α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus pulegioides, Phytotoxicity, and Antimicrobial Effects
Abstract
1. Introduction
2. Results
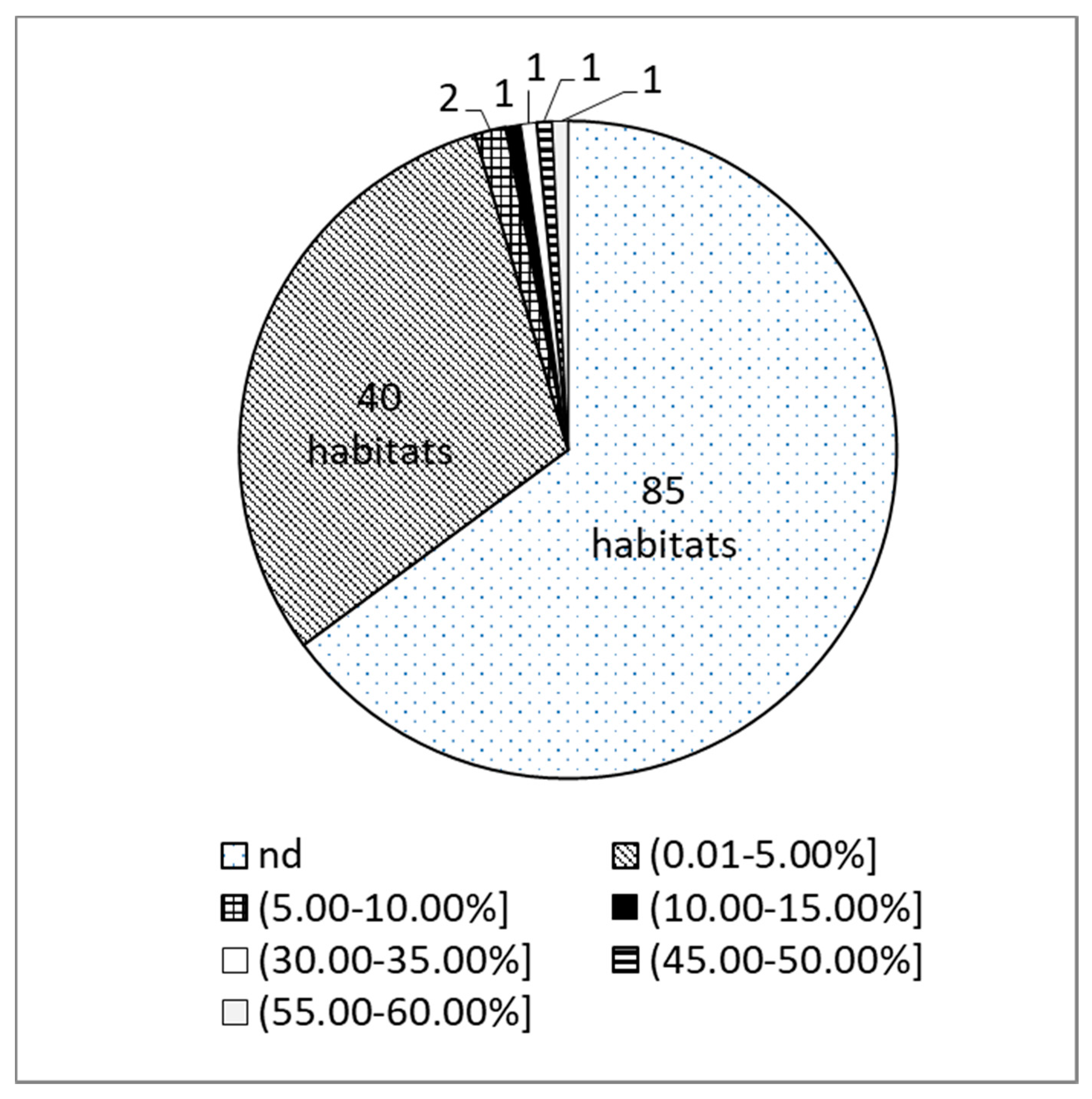
2.1. Occurence of α-Terpinyl Acetate Chemotype in Thymus pulegioides
2.2. Phytotoxic Effect of α-Terpinyl Acetate Essential Oil
2.3. Antimicrobial Effect of α-Terpinyl Acetate Essential Oil
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Isolation and Analysis of Essential Oils
4.3. Analysis of Phytotoxic Effect
4.4. Microorganisms
4.5. Analysis of Antimicrobial Effect
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kaskoos, R.A.; Ali, M.; Kapoor, R.; Akhtar, M.M.S.; Mir, S.R. Essential oil composition of the fruits of Eletteria cardamomum. J. Essent. Oil Bear. Plants 2006, 9, 81–84. [Google Scholar] [CrossRef]
- Moradalizadeh, M.; Akhgar, M.R.; Rajaei, P.; Faghihi-Zarandi, A. Chemical composition of the essential oils of Levisticum officinale growing wild in Iran. Chem. Nat. Compd. 2012, 47, 1007–1009. [Google Scholar] [CrossRef]
- Sangun, K.M.; Aydin, E.; Timur, M.; Karadeniz, H.; Caliskan, M.; Ozkan, A. Comparision of chemical composition of the essential oil of Laurus nobilis L. leaves and fruits of different region of Hatay, Turkey. J. Environ. Biol. 2007, 28, 731–733. [Google Scholar]
- Usai, M.; Marchetti, M.; Culeddu, N.; Mulas, M. Chemical Composition of Myrtle (Myrtus communis L.) Berries Essential Oils as Observed in a Collection of Genotype. Molecules 2018, 23, 2502. [Google Scholar] [CrossRef]
- Yang, J.K.; Choi, M.S.; Seo, W.T.; Rinker, D.L.; Han, S.W.; Cheong, G.W. Chemical composition and antimicrobial activity of Chamaecyparis obtusa leaf essential oil. Fitoterapia 2007, 78, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, J.P.; Costa, J.; Bianchini, A.; Bernardini, A.F.; Casanova, J. Composition of variability and of the essential oil of Stachys glutinosa L. from Corsica (France). Flav. Frag J. 1997, 12, 205–209. [Google Scholar] [CrossRef]
- Halabi, S.; Battah, A.A.; Aburjai, T.; Hudaib, M. Phytochemical and antiplatelet investigation of Gundelia tournifortii. Pharm. Biol. 2005, 43, 496–500. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Real, I.M. Essential oil of Chenopodium ambrosioides from Cuba. J. Essent. Oil Res. 2003, 15, 213–214. [Google Scholar] [CrossRef]
- Chemical Data Reporting under the Toxic Substances Control Act. Available online: https://www.epa.gov/chemical-data-reporting (accessed on 27 January 2021).
- GRAS Substances III. Recent progress in the consideration of flavor ingredients under the Food Additives Amendment (reprinted). Food Technol. 1965, 19, 151–197. [Google Scholar]
- Zarzuelo, A.; Crespo, E. The medicinal and non-medicinal uses of thyme. In Medicinal and Aromatic Plants—Industrial profiles; Stahl-Biskup, E., Sáez, F., Eds.; Taylor & Francis: London, UK, 2002; Volume 17, pp. 263–292. [Google Scholar]
- Thompson, J.D.; Manicacci, D.; Tarayre, M. Thirty-five years of thyme: A tale of two polymorphisms. Why so many females? Why so many chemotypes? BioScience 1998, 48, 805–815. [Google Scholar] [CrossRef]
- Sáez, F.; Stahl-Biskup, E. Essential oil polymorphism in the genus Thymus. In Thyme. The genus Thymus; Stahl-Biskup, E., Sáez, F., Eds.; CRC Press: London, UK; New York, UK, 2002; pp. 125–143. [Google Scholar]
- Başer, K.H.C.; Kirimer, N.; Ermin, N.; Kürkçüoglu, M. Essential oils from four chemotypes of Thymus zygioides Griseb, var. lycaonicus (Celak) Ronniger. J. Essent. Oil Res. 1996, 8, 615–618. [Google Scholar]
- Adzet, T.; Cañiguera, S.; Gabalda, N.; Ibañez, C.; Tomas, X.; Vila, R. Composition and variability of the essential oil of Thymus willkomii. Phytochemistry 1991, 30, 2289–2293. [Google Scholar] [CrossRef]
- García Martín, D.; García Vallejo, M.C. Chemotypes of Thymus zygis (Löfl.) L. of Guadarramma Sierra and other places in Castile (Spain). In Proceedings of the 9th International Essential Oil Congress, Singapore, 13–17 March 1983; pp. 134–140. [Google Scholar]
- Varga, E.; Bardocz, A.; Belak, A.; Maraz, A.; Boros, B.; Felinger, A.; Böszörményi, A.; Horváth, G. Antimicrobial activity and chemical composition of thyme essential oils and the polyphenolic content of different Thymus extracts. Farmacia 2015, 63, 357–361. [Google Scholar]
- Benjilali, B.; Hammoumi, M.; Richard, H. Polymorphisme chimique des huiles essentielles de Thym du Maroc. I. Caracterisation des composantes. Sci. Aliment. 1987, 7, 77–91. [Google Scholar]
- Başer, K.H.C.; Özek, T.; Kirimer, N.; Tümen, G. The occurrence of three chemotypes of Thymus longicaulis C. Presl. subsp. longicaulis in the same population. J. Essent. Oil Res. 1993, 5, 291–295. [Google Scholar]
- Tzakou, O.; Verykokidou, E.; Roussis, V.; Chinou, I. Chemical composition and antibacterial properties of Thymus longicaulis subsp. chaubardii oils: Three chemotypes in the same population. J. Essent. Oil Res. 1998, 10, 97–99. [Google Scholar] [CrossRef]
- Tümen, G.; Ermin, N.; Kürkçüoglu, M.; Başer, K.H.C. Essential oil of Thymus leucostomus Hausskn. et Velen. var. leucostomus. J. Essent. Oil Res. 1997, 9, 229–230. [Google Scholar]
- Başer, K.H.C.; Kürkçüoglu, M.; Ermin, N.; Tümen, G.; Malyer, H. Composition of the essential oil of Thymus pseudopulegioides Klokov et Des.-Shot. from Turkey. J. Essent. Oil Res. 1999, 11, 86–88. [Google Scholar] [CrossRef]
- Zouari, N.; Ayadi, I.; Fakhfakh, N.; Rebai, A.; Zouari, S. Variation of chemical composition of essential oils in wild populations of Thymus algeriensis Boiss. et Reut., a North African endemic Species. Lipids Health Dis. 2012, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Kulevanova, S.; Ristic, M.; Stafilov, T. The composition of the essential oils from Thymus macedonicus (Degen et Urumov) Ronn. subsp. macedonicus and Thymus tosevii Velen. subsp. tosevii growing in Macedonia. Farmacija 1995, 43, 13–14. [Google Scholar]
- Kulevanova, S.; Ristic, M.; Stafilov, T.; Matevski, V. Composition of the essential oils of Thymus jankae Chel. var. jancae, T. jancae var. pantotrichus Ronn. and T. jancae var. patentipilus Lyka from Macedonia. J. Essent. Oil Res. 1998, 10, 191–194. [Google Scholar] [CrossRef]
- Karuza-Stojakovic, L. Composition and yield of essential oils of various species of the genus Thymus L. Arh. Farm. 1989, 39, 105–111. [Google Scholar]
- Mockutė, D.; Bernotienė, G. The α-terpinyl acetate chemotype of essential oil of Thymus pulegioides L. Biochem. Syst. Ecol. 2001, 29, 69–73. [Google Scholar]
- Vaičiulytė, V.; Ložienė, K.; Taraškevičius, R.; Butkienė, R. Variation of essential oil composition of Thymus pulegioides in relation to soil chemistry. Ind. Crop. Prod. 2017, 95, 422–433. [Google Scholar] [CrossRef]
- Michet, A.; Chalchar, C.J.; Figueredo, G.; Thebaud, G.; Billy, F.; Petel, G. Chemotypes in the volatiles of wild thyme (Thymus pulegioides L.). J. Essent. Oil Res. 2008, 20, 101–103. [Google Scholar] [CrossRef]
- Mártonfi, P. Polymorfism of essential oil of Thymus pulegioides subc. chamaedrys in Slovakia. J. Essent. Oil Res. 1992, 4, 173–179. [Google Scholar]
- De Martino, L.; Bruno, M.; Formisano, K.; De Feo, V.; Napolitano, F.; Rosselli, S.; Senatore, F. Chemical composition and antimicrobial activity of the essential oils from two species of Thymus growing wild in souththern Italy. Molecules 2009, 14, 4614–4624. [Google Scholar] [CrossRef]
- Pavel, M.; Ristic, M.; Stevic, T. Essential oils of Thymus pulegioides and Thymus glabrescens from Romania: Chemical composition and antimicrobial activity. J. Serb. Chem. Soc. 2010, 75, 27–34. [Google Scholar] [CrossRef]
- Bukantis, A. Lithuanian Climate; Vilnius University: Vilnius, Lithuania, 1994; pp. 45–193. [Google Scholar]
- Dudai, N.; Larkov, O.; Putievski, E.; Lerner, H.R.; Ravid, U.; Lewinshon, E.; Mayer, A.M. Biotransformation of constituents of essential oils by germinating wheat seed. Phytochemistry 2000, 55, 375–382. [Google Scholar] [CrossRef]
- Groendahl, E.; Ehlers, K.B. Local adaption to biotic factors: Reciprocal transplants of four species associated with aromatic Thymus pulegioides and T. serpyllum. J. Ecol. 2008, 96, 981–992. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, H.P.; Mittal, S.; Batish, D.R.; Kohli, R.K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as bioherbicide. Ind. Crop. Prod. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Balevičienė, J.; Kizienė, B.; Lazdaukaitė, Ž.; Patalauskaitė, D.; Rašomavičius, V.; Sinkevičienė, Z.; Tučienė, A.; Venckus, Z. Vegetation of Lithuania. 1.; Šviesa: Kaunas, Lithuania; Vilnius, Lithuania, 1998; p. 269. [Google Scholar]
- Ložienė, K.; Vaičiulytė, V. Ecological characteristics of habitats and occurrence of Thymus pulegioides (Lamiaceae) in Lithuania. Thaiszia J. Bot. 2017, 27, 49–64. [Google Scholar]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crop. Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Parente-Rocha, J.A.; Bailão, A.B.; Amaral, A.C.; Taborda, C.P.; Paccez, J.D.; Borges, C.L.; Pereira, M. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: An overview about endemic dimorphic fungi. Mediat. Inflamm. 2017, 9870679. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Ilio, C.D. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Raho, B.; Abouni, B. Escherichia coli and Staphylococcus aureus most common source of infection. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances and Educational Program, 1st ed.; Méndez-Vilas, A., Ed.; Formatex Research Center S.L.: Badajoz, Spain, 2015; pp. 637–648. [Google Scholar]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. J. Clin. Microbiol. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural Products as Platforms To Overcome Antibiotic Resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Rios, A.C.; Moutinho, C.G.; Pinto, F.C.; Del Fiol, F.S.; Jozala, A.; Chaud, M.V.; Vila, M.M.; Teixeira, J.A.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007; pp. 104–510. [Google Scholar]
- Pinto, E.; Pina-Vaz, C.; Salgueiro, L.; Concalves, M.J.; Costa-de-Oliveira, S.; Cavaleiro, C.; Palmaeira, A.; Rodrigues, A.; Martinez-de-Oliveira, J. Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermophyte species. J. Med. Microb. 2006, 55, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Mockutė, D.; Bernotienė, G. The main citral-geranio and carvacrol chemotypes of essential oil of Thymus pulegioides growing wild in Vilnius district (Lithuania). J. Agric. Food. Chem. 1999, 47, 3787–3790. [Google Scholar] [CrossRef] [PubMed]
- Mockutė, D.; Bernotienė, G. Chemical composition of the essential oils and the odor of Thymus pulegioides L. growing wild in Vilnius. J. Essent. Oil Res. 2005, 17, 415–418. [Google Scholar] [CrossRef]
- Kustrak, D.; Martinis, Z.; Kuftinec, J.; Blazevic, N. Composition of essential oils of some Thymus and Thymbra species. Flav. Frag J. 1990, 5, 227–231. [Google Scholar] [CrossRef]
- Radulescu, V.; Pavel, M.; Teodor, A.; Tanase, A.; Ilies, D.C. Analysis from volatile compounds from infusio and hidrodistilate obtained from the species Thymus pulegioides (Lamiaceae). Farmacia 2009, 57, 282–289. [Google Scholar]
- Boz, I.; Gille, E.; Necula, R.; Dunca, S.; Zamfirache, M.M. Chemical composition and antibacterial activity of essential oils from five populations of Thymus pulegioides L. Cellulose Chem. Technol. 2015, 49, 169–174. [Google Scholar]
- Messerschmidt, W. Gas-und dünnschichtchromatographische untersuchungen der ätherischen öle einiger Thymus arten. Planta Medica 1965, 13, 56–72. [Google Scholar] [CrossRef]
- Kowal, T.; Krupinska, A. Antibacterial activity of essentila oil from Thymus pulegioides L. (in Polish). Herba Pol. 1979, 25, 303–310. [Google Scholar]
- Mastelic, J.; Gruznov, K.; Kravar, A. The chemical composition of terpene alcohols and phenols from the essential oil and terpene glycosides isolated from Thymus pulegioides growing wild in Dalmantia. Planta Med. 1992, 58, 679–680. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Gauthier, P.; Keefover-Ring, K.; Thompson, J.D. Variable phytotoxin effects of Thymus vulgaris (Lamiaceae) terpenes on associated species. Int. J. Plant Sci. 2015, 176, 20–30. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Thompson, J.D. Terpene-based selective herbivory by Helix aspersa (Mollusca) on Thymus vulgaris (Labiatae). Oecologia 1995, 102, 126–132. [Google Scholar] [CrossRef]
- Linhart, Y.B.; Thompson, J.D. Thyme is of the essence: Biochemical polymorphism and multi-species deterrence. Evol. Ecol. Res. 1999, 1, 151–171. [Google Scholar]
- Tarayre, M.; Thompson, J.D.; Escarre, J.; Linhart, Y.B. Intra-specific variation in the inhibitory effects of Thymus vulgaris (Labiatae) monoterpenes on seed germination. Oecologia 1995, 101, 110–118. [Google Scholar] [CrossRef]
- Ehlers, B.K. Soil microorganisms alleviate the allelochemical effects of a thyme monoterpene on the performance of an assoctiated grass species. PLoS ONE 2011, 6, e26321. [Google Scholar] [CrossRef]
- Ehlers, B.K.; Charpentier, A.; Grendahl, E. An allelopathic plant facilitates species richness in the Mediterranean garrigue. J. Ecol. 2014, 102, 176–185. [Google Scholar] [CrossRef]
- Vokou, D.; Douvli, P.; Blionis, G.J.; Halley, J.M. Effects of monoterpenoids, acting alone or in pairs, on seed germination and subsequent seedling growth. J. Chem. Ecol. 2003, 29, 2281–2301. [Google Scholar] [CrossRef]
- Alam, A.; Majumdar, R.S.; Alam, P. Systematics Evaluations of Morphological Traits, Chemical Composition, and Antimicrobial Properties of Selected Varieties of Elettaria cardamomum (L.) Maton. Nat. Prod. Commun. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Guido, F.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Lorenzi, V.; Muselli, A.; Bernardini, A.F.; Berti, L.; Pagès, J.M.; Amaral, L.; Bolla, J.M. Geraniol restores antibiotic activities against multidrug-resistant isolates from gram-negative species. Antimicrob. Agents Chemother. 2009, 53, 2209–2211. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.X.; Tao, N.G.; Jia, L.; Zhou, H.E. Influence of α-terpineol on the growth and morphogenesis of Penicillium digitatum. Bot. Stud. 2015, 56, 35. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; de Oliveira, F.L.; Bicas, J.L. Production, Properties, and Applications of α-Terpineol. Food Bioprocess Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Directorate for the Quality of Medicines and HealthCare of the Council of Europe (EDQM), 10th ed.; EDQM: Strasbourg, France, 2008; Volume 1, pp. 307–308. [Google Scholar]
- Espinel-Ingroff, A.; Canton, E.; Peman, J. Updates in antifungal susceptibility testing of filamentous fungi. Curr. Fung. Infect. Rep. 2009, 3, 133–141. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L.; Arendrup, M.C.; Barchiesi, F.; Bille, J.; Chryssanthou, E.; Cuenca-Estrella, M. Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST). EUCAST definitive document EDef 7.1: Method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163. [Google Scholar] [CrossRef] [PubMed]

| Species of Genus Thymus | Country | Percentage of α-Terpinyl Acetate in Essential Oil | Literature Source |
|---|---|---|---|
| Thymus praecox Opiz | Great Britain | 36 | [13] |
| Cyprus | 22.7 | ||
| Thymus zygioides Griseb. | Cyprus | 36.2 | [14] |
| Thymus willkommii Ronniger | Spain | 36–69 | [15] |
| Thymus zygis L. | Spain | 65.4–73.1 | [16] |
| Thymus serpyllum L. (syn. Thymus glabrescens Benth.) | Romania | 10.1 | [17] |
| Thymus glabrescens Willd. | Romania | 47.6 | [17] |
| Thymus munbyanus subsp. ciliatus (Desf.) Greuter & Burdet (syn. Thymus ciliatus (Desf.) Benth.) | Marocco | 42.9 | [18] |
| Cyprus | 82.1 | [19] | |
| Thymus longicaulis C. Presl | Greece | 20.4 | [20] |
| Thymus leucostomus Hausskn. & Velen. | Cyprus | 23.8 | [21] |
| Thymus nummularius M.Bieb. (syn. Thymus pseudopulegioides Klokov & Des.-Shost.) | Cyprus | 16.7 | [22] |
| Thymus algeriensis Boiss. & Reut. | North Africa | 14.92 | [23] |
| Thymus sibthorpii Benth. (syn. Thymus tosevii Velen., syn. Thymus macedonicus (Degen & Urum.) Ronniger) | Macedonia | 11.3 | [24] |
| Thymus tosevii Velen. subsp. tosevii | Macedonia | 13.6 | [24] |
| Thymus praecox subsp. jankae (Celak.) Jalas (syn. Thymus jankae Celak.) | Macedonia | 11.3 | [25] |
| Thymus striatus Vahl | Bosnia-Herzegowina | 8.1–11.2 | [26] |
| Thymus pulegioides L. | Lithuania | 50–70 | [27,28] |
| France | 64.8–88 | [29] |
| Chemical Compound | Min–Max, % | Mean ± SD, % |
|---|---|---|
| Carvacrol | 0.00–48.00 | 17.66 ± 9.43 |
| Thymol | 0.00–31.00 | 3.17 ± 5.11 |
| Geraniol | 0.00–39.87 | 6.57 ± 8.70 |
| Linalool | 0.00–57.75 | 1.66 ± 6.59 |
| α-Terpinyl acetate | 0.00–57.50 | 1.48 ± 6.96 |
| Compound | Identification Method | RI | GC Area, % | RT | |
|---|---|---|---|---|---|
| Calculated | Literature [46] | ||||
| 1-octen-3-ol | RI, MS | 983 | 974 | 0.09 | 3.06 |
| α-Terpinene | RI, MS, Std | 1024 | 1014 | 2.22 | 11.03 |
| p-Cymene | RI, MS, Std | 1030 | 1020 | 0.02 | 12.39 |
| Limonene | RI, MS, Std | 1034 | 1024 | 0.02 | 12.49 |
| (E)-β-Ocimene | RI, MS | 1054 | 1044 | 0.06 | 12.60 |
| γ-Terpinene | RI, MS, Std | 1064 | 1054 | 0.13 | 13.46 |
| Linalool | RI, MS, Std | 1105 | 1095 | 0.78 | 14.77 |
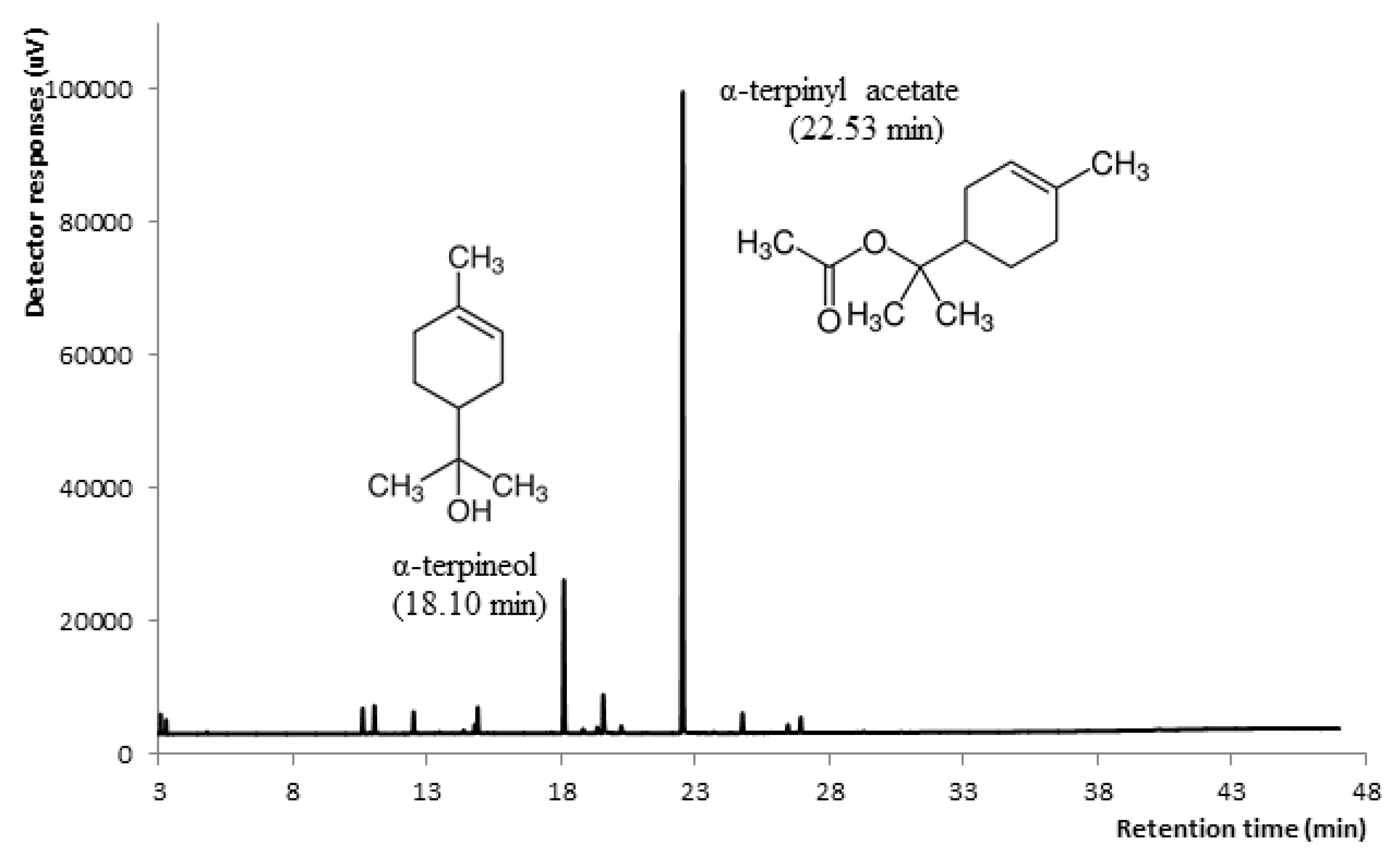
| α-Terpineol | RI, MS | 1197 | 1186 | 14.34 | 18.10 |
| Nerol | RI, MS, Std | 1238 | 1227 | 0.71 | 20.24 |
| Neral | RI, MS, | 1244 | 1235 | 0.59 | 19.34 |
| Geraniol | RI, MS, Std | 1260 | 1249 | 3.80 | 19.56 |
| Geranial | RI, MS, | 1275 | 1264 | 0.38 | 18.81 |
| α-Terpinyl acetate | RI, MS, Std | 1359 | 1346 | 64.22 | 22.53 |
| β-Bourbonene | RI, MS | 1399 | 1387 | 0.11 | 23.68 |
| β-Caryophyllene | RI, MS, Std | 1428 | 1417 | 1.95 | 24.76 |
| cis-β-Guaiene | RI, MS | 1503 | 1492 | 0.81 | 26.45 |
| β-Bisabolene | RI, MS | 1517 | 1505 | 1.47 | 26.93 |
| Caryophyllene oxide | RI, MS, Std | 1593 | 1582 | 0.14 | 29.28 |
| Monoterpene hydrocarbons | 2.45 | ||||
| Oxygenated monoterpenes | 84.82 | ||||
| Sesquiterpene hydrocarbons | 4.34 | ||||
| Oxygenated sesquiterpenes | 0.14 | ||||
| Other | 0.09 | ||||
| Total identified | 91.84 | ||||
| Plant Species | Effect | Chemical | GP | MGD, % | GI, Seeds/ Day | Radicle Development |
|---|---|---|---|---|---|---|
| Mean ± SD, % | Mean ± SD, mm | |||||
| Poa pratensis | Control | 51.67 ± 4.16 | 3.97 | 10.94 | 27.50 ± 5.78 | |
| Through air | Essential oil | 6.33 ± 4.46 a | 0.43 a | 0.60 a | 12.63 ± 4.11 a | |
| Standard | 3.60 ± 2.3 A | 0.26 A | 0.38 A | 14.94 ± 4.00 A | ||
| Through water | Essential oil | 0.40 ± 0.55 a | 0.03 a | 0.06 a | 0.55 ± 2.46 a | |
| Standard | 0.00 A | 0.00 A | 0.00 A | * | ||
| Phleum pratense | Control | 96.67 ± 0.50 | 12.13 | 31.38 | 9.77 ± 4.29 | |
| Through air | Essential oil | 88.50 ± 4.36 a | 8.80 a | 19.94 a | 4.07 ± 3.82 a | |
| Standard | 94.67 ± 1.15 | 7.5 A | 26.46 A | 7.00 ± 5.22 A | ||
| Through water | Essential oil | 94.67 ± 0.58 | 11.64 | 22.42 a | 7.23 ± 6.70 a | |
| Standard | 95.33 ± 1.10 | 6.90 A | 16.28 A | 16.68 ± 5.85 A | ||
| Trifolium pratense | Control | 75.00 ± 5.57 | 25.00 | 53.11 | 23.07 ± 6.75 | |
| Through air | Essential oil | 75.00 ± 1.73 | 25.00 | 43.89 | 15.20 ± 5.82 a | |
| Standard | 73.67 ± 2.89 | 24.56 | 44.89 | 21.17 ± 6.40 | ||
| Through water | Essential oil | 82.33 ± 1.53 | 27.44 | 41.11 | 17.20 ± 5.70 a | |
| Standard | 78.33 ± 10.69 | 26.11 | 37.44 A | 17.90 ± 5.73 A | ||
| Hypericum perforatum | Control | 46.67 ± 7.02 | 2.59 | 4.94 | 3.31 ± 1.24 | |
| Through air | Essential oil | 36.67 ± 1.53 a | 1.96 a | 2.97 a | 2.37 ± 0.78 a | |
| Standard | 37.33 ± 8.62 | 2.10 | 3.38 | 2.70 ± 0.87 | ||
| Through water | Essential oil | 38.67 ± 8.96 | 2.30 | 3.81 | 2.37 ± 0.78 a | |
| Standard | 8.33 ± 2.52 A | 0.48 A | 0.82 A | 1.77 ± 0.66 A | ||
| Thymus pulegioides | Control | 58.33 ± 13.43 | 5.83 | 21.18 | 11.60 ± 2.78 | |
| Through air | Essential oil | 58.20 ± 4.46 | 5.30 | 11.62 a | 12.40 ± 2.87 | |
| Standard | 64.33 ± 11.72 | 6.43 | 18.47 | 10.57 ± 2.17 | ||
| Through water | Essential oil | 53.33 ± 3.51 | 5.67 | 16.91 | 5.60 ± 2.28 a | |
| Standard | 52.00 ± 7.00 | 5.20 | 14.36 | 4.20 ± 2.32 A | ||
| Plant Species | Effect | Chemical | GP, p/U | MGD, p/U | GI p/U | Radicle Development, p/t |
|---|---|---|---|---|---|---|
| Poa pratensis | Through air | Essential oil | 0.02/0.00 | 0.02/0.00 | 0.02/0.00 | 0.00/16.32 |
| Standard | 0.03/0.00 | 0.03/0.00 | 0.02/0.00 | 0.00/18.49 | ||
| Through water | Essential oil | 0.03/0.00 | 0.03/0.00 | 0.04/0.00 | 0.00/16.15 | |
| Standard | 0.03/0.00 | 0.03/0.00 | 0.03/0.00 | * | ||
| Phleum pratense | Through air | Essential oil | 0.03/0.00 | 0.04/0.00 | 0.04/0.00 | 0.00/8.43 |
| Standard | 0.08/0.50 | 0.04/0.00 | 0.04/0.00 | 0.00/10.73 | ||
| Through water | Essential oil | 0.05/0.00 | 0.51/3.00 | 0.04/0.00 | 0.00/9.44 | |
| Standard | 0.13/1.00 | 0.04/0.00 | 0.04/0.00 | 0.00/17.45 | ||
| Trifolium pratense | Through air | Essential oil | 0.83/4.00 | 0.83/4.00 | 0.13/1.00 | 0.00/18.41 |
| Standard | 0.83/4.00 | 0.83/4.00 | 0.13/1.00 | 0.06/7.36 | ||
| Through water | Essential oil | 0.08/0.50 | 0.08/0.50 | 0.05/0.00 | 0.00/21.78 | |
| Standard | 0.51/3.00 | 0.51/3.00 | 0.04/0.00 | 0.00/21.78 | ||
| Hypericum perforatum | Through air | Essential oil | 0.04/0.00 | 0.04/0.00 | 0.04/0.00 | 0.00/7.76 |
| Standard | 0.13/1.00 | 0.28/2.00 | 0.13/1.00 | 0.06/7.78 | ||
| Through water | Essential oil | 0.13/1.00 | 0.51/3.00 | 0.13/1.00 | 0.00/9.64 | |
| Standard | 0.04/0.00 | 0.04/0.00 | 0.04/0.00 | 0.00/6.36 | ||
| Thymus pulegioides | Through air | Essential oil | 0.55/5.50 | 0.46/5.00 | 0.03/0.00 | 0.27/1.09 |
| Standard | 0.28/2.00 | 0.28/2.00 | 0.28/2.00 | 0.18/1.34 | ||
| Through water | Essential oil | 0.51/3.00 | 0.51/3.00 | 0.28/2.00 | 0.00/13.83 | |
| Standard | 0.51/3.00 | 0.51/3.00 | 0.13/1.00 | 0.00/10.90 |
| Microorganisms | α-Terpinyl Acetate | T. pulegioides α-Terpinyl Acetate Chemotype Essential Oil | Antibiotics/ Antifungals | ||
|---|---|---|---|---|---|
| MIC | MBC/MFC | MIC | MBC/MFC | MIC | |
| Mean ± SD, µg/mL | |||||
| Escherichia coli ATCC 25922 | 125.00 ± 0.50 | 125.00 ± 0.50 | 31.30 ± 0.10 | 31.30 ± 0.10 | 0.50 ± 0.00 a |
| Staphylococcus aureus ATCC 29213 | 31.30 ± 0.30 | 125.00 ± 1.50 | 31.30 ± 1.10 | 31.30 ± 0.30 | 0.75 ± 0.00 b |
| Aspergillus flavus CBS 120264 | 0.40 ± 0.10 | 25.00 ± 1.00 | 0.02 ± 0.00 | 25.00 ± 1.00 | 1.50 ± 0.00 c |
| Aspergillus fumigatus SC 6359 | 1.60 ± 0.20 | 6.30 ± 0.10 | 0.40 ± 0.00 | 6.30 ± 0.20 | 3.00 ± 0.00 c |
| Candida albicans CBS 2730 | 8.00 ± 0.20 | 31.30 ± 0.10 | 8.00 ± 0.15 | 31.30 ± 0.10 | 0.25 ± 0.00 c |
| Candida parapsilosis CBS 8836 | 2.00 ± 0.10 | 125.00 ± 1.00 | 8.00 ± 0.10 | 125.00 ± 1.00 | 32.00 ± 0.00 c |
| Trichophyton mentagrophytes ATCC 9533 | 1.60 ± 0.20 | 25.00 ± 1.00 | 0.02 ± 0.00 | 6.30 ± 0.20 | 32.00 ± 0.00 c |
| Trichophyton rubrum ATCC 28188 | 0.40 ± 0.10 | 0.40 ± 0.10 | 0.40 ± 0.10 | 1.60 ± 0.10 | 3.00 ± 0.00 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaičiulytė, V.; Ložienė, K.; Švedienė, J.; Raudonienė, V.; Paškevičius, A. α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus pulegioides, Phytotoxicity, and Antimicrobial Effects. Molecules 2021, 26, 1065. https://doi.org/10.3390/molecules26041065
Vaičiulytė V, Ložienė K, Švedienė J, Raudonienė V, Paškevičius A. α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus pulegioides, Phytotoxicity, and Antimicrobial Effects. Molecules. 2021; 26(4):1065. https://doi.org/10.3390/molecules26041065
Chicago/Turabian StyleVaičiulytė, Vaida, Kristina Ložienė, Jurgita Švedienė, Vita Raudonienė, and Algimantas Paškevičius. 2021. "α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus pulegioides, Phytotoxicity, and Antimicrobial Effects" Molecules 26, no. 4: 1065. https://doi.org/10.3390/molecules26041065
APA StyleVaičiulytė, V., Ložienė, K., Švedienė, J., Raudonienė, V., & Paškevičius, A. (2021). α-Terpinyl Acetate: Occurrence in Essential Oils Bearing Thymus pulegioides, Phytotoxicity, and Antimicrobial Effects. Molecules, 26(4), 1065. https://doi.org/10.3390/molecules26041065







