1. Introduction
Diabetes mellitus (DM) is a common chronic noninfectious disease, which can make the body experience continuous hyperglycemia and long-term metabolic disorder, and lead to the damage, dysfunction, and failure of the whole body’s tissues and organs [
1]. According to the International Diabetes Federation, 463 million people worldwide currently have diabetes. Further, this number is expected to increase to 592 million, implying that there will be around a 50% increase in diabetes by 2035 [
2]. DM is classified into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM), in which T2DM accounts for nearly 95% of individuals. As insulin resistance is a major characteristic of T2DM, improving insulin resistance is a primary strategy to improve metabolic control in subjects with type 2 diabetes [
3]. Glucose transporter type 4 (GLUT-4) is predominantly expressed in muscle cells and adipocytes [
4]. The insulin-stimulated glucose uptake is performed through the solute carrier family 2, facilitated glucose transporter type 4, which is rapidly translocated to the plasma membrane in response to the hormone [
5]. Therefore, this protein has a potential role in preventive or therapeutic approaches for diabetes.
Natural products (NPs), including herbal formulas and its extracts, have been used to treat human diseases with the unique system of theories and therapies for thousands of years, which have also been increasingly applied to treat T2DM. Much evidence has indicated that herbal medicines and their active ingredients possess anti-diabetics properties with less toxicity and fewer adverse effects [
6].
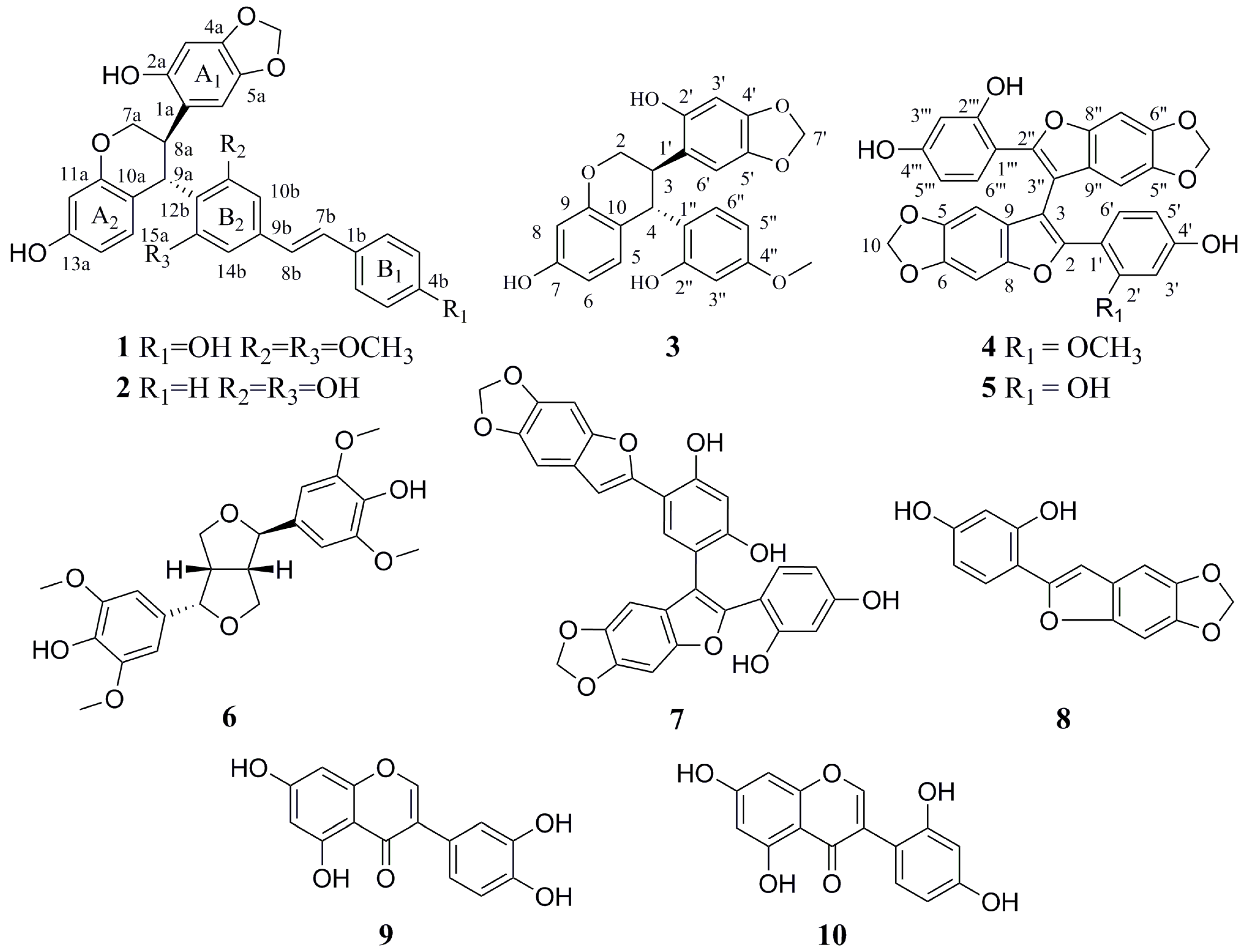
Sophora davidii (Franch.) Skeels (Fabaceae family) is a deciduous shrub or dunga-runga growing in valley scrub, hill slopes, and sandy places in valleys below 3400 m. It is mainly distributed in Gansu, Guangxi, Guizhou, Hebei, Henan, Hubei, Hunan, Jiangsu, Shaanxi, Sichuan, Xizang, Yunnan, and Zhejiang provinces of China [
7]. The roots of
S. davidii have been traditionally used to clear heat, sooth a sore throat, cool the blood and reduce swelling, as well as treat hematochezia, cough and dysentery, etc. [
8]. In the previous paper, we reported that the flavonoid-rich extract of
S. davidii showed a good effect in promoting GLUT-4 translocation and improving glucose uptake in L6 cells [
9]. Further, we isolated and determined five new compounds, davidones A-E, and one new isoflavonoid, cyclolicoisoflavones A
3, along with seven known compounds, leachianone A, brosimacutin C, crotalarin, gerontoisoflavone A, griffonianone H, acacetin, and pterostilbene from the roots of
S. davidii with some GLUT-4 translocation activities [
10]. As a continuation of our search for new bioactive natural chemical substances from
S. davidii, we further performed purification of an EtOAc fraction of the traditional herb that led to two new stilbene oligomers, Davidiol E–F (
1–
2), one new 4-aryl-substituted isoflavan Davidinin A (
3), and one new 2-arylbenzofuran dimer, Shandougenine C (
4), together with six known compounds (
Figure 1). In this paper, we described the isolation and structural elucidation of the four new compounds as well as the GLUT-4 translocation activities of compounds
1–
10.
2. Results and Discussion
The IR spectrum of showed Davidiol E (
1) the presence of hydroxyl (3327 cm
−1) and aromatic (1647 cm
−1 and 1450 cm
−1) structures. The UV spectrum showed
λmax (MeOH) (log
ε) at 230 (3.40) and 310 (3.84) nm [
11]. The
1H NMR spectrum (
Table 1) showed the presence of two
para-coupled aromatic proton moieties on ring A
1 at
δH 6.76 (1H, s, H-6a),
δH 6.30 (1H, s, H-3a), and an ABX spin system at δ
H 6.28 (1H, d,
J = 8.3 Hz, H-15a),
δH 6.17 (1H, d,
J = 1.7 Hz, H-12a), and
δH 6.11 (1H, dd,
J = 8.3, 1.7 Hz, H-14a) for ring A
2, and two groups of
meta-coupled aromatic protons belonging to rings B
2 at
δH 6.79 (1H, br s, H-10b),
δH 6.70 (1H, br s, H-14b), and 4-hydroxyphenyl group (ring B
1) at
δH 7.38 (2H, d,
J = 8.4 Hz, H-2b/6b),
δH 6.75 (2H, d,
J = 8.4 Hz, H-3b/5b). The
1H NMR spectrum also displayed the presence of a
trans-1,2-disubstituted vinyl group at
δH 7.12 (1H, d,
J = 16.3 Hz, H-7b),
δH 6.91 (1H, d,
J = 16.3 Hz, H-8b), and a methylenedioxy moiety with two non-equivalent protons at
δH 5.82 (1H, s, -OCH
2O-) and
δH 5.78 (1H, s, -OCH
2O-). The HMBC correlations of this -OCH
2O- group (
δH 5.82,
δH 5.78) with C-4a (
δC 145.4) and C-5a (
δC 139.6) indicated that the oxygen atoms were linked to carbons C-4a and C-5a in the tetrasubstituted aromatic ring A
1. The
13C NMR spectrum of
1 revealed the presence of two methoxy groups at
δC 56.2 and 55.8, four aliphatic carbons at
δC 100.3, 70.6, 34.7, and 34.4, besides 26 aromatic and olefinic carbons between
δC 97.3 and 158.9, and all protonated carbons were assigned from the HMQC spectrum. In the HMBC spectrum (
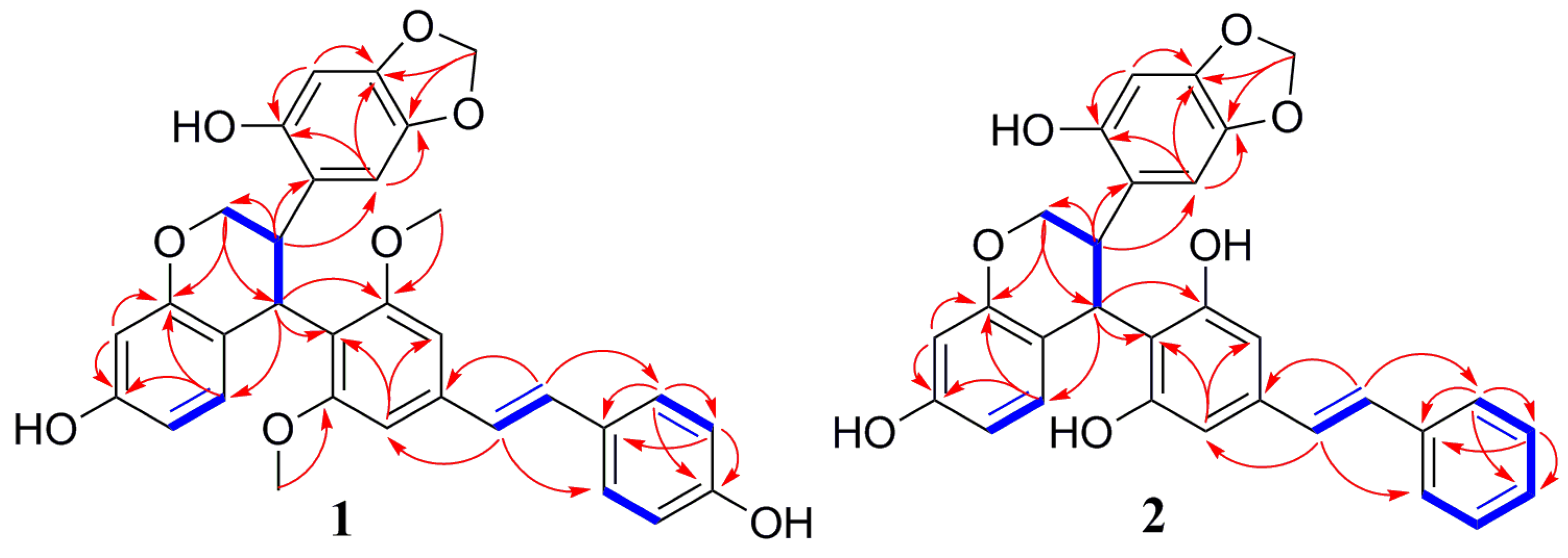
Figure 2), the long-range correlations of H-7b with C-2b, C-6b (
δC 127.8), and C-9b (
δC 137.3) and H-8b with C-1b (
δC 128.2), C-10b (
δC 102.3), and C-14b (
δC 104.0) indicated that the
trans-1,2-disubstituted vinyl group was attached to B
1 and B
2 rings. The correlations from H-7a to C-9a (
δC 34.7) and C-11a (
δC 154.5), from H-9a to C-10a (
δC 117.6), C-11a (
δC 154.5), C-14a (
δC 107.7), and C-15a (
δC 128.0), and the connections of H-7a/H-8a/H-9a were determined from the H
1-H
1 COSY, indicating that the H-7a, H-8a, and H-9a aliphatic protons could be assigned to the protons of the tetrahydrobenzopyran ring. The tetrahydrobenzopyran ring attached to A
1 ring and B
2 ring were also confirmed by the correlations between H-7a with C-1a (
δC 117.8) and between H-8a with C-1a (
δC 117.8), C-2a (
δC 150.0), and C-6a (
δC 107.3) and between H-9a with C-10b (
δC 102.3), C-11b (
δC 158.9). The relative configuration of H-8a and H-9a was inferred by the observed large coupling constants (
J = 11.4 Hz) for them and confirmed by correlations between H-8a with H-10b, and between H-9a with H-3a and H-6a in the ROESY spectrum. On the basis of these observations, H-8a and H-9a were in the opposite orientation [
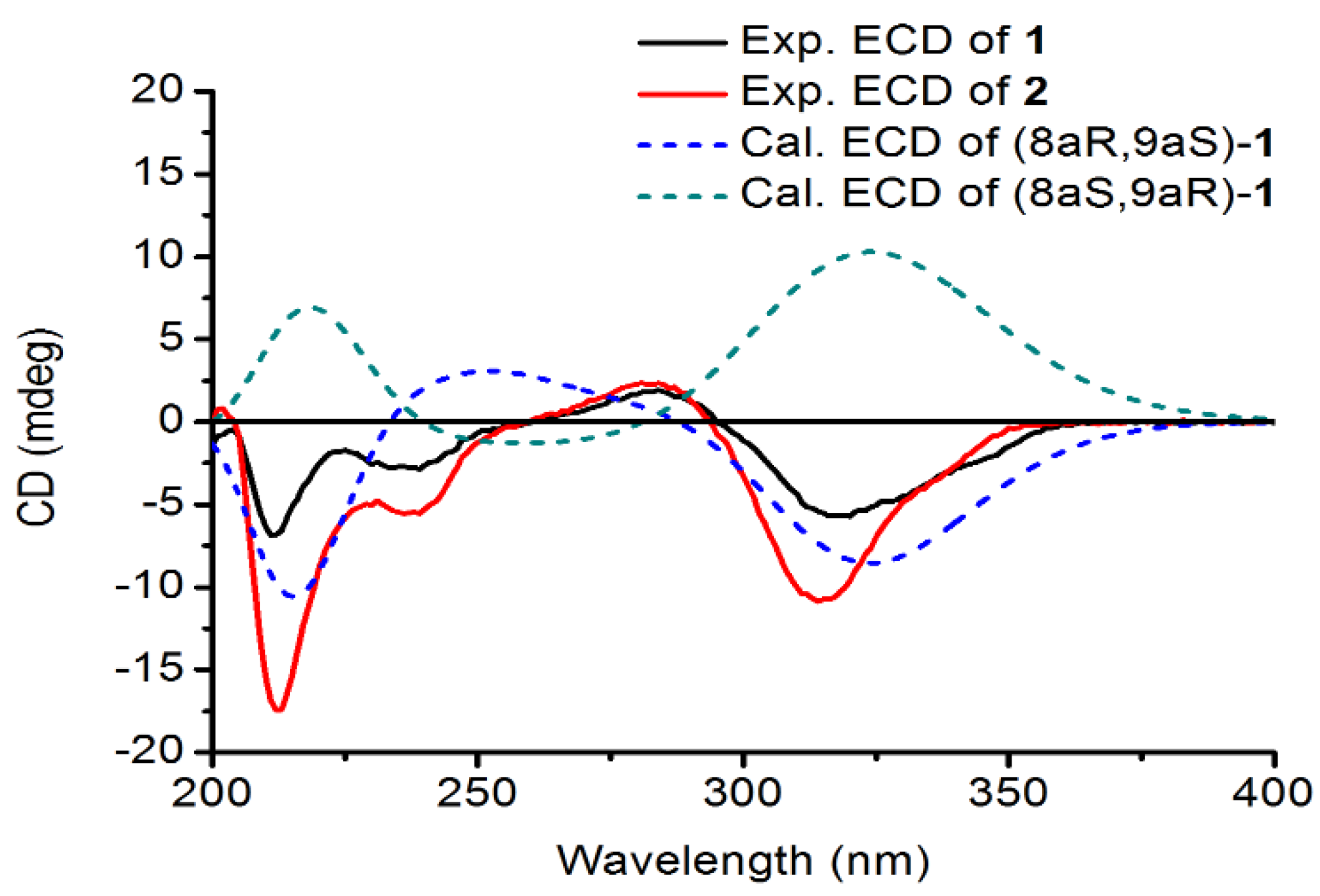
12]. As for the absolute configuration of
1, electronic circular dichroism (ECD) calculations of the enantiomers 8aR, 9aS-1, and 8aS, 9aR-1 were carried out using B3LYP/6-31G(d) optimized geometries at the B3LYP/6-311+G(d,p) level in MeOH. The experimental and calculated ECD spectra of
1 were in good agreement. The calculated ECD spectrum for
1 showed the positive Cotton effect at 281 nm and the negative Cotton effect around 320 nm in the ECD spectrum (
Figure 3). Therefore, Davidiol E was determined to be (8aR,9aS)-8a,9a-
trans-2a,13a-dihydroxy-4a,5a-methylenedioxy-9a-[(
E)-6-(3,5-dimethoxyphenyl)-5-(4’-hydroxyphenyl)-ethenyl]-isoflavan.
The
1H and
13C-NMR spectra data of Davidiol F (
2) (
Table 1) were very similar to those of
1, except for a monosubstituted phenyl group at
δH 7.55 (2H, d,
J = 7.4 Hz, H-2b/6b),
δH 7.34 (2H, t,
J = 7.8 Hz, H-3b/5b), and
δH 7.24 (1H, t,
J = 7.6 Hz, H-4b) for ring B
2. Meanwhile, the absence of two methoxy carbons at C-11b-OMe and C-13b-OMe was observed in the
13C NMR spectrum. Compound
2 had the same stereochemistry as
1, which was substantiated by the similar ROESY spectrum and the large coupling constant of H-8a/H-9a (
J = 11.7 Hz). In a similar manner to
1, the absolute configuration of
2 was established as 8aR, 9aS by the positive Cotton effect at 284 nm and the negative Cotton effect around 314 nm in the ECD spectrum (
Figure 3). Therefore, Davidiol F was determined to be (8a
R,9a
S)-8a,9a-
trans-2a,13a-dihydroxy-4a,5a-methylenedioxy-9a-[(
E)-6-(3,5-dihydroxyphenyl)-5-phenyl-ethenyl]-isoflavan.
The IR absorptions of Davidinin A (
3) suggested the presence of hydroxyl group (3370 cm
−1), aromatic (1655 and 1452 cm
−1), and methylenedioxy (-OCH
2O-) (1115 and 1032 cm
−1) group. The
1H NMR data of
3 (
Table 2) established characteristic resonances for two 1,2,4-trisubstituted benzene rings [
δH 6.67 (1H, d,
J = 8.5 Hz, H-6”),
δH 6.36 (1H, d,
J = 2.2 Hz, H-3”), and
δH 6.29 (1H, dd,
J = 8.5, 2.2 Hz, H-5”); and
δH 6.54 (1H, d,
J = 8.0 Hz, H-5),
δH 6.26 (1H, br s, H-8), and
δH 6.25 (1H, overlapped, H-6)], and a pair of isolated singlet signals at
δH 6.72 (1H, s, H-6’) and 6.32 (1H, s, H-3’). A methylenedioxy group signal at
δH 5.76, 5.75 (each 1H, br s, H-7”), a methoxyl group at
δH 3.70 (3H, s, 4”-OMe), and four aliphatic protons at
δH 4.51 (1H, d,
J = 7.3 Hz, H-4),
δH 4.20 (1H, dd,
J = 10.6 Hz, 3.1 Hz, H-2),
δH 4.08 (1H, dd,
J = 10.6 Hz, 7.1 Hz, H-2), and
δH 3.60 (1H, m, H-3) were observed in the
1H NMR spectrum. The
13C NMR, DEPT (
Table 2) and the HSQC spectra showed 23 carbon signals, including eighteen aromatic carbons, a methylenedioxy carbon at
δC 101.9 (C-7’), an oxymethylene carbon at
δC 69.1 (C-2), a methoxyl carbon at
δC 55.5 (4”-OMe), and two methines at
δC 39.2 (C-4) and
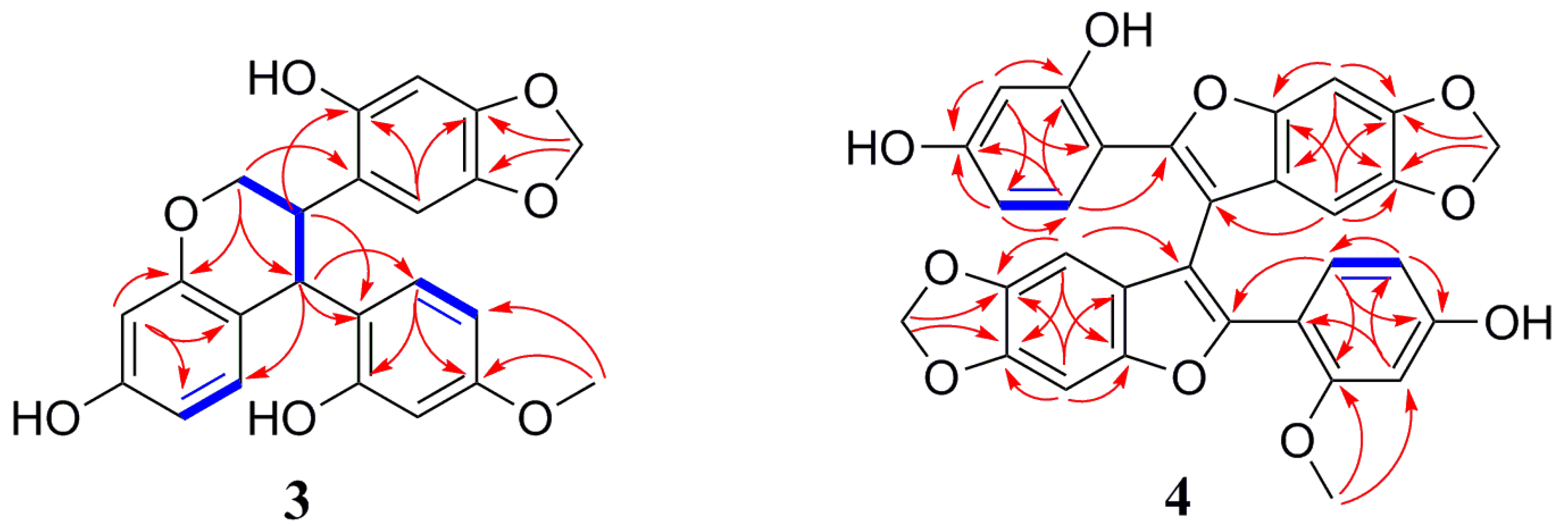
δC 38.9 (C-3). The presence of a benzotetrahydropyran ring was supported by HMBC correlations (
Figure 4) from H-2 to C-8 and C-4, and from H-4 to C-5, C-9 and C-10, along with the linkage moiety CH
2(2)-CH(3)-CH(4) from the
1H-
1H COSY correlations of H-2/H-3/H-4. Moreover, the HMBC correlations from a methylenedioxy proton (
δH 5.76, 5.75) with C-4’ (
δC 147.6) and C-5’ (
δC 142.0) suggested that the methylenedioxy group was located at C-5’, C-6’. The location of ring B and ring C were assigned at C-3 and C-4 by the key HMBC correlations from H-3 to C-1’ and C-2’, and from H-4 to C-1’, C-2’, and C-6’. Regarding the relative configuration of
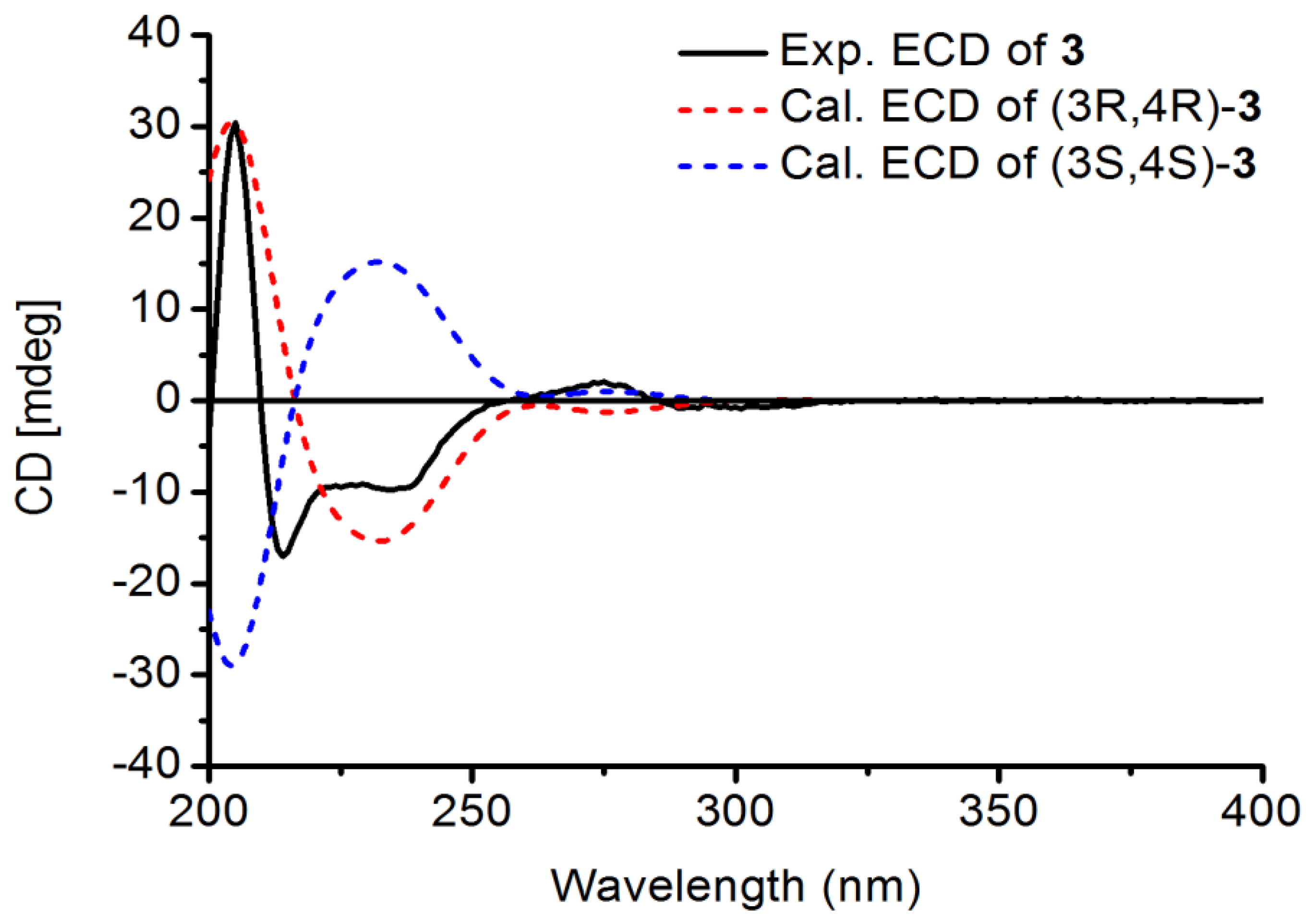
3, ROESY cross peaks between H-3/H-6” and H-4/H-6’ suggested that H-3 and H-4 were in the opposite orientation. Finally, the absolute configuration of
3 was determined as 3R, 4R by comparison of theoretical and experimental ECD spectra (
Figure 5). Its ECD spectrum displayed a negative Cotton effect at 240 nm and a positive Cotton effect in the range 260–280 nm, which was in agreement with those of Manuifolin Q [
13], an analogue whose stereochemistry as (3
R,4
R) has been unambiguously elucidated. Thus, Davidinin A was determined to be (3
R,4
R)-3,4-
trans-7,2’-dihydroxy-4’,5’-methylenedioxy-4-(4-methoxy-2-hydroxyphenyl)-isoflavan.
The IR spectrum of shandougenine C (
4) was similar to that of
3, also indicating that
4 had (3343 cm
−1), aromatic (1452 cm
−1), and methylenedioxy (1032 cm
−1) groups. The
1H NMR spectrum (
Table 2) exhibited two sets of ABX system signals at
δH 7.36 (1H, d,
J = 8.4 Hz, H-6’),
δH 6.31 (1H, d,
J = 2.2 Hz, H-3’), and
δH 6.42 (1H, dd,
J = 8.4, 2.2 Hz, H-5’); and at
δH 7.17 (1H, d,
J = 8.3 Hz, H-6‴),
δH 6.26 (1H, d,
J = 2.4 Hz, H-3‴), and
δH 6.24 (1H, dd,
J = 8.3, 2.4 Hz, H-5‴); four singlet signals [
δH 7.01 (1H, s, H-7),
δH 6.99 (1H, s, H-7”),
δH 6.38 (1H, s, H-4”), and
δH 6.72 (1H, s, H-4)], two methylenedioxy [
δH 5.88, 5.87 (each 2H, overlapped, H-10, H-10”)], and a methoxyl group [
δH 3.33 (3H, s, 2’-OMe)] were shown in the
1H NMR spectrum. Furthermore, two sets of similar carbon chemical shift values were found in the
13C NMR spectrum, which suggested that
4 might be a dimer. These spectroscopic characteristics of compound
4 were similar to the known compound shandougenine B [
14], a 2-arylbenzofuran dimer previously isolated from the roots of
Sophora tonkinensis. The difference was that 2’-OH was substituted by a methoxy group in compound
4. The correlation of H
3-2’-OMe to C-2’ (
δC 159.4) and C-3’ (
δC 100.2) suggested that 2’-OMe was linked to C-2’ according to the HMBC spectrum (
Figure 4). Thus, shandougenine C was determined to be 3,3”-bis[2-(2-methoxy-4-hydroxyphenyl)-2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran].
The known compounds Shandougenine A (
5) [
14], (+)-Lirioresinol-A (
6) [
15], Shandougenine B (
7) [
14], 2-(2’,4’-dihydroxyphenyl)-5,6-methylenedioxybenzofuran (
8) [
16], isoluteolin (
9) [
17], and 2’,4’,5,7-tetrahydroxyisoflavone (
10) [
18] were identified by comparison of their spectroscopic data with those in the literature.
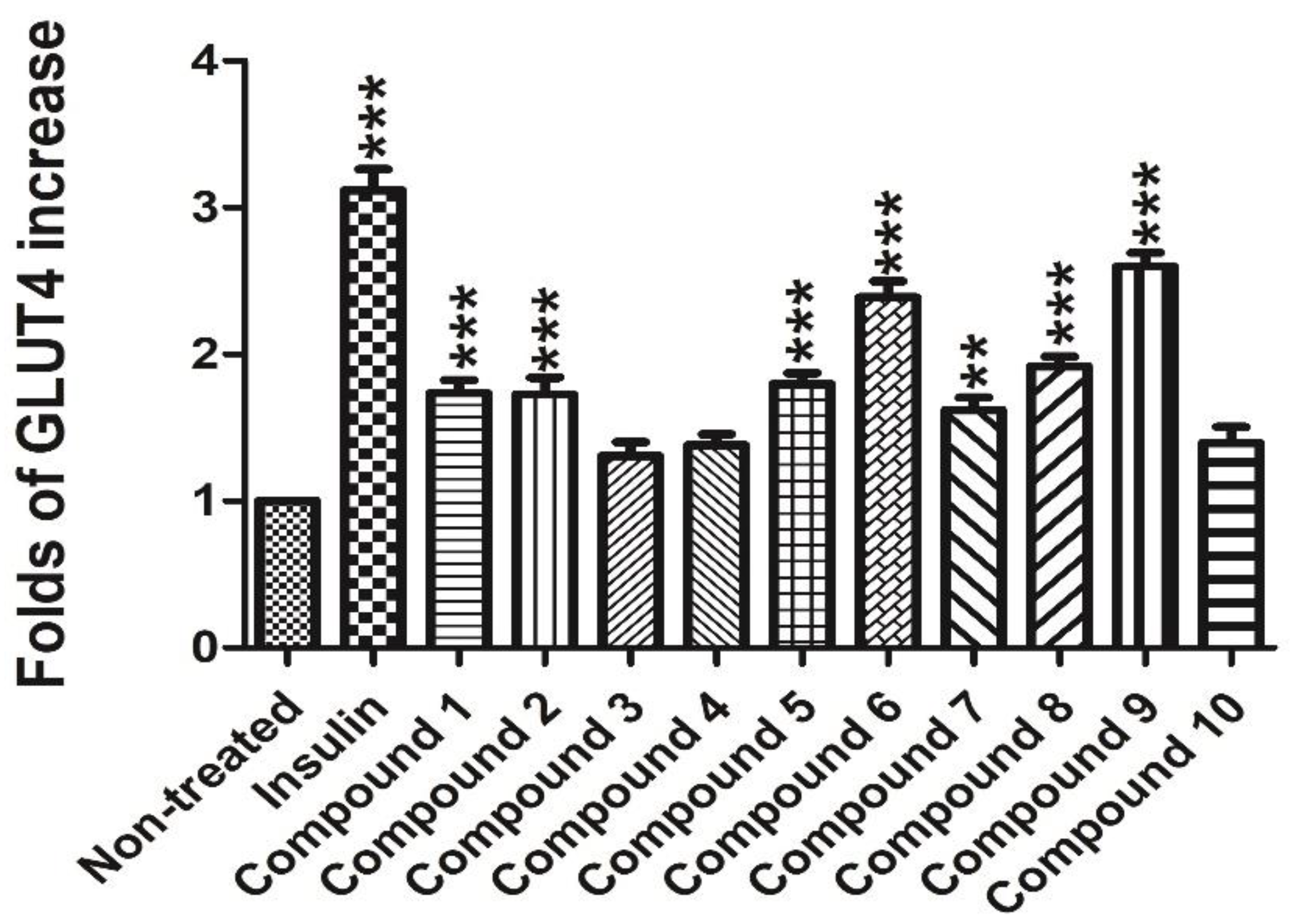
In order to test the potential GLUT-4 translocation activity of compounds
1–
10, a L6 cell line which stably expressed Myc-GLUT4-mOrange was used to evaluate the effects. Insulin (100 nM) was used as the positive control. The compounds Davidiol E-F (
1–2), Davidinin A (
3), Shandougenine C (
4), Shandougenine A (
5), Shandougenine B (
7), 2-(2’,4’-dihydroxyphenyl)-5,6-methylenedioxybenzofuran (
8) and 2’,4’,5,7-tetrahydroxyisoflavone (
10) exerted weak activity, increasing GLUT-4 translocation by 0.41–0.92 folds, respectively. (+)-Lirioresinol-A (
6) possesses a moderate effect on promoting GLUT-4 translocation, which increased GLUT-4 translocation to 2.39 folds. Isoluteolin (
9) was the most active compound, exhibiting good GLUT-4 translocation activity with 1.60-fold enhancement (
Figure 6). Compared with
10, they all exhibited a set of ABX system signals on B ring; the only difference was the hydroxyl group connected to 3’ and 4’ of B ring in compound
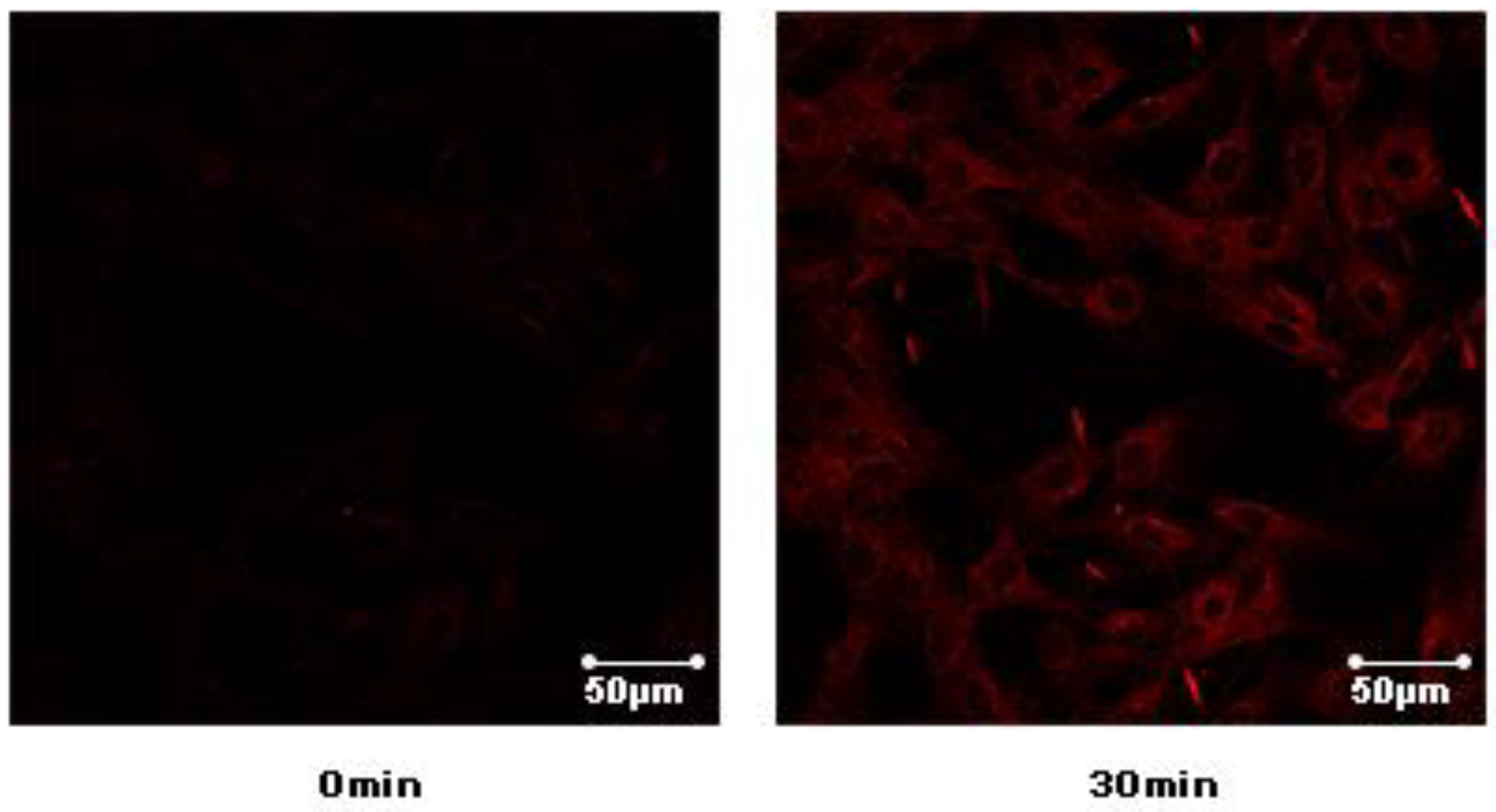
9, which may enhance the activities of GLUT-4 translocation. The laser-scanning confocal microscope LSM 700 (Carl Zeiss, Jena, Germany) was used to detect the fluorescence to indirectly reflect the content of GLUT4 on the plasma membrane in L6 cells (
Supplementary Materials S46). The greater intensity of the fluorescence reflects the greater content of GLUT4 on the plasma membrane. Confocal images in L6 cells incubated in the absence (basal) or presence of compound
9 for 30 min (L6 cells were infected with Myc-GLUT4-mOrange in order to detect externalized GLUT-4 by confocal microscopy) (
Figure 7).
3. Materials and Methods
3.1. General Information
UV and IR spectra were determined on a Shimadzu UV-250 spectrometer (Shimadzu (China) Co., Ltd., Shanghai, China) and a Shimadzu FTIR-8400S spectrometer (Shimadzu (China) Co., Ltd., Shanghai, China), respectively. Optical rotations were measured using an Autopol IV-T automatic polarimeter (Rudolph Research Analytical, Hackettstown, NJ, USA). Circular dichroism (CD) spectra were recorded on a JASCO J-720 W spectrophotometer (JASCO China (Shanghai) Co., Ltd., Shanghai, China). The HRESIMS data were recorded on a UHPLC System and the Q Exactive HF Mass Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). A Thermo 70105-159070 Betasil C18 column (5 μm, 10 mm × 150 mm, Thermo Fisher Scientific, Waltham, MA, USA) was used for semipreparative HPLC. A Waters 2535 HPLC fitted with a 2998 Photodiode Array Detector and a 2707 Autosampler (Waters, Milford, MA, USA) was used for the semipreparative separations. All the solvents used for chromatography were of HPLC-grade and all the other chemicals were of analytical reagent grade. HPLC-grade acetonitrile was purchased from Merck Chemical Company (Darmstadt, Germany). Silica gel (300–400 mesh) was used for medium-pressure column chromatography and GF254 for TLC (Qingdao HaiYang Chemical Group Co., Qingdao, China). Sephadex LH-20 (Amersham Pharmacia Biotech Co., Piscataway, NJ, USA) was also used for column chromatography.
3.2. Materials
The roots of S. davidii (Franch.) Skeels (age 12–15 years) were collected from Xiuwen county, Guizhou province, China (at altitudes of 1200 to 1300 m), in June 2014. The roots were dried at room temperature, macerated into a fine powder, and stored at room temperature. The identification was done by Professor Dingrong Wan of School of Pharmaceutical Sciences, South-Central University for Nationalities (SCUN), Wuhan, China. A voucher specimen (SC0801) is deposited in School of Pharmaceutical Sciences, SCUN, Wuhan, China.
3.3. Extraction and Isolation
Air-dried roots of Sophora davidii (18 kg) were triturated and then extracted with 80% EtOH (4 × 20 L, 3 days each) at room temperature. The EtOH extract (850 g) was suspended in H2O (2.0 L) and then partitioned successively with petroleum ether (PE) (4 × 10 L), ethyl acetate (EtOAc) (4 × 10 L), and n-butyl alcohol (n-BuOH) (4 ×10 L) to give a PE extract (90 g), EtOAc extract (215 g), and n-BuOH extract (110 g), respectively. The EtOAc extraction (200 g) was separated into sixteen fractions (F1–F16) by silica-gel column chromatography (300–400 mesh) eluting with a gradient solvent system of CH2Cl2/MeOH (200:1, 100:1, 80:1, 60:1, 40:1, 20:1, 10:1, 5:1, 3:1, 0:1, v/v). The fraction 5 (2.60 g) was subjected to a Sephadex LH-20 (eluted with MeOH) to afford six subfractions (F5-1 to F5-6). F5-2 was applied to semi-preparative HPLC (CNCH3/H2O, 15:85–25:75, 20 min) at a rate of 4 mL/min, an injection volume of 200 μL, and UV at 254 nm with column temperature at 30 °C to obtain compound 6 (tR = 16.25 min; 56.3 mg). The subfraction F5-6 was further purified by semi-preparative HPLC (CNCH3/H2O, 25:75–55:45, 20 min) at a rate of 4 mL/min, an injection volume of 100 μL, and UV at 254 nm with column temperature at 30 °C to give 8 (tR = 15.10 min; 5.3 mg). Fraction 8 (5.63 g) was chromatographed on a silica gel column (100–200 mesh) eluting with a gradient of CH2Cl2/MeOH (100:1–1:1, v/v) to afford five subfractions (F8-1 to F8-5). The subfraction F8-2 was separated by Sephadex LH-20 (eluted with 90% MeOH) and semi-preparative HPLC (CNCH3/H2O, 40:60–54:46, 20 min) at a rate of 4 mL/min, an injection volume of 100 μL, and UV at 254 nm with column temperature at 30 °C to give 3 (tR = 13.35 min; 2.7 mg). Fraction 9 (2.63 g) was subjected to Sephadex LH-20 with eluted with 90% MeOH to give six subfractions (F9-1 to F9-6). Subfraction F9-6 was purified using semi-preparative HPLC (CNCH3/H2O, 37:63–80:20, 20 min) at a rate of 4 mL/min, an injection volume of 150 μL, and UV at 254 nm with column temperature at 30 °C to yield 4 (tR = 16.65 min; 8.0 mg). Fraction 11 (2.03 g) was injected into a Sephadex LH-20 and eluted with MeOH and further separated by semi-preparative HPLC (CNCH3/H2O, 43:57–60:40, 20 min) at a rate of 4 mL/min, an injection volume of 200 μL, and UV at 254 nm with column temperature at 30 °C to give 1 (tR = 11.05 min; 23.5 mg). Fraction 12 (1.62 g) was subjected to Sephadex LH-20 to afford seven subfractions (F12-1 to F12-7). Fraction F12-2 was separated by semi-preparative HPLC (CNCH3/H2O, 10:90–45:55, 25 min), at a rate of 4 mL/min, an injection volume of 100 μL, and UV at 254 nm with column temperature at 30 °C to afford 10 (tR = 10.86 min; 5.7 mg). Similarly, fraction F12-4 was also separated by semi-preparative HPLC (CNCH3/H2O, 10:90–50:50, 25 min), at a rate of 4 mL/min, an injection volume of 150 μL, and UV at 254 nm with column temperature at 30 °C to obtain 2 (tR = 21.28 min; 7.3 mg). Fraction 14 (3.60 g) was chromatographed over Sephadex LH-20 eluting with MeOH and afforded six fractions (F14-1 to F14-6). F14-3 was isolated by semi-preparative HPLC (CNCH3/H2O, 45:55–77:23, 20 min) at a rate of 4 mL/min, an injection volume of 200 μL, and UV at 254 nm with column temperature at 30 °C to give 5 (tR = 17.34 min; 97.8 mg). Fr14-4 was separated by semi-preparative HPLC (CNCH3/H2O, 30:70–70:30, 20min) at a rate of 4 mL/min, an injection volume of 200 μL, and UV at 254 nm with column temperature at 30 °C to obtain 9 (tR = 11.18 min; 23.6 mg). Fr14-6 was further separated by semi-preparative HPLC (CNCH3/H2O, 50:50–60:40, 20min) at a rate of 4 mL/min, an injection volume of 150 μL, and UV at 254 nm with column temperature at 30 °C to obtain compound 7 (tR = 14.44 min; 12.5 mg).
3.3.1. Davidiol E (1)
− 294.2 (
c 0.50, MeOH); UV (MeOH) λ
max (log
ε) = 230 (3.40), 310 (3.84) nm; IR
νmax = 3327, 2953, 2843, 2115, 1647, 1450, 1111, 1016 cm
−1; ECD (
c 0.50, MeOH) λ
max (Δ
ε) = 204 (−0.15), 211 (−2.24), 225 (−0.57), 239 (−0.95), 284 (+0.64), 320 (−1.87) nm; For
1H NMR (600 MHz) and
13C NMR (150 MHz) data, see
Table 1; HRESIMS
m/z 541.1861 [M + H]
+ (calcd. for C
32H
29O
8 541.1857).
3.3.2. Davidiol F (2)
− 263.8 (
c 0.50, MeOH); UV (MeOH) λ
max (log
ε) = 210 (2.22), 305 (1.71) nm; IR
νmax = 3414, 2951, 2841, 2129, 1647, 1450, 1112, 1016 cm
−1; ECD (
c 0.50, MeOH) λ
max (Δ
ε) = 202 (+0.25), 212 (−5.25), 231 (−1.45), 239 (−1.67), 284 (+0.71), 314 (−3.27) nm; For
1H NMR (600 MHz) and
13C NMR (150 MHz) data, see
Table 1; HRESIMS
m/z 497.1592 [M + H]
+ (calcd. for C
30H
25O
7 497.1595).
3.3.3. Davidinin A (3)
− 79.8 (
c 0.50, MeOH); UV (MeOH) λ
max (log
ε) = 210 (2.18), 285 (0.60) nm; IR
νmax = 3370, 2947, 2833, 1655, 1452, 1115, 1032 cm
−1; ECD (
c 0.50, MeOH) λ
max (Δ
ε) = 205 (+7.52), 214 (−4.23), 275 (+0.51), 299 (−0.20) nm; For
1H NMR (600 MHz) and
13C NMR (150 MHz) data, see
Table 2; HRESIMS
m/z 409.1282 [M + H]
+ (calcd. for C
23H
21O
7 409.1282).
3.3.4. Shandougenine C (4)
UV (MeOH) λ
max (log
ε) = 210 (1.45), 325 (1.00) nm; IR
νmax = 3343, 2943, 2832, 1452, 1032 cm
−1; For
1H NMR (600 MHz) and
13C NMR (150 MHz) data, see
Table 2; HRESIMS
m/z 575.0949 [M + Na]
+ (calcd. for C
31H
20NaO
10, 575.0949).
3.4. ECD Calculations
The conformational search was performed on Spartan’14 using the MMFF (Merck molecular forcefield) [
19]. The conformers with a Boltzmann population of over 1% were chosen for further optimized by the DFT method at the B3LYP/6-31G(d) level in the gas phase. The calculation of ECD was conducted in MeOH using TDDFT at the B3LYP/6-311+G(d,p) level for all conformers of compounds
1 and
3. All theoretical calculations were performed using the Gaussian 09 program package [
20]. The IEF-PCM solvent model for MeOH was used [
21]. The ECD data were processed with SpecDis [
22] using σ value of 0.3 eV and UV correction of −3 nm.
3.5. GLUT-4 Translocation Assay
Construction of myc-GLUT4-mOrange plasmid and cell line were performed as described previously [
23]. Myc-GLUT4-mOrange-L6 cells were cultured on glass coverslips for 12 h, and then L6 myoblasts were differentiated to L6 myotubes. Cells were starved in a PSS solution for 2 h. After starvation, mOrange fluorescence was detected by laser-scanning confocal microscopy at an excitation wavelength of 555 nm. Cells were treated with 10 μg/mL tested samples and images were taken every 5 min over a period of 30 min. Zen 2010 Software (Carl Zeiss, Jena, Germany) was used to analyze the fluorescence intensity of mOrange. The detailed method of GLUT-4 fusion with the plasma membrane was described in previous reports [
24].