H2O-Induced Hydrophobic Interactions in MS-Guided Counter-Current Chromatography Separation of Anti-Cancer Mollugin from Rubia cordifolia
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Analyses of Crude Extracts and Purified Targets
2.2. H2O-Induced Significant Hydrophobic Roles both on the Solvents and Target
2.3. Preparative Aqueous CCC Isolation of Mollugin
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Apparatus and Agents
3.3. Preparation of the Crude Extract
3.4. Measurement of Two-Phase Volume Ratio and Partition Coefficient (K)
3.5. Preparation of Two-Phase Solvent Systems and Sample Solution
3.6. MS-Guided Preparative CCC Separation Procedure
3.7. HPLC Analysis and Identification of CCC Peak Fraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Friesen, J.B.; McAlpine, J.B.; Chen, S.-N.; Pauli, G.F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Miron, A.; Ignatova, S.; Skalicka-Wozniak, K. An overview of the two-phase solvent systems used in the countercurrent separation of phenylethanoid glycosides and iridoids and their biological relevance. Phytochem. Rev. 2019, 18, 377–403. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Kuang, P.; Guo, S.; Sun, Q.; Xue, T.; Li, H. An overview of recent progress in solvent systems, additives and modifiers of counter current chromatography. N. J. Chem. 2018, 42, 6584–6600. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Garrard, I. A comprehensive classification of solvent systems used for natural product purifications in countercurrent and centrifugal partition chromatography. Nat. Prod. Rep. 2015, 32, 1556–1561. [Google Scholar] [CrossRef]
- Pauli, G.F.; Pro, S.M.; Friesen, J.B. Countercurrent separation of natural products. J. Nat. Products 2008, 71, 1489–1508. [Google Scholar] [CrossRef]
- Wu, S.; Liang, J. Counter-current chromatography for high throughput analysis of natural products. Comb. Chem. High Throughput Screen. 2010, 13, 932–942. [Google Scholar] [CrossRef]
- Gong, Y.; Huang, X.-Y.; Pei, D.; Duan, W.-D.; Zhang, X.; Sun, X.; Di, D.-L. The applicability of high-speed counter current chromatography to the separation of natural antioxidants. J. Chromatogr. A 2020, 1623, 461150. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Fisher, D. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J. Chromatogr. A 2009, 1216, 740–753. [Google Scholar] [CrossRef]
- Guzlek, H.; Wood, P.L.; Janaway, L. Performance comparison using the GUESS mixture to evaluate counter-current chromatography instruments. J. Chromatogr. A 2009, 1216, 4181–4186. [Google Scholar] [CrossRef]
- Friesen, J.B.; Pauli, G.F. GUESS—A generally useful estimate of solvent systems in CCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2777–2806. [Google Scholar] [CrossRef]
- Liang, J.; Meng, J.; Wu, D.; Guo, M.; Wu, S. A novel 9 × 9 map-based solvent selection strategy for targeted counter-current chromatography isolation of natural products. J. Chromatogr. A 2015, 1400, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A.; Maryutina, T.; Spivakov, B.; Shpigun, O.; Sutherland, I.A. Countercurrent chromatography in analytical chemistry (IUPAC technical report). Pure Appl. Chem. 2009, 81, 355–387. [Google Scholar] [CrossRef] [Green Version]
- Berthod, A.; Ignatova, S.; Sutherland, I.A. Advantages of a small-volume counter-current chromatography column. J. Chromatogr. A 2009, 1216, 4169–4175. [Google Scholar] [CrossRef] [PubMed]
- Berthod, A. Countercurrent chromatography: From the milligram to the kilogram. In Advances in Chromatography; Grushka, E.G.N., Ed.; Marcel Dekker: New York, NY, USA, 2009; Volume 47, pp. 323–352. [Google Scholar]
- Sutherland, I.A. Recent progress on the industrial scale-up of counter-current chromatography. J. Chromatogr. A 2007, 1151, 6–13. [Google Scholar] [CrossRef]
- Wu, S.; Yang, L.; Gao, Y.; Liu, X.; Liu, F. Multi-channel counter-current chromatography for high-throughput fractionation of natural products for drug discovery. J. Chromatogr. A 2008, 1180, 99–107. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Pei, D.; Liu, J.-F.; Di, D.-L. A review on chiral separation by counter-current chromatography: Development, applications and future outlook. J. Chromatogr. A 2018, 1531, 1–12. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, P.; Han, R.; Wu, D.; Gao, J.-M.; Wu, S. Methanol linear gradient counter-current chromatography for the separation of natural products: Sinopodophyllum hexandrum as samples. J. Chromatogr. A 2019, 1603, 251–261. [Google Scholar] [CrossRef]
- Wu, S.H.; Wu, D.F.; Liang, J.L.; Berthod, A. Modeling gradient elution in countercurrent chromatography: Efficient separation of tanshinones from Salvia miltiorrhiza Bunge. J. Sep. Sci. 2012, 35, 964–976. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S. Hydrophobic and hydrophilic interactions in countercurrent chromatography. J. Chromatogr. A 2020, 1611, 460576. [Google Scholar] [CrossRef]
- Liu, J.G.; Qin, Z.F.; Wang, J.G. Liquid-liquid equilibria for methanol plus water plus hexane ternary mixtures. J. Chem. Eng. Data 2002, 47, 1243–1245. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Elution-extrusion countercurrent chromatography. Use of the liquid nature of the stationary phase to extend the hydrophobicity window. Anal. Chem. 2003, 75, 5886–5894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, M.Y.; Mi, C.; Wang, K.S.; Ma, J.; Jin, X. Mollugin has an anti-cancer therapeutic effect by inhibiting TNF-alpha-induced NF-kappa B activation. Int. J. Mol. Sci. 2017, 18, 1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, M.; Yu, S.; Yan, H.; Chen, P.; Zhang, L.; Ding, A. A Review of the botany, phytochemistry, pharmacology and toxicology of rubiae radix et rhizoma. Molecules 2016, 21, 1747. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, H.; Zhu, J.; Xu, J.; Ding, K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 2014, 450, 247–254. [Google Scholar] [CrossRef]
- Do, M.T.; Hwang, Y.P.; Kim, H.G.; Na, M.; Jeong, H.G. Mollugin inhibits proliferation and induces apoptosis by suppressing fatty acid synthase in HER2-overexpressing cancer cells. J. Cell. Physiol. 2013, 228, 1087–1097. [Google Scholar]
- Liu, R.; Lu, Y.; Wu, T.; Pan, Y. Simultaneous isolation and purification of mollugin and two anthraquinones from Rubia cordifolia by HSCCC. Chromatographia 2008, 68, 95–99. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, R.; Sun, C.; Pan, Y. An effective high-speed countercurrent chromatographic method for preparative isolation and purification of mollugin directly from the ethanol extract of the Chinese medicinal plant Rubia cordifolia. J. Sep. Sci. 2007, 30, 1313–1317. [Google Scholar] [CrossRef]
- Berthod, A.; Friesen, J.B.; Inui, T.; Pauli, G.F. Elution-extrusion countercurrent chromatography: Theory and concepts in metabolic analysis. Anal. Chem. 2007, 79, 3371–3382. [Google Scholar] [CrossRef] [Green Version]
- Friesent, J.B.; Pauli, G.F. Performance characteristics of countercurrent separation in analysis of natural products of agricultural significance. J. Agric. Food Chem. 2008, 56, 19–28. [Google Scholar] [CrossRef]
- Jerz, G.; Wybraniec, S.; Gebers, N.; Winterhalter, P. Target-guided separation of Bougainvillea glabra betacyanins by direct coupling of preparative ion-pair high-speed countercurrent chromatography and electrospray ionization mass-spectrometry. J. Chromatogr. A 2010, 1217, 4544–4554. [Google Scholar] [CrossRef] [PubMed]
- Gutzeit, D.; Winterhalter, P.; Jerz, G. Application of preparative high-speed counter-current chromatography/electrospray ionization mass spectrometry for a fast screening and fractionation of polyphenols. J. Chromatogr. A 2007, 1172, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Letsyo, E.; Beuerle, T.; Winterhalter, P.; Horn, G.; Jerz, G. High-performance countercurrent chromatography fractionation of epimeric pairs intermedine/lycopsamine and amabiline/supinine by an off-line electrospray mass spectrometry injection profiling of the roots of Lappula squarrosa. Microchem. J. 2020, 157, 104952. [Google Scholar] [CrossRef]
- dos Santos Grecco, S.; Letsyo, E.; Tempone, A.G.; Ghilardi Lago, J.H.; Jerz, G. Electrospray mass-spectrometry guided target isolation of neolignans from Nectandra leucantha (Lauraceae) by high performance- and spiral-coil countercurrent chromatography. J. Chromatogr. A 2019, 1608, 460422. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Mihara, K.; Takeya, K. Studies on a novel anthraquinone and its glycosides isolated from Rubia cordifolia and R. akane. Chem. Pharm. Bull. 1983, 31, 2353–2358. [Google Scholar] [CrossRef] [Green Version]
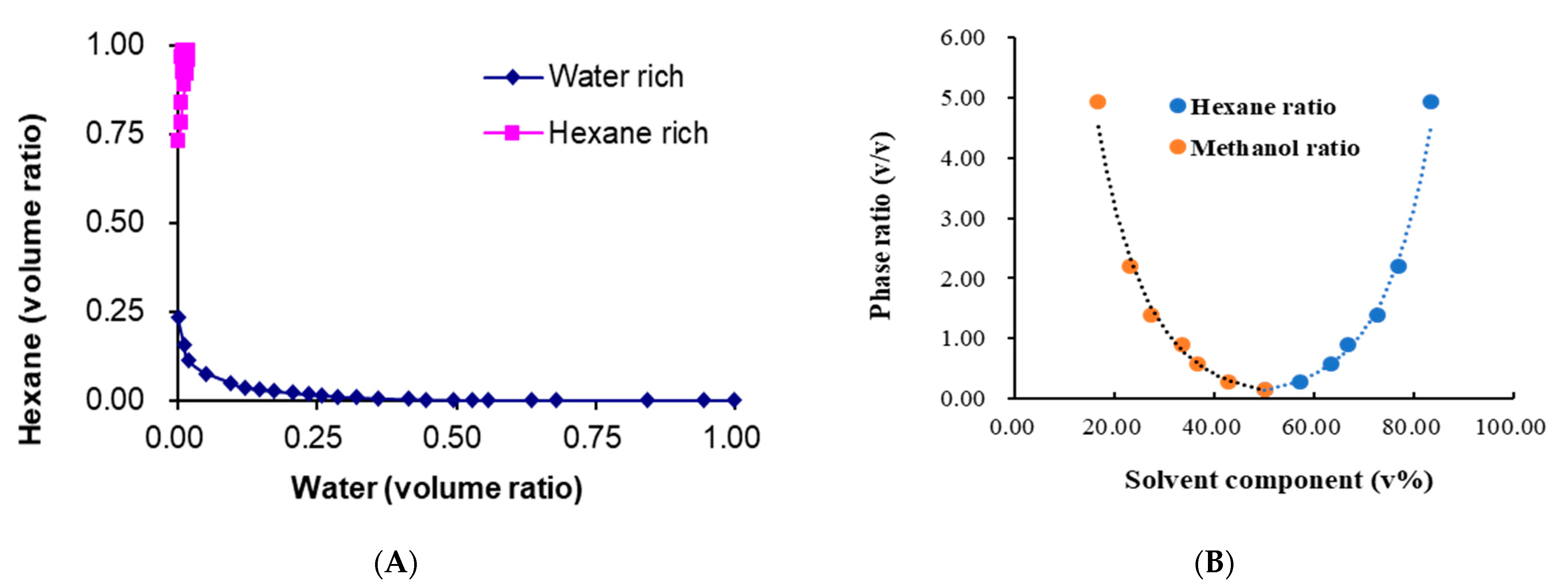
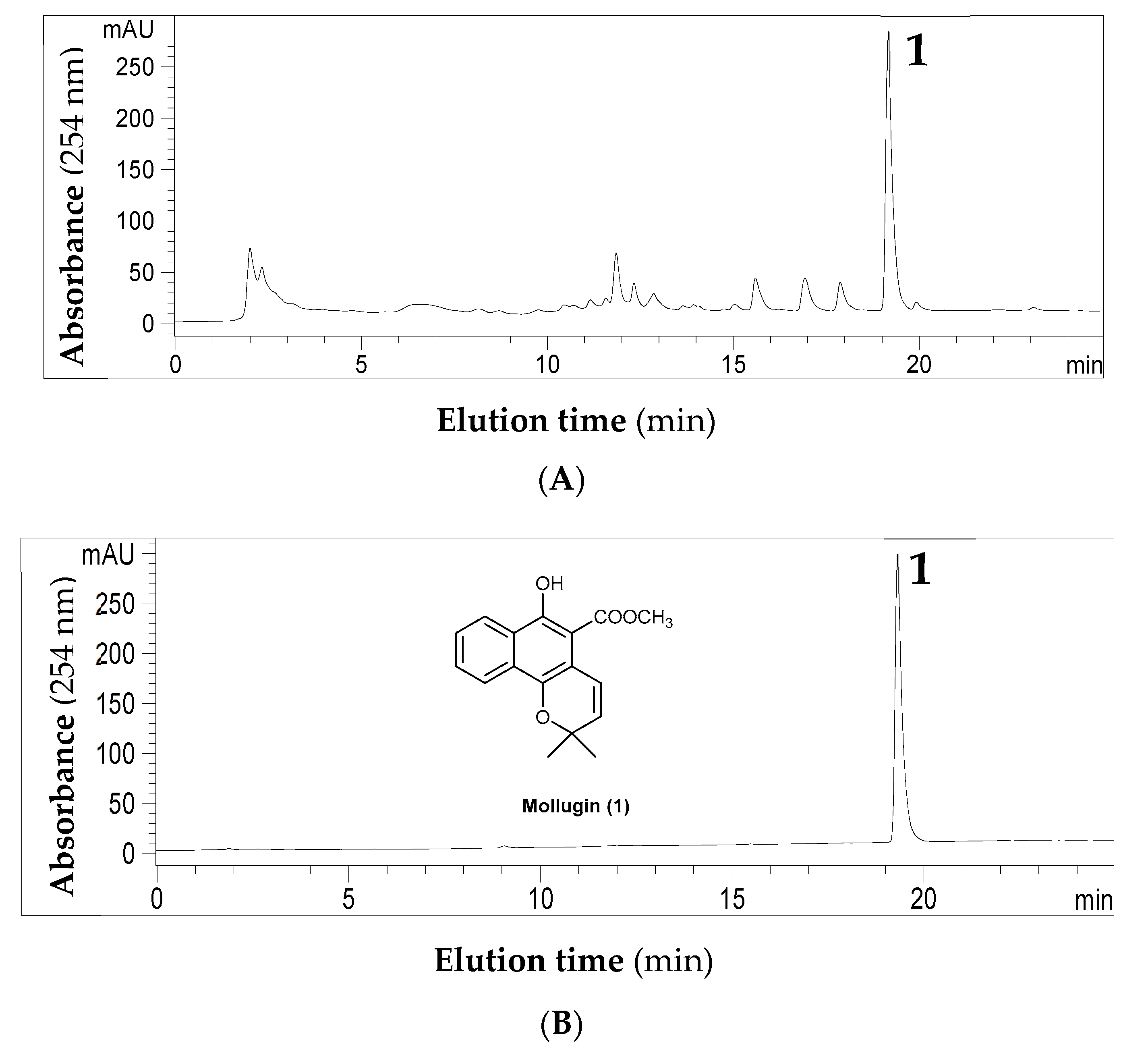
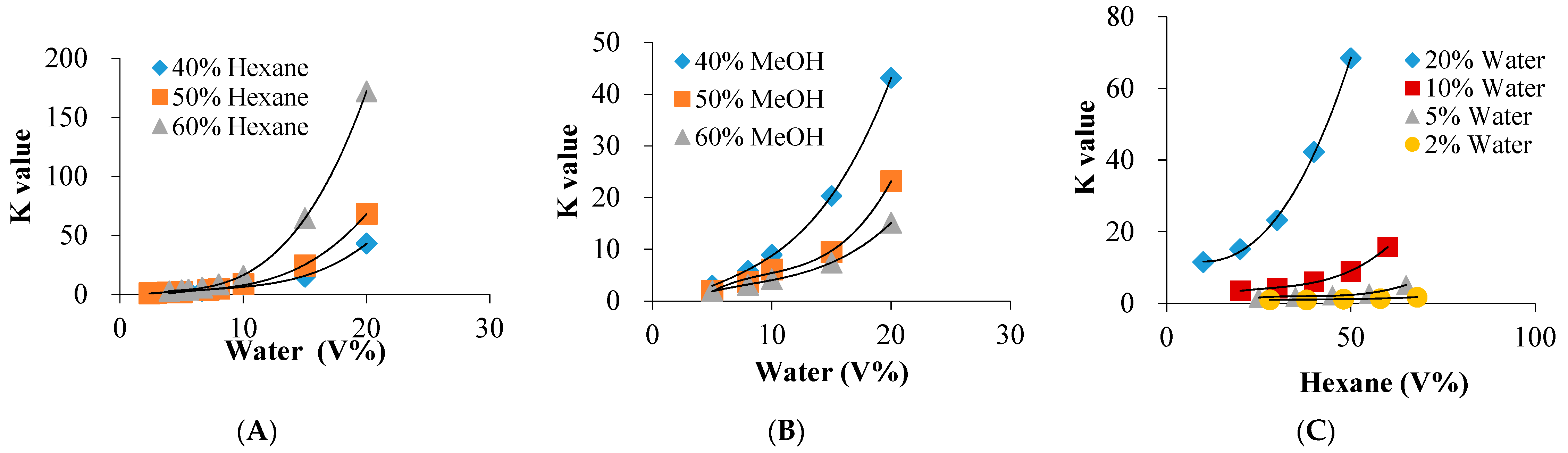
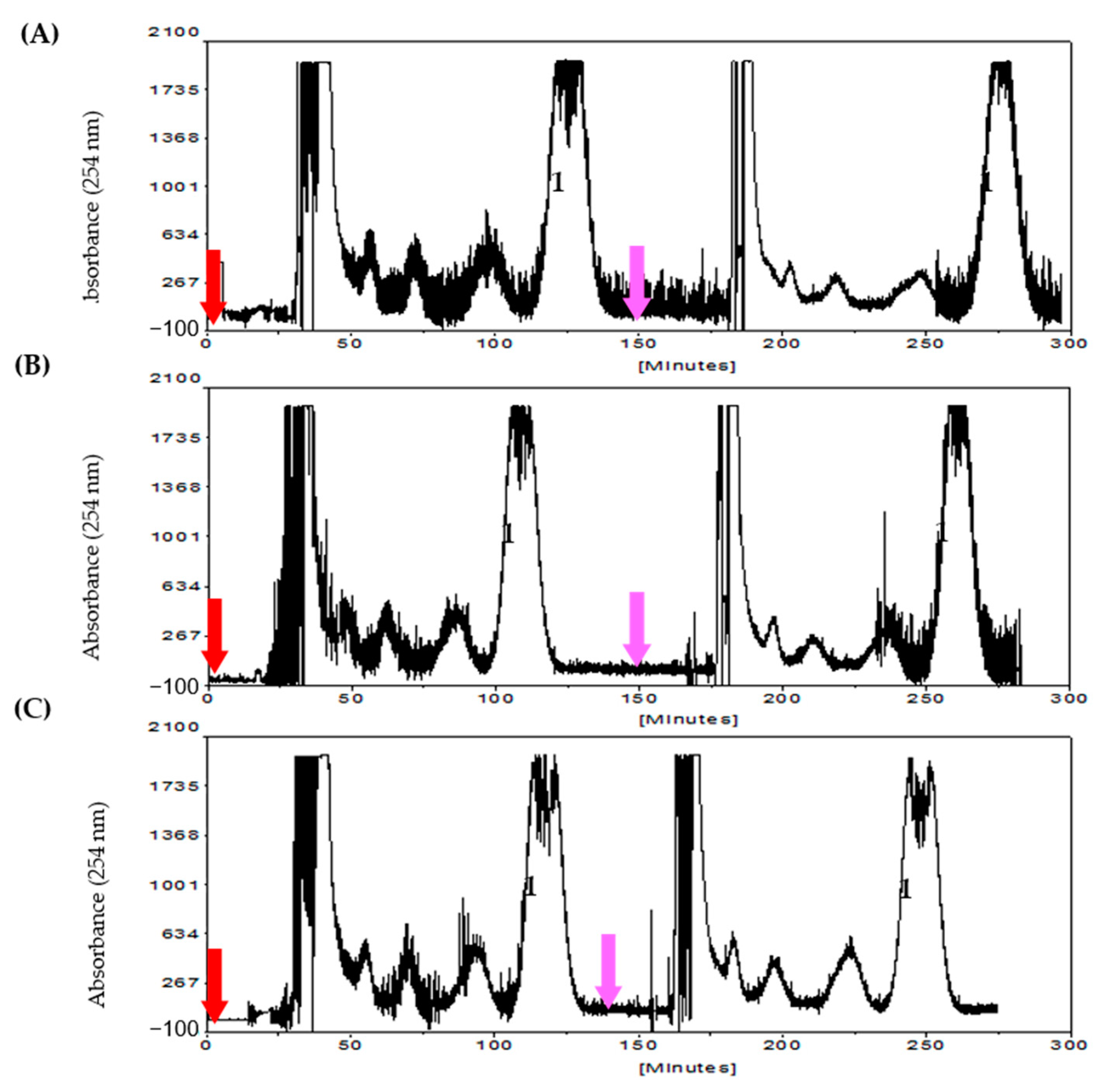
| Solvent System a | System Components (v %) | Phase Volume Ratio | Kb | |
|---|---|---|---|---|
| (Hexane:Methanol, v/v) | Hexane | Methanol | VU/VL | 1 |
| 3:3 | 50.00 | 50.00 | 0.16 | 0.85 |
| 4:3 | 57.14 | 42.86 | 0.28 | 0.83 |
| 1.9:1.1 | 63.33 | 36.67 | 0.58 | 0.80 |
| 6:3 | 66.67 | 33.33 | 0.81 | 0.82 |
| 8:3 | 72.73 | 27.27 | 1.39 | 0.79 |
| 10:3 | 76.92 | 23.08 | 2.20 | 0.79 |
| 15:3 | 83.33 | 16.67 | 4.94 | 0.78 |
| Solvent System | Solvent Components (v %) | Phase Ratio | Ka | Rf b (%) | TR/min | Duration (min) | Used Lower Phase (mL) | Purity by HPLC (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | MeOH | Water | n-Hexane | Methanol | Water | |||||||
| 9 | 10 | 1 | 45.00 | 50.00 | 5.00 | 0.67 | 2.63 | 66.67 | 188 | 208 | 624 | 97.32 |
| 10 | 11 | 1 | 45.45 | 50.00 | 4.55 | 0.76 | 2.36 | 65.19 | 170 | 199 | 597 | 97.41 |
| 10 | 12 | 1 | 43.48 | 52.17 | 4.35 | 0.61 | 2.23 | 66.30 | 140 | 157 | 550 | 97.27 |
| 13 | 15 | 1 | 44.83 | 51.72 | 3.45 | 0.61 | 2.12 | 64.81 | 133 | 147 | 515 | 98.43 |
| 12 | 14 | 1 | 44.44 | 51.85 | 3.70 | 0.63 | 1.92 | 63.33 | 122 | 146 | 511 | 98.42 |
| 13 | 16 | 1 | 43.33 | 53.33 | 3.33 | 0.55 | 1.94 | 59.26 | 120 | 140 | 490 | 97.96 |
| 14 | 18 | 1 | 42.42 | 54.55 | 3.03 | 0.51 | 1.65 | 55.55 | 105 | 121 | 424 | 99.08 |
| 13 | 20 | 1 | 38.24 | 58.82 | 2.94 | 0.40 | 1.79 | 55.55 | 111 | 131 | 459 | 97.10 |
| 15 | 20 | 1 | 41.67 | 55.56 | 2.78 | 0.47 | 1.61 | 61.11 | 106 | 127 | 445 | 97.38 |
| 17 | 20 | 1 | 44.74 | 52.63 | 2.63 | 0.57 | 1.69 | 54.44 | 106 | 120 | 420 | 97.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Xu, T.; Meng, J.; Wu, D.; Wu, S. H2O-Induced Hydrophobic Interactions in MS-Guided Counter-Current Chromatography Separation of Anti-Cancer Mollugin from Rubia cordifolia. Molecules 2021, 26, 751. https://doi.org/10.3390/molecules26030751
Zeng L, Xu T, Meng J, Wu D, Wu S. H2O-Induced Hydrophobic Interactions in MS-Guided Counter-Current Chromatography Separation of Anti-Cancer Mollugin from Rubia cordifolia. Molecules. 2021; 26(3):751. https://doi.org/10.3390/molecules26030751
Chicago/Turabian StyleZeng, Liping, Tianyi Xu, Jie Meng, Dingfang Wu, and Shihua Wu. 2021. "H2O-Induced Hydrophobic Interactions in MS-Guided Counter-Current Chromatography Separation of Anti-Cancer Mollugin from Rubia cordifolia" Molecules 26, no. 3: 751. https://doi.org/10.3390/molecules26030751
APA StyleZeng, L., Xu, T., Meng, J., Wu, D., & Wu, S. (2021). H2O-Induced Hydrophobic Interactions in MS-Guided Counter-Current Chromatography Separation of Anti-Cancer Mollugin from Rubia cordifolia. Molecules, 26(3), 751. https://doi.org/10.3390/molecules26030751







