Luminescent Ink Based on Upconversion of NaYF4:Er,Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment
Abstract
1. Introduction
2. Results and Discussion
2.1. Vibrational Characterization of UCNPs
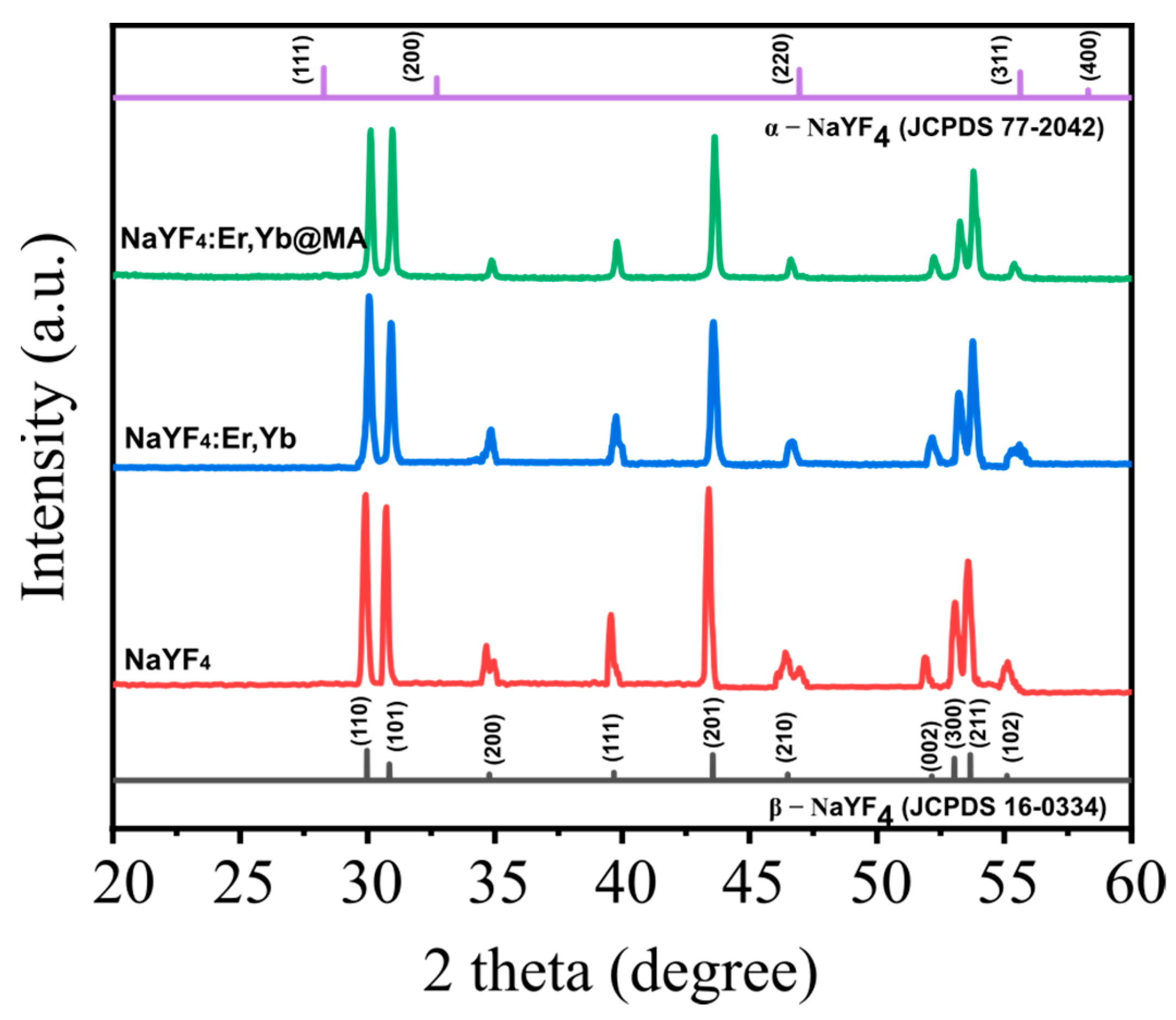
2.2. Structural Characterization of UCNPs
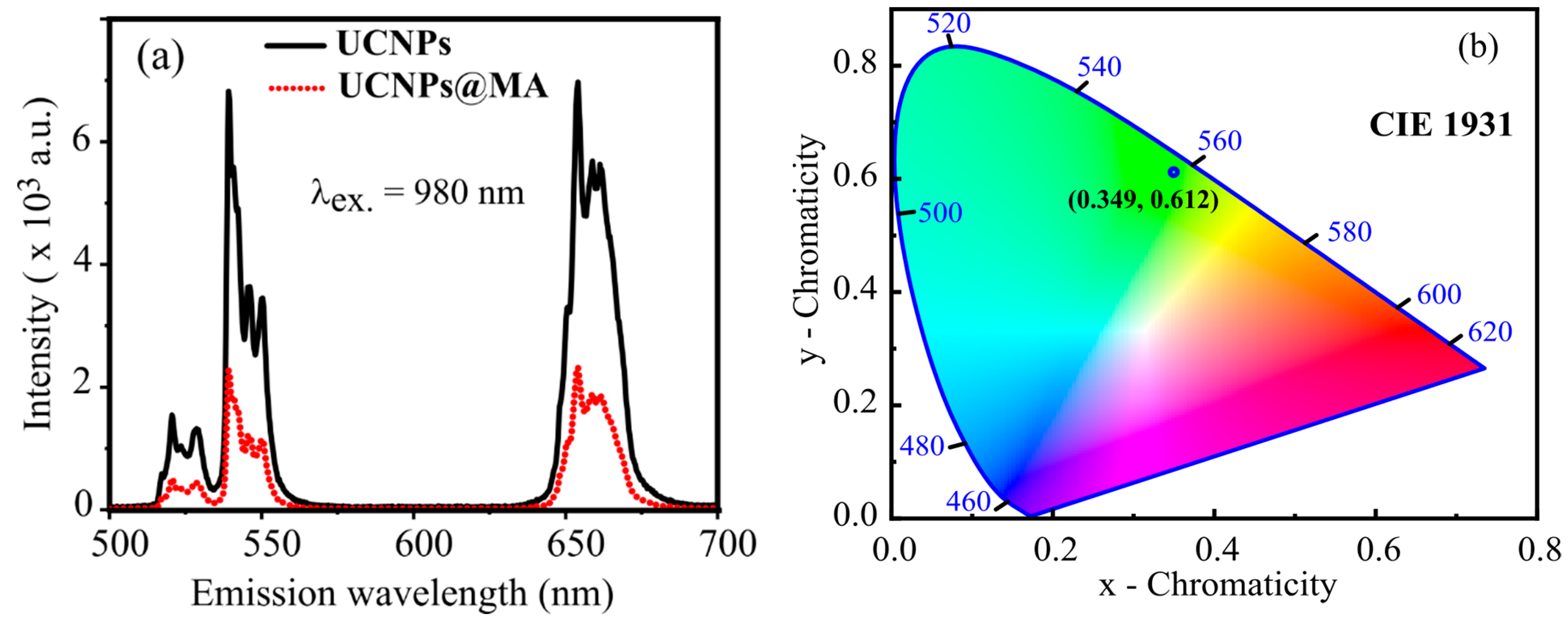
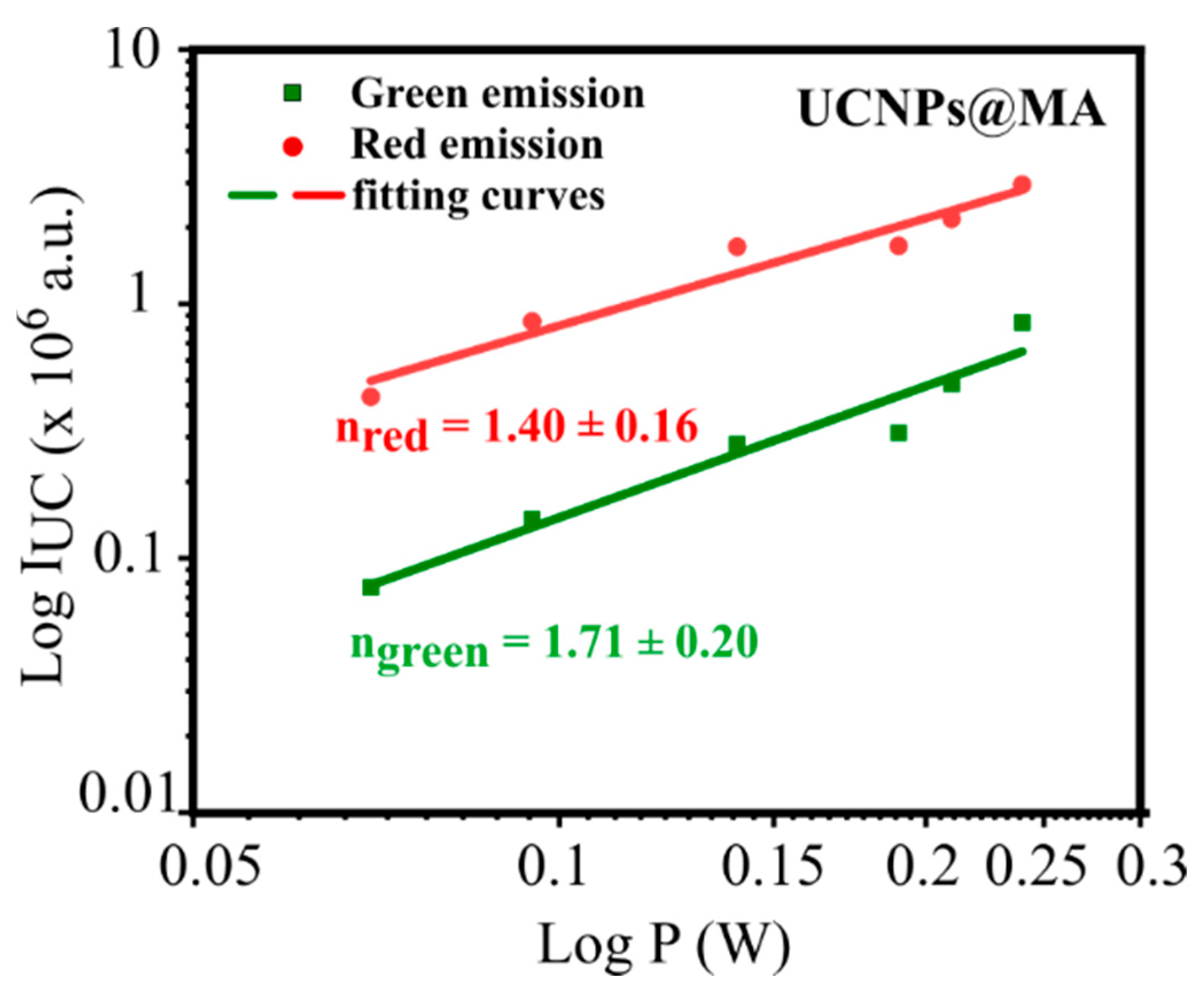
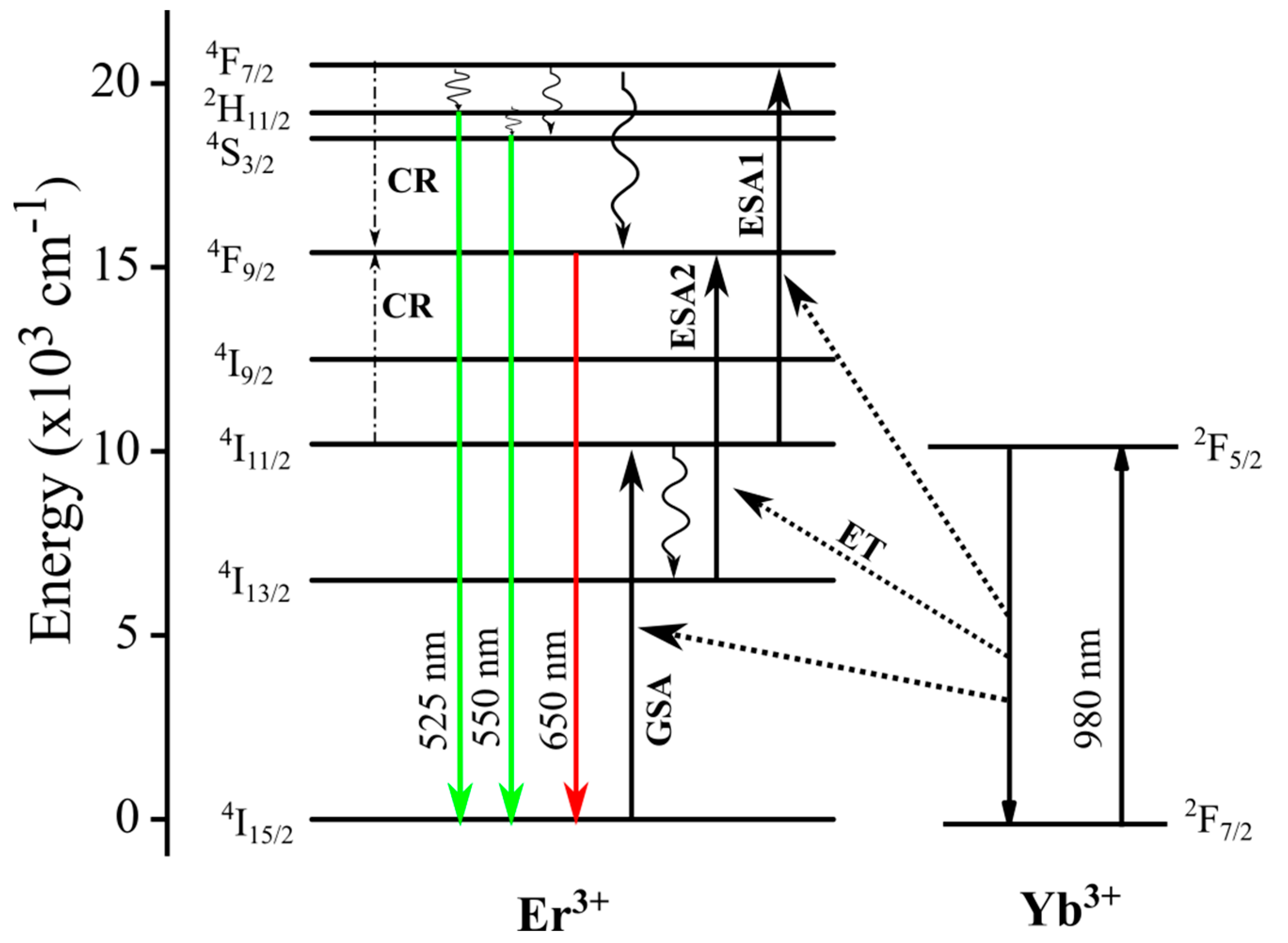
2.3. Optical Characterization
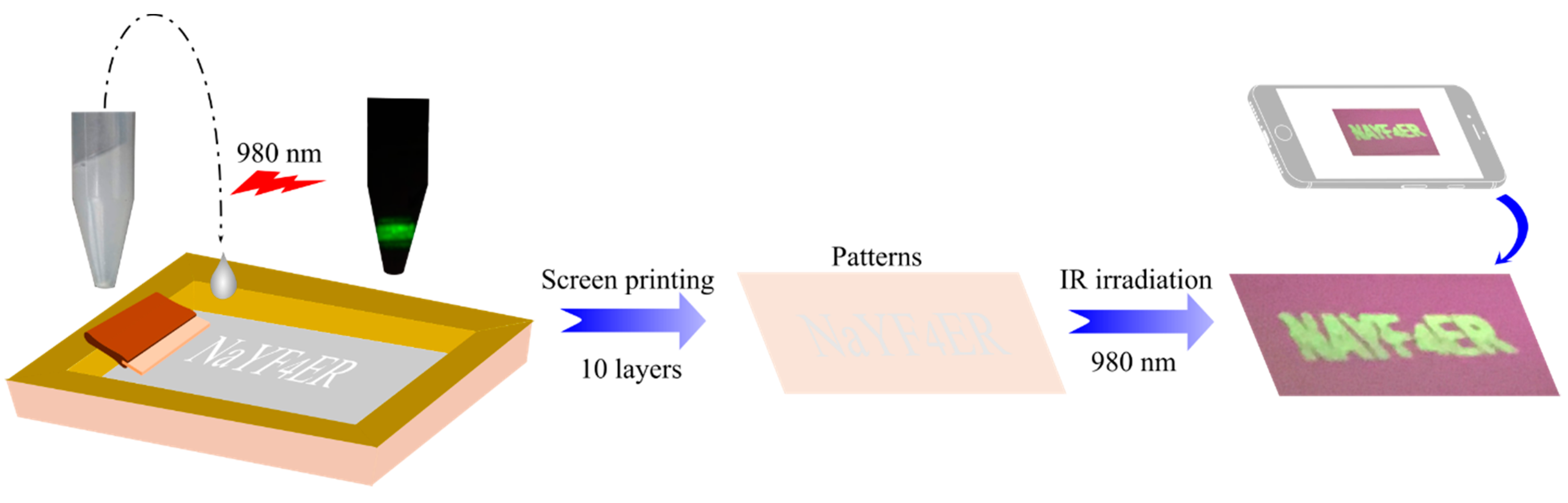
2.4. Screen Printing of UCNPs@MA for Anti-Counterfeiting Applications
3. Materials and Methods
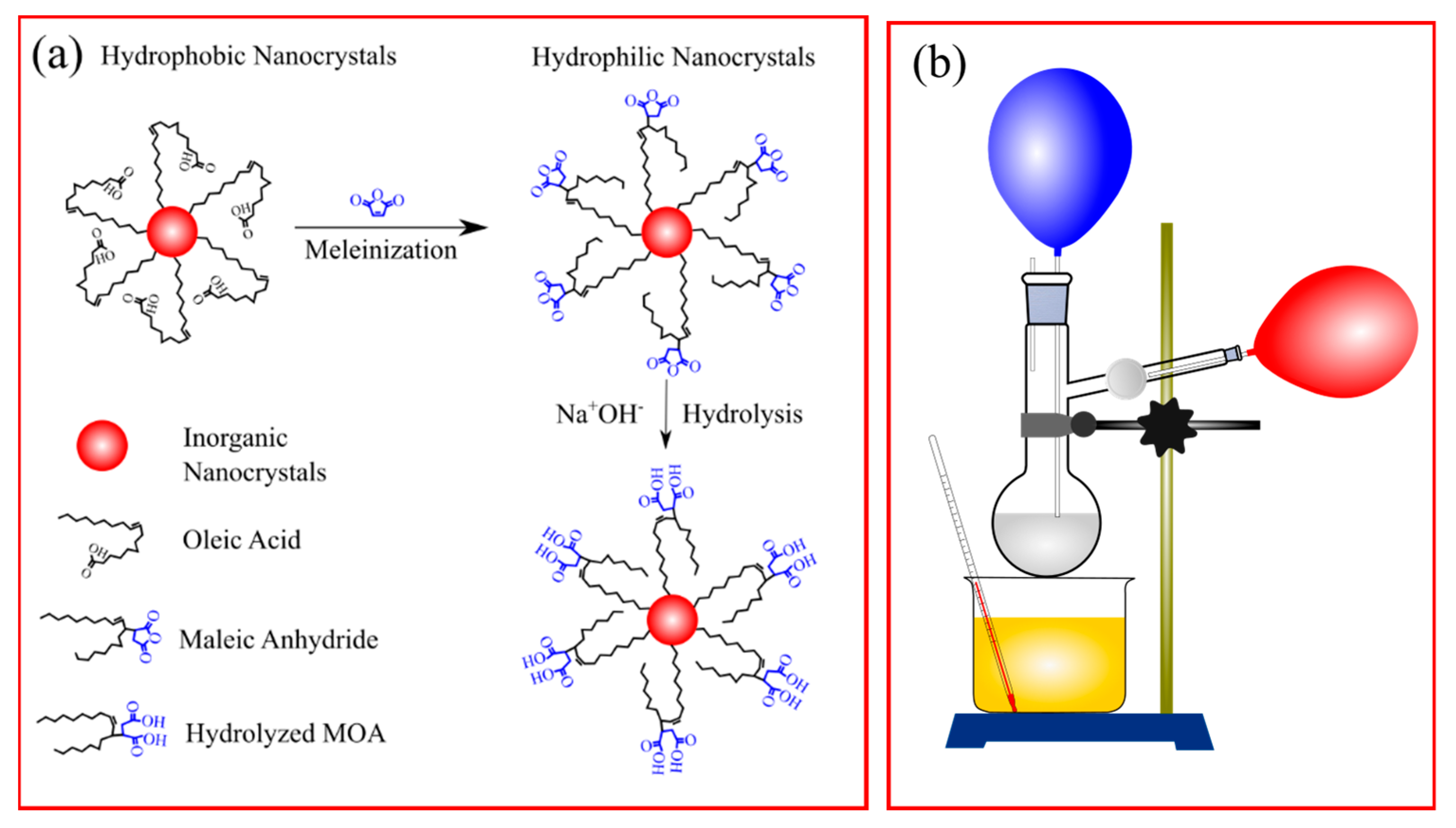
3.1. Synthesis of NaYF4:Er,Yb Upconversion Nanoparticles with Maleic Anhydride on the Surface
3.2. Ink Manufacturing Process
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cui, Y.; Ling, X.Y. Encoding molecular information in plasmonic nanostructures for anti-counterfeiting applications. Nanoscale 2014, 6, 282–288. [Google Scholar] [CrossRef]
- Han, S.; Park, W. Lithographically encoded polymer microtaggant using high-capacity and error-correctable QR code for anti-counterfeiting of drugs. Adv. Mater. 2012, 24, 5924–5929. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Designing lanthanide-doped nanocrystals with both up-and down-conversion luminescence for anti-counterfeiting. Nanoscale 2011, 3, 4804–4810. [Google Scholar] [CrossRef]
- Meruga, J.M.; Kellar, J.J. Security printing of covert quick response codes using upconverting nanoparticle inks. Nanotechnology 2012, 23, 395201. [Google Scholar] [CrossRef]
- Yoon, B.; Kim, J.M. Recent functional material based approaches to prevent and detect counterfeiting. J. Mater. Chem. C 2013, 1, 2388–2403. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, T. Inkjet-printed unclonable quantum dot fluorescent anti-counterfeiting labels with artificial intelligence authentication. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- Wei, W.; Zheng, G. Preparation of quantum dot luminescent materials through the ink approach. Mater. Des. 2016, 91, 165–170. [Google Scholar] [CrossRef]
- Ingrosso, C.; Brugger, J. Drop-on-demand inkjet printing of highly luminescent CdS and CdSe@ZnS nanocrystal based nanocomposites. Microelectron. Eng. 2009, 86, 1124–1126. [Google Scholar] [CrossRef]
- Blumenthal, T.; Meruga, J.; Luu, Q.A.N. Patterned direct-write and screen-printing of NIR-to-visible upconverting inks for security applications. Nanotechnology 2012, 23, 185305. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Xu, F. Inkjet printing of upconversion nanoparticles for anti-counterfeit applications. Nanoscale 2015, 7, 4423–4431. [Google Scholar] [CrossRef]
- Meruga, J.M.; May, P.S. Red-green-blue printing using luminescence-upconversion inks. J. Mater. Chem. C 2014, 2, 2221–2227. [Google Scholar] [CrossRef]
- Yao, W.; Wu, W. Dual upconversion nanophotoswitch for security encoding. Sci. China Mater. 2019, 62, 368–378. [Google Scholar] [CrossRef]
- Kumar, A.; Tiwari, S.P.; Kumar, K. Security writing application of thermal decomposition assisted NaYF4:Er3+/Yb3+ upconversion phosphor. Laser Phys. Lett. 2018, 15, 075901. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, C. Hydrothermal synthesis and inkjet printing of hexagonal-phase NaYF4:Ln3+ upconversion hollow microtubes for smart anti-counterfeiting encryption. Mater. Chem. Front. 2018, 2, 1997–2005. [Google Scholar] [CrossRef]
- Gong, G.; Zheng, J. Design of core/active-shell NaYF4: Ln3+@NaYF4:Yb3+ nanophosphors with enhanced red-green-blue upconversion luminescence for anti-counterfeiting printing. Compos. B Eng. 2019, 179, 107504. [Google Scholar] [CrossRef]
- Himmelstoß, S.F.; Hirsch, T. Long-Term Colloidal and Chemical Stability in Aqueous Media of NaYF4-Type Upconversion Nanoparticles Modified by Ligand-Exchange. Part. Part. Syst. Charact. 2019, 36, 1900235. [Google Scholar] [CrossRef]
- Huang, J.; Chen, N. Growth of β-NaYF4:Eu3+ Crystals by the Solvothermal Method with the Aid of Oleic Acid and Their Photoluminescence Properties. Materials 2019, 12, 3711. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, X. Solvothermal synthesis and modification of NaYF4:Yb/Er@ NaLuF4:Yb for enhanced up-conversion luminescence for bioimaging. RSC Adv. 2019, 9, 42163–42171. [Google Scholar] [CrossRef]
- Li, Z.; Tang, B. Fabrication of NaYF4:Yb,Er Nanoprobes for Cell Imaging Directly by Using the Method of Hydrion Rivalry Aided by Ultrasonic. Nanoscale Res. Lett. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Boyer, J.C.; Johnson, N.J.J.; Van Veggel, F.C.J.M. Upconverting lanthanide-doped NaYF4−PMMA polymer composites prepared by in situ polymerization. Chem. Mater. 2009, 21, 2010–2012. [Google Scholar] [CrossRef]
- Chai, R.; Lin, J. Preparation and characterization of upconversion luminescent NaYF4:Yb3+,Er3+(Tm3+)/PMMA bulk transparent nanocomposites through in situ photopolymerization. J. Phys. Chem. C 2010, 114, 610–616. [Google Scholar] [CrossRef]
- Liu, C.H.; Li, Z.P. Surface modification of hydrophobic NaYF4:Yb,Er upconversion nanophosphors and their applications for immunoassay. Sci. China Chem. 2011, 54, 1292. [Google Scholar] [CrossRef]
- Song, Y.; Xu, L. Synthesis and Inkjet Printing of NaYF4:Ln3+@NaYF4 Core–Shell Nanoparticles with Enhanced Upconversion Fluorescence for Anti-Counterfeiting Applications. J. Nanosci. Nanotechnol. 2020, 20, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, J. PEG-mediated solvothermal synthesis of NaYF4:Yb/Er superstructures with efficient upconversion luminescence. J. Alloys Compd. 2010, 506, L17–L21. [Google Scholar] [CrossRef]
- Peng, E.; Ding, J.; Xue, J.M. Succinic anhydride functionalized alkenoic ligands: A facile route to synthesize water dispersible nanocrystals. J. Mater. Chem. 2012, 22, 13832–13840. [Google Scholar] [CrossRef]
- Stefanoiu, F.; Borredon, E. Kinetics and mechanism of the reaction between maleic anhydride and fatty acid esters and the structure of the products. Eur. J. Lipid Sci. Technol. 2008, 110, 441–447. [Google Scholar] [CrossRef]
- Kellar, J.; Blumenthal, T. Systems and Methods for Printing Patterns Using Near Infrared Upconverting Inks. U.S. Patent US10358569B2, 23 July 2019. [Google Scholar]
- Cheng, Z.Y.; Chang, S.C. Authenticated RFID security mechanism based on chaotic maps. Secur. Commun. Netw. 2013, 6, 247–256. [Google Scholar] [CrossRef]
- You, M.; Xu, F. Three-dimensional quick response code based on inkjet printing of upconversion fluorescent nanoparticles for drug anti-counterfeiting. Nanoscale 2016, 8, 10096–10104. [Google Scholar] [CrossRef]
- Peng, H.; Bowman, C.N. Monochromatic visible light “photoinitibitor”: Janus-faced initiation and inhibition for storage of colored 3D images. J. Am. Chem. Soc. 2014, 136, 8855–8858. [Google Scholar] [CrossRef]
- Ni, M.; Xie, X. 3D image storage in photopolymer/ZnS nanocomposites tailored by “photoinitibitor”. Macromolecules 2015, 48, 2958–2966. [Google Scholar] [CrossRef]
- Yao, W.; Wu, W. Large-scale synthesis and screen printing of upconversion hexagonal-phase NaYF4:Yb3+,Tm3+/Er3+/Eu3+ plates for security applications. J. Mater. Chem. C 2016, 4, 6327–6335. [Google Scholar] [CrossRef]
- Li, D.; She, J. Screen printing of upconversion NaYF4:Yb3+/Eu3+ with Li+ doped for anti-counterfeiting application. Chin. Opt. Lett. 2020, 18, 110501. [Google Scholar] [CrossRef]
- Attia, M.S.; Abdel-Mottaleb, M.S.A. Inkjet Printable Luminescent Eu3+-TiO2 Doped in Sol Gel Matrix for Paper Tagging. J. Fluoresc. 2015, 25, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Labidi, N.S.; Iddou, A. Adsorption of oleic acid on quartz/water interface. J. Saudi Chem. Soc. 2007, 11, 221–234. [Google Scholar]
- Premaratne, W.A.P.J.; De Alwis, A.A.P. Synthesis of nanosilica from paddy husk ash and their surface functionalization. J. Sci. Univ. Kelaniya 2014, 8, 33–48. [Google Scholar] [CrossRef]
- Soliman, E.A.; Ibrahim, H.S. Synthesis and performance of maleic anhydride copolymers with alkyl linoleate or tetra-esters as pour point depressants for waxy crude oil. Fuel 2018, 211, 535–547. [Google Scholar] [CrossRef]
- Zhang, F. Photon Upconversion Nanomaterials. In Nanostructure Science and Technology; Lockwood, D.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-45596-8. [Google Scholar]
- Catunda, T.; Aegerter, M.A. Spectroscopic properties and upconversion mechanisms in Er3+ doped fluoroindate glasses. Phys. Rev. B 1996, 53, 6065–6070. [Google Scholar] [CrossRef]
- Reddy, B.R.; Venkateswarlu, P. Infrared to visible energy upconversion in Er3+ doped oxide glass. Appl. Phys. Lett. 1994, 64, 1327–1329. [Google Scholar] [CrossRef]
- Gonçalves, R.R.; Messaddeq, Y. Infrared-to-visible CW frequency upconversion in erbium activated silica–hafnia waveguides prepared by sol–gel route. J. Non-Cryst. Solids 2003, 322, 306–310. [Google Scholar] [CrossRef]
- Cao, T.M.D.; Le, T.T.G.; Tran, T.T.V. Investigating the effect of Yb3+ and Er3+ concentration on red/green luminescent ratio in β-NaYF4:Er,Yb nanocrystals using spectroscopic techniques. J. Mol. Struct. 2020, 1210, 128014. [Google Scholar] [CrossRef]
- Daly, R.; Njah, N. The studies of crystallite size and microstrains in aluminum powder prepared by mechanical milling. Phys. Stat. Sol. (C) 2006, 3, 3325–3331. [Google Scholar] [CrossRef]

| Weight of PVA (g) | Viscosity (cP) | Surface Tension (mN/m) |
|---|---|---|
| 1 | 7.8 | 59 |
| 2 | 68.5 | 58.6 |
| 3 | 392.5 | 62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, T.M.D.; Le, T.T.G.; Turrell, S.; Ferrari, M.; Lam, Q.V.; Tran, T.T.V. Luminescent Ink Based on Upconversion of NaYF4:Er,Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment. Molecules 2021, 26, 1041. https://doi.org/10.3390/molecules26041041
Cao TMD, Le TTG, Turrell S, Ferrari M, Lam QV, Tran TTV. Luminescent Ink Based on Upconversion of NaYF4:Er,Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment. Molecules. 2021; 26(4):1041. https://doi.org/10.3390/molecules26041041
Chicago/Turabian StyleCao, T. M. Dung, T. T. Giang Le, Sylvia Turrell, Maurizio Ferrari, Quang Vinh Lam, and T. T. Van Tran. 2021. "Luminescent Ink Based on Upconversion of NaYF4:Er,Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment" Molecules 26, no. 4: 1041. https://doi.org/10.3390/molecules26041041
APA StyleCao, T. M. D., Le, T. T. G., Turrell, S., Ferrari, M., Lam, Q. V., & Tran, T. T. V. (2021). Luminescent Ink Based on Upconversion of NaYF4:Er,Yb@MA Nanoparticles: Environmental Friendly Synthesis and Structural and Spectroscopic Assessment. Molecules, 26(4), 1041. https://doi.org/10.3390/molecules26041041







