Quo Vadis Advanced Prostate Cancer Therapy? Novel Treatment Perspectives and Possible Future Directions
Abstract
1. Introduction
2. Prostate Cancer Treatment
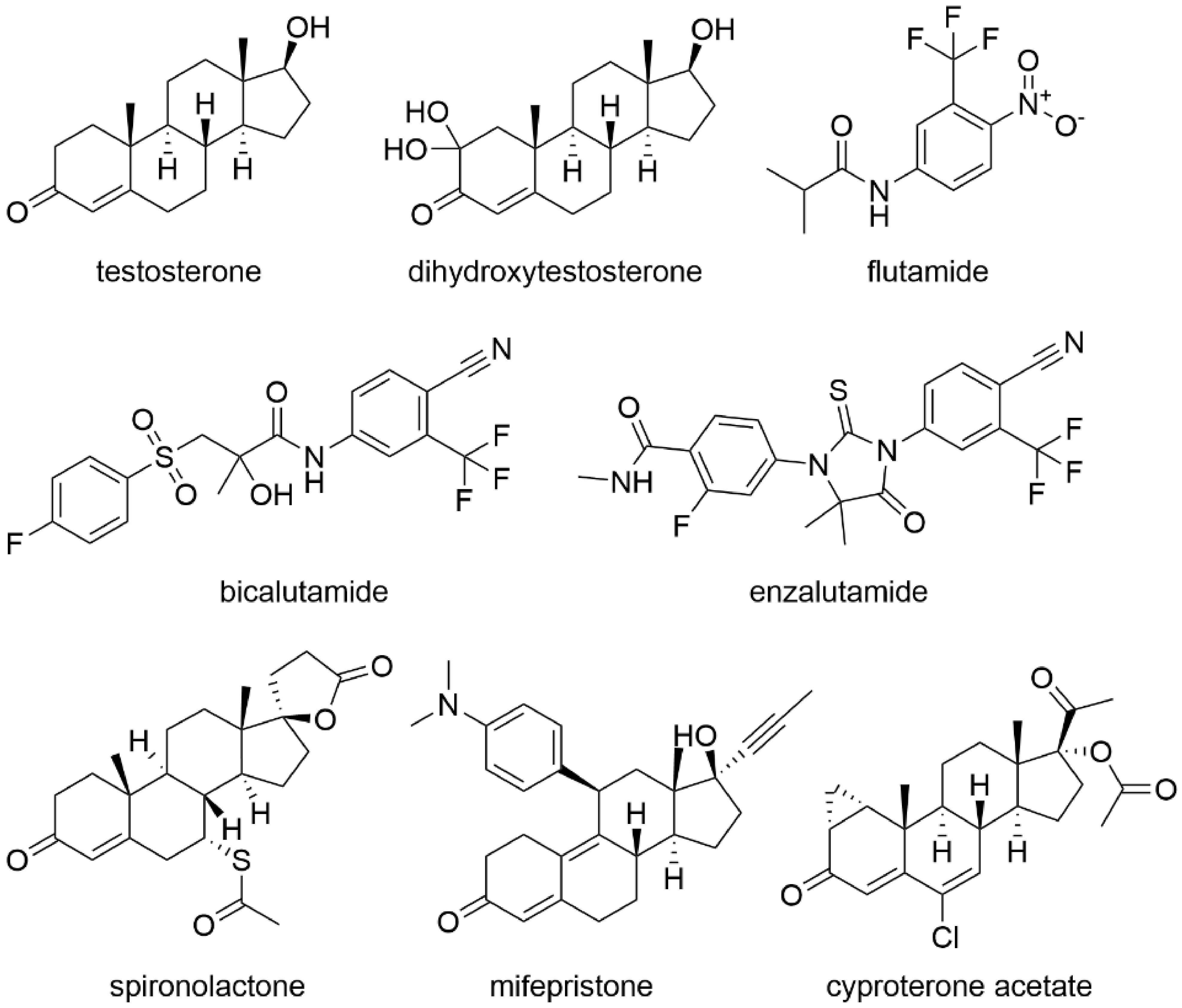
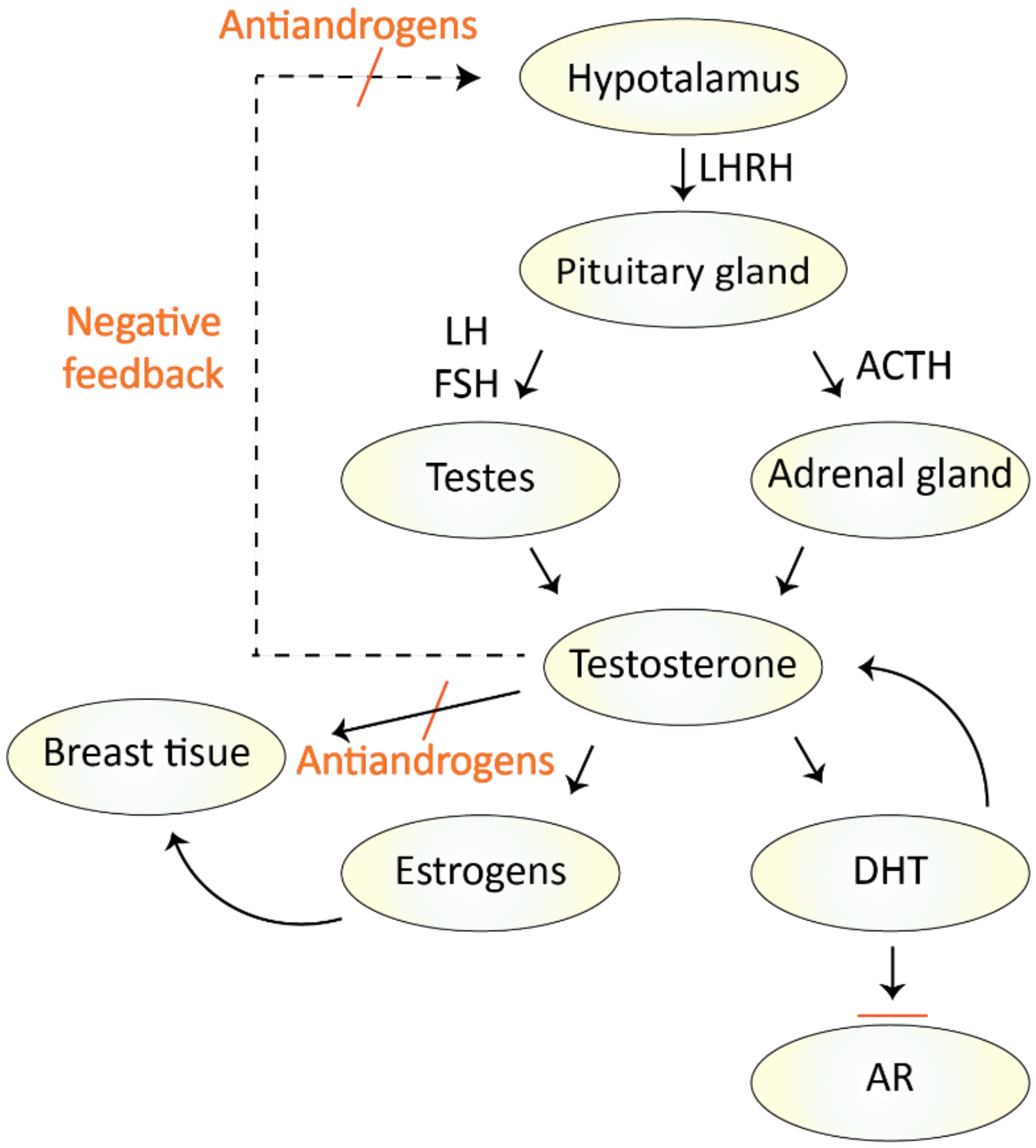
2.1. Treatment with Antiandrogens
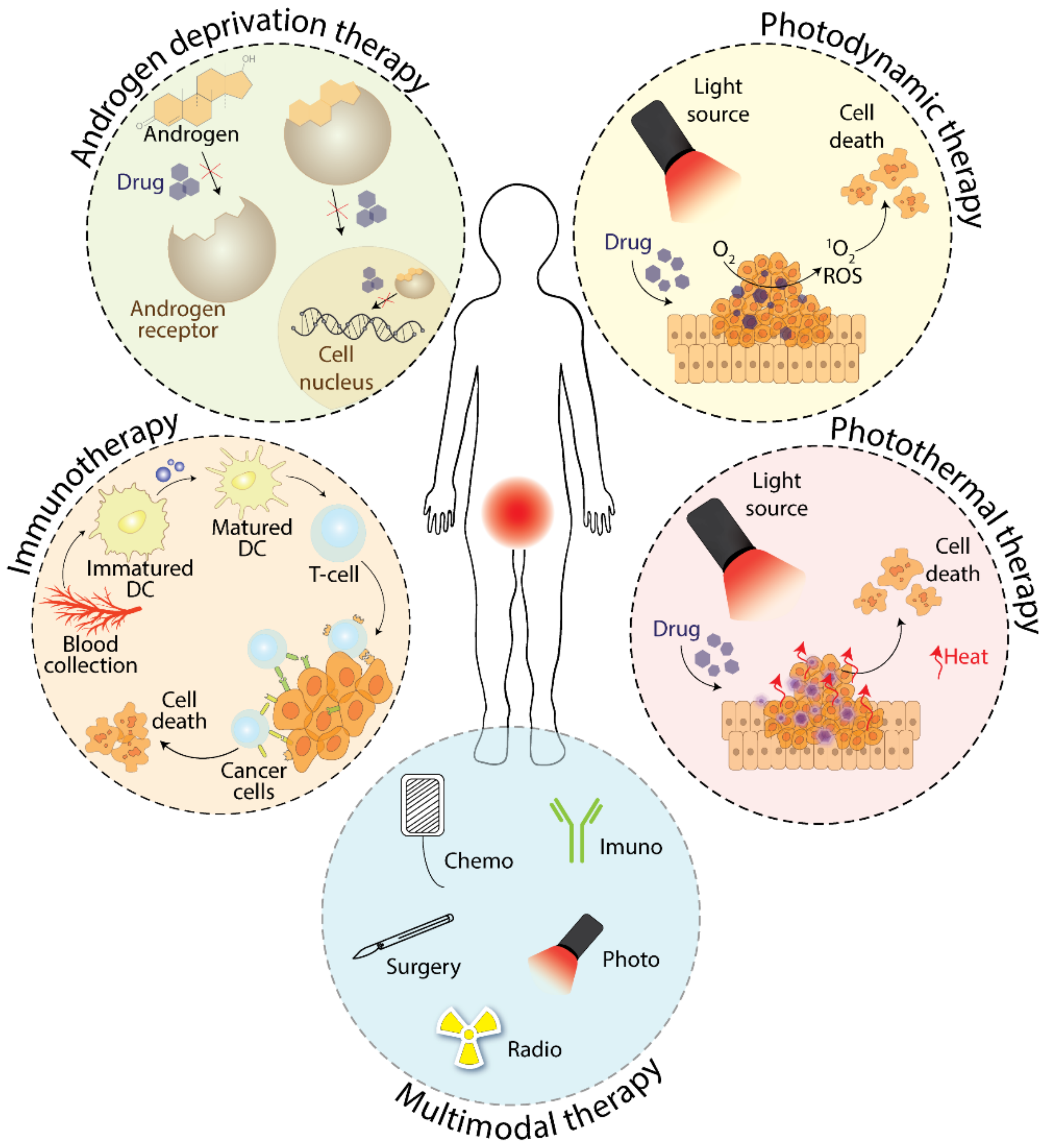
2.2. Treatment with Androgen Deprivation
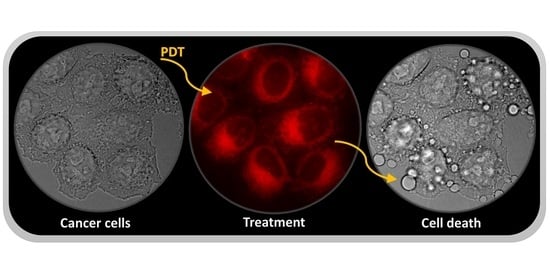
3. Phototherapy as a Tool for Prostate Cancer Treatment
3.1. Photodynamic Therapy
3.2. Photothermal Therapy
4. Immunotherapy in the Therapy of Prostate Cancer
5. Multimodal Therapy of Prostate Cancer
6. Poly(ADP-Ribose) Polymerase Inhibitors
7. Akt Inhibitors
8. Cyclin-Dependent Kinase Inhibitors
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADT | Androgen-deprivation therapy |
| Akt | Protein kinase B |
| AR | The androgen receptor |
| AuNC SiO2 | Silica-coated gold-nanoparticle clusters |
| CAR T | Chimeric antigen receptor T-cell therapy |
| CDK | Cyclin-dependent kinase |
| CPA | Cyproterone acetate |
| CRPC | Castration-resistant prostate cancer |
| CTLA-4 | Cytotoxic T-lymphocyte antigen 4 (CD152) |
| CYP17A1 | Cytochrome P450-17A1 |
| DC | Dendritic cells |
| DCVAC/PCa | Autologous active cellular immunotherapy based on activated dendritic cells |
| DHT | Dihydroxytestosterone |
| DU145 | Human cells from prostate carcinoma |
| EMA | European medicines agency |
| FDA | U.S. Food and Drug Administration |
| FSH | Follicle-stimulating hormone |
| GCPII | Glutamate carboxypeptidase II |
| GNPs | Gold nanoparticles |
| HER2/neu | Receptor tyrosine-protein kinase erbB-2 |
| LH | Luteinizing hormone |
| LHRH | Luteinizing hormone-releasing hormone |
| LNCaP | Human cells from prostate carcinoma |
| mCRPC | Metastatic CRPC |
| NNALADase I | N-acetyl-L-aspartyl-L-glutamate peptidase I |
| PAP | Prostatic acid phosphatase |
| PARP | Poly(ADP-ribose) polymerase |
| PARPi | Poly(ADP-ribose) polymerase inhibitors |
| PC-3 | Human cells from prostate carcinoma |
| PD-1 | Programmed cell death 1 receptor (CD279) |
| PD-L1 | Ligand 1 of programmed cell death receptor |
| PD-L2 | Ligand 2 of programmed cell death receptor |
| PDT | Photodynamic therapy |
| PIP3 | Phosphatidylinositol-3,4,5-trisphosphate |
| PS | Photosensitizer |
| PSA | Prostate-specific antigen |
| PSMA | Prostate-specific membrane antigen |
| PTEN | Phosphatase and tensin homologue |
| PTT | Photothermal therapy |
| ROS | Reactive oxygen species |
| SPECT | Single-photon emission computed tomography |
| VGFA | Vascular endothelial growth factor |
| VTP | Vascular-targeted photodynamic therapy |
References
- Lonergan, P.E.; Tindall, D.J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 2011, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Schaufele, F.; Carbonell, X.; Guerbadot, M.; Borngraeber, S.; Chapman, M.S.; Ma, A.A.; Miner, J.N.; Diamond, M.I. The structural basis of androgen receptor activation: Intramolecular and intermolecular amino-carboxy interactions. Proc. Natl. Acad. Sci. USA 2005, 102, 9802–9807. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J. The role of antiandrogen monotherapy in the treatment of prostate cancer. BJU Int. 2003, 91, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Potosky, A.L.; Knopf, K.; Clegg, L.X.; Albertsen, P.C.; Stanford, J.L.; Hamilton, A.S.; Gilliland, F.D.; Eley, J.W.; Stephenson, R.A.; Hoffman, R.M. Quality-of-life outcomes after primary androgen deprivation therapy: Results from the prostate cancer outcomes study. J. Clin. Oncol. 2001, 19, 3750–3757. [Google Scholar] [CrossRef]
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef]
- Iversen, P. Antiandrogen monotherapy: Indications and results. Urology 2002, 60, 64–71. [Google Scholar] [CrossRef]
- Singh, S.M.; Gauthier, S.; Labrie, F. Androgen receptor antagonists (antiandrogens): Structure-activity relationships. Curr. Med. Chem. 2000, 7, 211–247. [Google Scholar] [CrossRef]
- Rice, M.A.; Malhotra, S.V.; Stoyanova, T. Second-generation antiandrogens: From discovery to standard of care in castration resistant prostate cancer. Front Oncol. 2019, 9, 801. [Google Scholar] [CrossRef]
- Kenny, B.; Ballard, S.; Blagg, J.; Fox, D. Pharmacological options in the treatment of benign prostatic hyperplasia. J. Med. Chem. 1997, 40, 1293–1315. [Google Scholar] [CrossRef]
- Schellhammer, P.; Sharifi, R.; Block, N.; Soloway, M.; Venner, P.; Patterson, A.L.; Sarosdy, M.; Vogelzang, N.; Jones, J.; Kolvenbag, G. A controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy, in patients with advanced prostate cancer. Urology 1995, 45, 745–752. [Google Scholar] [CrossRef]
- Ito, Y.; Sadar, M.D. Enzalutamide and blocking androgen receptor in advanced prostate cancer: Lessons learnt from the history of drug development of antiandrogens. Res. Rep. Urol. 2018, 10, 23–32. [Google Scholar] [CrossRef]
- Rathkopf, D.; Scher, H.I. Androgen receptor antagonists in castration-resistant prostate cancer. Cancer, J. 2013, 19, 43–49. [Google Scholar] [CrossRef]
- Tan, P.S.; Haaland, B.; Montero, A.J.; Kyriakopoulos, C.E.; Lopes, G. Enzalutamide and abiraterone acetate in the treatment of metastatic castration-resistant prostate cancer (mCRPC) post-docetaxel-an indirect comparison. Clin. Med. Insights Oncol. 2014, 8, 29–36. [Google Scholar] [CrossRef]
- Tran, C.; Ouk, S.; Clegg, N.J.; Chen, Y.; Watson, P.A.; Arora, V.; Wongvipat, J.; Smith-Jones, P.M.; Yoo, D.; Kwon, A.; et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009, 324, 787–790. [Google Scholar] [CrossRef]
- FDA. Available online: http://www.fda.gov (accessed on 1 November 2019).
- Rathkopf, D.E.; Scher, H.I. Apalutamide for the treatment of prostate cancer. Expert Rev. Anticancer Ther. 2018, 18, 823–836. [Google Scholar] [CrossRef]
- Shore, N.D. Darolutamide (ODM-201) for the treatment of prostate cancer. Expert Opin. Pharmacother. 2017, 18, 945–952. [Google Scholar] [CrossRef]
- Attard, G.; Reid, A.H.; Yap, T.A.; Raynaud, F.; Dowsett, M.; Settatree, S.; Barrett, M.; Parker, C.; Martins, V.; Folkerd, E.; et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 2008, 26, 4563–4571. [Google Scholar] [CrossRef]
- Clegg, N.J.; Wongvipat, J.; Joseph, J.D.; Tran, C.; Ouk, S.; Dilhas, A.; Chen, Y.; Grillot, K.; Bischo, E.D.; Cail, L.; et al. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012, 72, 1494–1503. [Google Scholar] [CrossRef]
- Moilanen, A.M.; Riikonen, R.; Oksala, R.; Ravanti, L.; Aho, E.; Wohlfahrt, G.; Nykänen, P.S.; Törmäkangas, O.P.; Palvimo, J.J.; Kallioa, P.J. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci. Rep. 2015, 5, 12007. [Google Scholar] [CrossRef]
- Chen, Y.; Clegg, N.J.; Scher, H.I. Anti-androgens and androgen-depleting therapies in prostate cancer: New agents for an established target. Lancet Oncol. 2009, 10, 981–991. [Google Scholar] [CrossRef]
- Hammerer, P.; Madersbacher, S. Landmarks in hormonal therapy for prostate cancer. BJU Int. 2012, 110, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Staffurth, J. Hormonal therapy for cancer. Medicine 2016, 44, 30–33. [Google Scholar] [CrossRef]
- Pullar, B.; Shah, N. Prostate cancer. Surgery 2016, 34, 505–511. [Google Scholar] [CrossRef]
- Omlin, A.; Pezaro, C.; Mukherji, D.; Cassidy, A.M.; Sandhu, S.; Bianchini, D.; Olmos, D.; Ferraldeschi, R.; Maier, G.; Thompson, E.; et al. Improved survival in a cohort of trial participants with metastatic castration-resistant prostate cancer demonstrates the need for updated prognostic nomograms. Eur. Urol. 2013, 64, 300–306. [Google Scholar] [CrossRef]
- Schally, A.V.; Block, N.L.; Rick, F.G. New therapies for relapsed castration-resistant prostate cancer based on peptide analogs of hypothalamic hormones. Asian, J. Androl. 2015, 17, 925–928. [Google Scholar] [CrossRef]
- Kotake, T.; Usami, M.; Akaza, H.; Koiso, K.; Homma, Y.; Kawabe, K.; Aso, Y.; Orikasa, S.; Shimazaki, J.; Isaka, S.; et al. Goserelin acetate with or without antiandrogen or estrogen in the treatment of patients with advanced prostate cancer: A multicenter, randomized, controlled trial in Japan. Jpn. J. Clin. Oncol. 1999, 29, 562–570. [Google Scholar] [CrossRef][Green Version]
- FDA. Available online: http://www.accessdata.fda.gov (accessed on 6 November 2019).
- Heidenreich, A.; Bellmunt, J.; Bolla, M.; Joniau, S.; Mason, M.; Matveev, V.; Mottet, N.; Schmid, H.P.; van der Kwast, T.; Wiegel, T.; et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 2011, 59, 61–71. [Google Scholar] [CrossRef]
- Klotz, L.; Miller, K.; Crawford, E.D.; Shore, N.; Tombal, B.; Karup, C.; Malmberg, A.; Persson, B.E. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur. Urol. 2014, 66, 1101–1108. [Google Scholar] [CrossRef]
- Stricker, H.J. Luteinizing hormone–releasing hormone antagonists in prostate cancer. Urology 2001, 58, 24–27. [Google Scholar] [CrossRef]
- EMA. Available online: http://www.ema.eu (accessed on 6 November 2019).
- Kiratli, B.J.; Srinivas, S.; Perkash, I.; Terris, M.K. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology 2001, 57, 127–132. [Google Scholar] [CrossRef]
- Skolarus, T.A.; Caram, M.V.; Shahinian, V.B. Androgen-deprivation-associated bone disease. Curr. Opin. Urol. 2014, 24, 601–607. [Google Scholar] [CrossRef]
- Seidell, J.C.; Bjorntorp, P.; Sjostrom, L.; Kvist, H.; Sannerstedt, R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 1990, 39, 897–901. [Google Scholar] [CrossRef]
- Ferroni, C.; Del Rio, A.; Martini, C.; Manoni, E.; Varchi, G. Light-induced therapies for prostate cancer treatment. Front. Chem. 2019, 7, 719. [Google Scholar] [CrossRef]
- Lian, H.; Wu, J.; Hu, Y.; Guo, H. Self-assembled albumin nanoparticles for combination therapy in prostate cancer. Int. J. Nanomed. 2017, 12, 7777–7787. [Google Scholar] [CrossRef]
- Miller, J.D.; Baron, E.D.; Scull, H.; Hsia, A.; Berlin, J.C.; McCormick, T.; Colussi, V.; Kenney, M.E.; Cooper, K.D.; Oleinick, N.L. Photodynamic therapy with the phthalocyanine photosensitizer Pc 4: The case experience with preclinical mechanistic and early clinical-translational studies. Toxicol. Appl. Pharmacol. 2007, 224, 290–299. [Google Scholar] [CrossRef]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.T.; Heidi, A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photod. Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in photosensitizers and light delivery for photodynamic therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef]
- Darmostuk, M.; Rimpelova, S.; Gbelcova, H.; Ruml, T. Current approaches in SELEX: An update to aptamer selection technology. Biotechnol. Adv. 2015, 33, 1141–1161. [Google Scholar] [CrossRef]
- Pavlíčková, V.; Jurášek, M.; Rimpelová, S.; Záruba, K.; Sedlák, D.; Šimková, M.; Kodr, D.; Staňková, E.; Fähnrich, J.; Rottnerová, Z.; et al. Oxime-based 19-nortestosterone–pheophorbide a conjugate: Bimodal controlled release concept for PDT. J. Mater. Chem. B 2019, 7, 5465–5477. [Google Scholar] [CrossRef]
- Peterková, L.; Kmoníčková, E.; Ruml, T.; Rimpelová, S. Sarco/endoplasmic reticulum calcium ATPase inhibitors: Beyond anticancer perspective. J. Med. Chem. 2020, 63, 1937–1963. [Google Scholar] [CrossRef]
- Zimmermann, T.; Drašar, P.; Rimpelová, S.; Christensen, S.B.; Khripach, V.A.; Jurášek, M. Large scale conversion of trilobolide into the payload of mipsagargin: 8-O-(12-aminododecanoyl)-8-O-debutanoylthapsigargin. Biomolecules 2020, 10, 1640. [Google Scholar] [CrossRef]
- Tomanová, P.; Rimpelová, S.; Jurášek, M.; Buděšínský, M.; Vejvodová, L.; Ruml, T.; Kmoníčková, E.; Drašar, P.B. Trilobolide–porphyrin conjugates: On synthesis and biological effects evaluation. Steroids 2015, 97, 8–12. [Google Scholar] [CrossRef]
- Kozlowska, D.; Foran, P.; MacMahon, P.; Shelly, M.J.; Eustace, S.; O’Kennedy, R. Molecular and magnetic resonance imaging: The value of immunoliposomes. Adv. Drug Deliv. Rev. 2009, 61, 1402–1411. [Google Scholar] [CrossRef]
- Liu, T.; Wu, L.Y.; Choi, J.K.; Berkman, C.E. In vitro targeted photodynamic therapy with a pyropheophorbide-a conjugated inhibitor of prostate specific membrane antigen. Prostate 2009, 69, 585–594. [Google Scholar] [CrossRef]
- Wang, X.; Tsui, B.; Ramamurthy, G.; Zhang, P.; Meyers, J.; Kenney, M.E.; Kiechle, J.; Ponsky, L.; Basilion, J.P. Theranostic agents for photodynamic therapy of prostate cancer by targeting prostate-specific membrane antigen. Mol. Cancer Ther. 2016, 15, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007, 9, E128–E147. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Guo, S.; Lin, T.; Guo, H. Highly effective photothermal chemotherapy with pH-responsive polymer-coated drug-loaded melanin-like nanoparticles. Int. J. Nanomed. 2017, 12, 1827–1840. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, Y.; He, Z.; Jiang, L.P.; Zhu, J.J. NIR-triggered chemo-photothermal therapy by thermosensitive gold nanostarmesoporous silicaliposome-composited drug delivery systems. ACS Appl. Biomater. 2020, 3, 5322–5330. [Google Scholar] [CrossRef]
- Motlagh, N.S.J.; Parvin, P.; Mirzaie, Z.H.; Karimi, R.; Sanderson, J.H.; Atyabi, F. Synergistic performance of triggered drug release and photothermal therapy of MCF7 cells based on laser activated PEGylated GO + DOX. Biomed. Opt. Express 2020, 3783–3794. [Google Scholar] [CrossRef]
- Tran, V.A.; Vo, V.G.; Shim, K.; Lee, S.W.; An, S.S.A. Multimodal mesoporous silica nanocarriers for dual stimuli-responsive drug release and excellent photothermal ablation of cancer cells. Int. J. Nanomed. 2020, 15, 7667–7685. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Hua, M.; Yu, H.; Wei, S.; Wang, A.; Zhou, J. Photothermal-triggered controlled drug release from mesoporous silica nanoparticles based on base-pairing rules. ACS Biomater. Sci. Eng. 2019, 5, 2399–2408. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, X.; Shen, S.; Wang, X. Doxorubicin-loaded magnetic nanoparticle clusters for chemo-photothermal treatment of the prostate cancer cell line PC3. Biochem. Biophys. Res. Commun. 2015, 466, 278–282. [Google Scholar] [CrossRef]
- Qiang, L.; Cai, Z.; Jiang, W.; Liu, J.; Tai, Z.; Li, G.; Gong, C.; Gao, S.; Gao, Y. A novel macrophage-mediated biomimetic delivery system with NIR-triggered release for prostate cancer therapy. J. Nanobiotechnol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Doughty, A.C.; Hoover, A.R.; Layton, E.; Murray, C.K.; Howard, E.W.; Chen, W.R. Nanomaterial applications in photothermal therapy for cancer. Materials 2019, 12, 779. [Google Scholar] [CrossRef]
- Riley, R.S.; Day, E.S. Gold nanoparticle-mediated photothermal therapy: Applications and opportunities for multimodal cancer treatment. Wiley Interdiscip. Rev. Nanomed. 2017, 9, e1449. [Google Scholar] [CrossRef]
- Kim, J.; Chun, S.H.; Amornkitbamrung, L.; Song, C.; Yuk, J.S.; Ahn, S.Y.; Kim, B.W.; Lim, Y.T.; Oh, B.K.; Um, S.H. Gold nanoparticle clusters for the investigation of therapeutic efficiency against prostate cancer under near-infrared irradiation. Nano Converg. 2020, 7, 5. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef]
- Huang, L.; Xu, C.; Xu, P.; Qin, Y.; Chen, M.; Feng, Q.; Wen, X.; Wang, Y.; Shi, Y.; Cheng, Y. Intelligent photosensitive mesenchymal stem cells and cell-derived microvesicles for photothermal therapy of prostate cancer. Nanotheranostic 2019, 3, 41–53. [Google Scholar] [CrossRef]
- Stern, J.M.; Solomonov, V.V.K.; Sazykina, E.; Schwartz, J.A.; Gad, S.C.; Goodrich, G.P. Initial evaluation of the safety of nanoshell-directed photothermal therapy in the treatment of prostate disease. Int. J. Toxicol. 2016, 35, 38–46. [Google Scholar] [CrossRef]
- Gad, S.C.; Sharp, K.L.; Montgomery, C.; Payne, J.D.; Goodrich, G.P. Evaluation of the toxicity of intravenous delivery of auroshell particles (gold–silica nanoshells). Int. J. Toxicol. 2012, 31, 584–594. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Slovin, S. Chemotherapy and immunotherapy combination in advanced prostate cancer. Clin. Adv. Hematol. Oncol. 2012, 10, 90–100. [Google Scholar] [CrossRef]
- Cordes, L.M.; Gulley, J.L.; Madan, R.A. Perspectives on the clinical development of immunotherapy in prostate cancer. Asian J. Androl. 2018, 20, 253–259. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef]
- Steinman, R.M. Lasker basic medical research award. Dendritic cells: Versatile controllers of the immune system. Nat. Med. 2007, 13, 1155–1159. [Google Scholar] [CrossRef]
- Handy, C.E.; Antonarakis, E.S. Sipuleucel-T for the treatment of prostate cancer: Novel insights and future directions. Future Oncol. 2017, 14, 907–917. [Google Scholar] [CrossRef] [PubMed]
- FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/provenge-sipuleucel-t (accessed on 9 January 2019).
- Podrazil, M.; Horvath, R.; Becht, E.; Rozkova, D.; Bilkova, P.; Sochorova, K.; Hromadkova, H.; Kayserova, J.; Vavrova, K.; Lastovicka, J.; et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2015, 6, 18192–18205. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Cursano, M.C.; Casadei, C.; Menna, C.; Altavilla, A.; Lolli, C.; Cerchione, C.; Paganelli, G.; Santini, D.; Tonini, G.; et al. CAR-T cell therapy: A potential new strategy against prostate cancer. J. Immunother. Cancer 2019, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Farolfi, A.; Conteduca, V.; Martignano, F.; De Lisi, D.; Ravaglia, G.; Rossi, L.; Menna, C.; Bellia, R.S.; Barone, D.; et al. Immunotherapy for prostate cancer: Where we are headed. Int. J. Mol. Sci. 2017, 18, 2627. [Google Scholar] [CrossRef]
- Hansen, A.R.; Massard, C.; Ott, P.A.; Haas, N.B.; Lopez, J.S.; Ejadi, S.; Wallmark, J.M.; Keam, B.; Delord, J.P.; Aggarwal, R.; et al. Pembrolizumab for advanced prostate adenocarcinoma: Findings of the KEYNOTE-028 study. Ann. Oncol. 2018, 29, 1807–1813. [Google Scholar] [CrossRef]
- Adam, M.; Tennstedt, P.; Lanwehr, D.; Tilki, D.; Steuber, T.; Beyer, B.; Thederan, I.; Heinzer, H.; Haese, A.; Salomon, G.; et al. Functional outcomes and quality of life after radical prostatectomy only versus a combination of prostatectomy with radiation and hormonal therapy. Eur. Urol. 2017, 71, 330–336. [Google Scholar] [CrossRef]
- Seisen, T.; Abdollah, F. Surgery-based multimodal management of high-risk prostate cancer patients: What is the functional price to pay for optimal disease control? Eur. Urol. 2017, 71, 337–339. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, P.Y. Combinations in multimodality treatments and clinical outcomes during cancer. Oncol. Letters 2016, 12, 4301–4304. [Google Scholar] [CrossRef]
- Koupparis, A.; Gleave, M.E. Multimodal approaches to high-risk prostate cancer. Curr. Oncol. 2010, 17, S33–S37. [Google Scholar] [CrossRef]
- Lee, S.U.; Cho, K.H. Multimodal therapy for locally advanced prostate cancer: The roles of radiotherapy, androgen deprivation therapy, and their combination. Rad. Oncol. J. 2017, 35, 189–197. [Google Scholar] [CrossRef]
- Azzouzi, A.R.; Barret, E.; Moore, C.M.; Villers, A.; Allen, C.; Scherz, A.; Muir, G.; de Wildt, M.; Barber, N.J.; Lebdai, S.; et al. TOOKAD® Soluble vascular-targeted photodynamic (VTP) therapy: Determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013, 112, 766–774. [Google Scholar] [CrossRef]
- Borle, F.; Radu, A.; Fontolliet, C.; van den Bergh, H.; Monnier, P.; Wagnières, G. Selectivity of the photosensitiser TOOKAD® for photodynamic therapy evaluated in the Syrian golden hamster cheek pouch tumour model. Br. J. Cancer 2003, 89, 2320–2326. [Google Scholar] [CrossRef][Green Version]
- Koudinova, N.V.; Pinthus, J.H.; Brandis, A.; Brenner, O.; Bendel, P.; Ramon, J.; Eshhar, Z.; Scherz, A.; Salomon, Y. Photodynamic therapy with Pd-Bacteriopheophorbide (TOOKAD): Successful in vivo treatment of human prostatic small cell carcinoma xenografts. Int. J. Cancer 2003, 104, 782–789. [Google Scholar] [CrossRef]
- Azzouzi, A.R.; Lebdai, S.; Benzaghou, F.; Stief, C. Vascular-targeted photodynamic therapy with TOOKAD® Soluble in localized prostate cancer: Standardization of the procedure. World J. Urol. 2015, 33, 937–944. [Google Scholar] [CrossRef]
- Rapozzi, V.; Ragno, D.; Guerrini, A.; Ferroni, C.; della Pietra, E.; Cesselli, D.; Castoria, G.; Di Donato, M.; Saracino, E.; Benfenati, V.; et al. Androgen receptor targeted conjugate for bimodal photodynamic therapy of prostate cancer in vitro. Biocon. Chem. 2015, 26, 1662–1671. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-infrared photoimmunotherapy targeting prostate cancer with prostate-specific membrane antigen (PSMA) antibody. Mol. Cancer Res. 2017, 15, 1153–1162. [Google Scholar] [CrossRef]
- Fucikova, J.; Moserova, I.; Truxova, I.; Hermanova, I.; Vancurova, I.; Partlova, S.; Fialova, A.; Sojka, L.; Cartron, P.F.; Houska, M.; et al. High hydrostatic pressure induces immunogenic cell death in human tumor cells. Int. J. Cancer 2014, 135, 1165–1177. [Google Scholar] [CrossRef]
- Melichar, B.; Študentova, H.; Kalábová, H.; Vitásková, D.; Čermáková, P.; Hormychová, H.; Ryška, A. Predictive and prognostic significance of tumor-infiltrating lymphocytes in patients with breast cancer treated with neoadjuvant systemic therapy. Anticancer Res. 2014, 34, 1115–1125. [Google Scholar]
- McDonnell, A.M.; Nowak, A.K.; Lake, R.A. Contribution of the immune system to the chemotherapeutic response. Semin. Immunopathol. 2011, 33, 353–367. [Google Scholar] [CrossRef]
- Škubník, J.; Jurášek, M.; Ruml, T.; Rimpelová, S. Mitotic poisons in research and medicine. Molecules 2020, 25, 4632. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Vogelzang, N.J.; Chatta, G.S.; Fleming, M.T.; Smith, D.C.; Appleman, L.J.; Hussain, A.; Modiano, M.; Singh, P.; Tagawa, S.T.; et al. PSMA ADC monotherapy in patients with progressive metastatic castration-resistant prostate cancer following abiraterone and/or enzalutamide: Efficacy and safety in open-label single-arm phase 2 study. Prostate 2020, 80, 99–108. [Google Scholar] [CrossRef]
- Tekin, V.; Aweda, T.; Guldu, O.K.; Muftuler, Z.B.; Bartels, J.; Lapib, S.E.; Unak, P. A novel anti-angiogenic radio/photo sensitizer for prostate cancer imaging and therapy: 89Zr-Pt@TiO2-SPHINX, synthesis and in vitro evaluation. Nucl. Med. Biol. 2021, 94–95. [Google Scholar] [CrossRef]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef]
- Patel, A.G.; Sarkaria, J.N.; Kaufmanna, S.H. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc. Natl. Acad. Sci. USA 2011, 108, 3406–3411. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Boehler, C.; Barbat, J.G.; Bonnet, M.E.; Illuzzi, G.; Ronde, P.; Gauthier, L.R.; Magroun, N.; Rajendran, A.; Lopez, B.S.; et al. PARP3 affects the relative contribution of homologous recombination and nonhomologous end-joining pathways. Nucleic Acids Res. 2014, 42, 5616–5632. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02975934 (accessed on 15 February 2021).
- Smith, M.R.; Sandhu, S.K.; Kelly, W.K.; Scher, H.I.; Efstathiou, E.; Lara, P.N.; Yu, E.Y.; George, D.J.; Chi, K.N.; Saad, F.; et al. LBA50—Pre-specified interim analysis of GALAHAD: A phase II study of niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD). Ann. Oncol. 2019, 30, v884–v885. [Google Scholar] [CrossRef]
- De Bono, J.S.; Mehra, N.; Higano, C.S.; Saad, F.; Buttigliero, C.; Mata, M.; Chen, H.C.; Healy, C.G.; Paccagnella, M.L.; Czibere, A.; et al. TALAPRO-1: A phase II study of talazoparib (TALA) in men with DNA damage repair mutations (DDRmut) and metastatic castration-resistant prostate cancer (mCRPC)—First interim analysis (IA). J. Clin. Oncol. 2020, 38, 119. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03395197 (accessed on 15 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT01576172 (accessed on 15 February 2021).
- Clinical Trials. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03712930?term=pamiparib&cond=prostate&draw=2&rank=1 (accessed on 15 February 2021).
- Chowdhury, S.; Mateo, J.; Gross, M.; Armstrong, A.J.; Cruz-Correa, M.; Piulats, J.M.; Blay, J.Y.; Li, M.; Rivas, D.; Quintero, L.; et al. Pamiparib, an investigational PARP inhibitor, in patients with metastatic castration-resistant prostate cancer (mCRPC) and a circulating tumor cell (CTC) homologous recombination deficiency (HRD) phenotype or BRCA defects: A trial in progress. J. Clin. Oncol. 2019, 37 (Suppl 15). [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03572478 (accessed on 15 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03012321 (accessed on 15 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03732820 (accessed on 15 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03317392 (accessed on 15 February 2021).
- Nizialek, E.; Antonarakis, E.S. PARP inhibitors in metastatic prostate cancer: Evidence to date. Cancer Manag. Res. 2020, 12, 8105–8114. [Google Scholar] [CrossRef]
- Powers, E.; Karachaliou, G.S.; Kao, C.; Harrison, M.R.; Hoimes, C.J.; George, D.J.; Armstrong, A.J.; Zhang, T. Novel therapies are changing treatment paradigms in metastatic prostate cancer. J. Hematol. Oncol. 2020, 13, 144. [Google Scholar] [CrossRef]
- Pezaro, C. PARP inhibitor combinations in prostate cancer. Ther. Adv. Med. Oncol. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://www.clinicaltrials.gov/ (accessed on 9 April 2021).
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef]
- Braglia, L.; Zavatti, M.; Vinceti, M.; Martelli, A.M.; Marmiroli, S. Deregulated PTEN/PI3K/AKT/mTOR signaling in prostate cancer: Still a potential druggable target? Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118731. [Google Scholar] [CrossRef]
- Grünwald, V.; DeGraffenried, L.; Russel, D.; Friedrichs, W.E.; Ray, R.B.; Hidalgo, M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002, 62, 6141–6145. [Google Scholar] [CrossRef]
- Skvortsova, I.; Skvortsov, S.; Stasyk, T.; Raju, U.; Popper, B.A.; Schiestl, B.; von Guggenber, E.; Neher, A.; Bonn, G.K.; Huber, L.K.; et al. Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Cell Biol. 2008, 8, 4521–4533. [Google Scholar] [CrossRef]
- De Bono, J.S.; De Giorgi, U.; Rodrigues, D.N.; Massard, C.; Bracarda, S.; Font, A.; Arija, J.A.A.; Shih, K.C.; Radavoi, G.D.; Xu, N.; et al. Randomized phase II study evaluating Akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 2019, 25, 2019. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03072238 (accessed on 16 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03673787 (accessed on 16 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04404140 (accessed on 16 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04493853 (accessed on 16 February 2021).
- Clinical Trials. Available online: https://www.clinicaltrials.gov/ct2/show/NCT04087174 (accessed on 16 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02121639 (accessed on 16 February 2021).
- Crabb, S.J.; Griffiths, G.; Marwood, E.; Dunkley, D.; Downs, N.; Martin, K.; Light, M.; Northey, J.; Wilding, S.; Whitehead, A.; et al. Pan-AKT Inhibitor Capivasertib With Docetaxel and Prednisolone in Metastatic Castration-Resistant Prostate Cancer: A Randomized, Placebo-Controlled Phase II Trial (ProCAID). J. Clin. Oncol. 2021, 39, 190–201. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://repository.icr.ac.uk/bitstream/handle/internal/3528/jco.19.00368.pdf?sequence=2&isAllowed=y (accessed on 17 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02905318 (accessed on 17 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02059213 (accessed on 17 February 2021).
- Palmbos, P.L.; Tomlins, S.A.; Daignault, S.; Agarwal, N.; Twardowski, P.; Morgans, A.K.; Kelly, W.K.; Arora, V.; Antonarakis, E.S.; Siddiqui, J.; et al. Clinical outcomes and markers of treatment response in a randomized phase II study of androgen deprivation therapy with or without palbociclib in RB-intact metastatic hormone-sensitive prostate cancer (mHSPC). J. Clin. Oncol. 2020, 38, 5573. Available online: https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.5573 (accessed on 17 February 2021). [CrossRef]
- Palmbos, P.L.; Tomlins, S.A.; Agarwal, N.; Twardowski, P.; Morgans, A.K.; Kelly, W.K.; Arora, V.; Antonarakis, E.S.; Siddiqui, J.; Daignault, S.; et al. Cotargeting AR signaling and cell cycle: A randomized phase II study of androgen deprivation therapy with or without palbociclib in RB-positive metastatic hormone sensitive prostate cancer (mHSPC). J. Clin. Oncol. 2018, 36, 251. Available online: https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.6_suppl.251 (accessed on 17 February 2021). [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02555189?term=Ribociclib&cond=prostate+cancer&draw=2&rank=1 (accessed on 17 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02494921 (accessed on 17 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04408924?term=Abemaciclib&cond=Prostate+Cancer&draw=2&rank=3 (accessed on 17 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04298983 (accessed on 17 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT03706365 (accessed on 17 February 2021).
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT04751929?term=Abemaciclib&cond=Prostate+Cancer&draw=2&rank=5 (accessed on 17 February 2021).
| PARPi | Combination | Indication | Status | Phase | Clinical Trial Identifier |
|---|---|---|---|---|---|
| Olaparib | Monotherapy | mCRPC, a mutation in one of 15 genes involved in the HRR | n.r. | III | NTC02987543 |
| Monotherapy | Advanced CRPC | n.r. | II | NCT01682772 | |
| Monotherapy | mCRPC, after docetaxel treatment | n.r. | II | NCT03434158 | |
| Monotherapy | Non-mBRPC | Recruiting | II | NCT03047135 | |
| Prior to radical prostatectomy | localized PC | Terminated | II | NCT03570476 | |
| Prior to radical prostatectomy | Locally advanced PC with DNA repair defects | Recruiting | II | NCT03432897 | |
| Ceralasertib | mCRPC, with DNA repair-deficient | Recruiting | II | NCT03787680 | |
| Bromodomain and extraterminal inhibitor (AZD5153) | Malignant solid tumors | n.r. | I | NCT03205176 | |
| AA | mCRPC, prior chemotherapy containing docetaxel | n.r. | II | NCT01972217 | |
| AA, P | mCRPC, with DNA repair-deficient | Recruiting | II | NCT03012321 | |
| PLX2853, AA, P | mCRPC | Recruiting | Ib/IIa | NCT04556617 | |
| MEDI4736, cediranib | Advanced solid tumors | Recruiting | I/II | NCT02484404 | |
| AA | mCRPC, no prior chemotherapy or new hormonal agents | n.r. | III | NCT03732820 | |
| Testosterone | mCRPC | n.r. | II | NCT03516812 | |
| Degarelix, prior to radical prostatectomy | Intermediate/high risk PC | Completed | I | NCT02324998 | |
| Cediranib | mCRPC | n.r. | II | NCT02893917 | |
| Nanoparticle camptothecin | mCRPC, relapsed/refractory small cell lung cancer | Recruiting | I/II | NCT02769962 | |
| Carboplatin | mCRPC, tumors containing BRCA1, BRCA2 or PALB2 | Recruiting | II | NCT04038502 | |
| Carboplatin, cabazitaxel, P | Aggressive variant PC | n.r. | II | NCT03263650 | |
| Pembrolizumab | mCRPC, failed to respond to either AA or enzalutamide (but not both), and chemotherapy | Recruiting | III | NCT03834519 | |
| Radiation, ADT, AA, P | CSOmPC | n.y.r. | II | NCT04748042 | |
| Pembrolizumab | mCRPC | Recruiting | Ib/II | NCT02861573 | |
| Durvalumab | CSBR non-mPC, harboring mutations in DNA damage repair | Recruiting | II | NCT03810105 | |
| 177Lu-PSMA | mCRPC | Recruiting | I | NCT03874884 | |
| Ra-223 dichloride | mCRPC | Recruiting | I/II | NCT03317392 | |
| Rucaparib | Monotherapy | mCRPC, HRR gene deficiency | Recruiting | III | NCT02975934 |
| Monotherapy | mHSPC | Recruiting | II | NCT03413995 | |
| Monotherapy | Non-mHSPC | Recruiting | NCT03533946 | ||
| Monotherapy | mCRPC, ovarian, epithelial, peritoneal, fallopian tube cancer | n.y.r. | III | NCT04676334 | |
| Monotherapy | Solid tumors associated with deleterious mutations in HRR genes | Recruiting | II | NCT04171700 | |
| Monotherapy | mCRPC | n.r. | NCT02952534 | ||
| Nivolumab | mCRPC | n.r. | II | NCT03338790 | |
| Nivolumab | PC, endometrial cancer | Terminated | Ib/IIa | NCT03572478 | |
| Ipatasertib | Advanced BC, OC, PC | n.r. | I/II | NCT03840200 | |
| Enzalutamide, AA | mCRPC | n.r. | Ib | NCT04179396 | |
| Enzalutamide | mCRPC, resistant to testosterone-deprivation therapy | Suspended | III | NCT04455750 | |
| Docetaxel, carboplatin | mCRPC, HRR gene deficiency | Recruiting | II | NCT03442556 | |
| Copanlisib | mCRPC | Recruiting | Ib/II | NCT04253262 | |
| Niraparib | AA, P | mCRPC, HRR gene alteration | Recruiting | III | NCT03748641 |
| AA, P | mCSPC, deleterious germline or somatic HRR gene-mutated | Recruiting | III | NCT04497844 | |
| AA, P | mCRPC, with and without HRR gene alterations | Recruiting | I | NCT04577833 | |
| Cetrelimab, AA, P | mCRPC | Recruiting | I/II | NCT03431350 | |
| Monotherapy | Platinum sensitive CRPC | Recruiting | II | NCT04288687 | |
| Apalutamide, AA, P | mCRPC | Completed | I | NCT02924766 | |
| Monotherapy | mCRPC and DNA repair anomalies | n.r. | II | NCT02854436 | |
| Monotherapy | Advanced solid tumor or hematologic malignancies | Completed | I | NCT00749502 | |
| Prostatectomy | Non-metastatic high-risk PC | Recruiting | II | NCT04030559 | |
| Cabazitaxel, carboplatin, cetrelimab | Aggressive variant mPC | Recruiting | II | NCT04592237 | |
| Leuprolide, AA, P, radiotherapy | High risk and node-positive PC | Recruiting | I/II | NCT04194554 | |
| ATR inhibitor (BAY 1895344) | Advanced solid tumors, OC | Recruiting | Ib | NCT04267939 | |
| Radium 223 | mPC, hormone refractory PC, stage IV PC | n.r. | Ib | NCT03076203 | |
| Radiotherapy, antiandrogen therapy | high-risk PC | Recruiting | II | NCT04037254 | |
| Talazoparib | Monotherapy | mCRPC, previously received taxane-based chemotherapy and progressed on at least 1 novel hormonal agent | n.r. | II | NCT03148795 |
| Monotherapy | Advanced cancer, a mutation in DNA damage response genes | Recruiting | II | NCT04550494 | |
| Enzalutamide | mCRPC | Recruiting | III | NCT03395197 | |
| Enzalutamide | mHSPC | Recruiting | II | NCT04332744 | |
| Enzalutamide | mCSPC | n.y.r. | III | NCT04821622 | |
| Glutaminase inhibitor telaglenastat | mCRPC | n.y.r. | II | NCT04824937 | |
| Temozolomide | PC | Recruiting | Ib/II | NCT04019327 | |
| belinostat | mCRPC, mBC, OC | n.y.r. | I | NCT04703920 | |
| ADT | mCSPC | n.y.r. | II | NCT04734730 | |
| Avelumab, bempegaldesleukin | Advanced squamous cell carcinoma of the head and neck, mCRPC | Terminated | II | NCT04052204 | |
| Avelumab | Advanced or metastatic solid tumors | n.r. | Ib/II | NCT03330405 | |
| Monotherapy | Advanced or recurrent solid tumors | Completed | I | NCT01286987 | |
| Monotherapy | Metastatic solid tumors | Withdrawn | I | NCT02567396 | |
| Veliparib | AA, P | mCRPC | Completed | II | NCT01576172 |
| Temozolomide | mPC | Completed | I | NCT01085422 | |
| Monotherapy | Malignant solid tumors | Completed | I | NCT00892736 | |
| Pamiparib | Monotherapy | mCRPC | Terminated | II | NCT03712930 |
| Temozolomide | Locally advanced or metastatic solid tumors | Recruiting | I/II | NCT03150810 |
| Akt Inhibitor | Combination | Indication | Status | Phase | Clinical Trial Identifier |
|---|---|---|---|---|---|
| Ipatasertib | AA | mCRP | n.r. | III | NCT03072238 |
| AA | mCRP, previous treatment with docetaxel | n.r. | I/II | NCT01485861 | |
| Androgen deprivation therapy, darolutamide | Localized, high-risk PC | n.y.r. | I/II | NCT04737109 | |
| Atezolizumab | Advanced solid tumors, glioblastoma multiforme, PC | Recruiting | I/II | NCT03673787 | |
| Atezolizumab, docetaxel | mCRPC previously treated with second-generation AR targeted therapy | Recruiting | Ib | NCT04404140 | |
| Rucaparib | Advanced BC, OC, PC | n.r. | I/II | NCT03840200 | |
| Monotherapy | PC | Recruiting | II | NCT03385655 | |
| Monotherapy | Solid tumors, lymphomas, multiple myeloma | ||||
| Capivasertib | AA, P | mHSPC | Recruiting | III | NCT04493853 |
| AA, enzalutamide | mCRPC | n.r. | I | NCT04087174 | |
| Monotherapy | Solid tumors, lymphomas, multiple myeloma | Recruiting | II | NCT02465060 | |
| Docetaxel, P | mCRPC | n.r. | I/II | NCT02121639 | |
| Monotherapy | mCRPC | Completed | I | NCT01692262 | |
| Enzalutamid | mCRPC | Unknown | II | NCT02525068 | |
| Enzalutamide, fulVestrant | Advanced solid tumors (PC, BC) | n.r. | I | NCT03310541 | |
| Itraconazole | Healthy volunteers (intended indication: metastatic triple negative/HR+ BC, mHSPC) | n.r. | I | NCT04712396 |
| CDK Inhibitor | Combination | Indication | Status | Phase | Clinical Trial Indetifier |
|---|---|---|---|---|---|
| Palbociclib | Monotherapy | mCRPC | Recruiting | II | NCT02905318 |
| bicalutemide, zoladex, lupron depot | Retinoblastoma protein-positive mPC | Completed | II | NCT02059213 | |
| KAT6 inhibitor (PF-07248144) | Advanced or metastatic solid tumors (BC, PC, lung cancer) | Recruiting | I | NCT04606446 | |
| Monotherapy | Advanced refractory solid tumors, lymphomas, multiple myeloma | Recruiting | II | NCT02465060 | |
| Combinations of drug | Metastatic solid tumor or hematological malignancy | Recruiting | Ib | NCT03878524 | |
| Ribociclib | Enzalutamide | HRPC, mPC | Recruiting | Ib/II | NCT02555189 |
| Docetaxel, P | mCRPC | n.r. | II | NCT02494921 | |
| Abemaciclib | Monotherapy | mCRPC previously treated hormonally or with taxane chemotherapy | Recruiting | II | NCT04408924 |
| Androgen deprivation therapy, radiation therapy | Locally advanced PC | Recruiting | II | NCT04298983 | |
| Atezolizumab | mCRPC | Withdrawn | II | NCT04272645 | |
| AA, P | mCRPC | n.r. | II | NCT03706365 | |
| Atezolizumab | mCRPC | n.y.r. | II | NCT04751929 | |
| Combinations of drug | Metastatic solid tumor or hematological malignancy | Recruiting | Ib | NCT03878524 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvízová, J.; Pavlíčková, V.; Kmoníčková, E.; Ruml, T.; Rimpelová, S. Quo Vadis Advanced Prostate Cancer Therapy? Novel Treatment Perspectives and Possible Future Directions. Molecules 2021, 26, 2228. https://doi.org/10.3390/molecules26082228
Kvízová J, Pavlíčková V, Kmoníčková E, Ruml T, Rimpelová S. Quo Vadis Advanced Prostate Cancer Therapy? Novel Treatment Perspectives and Possible Future Directions. Molecules. 2021; 26(8):2228. https://doi.org/10.3390/molecules26082228
Chicago/Turabian StyleKvízová, Jana, Vladimíra Pavlíčková, Eva Kmoníčková, Tomáš Ruml, and Silvie Rimpelová. 2021. "Quo Vadis Advanced Prostate Cancer Therapy? Novel Treatment Perspectives and Possible Future Directions" Molecules 26, no. 8: 2228. https://doi.org/10.3390/molecules26082228
APA StyleKvízová, J., Pavlíčková, V., Kmoníčková, E., Ruml, T., & Rimpelová, S. (2021). Quo Vadis Advanced Prostate Cancer Therapy? Novel Treatment Perspectives and Possible Future Directions. Molecules, 26(8), 2228. https://doi.org/10.3390/molecules26082228