Challenges and Perspectives of Standard Therapy and Drug Development in High-Grade Gliomas
Abstract
1. Introduction
2. Overview of Standard Therapy in HGG
2.1. Surgical Resection
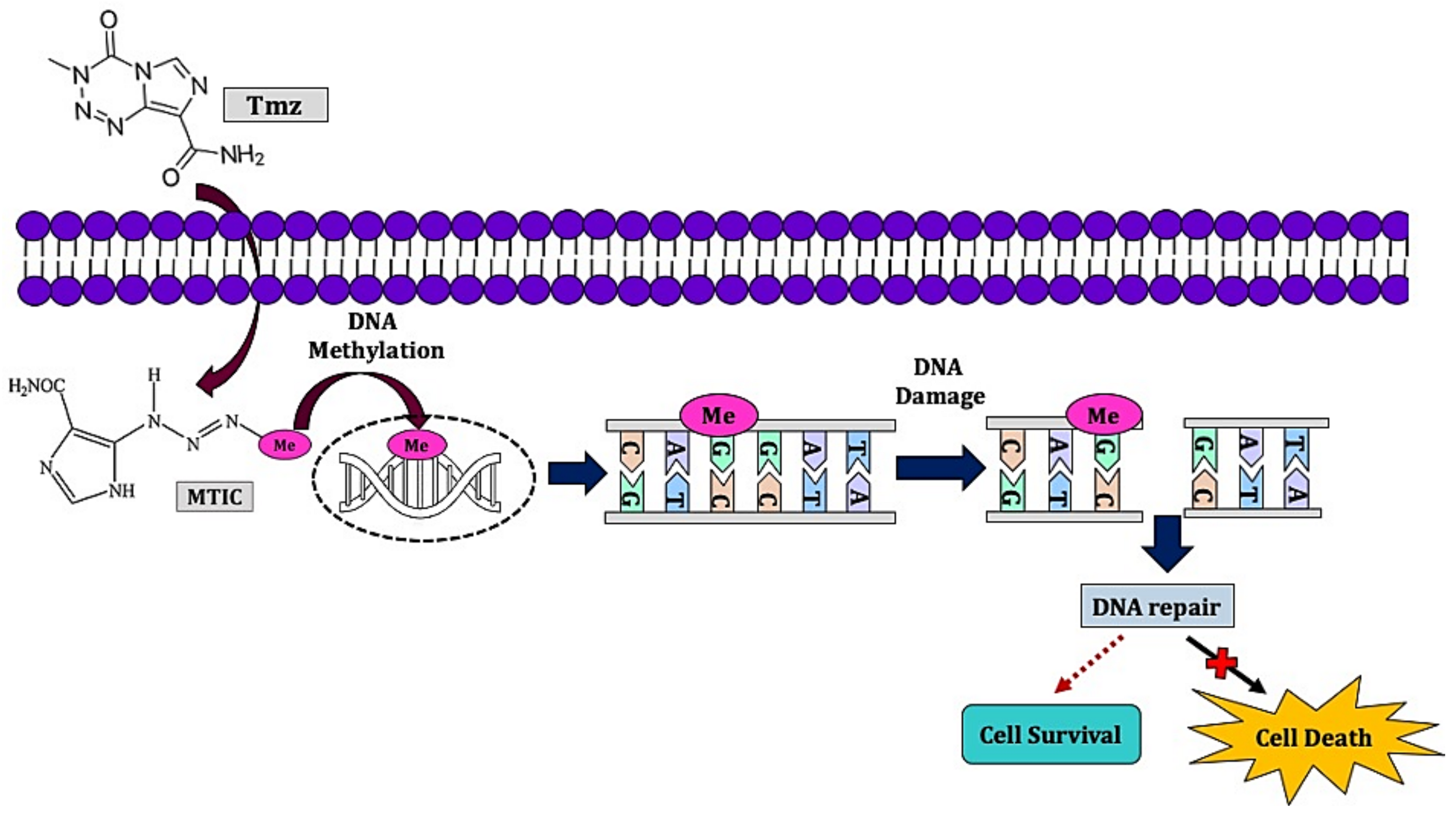
2.2. Chemotherapy
2.3. Radiotherapy
3. Challenges in HGG Standard Therapy
4. Drug Development for HGG: Advancements and Challenges
4.1. Gene Therapy
| Vector | Findings |
|---|---|
| Herpesvirus and Retrovirus | The use of herpes simplex virus as suicide gene therapy by converting antiviral drugs which prolonged prodrug treatment, improved survival and inhibited proliferation as well as tumor growth [171,172,173]. |
| TOCA 511 resulted in the promotion of T cell expansion (Th1, Th2 in CD4+, CD8+), mediated antitumor immune response, and concentrated the effect of drugs at the tumor site which increased direct tumor cell death, alterations in immune cell infiltration, and improved survival [174,175,176,177,178]. | |
| Retroviral replicating vectors (RRV) based on gibbon ape leukemia virus enabled high-efficiency gene transfer and persistent expression of E. coli nitroreductase prodrug activator genes, resulting in efficient cell killing, suppression of tumor growth, and prolonged survival upon CB1954 administration [166]. | |
| Semi- and pseudotyped-RRV system harboring two suicide genes—HSV1 thymidine kinase and yeast cytosine deaminase and prodrug demonstrated high oncolytic capability against extremely heterogeneous and treatment-refractory GBM which promoted the inhibition of cell proliferation, angiogenesis, increased apoptosis, and the depletion of tumor-associated macrophages in orthotopic GBM [179]. | |
| Adenovirus | The replication-deficient adenovirus mutant thymidine kinase (ADV-TK) in combination with ganciclovir improved recurrent patients’ survival, integrin antagonist cRGD (EMD121974) promoted adenovirus-mediated REIC/Dkk-3 reduction of cell proliferation and mice survival. Adenovirus is also used to transfect p53 gene, mediated cytotoxic immune therapy of prodrug and PTEN, PI3K inhibitors [180,181,182,183]. |
| Vector | Benefits | Challenges |
|---|---|---|
| Adenovirus |
|
|
| Adeno-associated virus |
|
|
| Retrovirus |
|
|
| Gold nanoparticles |
|
|
| Polymeric micelles |
|
|
| Dendrimer and Dendrigraft |
|
|
| Poly(β-amino ester) |
|
|
| Study Reference | WHO Classification of Tumor | Phase of the Clinical Trial | Total Patients | Outcome | |
|---|---|---|---|---|---|
| Experimental Group | Placebo Group | ||||
| Rainov et al. [184] | IV (GBM) | III | 111 | 103 | OS, PFS |
| Stragliatto et al. [185] | IV (GBM) | I/II | 22 | 20 | OS, PFS |
| Westphal et al. [186] | IV (GBM) | III | 119 | 117 | OS |
| Wheeler et al. [187] | III (AA,AO), IV (GBM) | Ib/IIb | 48 | 134 | OS, PFS |
4.2. Immunotherapy
| Immunotherapy | Description |
|---|---|
| Bevacizumab |
|
| Depatuxizumab mafodotin (ABT-414) | |
| Peptide vaccine |
|
| Heat Shock Protein (HSP) vaccine |
|
| Dendritic cell (DC) vaccine |
|
| Study Reference | WHO Classification of Tumor | Phase of the Clinical Trial | Total Patients | Outcome | |
|---|---|---|---|---|---|
| DC Vaccine | Placebo | ||||
| Wheeler et al. [232] | IV (GBM) | IA/IB/II | 13 | 13 | OS |
| Yu et al. [233] | III (AA), IV (GBM) | I | 8 | 26 | OS |
| Batich et al. [234] | IV (GBM) | I | 11 | 23 | OS |
| Der-Yang Co et al. [231] | IV (GBM) | II | 18 | 16 | OS, PFS |
| Chang et al. [235] | III (AA, AO), IV (GBM) | I/II | 16 | 63 | OS |
| Yamanaka et al. [236] | IV (GBM) | I/II | 18 | 27 | OS |
| Jie et al. [237] | IV (GBM) | I/II | 13 | 12 | OS |
| Vik-Mo et al. [238] | IV (GBM) | I/II | 7 | 10 | OS, PFS |
4.3. Tumor-Treating Field (TTF)
5. Repurposing Drugs for HGG
6. Phytochemicals and Nanoparticles in HGG
6.1. Flavonoids
6.2. Polysaccharides
6.3. Cannabinoids
6.4. Thymoquinone
6.5. Potential and Challenges of Phytochemicals and Nanoparticles
| Phytochemical | Study Design | Observations |
|---|---|---|
| Curcumin | U118, U87, U251MG-100 µM nimustine hydrochloride + 20 µM curcumin | Enhanced anti-proliferation, anti-migration, and proapoptotic activities of nimustine hydrochloride [20]. |
| Patient-derived GSCs (Glio 3, Glio 9)—25 µM curcumin | Reduced cell viability of GSCs via ROS-dependent mechanism, MAPK-pathway activation and downregulation of STAT3 and IAPs [281]. | |
| U87-miR-378-50 µM c SCID mice-30, 60, 120 mg/kg | miR-378 sensitized GBM toward curcumin, inhibited tumor growth, cell proliferation, and induce apoptosis [285]. | |
| Thymoquinone | U87MG-50 µM TQ + 100 µM Tmz | Decreased cell migration and invasion [334]. |
| Plumbagin | A172, U251-5.5 μM (IC50) | Cell cycle arrestment at G2/M phase. Apoptotic induction with minimal necrotic cell death. PTEN overexpression and downregulation of E2F1, MDM2, cyclin B1, surviving, Bcl-2 protein, and PARP-1. Inhibition of telomerase activity [351]. |
| Sativex | NCT01812603 Phase I and Phase II (n = 21 GBM) with Karnofsky performance scale ≥60% 100 µL (12 spray/day) Sativex (27 mg/mL THC + 25 mg/mL CBD) orally + Tmz Control: Tmz alone | 83% of one year survival rate in Sativex + Tmz group compared to 44% in Tmz alone [328]. |
| Quercetin | T98G-50 µM quercetin + 20 µM chloroquine | Induced autophagy and ER stress [294]. |
| C6, T98G-25 µM quercetin + 1mM NaB | Promoted apoptosis via increased expression of Bax, caspase 3, downregulation of Bcl-2, surviving and PARP degradation [295]. | |
| Resveratrol | C6-50,100,150 µM | Inhibited cell proliferation, cell cycle arrestment at s-phase, apoptotic induction, downregulation of miR-21, miR-19 and miR30a-5p [296]. |
| RG-2-25 µM Resveratrol + 250 µM Tmz LN18, LN428-75 µM Resveratrol + 750 µM Tmz | Inhibition of MGMT expression, downregulation of STAT3/Bcl-2/surviving, apoptosis and cell cycle arrestment (G1 or S-phase) [297]. | |
| Galangin | U87MG and U251-100 µM | Apoptosis, cell cycle arrest G0/G1 pytoptosis, and protective autophagy. Enhanced chloroquine-suppressed tumor growth compared to galangin monotherapy [276]. |
| Male BALB/c athymic mice, 4 weeks old; 14–17 g) (orthotopic U87MG xenograft) 100 mg/kg/day GG + 25 mg/kg/day chloroquine; control: DMSO | ||
| Schizophyllan | CNS-1-40 and 60 mg/L Schizophyllan | Apoptosis and cell cycle arrest at G0/G1 phase. Tumor growth inhibited [310]. |
| Sprague Dawley male rats (n = 40) (in situ intracranial tumors, CNS-1) 20, 40, 60 mg/kg; control 0.9% NaCl | ||
| Icariin | U87MG-10 µM ICA + 200 µM Tmz | Synergistically decreased cell proliferation, sensitized GBM cell by enhanced apoptosis by increased caspase-3 and cleaved PARP expression. Inhibited cell migration, invasion via suppression of NF-κB activity [352]. |
| Silbinin (Silybum) | A172, SR-50, 100, 150 µM s | Apoptotic induction via caspase-3 activation and PARP-1 cleavage. Enhanced autophagic flux via LC3-I to LC3-II conversion and P62 degradation. Inhibition of mTOR and downregulation of YAP [353]. |
| Luteolin | U251, LN229-10, 20 30 µM | Inhibited cell proliferation. Apoptotic induction via MAPK by activation of FADD, upregulation of cleaved PARP, cleaved caspase-8, and cleaved caspase-3. Increased expression of Bax to Bcl2 ratio. Autophagy induction promoting miR-124-3p expression [354]. |
| Silbinin + Luteolin | U87, T98G-50 µM SIL + 20 µM | Synergistically inhibited cell proliferation, invasion, and migration. Apoptosis induction and inhibition of rapamycin (RAPA)-induced autophagy via iNOS downregulation, PKCα suppression, and miR-7-1-3p upregulation [355]. |
| Female nude mice (nu/nu) (subcutaneous U87MG, T98G xenografts) Silbinin (200 mg/kg/day) + Luteolin (10 mg/kg/day) | ||
| Oligo-fucoidan | GBM8401, U87MG-50, 100, 200 µg/mL | Cell cycle arresting at G1/S phase induced cell differentiation, inhibited DNA Methyltransferases, and decreased p21 methylation [249]. |
| G. lucidum polysaccharides (GL-PS) | U251- 50, 100, 200, 400 or 800 μg/mL | Inhibited cell proliferation, cell cycle arrestment at G0/G1 phase, promote apoptosis via caspase 3 activation. Increased IL-2, TNF-α, INF-γ. Enhanced cytotoxicity of NK and T cells. Inhibited tumor growth and prolonged rat survival [316]. |
| Male Fischer rats (200-250G)- 50, 100, and 200 mg/(kg d) GL-PS; control: saline | ||
| Saponin D (Pulsatilla koreana) | U87 MG-10 μM SB365 | Inhibited cell proliferation. Alteration in mitochondrial membrane potential (MMP), neutralization of lysosomal pH Increased ratio of LC3-II/I and p26 in cell indicating Inhibition of autophagic influx mediated by cathepsin B and mainly ROS. Co-treatment of SB365 and Tmz exerted an additive effect. Suppression of tumor growth in xenograft model [356]. |
| Nude mice-SB365 (5 mg/kg/every other day, intratumoral) + Tmz (2.5 mg/kg/day, i.p., U87 xenograft) | ||
| Toosendanin | U87, C6, T98G-10 nM | Inhibited cell proliferation and induced apoptosis in vitro and in vivo. Reduce tumor progression via apoptosis. Reduced tumor weight. Increased expression of Bax, cleaved caspase-3, and reduction in Bcl-2 expression. No cytotoxic effect in T98G. Apoptosis induced via increased expression of estrogen receptor β and p53 [357]. |
| Athymic nude mice—6 weeks old (n = 10), (U87-Luc xenograft, subcutaneous) 1 mg/kg qd (orally) | ||
| Coronarin D | U251-10, 20, 40 μM | Cell cycle arrest at G1 phase, induced caspase-dependent mitochondrial-mediated apoptosis by increasing phosphorylated ERK, p-H2AX histone, and overexpression of p21 [358]. |
| Carvacrol | U87-500 μM | Inhibition of TRPM7. Reduction in cell viability, migration, invasion, and MMP-2. Promotion of cofilin phosphorylation and inhibition of Ras/MEK/MAPK and PI3K/Akt. TRPM7 [359]. |
| Lentinan | C6- 20, 40, 80 mg/L | Inhibited tumor growth, cell proliferation, cell cycle arrestment at G0/G1 phase, and promoted apoptosis [323]. |
| SD male rats-20, 40, 80 mg/kg/d; control: 0.9% Nacl | ||
| Ficus carica | U138 MG, T98G, U87 MG-0.25 mg/mL | Inhibited GBM cell proliferation, and stimulated apoptosis. Inhibit cell invasion via reduction in VEGF expression. Synergistic inhibition in GBM cell proliferation. The co-treatment increased miRNA expression (let-7d) in T98G cells modulating GBM progression via miRNA [360]. |
| U138 MG, T98G-0.25 mg/mL + 450 μM Tmz U87 MG-0.25 mg/mL + 25μM Tmz | ||
| Celastrus orbiculatus | U87, U251-20, 40, 80 μg/mL | Inhibition of cell adhesion, migration, and invasion. Reduction in N-cadherin, vimentin, MMP-2, and MMP-9 expression. Upregulation of E-cadherin. Inhibition in actin assembly. [361]. |
| Tetrandrine (Stephania tetrandra) | U87, U251-4 μM Tet + 2 Gy | Enhanced radiosensitivity of the cell. Inhibited cell proliferation by decreasing phosphorylated ERK expression. Cell cycle arrestment at G0/G1 phase [362]. |
| Osthole | U87-50, 100, 200 μM | Inhibited cell proliferation and enhanced apoptosis in cells. Increased expression of miR-16 precursor and decreased expression of MMP-9 [363]. |
| Trichosanthin | U87, U251-10, 20 μM | Inhibited cell proliferation, invasion and migration. Induced apoptosis and inhibited LGR5 expression suggesting repression in Wnt/β - catenin signaling pathway [364]. |
7. Precision Medicine
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.F.; Juweid, M. Epidemiology and outcome of glioblastoma. Exon Publ. 2017, 143–153. [Google Scholar] [CrossRef]
- Diwanji, T.P.; Engelman, A.; Snider, J.W.; Mohindra, P. Epidemiology, diagnosis, and optimal management of glioma in adolescents and young adults. Adolesc. Health Med. Ther. 2017, 8, 99. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Cahill, D.; Turcan, S. Origin of gliomas. In Seminars in Neurology; Thieme Medical Publishers: New York, NY, USA, 2018; Volume 38, pp. 5–10. [Google Scholar]
- Hervey-Jumper, S.L.; Berger, M.S. Maximizing safe resection of low-and high-grade glioma. J. Neuro Oncol. 2016, 130, 269–282. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Xu, J.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol. 2016, 18, v1–v75. [Google Scholar] [CrossRef]
- Fangusaro, J.; Bandopadhayay, P. The “Risk” in Pediatric Low-Grade Glioma. Cancer Cell 2020, 37, 424–425. [Google Scholar] [CrossRef]
- De Blank, P.; Bandopadhayay, P.; Haas-Kogan, D.; Fouladi, M.; Fangusaro, J. Management of pediatric low-grade glioma. Curr. Opin. Pediatr. 2019, 31, 21. [Google Scholar] [CrossRef]
- Barnholtz-Sloan, J.S.; Ostrom, Q.T.; Cote, D. Epidemiology of brain tumors. Neurol. Clin. 2018, 36, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Pretanvil, J.-A.; Salinas, I.Q.; Piccioni, D.E. Glioblastoma in the elderly: Treatment patterns and survival. CNS Oncol. 2017, 6, 19–28. [Google Scholar] [CrossRef]
- Bauchet, L.; Ostrom, Q.T. Epidemiology and molecular epidemiology. Neurosurg. Clin. 2019, 30, 1–16. [Google Scholar] [CrossRef]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef]
- Marra, J.S.; Mendes, G.P.; Yoshinari, G.H., Jr.; da Silva Guimarães, F.; Mazin, S.C.; de Oliveira, H.F. Survival after radiation therapy for high-grade glioma. Rep. Pract. Oncol. Radiother. 2019, 24, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Sharma, A.; Pitz, M.; Loewen, S.; Quon, H.; Poulin, A.; Essig, M. High-grade glioma management and response assessment—recent advances and current challenges. Curr. Oncol. 2016, 23, e383. [Google Scholar] [CrossRef] [PubMed]
- Harder, B.; Blomquist, M.; Wang, J.W.; Kim, A.; Woodworth, G.; Winkles, J.; Loftus, J.; Tran, N. Developments in Blood-Brain Barrier Penetrance and Drug Repurposing for Improved Treatment of Glioblastoma. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, J.; Lv, X.; Xing, J.; Liu, S.; Chen, C.; Xu, Y. Curcumin potentiates the potent antitumor activity of ACNU against glioblastoma by suppressing the PI3K/AKT and NF-KB/COX-2 signaling pathways. OncoTargets Ther. 2017, 10, 5471. [Google Scholar] [CrossRef]
- Abbas, M.; Kausar, S.; Cui, H. Therapeutic potential of natural products in glioblastoma treatment: Targeting key glioblastoma signaling pathways and epigenetic alterations. Clin. Transl. Oncol. 2020, 22, 963–977. [Google Scholar] [CrossRef]
- Jain, K.K. A critical overview of targeted therapies for glioblastoma. Front. Oncol. 2018, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Darko, M.; Amuah, J.E.; Kelly, J.J.P. Surgical Resection of Anterior and Posterior Butterfly Glioblastoma. World Neurosurg. 2018, 110, e612–e620. [Google Scholar] [CrossRef]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in brain tumor surgery for glioblastoma in adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Siglin, J.; Yamoah, K.; Dan, T.; Champ, C.E.; Bar-Ad, V.; Werner-Wasik, M.; Evans, J.J.; Kim, L.; Glass, J. Re-resection for recurrent high-grade glioma in the setting of re-irradiation: More is not always better. J. Neuro Oncol. 2015, 124, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Eseonu, C.I.; Rincon-Torroella, J.; ReFaey, K.; Lee, Y.M.; Nangiana, J.; Vivas-Buitrago, T.; Quiñones-Hinojosa, A. Awake craniotomy vs craniotomy under general anesthesia for perirolandic gliomas: Evaluating perioperative complications and extent of resection. Neurosurgery 2017, 81, 481–489. [Google Scholar] [CrossRef]
- Eseonu, C.I.; Eguia, F.; ReFaey, K.; Garcia, O.; Rodriguez, F.J.; Chaichana, K.; Quinones-Hinojosa, A. Comparative volumetric analysis of the extent of resection of molecularly and histologically distinct low grade gliomas and its role on survival. J. Neuro Oncol. 2017, 134, 65–74. [Google Scholar] [CrossRef]
- Eseonu, C.I.; Rincon-Torroella, J.; ReFaey, K.; Quiñones-Hinojosa, A. The cost of brain surgery: Awake vs asleep craniotomy for perirolandic region tumors. Neurosurgery 2017, 81, 307–314. [Google Scholar] [CrossRef]
- Lakomkin, N.; Hadjipanayis, C.G. Fluorescence-guided surgery for high-grade gliomas. J. Surg. Oncol. 2018, 118, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Wirtz, C.R. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: A systematic review. J. Neuro Oncol. 2019, 141, 533–546. [Google Scholar] [CrossRef]
- Senders, J.T.; Muskens, I.S.; Schnoor, R.; Karhade, A.V.; Cote, D.J.; Smith, T.R.; Broekman, M.L. Agents for fluorescence-guided glioma surgery: A systematic review of preclinical and clinical results. Acta Neurochir. 2017, 159, 151–167. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Tsien, R.Y. Fluorescence-guided surgery with live molecular navigation—A new cutting edge. Nat. Rev. Cancer 2013, 13, 653–662. [Google Scholar] [CrossRef]
- Pirro, V.; Alfaro, C.M.; Jarmusch, A.K.; Hattab, E.M.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 6700–6705. [Google Scholar] [CrossRef] [PubMed]
- Eberlin, L.S.; Dill, A.L.; Golby, A.J.; Ligon, K.L.; Wiseman, J.M.; Cooks, R.G.; Agar, N.Y. Discrimination of human astrocytoma subtypes by lipid analysis using desorption electrospray ionization imaging mass spectrometry. Angew. Chem. 2010, 122, 6089–6092. [Google Scholar] [CrossRef]
- Brown, H.M.; Pu, F.; Dey, M.; Miller, J.; Shah, M.V.; Shapiro, S.A.; Ouyang, Z.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative detection of isocitrate dehydrogenase mutations in human gliomas using a miniature mass spectrometer. Anal. Bioanal. Chem. 2019, 411, 7929–7933. [Google Scholar] [CrossRef]
- Young, R.M.; Jamshidi, A.; Davis, G.; Sherman, J.H. Current trends in the surgical management and treatment of adult glioblastoma. Ann. Transl. Med. 2015, 3, 121121. [Google Scholar] [CrossRef]
- Alfaro, C.M.; Pirro, V.; Keating, M.F.; Hattab, E.M.; Cooks, R.G.; Cohen-Gadol, A.A. Intraoperative assessment of isocitrate dehydrogenase mutation status in human gliomas using desorption electrospray ionization–mass spectrometry. J. Neurosurg. 2019, 132, 180–187. [Google Scholar] [CrossRef]
- Ashby, L.S.; Smith, K.A.; Stea, B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: A systematic literature review. World J. Surg. Oncol. 2016, 14, 225. [Google Scholar] [CrossRef]
- Champeaux, C.; Weller, J. Implantation of carmustine wafers (Gliadel®) for high-grade glioma treatment. A 9-year nationwide retrospective study. J. Neuro Oncol. 2020, 147, 159–169. [Google Scholar] [CrossRef]
- Fernandes, C.; Costa, A.; Osório, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current standards of care in glioblastoma therapy. Exon Publ. 2017, 197–241. [Google Scholar] [CrossRef]
- Jakobsen, J.; Urup, T.; Grunnet, K.; Toft, A.; Johansen, M.; Poulsen, S.; Christensen, I.; Muhic, A.; Poulsen, H. Toxicity and efficacy of lomustine and bevacizumab in recurrent glioblastoma patients. J. Neuro Oncol. 2018, 137, 439–446. [Google Scholar] [CrossRef]
- Parasramka, S.; Talari, G.; Rosenfeld, M.; Guo, J.; Villano, J.L. Procarbazine, lomustine and vincristine for recurrent high-grade glioma. Cochrane Database Syst. Rev. 2017, 7. [Google Scholar] [CrossRef]
- Weller, M.; van Den Bent, M.; Tonn, J.C.; Stupp, R.; Preusser, M.; Cohen-Jonathan-Moyal, E.; Henriksson, R.; Rhun, E.L.; Balana, C.; Chinot, O.; et al. European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol. 2017, 18, e315–e329. [Google Scholar] [CrossRef]
- Weller, M.; Le Rhun, E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat. Rev. 2020, 87, 102029. [Google Scholar] [CrossRef]
- Wheeler, G.P.; Bowdon, B.J.; Struck, R.F. Carbamoylation of Amino Acids, Peptides, and Proteins by Nitrosoureas. Cancer Res. 1975, 35, 2974–2984. [Google Scholar]
- Kohn, K.W. Interstrand cross-linking of DNA by 1,3-bis(2-chloroethyl)-1-nitrosourea and other 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. 1977, 37, 1450. [Google Scholar]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.-D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Stritzelberger, J.; Distel, L.; Buslei, R.; Fietkau, R.; Putz, F. Acquired temozolomide resistance in human glioblastoma cell line U251 is caused by mismatch repair deficiency and can be overcome by lomustine. Clin. Transl. Oncol. 2018, 20, 508–516. [Google Scholar] [CrossRef]
- Wick, W.; Puduvalli, V.K.; Chamberlain, M.C.; van Den Bent, M.J.; Carpentier, A.F.; Cher, L.M.; Mason, W.; Weller, M.; Hong, S.; Musib, L.; et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J. Clin. Oncol. 2010, 28, 1168. [Google Scholar] [CrossRef] [PubMed]
- Yung, W.K.A.; Albright, R.E.; Olson, J.; Fredericks, R.; Fink, K.; Prados, M.D.; Brada, M.; Spence, A.; Hohl, R.J.; Shapiro, W.; et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br. J. Cancer 2000, 83, 588. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, J.R.; Rajdev, P.; Mukherji, S.K. Temozolomide (Temodar). Ajnr Am. J. Neuroradiol. 2010, 31, 1383. [Google Scholar] [CrossRef] [PubMed]
- Belter, A.; Barciszewski, J.; Barciszewska, A.-M. Revealing the epigenetic effect of temozolomide on glioblastoma cell lines in therapeutic conditions. PLoS ONE 2020, 15, e0229534. [Google Scholar] [CrossRef]
- Koukourakis, G.V.; Kouloulias, V.; Zacharias, G.; Papadimitriou, C.; Pantelakos, P.; Maravelis, G.; Fotineas, A.; Beli, I.; Chaldeopoulos, D.; Kouvaris, J. Temozolomide with radiation therapy in high grade brain gliomas: Pharmaceuticals considerations and efficacy; a review article. Molecules 2009, 14, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Schreck, K.C.; Grossman, S.A. Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology 2018, 32, 555–569. [Google Scholar] [PubMed]
- Portnow, J.; Badie, B.; Chen, M.; Liu, A.; Blanchard, S.; Synold, T.W. The Neuropharmacokinetics of Temozolomide in Patients with Resectable Brain Tumors: Potential Implications for the Current Approach to Chemoradiation. Clin. Cancer Res. 2009, 15, 7092–7098. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, S. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef]
- Zhang, J.; FGStevens, M.; DBradshaw, T. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Malmström, A.; Poulsen, H.S.; Grønberg, B.H.; Stragliotto, G.; Hansen, S.; Asklund, T.; Holmlund, B.; Łysiak, M.; Dowsett, J.; Kristensen, B.W.; et al. Postoperative neoadjuvant temozolomide before radiotherapy versus standard radiotherapy in patients 60 years or younger with anaplastic astrocytoma or glioblastoma: A randomized trial. Acta Oncol. 2017, 56, 1776–1785. [Google Scholar] [CrossRef] [PubMed]
- Jiapaer, S.; Furuta, T.; Tanaka, S.; Kitabayashi, T.; Nakada, M. Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol. Med. Chir. 2018, 58, 405. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Duwa, R.; Emami, F.; Lee, S.; Jeong, J.-H.; Yook, S. Polymeric and lipid-based drug delivery systems for treatment of glioblastoma multiforme. J. Ind. Eng. Chem. 2019, 79, 261–273. [Google Scholar] [CrossRef]
- Grimm, S.A.; Chamberlain, M.C. Anaplastic astrocytoma. CNS Oncol. 2016, 5, 145–157. [Google Scholar] [CrossRef] [PubMed]
- McTyre, E.; Lucas, J.T.; Helis, C.; Farris, M.; Soike, M.; Mott, R.; Laxton, A.W.; Tatter, S.B.; Lesser, G.J.; Strowd, R.E. Outcomes for anaplastic glioma treated with radiation therapy with or without concurrent temozolomide. Am. J. Clin. Oncol. 2018, 41, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.I.; Mason, W.P. Temozolomide: The evidence for its therapeutic efficacy in malignant astrocytomas. Core Evid. 2009, 4, 93. [Google Scholar]
- Wei, W.; Chen, X.; Ma, X.; Wang, D.; Guo, Z. The efficacy and safety of various dose-dense regimens of temozolomide for recurrent high-grade glioma: A systematic review with meta-analysis. J. Neuro Oncol. 2015, 125, 339–349. [Google Scholar] [CrossRef]
- Garcia, C.R.; Slone, S.A.; Morgan, R.M.; Gruber, L.; Kumar, S.S.; Lightner, D.D.; Villano, J.L. Dose-dense temozolomide for recurrent high-grade gliomas: A single-center retrospective study. Med. Oncol. 2018, 35, 136. [Google Scholar] [CrossRef]
- Ruff, M.W.; Buckner, J.C. The Use of PCV Chemotherapy in Oligodendrogliomas; Elsevier: Amsterdam, The Netherlands, 2019; pp. 331–339. [Google Scholar] [CrossRef]
- Hafazalla, K.; Sahgal, A.; Jaja, B.; Perry, J.R.; Das, S. Procarbazine, CCNU and vincristine (PCV) versus temozolomide chemotherapy for patients with low-grade glioma: A systematic review. Oncotarget 2018, 9, 33623. [Google Scholar] [CrossRef]
- Wei, W.; Jia, Y.; Hui, C. Radiotherapy plus procarbazine, lomustine, and vincristine versus radiotherapy alone for glioma: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2017, 10, 6810–6818. [Google Scholar]
- Ruff, M.W.; Uhm, J. Anaplastic glioma: Treatment approaches in the era of molecular diagnostics. Curr. Treat. Options Oncol. 2018, 19, 61. [Google Scholar] [CrossRef]
- Ruff, M.W.; Buckner, J.C.; Johnson, D.R.; Van Den Bent, M.J.; Geurts, M. Neuro-Oncology Clinical Debate: PCV or temozolomide in combination with radiation for newly diagnosed high-grade oligodendroglioma. Neuro Oncol. Pract. 2019, 6, 17–21. [Google Scholar] [CrossRef]
- González-Aguilar, A.; Reyes-Moreno, I.; Peiro-Osuna, R.P.; Hernández-Hernández, A.; Gutiérrez-Aceves, A.; Santos-Zambrano, J.; Guerrero-Juárez, V.; López-Martínez, M.; Castro-Martínez, E. Radiotherapy plus temozolomide or PCV in patients with anaplastic oligodendroglioma 1p19q codeleted. Rev. Neurol. 2018, 67, 293–297. [Google Scholar]
- Iwadate, Y.; Matsutani, T.; Hara, A.; Hirono, S.; Ikegami, S.; Kobayashi, M.; Ito, D.; Kawauchi, D.; Horiguchi, K.; Tamiya, A. Eighty percent survival rate at 15 years for 1p/19q co-deleted oligodendroglioma treated with upfront chemotherapy irrespective of tumor grade. J. Neuro Oncol. 2019, 141, 205–211. [Google Scholar] [CrossRef]
- McNamara, M.G.; Sahebjam, S.; Mason, W.P. Anaplastic oligodendroglioma: Advances and treatment options. Curr. Treat. Options Neurol. 2013, 15, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Blakeley, J.; Grossman, S. Anaplastic oligodendroglioma. Curr. Treat. Options Neurol. 2008, 10, 295. [Google Scholar] [CrossRef]
- Alba, A.B.; Alicia, T.; Giovanna, C.; Michele, R.; Enrico, F.; Laura, B.; Roberta, B.; Marina, G.; Claudio, G.; Paolo, I.; et al. Correlations Between O6-Methylguanine DNA Methyltransferase Promoter Methylation Status, 1p and 19q Deletions, and Response to Temozolomide in Anaplastic and Recurrent Oligodendroglioma: A Prospective GICNO Study. J. Clin. Oncol. 2006, 24, 4746–4753. [Google Scholar]
- Boots-Sprenger, S.H.; Sijben, A.; Rijntjes, J.; Tops, B.B.; Idema, A.J.; Rivera, A.L.; Bleeker, F.E.; Gijtenbeek, A.M.; Diefes, K.; Heathcock, L. Significance of complete 1p/19q co-deletion, IDH1 mutation and MGMT promoter methylation in gliomas: Use with caution. Mod. Pathol. 2013, 26, 922–929. [Google Scholar] [CrossRef]
- Bell, E.H.; Zhang, P.; Fisher, B.J.; Macdonald, D.R.; McElroy, J.P.; Lesser, G.J.; Fleming, J.; Chakraborty, A.R.; Liu, Z.; Becker, A.P.; et al. Association of MGMT Promoter Methylation Status With Survival Outcomes in Patients With High-Risk Glioma Treated With Radiotherapy and Temozolomide: An Analysis From the NRG Oncology/RTOG 0424 Trial. JAMA Oncol. 2018, 4, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Gwak, H.-S.; Yee, G.T.; Park, C.-K.; Kim, J.W.; Hong, Y.-K.; Kang, S.-G.; Kim, J.H.; Seol, H.J.; Jung, T.-Y.; Chang, J.H. Temozolomide salvage chemotherapy for recurrent anaplastic oligodendroglioma and oligo-astrocytoma. J. Korean Neurosurg. Soc. 2013, 54, 489. [Google Scholar] [CrossRef]
- Chinot, O.-L.; Honore, S.; Dufour, H.; Barrie, M.; Figarella-Branger, D.; Muracciole, X.; Braguer, D.; Martin, P.-M.; Grisoli, F. Safety and efficacy of temozolomide in patients with recurrent anaplastic oligodendrogliomas after standard radiotherapy and chemotherapy. J. Clin. Oncol. 2001, 19, 2449–2455. [Google Scholar] [CrossRef]
- Lukas, R.V.; Wainwright, D.A.; Ladomersky, E.; Sachdev, S.; Sonabend, A.M.; Stupp, R. Newly Diagnosed Glioblastoma: A Review on Clinical Management. Oncology 2019, 33, 91. [Google Scholar]
- Stupp, R.; Mason, W.P.; van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Szopa, W.; Burley, T.A.; Kramer-Marek, G.; Kaspera, W. Diagnostic and Therapeutic Biomarkers in Glioblastoma: Current Status and Future Perspectives. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir-Kaynak, E.; Qutub, A.A.; Yesil-Celiktas, O. Advances in Glioblastoma Multiforme Treatment: New Models for Nanoparticle Therapy. Front. Physiol. 2018, 9, 170. [Google Scholar] [CrossRef]
- Wick, W.; Roth, P.; Hartmann, C.; Hau, P.; Nakamura, M.; Stockhammer, F.; Sabel, M.C.; Wick, A.; Koeppen, S.; Ketter, R. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016, 18, 1529–1537. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, W.; Wang, Y.; Peng, X.; Chen, B.; Qiu, X.; Li, G.; Li, S.; Wu, C.; Yao, K. IDH mutation and MGMT promoter methylation in glioblastoma: Results of a prospective registry. Oncotarget 2015, 6, 40896. [Google Scholar] [CrossRef] [PubMed]
- Janaki, M.; Arunmohan, P.; Harshitha, M.J. Improved Survival In A Patient Of Anaplastic Astrocytoma With Re-Irradiation: A Case Report. J. Cancer Res. Ther. 2017, 13, S280. [Google Scholar]
- Krauze, A.V.; Attia, A.; Braunstein, S.; Chan, M.; Combs, S.E.; Fietkau, R.; Fiveash, J.; Flickinger, J.; Grosu, A.; Howard, S. Expert consensus on re-irradiation for recurrent glioma. Radiat. Oncol. 2017, 12, 194. [Google Scholar] [CrossRef]
- Cairncross, J.G.; Wang, M.; Jenkins, R.B.; Shaw, E.G.; Giannini, C.; Brachman, D.G.; Buckner, J.C.; Fink, K.L.; Souhami, L.; Laperriere, N.J. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J. Clin. Oncol. 2014, 32, 783. [Google Scholar] [CrossRef]
- Speirs, C.K.; Simpson, J.R.; Robinson, C.G.; DeWees, T.A.; Tran, D.D.; Linette, G.; Chicoine, M.R.; Dacey, R.G.; Rich, K.M.; Dowling, J.L. Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 268–276. [Google Scholar] [CrossRef]
- Sarmiento, J.M.; Venteicher, A.S.; Patil, C.G. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst. Rev. 2015, 6. [Google Scholar] [CrossRef]
- Dhawan, S.; Patil, C.G.; Chen, C.; Venteicher, A.S. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst. Rev. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Im, J.H.; Hong, J.B.; Kim, S.H.; Choi, J.; Chang, J.H.; Cho, J.; Suh, C.-O. Recurrence patterns after maximal surgical resection and postoperative radiotherapy in anaplastic gliomas according to the new 2016 WHO classification. Sci. Rep. 2018, 8, 777. [Google Scholar] [CrossRef]
- Torensma, R. The dilemma of cure and damage in oligodendroglioma: Ways to tip the balance away from the damage. Cancers 2018, 10, 431. [Google Scholar] [CrossRef]
- Venteicher, A.S.; Tirosh, I.; Hebert, C.; Yizhak, K.; Neftel, C.; Filbin, M.G.; Hovestadt, V.; Escalante, L.E.; Shaw, M.L.; Rodman, C.; et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017, 355, 6332. [Google Scholar] [CrossRef]
- Zhao, J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol. Ther. 2016, 160, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.D.; Gilbert, M.R. Clinical discussion of the management of anaplastic oligodendroglioma/oligoastrocytoma (both codeleted and nondeleted). J. Natl. Compr Canc Netw. 2014, 12, 665–672. [Google Scholar] [CrossRef]
- Lassman, A.B. Procarbazine, lomustine and vincristine or temozolomide: Which is the better regimen? CNS Oncol. 2015, 4, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bent, J.M. Chemotherapy for low-grade glioma: When, for whom, which regimen? Curr. Opin. Neurol. 2015, 28, 633–938. [Google Scholar] [CrossRef]
- Wick, W.; Hartmann, C.; Engel, C.; Stoffels, M.; Felsberg, J.; Stockhammer, F.; Sabel, M.C.; Koeppen, S.; Ketter, R.; Meyermann, R.; et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J. Clin. Oncol. 2009, 27, 5874. [Google Scholar] [CrossRef] [PubMed]
- Wick, W.; Roth, P.; Wiestler, B.; Hartmann, C.; Hau, P.; Nakamura, M.; Stockhammer, F.; Sabel, M.; Koeppen, S.; Ketter, R.; et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. J. Clin. Oncol. 2015, 33, 15. [Google Scholar] [CrossRef]
- Wick, W.; Winkler, F. Regimen of procarbazine, lomustine, and vincristine versus temozolomide for gliomas. Cancer 2018, 124, 2674–2676. [Google Scholar] [CrossRef]
- Christians, A.; Adel-Horowski, A.; Banan, R.; Lehmann, U.; Bartels, S.; Behling, F.; Barrantes-Freer, A.; Stadelmann, C.; Rohde, V.; Stockhammer, F.; et al. The prognostic role of IDH mutations in homogeneously treated patients with anaplastic astrocytomas and glioblastomas. Acta Neuropathol. Commun. 2019, 7, 156. [Google Scholar] [CrossRef]
- Picca, A.; Berzero, G.; Sanson, M. Current therapeutic approaches to diffuse grade II and III gliomas. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617752039. [Google Scholar] [CrossRef] [PubMed]
- Waitkus, M.S.; Diplas, B.H.; Yan, H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016, 18, 16–26. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.-H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.-T. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Liu, Y.; Lang, F.; Chou, F.-J.; Zaghloul, K.A.; Yang, C. Isocitrate Dehydrogenase Mutations in Glioma: Genetics, Biochemistry, and Clinical Indications. Biomedicines 2020, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohle, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 2012, 483, 474–478. [Google Scholar] [CrossRef]
- Wang, P.; Wu, J.; Ma, S.; Zhang, L.; Yao, J.; Hoadley, K.A.; Wilkerson, M.D.; Perou, C.M.; Guan, K.-L.; Ye, D. Oncometabolite D-2-hydroxyglutarate inhibits ALKBH DNA repair enzymes and sensitizes IDH mutant cells to alkylating agents. Cell Rep. 2015, 13, 2353–2361. [Google Scholar] [CrossRef]
- Inoue, S.; Li, W.Y.; Tseng, A.; Beerman, I.; Elia, A.J.; Bendall, S.C.; Lemonnier, F.; Kron, K.J.; Cescon, D.W.; Hao, Z. Mutant IDH1 downregulates ATM and alters DNA repair and sensitivity to DNA damage independent of TET2. Cancer Cell 2016, 30, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Pellerino, A.; Bertero, L.; Rudà, R.; Soffietti, R. Choosing appropriate chemotherapy for diffusely infiltrating WHO grade II gliomas in adults. Expert Opin. Pharmacother. 2020, 21, 613–615. [Google Scholar] [CrossRef]
- Valiyaveettil, D.; Malik, M.; Joseph, D.; Ahmed, S.F.; Kothwal, S.A. Prognostic factors and outcomes in anaplastic gliomas: An institutional experience. S. Asian J. Cancer 2018, 7, 1. [Google Scholar]
- Jovanović, N.; Mitrović, T.; Cvetković, V.J.; Tošić, S.; Vitorović, J.; Stamenković, S.; Nikolov, V.; Kostić, A.; Vidović, N.; Krstić, M.; et al. The Impact of Promoter Methylation and Temozolomide Treatment in Serbian Patients with Primary Glioblastoma. Medicina 2019, 55, 2. [Google Scholar]
- Yi, G.Z.; Huang, G.; Guo, M.; Zhang, X.; Wang, H.; Deng, S.; Li, Y.; Xiang, W.; Chen, Z.; Pan, J.; et al. Acquired temozolomide resistance in MGMT-deficient glioblastoma cells is associated with regulation of DNA repair by DHC2. Brain 2019, 142, 2352–2366. [Google Scholar] [CrossRef]
- Bienkowski, M.; Berghoff, A.S.; Marosi, C.; Wöhrer, A.; Heinzl, H.; Hainfellner, J.A.; Preusser, M. Clinical Neuropathology practice guide 5–2015: MGMT methylation pyrosequencing in glioblastoma: Unresolved issues and open questions. Clin. Neuropathol. 2015, 34, 250–257. [Google Scholar] [CrossRef]
- Malley, D.S.; Hamoudi, R.A.; Kocialkowski, S.; Pearson, D.M.; Collins, V.P.; Ichimura, K. A distinct region of the MGMT CpG island critical for transcriptional regulation is preferentially methylated in glioblastoma cells and xenografts. Acta Neuropathol. 2011, 121, 651–661. [Google Scholar] [CrossRef]
- Mansouri, A.; Hachem, L.D.; Mansouri, S.; Nassiri, F.; Laperriere, N.J.; Xia, D.; Lindeman, N.I.; Wen, P.Y.; Chakravarti, A.; Mehta, M.P.; et al. MGMT promoter methylation status testing to guide therapy for glioblastoma: Refining the approach based on emerging evidence and current challenges. Neuro Oncol. 2019, 21, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef]
- Hombach-Klonisch, S.; Mehrpour, M.; Shojaei, S.; Harlos, C.; Pitz, M.; Hamai, A.; Siemianowicz, K.; Likus, W.; Wiechec, E.; Toyota, B.D.; et al. Glioblastoma and chemoresistance to alkylating agents: Involvement of apoptosis, autophagy, and unfolded protein response. Pharmacol. Ther. 2018, 184, 13–41. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, J.; Wang, W.; Wang, D. Relationship between MGMT gene expression and treatment effectiveness and prognosis in glioma. Oncol. Lett. 2017, 14, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Rabé, M.; Dumont, S.; Álvarez-Arenas, A.; Janati, H.; Belmonte-Beitia, J.; Calvo, G.F.; Thibault-Carpentier, C.; Séry, Q.; Chauvin, C.; Joalland, N.; et al. Identification of a transient state during the acquisition of temozolomide resistance in glioblastoma. Cell Death Dis. 2020, 11, 19. [Google Scholar]
- Woo, P.; Li, Y.; Chan, A.; Ng, S.; Loong, H.; Chan, D.; Wong, G.; Poon, W.-S. A multifaceted review of temozolomide resistance mechanisms in glioblastoma beyond O-6-methylguanine-DNA methyltransferase. Glioma 2019, 2, 68–82. [Google Scholar]
- Chen, X.; Zhang, M.; Gan, H.; Wang, H.; Jeong-Heon, L.; Fang, D.; Kitange, G.; He, L.; Hu, Z.; Parney, I.; et al. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat. Commun. 2018, 9, 2949. [Google Scholar] [CrossRef]
- Low, S.Y.Y.; Ho, Y.K.; Too, H.-P.; Yap, C.T.; Ng, W.H. MicroRNA as potential modulators in chemoresistant high-grade gliomas. J. Clin. Neurosci. 2014, 21, 395–400. [Google Scholar] [CrossRef]
- Kaina, B.; Christmann, M.; Naumann, S.; Roos, W.P. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 2007, 6, 1079–1099. [Google Scholar] [CrossRef]
- Aasland, D.; Götzinger, L.; Hauck, L.; Berte, N.; Meyer, J.; Effenberger, M.; Schneider, S.; Reuber, E.E.; Roos, W.P.; Tomicic, M.T.; et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR-CHK1, p21, and NF-κB. Cancer Res. 2019, 79, 99. [Google Scholar] [CrossRef] [PubMed]
- McFaline-Figueroa, J.L.; Braun, C.J.; Stanciu, M.; Nagel, Z.D.; Mazzucato, P.; Sangaraju, D.; Cerniauskas, E.; Barford, K.; Vargas, A.; Chen, Y.; et al. Minor Changes in Expression of the Mismatch Repair Protein MSH2 Exert a Major Impact on Glioblastoma Response to Temozolomide. Cancer Res. 2015, 75, 3127. [Google Scholar] [CrossRef]
- Erasimus, H.; Gobin, M.; Niclou, S.; Van Dyck, E. DNA repair mechanisms and their clinical impact in glioblastoma. Mutat. Res. Rev. Mutat. Res. 2016, 769, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.J.; Ng, W.; Stylli, S.S.; Hovens, C.M.; Kaye, A.H. Repair mechanisms help glioblastoma resist treatment. J. Clin. Neurosci. 2015, 22, 14–20. [Google Scholar] [CrossRef]
- Angeli, J.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Guo, N.; Xu, H.; Pan, T.; Lei, H.; Yan, A.; Mi, Y.; Xu, L. Ibuprofen induces ferroptosis of glioblastoma cells via downregulation of nuclear factor erythroid 2-related factor 2 signaling pathway. Anti Cancer Drugs 2020, 31, 27–34. [Google Scholar] [CrossRef]
- Hirose, Y.; Berger, M.S.; Pieper, R.O. p53 effects both the duration of G 2 /M arrest and the fate of temozolomide-treated human glioblastoma cells. Cancer Res. 2001, 61, 1957–1963. [Google Scholar] [PubMed]
- Chien, C.-H.; Hsueh, W.-T.; Chuang, J.-Y.; Chang, K.-Y. Role of autophagy in therapeutic resistance of glioblastoma. J. Cancer Metastas. Treat. 2019, 5, 66. [Google Scholar] [CrossRef]
- Buccarelli, M.; Marconi, M.; Pacioni, S.; De Pascalis, I.; D’Alessandris, Q.G.; Martini, M.; Ascione, B.; Malorni, W.; Larocca, L.M.; Pallini, R.; et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018, 9, 841. [Google Scholar] [CrossRef]
- Yang, K.; Niu, L.; Bai, Y.; Le, W. Glioblastoma: Targeting the autophagy in tumorigenesis. Brain Res. Bull. 2019, 153, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Das, C.K.; Mandal, M.; Kögel, D. Pro-survival autophagy and cancer cell resistance to therapy. Cancer Metastas. Rev. 2018, 37, 749–766. [Google Scholar] [CrossRef]
- Ambrosio, S.; Majello, B. Autophagy Roles in Genome Maintenance. Cancers 2020, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol. Lett. 2016, 12, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.; Unger, E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomed. 2018, 13, 6049. [Google Scholar] [CrossRef] [PubMed]
- Colwell, N.; Larion, M.; Giles, A.J.; Seldomridge, A.N.; Sizdahkhani, S.; Gilbert, M.R.; Park, D.M. Hypoxia in the glioblastoma microenvironment: Shaping the phenotype of cancer stem-like cells. Neuro Oncol. 2017, 19, 887–896. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The role of hypoxia in glioblastoma invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef]
- Lo Dico, A.; Martelli, C.; Diceglie, C.; Lucignani, G.; Ottobrini, L. Hypoxia-inducible factor-1α activity as a switch for glioblastoma responsiveness to temozolomide. Front. Oncol. 2018, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, D.; Shen, L.; Dong, K.; Wu, M.; Ou, Z.; Shi, D. Redox homeostasis protects mitochondria through accelerating ROS conversion to enhance hypoxia resistance in cancer cells. Sci. Rep. 2016, 6, 22831. [Google Scholar] [CrossRef]
- Chen, R.; Lai, U.H.; Zhu, L.; Singh, A.; Ahmed, M.; Forsyth, N.R. Reactive oxygen species formation in the brain at different oxygen levels: The role of hypoxia inducible factors. Front. Cell Dev. Biol. 2018, 6, 132. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic radioresistance: Can ROS be the key to overcome it? Cancers 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Stępień, K.; Ostrowski, R.P.; Matyja, E. Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med. Oncol. 2016, 33, 101. [Google Scholar]
- Yahara, K.; Ohguri, T.; Udono, H.; Yamamoto, J.; Tomura, K.; Onoda, T.; Imada, H.; Nishizawa, S.; Korogi, Y. Radiotherapy using IMRT boosts after hyperbaric oxygen therapy with chemotherapy for glioblastoma. J. Radiat. Res. 2017, 58, 351–356. [Google Scholar] [CrossRef]
- Buehler, H.; Strohm, G.L.; Nguemgo-Kouam, P.; Lamm, H.; Fakhrian, K.; Adamietz, I.A. The therapeutic effect of photon irradiation on viable glioblastoma cells is reinforced by hyperbaric oxygen. Anticancer Res. 2015, 35, 1977–1983. [Google Scholar]
- Clarke, R.H.; Moosa, S.; Anzivino, M.; Wang, Y.; Floyd, D.H.; Purow, B.W.; Lee, K.S. Sustained radiosensitization of hypoxic glioma cells after oxygen pretreatment in an animal model of glioblastoma and in vitro models of tumor hypoxia. PLoS ONE 2014, 9, e111199. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Ma, J.; Liu, B.; Dai, C.; Xie, T.; Ma, X.; Li, M.; Dong, J.; Lan, Q.; Huang, Q. Hyperbaric oxygen therapy sensitizes nimustine treatment for glioma in mice. Cancer Med. 2016, 5, 3147–3155. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zeng, X.; Wu, X.; Hu, J.; Zhu, Y.; Yang, X. Hyperbaric oxygen as an adjuvant to temozolomide nanoparticle inhibits glioma growth by inducing G2/M phase arrest. Nanomedicine 2018, 13, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Dutta, P.; Parihar, V.K.; Chamallamudi, M.R.; Kumar, N. Radiotherapy and Its Impact on the Nervous System of Cancer Survivors. CNS Neurol. Disord. Drug Targets 2020, 19, 374–385. [Google Scholar] [CrossRef]
- Toussaint, L.; Indelicato, D.J.; Stokkevåg, C.H.; Lassen-Ramshad, Y.; Pedro, C.; Mikkelsen, R.; Di Pinto, M.; Li, Z.; Flampouri, S.; Vestergaard, A. Radiation doses to brain substructures associated with cognition in radiotherapy of pediatric brain tumors. Acta Oncol. 2019, 58, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Makale, M.T.; McDonald, C.R.; Hattangadi-Gluth, J.A.; Kesari, S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017, 13, 52. [Google Scholar] [CrossRef]
- Ghia, A.J. Fractionated radiotherapy of intracranial gliomas. In Intracranial Gliomas Part II-Adjuvant Therapy; Karger Publishers: Basel, Switzerland, 2018; Volume 31, pp. 38–47. [Google Scholar]
- Song, A.; Andrews, D.W.; Werner-Wasik, M.; Kim, L.; Glass, J.; Bar-Ad, V.; Evans, J.J.; Farrell, C.J.; Judy, K.D.; Daskalakis, C. Phase I trial of alisertib with concurrent fractionated stereotactic re-irradiation for recurrent high grade gliomas. Radiother. Oncol. 2019, 132, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Nachbichler, S.B.; Kreth, F.-W. Brachytherapy of intracranial gliomas. In Intracranial Gliomas Part II-Adjuvant Therapy; Karger Publishers: Basel, Switzerland, 2018; Volume 31, pp. 72–86. [Google Scholar]
- Bartek, J.; Alattar, A.A.; Dhawan, S.; Ma, J.; Koga, T.; Nakaji, P.; Dusenbery, K.E.; Chen, C.C. Receipt of brachytherapy is an independent predictor of survival in glioblastoma in the Surveillance, Epidemiology, and End Results database. J. Neuro Oncol. 2019, 145, 75–83. [Google Scholar] [CrossRef]
- Barbarite, E.; Sick, J.T.; Berchmans, E.; Bregy, A.; Shah, A.H.; Elsayyad, N.; Komotar, R.J. The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg. Rev. 2017, 40, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.; Kirkpatrick, J.; Fiveash, J.; Shih, H.; Koay, E.; Lutz, S.; Reardon, D.; Petit, J.; Chao, S.; Brown, P. Radiation therapy for glioblastoma: An astro evidence-based clinical practice guideline. Pract. Radiat Oncol. 2016, 6, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Barney, C.; Shukla, G.; Bhamidipati, D.; Palmer, J.D. Re-irradiation for recurrent glioblastoma multiforme. Chin. Clin. Oncol. 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Darakchiev, B.J.; Albright, R.E.; Breneman, J.C.; Warnick, R.E. Safety and efficacy of permanent iodine-125 seed implants and carmustine wafers in patients with recurrent glioblastoma multiforme. J. Neurosurg. 2008, 108, 236–242. [Google Scholar] [CrossRef]
- Caffery, B.; Lee, J.S.; Alexander-Bryant, A.A. Vectors for glioblastoma gene therapy: Viral & non-viral delivery strategies. Nanomaterials 2019, 9, 105. [Google Scholar]
- Hossain, J.A.; Marchini, A.; Fehse, B.; Bjerkvig, R.; Miletic, H. Suicide gene therapy for the treatment of high-grade glioma: Past lessons, present trends, and future prospects. Neuro Oncol. Adv. 2020, 2, vdaa013. [Google Scholar] [CrossRef]
- Portnow, J.; Synold, T.W.; Badie, B.; Tirughana, R.; Lacey, S.F.; D’Apuzzo, M.; Metz, M.Z.; Najbauer, J.; Bedell, V.; Vo, T. Neural stem cell–based anticancer gene therapy: A first-in-human study in recurrent high-grade glioma patients. Clin. Cancer Res. 2017, 23, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Sun, J.-M.; Chen, B.-M.; Lin, S.-C.; Chang, H.-F.; Collins, S.; Chang, D.; Wu, S.-F.; Lu, Y.-C.; Wang, W. Efficient prodrug activator gene therapy by retroviral replicating vectors prolongs survival in an immune-competent intracerebral glioma model. Int. J. Mol. Sci. 2020, 21, 1433. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, C.; Kaushik, A.; Sen, D. Viral vector: Potential therapeutic for glioblastoma multiforme. Cancer Gene Ther. 2019, 27, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Artene, S.-A.; Turcu-Stiolica, A.; Ciurea, M.E.; Folcuti, C.; Tataranu, L.G.; Alexandru, O.; Purcaru, O.S.; Tache, D.E.; Boldeanu, M.V.; Silosi, C. Comparative effect of immunotherapy and standard therapy in patients with high grade glioma: A meta-analysis of published clinical trials. Sci. Rep. 2018, 8, 11800. [Google Scholar] [CrossRef] [PubMed]
- Nduom, E.K.; Weller, M.; Heimberger, A.B. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015, 17, vii9–vii14. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, M.M.; Sankey, E.W.; Ryan, K.J.; Chongsathidkiet, P.; Lorrey, S.J.; Wilkinson, D.S.; Fecci, P.E. Immune suppression in gliomas. J. Neuro Oncol. 2020, 151, 3–12. [Google Scholar] [CrossRef]
- Hossain, J.A.; Latif, M.A.; Ystaas, L.A.; Ninzima, S.; Riecken, K.; Muller, A.; Azuaje, F.; Joseph, J.V.; Talasila, K.M.; Ghimire, J. Long-term treatment with valganciclovir improves lentiviral suicide gene therapy of glioblastoma. Neuro Oncol. 2019, 21, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Stedt, H.; Samaranayake, H.; Kurkipuro, J.; Wirth, G.; Christiansen, L.; Vuorio, T.; Määttä, A.; Piškur, J.; Ylä-Herttuala, S. Tomato thymidine kinase-based suicide gene therapy for malignant glioma—An alternative for Herpes Simplex virus-1 thymidine kinase. Cancer Gene Ther. 2015, 22, 130–137. [Google Scholar] [CrossRef]
- Dührsen, L.; Hartfuß, S.; Hirsch, D.; Geiger, S.; Maire, C.L.; Sedlacik, J.; Guenther, C.; Westphal, M.; Lamszus, K.; Hermann, F.G. Preclinical analysis of human mesenchymal stem cells: Tumor tropism and therapeutic efficiency of local HSV-TK suicide gene therapy in glioblastoma. Oncotarget 2019, 10, 6049. [Google Scholar]
- Huang, T.T.; Parab, S.; Burnett, R.; Diago, O.; Ostertag, D.; Hofman, F.M.; Espinoza, F.L.; Martin, B.; Ibanez, C.E.; Kasahara, N. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum. Gene Ther. 2015, 26, 82–93. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Lopez Espinoza, F.; Mendoza, D.; Kato, Y.; Inagaki, A.; Hiraoka, K.; Kasahara, N.; Gruber, H.E.; Jolly, D.J.; Robbins, J.M. Toca 511 gene transfer and treatment with the prodrug, 5-fluorocytosine, promotes durable antitumor immunity in a mouse glioma model. Neuro Oncol. 2017, 19, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Philbrick, B.D.; Adamson, D.C. Early clinical trials of Toca 511 and Toca FC show a promising novel treatment for recurrent malignant glioma. Expert Opin. Investig. Drugs 2019, 28, 207–216. [Google Scholar] [CrossRef]
- Accomando, W.P.; Rao, A.R.; Hogan, D.J.; Newman, A.M.; Nakao, A.; Alizadeh, A.A.; Diehn, M.; Diago, O.R.; Gammon, D.K.; Haghighi, A. Molecular and immunological signatures are related to clinical benefit from treatment with Vocimagene amiretrorepvec (Toca 511) and 5-fluorocytosine (Toca FC) in patients with glioma. Clin. Cancer Res. 2020, 26, 6176–6186. [Google Scholar] [CrossRef]
- Yagiz, K.; Huang, T.T.; Lopez Espinoza, F.; Mendoza, D.; Ibanez, C.E.; Gruber, H.E.; Jolly, D.J.; Robbins, J.M. Toca 511 plus 5-fluorocytosine in combination with lomustine shows chemotoxic and immunotherapeutic activity with no additive toxicity in rodent glioblastoma models. Neuro Oncol. 2016, 18, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, Y.-S.; Lee, K.; Kang, M.; Shin, H.; Oh, J.-W.; Koo, H.; Kim, D.; Kim, Y.; Kong, D.-S. Novel Semi-Replicative Retroviral Vector Mediated Double Suicide Gene Transfer Enhances Antitumor Effects in Patient-Derived Glioblastoma Models. Cancers 2019, 11, 1090. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Weng, D.; Liu, C.; Gu, Z.; Chen, S.; Guo, Y.; Fan, Z.; Wang, X.; Chen, J.; Zhao, Y. Adenovirus-mediated delivery of herpes simplex virus thymidine kinase administration improves outcome of recurrent high-grade glioma. Oncotarget 2016, 7, 4369. [Google Scholar] [CrossRef]
- Shimazu, Y.; Kurozumi, K.; Ichikawa, T.; Fujii, K.; Onishi, M.; Ishida, J.; Oka, T.; Watanabe, M.; Nasu, Y.; Kumon, H. Integrin antagonist augments the therapeutic effect of adenovirus-mediated REIC/Dkk-3 gene therapy for malignant glioma. Gene Ther. 2015, 22, 146–154. [Google Scholar] [CrossRef][Green Version]
- Kiyokawa, J.; Wakimoto, H. Preclinical and clinical development of oncolytic adenovirus for the treatment of malignant glioma. Oncolytic Virother. 2019, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.; Guo, L.; Song, Y.; Wang, L.; Yu, K.; Huang, Q.; Zhong, Y. Combinatorial therapy with adenoviral-mediated PTEN and a PI3K inhibitor suppresses malignant glioma cell growth in vitro and in vivo by regulating the PI3K/AKT signaling pathway. J. Cancer Res. Clin. Oncol. 2017, 143, 1477–1487. [Google Scholar] [CrossRef]
- Rainov, N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum. Gene Ther. 2000, 11, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- Stragliotto, G.; Rahbar, A.; Solberg, N.W.; Lilja, A.; Taher, C.; Orrego, A.; Bjurman, B.; Tammik, C.; Skarman, P.; Peredo, I. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: A randomized, double-blind, hypothesis-generating study. Int. J. Cancer 2013, 133, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Ylä-Herttuala, S.; Martin, J.; Warnke, P.; Menei, P.; Eckland, D.; Kinley, J.; Kay, R.; Ram, Z.; Group, A.S. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): A randomised, open-label, phase 3 trial. Lancet Oncol. 2013, 14, 823–833. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Manzanera, A.G.; Bell, S.D.; Cavaliere, R.; McGregor, J.M.; Grecula, J.C.; Newton, H.B.; Lo, S.S.; Badie, B.; Portnow, J. Phase II multicenter study of gene-mediated cytotoxic immunotherapy as adjuvant to surgical resection for newly diagnosed malignant glioma. Neuro Oncol. 2016, 18, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Zhang, H.; Gu, L.; Ye, B.; Jian, Z.; Stary, C.; Xiong, X.X. Advances in Immunotherapy for Glioblastoma Multiforme. J. Immunol. Res. 2017, 2017, 3597613. [Google Scholar] [CrossRef] [PubMed]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar]
- Tonigold, M.; Simon, J.; Estupiñán, D.; Kokkinopoulou, M.; Reinholz, J.; Kintzel, U.; Kaltbeitzel, A.; Renz, P.; Domogalla, M.P.; Steinbrink, K. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat. Nanotechnol. 2018, 13, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Ramalho, M.J.; Carmo Pereira, M.D. Immuno-nanocarriers for brain delivery: Limitations from in vitro to preclinical and clinical studies. Nanomedicine 2020, 15, 543–545. [Google Scholar] [CrossRef]
- Ampie, L.; Choy, W.; Lamano, J.B.; Fakurnejad, S.; Bloch, O.; Parsa, A.T. Heat shock protein vaccines against glioblastoma: From bench to bedside. J. Neuro Oncol. 2015, 123, 441–448. [Google Scholar] [CrossRef]
- Weller, M.; Roth, P.; Preusser, M.; Wick, W.; Reardon, D.A.; Platten, M.; Sampson, J.H. Vaccine-based immunotherapeutic approaches to gliomas and beyond. Nat. Rev. Neurol. 2017, 13, 363–374. [Google Scholar] [CrossRef]
- Rahat, M.A. Targeting angiogenesis with peptide vaccines. Front. Immunol. 2019, 10, 1924. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Reardon, D.A.; Armstrong, T.S.; Phuphanich, S.; Aiken, R.D.; Landolfi, J.C.; Curry, W.T.; Zhu, J.-J.; Glantz, M.; Peereboom, D.M. A randomized double-blind placebo-controlled phase II trial of dendritic cell vaccine ICT-107 in newly diagnosed patients with glioblastoma. Clin. Cancer Res. 2019, 25, 5799–5807. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Schneider, J.; Boockvar, J.A. Transdifferentiation induced neural stem cells for the treatment of malignant gliomas. Neurosurgery 2016, 79, N17–N18. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Butowski, N.; Tran, D.D.; Recht, L.D.; Lim, M.; Hirte, H.; Ashby, L.; Mechtler, L.; Goldlust, S.A.; Iwamoto, F. Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): A randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017, 18, 1373–1385. [Google Scholar] [CrossRef]
- Bagley, S.J.; Desai, A.S.; Linette, G.P.; June, C.H.; O’Rourke, D.M. CAR T-cell therapy for glioblastoma: Recent clinical advances and future challenges. Neuro Oncol. 2018, 20, 1429–1438. [Google Scholar] [CrossRef]
- Li, L.; Zhu, X.; Qian, Y.; Yuan, X.; Ding, Y.; Hu, D.; He, X.; Wu, Y. Chimeric Antigen Receptor T-Cell Therapy in Glioblastoma: Current and Future. Front. Immunol. 2020, 11, 2837. [Google Scholar] [CrossRef]
- Migliorini, D.; Dietrich, P.-Y.; Stupp, R.; Linette, G.P.; Posey, A.D.; June, C.H. CAR T-cell therapies in glioblastoma: A first look. Clin. Cancer Res. 2018, 24, 535–540. [Google Scholar] [CrossRef]
- Prinzing, B.L.; Gottschalk, S.M.; Krenciute, G. CAR T-cell therapy for glioblastoma: Ready for the next round of clinical testing? Expert Rev. Anticancer Ther. 2018, 18, 451–461. [Google Scholar] [CrossRef]
- Burger, M.C.; Zhang, C.; Harter, P.N.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.P.; Wels, W.S. CAR-engineered NK cells for the treatment of glioblastoma: Turning innate effectors into precision tools for cancer immunotherapy. Front. Immunol. 2019, 10, 2683. [Google Scholar] [CrossRef]
- Jin, L.; Ge, H.; Long, Y.; Yang, C.; Chang, Y.; Mu, L.; Sayour, E.J.; De Leon, G.; Wang, Q.J.; Yang, J.C. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro Oncol. 2018, 20, 55–65. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, S.; Zhang, Y.; Wang, Y.; Zhang, Z.; Yang, M.; Zhu, Y.; Zhang, G.; Guo, G.; Tong, A. B7-H3 as a novel CAR-T therapeutic target for glioblastoma. Mol. Ther. Oncolytics 2019, 14, 279–287. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Chen, G.; Wei, X.; Wang, C.; Zhou, S.; Huang, A.; Zhang, Z.; Zhan, C.; Wu, Y. Arming Anti-EGFRvIII CAR-T With TGFβ Trap Improves Antitumor Efficacy in Glioma Mouse Models. Front. Oncol. 2020, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Bielamowicz, K.; Fousek, K.; Byrd, T.T.; Samaha, H.; Mukherjee, M.; Aware, N.; Wu, M.-F.; Orange, J.S.; Sumazin, P.; Man, T.-K. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018, 20, 506–518. [Google Scholar] [CrossRef]
- Weiss, T.; Weller, M.; Guckenberger, M.; Sentman, C.L.; Roth, P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018, 78, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, C.M.; Desai, R.; Abel, M.L.; Riccione, K.A.; Batich, K.A.; Shen, S.H.; Chongsathidkiet, P.; Gedeon, P.C.; Elsamadicy, A.A.; Snyder, D.J. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 2018, 7, e1434464. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; Macarena, I.; Ivan, M.E.; Komotar, R.J. The role of bevacizumab in the treatment of glioblastoma. J. Neuro Oncol. 2017, 133, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Brugnara, G.; Hansen, M.B.; Nowosielski, M.; Pflüger, I.; Schell, M.; Isensee, F.; Foltyn, M.; Neuberger, U.; Kessler, T. Noninvasive Characterization of Tumor Angiogenesis and Oxygenation in Bevacizumab-treated Recurrent Glioblastoma by Using Dynamic Susceptibility MRI: Secondary Analysis of the European Organization for Research and Treatment of Cancer 26101 Trial. Radiology 2020, 297, 164–175. [Google Scholar] [CrossRef]
- Clarke, J.; Neil, E.; Terziev, R.; Gutin, P.; Barani, I.; Kaley, T.; Lassman, A.B.; Chan, T.A.; Yamada, J.; DeAngelis, L. Multicenter, phase 1, dose escalation study of hypofractionated stereotactic radiation therapy with bevacizumab for recurrent glioblastoma and anaplastic astrocytoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 797–804. [Google Scholar] [CrossRef]
- Kreisl, T.N.; Zhang, W.; Odia, Y.; Shih, J.H.; Butman, J.A.; Hammoud, D.; Iwamoto, F.M.; Sul, J.; Fine, H.A. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011, 13, 1143–1150. [Google Scholar] [CrossRef]
- Lee, E.Q.; Reardon, D.A.; Schiff, D.; Drappatz, J.; Muzikansky, A.; Grimm, S.A.; Norden, A.D.; Nayak, L.; Beroukhim, R.; Rinne, M.L. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015, 17, 862–867. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Johnston, S. Salvage chemotherapy with bevacizumab for recurrent alkylator-refractory anaplastic astrocytoma. J. Neuro Oncol. 2009, 91, 359. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bent, M.; Gan, H.K.; Lassman, A.B.; Kumthekar, P.; Merrell, R.; Butowski, N.; Lwin, Z.; Mikkelsen, T.; Nabors, L.B.; Papadopoulos, K.P. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: Results from a multi-center, international study. Cancer Chemother. Pharmacol. 2017, 80, 1209–1217. [Google Scholar] [CrossRef]
- Narita, Y.; Muragaki, Y.; Maruyama, T.; Kagawa, N.; Asai, K.; Kuroda, J.; Kurozumi, K.; Nagane, M.; Matsuda, M.; Ueki, K. Phase I/II study of depatuxizumab mafodotin (ABT-414) monotherapy or combination with temozolomide in Japanese patients with/without EGFR-amplified recurrent glioblastoma. J. Clin. Oncol. 2019, 37, 15. [Google Scholar] [CrossRef]
- Von Achenbach, C.; Silginer, M.; Blot, V.; Weiss, W.A.; Weller, M. Depatuxizumab mafodotin (ABT-414)-induced glioblastoma cell death requires EGFR overexpression, but not EGFRY1068 phosphorylation. Mol. Cancer Ther. 2020, 19, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Gatson, N.T.N.; Weathers, S.-P.S.; de Groot, J.F. ReACT Phase II trial: A critical evaluation of the use of rindopepimut plus bevacizumab to treat EGFRvIII-positive recurrent glioblastoma. CNS Oncol. 2016, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Desjardins, A.; Vredenburgh, J.J.; O’Rourke, D.M.; Tran, D.D.; Fink, K.L.; Nabors, L.B.; Li, G.; Bota, D.A.; Lukas, R.V. Rindopepimut with bevacizumab for patients with relapsed EGFRvIII-expressing glioblastoma (ReACT): Results of a double-blind randomized phase II trial. Clin. Cancer Res. 2020, 26, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Paff, M.; Alexandru-Abrams, D.; Hsu, F.P.; Bota, D.A. The evolution of the EGFRvIII (rindopepimut) immunotherapy for glioblastoma multiforme patients. Hum. Vaccines Immunother. 2014, 10, 3322–3331. [Google Scholar] [CrossRef]
- Elsamadicy, A.A.; Chongsathidkiet, P.; Desai, R.; Woroniecka, K.; Farber, S.H.; Fecci, P.E.; Sampson, J.H. Prospect of rindopepimut in the treatment of glioblastoma. Expert Opin. Biol. Ther. 2017, 17, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bloch, O.; Crane, C.A.; Fuks, Y.; Kaur, R.; Aghi, M.K.; Berger, M.S.; Butowski, N.A.; Chang, S.M.; Clarke, J.L.; McDermott, M.W. Heat-shock protein peptide complex–96 vaccination for recurrent glioblastoma: A phase II, single-arm trial. Neuro Oncol. 2014, 16, 274–279. [Google Scholar] [CrossRef]
- Bloch, O.; Shi, Q.; Anderson, S.K.; Knopp, M.; Raizer, J.; Clarke, J.; Waziri, A.; Colman, H.; Bruce, J.; Olson, J.J. ATIM-14. Alliance A071101: A phase II randomized trial comparing the efficacy of heat shock protein peptide complex-96 (HSPPC-96) vaccine given with bevacizumab versus bevacizumab alone in the treatment of surgically resectable recurrent glioblastoma. Neuro Oncol. 2017, 19, vi29. [Google Scholar] [CrossRef]
- Ji, N.; Zhang, Y.; Liu, Y.; Xie, J.; Wang, Y.; Hao, S.; Gao, Z. Heat shock protein peptide complex-96 vaccination for newly diagnosed glioblastoma: A phase I, single-arm trial. JCI Insight 2018, 3, e99145. [Google Scholar] [CrossRef]
- Srivastava, S.; Jackson, C.; Kim, T.; Choi, J.; Lim, M. A characterization of dendritic cells and their role in immunotherapy in glioblastoma: From preclinical studies to clinical trials. Cancers 2019, 11, 537. [Google Scholar] [CrossRef]
- Malo, C.S.; Khadka, R.H.; Ayasoufi, K.; Jin, F.; AbouChehade, J.E.; Hansen, M.J.; Iezzi, R.; Pavelko, K.D.; Johnson, A.J. Immunomodulation mediated by anti-angiogenic therapy improves CD8 T cell immunity against experimental glioma. Front. Oncol. 2018, 8, 320. [Google Scholar] [CrossRef]
- Mosaheb, M.M.; Dobrikova, E.Y.; Brown, M.C.; Yang, Y.; Cable, J.; Okada, H.; Nair, S.K.; Bigner, D.D.; Ashley, D.M.; Gromeier, M. Genetically stable poliovirus vectors activate dendritic cells and prime antitumor CD8 T cell immunity. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Prins, R.M.; Soto, H.; Konkankit, V.; Odesa, S.K.; Eskin, A.; Yong, W.H.; Nelson, S.F.; Liau, L.M. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin. Cancer Res. 2011, 17, 1603–1615. [Google Scholar] [CrossRef]
- Antonios, J.; Everson, R.; Soto, H.; Khattab, S.; Bethel, J.; Sun, M.; Mochizuki, A.; Lee, A.; Odesa, S.; Billingslea-Yoon, E. Atim-39. Improved survival noted in glioblastoma patients treated with adjuvant tlr-3 agonist in setting of autologous lysate-pulsed dc vaccination. Neuro Oncol. 2018, 20, vi10. [Google Scholar] [CrossRef][Green Version]
- Cho, D.-Y.; Yang, W.-K.; Lee, H.-C.; Hsu, D.-M.; Lin, H.-L.; Lin, S.-Z.; Chen, C.-C.; Harn, H.-J.; Liu, C.-L.; Lee, W.-Y. Adjuvant immunotherapy with whole-cell lysate dendritic cells vaccine for glioblastoma multiforme: A phase II clinical trial. World Neurosurg. 2012, 77, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.J.; Das, A.; Liu, G.; John, S.Y.; Black, K.L. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin. Cancer Res. 2004, 10, 5316–5326. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Liu, G.; Ying, H.; Yong, W.H.; Black, K.L.; Wheeler, C.J. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004, 64, 4973–4979. [Google Scholar] [CrossRef] [PubMed]
- Batich, K.A.; Reap, E.A.; Archer, G.E.; Sanchez-Perez, L.; Nair, S.K.; Schmittling, R.J.; Norberg, P.; Xie, W.; Herndon, J.E.; Healy, P., 2nd; et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin. Cancer Res. 2017, 23, 1898–1909. [Google Scholar] [CrossRef]
- Chang, C.-N.; Huang, Y.-C.; Yang, D.-M.; Kikuta, K.; Wei, K.-J.; Kubota, T.; Yang, W.-K. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J. Clin. Neurosci. 2011, 18, 1048–1054. [Google Scholar] [CrossRef]
- Yamanaka, R.; Homma, J.; Yajima, N.; Tsuchiya, N.; Sano, M.; Kobayashi, T.; Yoshida, S.; Abe, T.; Narita, M.; Takahashi, M. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: Results of a clinical phase I/II trial. Clin. Cancer Res. 2005, 11, 4160–4167. [Google Scholar] [CrossRef]
- Jie, X.; Hua, L.; Jiang, W.; Feng, F.; Feng, G.; Hua, Z. Clinical application of a dendritic cell vaccine raised against heat-shocked glioblastoma. Cell Biochem. Biophys. 2012, 62, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Vik-Mo, E.O.; Nyakas, M.; Mikkelsen, B.V.; Moe, M.C.; Due-Tønnesen, P.; Suso, E.M.I.; Sæbøe-Larssen, S.; Sandberg, C.; Brinchmann, J.E.; Helseth, E. Therapeutic vaccination against autologous cancer stem cells with mRNA-transfected dendritic cells in patients with glioblastoma. Cancer Immunol. Immunother. 2013, 62, 1499–1509. [Google Scholar] [CrossRef]
- Branter, J.; Basu, S.; Smith, S. Tumour treating fields in a combinational therapeutic approach. Oncotarget 2018, 9, 36631–36644. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xu, S.; Dai, G.; Xiao, Z.; Chen, L.; Liu, Z. Tumor treating fields for high-grade gliomas. BioMed Pharm. 2020, 127, 110193. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Guzauskas, G.F.; Pollom, E.L.; Stieber, V.W.; Wang, B.C.; Garrison, L.P., Jr. Tumor treating fields and maintenance temozolomide for newly-diagnosed glioblastoma: A cost-effectiveness study. J. Med. Econ. 2019, 22, 1006–1013. [Google Scholar] [CrossRef]
- Lu, G.; Rao, M.; Zhu, P.; Liang, B.; El-Nazer, R.T.; Fonkem, E.; Bhattacharjee, M.B.; Zhu, J.-J. Triple-drug therapy with bevacizumab, irinotecan, and temozolomide plus tumor treating fields for recurrent glioblastoma: A retrospective study. Front. Neurol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Robins, H.I.; Nguyen, H.N.; Field, A.; Howard, S.; Salamat, S.; Deming, D.A. Molecular evolution of a glioblastoma controlled with tumor treating fields and concomitant temozolomide. Front. Oncol. 2018, 8, 451. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Lai, I.C.; Kuo, H.-C.; Chuang, S.-E.; Lee, H.-L.; Whang-Peng, J.; Yao, C.-J.; Lai, G.-M. Epigenetic Modification and Differentiation Induction of Malignant Glioma Cells by Oligo-Fucoidan. Mar. Drugs 2019, 17, 525. [Google Scholar] [CrossRef] [PubMed]
- Guzauskas, G.F.; Salzberg, M.; Wang, B.C. Estimated lifetime survival benefit of tumor treating fields and temozolomide for newly diagnosed glioblastoma patients. CNS Oncol. 2018, 7, CNS23. [Google Scholar] [CrossRef] [PubMed]
- Fabian, D.; Guillermo Prieto Eibl, M.D.; Alnahhas, I.; Sebastian, N.; Giglio, P.; Puduvalli, V.; Gonzalez, J.; Palmer, J.D. Treatment of Glioblastoma (GBM) with the Addition of Tumor-Treating Fields (TTF): A Review. Cancers 2019, 11, 174. [Google Scholar] [CrossRef]
- Bernard-Arnoux, F.; Lamure, M.; Ducray, F.; Aulagner, G.; Honnorat, J.; Armoiry, X. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016, 18, 1129–1136. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Demireva, P.; Kanner, A.A.; Pannullo, S.; Mehdorn, M.; Avgeropoulos, N.; Salmaggi, A.; Silvani, A.; Goldlust, S.; David, C. Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma. J. Neuro Oncol. 2017, 135, 545–552. [Google Scholar] [CrossRef]
- Connock, M.; Auguste, P.; Dussart, C.; Guyotat, J.; Armoiry, X. Cost-effectiveness of tumor-treating fields added to maintenance temozolomide in patients with glioblastoma: An updated evaluation using a partitioned survival model. J. Neuro Oncol. 2019, 143, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.; Bernstock, J.D.; Ilyas, A.; Ibrahim, A.; Yamashita, D.; Markert, J.M.; Nakano, I. Current Approaches and Challenges in the Molecular Therapeutic Targeting of Glioblastoma. World Neurosurg. 2019, 129, 90–100. [Google Scholar] [CrossRef]
- Mu, Q.; Jeon, M.; Hsiao, M.H.; Patton, V.K.; Wang, K.; Press, O.W.; Zhang, M. Stable and Efficient Paclitaxel Nanoparticles for Targeted Glioblastoma Therapy. Adv. Healthc. Mater. 2015, 4, 1236–1245. [Google Scholar] [CrossRef]
- Mu, Q.; Lin, G.; Patton, V.K.; Wang, K.; Press, O.W.; Zhang, M. Gemcitabine and chlorotoxin conjugated iron oxide nanoparticles for glioblastoma therapy. J. Mater. Chem. 2015, 4, 32–36. [Google Scholar] [CrossRef]
- Hori, Y.S.; Hosoda, R.; Akiyama, Y.; Sebori, R.; Wanibuchi, M.; Mikami, T.; Sugino, T.; Suzuki, K.; Maruyama, M.; Tsukamoto, M. Chloroquine potentiates temozolomide cytotoxicity by inhibiting mitochondrial autophagy in glioma cells. J. Neuro Oncol. 2015, 122, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Q.; Wang, S.B.; Shao, Y.F.; Shi, J.N.; Wang, W.; Chen, W.Y.; Ye, Z.Q.; Jiang, J.Y.; Fang, Q.X.; Zhang, G.B.; et al. Hydroxychloroquine potentiates the anti-cancer effect of bevacizumab on glioblastoma via the inhibition of autophagy. Biomed. Pharmacother. 2019, 118, 109339. [Google Scholar] [CrossRef]
- Ye, H.; Chen, M.; Cao, F.; Huang, H.; Zhan, R.; Zheng, X. Chloroquine, an autophagy inhibitor, potentiates the radiosensitivity of glioma initiating cells by inhibiting autophagy and activating apoptosis. BMC Neurol. 2016, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.M.; Agnes, J.P.; Delgobo, M.; de Souza, P.O.; Thomé, M.P.; Heimfarth, L.; Lenz, G.; Moreira, J.C.F.; Zanotto-Filho, A. Late autophagy inhibitor chloroquine improves efficacy of the histone deacetylase inhibitor SAHA and temozolomide in gliomas. Biochem. Pharmacol. 2019, 163, 440–450. [Google Scholar] [CrossRef]
- Roy, L.-O.; Poirier, M.-B.; Fortin, D. Chloroquine inhibits the malignant phenotype of glioblastoma partially by suppressing TGF-beta. Investig. New Drugs 2015, 33, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.R.; Ye, X.; Supko, J.G.; Desideri, S.; Grossman, S.A.; Brem, S.; Mikkelson, T.; Wang, D.; Chang, Y.C.; Hu, J. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy 2014, 10, 1359–1368. [Google Scholar] [CrossRef]
- Compter, I.; Eekers, D.B.; Hoeben, A.; Rouschop, K.M.; Reymen, B.; Ackermans, L.; Beckervordersantforth, J.; Bauer, N.J.; Anten, M.M.; Wesseling, P. Chloroquine combined with concurrent radiotherapy and temozolomide for newly diagnosed glioblastoma: A phase IB trial. Autophagy 2020. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Tchokonte-Nana, V.; Lotfi, M.; Lotfi, M.; Ghorbani, A.; Sadeghnia, H.R. Wnt/beta-catenin and PI3K/Akt/mtor Signaling Pathways in Glioblastoma: Two main targets for drug design: A Review. Curr. Pharm. Des. 2020, 26, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Seliger, C.; Luber, C.; Gerken, M.; Schaertl, J.; Proescholdt, M.; Riemenschneider, M.J.; Meier, C.R.; Bogdahn, U.; Leitzmann, M.F.; Klinkhammer-Schalke, M. Use of metformin and survival of patients with high-grade glioma. Int. J. Cancer 2019, 144, 273–280. [Google Scholar] [CrossRef]
- Mazurek, M.; Litak, J.; Kamieniak, P.; Kulesza, B.; Jonak, K.; Baj, J.; Grochowski, C. Metformin as Potential Therapy for High-Grade Glioma. Cancers 2020, 12, 210. [Google Scholar] [CrossRef]
- Kamarudin, M.N.; Parhar, I. Emerging therapeutic potential of anti-psychotic drugs in the management of human glioma: A comprehensive review. Oncotarget 2019, 10, 3952. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Jermakowicz, A.; Mookhtiar, A.K.; Nemeroff, C.B.; Schürer, S.C.; Ayad, N.G. Drug repositioning in glioblastoma: A pathway perspective. Front. Pharmacol. 2018, 9, 218. [Google Scholar] [CrossRef]
- Rundle-Thiele, D.; Head, R.; Cosgrove, L.; Martin, J.H. Repurposing some older drugs that cross the blood–brain barrier and have potential anticancer activity to provide new treatment options for glioblastoma. Br. J. Clin. Pharmacol. 2016, 81, 199–209. [Google Scholar] [CrossRef]
- Adamski, V.; Schmitt, C.; Ceynowa, F.; Adelung, R.; Lucius, R.; Synowitz, M.; Hattermann, K.; Held-Feindt, J. Effects of sequentially applied single and combined temozolomide, hydroxychloroquine and AT101 treatment in a long-term stimulation glioblastoma in vitro model. J. Cancer Res. Clin. Oncol. 2018, 144, 1475–1485. [Google Scholar] [CrossRef]
- Boojar, M. An Overview of the Cellular Mechanisms of Flavonoids Radioprotective Effects. Adv. Pharm. Bull. 2020, 10, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, E.; Hassanzadeh, A.; Marofi, F.; Alivand, M.R.; Solali, S. Flavonoid-Based Cancer Therapy: An Updated Review. Anti Cancer Agents Med. Chem. 2020, 20, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Chabot, G.G.; Touil, Y.S.; Pham, M.H.; Dauzonne, D. Flavonoids in Cancer Prevention and Therapy: Chemistry, Pharmacology, Mechanisms of Action, and Perspectives for Cancer Drug Discovery. In Alternative and Complementary Therapies for Cancer; Springer: Boston, MA, USA, 2010; pp. 583–612. [Google Scholar]
- Atiq, A.; Parhar, I. Anti-neoplastic Potential of Flavonoids and Polysaccharide Phytochemicals in Glioblastoma. Molecules 2020, 25, 4895. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A. Anti-cancer potential of flavonoids: Recent trends and future perspectives. Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef]
- Tavana, E.; Mollazadeh, H.; Mohtashami, E.; Modaresi, S.M.S.; Hosseini, A.; Sabri, H.; Soltani, A.; Javid, H.; Afshari, A.R.; Sahebkar, A. Quercetin: A promising phytochemical for the treatment of glioblastoma multiforme. BioFactors 2020, 46, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Xiong, Z.; Xu, J.; Yin, J.; Luo, R. Chemopreventive mechanisms of galangin against hepatocellular carcinoma: A review. Biomed. Pharmacother. 2019, 109, 2054–2061. [Google Scholar] [CrossRef]
- Kong, Y.; Feng, Z.; Chen, A.; Qi, Q.; Han, M.; Wang, S.; Zhang, Y. The Natural Flavonoid Galangin Elicits Apoptosis, Pyroptosis, and Autophagy in Glioblastoma. Front. Oncol. 2019, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Shi, M.D.; Lee, Y.; Te, C.; Shih, Y. Galangin, a novel dietary flavonoid, attenuates metastatic feature via PKC/ERK signaling pathway in TPA-treated liver cancer HepG2 cells. Cancer Cell Int. 2015, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.; Aggarwal, B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Wang, L.X.; Ye, X.; Cai, X.; Su, J.; Ma, R.; Yin, X.; Zhou, X.X.; Li, H.; Wang, Z. Curcumin suppresses cell growth and invasion and induces apoptosis by down-regulation of Skp2 pathway in glioma cellsE. Oncotarget 2015, 6, 18027–18037. [Google Scholar] [CrossRef]
- Fratantonio, D.; Molonia, M.S.; Bashllari, R.; Muscarà, C.; Ferlazzo, G.; Costa, G.; Saija, A.; Cimino, F.; Speciale, A. Curcumin potentiates the antitumor activity of Paclitaxel in rat glioma C6 cells. Phytomedicine 2019, 55, 23–30. [Google Scholar] [CrossRef]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin decreases malignant characteristics of glioblastoma stem cells via induction of reactive oxygen species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Li, S.; He, H.; Sun, F.; Wang, C.; Lu, Y.; Wang, X.; Tao, B. Decreased expression of miR-378 correlates with tumor invasiveness and poor prognosis of patients with glioma. Int. J. Clin. Exp. Pathol. 2015, 8, 7016–7021. [Google Scholar] [PubMed]
- Kutanzi, K.R.; Yurchenko, O.V.; Beland, F.A.; Checkhun, V.F.; Pogribny, I.P. MicroRNA-mediated drug resistance in breast cancer. Clin. Epigenet. 2011, 2, 171–185. [Google Scholar] [CrossRef]
- Ma, J.; Dong, C.; Ji, C. MicroRNA and drug resistance. Cancer Gene Ther. 2010, 17, 523–531. [Google Scholar] [CrossRef]
- Li, W.; Yang, W.; Liu, Y.; Chen, S.; Chin, S.M.; Qi, X.L.; Zhao, Y.; Liu, H.; Wang, J.; Mei, X.; et al. MicroRNA-378 enhances inhibitory effect of curcumin on glioblastoma. Oncotarget 2017, 8, 73938–73946. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood–brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. ApoE3 mediated polymeric nanoparticles containing curcumin: Apoptosis induced in vitro anticancer activity against neuroblastoma cells. Int. J. Pharm. 2012, 437, 29–41. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, L.; Jiang, M.; Li, D.; Jin, L. Nanostructured lipid carriers enhance the bioavailability and brain cancer inhibitory efficacy of curcumin both in vitro and in vivo. Drug Deliv. 2016, 23, 1383–1392. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Khan, F.; Niaz, K.; Maqbool, F.; Hassan, F.; Abdollahi, M.; Venkata, K.; Nabavi, S.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Carrasco-Pozo, C. The Anti-Cancer Effect of Quercetin: Molecular Implications in Cancer Metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Sang, D.P.; Li, R.J.; Lan, Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol. Sin. 2014, 35, 832–838. [Google Scholar] [CrossRef]
- Jang, E.; Kim, I.Y.; Kim, H.; Lee, D.M.; Seo, D.Y.; Lee, J.A.; Choi, K.S.; Kim, E. Quercetin and chloroquine synergistically kill glioma cells by inducing organelle stress and disrupting Ca2+ homeostasis. Biochem. Pharmacol. 2020, 178, 114098. [Google Scholar] [CrossRef]
- Taylor, M.; Khathayer, F.; Ray, S. Quercetin and Sodium Butyrate Synergistically Increase Apoptosis in Rat C6 and Human T98G Glioblastoma Cells Through Inhibition of Autophagy. Neurochem. Res. 2019, 44, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Dai, F.; Yu, K.; Jia, Z.; Zhang, A.; Huang, Q.; Kang, C.; Jiang, H.; Pu, P. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int. J. Oncol. 2015, 46, 1739–1747. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic Effects of Resveratrol and Temozolomide Against Glioblastoma Cells: Underlying Mechanism and Therapeutic Implications. Cancer Manag. Res. 2020, 12, 8341–8354. [Google Scholar] [CrossRef]
- Richard, S. The Therapeutic Potential of Resveratrol in Gliomas. Adv. Biosci. Clin. Med. 2019, 7, 44–59. [Google Scholar] [CrossRef][Green Version]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef]
- Yuan, Y.; Xue, X.; Guo, R.B.; Sun, X.L.; Hu, G. Resveratrol Enhances the Antitumor Effects of Temozolomide in Glioblastoma via ROS-dependent AMPK-TSC-mTOR Signaling Pathway. CNS Neurosci. Ther. 2012, 18, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.H.; Wang, L.L.; Li, H.; Song, X.; Shi, S.; Gu, J.Y.; Wu, M.L.; Chen, X.Y.; Kong, Q.Y.; Liu, J. Diffusion Efficiency and Bioavailability of Resveratrol Administered to Rat Brain by Different Routes: Therapeutic Implications. Neurotherapeutics 2015, 12, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shu, X.-H.; Wu, M.-L.; Zheng, X.; Jia, B.; Kong, Q.-Y.; Liu, J.; Li, H. Postoperative resveratrol administration improves prognosis of rat orthotopic glioblastomas. BMC Cancer 2018, 18, 871. [Google Scholar] [CrossRef]
- Vijayakumar, M.R.; Vajanthri, K.Y.; Balavigneswaran, C.K.; Mahto, S.K.; Mishra, N.; Muthu, M.S.; Singh, S. Pharmacokinetics, biodistribution, in vitro cytotoxicity and biocompatibility of Vitamin E TPGS coated trans resveratrol liposomes. Coll. Surf. 2016, 145, 479–491. [Google Scholar] [CrossRef]
- Johnsen, K.; Burkhart, A.; Melander, F.; Kempen, P.; Vejlebo, J.; Siupka, P.; Nielsen, M.; Andresen, T.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Mukherjee, S.; Baidoo, J.N.E.; Sampat, S.; Mancuso, A.; David, L.; Cohen, L.S.; Zhou, S.; Banerjee, P. Liposomal TriCurin, A Synergistic Combination of Curcumin, Epicatechin Gallate and Resveratrol, Repolarizes Tumor-Associated Microglia/Macrophages, and Eliminates Glioblastoma (GBM) and GBM Stem Cells. Molecules 2018, 23, 201. [Google Scholar] [CrossRef]
- Ravi, R.; Bedi, A. NF-kappa B in cancer-a friend turned foe. Drug Resist. Update 2004, 7, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp. Oncol. 2012, 16, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Date, A.; Chawda, H.; Patel, K. Polysaccharides as potential anticancer agents—A review of their progress. Carbohydr. Polym. 2019, 210, 412–428. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Fu, Q.; Song, S.S.; Zheng, H.L.; Wei, Y.Z. Inhibitory effect of schizophyllan on rat glioma cells. Bangladesh J. Pharmacol. 2015, 10, 759–764. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Yoo, H.J.; You, D.J.; Lee, K.W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Q.; Zhou, C.; Jia, W.; Da Silva, L.; Nguyen, L.D.; Reutter, W.; Fan, H. GLIS, a bioactive proteoglycan fraction from Ganoderma lucidum, displays anti-tumour activity by increasing both humoral and cellular immune response. Life Sci. 2010, 87, 628–637. [Google Scholar] [CrossRef]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.; Wang, R.; Wang, S.; Chen, C. Antitumor and Immunomodulatory Activities of Ganoderma lucidum Polysaccharides in Glioma-Bearing Rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef]
- Li, A.; Shuai, X.; Jia, Z.; Li, H.; Liang, X.; Su, D.; Guo, W. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J. Transl. Med. 2015, 13, 100. [Google Scholar] [CrossRef]
- Wang, C.; Lin, D.; Chen, Q.; Lin, S.; Shi, S.; Chen, C. Polysaccharide peptide isolated from grass-cultured induces anti-proliferative and pro-apoptotic effects in the human U251 glioma cell line. Oncol. Lett. 2018, 15, 4330. [Google Scholar] [CrossRef]
- Wang, K.-P.; Zhang, Q.-L.; Liu, Y.; Wang, J.; Cheng, Y.; Zhang, Y. Structure and Inducing Tumor Cell Apoptosis Activity of Polysaccharides Isolated from Lentinus edodes. J. Agric. Food Chem. 2013, 61, 9849–9858. [Google Scholar] [CrossRef]
- Chen, S.-N.; Ching-Sheng, C.; Chen, S.; Wang, W.; Cheng-Jeng, T.; Chung-Lun, L.; Kim, H. The Effect of Mushroom Beta-Glucans from Solid Culture of Ganoderma lucidum on Inhibition of the Primary Tumor Metastasis. Evid. Based Complement. Altern. Med. 2014, 2014, 252171. [Google Scholar]
- Huijeong, A.; Eunsaem, J.; Jin-Chul, K.; Seung Goo, K.; Sung-Il, Y.; Hyun-Jeong, K.; Pyeung-Hyeun, K.; Geun-Shik, L. Lentinan from shiitake selectively attenuates AIM2 and non-canonical inflammasome activation while inducing pro-inflammatory cytokine production. Sci. Rep. 2017, 7, 1. [Google Scholar]
- Xu, H.L.; Dai, J.H.; Hu, T.; Liao, Y.F. Lentinan up-regulates microRNA-340 to promote apoptosis and autophagy of human osteosarcoma cells. Int. J. Clin. Exp. Pathol. 2018, 11, 3876–3883. [Google Scholar]
- Xu, H.; Zou, S.; Xu, X. The β-glucan from Lentinus edodes suppresses cell proliferation and promotes apoptosis in estrogen receptor positive breast cancers. Oncotarget 2017, 8, 86693–86709. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Li, W.; Wang, Q.; Zhang, H. Combined Effect of Lentinan and Cisplatin on Cytokines IL-6, TNF-alpha, and TGF-beta in Tumor Therapy. Int. J. Polym. Sci. 2019, 2019, 4064703. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M. In vitro inhibitory effects of lentinan on rat glioma cells. Biomed. Res. 2014, 25, 1. [Google Scholar]
- Mortimer, T.; Mabin, T.; Engelbrecht, A. Cannabinoids: The lows and the highs of chemotherapy-induced nausea and vomiting. Future Oncol. 2019, 15, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, D.; Marnett, L. Cannabinoids, endocannabinoids, and cancer. Cancer Metastas. Rev. 2011, 30, 599–612. [Google Scholar] [CrossRef]
- Gado, F.; Digiacomo, M.; Macchia, M.; Bertini, S.; Manera, C. Traditional Uses of Cannabinoids and New Perspectives in the Treatment of Multiple Sclerosis. Medicines 2018, 5, 91. [Google Scholar] [CrossRef] [PubMed]
- Erices, J.I.; Torres, Á.; Niechi, I.; Bernales, I.; Quezada, C. Current natural therapies in the treatment against glioblastoma. Phytother. Res. 2018, 32, 2191–2201. [Google Scholar] [CrossRef]
- Dumitru, C.; Sandalcioglu, I.; Karsak, M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front. Mol. Neurosci. 2018, 11, 159. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The combination of cannabidiol and Δ 9 -tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef]
- Torres, S.; Lorente, M.; Rodríguez-Fornés, F.; Hernández-Tiedra, S.; Salazar, M.; García-Taboada, E.; Barcia, J.; Guzmán, M.; Velasco, G. A combined preclinical therapy of cannabinoids and temozolomide against glioma. Mol. Cancer Ther. 2011, 10, 90. [Google Scholar] [CrossRef]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M. Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.; Beyer, M. GW Pharmaceuticals Achieves Positive Results in Phase 2 Proof of Concept Study in Glioma; GW Pharmaceuticals: Cambridge, UK, 2017; Available online: http://ir.gwpharm.com/static-files/cde942fe-555c-4b2f-9cc9-f34d24c7ad27 (accessed on 7 February 2017).
- Goyal, S.; Prajapati, C.P.; Gore, P.; Patil, C.R.; Mahajan, U.; Sharma, C.; Talla, S.; Ojha, S. Therapeutic Potential and Pharmaceutical Development of Thymoquinone: A Multitargeted Molecule of Natural Origin. Front. Pharmacol. 2017, 8, 656. [Google Scholar] [CrossRef] [PubMed]
- Pazhouhi, M.; Sariri, R.; Khazaei, M.; Moradi, M.; Khazaei, M. Synergistic effect of temozolomide and thymoquinone on human glioblastoma multiforme cell line (U87MG). J. Cancer Res. Ther. 2018, 14, 1023. [Google Scholar]
- Khan, M.A.; Tania, M.; Wei, C.; Mei, Z.; Fu, S.; Cheng, J.; Xu, J.; Fu, J. Thymoquinone inhibits cancer metastasis by downregulating TWIST1 expression to reduce epithelial to mesenchymal transition. Oncotarget 2015, 6, 19580–19591. [Google Scholar] [CrossRef]
- Peng, L.; Liu, A.; Shen, Y.; Xu, H.-Z.; Yang, S.-Z.; Ying, X.-Z.; Liao, W.; Liu, H.-X.; Lin, Z.-Q.; Chen, Q.-Y.; et al. Antitumor and anti-angiogenesis effects of thymoquinone on osteosarcoma through the NF-κB pathway. Oncol. Rep. 2013, 29, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef]
- Racoma, I.O.; Meisen, W.H.; Wang, Q.-E.; Kaur, B.; Wani, A.A. Thymoquinone Inhibits Autophagy and Induces Cathepsin-Mediated, Caspase-Independent Cell Death in Glioblastoma Cells. PLoS ONE 2013, 8, e72882. [Google Scholar] [CrossRef] [PubMed]
- Khan, H. Medicinal Plants in Light of History: Recognized Therapeutic Modality. J. Evid. Based Complement. Altern. Med. 2014, 19, 216–219. [Google Scholar] [CrossRef]
- Yool, A.J.; Ramesh, S. Molecular Targets for Combined Therapeutic Strategies to Limit Glioblastoma Cell Migration and Invasion. Front. Pharmacol. 2020, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef] [PubMed]
- Navya, P.; Kaphle, A.; Srinivas, S.; Bhargava, S.; Rotello, V.; Daima, H. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Min, Y.; Caster, J.M.; Eblan, M.J.; Wang, A.Z. Clinical Translation of Nanomedicine. Chem. Rev. 2015, 115, 11147–11190. [Google Scholar] [CrossRef]
- Michael, J.S.; Lee, B.-S.; Zhang, M.; Yu, J.S. Nanotechnology for Treatment of Glioblastoma Multiforme. J. Transl. Intern. Med. 2018, 6, 128. [Google Scholar] [CrossRef]
- Miranda, A.; Blanco-Prieto, M.J.; Sousa, J.; Pais, A.; Vitorino, C. Breaching barriers in glioblastoma. Part II: Targeted drug delivery and lipid nanoparticles. Int. J. Pharm. 2017, 531, 389–410. [Google Scholar] [CrossRef]
- Šamec, N.; Zottel, A.; Videtič Paska, A.; Jovčevska, I. Nanomedicine and immunotherapy: A step further towards precision medicine for glioblastoma. Molecules 2020, 25, 490. [Google Scholar]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Ananda, S.; Nowak, A.K.; Cher, L.; Dowling, A.; Brown, C.; Simes, J.; Rosenthal, M.A. Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J. Clin. Neurosci. 2011, 18, 1444–1448. [Google Scholar] [CrossRef]
- Nordling-David, M.M.; Yaffe, R.; Guez, D.; Meirow, H.; Last, D.; Grad, E.; Salomon, S.; Sharabi, S.; Levi-Kalisman, Y.; Golomb, G. Liposomal temozolomide drug delivery using convection enhanced delivery. J. Control. Release 2017, 261, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Moses-Gardner, A.; Huang, J.; Smith, E.R.; Moses, M.A. ITGA2 as a potential nanotherapeutic target for glioblastoma. Sci. Rep. 2019, 9, 6195. [Google Scholar]
- Khaw, A.K.; Sameni, S.; Venkatesan, S.; Kalthur, G.; Hande, M.P. Plumbagin alters telomere dynamics, induces DNA damage and cell death in human brain tumour cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 793, 86–95. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Guo, H.; Guo, M. Synergistic Anti-Cancer Effects of Icariin and Temozolomide in Glioblastoma. Cell Biochem. Biophys. 2015, 71, 1379–1385. [Google Scholar] [CrossRef]
- Bai, Z.-L.; Tay, V.; Guo, S.-Z.; Ren, J.; Shu, M.-G. Silibinin Induced Human Glioblastoma Cell Apoptosis Concomitant with Autophagy through Simultaneous Inhibition of mTOR and YAP. BioMed Res. Int. 2018, 2018, 6165192. [Google Scholar] [CrossRef]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma. OncoTargets Ther. 2019, 12, 2383–2396. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Ray, S. Anti-tumor activities of luteolin and silibinin in glioblastoma cells: Overexpression of miR-7–1-3p augmented luteolin and silibinin to inhibit autophagy and induce apoptosis in glioblastoma in vivo. Int. J. Program. Cell Death 2016, 21, 312–328. [Google Scholar] [CrossRef]
- Hong, J.-M.; Kim, J.-H.; Kim, H.; Lee, W.J.; Hwang, Y.-I. SB365, Saponin D Induces Caspase-Independent Cell Death and Augments the Anticancer Effect of Temozolomide in Glioblastoma Multiforme Cells. Molecules 2019, 24, 3230. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Qu, D.; Wang, H.; Zhang, S.; Jia, C.; Shi, Z.; Wang, Z.; Zhang, J.; Ma, J. Toosendanin exerts an anti-cancer effect in glioblastoma by inducing estrogen receptor β- and p53-mediated apoptosis. Int. J. Mol. Sci. 2016, 17, 1928. [Google Scholar] [CrossRef] [PubMed]
- Franco, Y.; Okubo, M.; Torre, A.; Paiva, P.; Rosa, M.; Silva, V.; Reis, R.; Imamura, P.; de Carvalho, J.; Longato, G. Coronarin D Induces Apoptotic Cell Death and Cell Cycle Arrest in Human Glioblastoma Cell Line. Molecules 2019, 24, 4498. [Google Scholar] [CrossRef]
- Chen, W.-L.; Barszczyk, A.; Turlova, E.; Deurloo, M.; Liu, B.; Yang, B.B.; Rutka, J.T.; Feng, Z.-P.; Sun, H.-S. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget 2015, 6, 16321–16340. [Google Scholar] [CrossRef]
- Tezcan, G.; Tunca, B.; Bekar, A.; Yalcin, M.; Sahin, S.; Budak, F.; Cecener, G.; Egeli, U.; Demir, C.; Guvenc, G.; et al. Ficus carica Latex Prevents Invasion Through Induction of Let-7d Expression in GBM Cell Lines. Cell. Mol. Neurobiol. 2015, 35, 175–187. [Google Scholar] [CrossRef]
- Gu, H.; Feng, J.; Wang, H.; Qian, Y.; Yang, L.; Chen, J.; Jin, F.; Shi, Y.; Lu, S.; Liu, Y. Celastrus orbiculatus extract inhibits the migration and invasion of human glioblastoma cells in vitro. BMC Complement. Altern. Med. 2016, 16, 387. [Google Scholar] [CrossRef]
- Ma, J.-W.; Zhang, Y.; Ye, J.-C.; Li, R.; Wen, Y.-L.; Huang, J.-X.; Zhong, X.-Y. Tetrandrine Exerts a Radiosensitization Effect on Human Glioma through Inhibiting Proliferation by Attenuating ERK Phosphorylation. Biomol. Ther. 2017, 25, 186. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Gao, Z.; Shang, B.; Sui, S.; Fu, Q. Osthole suppresses the proliferation and accelerates the apoptosis of human glioma cells via the upregulation of microRNA-16 and downregulation of MMP-9. Mol. Med. Rep. 2015, 12, 4592. [Google Scholar] [CrossRef]
- Miao, J.; Jiang, Y.; Wang, D.; Zhou, J.; Fan, C.; Jiao, F.; Liu, B.; Zhang, J.; Wang, Y.; Zhang, Q. Trichosanthin suppresses the proliferation of glioma cells by inhibiting LGR5 expression and the Wnt/β-catenin signaling pathway. Oncol. Rep. 2015, 34, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, Z.; Wang, B.; Xu, R. Towards precision medicine-based therapies for glioblastoma: Interrogating human disease genomics and mouse phenotypes. BMC Genom. 2016, 17, 516. [Google Scholar] [CrossRef]
- Logun, M.; Zhao, W.; Mao, L.; Karumbaiah, L. Microfluidics in malignant glioma research and precision medicine. Adv. Biosyst. 2018, 2, 1700221. [Google Scholar] [CrossRef]
- Fan, Y.; Nguyen, D.T.; Akay, Y.; Xu, F.; Akay, M. Engineering a brain cancer chip for high-throughput drug screening. Sci. Rep. 2016, 6, 25062. [Google Scholar] [CrossRef]
- Akay, M.; Hite, J.; Avci, N.G.; Fan, Y.; Akay, Y.; Lu, G.; Zhu, J.-J. Drug screening of human GBM spheroids in brain cancer chip. Sci. Rep. 2018, 8, 15423. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Perez, D.; Chang, L.; Shi, J.; Ma, J.; Kim, S.-H.; Zhao, X.; Malkoc, V.; Wang, X.; Minata, M.; Kwak, K.J. On-chip clonal analysis of glioma-stem-cell motility and therapy resistance. Nano Lett. 2016, 16, 5326–5332. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.F. Glioma on Chips Analysis of Glioma Cell Guidance and Interaction in Microfluidic-Controlled Microenvironment Enabled by Machine Learning. Ph.D. Thesis, Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan, 29 February 2020. [Google Scholar]
- Bagó, J.R.; Alfonso-Pecchio, A.; Okolie, O.; Dumitru, R.; Rinkenbaugh, A.; Baldwin, A.S.; Miller, C.R.; Magness, S.T.; Hingtgen, S.D. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat. Commun. 2016, 7, 10593. [Google Scholar]
- Ramakrishna, R.; Pisapia, D. Recent molecular advances in our understanding of glioma. Cureus 2015, 7, e287. [Google Scholar] [CrossRef]
- Karsy, M.; Guan, J.; Cohen, A.L.; Jensen, R.L.; Colman, H. New molecular considerations for glioma: IDH, ATRX, BRAF, TERT, H3 K27M. Curr. Neurol. Neurosci. Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- Khani, P.; Nasri, F.; Khani Chamani, F.; Saeidi, F.; Sadri Nahand, J.; Tabibkhooei, A.; Mirzaei, H. Genetic and epigenetic contribution to astrocytic gliomas pathogenesis. J. Neurochem. 2019, 148, 188–203. [Google Scholar] [CrossRef]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the molecular genetics of gliomas—implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Prados, M.D.; Byron, S.A.; Tran, N.L.; Phillips, J.J.; Molinaro, A.M.; Ligon, K.L.; Wen, P.Y.; Kuhn, J.G.; Mellinghoff, I.K.; De Groot, J.F. Toward precision medicine in glioblastoma: The promise and the challenges. Neuro Oncol. 2015, 17, 1051–1063. [Google Scholar] [CrossRef]


| Innovation | Description |
|---|---|
| Awake Craniotomy |
|
| 5-Aminolevulinic acid (5-ALA) | |
| Intraoperative mass spectrometry (MS) and Desorption electrospray ionization (DESI) | |
| Carmustine (BCNU) wafers (Gliadel®) |
| Study Design | Treatment Intervention | Outcomes | |
|---|---|---|---|
| Dendritic cell (DC) vaccine | Phase II—randomized, double-blind, controlled study (n = 124 newly diagnosed GBM without chemoradiation) NCT01280552 | Patients ratio, 2:1
| 18.3 months overall survival for ICT-7 group vs. 16.7 months control group [252]. |
| Phase III—a randomized trial (n = 331 GBM post-surgery and chemoradiation) NCT00045968 | Patients ratio, 2:1
| Median overall survival of methylated MGMT—34.7 months, with 3 years OS (46.4%) [189]. | |
| Tumor-treating fields (TTF) | Phase III—randomized, open label-trial (n = 695 GBM with resected tumors and completed chemoradiation) | Patients ratio, 2:1
| TTF with chemoradiation increased overall survival from 16 months (Tmz alone) to 20.9 months [242]. |
| Nanoparticles | In vitro SF-763, and U-118MG cell lines | Iron Oxide Nanoparticle conjugated with Cyclodextrin and Chlorotoxin and loaded with fluorescein and paclitaxel | Selectively targeted GBM cell line, effectively killing MGMT-resistant GBM cells [253]. |
| In vivo Wild type mice IV administration | Gemcitabine + Chlorotoxin Conjugated Iron Oxide Nanoparticle + Hyaluronic acid | Increased half-life (blood) 2.8 h, 10-folds higher than free GEM mice [254]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chelliah, S.S.; Paul, E.A.L.; Kamarudin, M.N.A.; Parhar, I. Challenges and Perspectives of Standard Therapy and Drug Development in High-Grade Gliomas. Molecules 2021, 26, 1169. https://doi.org/10.3390/molecules26041169
Chelliah SS, Paul EAL, Kamarudin MNA, Parhar I. Challenges and Perspectives of Standard Therapy and Drug Development in High-Grade Gliomas. Molecules. 2021; 26(4):1169. https://doi.org/10.3390/molecules26041169
Chicago/Turabian StyleChelliah, Shalini Sundramurthi, Ervin Ashley Lourdes Paul, Muhamad Noor Alfarizal Kamarudin, and Ishwar Parhar. 2021. "Challenges and Perspectives of Standard Therapy and Drug Development in High-Grade Gliomas" Molecules 26, no. 4: 1169. https://doi.org/10.3390/molecules26041169
APA StyleChelliah, S. S., Paul, E. A. L., Kamarudin, M. N. A., & Parhar, I. (2021). Challenges and Perspectives of Standard Therapy and Drug Development in High-Grade Gliomas. Molecules, 26(4), 1169. https://doi.org/10.3390/molecules26041169






