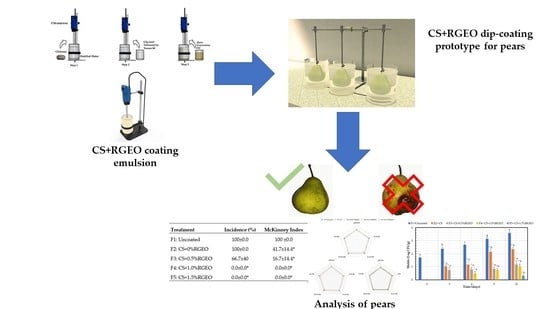
Packham’s Triumph Pears (Pyrus communis L.) Post-Harvest Treatment during Cold Storage Based on Chitosan and Rue Essential Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical-Chemical Analysis of Fruits after CS + RGEO Coating
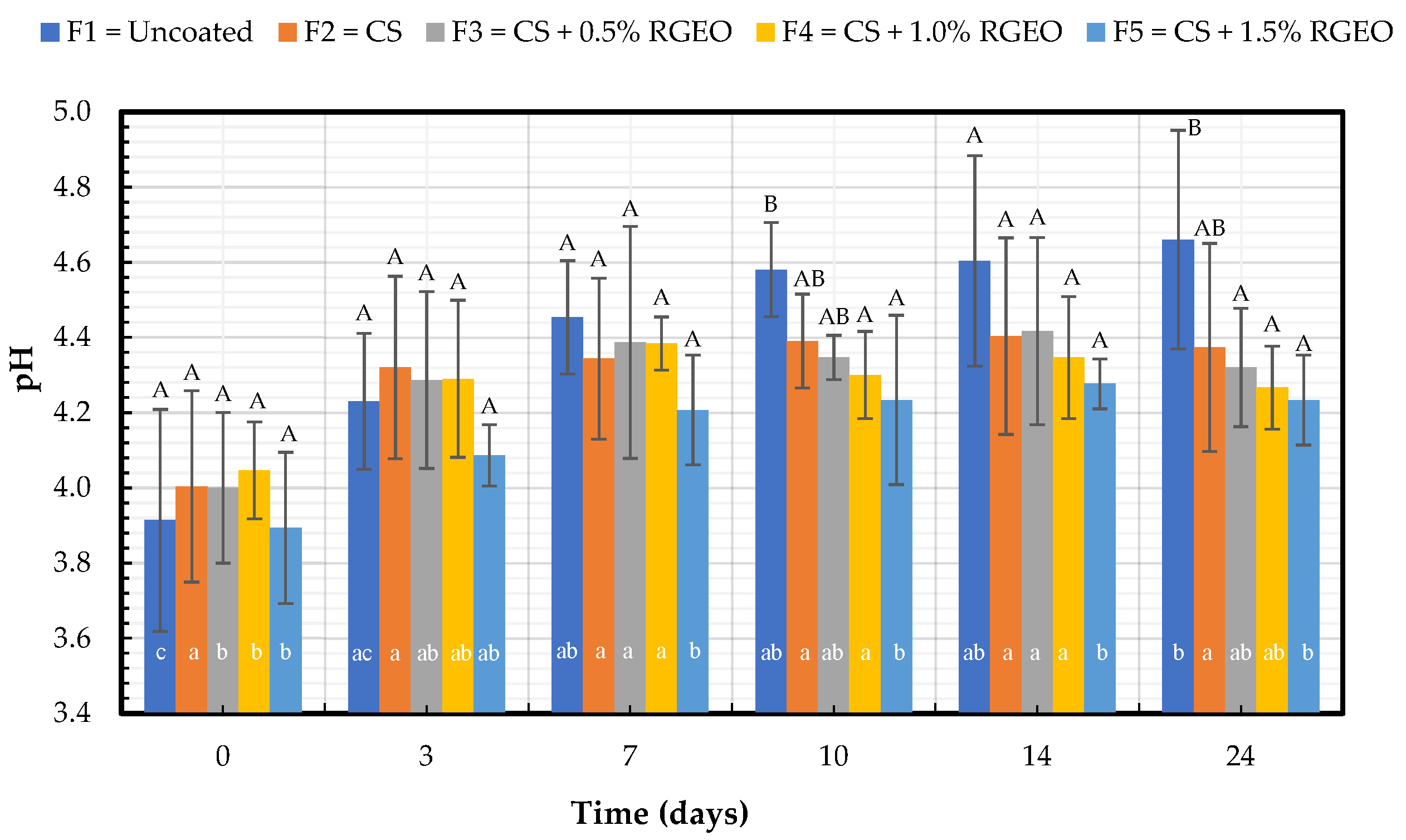
2.1.1. pH Analysis
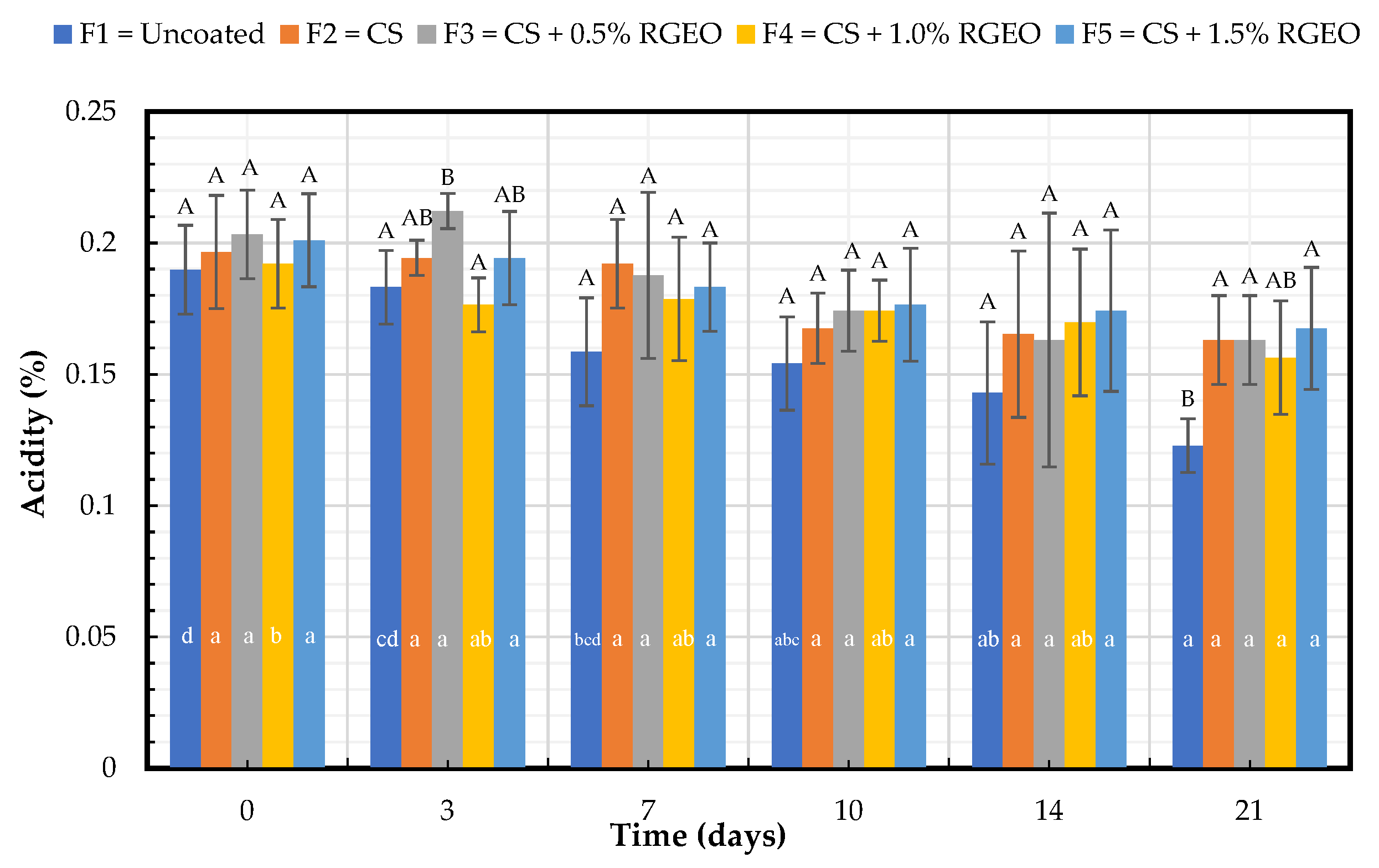
2.1.2. Titratable Acidity (TA)
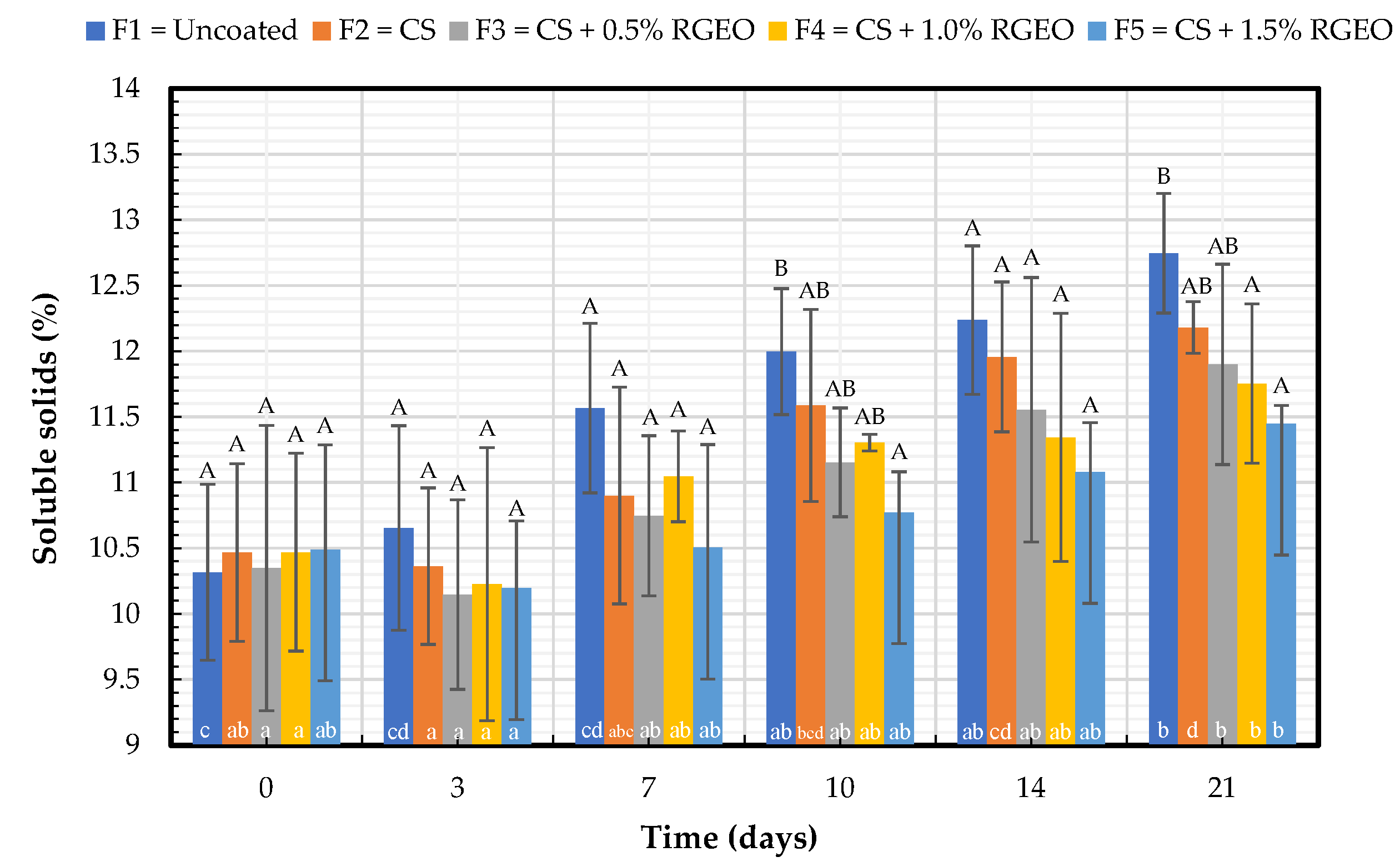
2.1.3. Soluble Solids Content (SS)
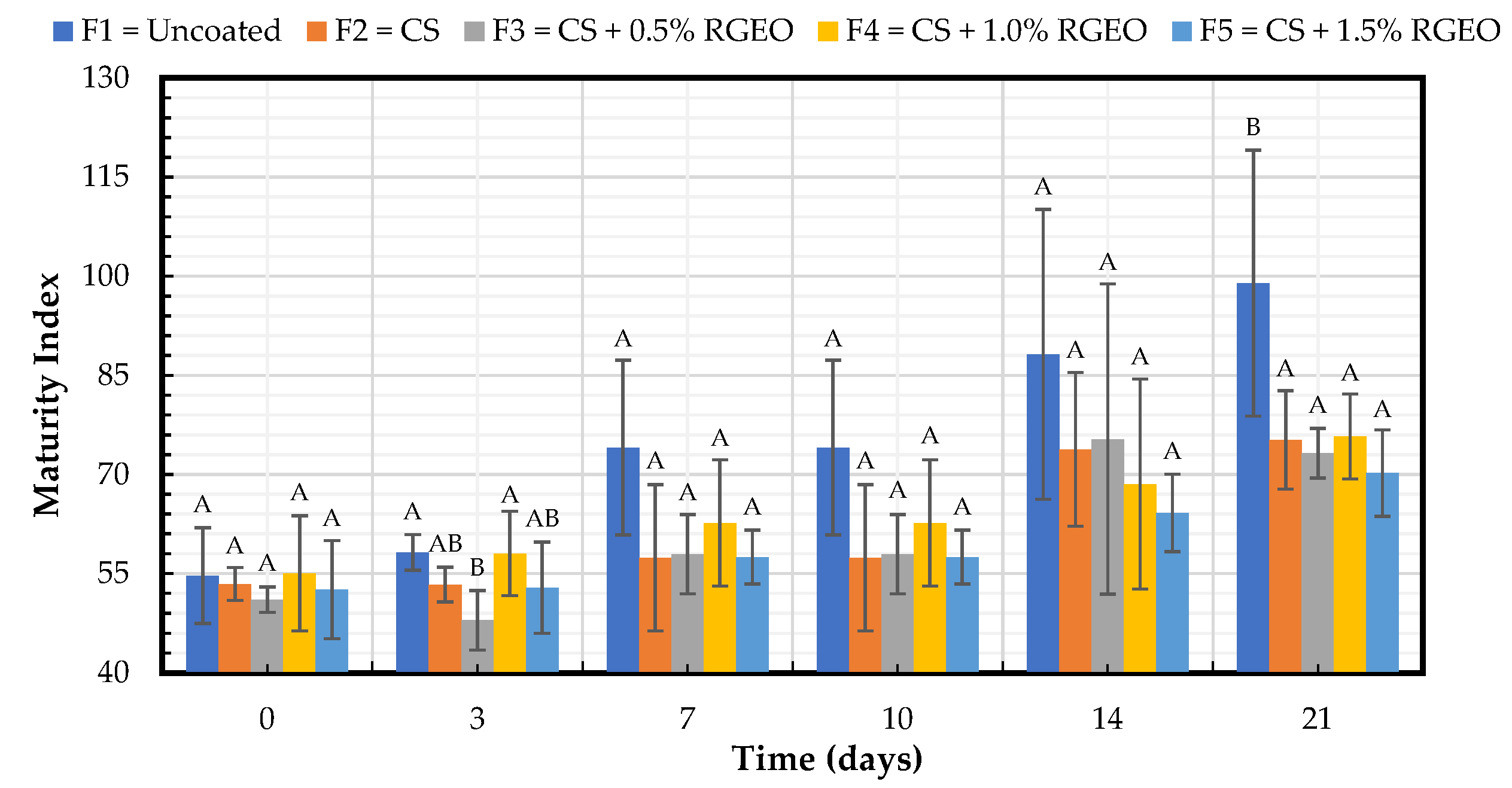
2.1.4. Maturity Index
2.1.5. Weight Loss
2.1.6. CO2 Respiration Rate
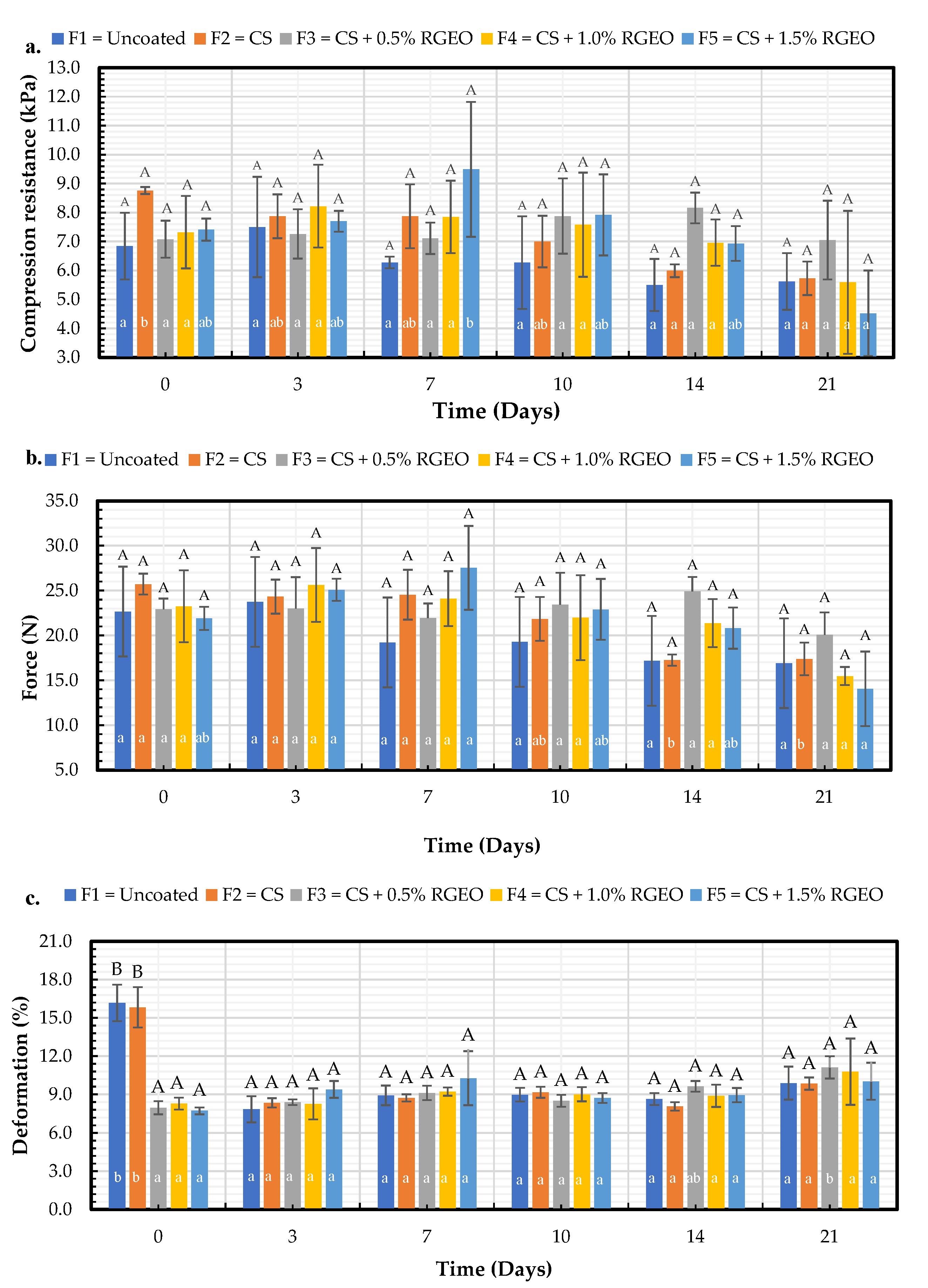
2.1.7. Mechanical Properties
2.1.8. Color Parameters
2.2. Decay Evaluation
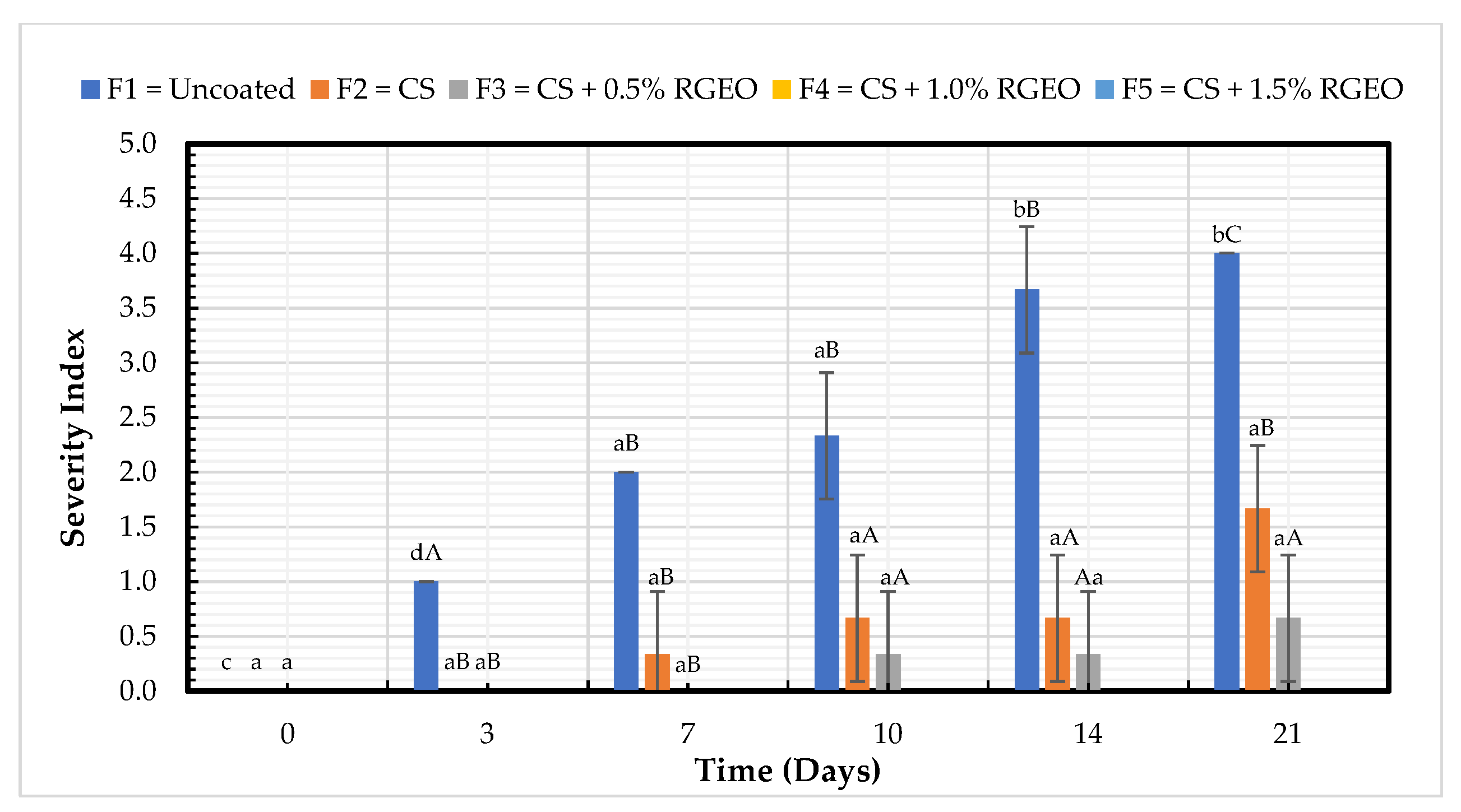
2.2.1. Severity Index (SI)
2.2.2. Disease Damage Incidence and Infection Index
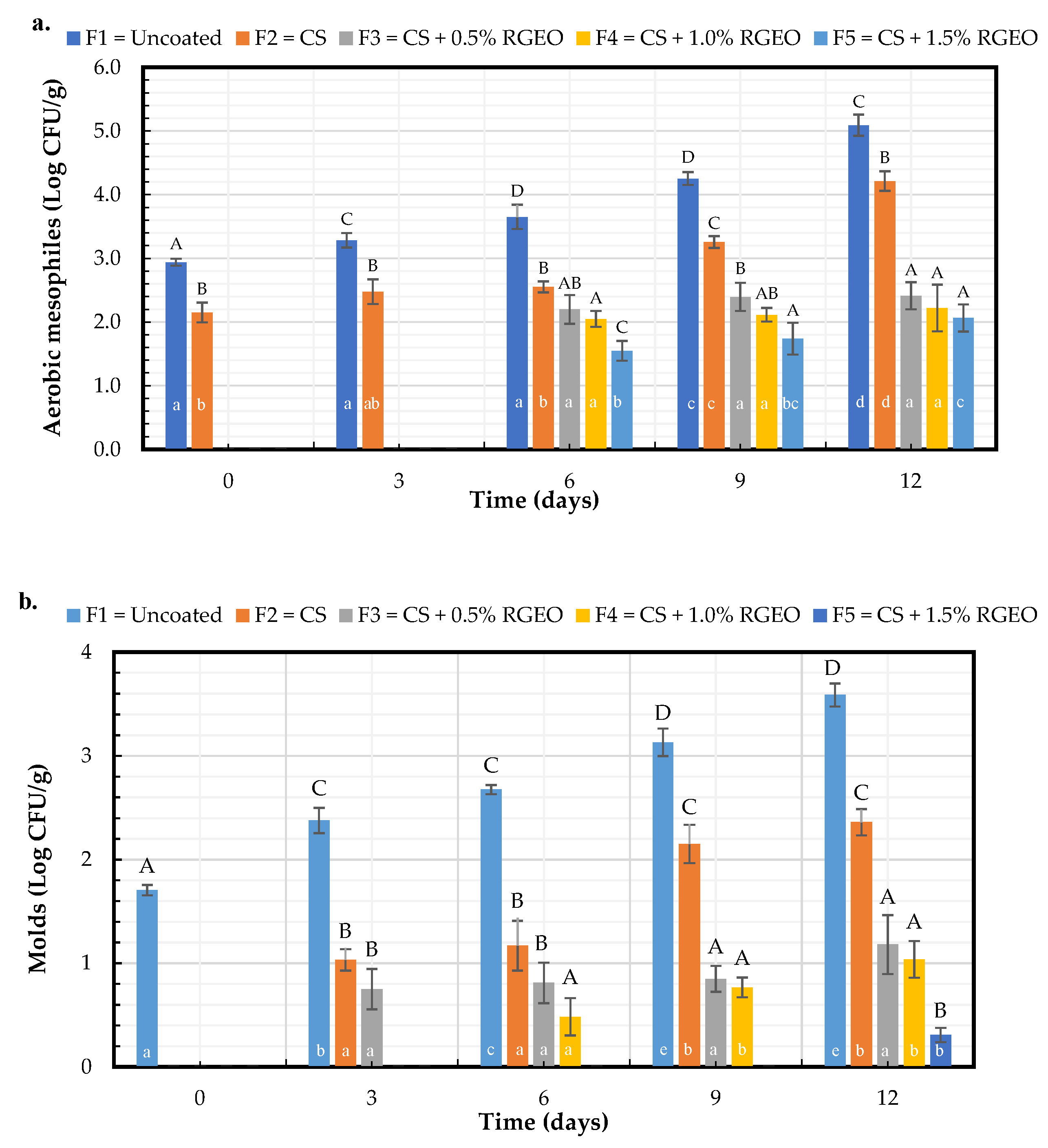
2.2.3. Microbiological Analysis
2.3. Sensory Properties
3. Materials and Methods
3.1. Fruit Samples
3.2. Preparation of Edible Coatings
3.3. Coating of the Fruits
3.4. Post-Harvest Quality Properties of Pears
3.4.1. pH and Soluble Solids (SS)
3.4.2. Titratable Acidity (TA)
3.4.3. Maturity Index (MI)
3.4.4. Weight Loss Percentage
3.4.5. Color Analysis
3.4.6. CO2 Respiration Rate
3.4.7. Mechanical Properties of Pears
3.5. Decay Evaluation
3.5.1. Antimicrobial Assay
3.5.2. Severity Index (SI)
3.5.3. Disease Damage Incidence
3.5.4. Infection Index
3.6. Sensorial Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- United States Department of Agriculture. Fresh Apples, Grapes, and Pears: World Markets and Trade. Available online: https://www.fas.usda.gov/data/fresh-apples-grapes-and-pears-world-markets-and-trade (accessed on 21 December 2020).
- Mari, M.; Bertolini, P.; Pratella, G.C. Non-conventional methods for the control of post-harvest pear diseases. J. Appl. Microbiol. 2003, 94, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Sardella, D.; Muscat, A.; Brincat, J.-P.; Gatt, R.; Decelis, S.; Valdramidis, V. A Comprehensive Review of the Pear Fungal Diseases. Int. J. Fruit Sci. 2016, 16, 351–377. [Google Scholar] [CrossRef]
- Dave, R.K.; Rao, T.V.R.; Nandane, A.S. Improvement of post-harvest quality of pear fruit with optimized composite edible coating formulations. J. Food Sci. Technol. 2017, 54, 3917–3927. [Google Scholar] [CrossRef] [PubMed]
- Scramin, J.A.; de Britto, D.; Forato, L.A.; Bernardes-Filho, R.; Colnago, L.A.; Assis, O.B.G. Characterisation of zein–oleic acid films and applications in fruit coating. Int. J. Food Sci. Technol. 2011, 46, 2145–2152. [Google Scholar] [CrossRef]
- Ju, Z.; Duan, Y.; Ju, Z. Plant oil emulsion modifies internal atmosphere, delays fruit ripening, and inhibits internal browning in Chinese pears. Postharvest Biol. Technol. 2000, 20, 243–250. [Google Scholar] [CrossRef]
- Hussain, P.R.; Meena, R.S.; Dar, M.A.; Wani, A.M. Carboxymethyl cellulose coating and low-dose gamma irradiation improves storage quality and shelf life of pear (Pyrus communis L., Cv. Bartlett/William). J. Food Sci. 2010, 75, M586–M596. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Baños, S.B.; Sivakumar, D. Shelf life extension of fresh fruit and vegetables by chitosan treatment. Crit. Rev. Food Sci. Nutr. 2017, 57, 579–601. [Google Scholar] [CrossRef]
- Rajestary, R.; Landi, L.; Romanazzi, G. Chitosan and postharvest decay of fresh fruit: Meta-analysis of disease control and antimicrobial and eliciting activities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 563–582. [Google Scholar]
- Deng, Z.; Jung, J.; Simonsen, J.; Zhao, Y. Cellulose nanocrystals Pickering emulsion incorporated chitosan coatings for improving storability of postharvest Bartlett pears (Pyrus communis) during long-term cold storage. Food Hydrocoll. 2018, 84, 229–237. [Google Scholar] [CrossRef]
- Rosenbloom, R.A.; Wang, W.; Zhao, Y. Delaying ripening of ‘Bartlett’ pears (Pyrus communis) during long-term simulated industrial cold storage: Mechanisms and validation of chitosan coatings with cellulose nanocrystals Pickering emulsions. LWT 2020, 122, 109053. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Wolters, B.; Eilert, U. Antimicrobial substances in callus cultures of Ruta graveolens. Planta Med. 1981, 43, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Haddouchi, F.; Chaouche, T.M.; Zaouali, Y.; Ksouri, R.; Attou, A.; Benmansour, A. Chemical composition and antimicrobial activity of the essential oils from four Ruta species growing in Algeria. Food Chem. 2013, 141, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Ruiz, Y.; Grande Tovar, C.G.; Sinning-Mangonez, A.; Bermont, D.; Pérez Cordero, A.; Paparella, A.; Chaves-López, C. Colletotrichum gloesporioides inhibition using chitosan-Ruta graveolens L essential oil coatings: Studies in vitro and in situ on Carica papaya fruit. Int. J. Food Microbiol. 2020, 326, 108649. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Nanoencapsulation of Zataria multiflora essential oil preparation and characterization with enhanced antifungal activity for controlling Botrytis cinerea, the causal agent of gray mould disease. Innov. food Sci. Emerg. Technol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Paparella, A. Chitosan coatings enriched with essential oils: Effects on fungi involve in fruit decay and mechanisms of action. Trends Food Sci. Technol. 2018, 78, 61–71. [Google Scholar] [CrossRef]
- Grande Tovar, C.D.; Delgado-Ospina, J.; Navia Porras, D.P.; Peralta-Ruiz, Y.; Cordero, A.P.; Castro, J.I.; Valencia, C.; Noé, M.; Mina, J.H.; Chaves López, C. Colletotrichum gloesporioides Inhibition In Situ by Chitosan-Ruta graveolens Essential Oil Coatings: Effect on Microbiological, Physicochemical, and Organoleptic Properties of Guava (Psidium guajava L.) during Room Temperature Storage. Biomolecules 2019, 9, 399. [Google Scholar] [CrossRef]
- González-Locarno, M.; Maza Pautt, Y.; Albis, A.; Florez López, E.; Grande Tovar, D.C. Assessment of Chitosan-Rue (Ruta graveolens L.) Essential Oil-Based Coatings on Refrigerated Cape Gooseberry (Physalis peruviana L.) Quality. Appl. Sci. 2020, 10, 2684. [Google Scholar] [CrossRef]
- Peralta-Ruiz, Y.; Grande Tovar, C.D.; Sinning-Mangonez, A.; Coronell, E.A.; Marino, M.F.; Chaves-Lopez, C. Reduction of Postharvest Quality Loss and Microbiological Decay of Tomato “Chonto”(Solanum lycopersicum L.) Using Chitosan-E Essential Oil-Based Edible Coatings under Low-Temperature Storage. Polymers 2020, 12, 1822. [Google Scholar] [CrossRef]
- Xiao, C.; Zhu, L.; Luo, W.; Song, X.; Deng, Y. Combined action of pure oxygen pretreatment and chitosan coating incorporated with rosemary extracts on the quality of fresh-cut pears. Food Chem. 2010, 121, 1003–1009. [Google Scholar] [CrossRef]
- Osorio, S.; Scossa, F.; Fernie, A. Molecular regulation of fruit ripening. Front. Plant Sci. 2013, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- Lira, B.S.; Rosado, D.; Almeida, J.; de Souza, A.P.; Buckeridge, M.S.; Purgatto, E.; Guyer, L.; Hörtensteiner, S.; Freschi, L.; Rossi, M. Pheophytinase knockdown impacts carbon metabolism and nutraceutical content under normal growth conditions in tomato. Plant Cell Physiol. 2016, 57, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; Luo, M.; Wu, Y.; Duan, S.; Li, J.; Wang, L.; Song, S.; Xu, W.; Wang, S. Proteomic analysis of pear (Pyrus pyrifolia) ripening process provides new evidence for the sugar/acid metabolism difference between core and mesocarp. Proteomics 2016, 16, 3025–3041. [Google Scholar] [CrossRef]
- Lin, L.; Wang, B.; Wang, M.; Cao, J.; Zhang, J.; Wu, Y.; Jiang, W. Effects of a chitosan-based coating with ascorbic acid on post-harvest quality and core browning of ‘Yali’pears (Pyrus bertschneideri Rehd.). J. Sci. Food Agric. 2008, 88, 877–884. [Google Scholar] [CrossRef]
- Arabpoor, B.; Yousefi, S.; Weisany, W.; Ghasemlou, M. Multifunctional coating composed of Eryngium campestre L. essential oil encapsulated in nano-chitosan to prolong the shelf-life of fresh cherry fruits. Food Hydrocoll. 2021, 111, 106394. [Google Scholar] [CrossRef]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or Causation? Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Barreto, T.A.; Andrade, S.C.A.; Maciel, J.F.; Arcanjo, N.M.O.; Madruga, M.S.; Meireles, B.; Cordeiro, Â.M.T.; Souza, E.L.; Magnani, M. A Chitosan Coating Containing Essential Oil from Origanum vulgare L. to Control Postharvest Mold Infections and Keep the Quality of Cherry Tomato Fruit. Front. Microbiol. 2016, 7, 1724. [Google Scholar] [CrossRef]
- Sabır, A.; Sabır, F.K.; Kara, Z. Effects of modified atmosphere packing and honey dip treatments on quality maintenance of minimally processed grape cv. Razaki (V. vinifera L.) during cold storage. J. Food Sci. Technol. 2011, 48, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cosme Silva, G.M.; Silva, W.B.; Medeiros, D.B.; Salvador, A.R.; Cordeiro, M.H.M.; da Silva, N.M.; Santana, D.B.; Mizobutsi, G.P. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017, 237, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Martínez, K.; Ortiz, M.; Albis, A.; Gilma Gutiérrez Castañeda, C.; Valencia, E.M.; Grande Tovar, D.C. The Effect of Edible Chitosan Coatings Incorporated with Thymus capitatus Essential Oil on the Shelf-Life of Strawberry (Fragaria x ananassa) during Cold Storage. Biomolecules 2018, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Nicolaï, B.M. A model for gas transport in pear fruit at multiple scales. J. Exp. Bot. 2010, 61, 2071–2081. [Google Scholar] [CrossRef]
- Ho, Q.T.; Hertog, M.L.A.T.M.; Verboven, P.; Ambaw, A.; Rogge, S.; Verlinden, B.E.; Nicolaï, B.M. Down-regulation of respiration in pear fruit depends on temperature. J. Exp. Bot. 2018, 69, 2049–2060. [Google Scholar] [CrossRef]
- Armstrong, W.; Beckett, P.M. Experimental and modelling data contradict the idea of respiratory down-regulation in plant tissues at an internal [O2] substantially above the critical oxygen pressure for cytochrome oxidase. New Phytol. 2011, 190, 431–441. [Google Scholar] [CrossRef]
- Oliveira, M.; Abadias, M.; Usall, J.; Torres, R.; Teixidó, N.; Viñas, I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables–A review. Trends Food Sci. Technol. 2015, 46, 13–26. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Li, W.; Tang, Y.; Yun, J. Effects of ascorbic acid and high oxygen modified atmosphere packaging during storage of fresh-cut eggplants. Food Sci. Technol. Int. 2014, 20, 99–108. [Google Scholar] [CrossRef]
- Teixeira, G.H.A.; Cunha Júnior, L.C.; Ferraudo, A.S.; Durigan, J.F. Quality of guava (Psidium guajava L. cv. Pedro Sato) fruit stored in low-O2 controlled atmospheres is negatively affected by increasing levels of CO2. Postharvest Biol. Technol. 2016, 111, 62–68. [Google Scholar] [CrossRef]
- Sahraei Khosh Gardesh, A.; Badii, F.; Hashemi, M.; Ardakani, A.Y.; Maftoonazad, N.; Gorji, A.M. Effect of nanochitosan based coating on climacteric behavior and postharvest shelf-life extension of apple cv. Golab Kohanz. LWT 2016, 70, 33–40. [Google Scholar] [CrossRef]
- Eshghi, S.; Hashemi, M.; Mohammadi, A.; Badii, F.; Mohammadhoseini, Z.; Ahmadi, K. Effect of Nanochitosan-Based Coating With and Without Copper Loaded on Physicochemical and Bioactive Components of Fresh Strawberry Fruit (Fragaria x ananassa Duchesne) During Storage. Food Bioprocess Technol. 2014, 7, 2397–2409. [Google Scholar] [CrossRef]
- Gao, P.; Zhu, Z.; Zhang, P. Effects of chitosan–glucose complex coating on postharvest quality and shelf life of table grapes. Carbohydr. Polym. 2013, 95, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Yahia, E.M.; Carrillo-Lopez, A. Postharvest physiology and biochemistry of fruits and vegetables; Woodhead Publishing: Cambridge, UK, 2018; ISBN 0128132795. [Google Scholar]
- Dai, L.; Zhang, J.; Cheng, F. Cross-linked starch-based edible coating reinforced by starch nanocrystals and its preservation effect on graded Huangguan pears. Food Chem. 2020, 311, 125891. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.P.N.; Suárez, M.G.; Umaña, J.H.; Perdomo, L.G.P. Optimization of Physical, Optical and Barrier Properties of Films Made from Cassava Starch and Rosemary Oil. J. Polym. Environ. 2019, 27, 127–140. [Google Scholar] [CrossRef]
- Zhou, R.; Mo, Y.; Li, Y.; Zhao, Y.; Zhang, G.; Hu, Y. Quality and internal characteristics of Huanghua pears (Pyrus pyrifolia Nakai, cv. Huanghua) treated with different kinds of coatings during storage. Postharvest Biol. Technol. 2008, 49, 171–179. [Google Scholar] [CrossRef]
- Calvo, G.; Candan, A.P. Guía Para la Identificación de Fisiopatías en Peras y Manzanas, 1st ed.; INTA, Ed.; Ministerio de Agroindustria: Alto Valle, Argentina, 2010.
- Adi, D.D.; Oduro, I.N.; Tortoe, C. Physicochemical changes in plantain during normal storage ripening. Sci. African 2019, 6. [Google Scholar] [CrossRef]
- Salehi, F.; Kashaninejad, M. Modeling of moisture loss kinetics and color changes in the surface of lemon slice during the combined infrared-vacuum drying. Inf. Process. Agric. 2018, 5, 516–523. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Serio, A.; Delgado-Ospina, J.; Paparella, A.; Rossi, C.; Chaves-López, C. Chitosan films incorporated with Thymus capitatus essential oil: Mechanical properties and antimicrobial activity against degradative bacterial species isolated from tuna (Thunnus sp.) and swordfish (Xiphias gladius). J. Food Sci. Technol. 2018, 55. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, I.H.; Dai, L.; Khan, A.; An, X. Chitosan as A Preservative for Fruits and Vegetables: A Review on Chemistry and Antimicrobial Properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Perdones, A.; Sánchez-González, L.; Chiralt, A.; Vargas, M. Effect of chitosan–lemon essential oil coatings on storage-keeping quality of strawberry. Postharvest Biol. Technol. 2012, 70, 32–41. [Google Scholar] [CrossRef]
- Chiralt, A.; Martínez-Navarrete, N.; Martínez-Monzó, J.; Talens, P.; Moraga, G.; Ayala, A.; Fito, P. Changes in mechanical properties throughout osmotic processes: Cryoprotectant effect. J. Food Eng. 2001, 49, 129–135. [Google Scholar] [CrossRef]
- Grande-tovar, C.D.; Chaves-Lopez, C.; Viuda-Martos, M.; Serio, A.; Delgado-Ospina, J.; Perez-Alvarez, J.A.; Ospina, N.; Palmieri, S.; Paparella, A.; la Tora, S.; et al. Sub-lethal concentrations of Colombian Austroeupatorium inulifolium (H. B. K.) essential oil and its effect on fungal growth and the production of enzymes. Ind. Crops Prod. 2016, 87, 315–323. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, Y.; Che, Z.; Guan, T.; Zhang, L.; Bai, Y.; Gong, L. Effect of chitosan coating and oil fumigation on the microbiological and quality safety of fresh-cut pear. J. Food Saf. 2013, 33, 179–189. [Google Scholar] [CrossRef]
- dos Santos, N.S.T.; Athayde Aguiar, A.J.A.; Vasconcelos de Oliveira, C.E.; Veríssimo de Sales, C.; de Melo e Silva, S.; Sousa da Silva, R.; Montenegro Stamford, T.C.; de Souza, E.L. Efficacy of the application of a coating composed of chitosan and Origanum vulgare L. essential oil to control Rhizopus stolonifer and Aspergillus niger in grapes (Vitis labrusca L.). Food Microbiol. 2012, 32, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.D.L.; de Oliveira, K.Á.R.; dos Santos Vieira, W.A.; Câmara, M.P.S.; de Souza, E.L. Control of anthracnose caused by Colletotrichum species in guava, mango and papaya using synergistic combinations of chitosan and Cymbopogon citratus (D.C. ex Nees) Stapf. essential oil. Int. J. Food Microbiol. 2018, 266, 87–94. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H. Chromoplast biogenesis and carotenoid accumulation. Arch. Biochem. Biophys. 2013, 539, 102–109. [Google Scholar] [CrossRef]
- Feliziani, E.; Landi, L.; Romanazzi, G. Preharvest treatments with chitosan and other alternatives to conventional fungicides to control postharvest decay of strawberry. Carbohydr. Polym. 2015, 132, 111–117. [Google Scholar] [CrossRef]


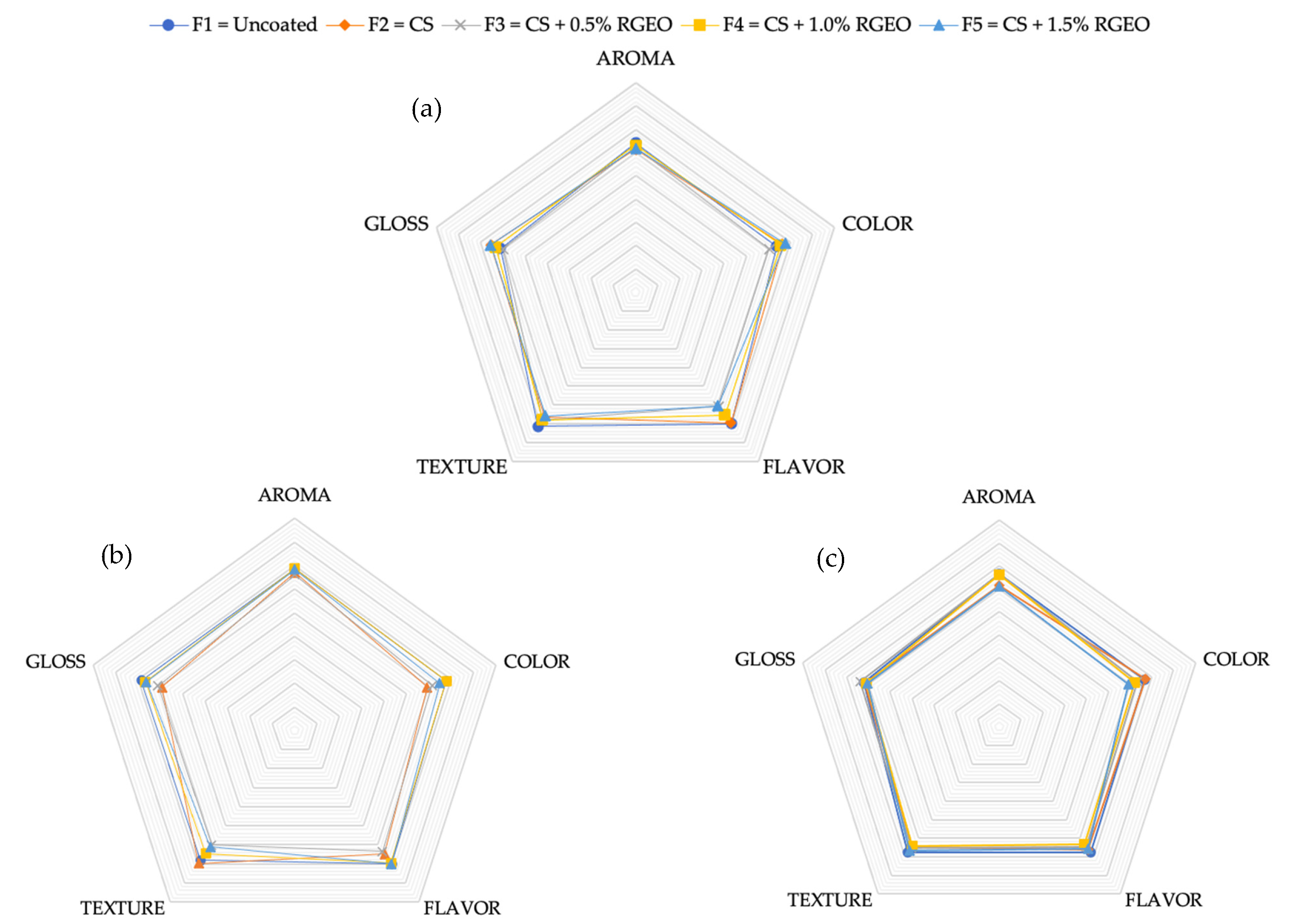

| Days | 0 | 3 | 7 | 10 | 14 | 21 |
|---|---|---|---|---|---|---|
| Treatment | Weight Loss (%) | |||||
| Control | 0 | 5.4 ± 1.8 aA | 10.4 ± 1.0 abA | 15.6 ± 2.6 bA | 23.2 ± 1.7 cA | 40.2 ± 5.3 dB |
| CS + 0% RGEO | 0 | 4.4 ± 0.4 aA | 9.7 ± 0.8 abA | 14.7 ± 1.3 bcA | 21.5 ± 0.8 cAB | 29.8 ± 9.6 dAB |
| CS + 0.5% RGEO | 0 | 5.7 ± 1.3 bA | 9.4 ± 1.4 cA | 15.2 ± 0.8 dA | 21.9 ± 0.8 aA | 20.3 ± 3.9 aA |
| CS + 1.0% RGEO | 0 | 4.4 ± 0.2 bA | 9.2 ± 0.5 bcA | 15.5 ± 2.3 acA | 21.6 ± 1.6 aAB | 21.3 ± 8.5 aA |
| CS + 1.5% RGEO | 0 | 4.4 ± 1.1 aA | 9.3 ± 0.8 abA | 14.7 ± 1.0 bcA | 16.6 ± 5.3 cB | 23.8 ± 5.6 dA |
| Treatment | Incidence (%) | McKinney Index |
|---|---|---|
| F1: Uncoated | 100 ± 0.0 A | 100 ± 0.0 C |
| F2: CS + 0% RGEO | 100 ± 0.0 A | 41.7 ± 14.4 B |
| F3: CS + 0.5% RGEO | 66.7 ± 40 A | 16.7 ± 14.4 C |
| F4: CS + 1.0% RGEO | 0.0 ± 0.0 B | 0.0 ± 0.0 C |
| F5: CS + 1.5% RGEO | 0.0 ± 0.0 B | 0.0 ± 0.0 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta-Ruiz, Y.; Grande-Tovar, C.D.; Navia Porras, D.P.; Sinning-Mangonez, A.; Delgado-Ospina, J.; González-Locarno, M.; Maza Pautt, Y.; Chaves-López, C. Packham’s Triumph Pears (Pyrus communis L.) Post-Harvest Treatment during Cold Storage Based on Chitosan and Rue Essential Oil. Molecules 2021, 26, 725. https://doi.org/10.3390/molecules26030725
Peralta-Ruiz Y, Grande-Tovar CD, Navia Porras DP, Sinning-Mangonez A, Delgado-Ospina J, González-Locarno M, Maza Pautt Y, Chaves-López C. Packham’s Triumph Pears (Pyrus communis L.) Post-Harvest Treatment during Cold Storage Based on Chitosan and Rue Essential Oil. Molecules. 2021; 26(3):725. https://doi.org/10.3390/molecules26030725
Chicago/Turabian StylePeralta-Ruiz, Yeimmy, Carlos David Grande-Tovar, Diana Paola Navia Porras, Angie Sinning-Mangonez, Johannes Delgado-Ospina, María González-Locarno, Yarley Maza Pautt, and Clemencia Chaves-López. 2021. "Packham’s Triumph Pears (Pyrus communis L.) Post-Harvest Treatment during Cold Storage Based on Chitosan and Rue Essential Oil" Molecules 26, no. 3: 725. https://doi.org/10.3390/molecules26030725
APA StylePeralta-Ruiz, Y., Grande-Tovar, C. D., Navia Porras, D. P., Sinning-Mangonez, A., Delgado-Ospina, J., González-Locarno, M., Maza Pautt, Y., & Chaves-López, C. (2021). Packham’s Triumph Pears (Pyrus communis L.) Post-Harvest Treatment during Cold Storage Based on Chitosan and Rue Essential Oil. Molecules, 26(3), 725. https://doi.org/10.3390/molecules26030725