technol. 2013, 139, 308–315. [Google Scholar] [CrossRef]
- Mani Rathnam, V.; Madras, G. Conversion of Shizochitrium limacinum microalgae to biodiesel by non-catalytic transesterification using various supercritical fluids. Bioresour. Technol. 2019. [Google Scholar] [CrossRef]
- Xie, Q.; Cai, L.; Xia, F.; Liang, X.; Zhenyu, W.; Liu, Y.; Li, X.; Lu, M.; Nie, Y.; Ji, J. High vacuum distillation for low-sulfur biodiesel production: From laboratory to large scale. J. Clean. Prod. 2019, 223. [Google Scholar] [CrossRef]
- Tong, F.; Deng, L.; Sun, R.; Zhong, G. Effect of octenyl succinic anhydride starch ester by semi-dry method with vacuum-microwave assistant. Int. J. Biol. Macromol. 2019, 141, 1128–1136. [Google Scholar] [CrossRef]
- Özgül-Yücel, S.; Türkay, S. Variables affecting the yields of methyl esters derived from in situ esterification of rice bran oil. J. Am. Oil Chem. Soc. 2002, 79, 611–614. [Google Scholar] [CrossRef]
- Ookjantra, K.; Wongwuttanasatian, T. Optimisation of biodiesel production from refined palm oil with heterogeneous CaO catalyst using pulse ultrasonic waves under a vacuum condition. Energy Convers. Manag. 2017, 154, 1–10. [Google Scholar] [CrossRef]
- Khalfan, H.; Dwivedi, P. Microwave assisted trans-esterification of waste cooking oil in presence of alkali catalyst. Int. J. Students Res. Technol. Manag. 2014, 2, 145–148. [Google Scholar]
- Bahrani, S.A.; Loisel, C.; Rezzoug, S.-A.; Doublier, J.-L.; Maache-Rezzoug, Z. Role of vacuum steps added before and after steaming treatment of maize starch. Impact on pasting, morphological and rheological properties. Carbohydr. Polym. 2012, 89, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Phromphithak, S.; Meepowpan, P.; Shimpalee, S.; Tippayawong, N. Transesterification of palm oil into biodiesel using ChOH ionic liquid in a microwave heated continuous flow reactor. Renew. Energy 2020, 154, 925–936. [Google Scholar] [CrossRef]
- Balaraman, H.B.; Rathnasamy, S.K. Kinetics and microwave-assisted extractive transesterification studies of high octane methyl esters (HOME) from karanja and chicken lard oil using protic deep eutectic solvent. Fuel 2020, 268, 117299. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Li, K.; Zhang, H.; Fan, L.; Lu, Z. Efficient production of biodiesel from ionic liquid catalyzed esterification using ultrasonic-microwave combined intensification. Chem. Eng. Process. Process Intensif. 2020, 149, 107870. [Google Scholar] [CrossRef]
- Ding, H.; Ye, W.; Wang, Y.; Wang, X.; Li, L.; Liu, D.; Gui, J.; Song, C.; Ji, N. Process intensification of transesterification for biodiesel production from palm oil: Microwave irradiation on transesterification reaction catalyzed by acidic imidazolium ionic liquids. Energy 2018. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Yusof, N.M.; Kamis, N.H.H.; Shaleh, S.R.M. Optimization of the ionic liquid-microwave assisted one-step biodiesel production process from wet microalgal biomass. Energy Convers. Manag. 2018, 171, 1397–1404. [Google Scholar] [CrossRef]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. Ionic liquid as a promising biobased green solvent in combination with microwave irradiation for direct biodiesel production. Bioresour. Technol. 2016, 206, 150–154. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Plechkova, N.V.; Fabregat, A.; Stüber, F.; Fortuny, A.; Font, J.; Bengoa, C. Efficient extraction of lipids from primary sewage sludge using ionic liquids for biodiesel production. Sep. Purif. Technol. 2015, 153, 118–125. [Google Scholar] [CrossRef]
- Lin, Y.C.; Chen, S.C.; Wu, T.Y.; Yang, P.M.; Jhang, S.R.; Lin, J.F. Energy-saving and rapid transesterification of jatropha oil using a microwave heating system with ionic liquid catalyst. J. Taiwan Inst. Chem. Eng. 2015, 49, 72–78. [Google Scholar] [CrossRef]
- Lin, Y.C.; Yang, P.M.; Chen, S.C.; Lin, J.F. Improving biodiesel yields from waste cooking oil using ionic liquids as catalysts with a microwave heating system. Fuel Process. Technol. 2013, 115, 57–62. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. The combined use of microwaves and ultrasound: Improved tools in process chemistry and organic synthesis. Chem. A Eur. J. 2007, 13, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Kiss, A.A.; Geertman, R.; Wierschem, M.; Skiborowski, M.; Gielen, B.; Jordens, J.; John, J.J.; Van Gerven, T. Ultrasound-assisted emerging technologies for chemical processes. J. Chem. Technol. Biotechnol. 2018, 93, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, K.; Idlan, M.K.; Saifudin, N. Preliminary evaluation of the effectiveness of moisture removal and energy usage in pretreatment module of waste cooking oil for biodiesel production. IOP Conf. Ser. Earth Environ. Sci. 2013, 16, 12053. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Dokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Gogate, P.R.; Mujumdar, S.; Pandit, A.B. Large-scale sonochemical reactors for process intensification: Design and experimental validation. J. Chem. Technol. Biotechnol. 2003, 78, 685–693. [Google Scholar] [CrossRef]
- Rybicka, J.; Tiwari, A.; Leeke, G.A. Technology readiness level assessment of composites recycling technologies. J. Clean. Prod. 2016, 112, 1001–1012. [Google Scholar] [CrossRef]

| Features | Conventional | Microwave |
|---|---|---|
| Reaction time | Longer processing times | Very short, instant heating |
| Solvent requirement | No or low solvent | No or low solvent |
| Product quality | Vary | Better |
| Product yield | Vary | Higher |
| Separation time | Long time | Very short time |
| Temperature (°C) | Catalyst | Molar Ratio Reactant | Product | Yield (%) | Conversion (%) | Reference |
|---|---|---|---|---|---|---|
| 60 | 1% Sodium methoxide | Oil:methanol (1:6) | Methyl ester | 99.7 in 5 min | 98.9 | [45] |
| 60 | 1.5% KOH | Oil:ethanol (1:10) | Ethyl ester | 80.1 in 10 min | NA | [47] |
| 60 | 0.5% NaOH and 1% KOH | Oil:methanol (1:6) | Methyl ester | 97.5 in 10 min | NA | [48] |
| 60 | Heteropoly acid | Yellow horn oil:methanol (1:12) | Methyl ester | 96.2 in 10 min | NA | [42] |
| 65 | 3% Calcium oxide | Soybean oil:methanol (1:7) | Methyl ester | NA | 96.6 in 60 min | [49] |
| 70 | 250 mg Novozym 435 | 1,2 propanediol:dimethyl carbonate (1:3) | Propylene carbonate | NA | 93 in 6 h | [44] |
| 80 | 4% Tungstated zirconia | Oil:methanol (1:45) | Methyl ester | NA | 51.94 in 20 min | [46] |
| 90 | 3% H2SO4 | Oil:methanol (1:8) | Methyl ester | 86.74 in 30 min | NA | [41] |
| 120 | 1.0% Sodium methoxide | Oil:methanol (1:6) | Methyl ester | NA | 96 in 30 s | [50] |
| 170 | 10% Montmorillonite KSF | Rapeseed oil:methanol (1:18) | Methyl ester | 57 in 60 min | NA | [51] |
| Not mentioned | 4.86% Calcium oxide | Nyamplung oil:methanol | Methyl ester | 93 in 10 min | NA | [52] |
| Issues | References |
|---|---|
| Arching | [61,63] |
| Overheating and explosion | [62] |
| Expensive fiber optic thermocouple and difficult measurement of block temperature | [63] |
| Non-uniform heating, hotspot | [7,61,63,64] |
| Scalability, limited field of applications and health hazard | [65] |
| Material | Operating Conditions | Ester Content | Energy Assessment | Reference |
|---|---|---|---|---|
| Nagchampa oil Catalyst: Potassium hydroxide | 15 min (esterification) and 6 min (transesterification), 100–140 W (MW) 20 kHz 120 W (US) and molar ratio of 1:2 | NA | 2.5 × 102 kJ/kg | [76] |
| Waste oils Catalyst: Sodium hydroxide, NaOH | 2 min, 300 W (MW-US) and molar ratio of 9:1 (alcohol to oil) | 98% biodiesel yield | NA | [78] |
| Palm oil Catalyst: KOH | 2.2 min, molar ratio of 7:3.1 methanol/oil, 58.4 °C | 97.5% biodiesel yield | 0.36 MJ/L | [77] |
| Soybean oil Catalyst: KOH | 6 min, 65 °C and 700 W (MW) 800 W (US) | 98.0% FAME yield | NA | [69] |
| Material | Operating Condition | Ester Content | Energy Assessment | Reference |
|---|---|---|---|---|
| Palm oil Solvent: dimethyl carbonate | DMC to oil 9.5:1 167 °C 2.5 h, 5 bar | 86% yield | Microwave-assisted Ea: 44.88 kJ/mol | [85] |
| Waste cooking oil Solvent: methanol | Methanol to oil 37:1 253.5 °C, 198.5 bar, 14.8 min | 91% yield | Without microwave Ea: 50.5 kJ/mol | [84] |
| Wet whole kernel Solvent: subcritical methanol and acetic acid | 1st step: supercritical water treatment on kernel 488 K, 2 MPa, 15 min 2nd step: subcritical methanol and acetic acid 523 K (250 °C), 13 MPa, 105 min | 65.1% yield | Without microwave (2-step approach) | [86] |
| Algae Solvent: ethanol | 1:9 (wt./vol) ratio of ethanol, 265 °C, 80 bar, 20 min | 30% yield | Microwave-assisted Heat energy supplied: 1560 kJ Thermal energy associated with sample volume with wet algal: 35.03 kJ | [94] |
| Microalgae Solvent: methanol, dimethyl carbonate and methyl acetate | Methylating agent to algae 10:1 518 to 643 K, 20 MPa, 10 to 80 min | >90% (methanol) 50% (DMC) 40% (methylacetate) | Without microwave | [95] |
| Rapeseed oil Solvent: dimethyl carbonate | DMC to oil 42:1 300 °C, 20 MPa, 20 min | 97.4% yield | Without microwave | [91] |
| Soybean oil Solvent: supercritical methanol, carbon dioxide and acetic acid | Methanol to oil 60: 1, acetic acid to oil 3:1 280 °C, 20 MPa, 90 min | 97.8% yield | Without microwave | [90] |
| Rapeseed oil Solvent: subcritical acetic acid and supercritical methanol | 1st step: acetic acid to oil 54:1 300 °C, 20 MPa, 30 min treatment 2nd step: reactant to methanol 1:1.6 270 °C, 17 MPa, 15 min | 97% yield | Without microwave (2-step approach) | [89] |
| Palm oil Solvent: dimethyl carbonate | DMC to oil 39:1 380 °C, 20 MPa, 30 min | 91% yield | Without microwave | [92] |
| Material | Operating Condition | Ester Content | Energy Assessment | Reference |
|---|---|---|---|---|
| Oleic acid and methanol esterification Catalyst: 1-(4-sulfobutyil)-3-methylmidazolium hydrosulfate, [Bsmim]H2SO4 | 10–20 min 100–140 W (MW) 40 kHz 50 W (US) MR of M:O 20–24 Mass ratio IL:O 8–12 | 97. 85% of oleic acid conversion (17 min, 118 W, M:O 22, mass of IL:O 11) | 1.75 × 104 kJ/kg | [104] |
| Palm oil and methanol Catalyst: Choline hydroxide (ChOH) | 400–800 W ChOH 2–6% (w/w) M:O 9:1–15:1 | 90% of biodiesel yield, 800 W, M:O 13.2:1, IL: 6% (w/w) | NA Catalyst can be reused | [102] |
| Chicken lard and karanja oil Solvent: protic deep eutectic from benzyl trimethyl ammonium chloride (BMC) and various organic acids | 140–240 W, 10–30 min M:O 1:1–6:1 Solvent: 1–10% (w/w) Enzyme: 5% (w/w) | 240 W, 30 min Met: Oil 6:1 Solvent: 8% (w/w) CL: 96.4% KO: 88.6% Enzyme: 5% (w/w) CL: 91% KO: 91.2% | For pDes treatment CL: 43.1 MJ/kg KO: 35.8 MJ/kg For enzyme catalyst CL: 39.4 MJ/kg KO: 24.7 MJ/kg | [103] |
| Palm oil and methanol transesterification Catalyst: imidazolium IL, [HSO3-Bmim] H2SO4 | 60–120 W 1–8 h MR of M:O 3–18 IL 4–14% | 98.9% of biodiesel yield 6.43 h, 168 W, M:O 11, IL: 9.2%, at 108 °C | Save up to 44% compared to conventional | [105] |
| Algae Nannochloropsis sp. and methanol direct transesterification Catalyst: 1-ethyl-3-methylimmidazolium methyl sulfate, [EMIM][MeSO4] | MR of Algae to M 1:4–1:12 M to O 1:0.5–1:1 5–25 min Temp. 65–95 °C | 42.4% of biodiesel per dried biomass 14 min, Algae to M 1:4 M to O 1:0.5 At 65 °C | NA | [106] |
| Algae Nannochloropsis sp. and methanol direct transesterification Solvent: methanol and 1-ethyl-3-methylimmidazolium methyl sulfate, [EMIM][MeSO4] | 5–15 min Temp. 65 °C Difference type of solvent MR of M:O 6–12 | 36.8% of biodiesel per dried biomass, 15 min, At 65 °C, A:M = 1:4 M:IL = 1:0.5 | NA | [107] |
| Primary sewage sludge and methanol Solvent: tetrakis(hydroxymethyl) phosphonium chloride, [P(CH2OH)4]CL Catalyst: 1v% of sulfuric acid | MR of sludge: IL 1:5–1:20 (g/TS: cm3/IL) 25–100 °C | 23.4% (dried sludge) and 27.6% (raw sludge) lipid 17% (dried sludge) and 19.8% (raw sludge) biodiesel 3 h extraction, 1:5 (sludge: IL) 100 °C | Energy required to extract 1 kg of lipids was estimated to be 238 MJ/kglipid | [108] |
| Jatropha oil and methanol transesterification Waste cooking oil Soy oil Catalyst: 4-allyl-4-methylmorpholin-4-ium bromine, [MorMeA][Br] and Sodium hydroxide, NaOH | 3–8 min MR of M:O 6–12 NaOH: 0.5–1.5% IL: 0.5–1.5% Temp. 40–90 °C | 98.5% 89.1% 99.4% in 6 min M:O is 9:1 1 wt.% IL + 0.75 wt.% NaOH At 70 °C | 0.254 kWh 23 times lower than conventional | [109,110] |
| Type of Combined Technology | Advantages | Disadvantages |
|---|---|---|
| Microwave and acoustic cavitation | Improved heat and mass transfer [111] Short transesterification reaction time and high yields [77] Minimize the energy consumption and the use of hazardous chemicals [112] | Industrial scale-up difficulty [113] Inefficient ultrasound generation and high operating costs [113] |
| Microwave and supercritical/ subcritical | Reduction of cost for catalyst Eliminate separation and purification steps [86] | Large excess of methanol/ ethanol [86] Huge energy consumption for a reaction at supercritical condition at industry scale [94] |
| Microwave and vacuum | Low boiling point of the by-product, easy purification [58,96] Promotes the diffusion of reagents into the substance, increasing the contact area and accelerating the reaction [97] | High vacuum instrument consumes high energy [114] |
| Microwave and ionic solvent | Simplify processing step [115] Can be reused and recycle [105,106,115] Reduce amount of wastewater [115] | Expensive ionic solvent [108] Costly for traditional recovery and purification method [115] Insufficient information for whole life cycle assessment [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad Aziz, N.A.; Yunus, R.; Kania, D.; Abd Hamid, H. Prospects and Challenges of Microwave-Combined Technology for Biodiesel and Biolubricant Production through a Transesterification: A Review. Molecules 2021, 26, 788. https://doi.org/10.3390/molecules26040788
Mohamad Aziz NA, Yunus R, Kania D, Abd Hamid H. Prospects and Challenges of Microwave-Combined Technology for Biodiesel and Biolubricant Production through a Transesterification: A Review. Molecules. 2021; 26(4):788. https://doi.org/10.3390/molecules26040788
Chicago/Turabian StyleMohamad Aziz, Nur Atiqah, Robiah Yunus, Dina Kania, and Hamidah Abd Hamid. 2021. "Prospects and Challenges of Microwave-Combined Technology for Biodiesel and Biolubricant Production through a Transesterification: A Review" Molecules 26, no. 4: 788. https://doi.org/10.3390/molecules26040788
APA StyleMohamad Aziz, N. A., Yunus, R., Kania, D., & Abd Hamid, H. (2021). Prospects and Challenges of Microwave-Combined Technology for Biodiesel and Biolubricant Production through a Transesterification: A Review. Molecules, 26(4), 788. https://doi.org/10.3390/molecules26040788







