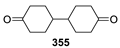
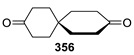
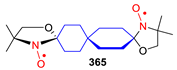
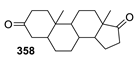
Spirocyclic Nitroxides as Versatile Tools in Modern Natural Sciences: From Synthesis to Applications. Part I. Old and New Synthetic Approaches to Spirocyclic Nitroxyl Radicals
Abstract
1. Introduction
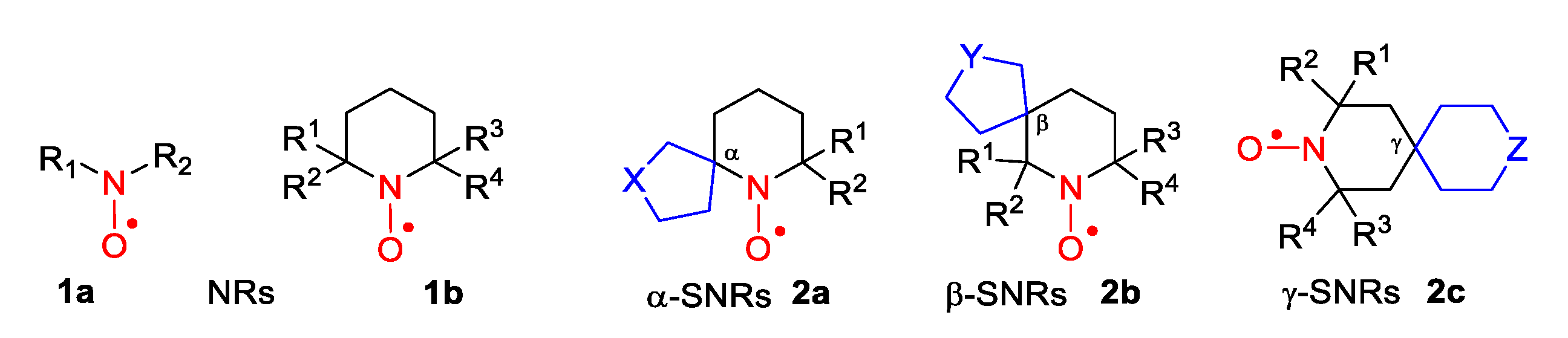
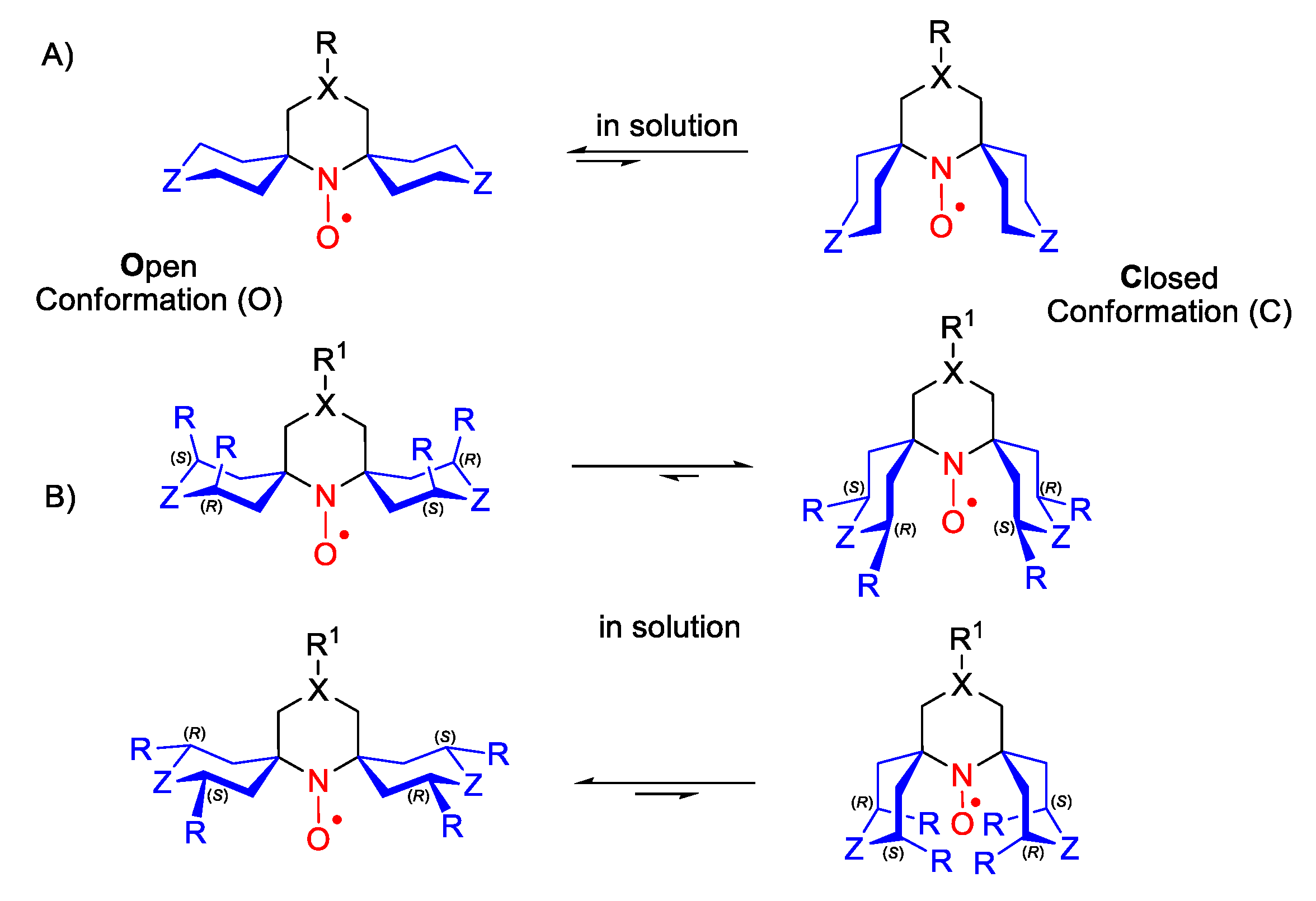
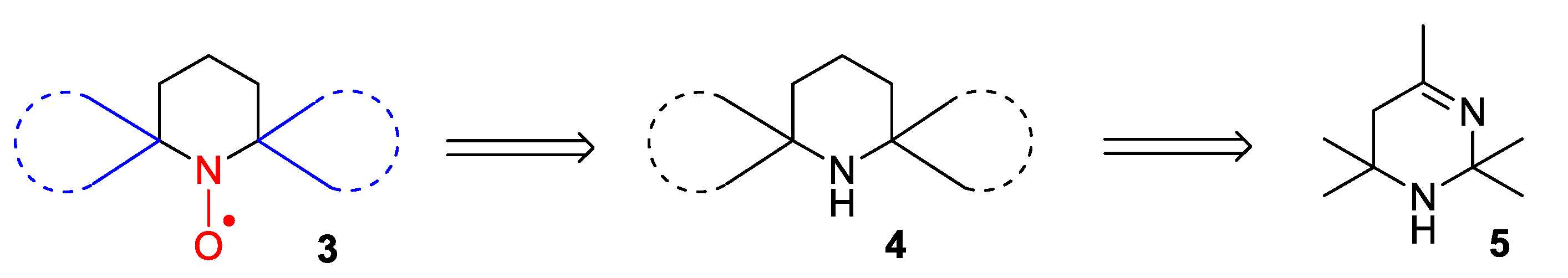
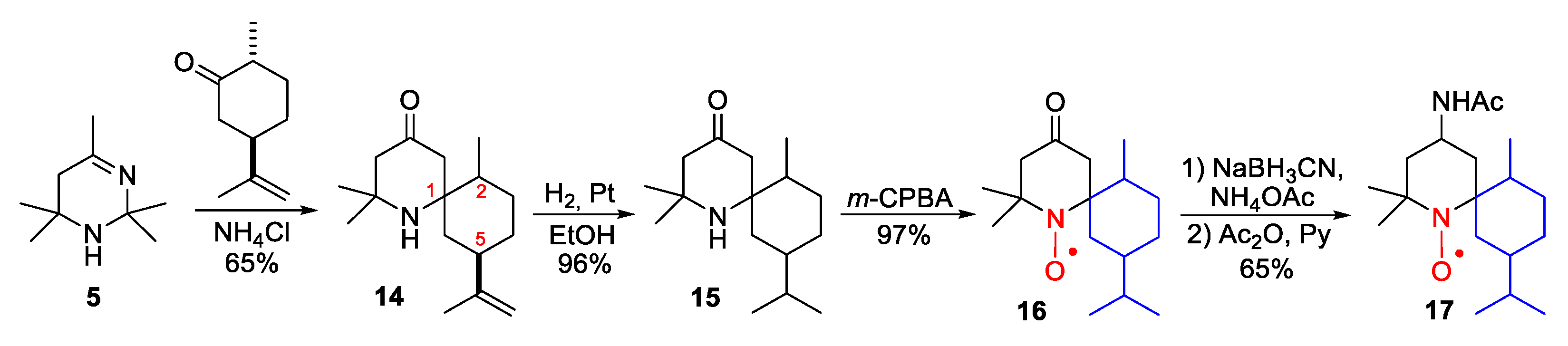
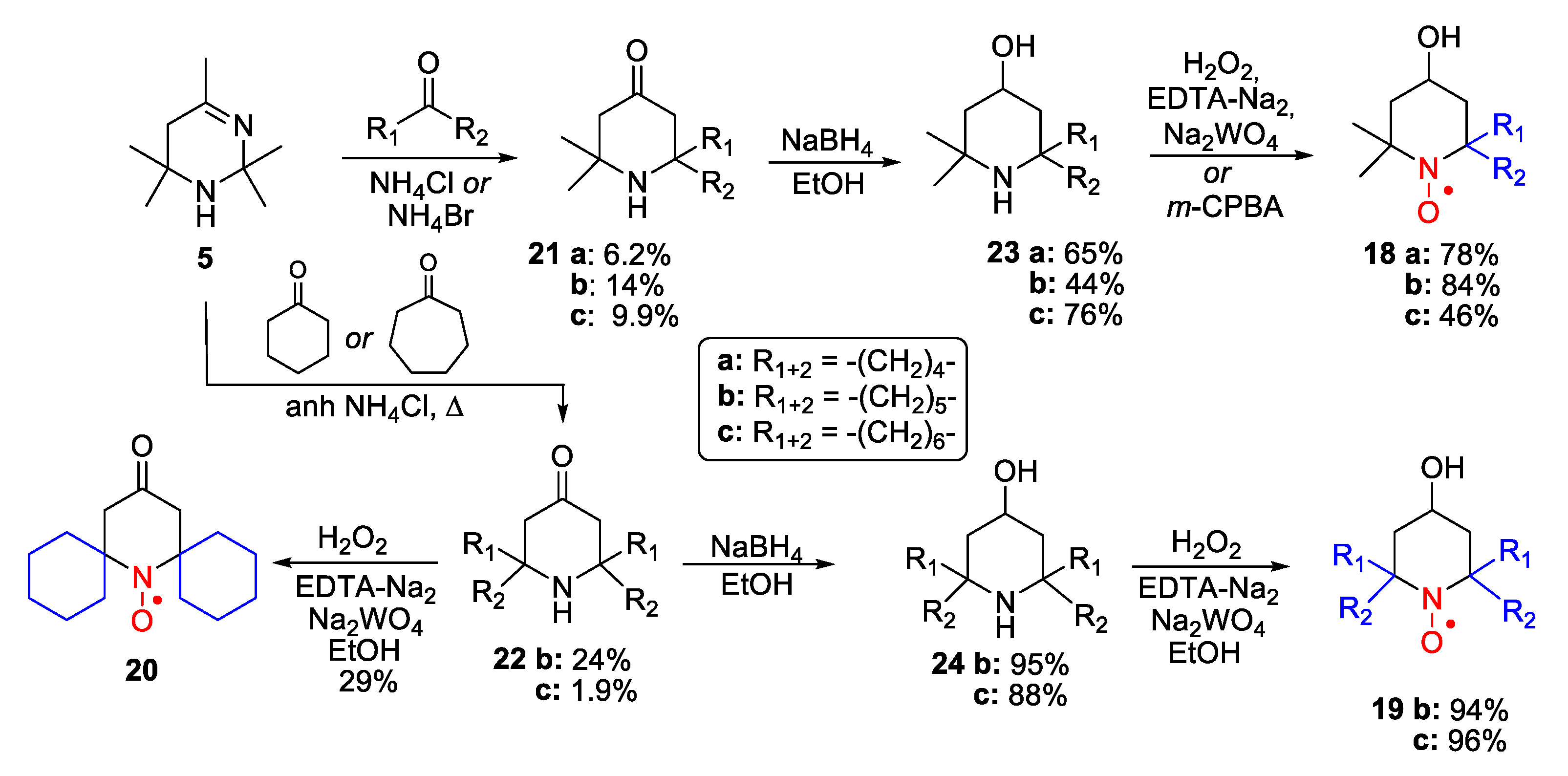
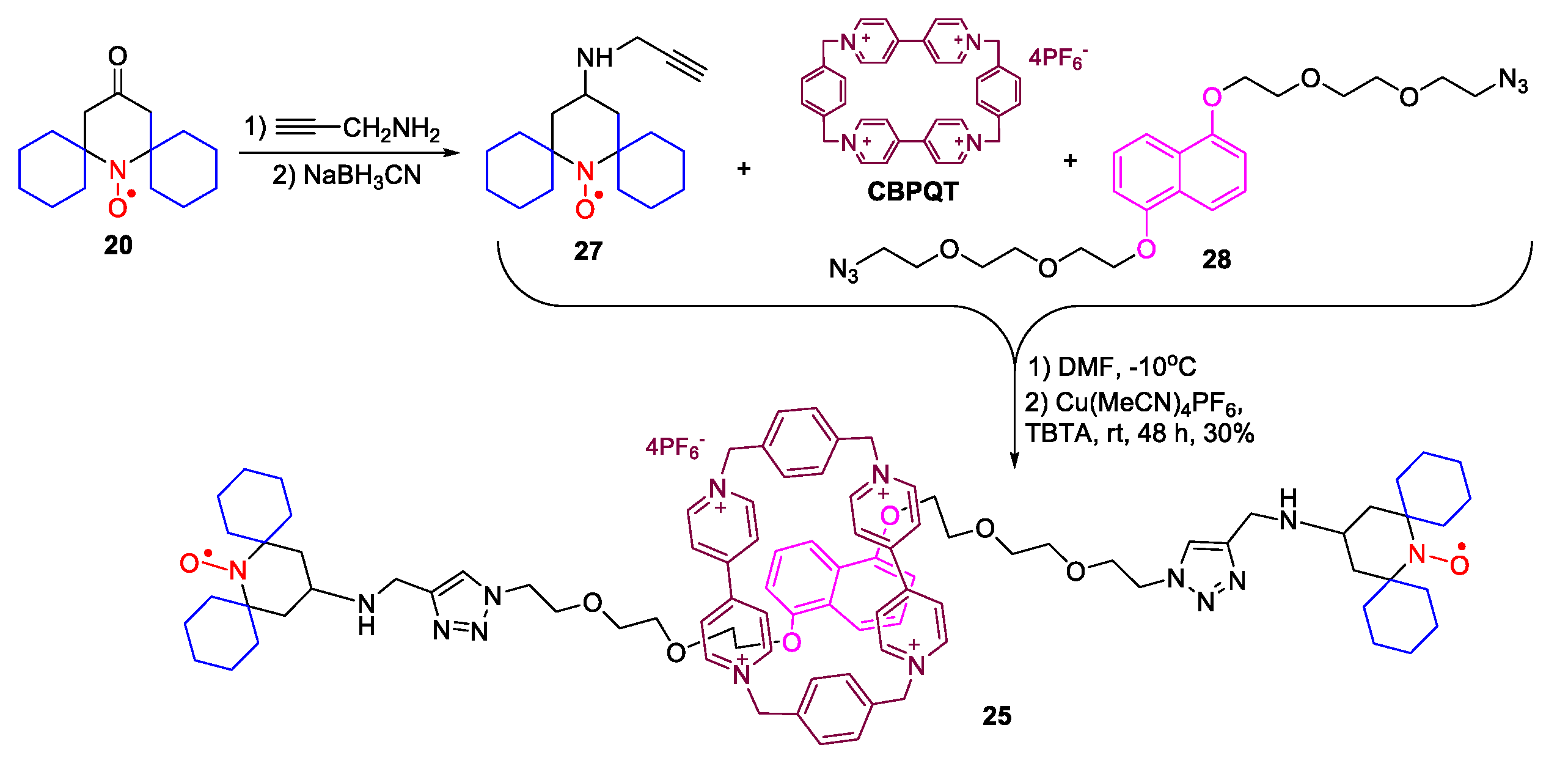
2. Piperidine Nitroxide Radicals (TEMPO Type)
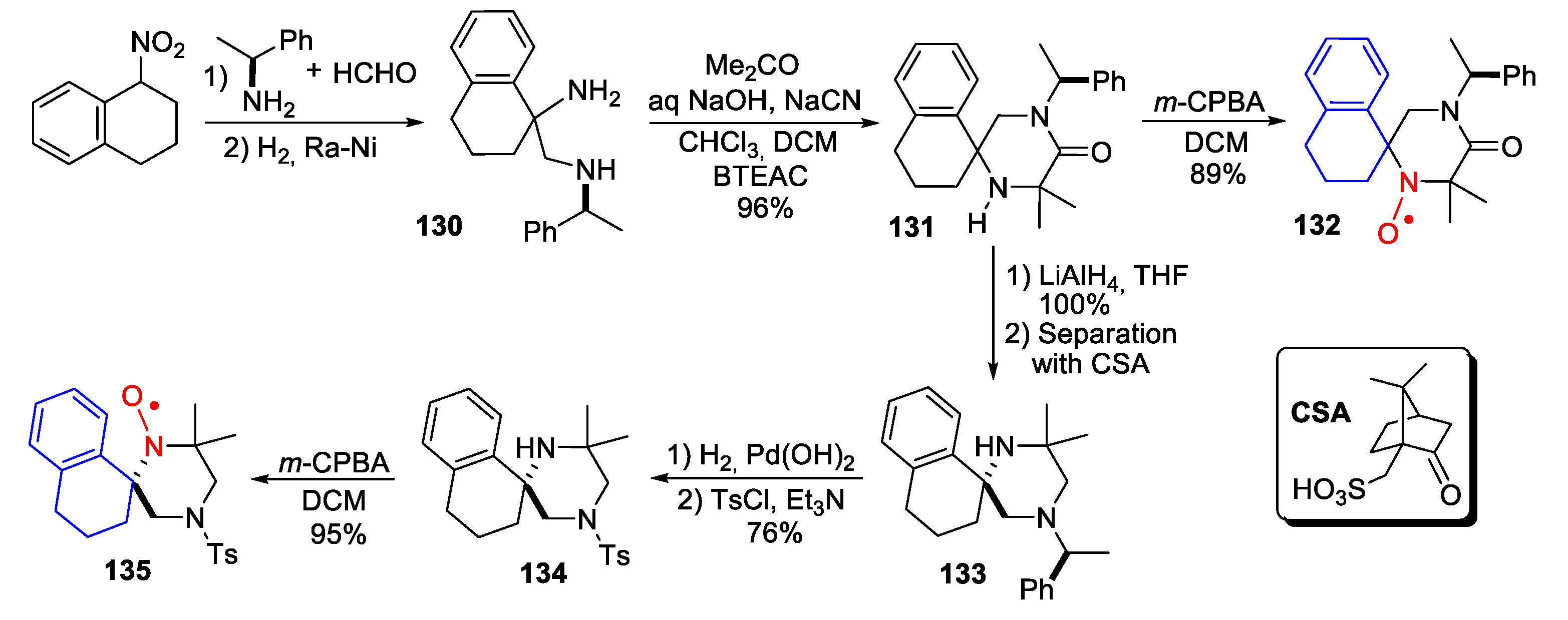
3. Benzoannelated Derivatives of Piperidine-Type SNRs
3.1. SNRs of 1,2,3,4-Tetrahydroquinolines Series
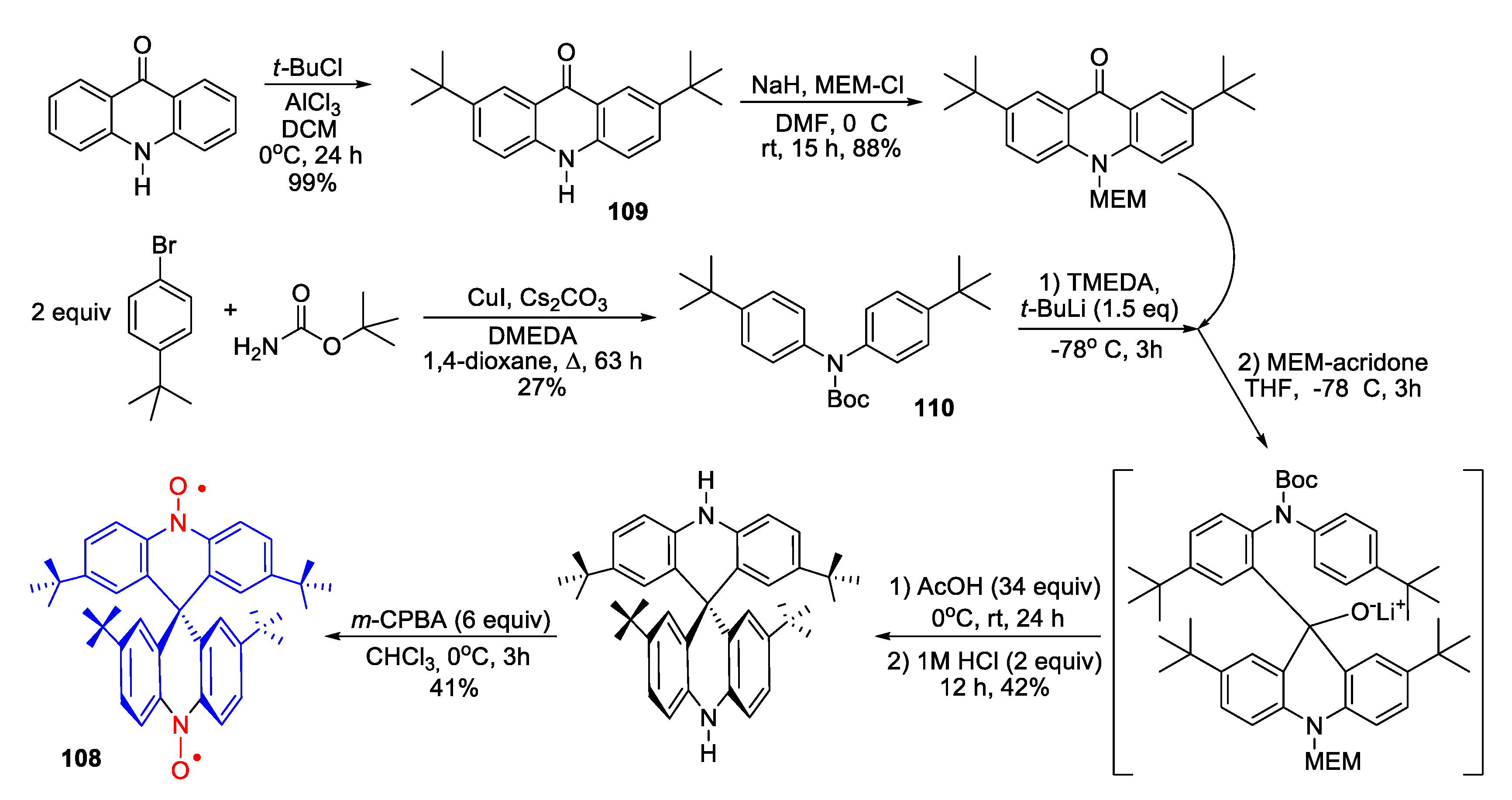
3.2. SNRs of the 10H,10′H-9,9′-Spirobi[acridine] Series
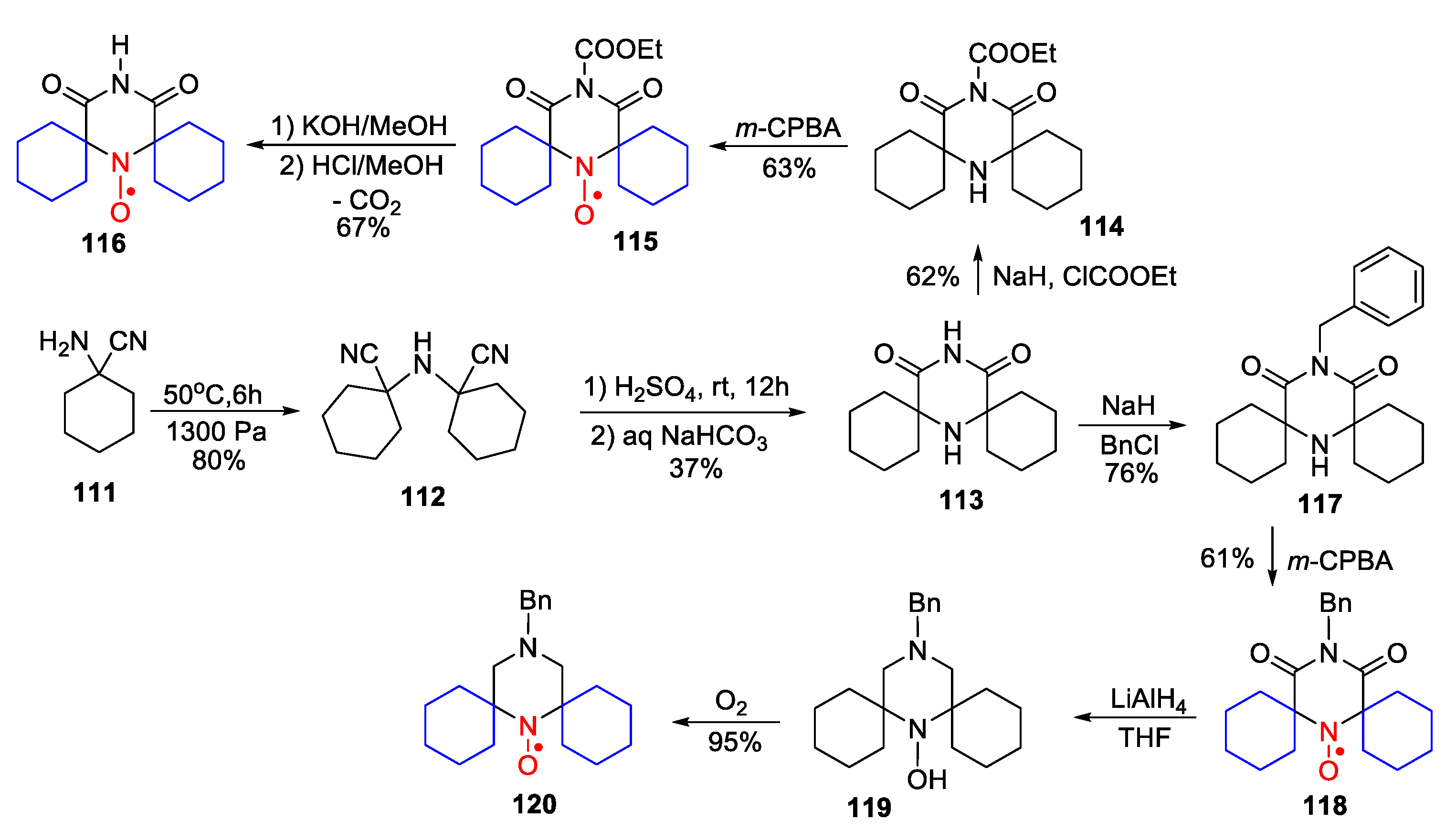
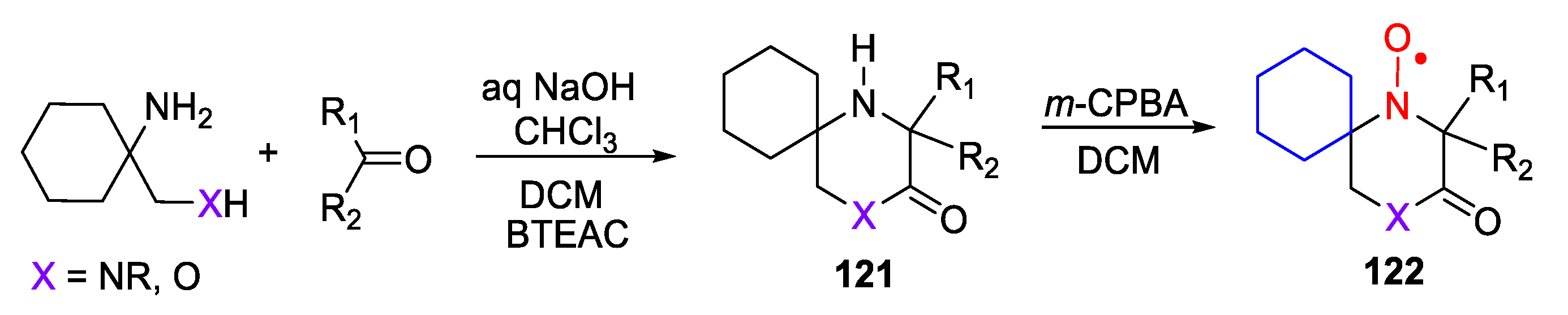
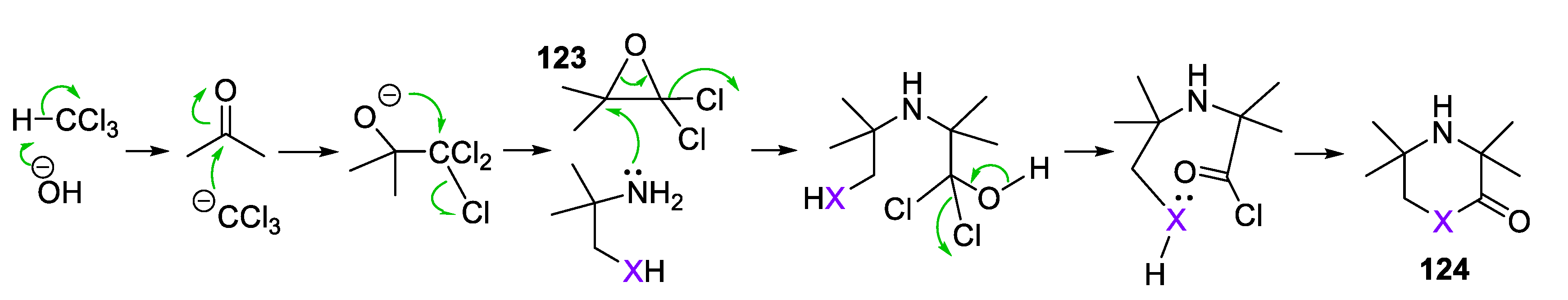
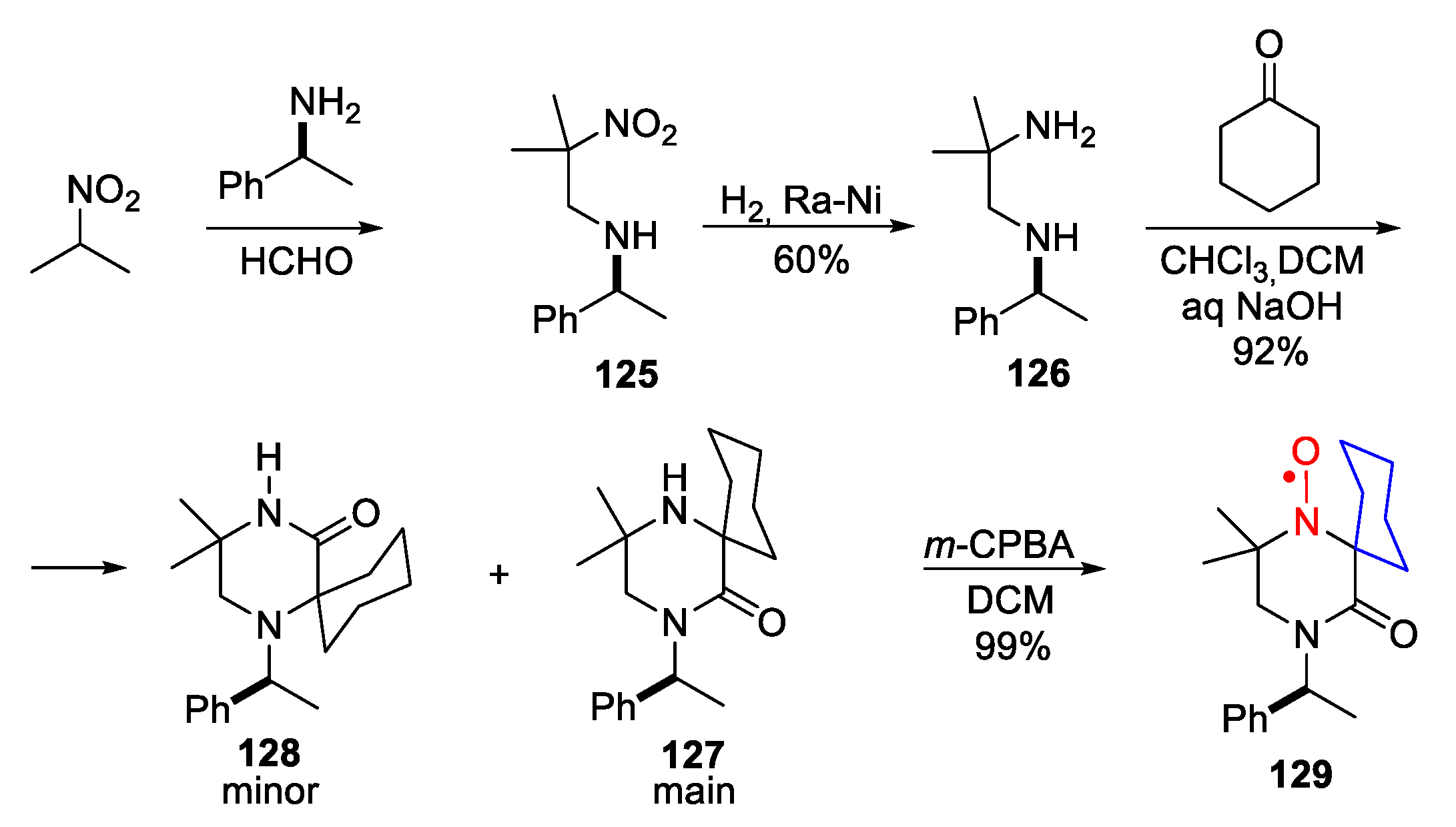
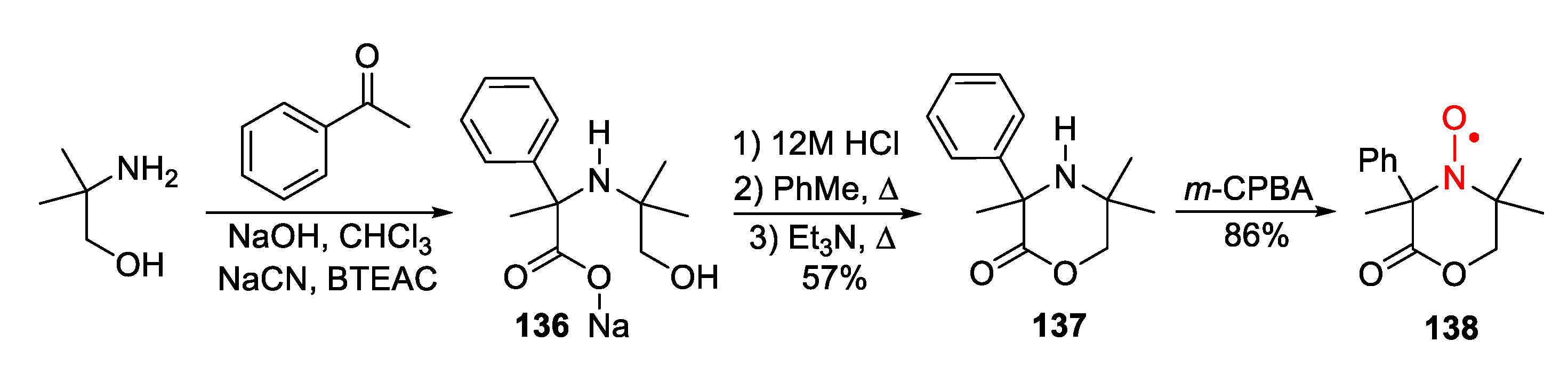
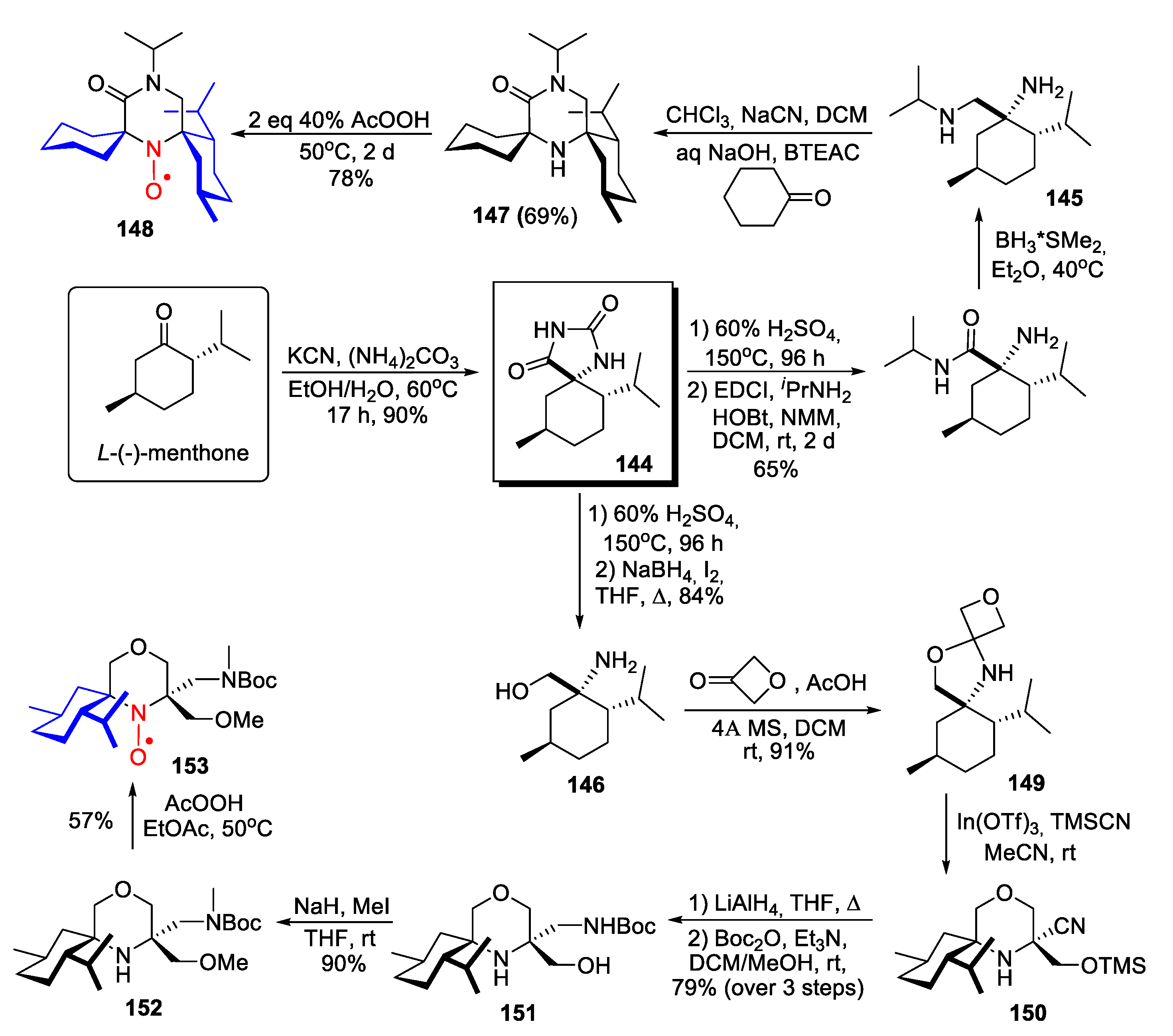
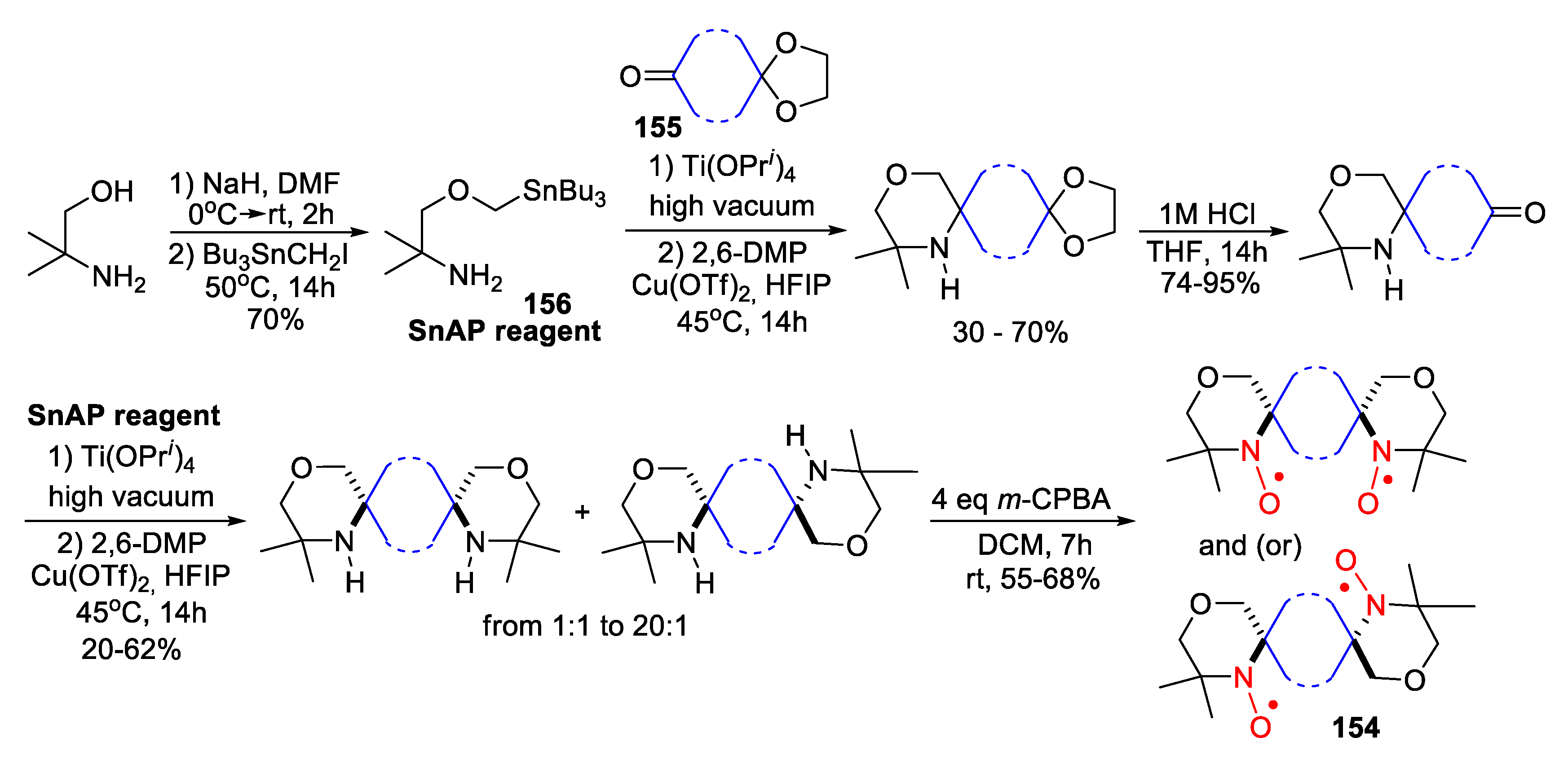
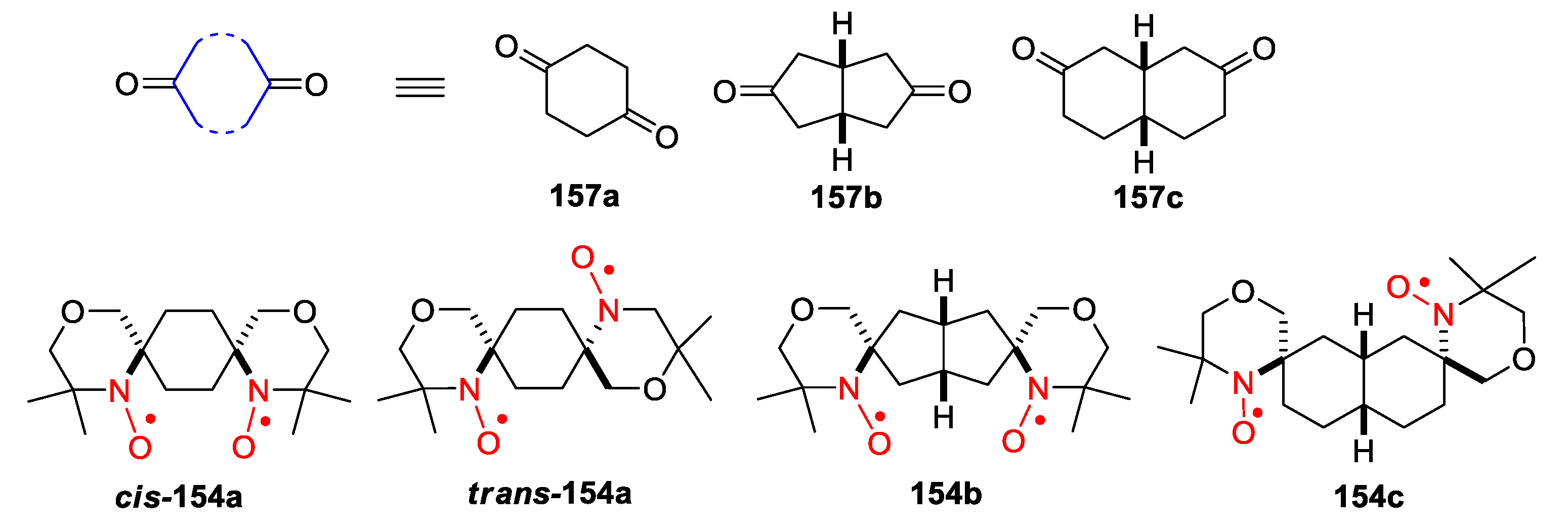
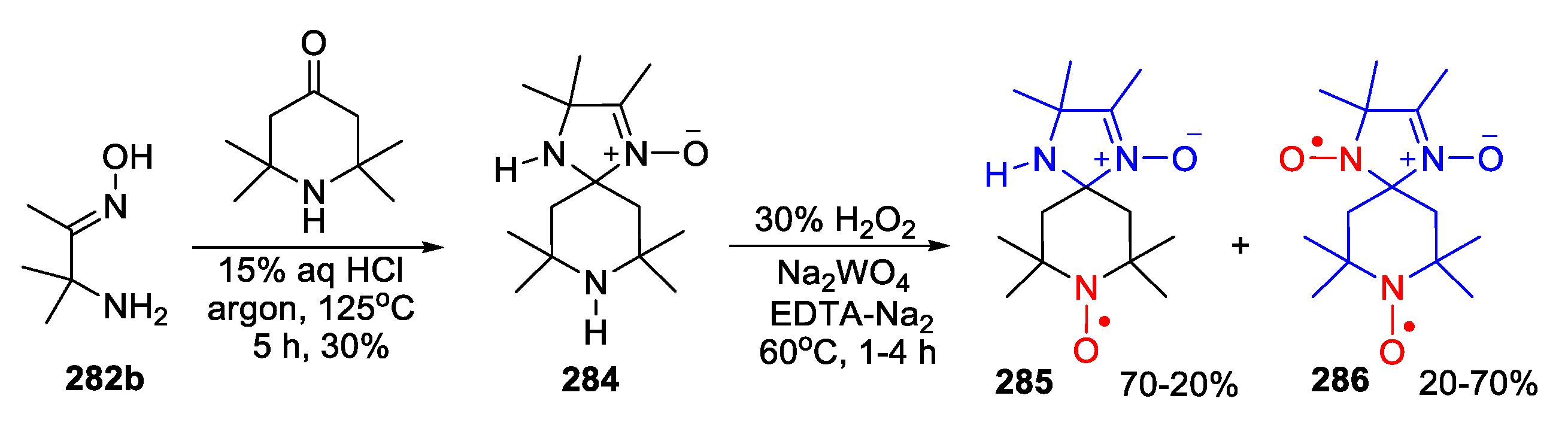
4. Piperazine- and Morpholine-Type SNRs
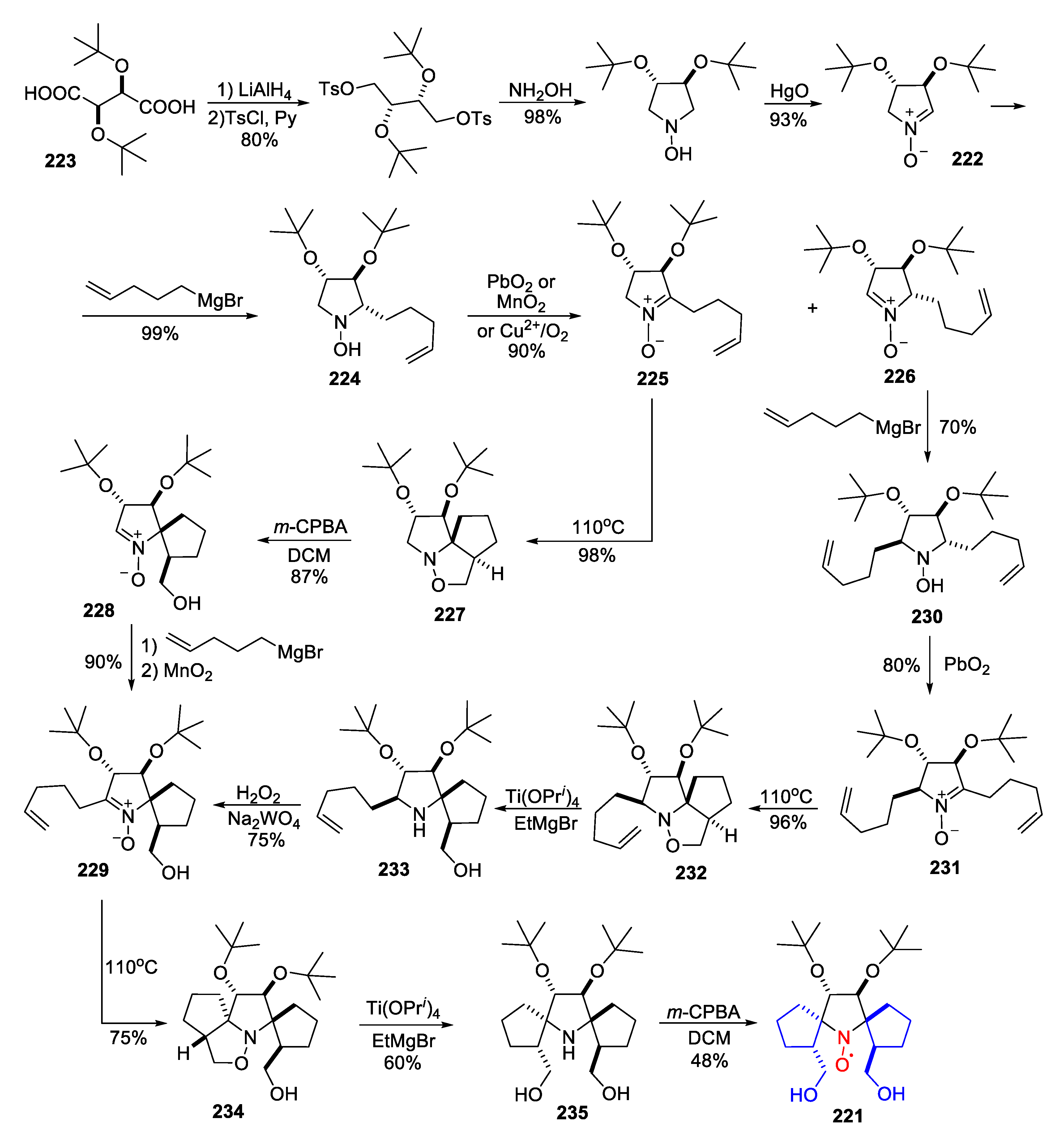
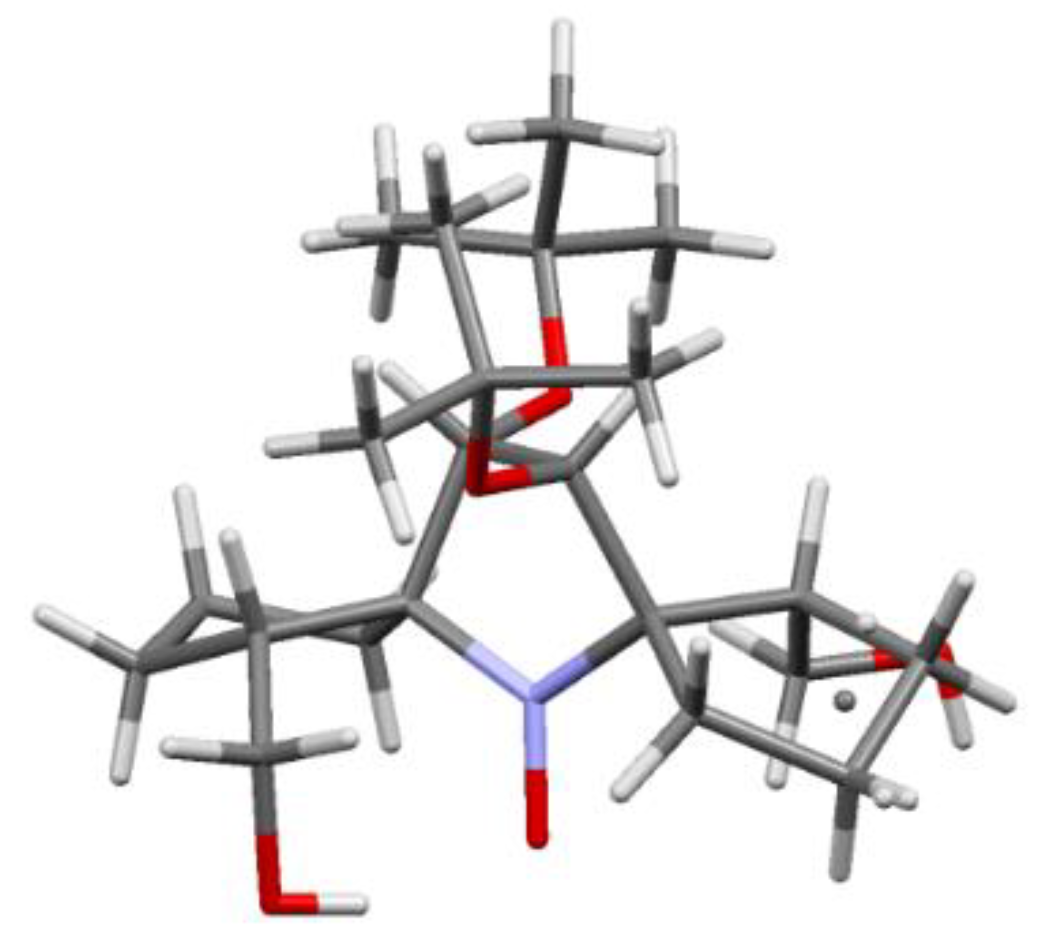
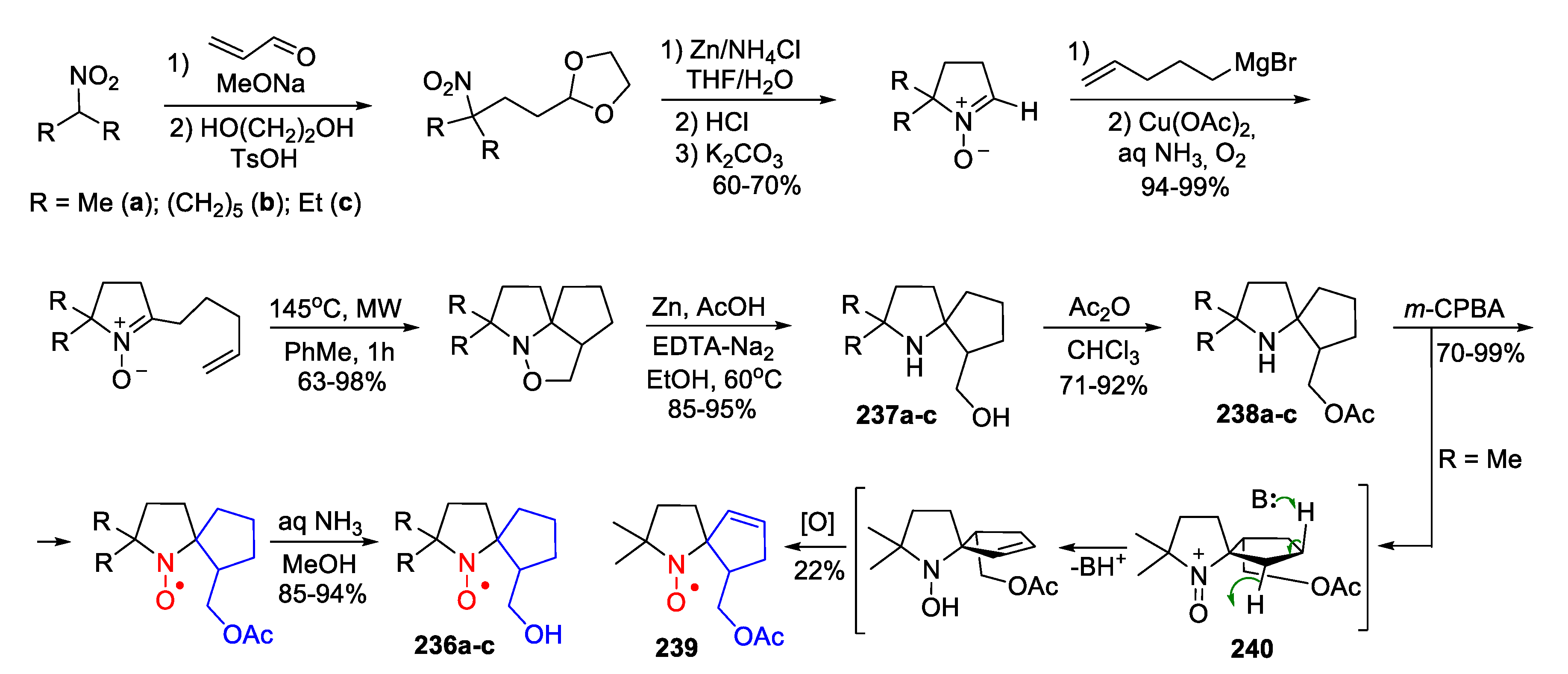
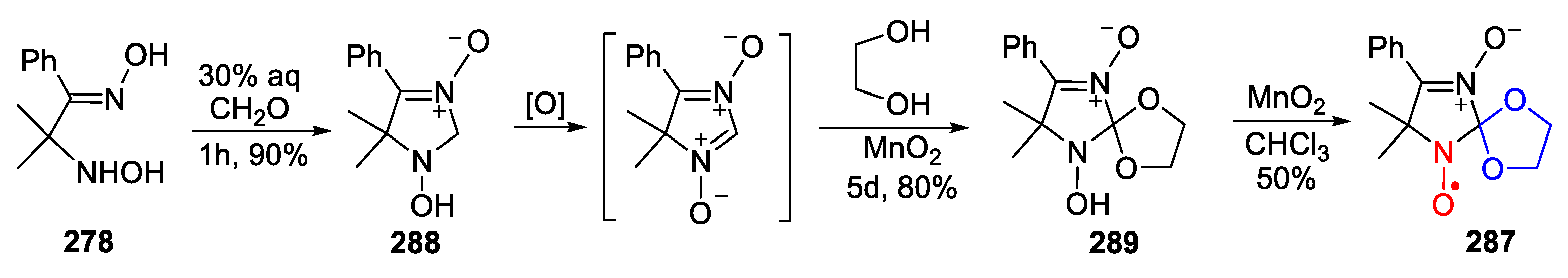
5. 2,5-Dihydropyrrole (3-Pyrroline)- and Pyrrolidine (PROXYL)-Type SNRs
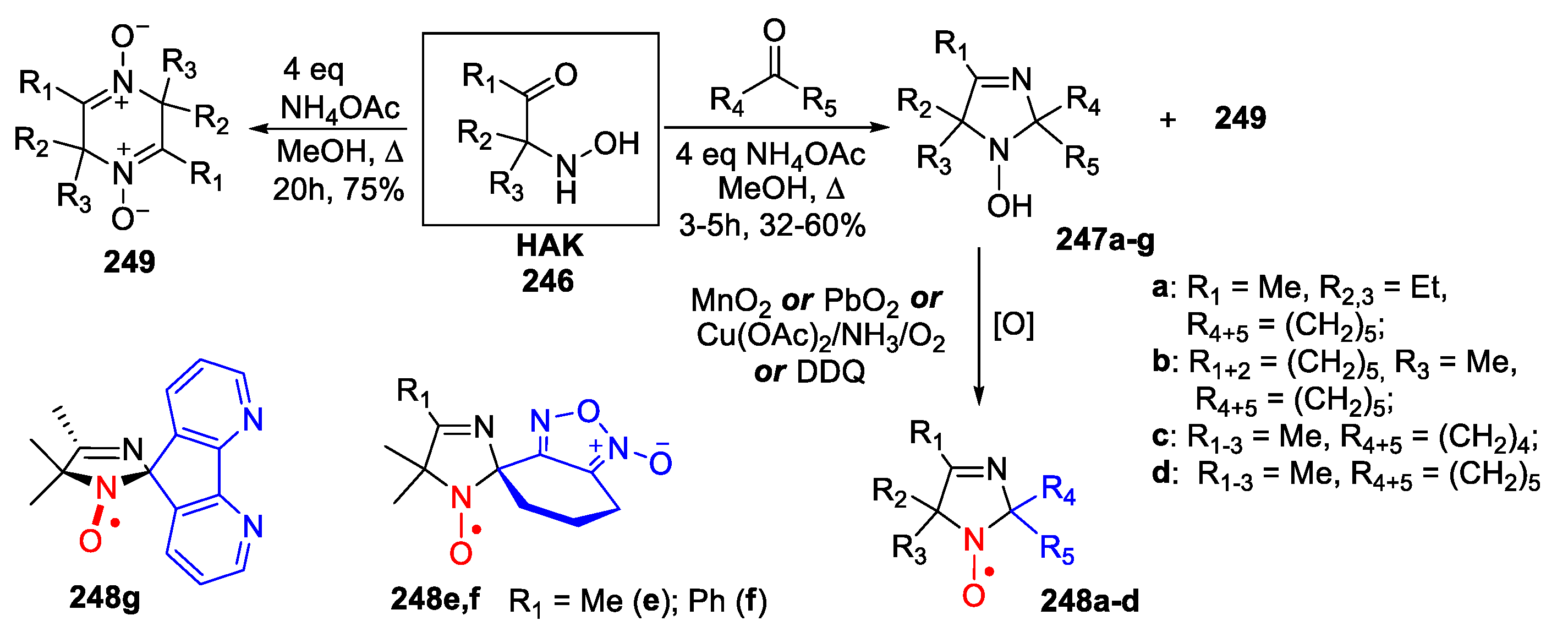
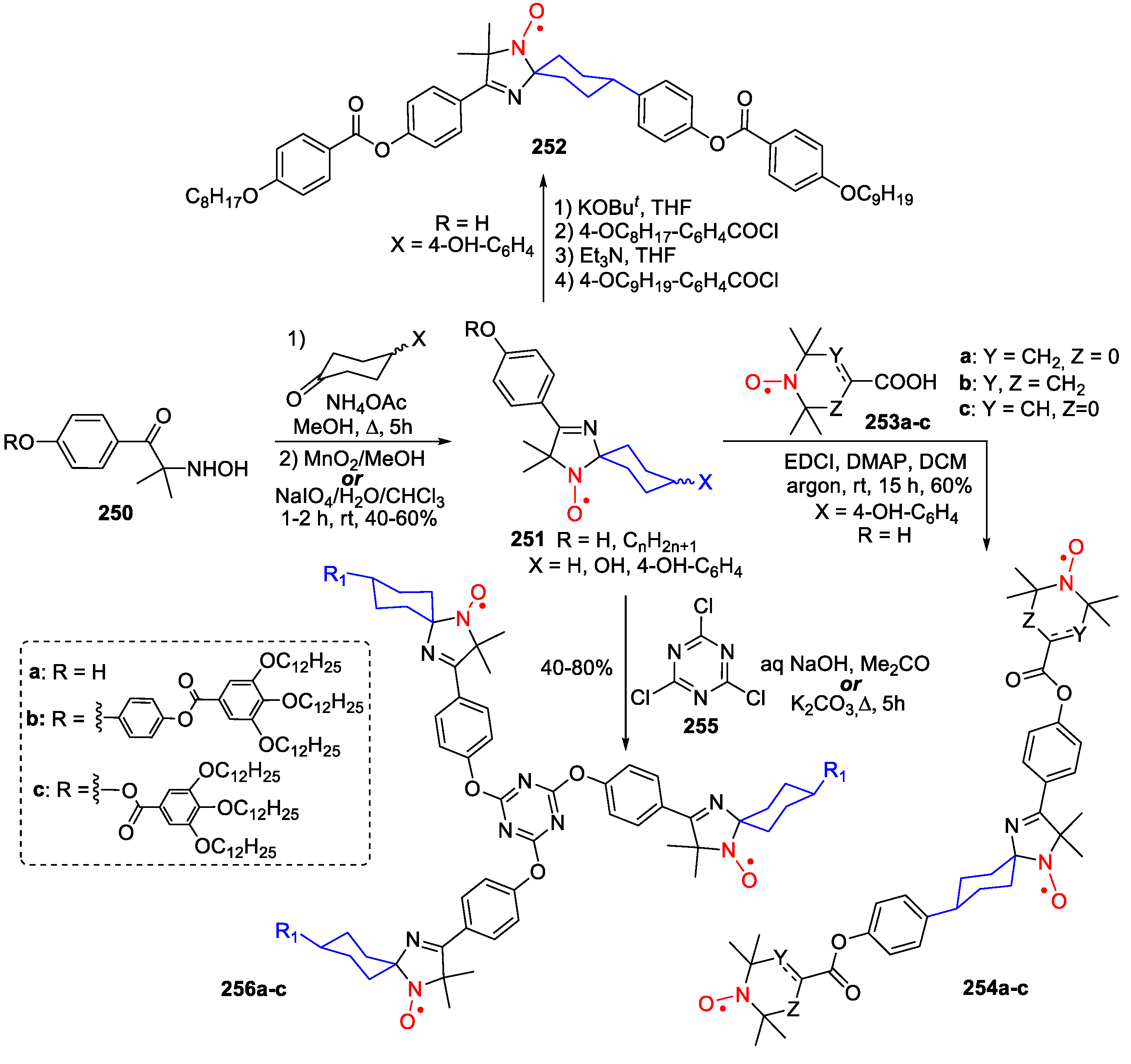
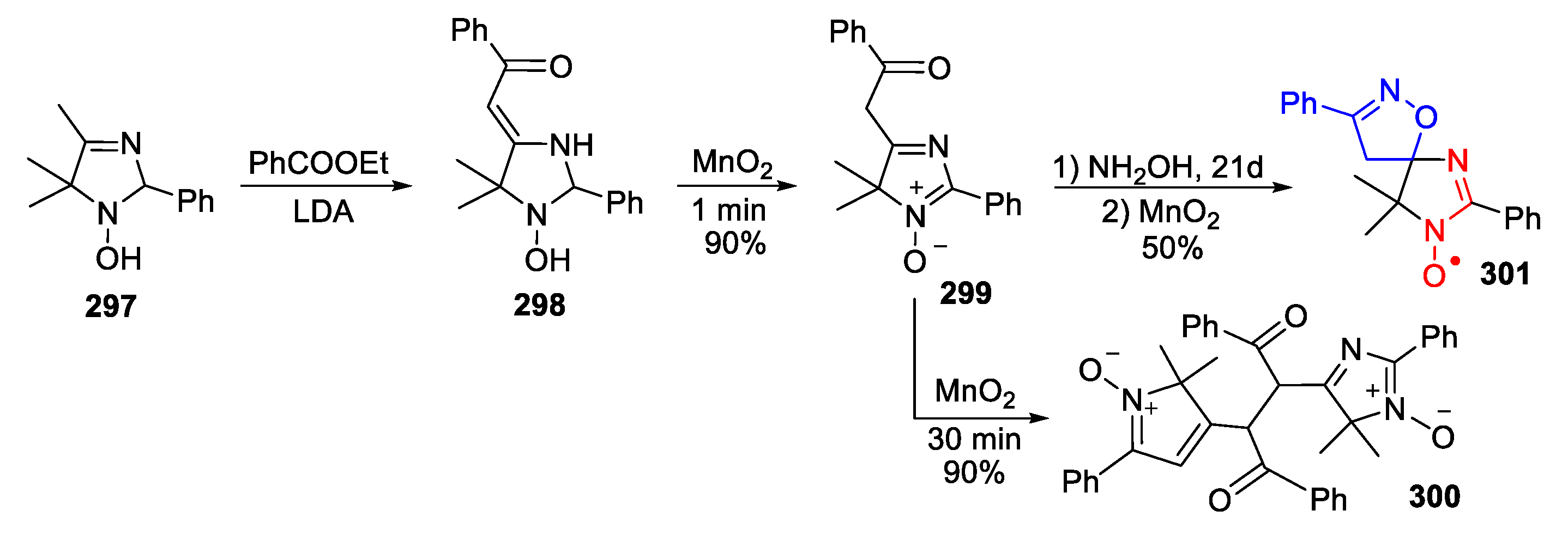
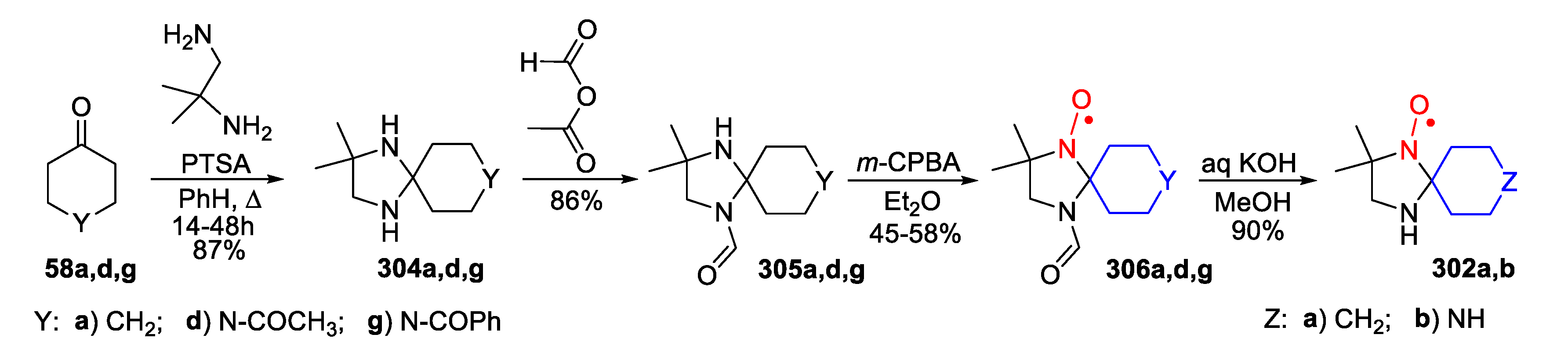
6. 2,5-Dihydroimidazole (3-Imidazoline)-Type SNRs
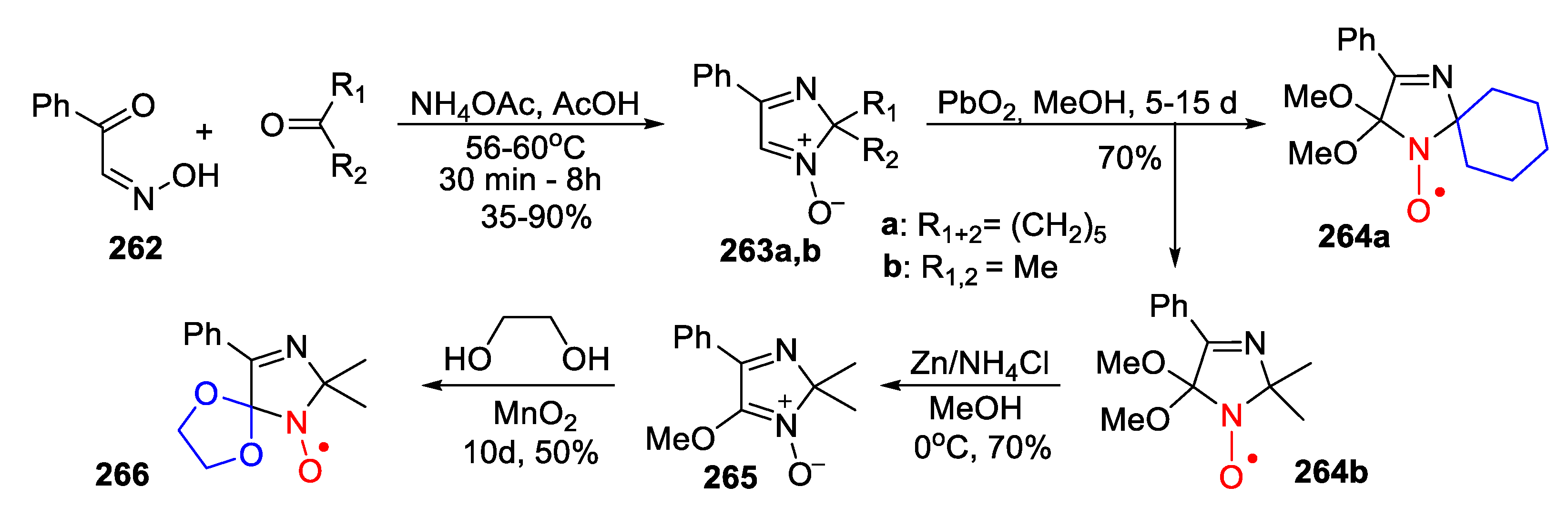
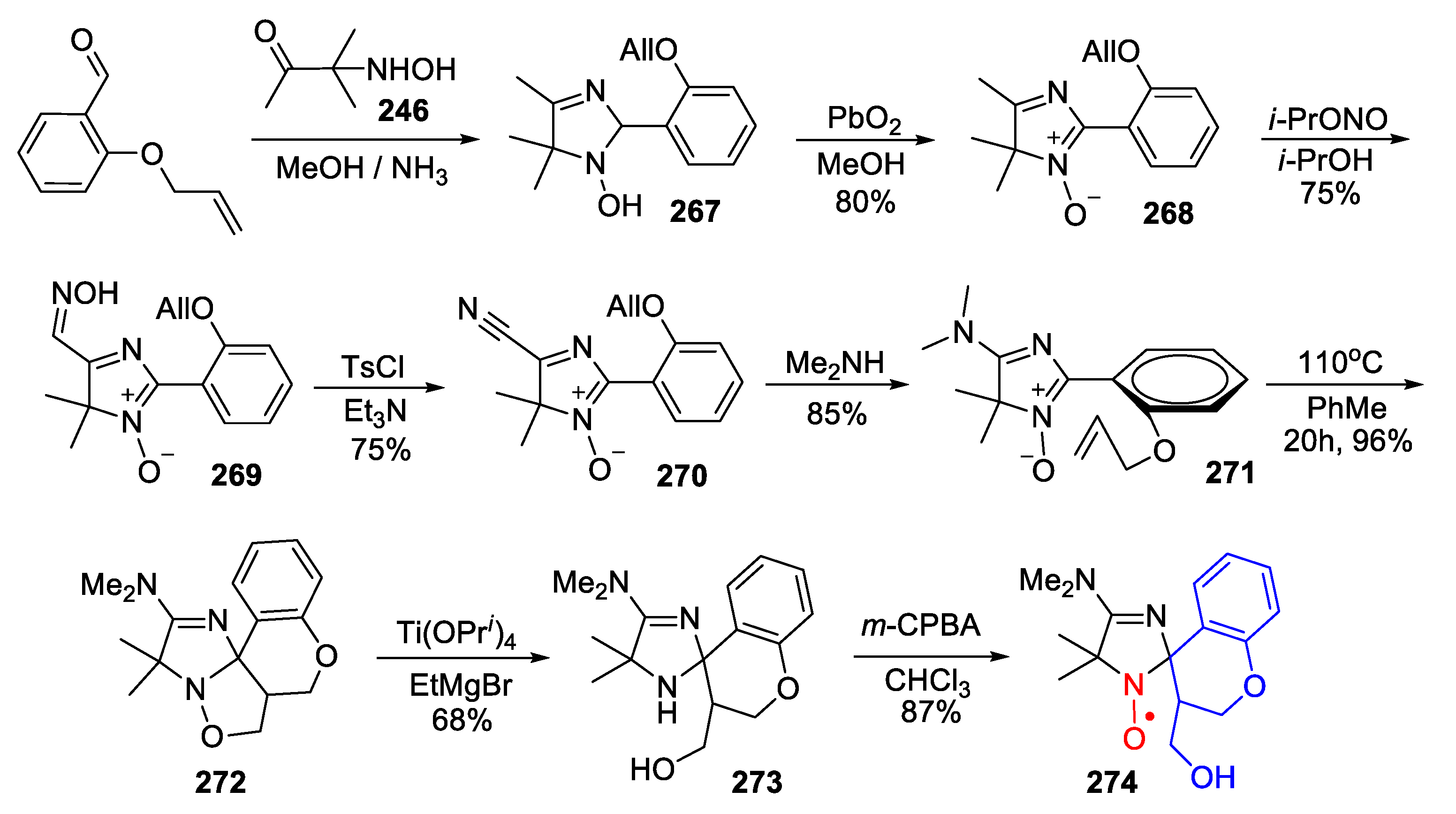
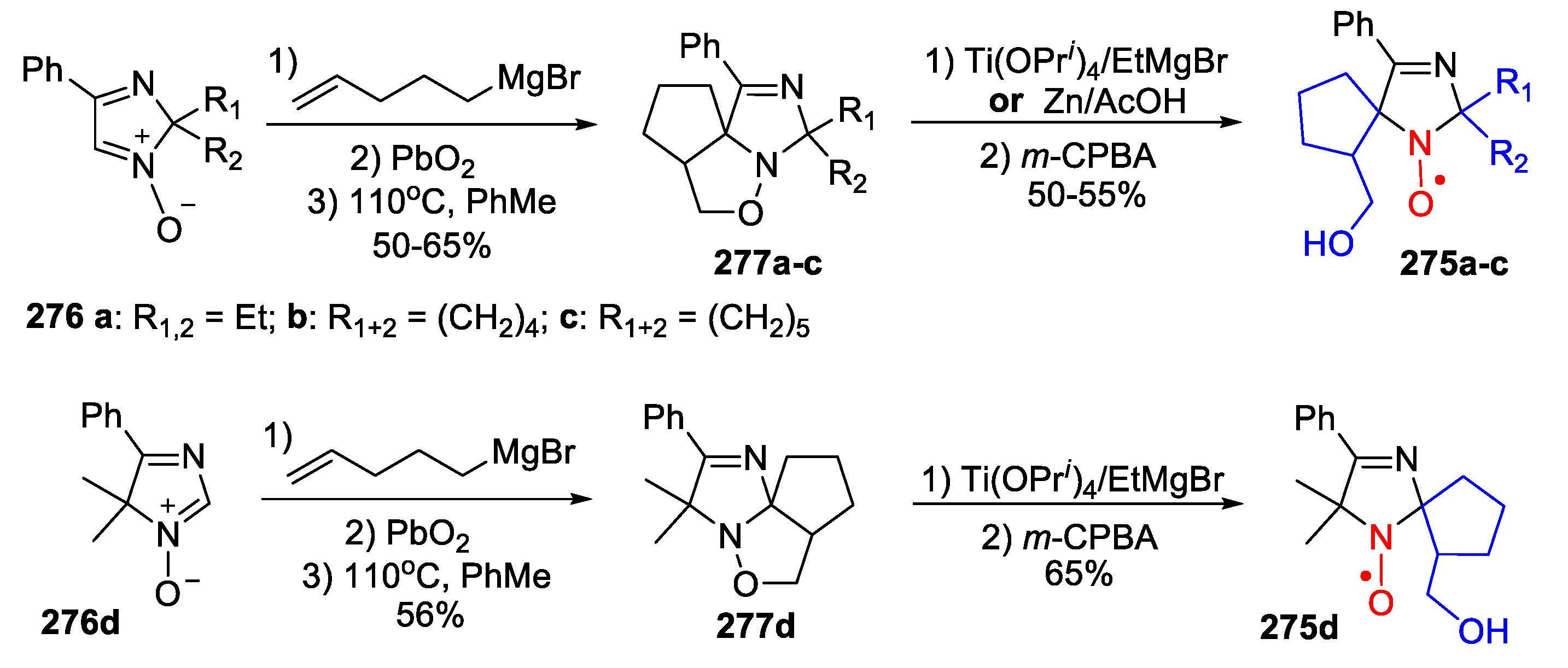
7. 4,5-Dihydroimidazole (2-Imidazoline)-Type SNRs
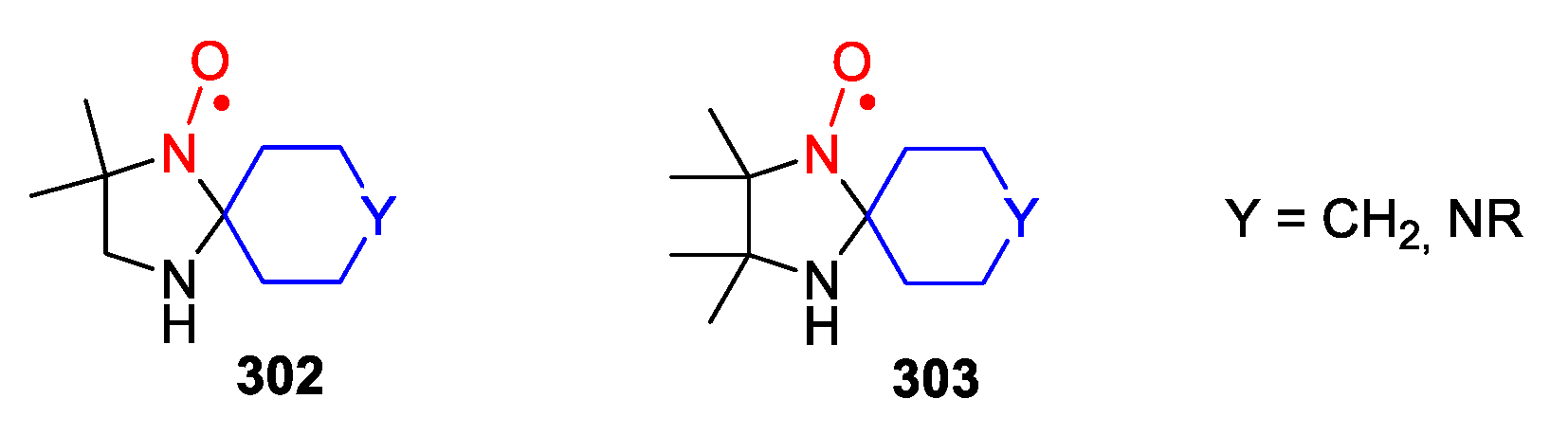
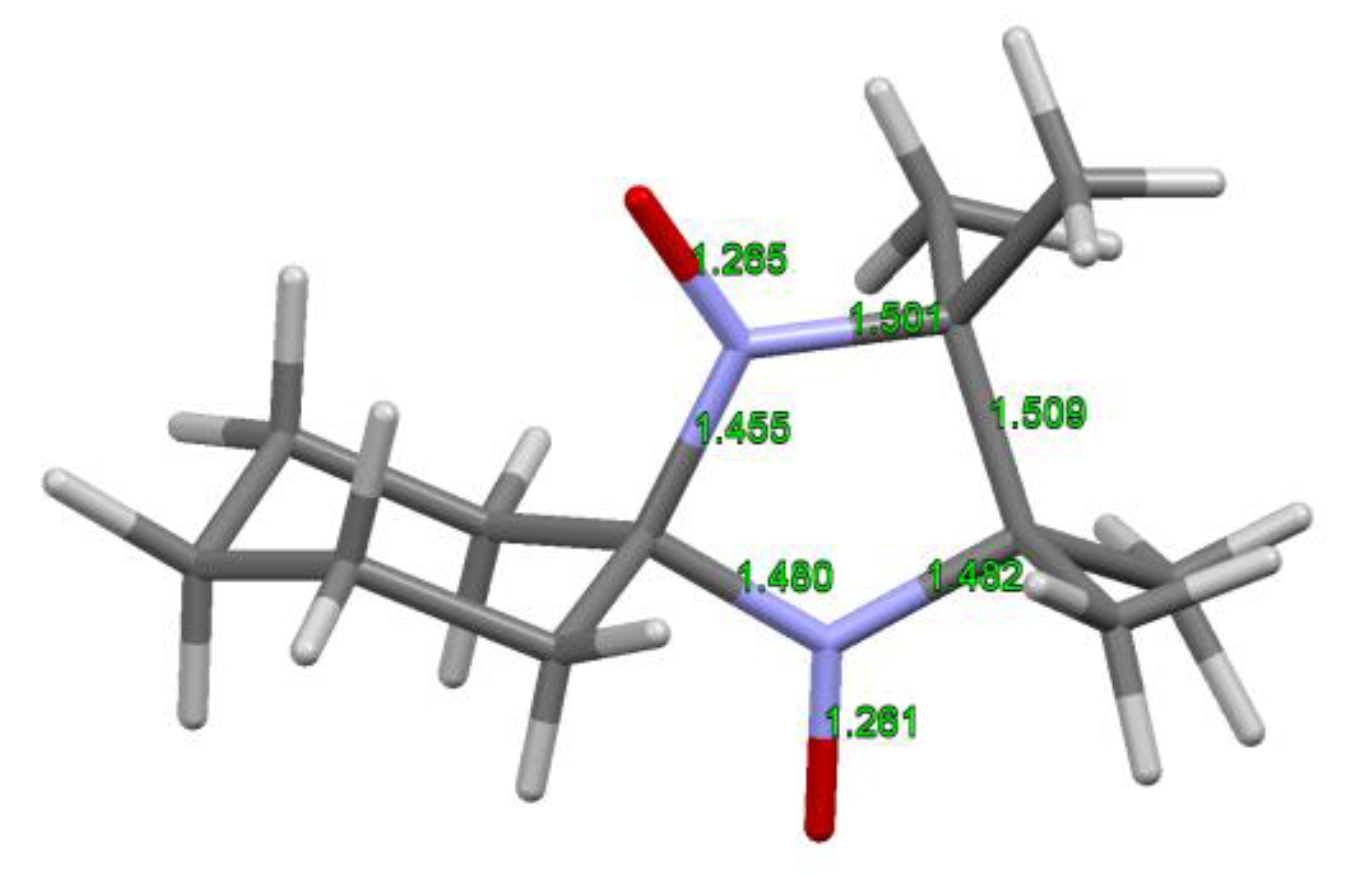
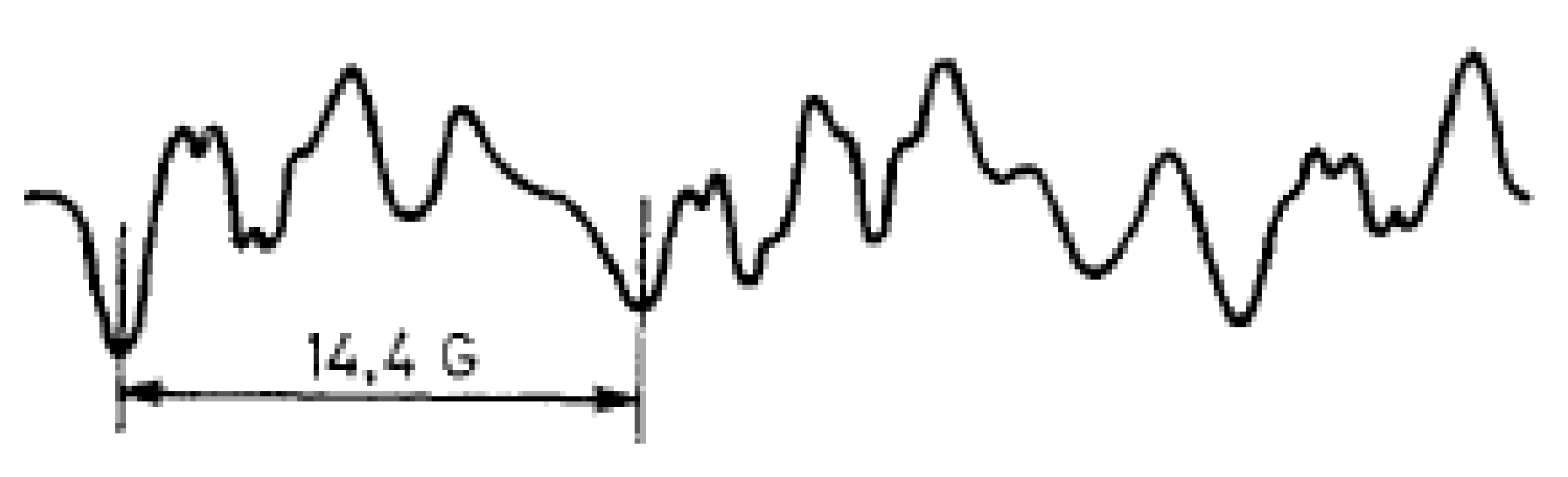
8. Imidazolidine-Type SNRs
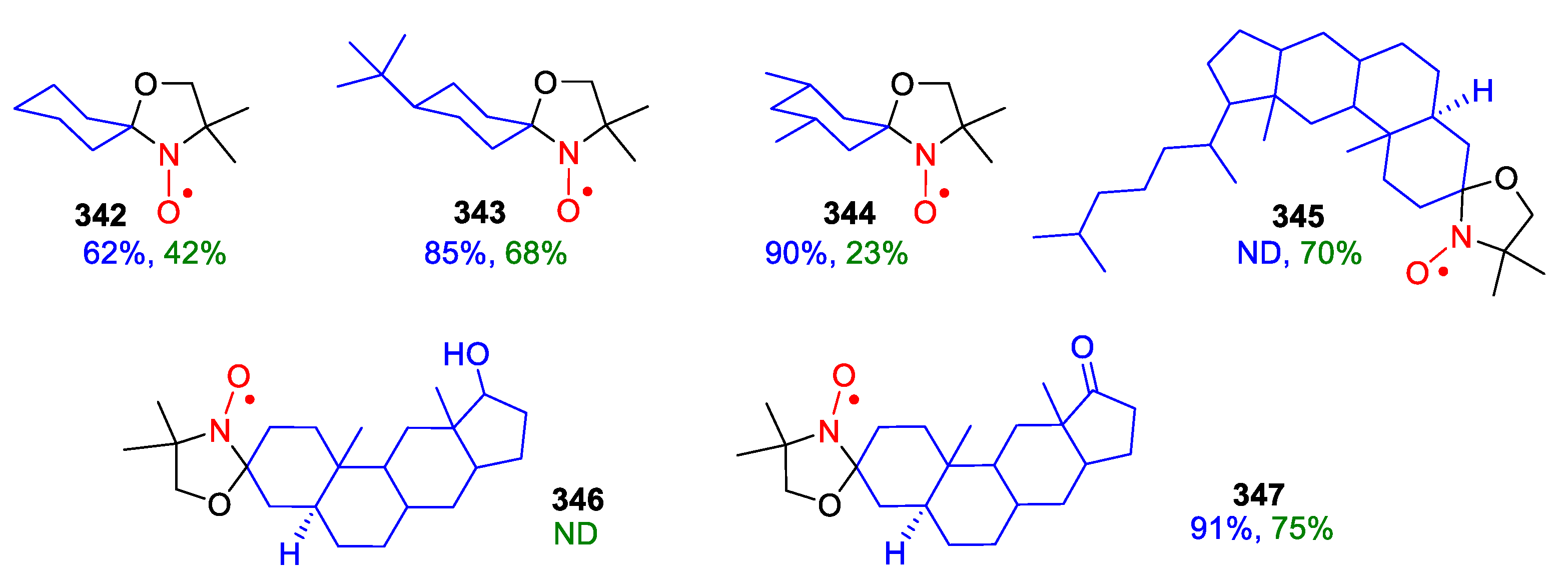
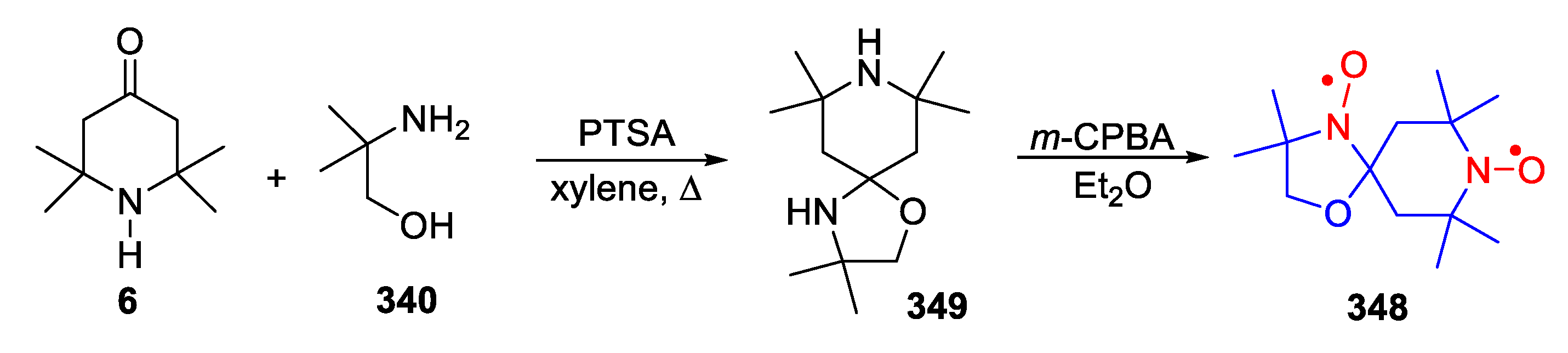
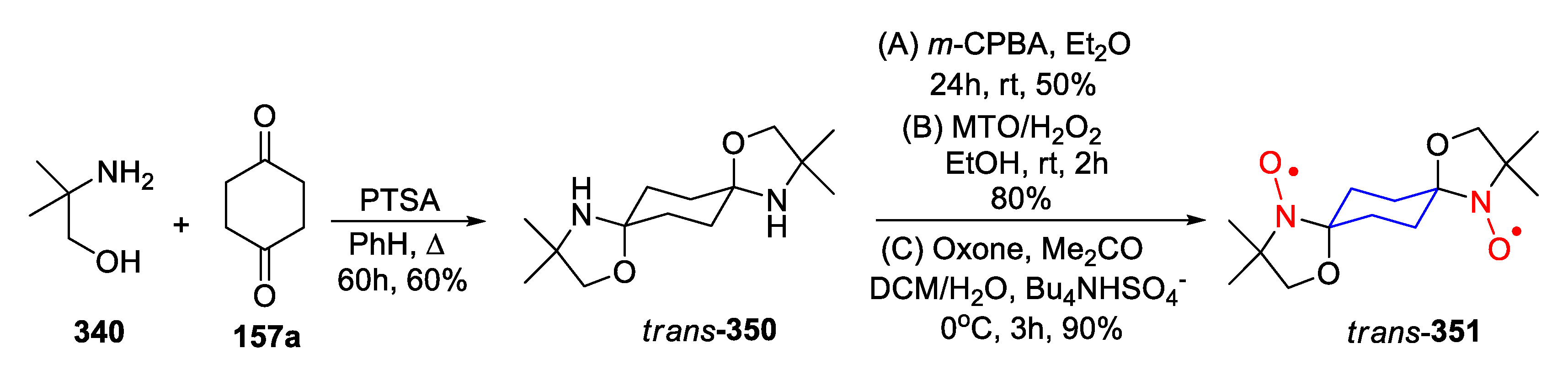
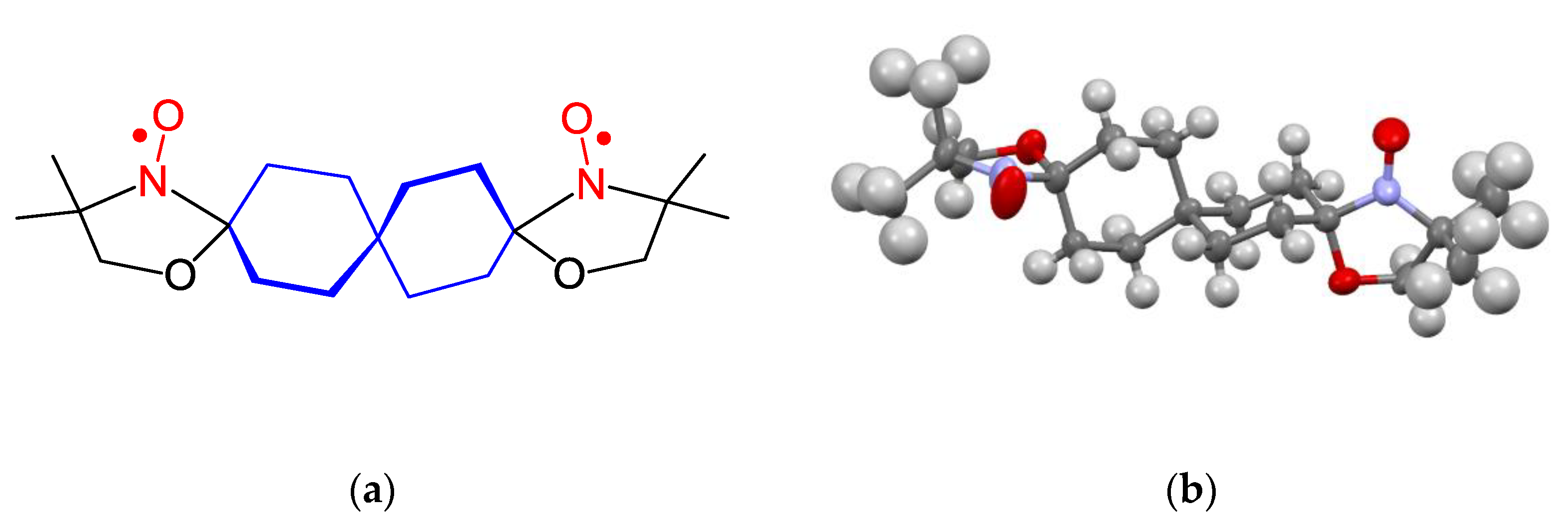
9. Oxazolidine (DOXYL) SNRs
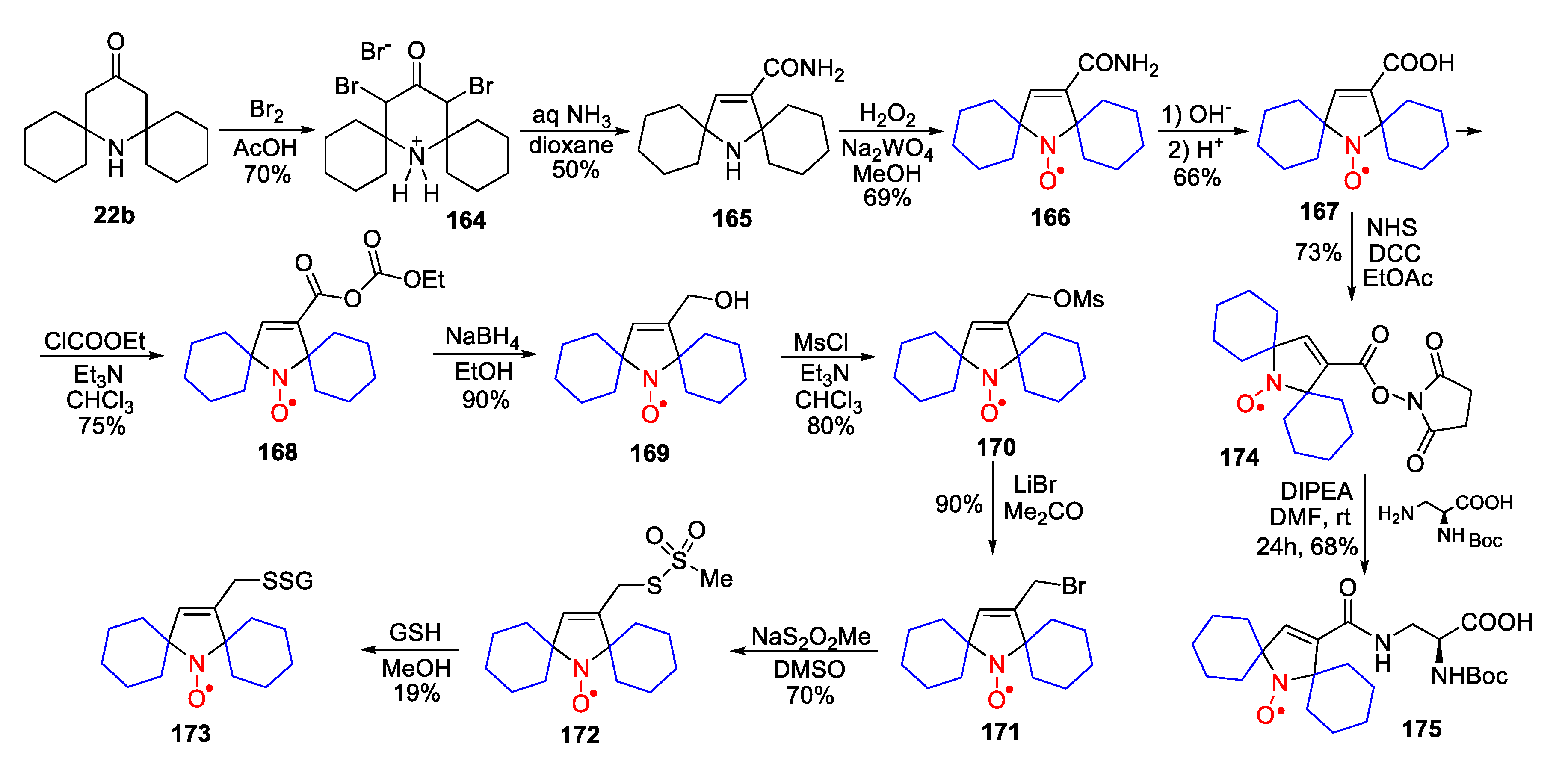
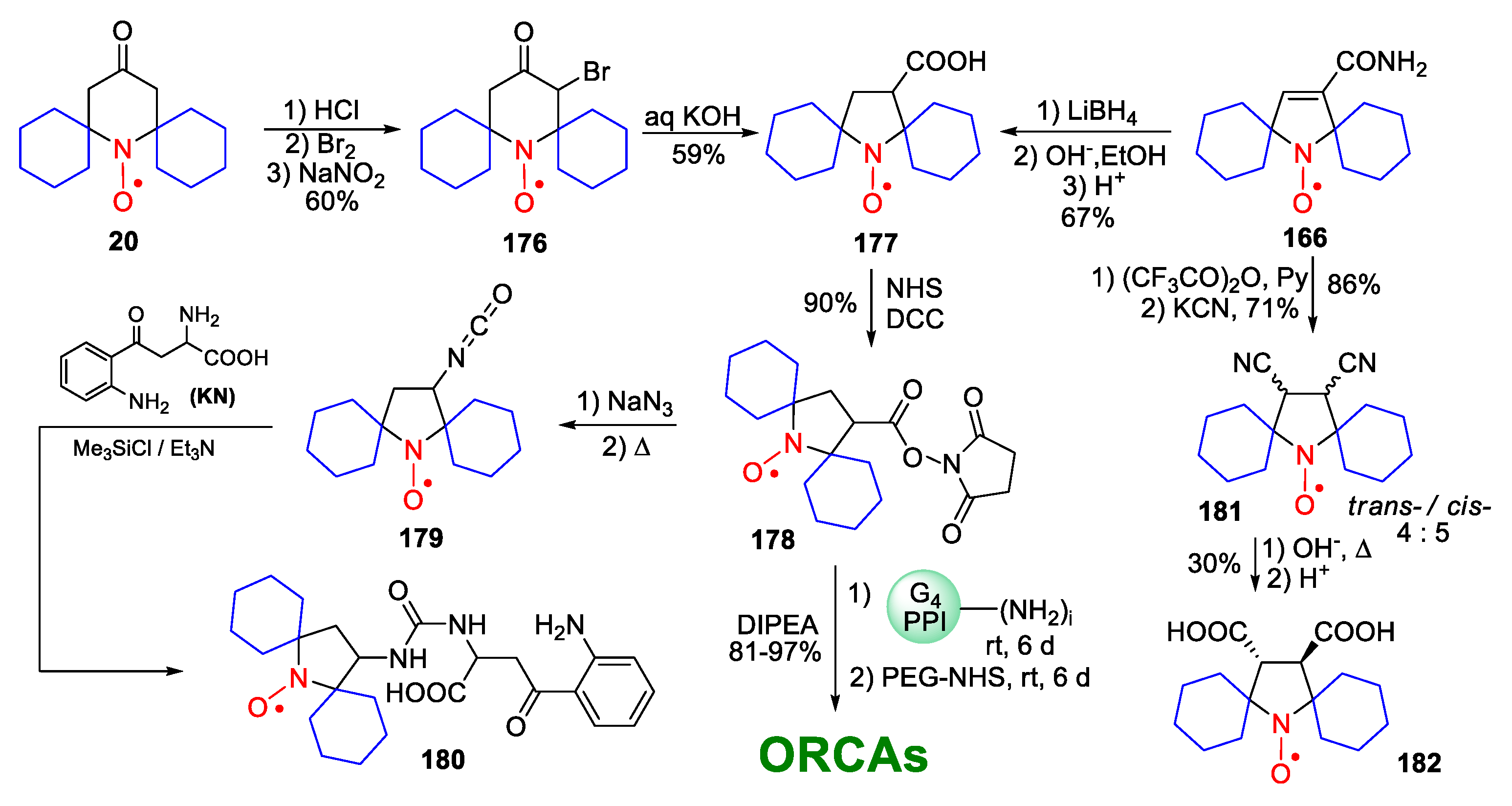
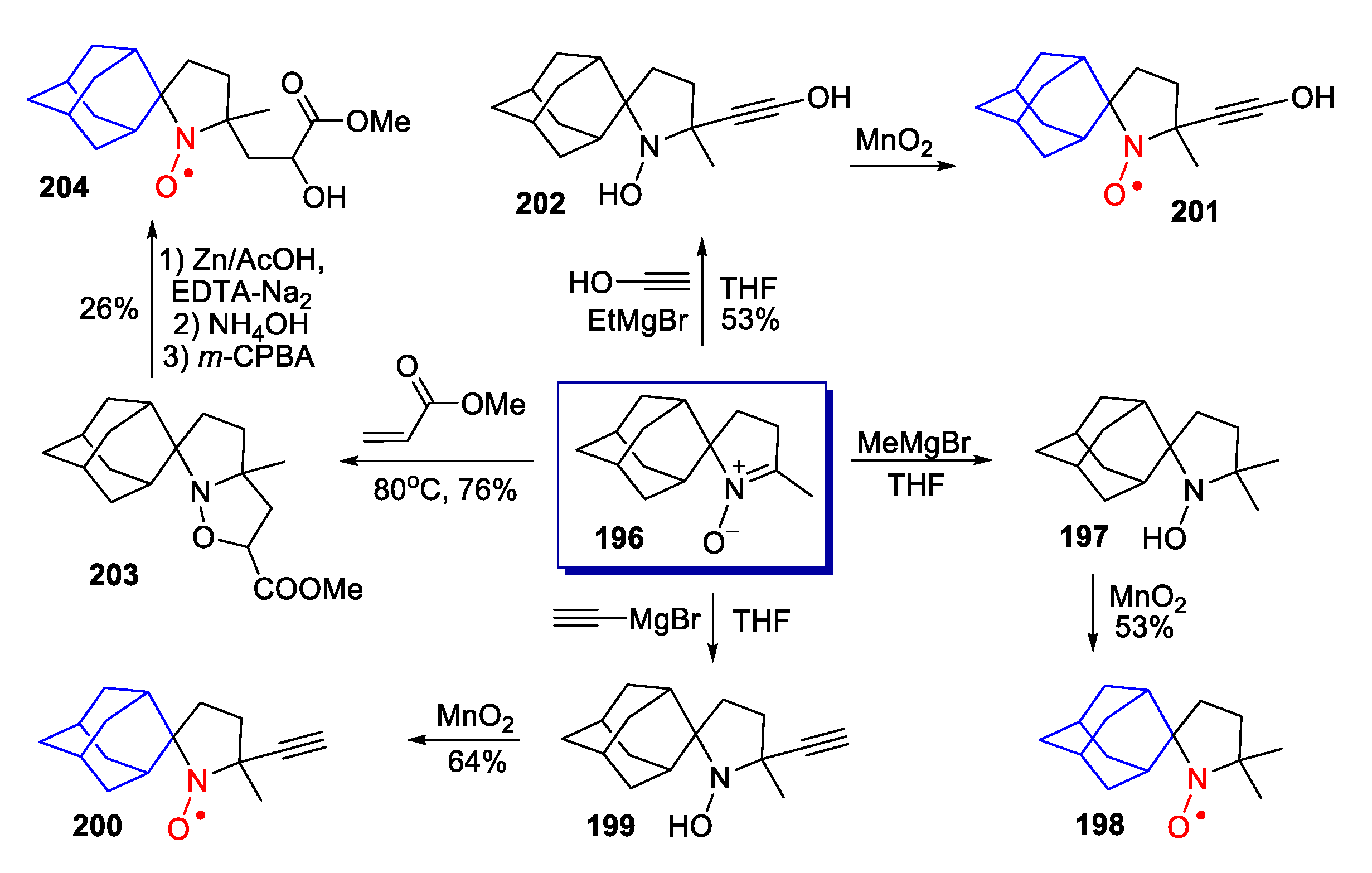
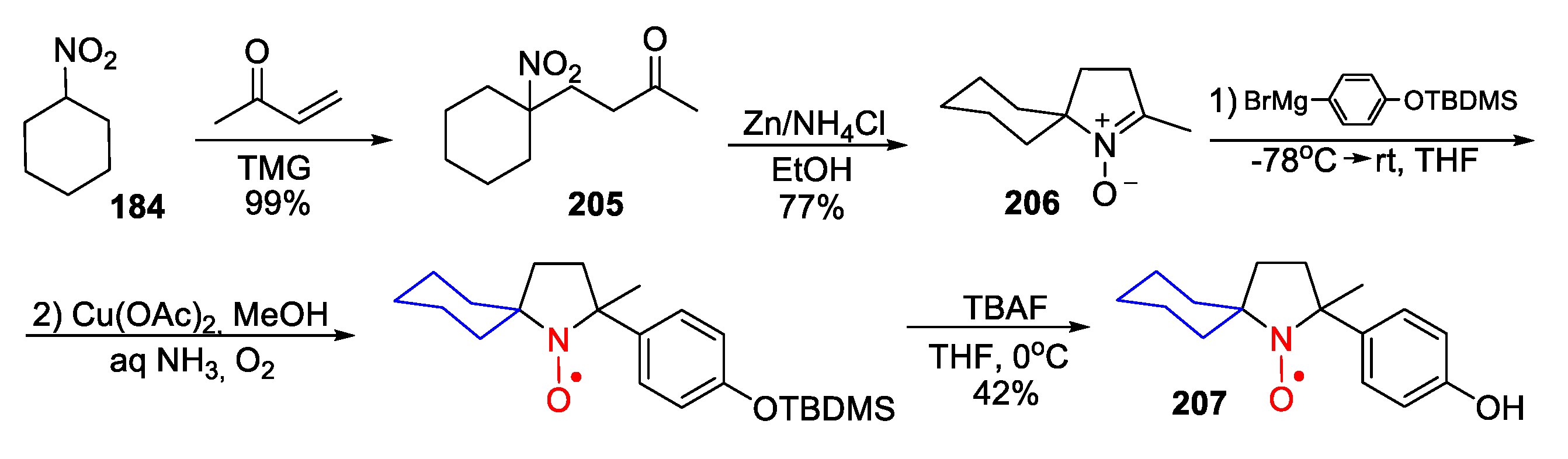
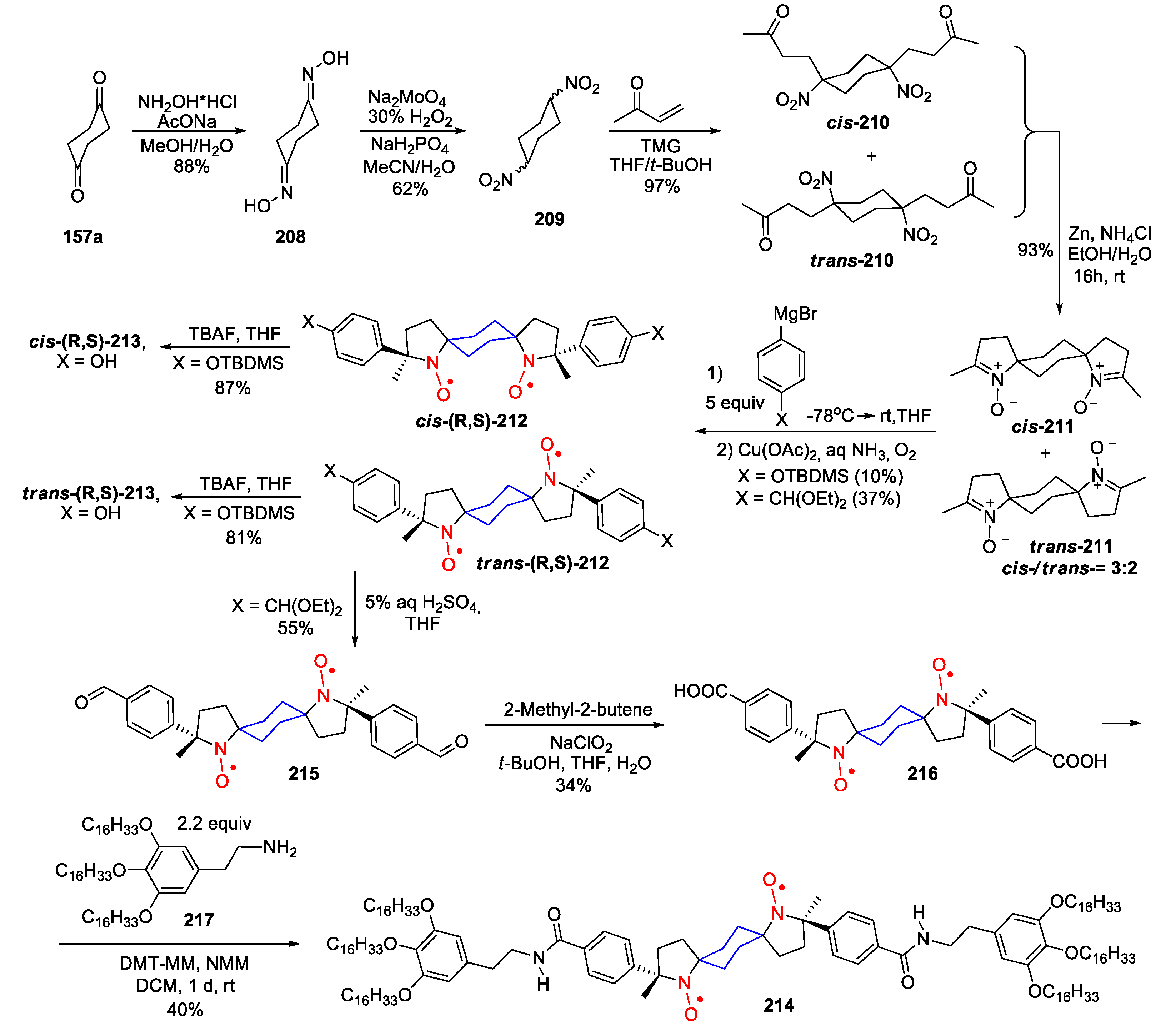
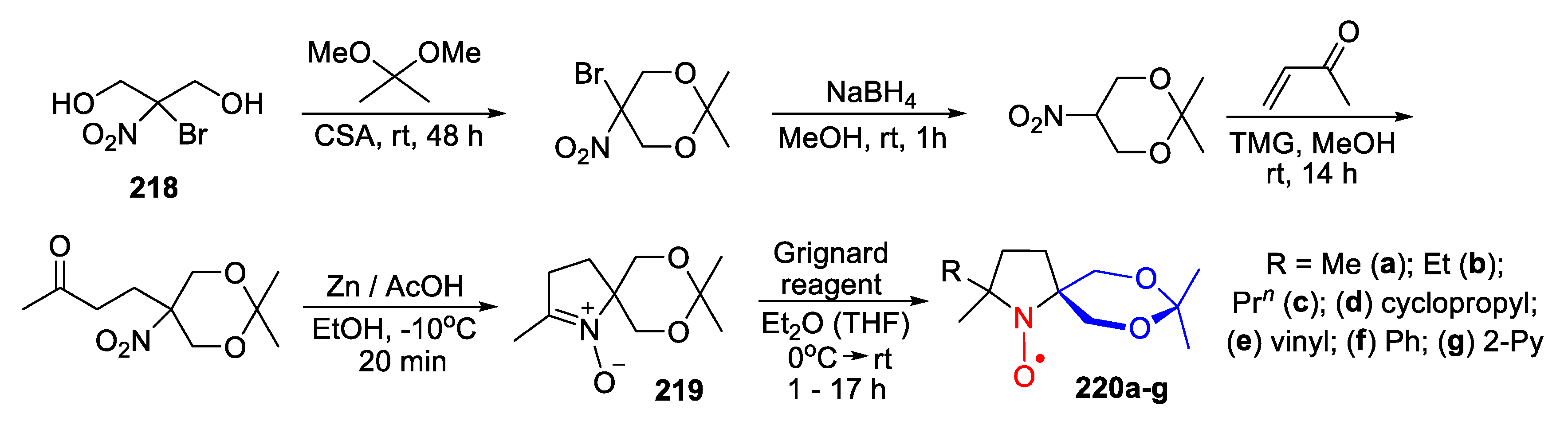
10. SNRs of Other Types
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMUPol | (15-{[(7-Oxyl-3,11-dioxa-7-azadispiro[5.1.5.3]hexadec-15-yl)carbamoyl][2-(2,5,8,11-tetraoxatridecan-13-ylamino)}-[3,11-dioxa-7-azadispiro[5.1.5.3]hexadec-7-yl])oxidanyl; |
| BCEDIPPA | Bis(2-cyanoethyl)-N,N-diisopropylphosphoramidite; |
| bcTol | [Bis(spirocyclohexyl-TEMPO-alcohol)urea]; |
| BINAP | 2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl; |
| BMS | Borane dimethylsulfide; |
| Boc2O | Di-tert-butyl dicarbonate, (ButOCO)2O; |
| bTbK | Bis-TEMPO-bis-ketal; |
| BTC | Bis(trichloromethyl) carbonate (Triphosgene); |
| BTEAC | Benzyltriethylammonium chloride; |
| BTT | 5-(Benzylthio)-1H-tetrazole; |
| m-CPBA | meta-Chloroperoxybenzoic acid; |
| CDI | 1,1′-Carbonyldiimidazole; |
| CSA | Camphorsulfonic acid, (7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonic acid; |
| cyolyl-TOTAPOL | [Spirocyclohexanolyl-1-(TEMPO-4-oxy)-3-(TEMPO-4-amino)propan-2-ol]; |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene; |
| DCC | N,N′-Dicyclohexylcarbodiimide; |
| DCM | Dichloromethane; |
| DDQ | 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone; |
| DEER | Double electron-electron resonance; |
| DIBAL-H | Diisobutylaluminium hydride; |
| DIPEA | N,N-Diisopropylethylamine (Hünig’s base); |
| DMAP | 4-Dimethylaminopyridine; |
| DMDO | Dimethyldioxirane; |
| DME | Dimethoxyethane; |
| DMEDA | N,N′-Dimethylethylenediamine; |
| 2,6-DMP | 2,6-Dimethoxypyridine; |
| DMP | Dess–Martin periodinane (3-Oxo-1,3-dihydro-1λ5,2-benziodoxole-1,1,1-triyl triacetate); |
| DMT-MM | 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride; |
| DOXYL | Oxazolidine-3-oxyl; |
| EDCI | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide; |
| EDTA-Na2 | Ethylenediaminetetraacetic acid, disodium salt; |
| Fmoc-Cl | 9-Fluorenylmethoxycarbonyl chloride; |
| Fmoc-OSu | N-(9-Fluorenylmethoxycarbonyloxy)succinimide; |
| GSH | Glutathione; |
| HAK | 2-Hydroxylaminoketones, R1-CO-CR2R3-NHOH; |
| HFIP | 1,1,1,3,3,3-Hexafluoro-2-propanol; |
| HOBt | 1-Hydroxybenzotriazole; |
| KN | Kynurenine, (S)-2-Amino-4-(2-aminophenyl)-4-oxo-butanoic acid; |
| LAH | Lithium aluminium hydride, LiAlH4; |
| LVT-reagent | Low valent titanium species; |
| MEM-Cl | 2-Methoxyethoxymethyl chloride; |
| MTO | Methyltrioxorhenium, CH3ReO3; |
| MS | Molecular sieves; |
| NBS | N-Bromosuccinimide; |
| NHS | N-Hydroxysuccinimide; |
| NMM | N-Methylmorpholine; |
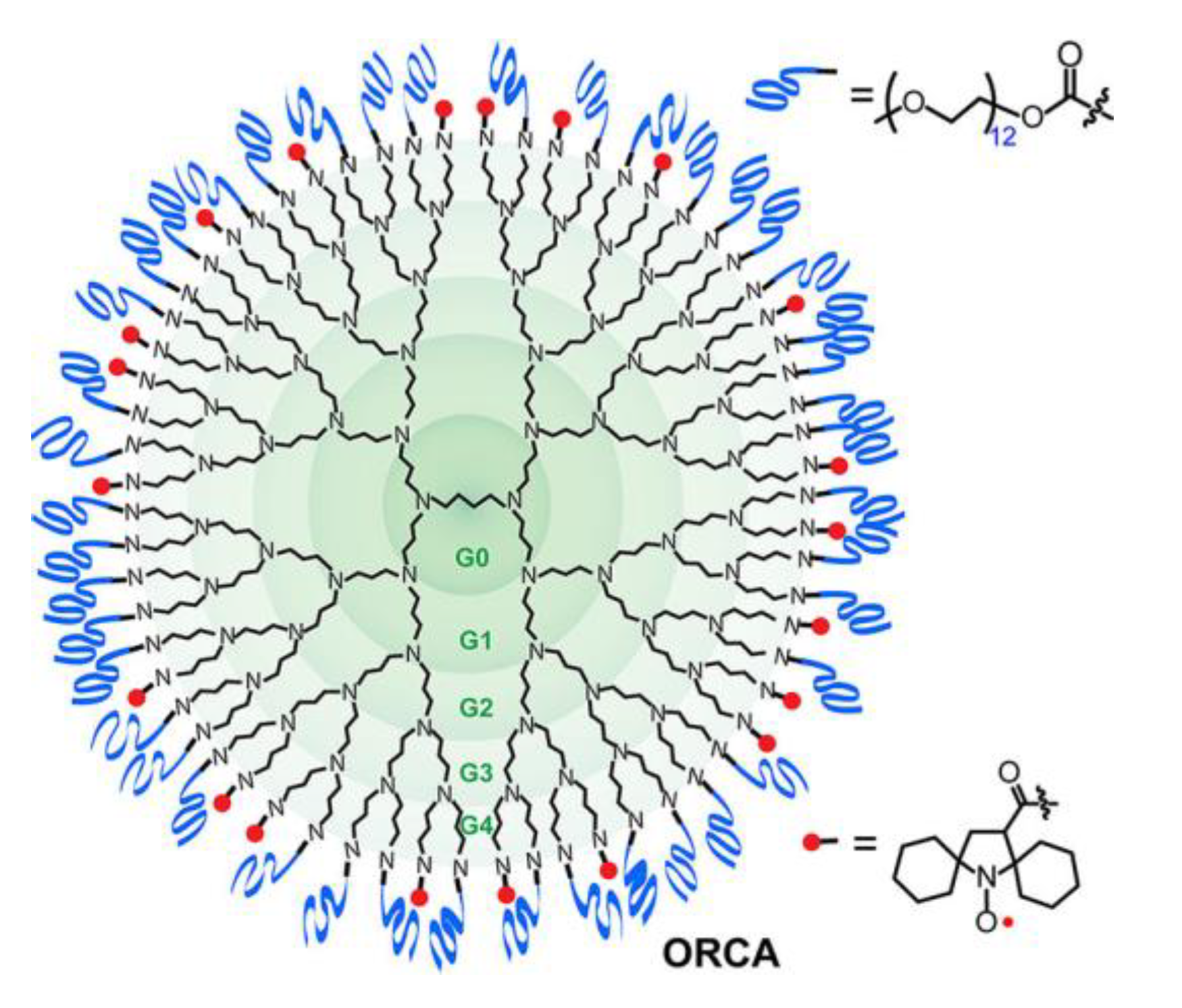
| ORCA | Organic radical contrast agent; |
| PCC | Pyridinium chlorochromate; |
| PivOH | Pivalic acid; |
| PROXYL | Pyrrolidine-1-oxyl; |
| PTSA | p-Toluenesulfonic acid, TsOH; |
| PyPol | (15-{[(7-Oxyl-3,11-dioxa-7-azadispiro[5.1.5.3]hexadec-15-yl)carbamoyl]amino}-[3,11-dioxa-7-azadispiro[5.1.5.3]hexadec-7-yl])oxidanyl); |
| Ra-Ni | Raney nickel; |
| SDSL | Site-directed spin labeling; |
| TBAF | Tetra-n-butylammonium fluoride; |
| TBAHS | Tetrabutylammonium hydrogen sulfate; |
| TBDMS | tert-Butyldimethylsilyl; |
| TBTA | Tris((1-benzyl-4-triazolyl)methyl)amine; |
| TEA | Triethylamine; |
| TEKPOL | Bis-phenylcyclohexyl-TEMPO-bis-ketal; |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxyl; |
| TEMPOL | 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl; |
| TEMPON | 2,2,6,6-Tetramethyl-4-oxypiperidine-1-oxyl; |
| TMEDA | N,N,N′,N′-Tetramethylethylenediamine; |
| TMG | 1,1,3,3-Tetramethylguanidine; |
| TMP | 2,2,6,6-Tetramethylpiperidine; |
| TMSCN | Trimethylsilyl cyanide; |
| TMSOTf | Trimethylsilyl trifluoromethanesulfonate, CF3SO3SiMe3; |
| TMSSPh | Trimethyl(phenylthio)silane, PhS-SiMe3; |
| TOAC | 2,2,6,6-Tetramethylpiperidine-1-oxyl-4-amino-4-carboxylic acid; |
| TosMIC | Toluenesulfonylmethyl isocyanide; |
| UHP | Urea hydrogen peroxide |
References
- Lebedev, O.L.; Kazarnovskii, S.N. Catalytic oxidation of aliphatic amines with hydrogen peroxide. Zhur. Obshch. Khim. 1960, 30, 1631–1635, Chem. Abstr. 1961, 55, 7792. [Google Scholar]
- Likhtenshtein, G.I. Nitroxides: Brief History, Fundamentals, and Recent Developments; Springer Nature: Cham, Switzerland, 2020; pp. 1–316. [Google Scholar] [CrossRef]
- Likhtenshtein, G.I.; Yamauchi, J.; Nakatsuji, S.; Smirnov, A.I.; Tamura, R. Nitroxides: Applications in Chemistry, Biomedicine and Material Science; Wiley: Weinheim, Germany, 2008; pp. 1–419. [Google Scholar]
- Kokorin, A.I. Nitroxides-Theory, Experiment and Applications; IntechOpen: Rijeka, Croatia, 2012; pp. 1–436. [Google Scholar] [CrossRef]
- Karoui, H.; Le Moigne, F.; Ouari, O.; Tordo, P. Nitroxide Radicals: Properties, Synthesis and Applications. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Hicks, R.G., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 173–229. [Google Scholar] [CrossRef]
- Brückner, C. Nitroxide-Catalyzed Alcohol Oxidations in Organic Synthesis. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Hicks, R.G., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 433–460. [Google Scholar] [CrossRef]
- Hansen, K.-A.; Blinco, J.P. Nitroxide radical polymers-a versatile material class for high-tech applications. Polym. Chem. 2018, 9, 1479–1516. [Google Scholar] [CrossRef]
- Tretyakov, E.V.; Ovcharenko, V.I. The chemistry of nitroxide radicals in the molecular design of magnets. Russ. Chem. Rev. 2009, 78, 971–1012. [Google Scholar] [CrossRef]
- Oyaizu, K.; Nishide, N. Polyradicals in Batteries. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Tamura, R.; Uchida, Y.; Suzuki, K. Magnetic Properties of Organic Radical Liquid Crystals and Metallomesogens. In Handbook of Liquid Crystals: 8 Volume Set, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H.F., Raynes, P., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014; Volume 8, Ch. 28; pp. 1–28. [Google Scholar] [CrossRef]
- Tebben, L.; Studer, A. Nitroxides: Applications in Synthesis and in Polymer Chemistry. Angew. Chem. Int. Ed. 2011, 50, 5034–5068. [Google Scholar] [CrossRef] [PubMed]
- Sciannamea, V.; Jerome, R.; Detrembleur, C. In-situ nitroxide-mediated radical polymerization (NMP) processes: Their understanding and optimization. Chem. Rev. 2008, 108, 1104–1126. [Google Scholar] [CrossRef] [PubMed]
- Bagryanskaya, E.G.; Marque, S.R.A. Scavenging of Organic C-Centered Radicals by Nitroxides. Chem. Rev. 2014, 114, 5011–5056. [Google Scholar] [CrossRef] [PubMed]
- Edeleva, M.V.; Marque, S.R.A.; Bagryanskaya, E.G. Imidazoline and imidazolidine nitroxides as controlling agents in nitroxide-mediated pseudoliving radical polymerization. Russ. Chem. Rev. 2018, 87, 328–349. [Google Scholar] [CrossRef]
- Khramtsov, V.V.; Zweier, J.L. Functional in vivo EPR Spectroscopy and Imaging Using Nitroxide and Trityl Radicals. In Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds; Hicks, R.G., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 537–566. [Google Scholar] [CrossRef]
- Bardelang, D.; Hardy, M.; Ouari, O.; Tordo, P. Spin Labels and Spin Probes. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Haugland, M.M.; Lovett, J.E.; Anderson, E.A. Advances in the synthesis of nitroxide radicals for use in biomolecule spin labelling. Chem. Soc. Rev. 2018, 47, 668–680. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as Antioxidants and Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef]
- Soule, B.P.; Hyodo, F.; Matsumoto, K.; Simone, N.L.; Cook, J.A.; Krishna, M.C.; Mitchell, J.B. The chemistry and biology of nitroxide compounds. Free Rad. Biol. Med. 2007, 42, 1632–1650. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pearlman, A. Chemistry and Antihypertensive Effects of Tempol and Other Nitroxides. Pharmacol. Rev. 2008, 60, 418–469. [Google Scholar] [CrossRef] [PubMed]
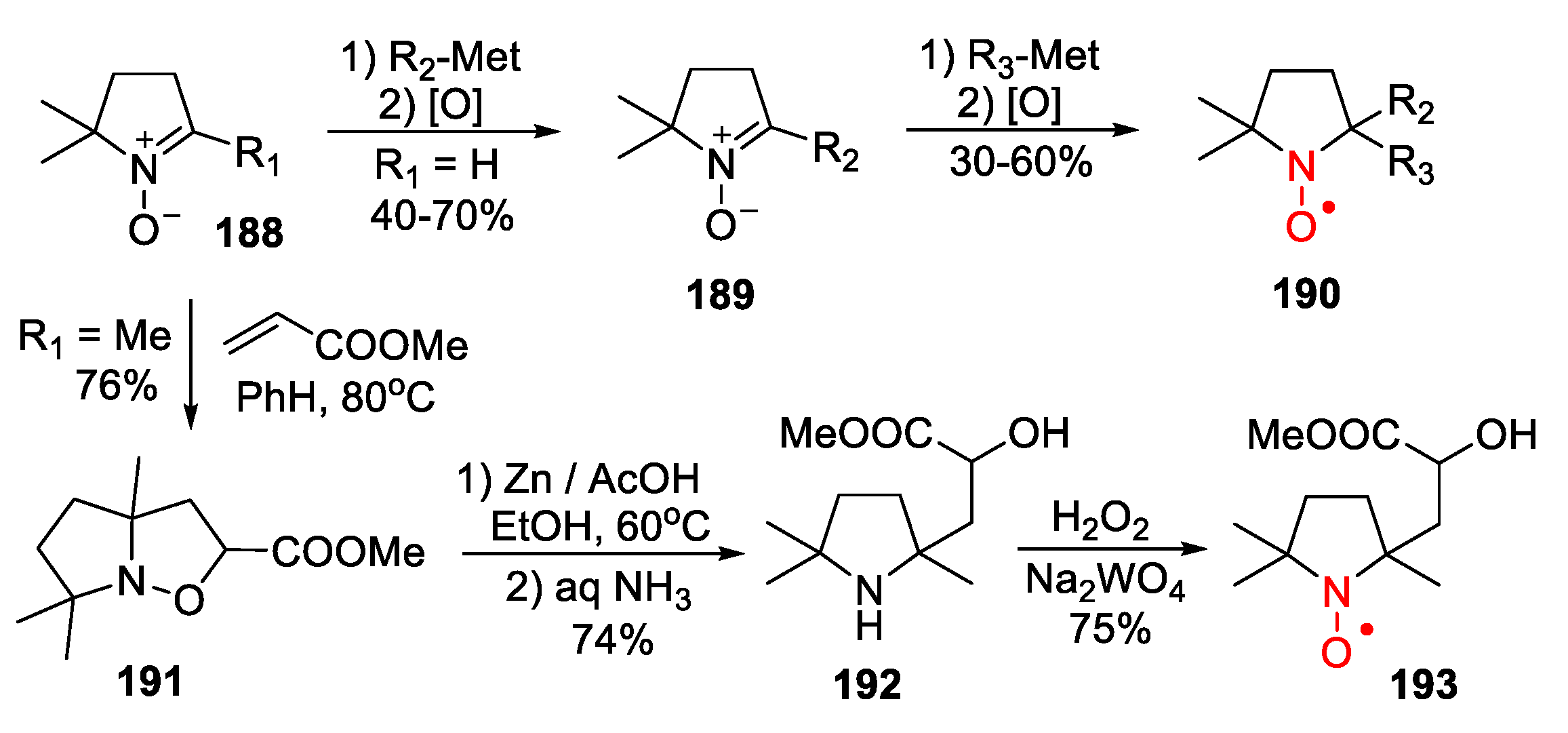
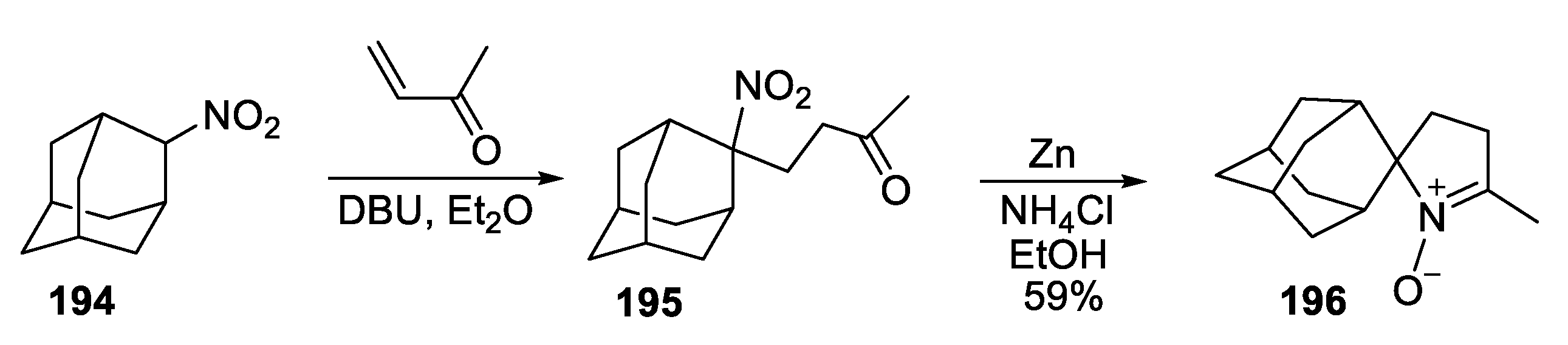
- Kirilyuk, I.A.; Polienko, Y.F.; Krumkacheva, O.A.; Strizhakov, R.K.; Gatilov, Y.V.; Grigor’ev, I.A.; Bagryanskaya, E.G. Synthesis of 2,5-Bis(spirocyclohexane)-Substituted Nitroxides of Pyrroline and Pyrrolidine series, Including Thiol-Specific Spin Label: An Analogue of MTSSL with Long Relaxation Time. J. Org. Chem. 2012, 77, 8016–8027. [Google Scholar] [CrossRef] [PubMed]
- Sar, C.P.; Osz, E.; Jeko, J.; Hideg, K. Synthesis of Spiro[pyrrolidine-2,2‘-adamantane]Nitrones and Nitroxides. Synthesis 2005, 255–259. [Google Scholar] [CrossRef]
- Morozov, D.A.; Kirilyuk, I.A.; Komarov, D.A.; Goti, A.; Bagryanskaya, I.Y.; Kuratieva, N.V.; Grigor’ev, I.A. Synthesis of a Chiral C2-Symmetric Sterically Hindered Pyrrolidine Nitroxide Radical via Combined Iterative Nucleophilic Additions and Intramolecular 1,3- Dipolar Cycloadditions to Cyclic Nitrones. J. Org. Chem. 2012, 77, 10688–10698. [Google Scholar] [CrossRef] [PubMed]
- Rajca, A.; Kathirvelu, V.; Roy, S.K.; Pink, M.; Rajca, S.; Sarkar, S.; Eaton, S.S.; Eaton, G.R. A Spirocyclohexyl Nitroxide Amino Acid Spin Label for Pulsed EPR Spectroscopy Distance Measurements. Chem. Eur. J. 2010, 16, 5778–5782. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.F.W.; Norton, R.S.; Morello, M.; Van Engen, D.; Clardy, J. Mononitroxides and Proximate Dinitroxides Derived by Oxidation of 2,2,4,4,5,5- Hexasubstituted Imidazolidines. A New Series of Nitroxide and Dinitroxide Spin Labels. J. Am. Chem. Soc. 1978, 100, 934–937. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Acarregui, M.J.; Boyle, S.L.M. 2,2-Disubstituted-4,4-dimethylimidazolidinyl-3-oxy Nitroxides: Indicators of Aqueous Acidity through Variation of aN with pH. J. Am. Chem. Soc. 1982, 104, 827–830. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Prabhu, V.S.; Shen, D.K. Synthesis of Spiro Heterocyclic Nitroxides Derived from 4-Piperidone. J. Org. Chem. 1988, 53, 2365–2367. [Google Scholar] [CrossRef]
- Okazaki, S.; Mannan, A.; Sawai, K.; Masumizu, T.; Miura, Y.; Takeshita, K. Enzymatic reduction–resistant nitroxyl spin probes with spirocyclohexyl rings. Free Rad. Res. 2007, 41, 1069–1077. [Google Scholar] [CrossRef]
- Morozov, D.A.; Kirilyuk, I.A.; Gatilov, Y.V.; Bagryanskaya, I.Y.; Bozhko, J.Y.; Komarov, D.A.; Grigor’ev, I.A. Intramolecular 1,3-Dipolar Cycloaddition of Alkenylnitrones of the 4H-Imidazole Series: Synthesis of a New Nitroxide pH-Sensitive Spin Probe. Synthesis 2010, 343–348. [Google Scholar] [CrossRef]
- Keana, J.F.W.; Keana, S.B.; Beetham, D. New versatile ketone spin label. J. Am. Chem. Soc. 1967, 89, 3055–3056. [Google Scholar] [CrossRef]
- Hubbell, W.L.; McConnell, H.M. Motion of steroid spin labels in membranes. Proc. Natl. Acad. Sci. USA 1969, 63, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Keana, J.W.F.; Dinerstein, R.J. New highly anisotropic dinitroxide ketone spin label. Sensitive probe for membrane structure. J. Am. Chem. Soc. 1971, 93, 2808–2810. [Google Scholar] [CrossRef]
- Michon, P.; Rassat, A. Nitroxides. LVIII. Structure of steroidal spin labels. J. Org. Chem. 1974, 39, 2121–2124. [Google Scholar] [CrossRef] [PubMed]
- Kathirvelu, V.; Smith, C.; Parks, C.; Mannan, M.A.; Miura, Y.; Takeshita, K.; Eaton, S.S.G.; Eaton, R. Relaxation rates for spirocyclohexyl nitroxyl radicals are suitable for interspin distance measurements at temperatures up to about 125 K. Chem. Commun. 2009, 454–456. [Google Scholar] [CrossRef]
- Yu, Z.; Quine, R.W.; Rinard, G.A.; Tseitlin, M.; Elajaili, H.; Kathirvelu, V.; Clouston, L.J.; Boratyński, P.J.; Rajca, A.; Stein, R.; et al. Rapid-scan EPR of immobilized nitroxides. J. Magn. Reson. 2014, 247, 67–71. [Google Scholar] [CrossRef]
- Fielding, A.J.; Concilio, M.G.; Heaven, G.; Hollas, M.A. New Developments in Spin Labels for Pulsed Dipolar EPR. Molecules 2014, 19, 16998–17025. [Google Scholar] [CrossRef]
- Babaylova, E.S.; Ivanov, A.V.; Malygin, A.A.; Vorobjeva, M.A.; Venyaminova, A.G.; Polienko, Y.F.; Kirilyuk, I.A.; Krumkacheva, O.A.; Fedin, M.V.; Karpova, G.G.; et al. A versatile approach for site-directed spin labeling and structural EPR studies of RNAs. Org. Biomol. Chem. 2014, 12, 3129–3136. [Google Scholar] [CrossRef]
- Meyer, V.; Swanson, M.A.; Clouston, L.J.; Boratyński, P.J.; Stein, R.A.; Mchaourab, H.S.; Rajca, A.; Eaton, S.S.; Eaton, G.R. Room-Temperature Distance Measurements of Immobilized Spin-Labeled Protein by DEER/PELDOR. Biophys. J. 2015, 108, 1213–1219. [Google Scholar] [CrossRef][Green Version]
- Bagryanskaya, E.G.; Krumkacheva, O.A.; Fedin, M.V.; Marque, S.R.A. Development and Application of Spin Traps, Spin Probes, and Spin Labels. Methods Enzymol. 2015, 563, 365–396. [Google Scholar] [CrossRef]
- Haugland, M.M.; El-Sagheer, A.H.; Porter, R.J.; Peña, J.; Brown, T.; Anderson, E.A.; Lovett, J.E. 2′-Alkynylnucleotides: A Sequence- and Spin Label-Flexible Strategy for EPR Spectroscopy in DNA. J. Am. Chem. Soc. 2016, 138, 9069–9072. [Google Scholar] [CrossRef] [PubMed]
- Krumkacheva, O.; Bagryanskaya, E. EPR-based distance measurements at ambient temperature. J. Magn. Reson. 2017, 280, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, Y.; Maly, T.; Ouari, O.; Karoui, H.; Le Moigne, F.; Rizatto, E.; Lyubenova, S.; Herzfeld, J.; Prisner, T.; Tordo, P.; et al. Dynamic Nuclear Polarization with a Rigid Biradical. Angew. Chem. Int. Ed. 2009, 48, 4996–5000. [Google Scholar] [CrossRef] [PubMed]
- Ysacco, C.; Rizzato, E.; Virolleaud, M.A.; Karoui, H.; Rockenbauer, A.; Le Moigne, F.; Siri, D.; Ouari, O.; Griffin, R.G.; Tordo, P. Properties of dinitroxides for use in dynamic nuclear polarization. Phys. Chem. Chem. Phys. 2010, 12, 5841–5845. [Google Scholar] [CrossRef]
- Rossini, A.J.; Zagdoun, A.; Lelli, M.; Canivet, J.; Aguado, S.; Ouari, O.; Tordo, P.; Rosay, M.; Maas, W.E.; Copéret, C.; et al. Dynamic Nuclear Polarization Enhanced Solid-State NMR Spectroscopy of Functionalized Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2012, 51, 123–127. [Google Scholar] [CrossRef]
- Zagdoun, A.; Casano, G.; Ouari, O.; Lapadula, G.; Rossini, A.J.; Lelli, M.; Baffert, M.; Gajan, D.; Veyre, L.; Maas, W.E.; et al. A Slowly Relaxing Rigid Biradical for Efficient Dynamic Nuclear Polarization Surface-Enhanced NMR Spectroscopy: Expeditious Characterization of Functional Group Manipulation in Hybrid Materials. J. Am. Chem. Soc. 2012, 134, 2284–2291. [Google Scholar] [CrossRef]
- Dane, E.L.; Corzilius, B.; Rizzato, E.; Stocker, P.; Maly, T.; Smith, A.A.; Griffin, R.G.; Ouari, O.; Tordo, P.; Swager, T.M. Rigid Orthogonal Bis-TEMPO Biradicals with Improved Solubility for Dynamic Nuclear Polarization. J. Org. Chem. 2012, 77, 1789–1797. [Google Scholar] [CrossRef]
- Kiesewetter, M.K.; Corzilius, B.; Smith, A.A.; Griffin, R.G.; Swager, T.M. Dynamic Nuclear Polarization with a Water-Soluble Rigid Biradical. J. Am. Chem. Soc. 2012, 134, 4537–4540. [Google Scholar] [CrossRef]
- Rossini, A.J.; Zagdoun, A.; Hegner, F.; Schwarzwälder, M.; Gajan, D.; Copéret, C.; Lesage, A.; Emsley, L. Dynamic Nuclear Polarization NMR Spectroscopy of Microcrystalline Solids. J. Am. Chem. Soc. 2012, 134, 16899–16908. [Google Scholar] [CrossRef]
- Salnikov, E.S.; Ouari, O.; Koers, E.; Sarrouj, H.; Franks, T.; Rosay, M.; Pawsey, S.; Reiter, C.; Bandara, P.; Oschkinat, H.; et al. Developing DNP/Solid-State NMR Spectroscopy of Oriented Membranes. Appl. Magn. Reson. 2012, 43, 91–106. [Google Scholar] [CrossRef]
- Ysacco, C.; Karoui, H.; Casano, G.; Le Moigne, F.; Combes, S.; Rockenbauer, A.; Rosay, M.; Maas, W.; Ouari, O.; Tordo, P. Dinitroxides for Solid State Dynamic Nuclear Polarization. Appl. Magn. Reson. 2012, 43, 251–261. [Google Scholar] [CrossRef]
- Blanc, F.; Sperrin, L.; Jefferson, D.A.; Pawsey, S.; Rosay, M.; Grey, C.P. Dynamic Nuclear Polarization Enhanced Natural Abundance 17O Spectroscopy. J. Am. Chem. Soc. 2013, 135, 2975–2978. [Google Scholar] [CrossRef] [PubMed]
- Zagdoun, A.; Casano, G.; Ouari, O.; Schwaerzwalder, M.; Rossini, A.J.; Aussenac, F.; Yulikov, M.; Jeschke, G.; Coperet, C.; Lesage, A.; et al. Large Molecular Weight Nitroxide Biradicals Providing Efficient Dynamic Nuclear Polarization at Temperatures up to 200 K. J. Am. Chem. Soc. 2013, 135, 12790–12797. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Akhmetzyanov, D.; Ouari, O.; Denysenkov, V.; Corzilius, B.; Plackmeyer, J.; Tordo, P.; Prisner, T.F.; Glaubitz, C. Host−Guest Complexes as Water-Soluble High-Performance DNP Polarizing Agents. J. Am. Chem. Soc. 2013, 135, 19275–19281. [Google Scholar] [CrossRef] [PubMed]
- Van der Cruijsen, K.E.J.; Sauvée, C.; Hulse, R.E.; Weingarth, M.; Ouari, O.; Perozo, E.; Tordo, P.; Baldus, M. Biomolecular DNP-Supported NMR Spectroscopy using Site-Directed Spin Labeling. Chem. Eur. J. 2015, 21, 12971–12977. [Google Scholar] [CrossRef]
- Salnikov, E.S.; Sarrouj, H.; Reiter, C.; Aisenbrey, C.; Purea, A.; Aussenac, F.; Ouari, O.; Tordo, P.; Fedotenko, I.; Engelke, F.; et al. Solid-State NMR/Dynamic Nuclear Polarization of Polypeptides in Planar Supported Lipid Bilayers. J. Phys. Chem. B 2015, 119, 14574–14583. [Google Scholar] [CrossRef]
- Lelli, M.; Chaudhari, S.R.; Gajan, D.; Casano, G.; Rossini, A.J.; Ouari, O.; Tordo, P.; Lesage, A.; Emsley, L. Solid-State Dynamic Nuclear Polarization at 9.4 and 18.8 T from 100 K to Room Temperature. J. Am. Chem. Soc. 2015, 137, 14558–14561. [Google Scholar] [CrossRef]
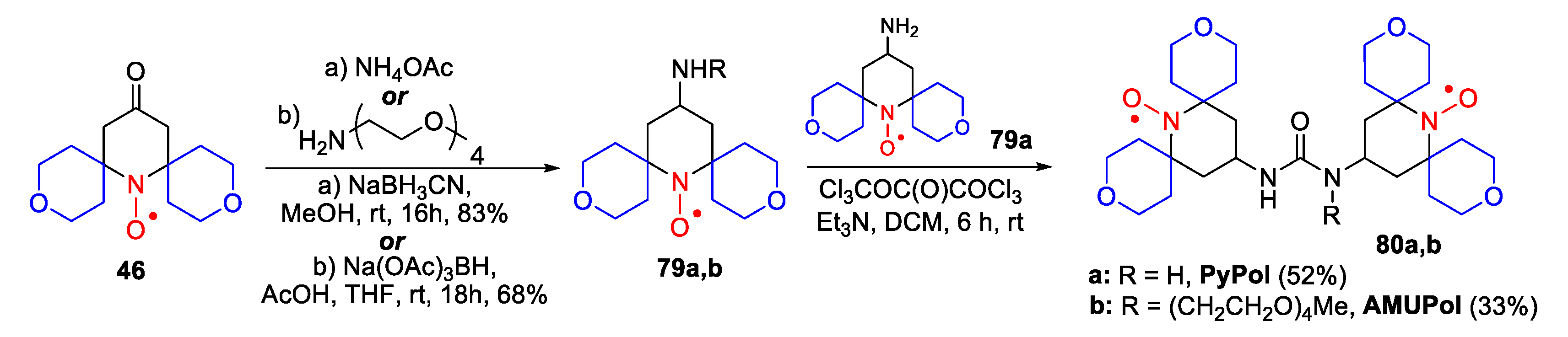
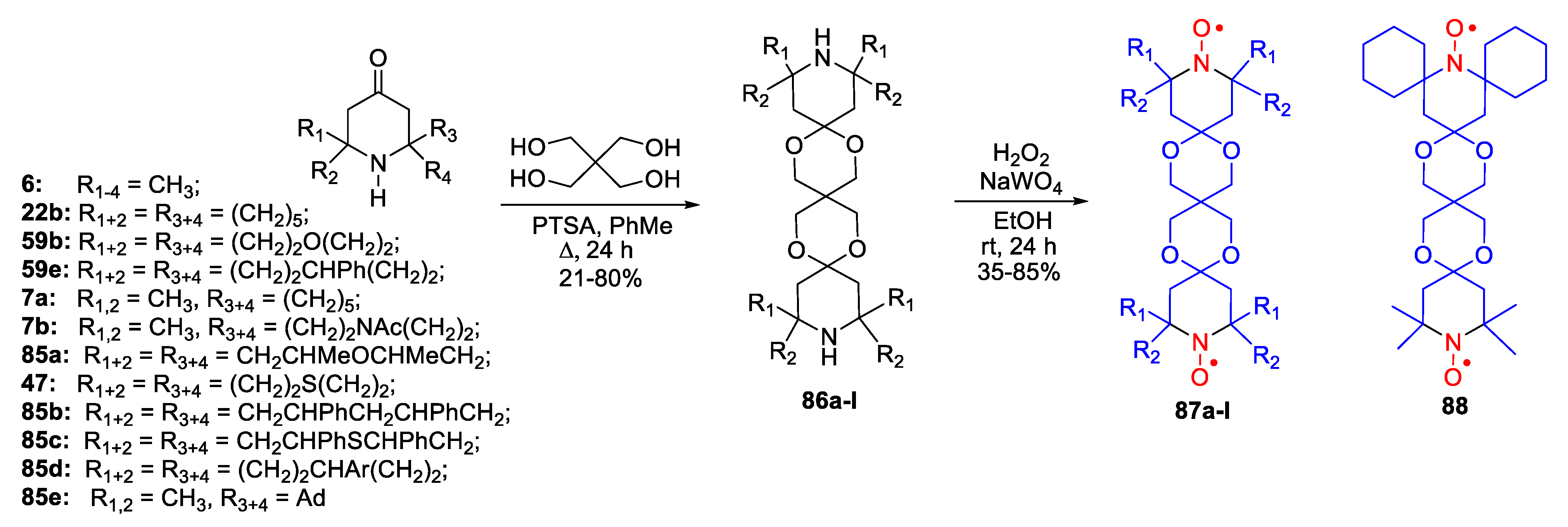
- Sauvée, C.; Casano, G.; Abel, S.; Rockenbauer, A.; Akhmetzyanov, D.; Karoui, H.; Siri, D.; Aussenac, F.; Maas, W.; Weber, R.T.; et al. Tailoring of Polarizing Agents in the bTurea Series for Cross-Effect Dynamic Nuclear Polarization in Aqueous Media. Chem. Eur. J. 2016, 22, 5598–5606. [Google Scholar] [CrossRef]
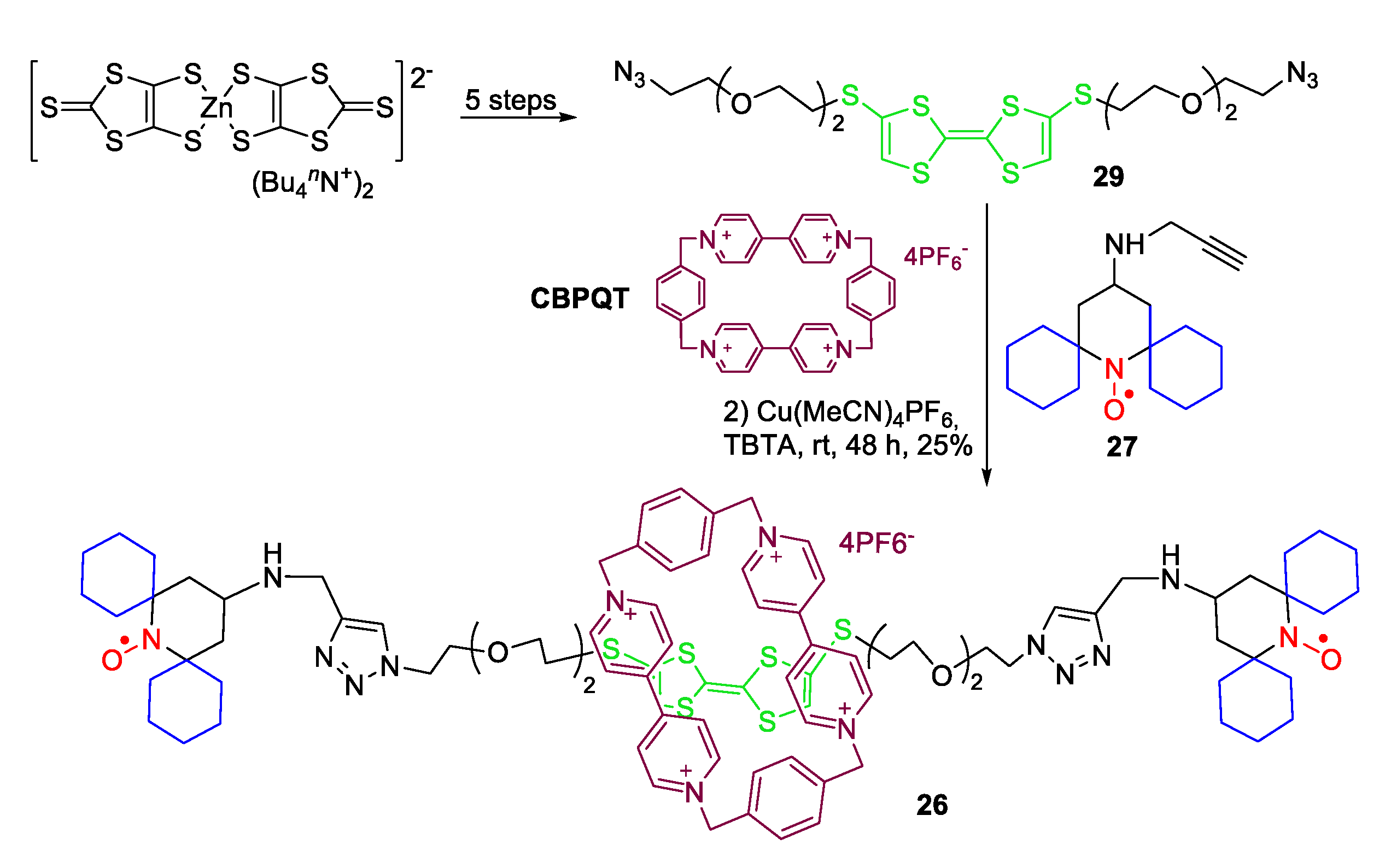
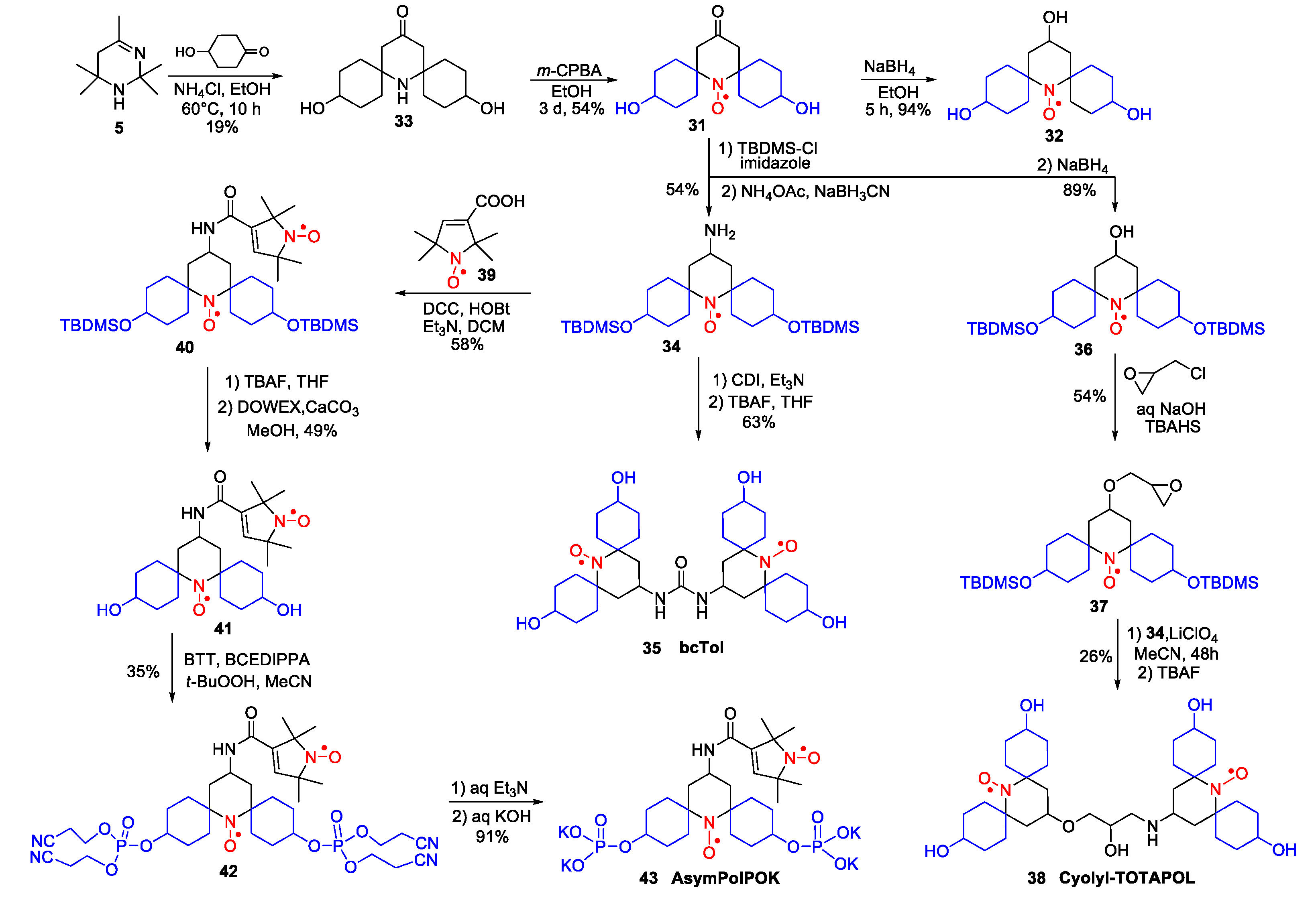
- Jagtap, A.P.; Geiger, M.-A.; Stöppler, D.; Orwick-Rydmark, M.; Oschkinat, H.; Sigurdsson, S.T. bcTol: A Highly Water-Soluble Biradical for Efficient Dynamic Nuclear Polarization of Biomolecules. Chem. Commun. 2016, 52, 7020–7023. [Google Scholar] [CrossRef]
- Kubicki, D.J.; Casano, G.; Schwarzwälder, M.; Abel, S.; Sauvée, C.; Ganesan, K.; Yulikov, M.; Rossini, A.J.; Jeschke, G.; Copéret, C.; et al. Rational design of dinitroxide biradicals for efficient cross-effect dynamic nuclear polarization. Chem. Sci. 2016, 7, 550–558. [Google Scholar] [CrossRef]
- Mentink-Vigier, F.; Marin-Montesinos, I.; Jagtap, A.P.; Halbritter, T.; van Tol, J.; Hediger, S.; Lee, D.; Sigurdsson, S.T.; De Paëpe, G. Computationally Assisted Design of Polarizing Agents for Dynamic Nuclear Polarization Enhanced NMR: The AsymPol Family. J. Am. Chem. Soc. 2018, 140, 11013–11019. [Google Scholar] [CrossRef] [PubMed]
- Wisser, D.; Karthikeyan, G.; Lund, A.; Casano, G.; Karoui, H.; Yulikov, M.; Menzildjian, G.; Pinon, A.C.; Purea, A.; Engelke, F.; et al. BDPA-Nitroxide Biradicals Tailored for Efficient Dynamic Nuclear Polarization Enhanced Solid-State NMR at Magnetic Fields up to 21.1 T. J. Am. Chem. Soc. 2018, 140, 13340–13349. [Google Scholar] [CrossRef] [PubMed]
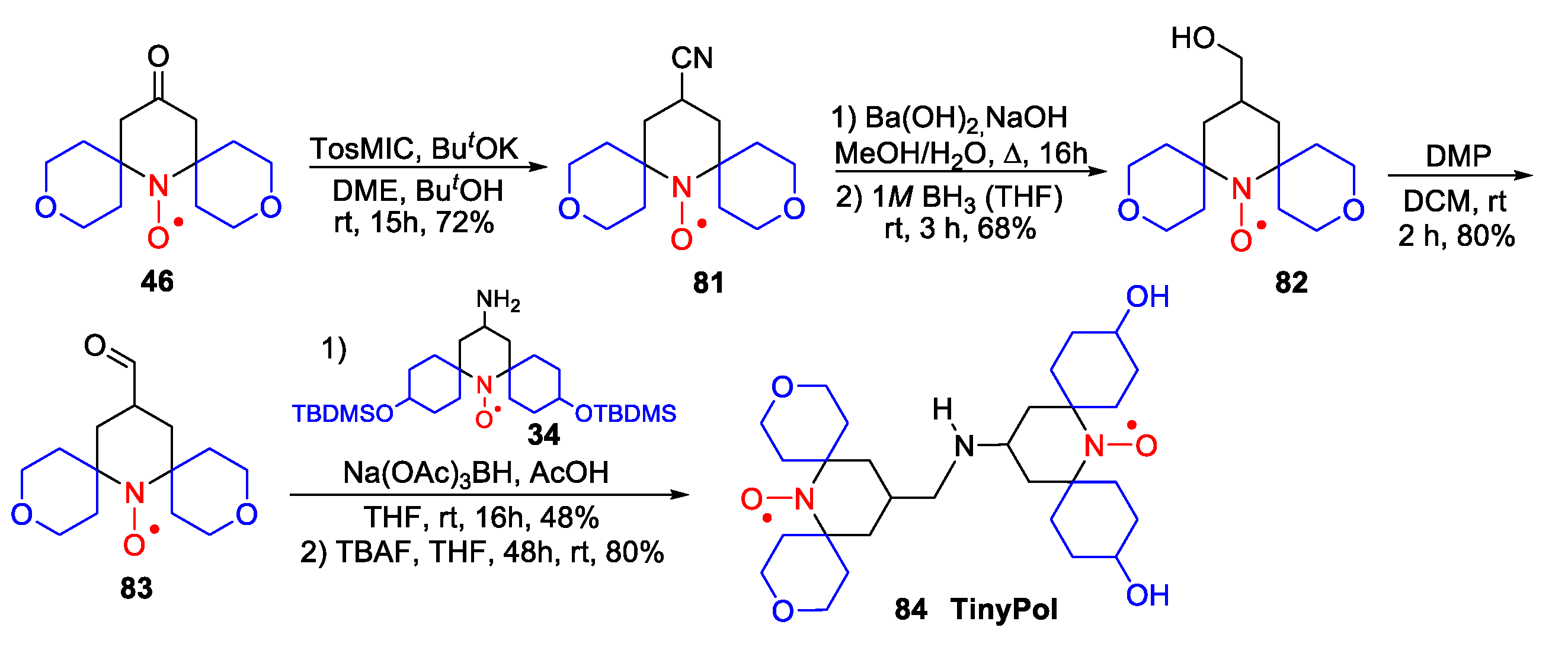
- Lund, A.; Casano, G.; Menzildjian, G.; Kaushik, M.; Stevanato, G.; Yulikov, M.; Jabbour, R.; Wisser, D.; Renom-Carrasco, M.; Thieuleux, C.; et al. TinyPols: A family of water-soluble binitroxides tailored for dynamic nuclear polarization enhanced NMR spectroscopy at 18.8 and 21.1 T. Chem. Sci. 2020, 11, 2810–2818. [Google Scholar] [CrossRef]
- Stevanato, G.; Casano, G.; Kubicki, D.J.; Rao, Y.; Hofer, L.E.; Menzildjian, G.; Karoui, H.; Siri, D.; Cordova, M.; Yulikov, M.; et al. Open and Closed Radicals: Local Geometry Around Unpaired Electrons Governs MAS DNP Performance. J. Am. Chem. Soc. 2020, 142, 16587–16599. [Google Scholar] [CrossRef] [PubMed]
- Mentink-Vigier, F. Optimizing nitroxide biradicals for cross-effect MAS-DNP: The role of g-tensors’ distance. Phys. Chem. Chem. Phys. 2020, 22, 3643–3652. [Google Scholar] [CrossRef] [PubMed]
- Berruyer, P.; Björgvinsdóttir, S.; Bertarello, A.; Stevanato, G.; Rao, Y.; Karthikeyan, G.; Casano, G.; Ouari, O.; Lelli, M.; Reiter, C.; et al. Dynamic Nuclear Polarization Enhancement of 200 at 21.15 T Enabled by 65 kHz Magic Angle Spinning. J. Phys. Chem. Lett. 2020, 11, 8386–8391. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Mibae, S.; Moto, H.; Nakamura, N.; Yamada, B. Controlled radical polymerization of styrene with an oxazolidinyl-N-oxyl stable free radical. Polym. Bull. 1999, 42, 17–24. [Google Scholar] [CrossRef]
- Miura, Y.; Nakamura, N.; Taniguchi, I. Low-Temperature “Living” Radical Polymerization of Styrene in the Presence of Nitroxides with Spiro Structures. Macromolecules 2001, 34, 447–455. [Google Scholar] [CrossRef]
- Huang, W.; Chiarelli, R.; Charleux, B.; Rassat, A.; Vairon, J.-P. Unique Behavior of Nitroxide Biradicals in the Controlled Radical Polymerization of Styrene. Macromolecules 2002, 35, 2305–2317. [Google Scholar] [CrossRef]
- Miura, Y.; Ichikawa, A.; Taniguchi, I. ‘Living’ radical polymerization of styrene mediated by spiro ring-substituted piperidinyl-N-oxyl radicals. The effect of the spiro rings on the control of polymerization. Polymer 2003, 44, 5187–5194. [Google Scholar] [CrossRef]
- Aldabbagh, F.; Dervan, P.; Phelan, M.; Gilligan, K.; Cunningham, D.; McArdle, P.; Zetterlund, P.B.; Yamada, B. Influence of Nitroxide Structure on the 2,5- and 2,6-Spirodicyclohexyl Substituted Cyclic Nitroxide-Mediated Free-Radical Polymerization of Styrene. J. Polym. Sci. A Polym. Chem. 2003, 41, 3892–3900. [Google Scholar] [CrossRef]
- Mannan, A.; Ichikawa, A.; Miura, Y. Living radical polymerization of styrene mediated by a piperidinyl-N-oxyl radical having very bulky substituents. Polymer 2007, 48, 743–749. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Parkhomenko, D.A.; Morozov, D.A.; Dobrynin, S.A.; Trofimov, D.G.; Kanagatov, B.; Kirilyuk, I.A.; Bagryanskaya, E.G. Controlled/living polymerization of methyl methacrylate using new sterically hindered imidazoline nitroxides prepared via intramolecular 1,3-dipolar cycloaddition reaction. J. Polym. Sci. A Polym. Chem. 2014, 52, 929–943. [Google Scholar] [CrossRef]
- Jing, Y.; Mardyukov, A.; Bergander, K.; Daniliuc, C.G.; Studer, A. Synthesis of Bulky Nitroxides, Characterization, and Their Application in Controlled Radical Polymerization. Macromolecules 2014, 47, 3595–3602. [Google Scholar] [CrossRef]
- Jing, Y.; Tesch, M.; Wang, L.; Daniliuc, S.G.; Studer, A. Synthesis of a bulky nitroxide and its application in the nitroxide mediated radical polymerization. Tetrahedron 2016, 72, 7665–7671. [Google Scholar] [CrossRef]
- Zaremski, M.Y.; Odintsova, V.V. Kinetic Features of Elementary Events in the Radical Polymerization of Methyl Methacrylate under Conditions of Nitroxide-Mediated Reversible Inhibition. Polymer Sci. Ser. B 2020, 62, 1–13. [Google Scholar] [CrossRef]
- Ma, Z.; Huang, Q.; Bobbitt, J.M. Oxoammonium salts. 5. A new synthesis of hindered piperidines leading to unsymmetrical TEMPO-type nitroxides. Synthesis and enantioselective oxidations with chiral nitroxides and chiral oxoammonium salts. J. Org. Chem. 1993, 58, 4837–4843. [Google Scholar] [CrossRef]
- Nesvadba, P.; Bugnon, L.; Maire, P.; Noval, P. Synthesis of A Novel Spirobisnitroxide Polymer and its Evaluation in an Organic Radical Battery. Chem. Mater. 2010, 22, 783–788. [Google Scholar] [CrossRef]
- Ishida, T.; Ooishi, M.; Ishii, N.; Mori, H.; Nogami, T. Mono- and dinitroxide radicals from 9,9′(10H,10H’)-spirobiacridine: An approach to a D2d triplet biradical. Polyhedron 2007, 26, 1793–1799. [Google Scholar] [CrossRef]
- Kanetomo, T.; Ichihashi, K.; Enomoto, M.; Ishi da, T. Ground Triplet Spirobiradical: 2,2′,7,7′-Tetra(tert-butyl)-9,9′(10H,10′H)-spirobiacridine-10,10′-dioxyl. Org. Lett. 2019, 21, 3909–3912. [Google Scholar] [CrossRef]
- Rajca, A.; Takahashi, M.; Pink, M.; Spagnol, G.; Rajca, S. Conformationally Constrained, Stable, Triplet Ground State (S = 1) Nitroxide Diradicals. Antiferromagnetic Chains of S = 1 Diradicals. J. Am. Chem. Soc. 2007, 129, 10159–10170. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Uchida, Y.; Tamura, R. Spin Symmetry Breaking: Superparamagnetic and Spin Glass-Like Behavior Observed in Rod-Like Liquid Crystalline Organic Compounds Contacting Nitroxide Radical Spins. Symmetry 2020, 12, 1910. [Google Scholar] [CrossRef]
- Rajca, A.; Wang, Y.; Boska, M.; Paletta, J.T.; Olankitwanit, A.; Swanson, M.A.; Mitchell, D.G.; Eaton, S.S.; Eaton, G.R.; Rajca, S. Organic Radical Contrast Agents for Magnetic Resonance Imaging. J. Am. Chem. Soc. 2012, 134, 15724–15727. [Google Scholar] [CrossRef]
- Paletta, J.T.; Pink, M.; Foley, B.; Rajca, S.; Rajca, A. Synthesis and Reduction Kinetics of Sterically Shielded Pyrrolidine Nitroxides. Org. Lett. 2012, 14, 5322–5325. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.A.; McCombs, J.R.; Wang, Y.; Paletta, J.T.; Morton, S.W.; Dreaden, E.C.; Boska, M.D.; Ottaviani, M.F.; Hammond, P.T.; Rajca, A.; et al. Redox-responsive branched-bottlebrush polymers for in vivo MRI and fluorescence imaging. Nat. Commun. 2014, 5, 5460. [Google Scholar] [CrossRef]
- Nguyen, H.V.-T.; Chen, Q.; Paletta, J.T.; Harvey, P.; Jiang, Y.; Zhang, H.; Boska, M.D.; Ottaviani, M.F.; Jasanoff, A.; Rajca, A.; et al. Nitroxide-Based Macromolecular Contrast Agents with Unprecedented Transverse Relaxivity and Stability for Magnetic Resonance Imaging of Tumors. ACS Cent. Sci. 2017, 3, 800–811. [Google Scholar] [CrossRef]
- Nguyen, H.V.-T.; Detappe, A.; Gallagher, N.; Zhang, H.; Harvey, P.; Yan, C.; Mathieu, C.; Golder, M.R.; Jiang, Y.; Ottaviani, M.F.; et al. Triply Loaded Nitroxide Brush-Arm Star Polymers Enable Metal-Free Millimetric Tumor Detection by Magnetic Resonance Imaging. ACS Nano 2018, 12, 11343–11354. [Google Scholar] [CrossRef]
- Akakuru, O.U.; Iqbal, M.Z.; Saeed, M.; Liu, C.; Paunesku, T.; Woloschak, G.; Hosmane, N.S.; Wu, A. The Transition from Metal-Based to Metal-Free Contrast Agents for T1 Magnetic Resonance Imaging Enhancement. Bioconjugate Chem. 2019, 30, 2264–2286. [Google Scholar] [CrossRef]
- Shiraishi, R.; Matsumoto, S.; Fuchi, Y.; Naganuma, T.; Yoshihara, D.; Usui, K.; Yamada, K.; Karasawa, S. Characterization and Water-Proton Longitudinal Relaxivities of Liposome-Type Radical Nanoparticles Prepared via a Supramolecular Approach. Langmuir 2020, 36, 5280–5286. [Google Scholar] [CrossRef]
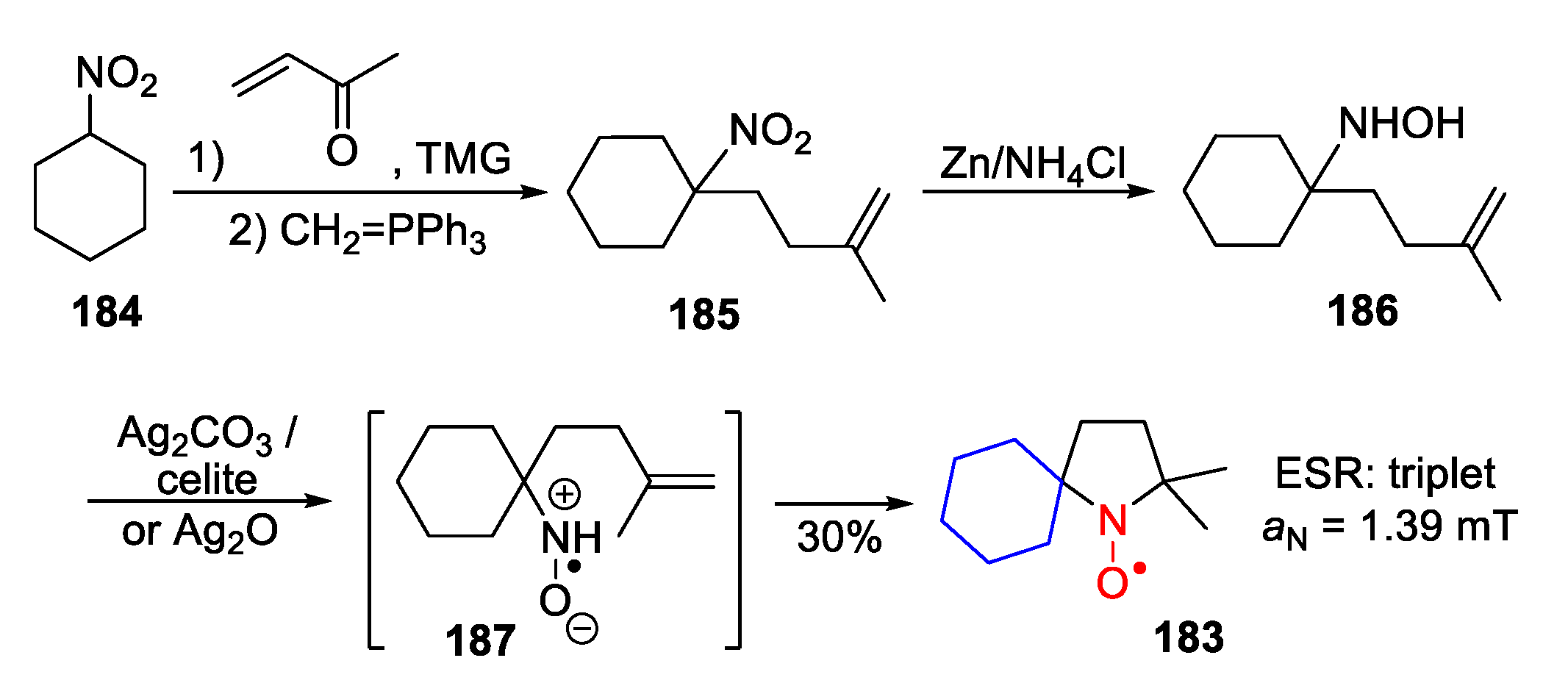
- Nguyen, H.V.-T.; Detappe, A.; Harvey, P.; Gallagher, N.; Mathieu, C.; Agius, M.P.; Zavidij, O.; Wang, W.; Jiang, Y.; Rajca, A.; et al. Pro-organic radical contrast agents (“pro-ORCAs”) for real-time MRI of pro-drug activation in biological systems. Polym. Chem. 2020, 11, 4768–4779. [Google Scholar] [CrossRef]
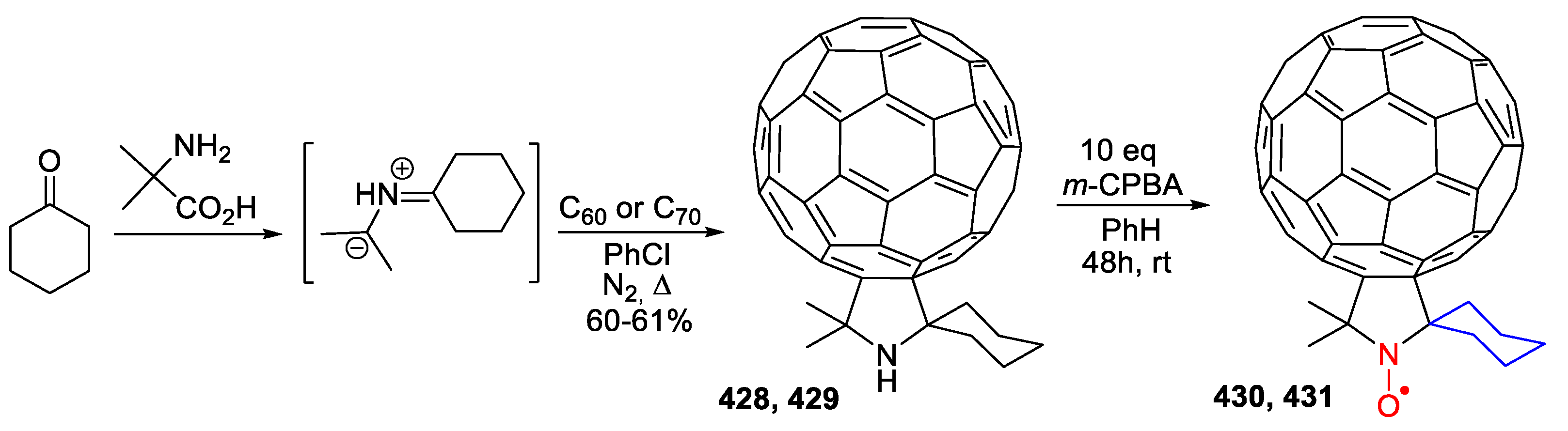
- Bardelang, D.; Giorgi, M.; Pardanaud, C.; Hornebecq, V.; Rizzato, E.; Tordo, P.; Ouari, O. Organic multishell isostructural host–guest crystals: Fullerenes C60 inside a nitroxide open framework. Chem. Commun. 2013, 49, 3519–3521. [Google Scholar] [CrossRef] [PubMed]
- Bardelang, D.; Giorgi, M.; Hornebecq, V.; Stepanov, A.; Hardy, M.; Rizzato, E.; Monnier, V.; Zaman, B.; Chan, G.; Udachin, K.; et al. Hosting Various Guests Including Fullerenes and Free Radicals in Versatile Organic Paramagnetic bTbk Open Frameworks. Cryst. Growth Des. 2014, 14, 467–476. [Google Scholar] [CrossRef]
- Manoni, R.; Romano, F.; Casati, C.; Franchi, P.; Mezzina, E.; Lucarini, M. Synthesis and characterization of spin-labelled [2]rotaxanes containing tetrathiafulvalene and 1,5-dioxynaphthalene molecular stations. Org. Chem. Front. 2014, 1, 477–483. [Google Scholar] [CrossRef]
- Ayhan, M.M.; Casano, G.; Karoui, H.; Rockenbauer, A.; Monnier, V.; Hardy, M.; Tordo, P.; Bardelang, D.; Ouari, O. EPR Studies of the Binding Properties, Guest Dynamics, and Inner-Space Dimensions of a Water-Soluble Resorcinarene Capsule. Chem. Eur. J. 2015, 21, 16404–16410. [Google Scholar] [CrossRef]
- Casano, G.; Poulhès, F.; Tran, T.K.; Ayhan, M.M.; Karoui, H.; Siri, D.; Gaudel-Siri, A.; Rockenbauer, A.; Jeschke, G.; Bardelang, D.; et al. High binding yet accelerated guest rotation within a cucurbit[7]uril complex. Toward paramagnetic gyroscopes and rolling nanomachines. Nanoscale 2015, 7, 12143–12150. [Google Scholar] [CrossRef]
- Bleve, V.; Franchi, P.; Gualandi, L.; Romano, F.; Mezzina, E.; Lucarini, M. Synthesis and characterization of a doubly spin-labelled electrochemically driven molecular shuttle. Org. Chem. Front. 2018, 5, 1579–1585. [Google Scholar] [CrossRef]
- Ouari, O.; Bardelang, D. Nitroxide Radicals with Cucurbit[n]urils and Other Cavitands (Review). Isr. J. Chem. 2018, 58, 343–356. [Google Scholar] [CrossRef]
- Combes, S.; Tran, K.T.; Ayhan, M.M.; Karoui, H.; Rockenbauer, A.; Tonetto, A.; Monnier, V.; Charles, L.; Rosas, R.; Viel, S.; et al. Triangular Regulation of Cucurbit[8]uril 1:1 Complexes. J. Am. Chem. Soc. 2019, 141, 5897–5907. [Google Scholar] [CrossRef]
- Liu, F.; Karoui, H.; Rockenbauer, A.; Liu, S.; Ouari, O.; Bardelang, D. EPR Spectroscopy: A Powerful Tool to Analyze Supramolecular Host•Guest Complexes of Stable Radicals with Cucurbiturils. Molecules 2020, 25, 776. [Google Scholar] [CrossRef]
- Lucarini, M. Improving Spin Probe Methodologies to Investigate Supramolecular Assemblies (Minireview). Eur. J. Org. Chem. 2020, 2995–3008. [Google Scholar] [CrossRef]
- Carr, S.G.; Khoo, S.K.; Luckhurst, G.R.; Zannoni, C. On the Ordering Matrix for the Spin Probe (3-spiro[2′-N-oxyl-3′,3′-dimethyloxazolidine])-5α-cholestane, in the Nematic Mesophase of 4,4′-dimethoxyazoxybenzene. Mol. Cryst. Liq. Cryst. 1976, 35, 7–13. [Google Scholar] [CrossRef]
- Chumakova, N.A.; Yankova, T.S.; Fairfull-Smith, K.E.; Bottle, S.E.; Vorobiev, A.K. Molecular Orientational Order of Nitroxide Radicals in Liquid Crystalline Media. J. Phys. Chem. B 2014, 118, 5589–5599. [Google Scholar] [CrossRef]
- Bogdanov, A.V.; Tamura, R.; Vorobiev, A.K. Novel nitroxide biradical probe with spiro-fused rigid core for EPR determination of rotational mobility and orientational order of soft materials. Chem. Phys. Lett. 2020, 749, 137432. [Google Scholar] [CrossRef]
- Chumakova, N.A.; Vorobiev, A.K. Orientation Distribution of Molecules: Characterization and Experimental Determination by Means of Magnetic Resonance. Appl. Magn. Reson. 2020. [Google Scholar] [CrossRef]
- Keana, J.F.W. Newer aspects of the synthesis and chemistry of nitroxide spin labels. Chem. Rev. 1978, 78, 37–64. [Google Scholar] [CrossRef]
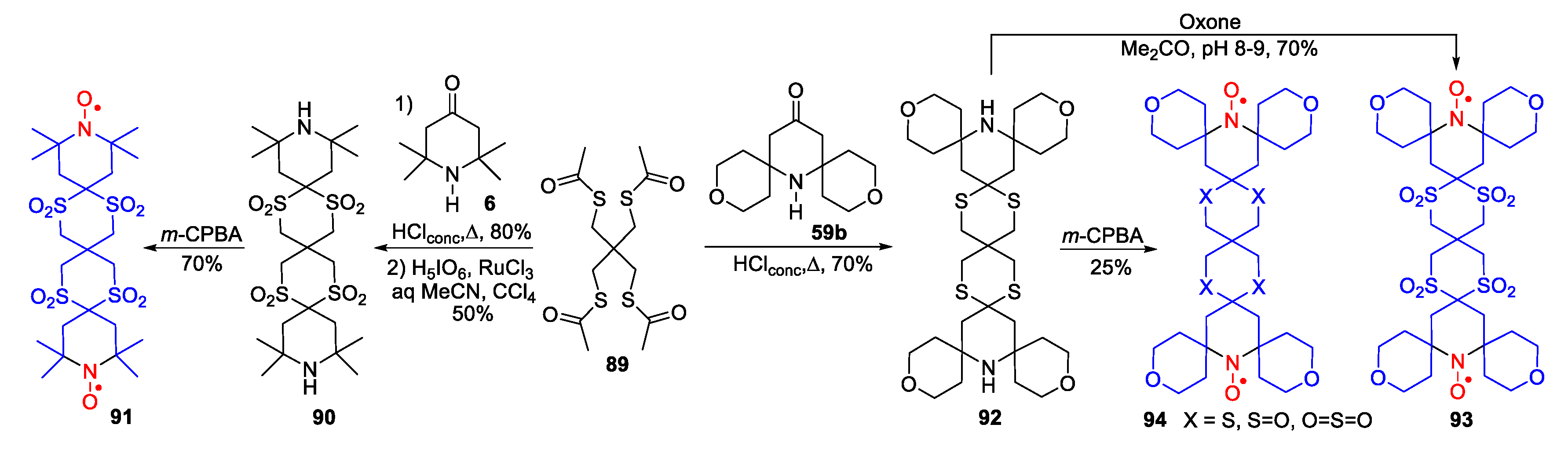
- Brik, M.E. Oxidation of secondary amines to nitroxides with oxone in aqueous buffered solution. Tetrahedron Lett. 1995, 36, 5519–5522. [Google Scholar] [CrossRef]
- Murray, R.W.; Iyanar, K. Nitroxide Synthesis Using the Methyltrioxorhenium/Hydrogen Peroxide System. Heteroatom Chem. 1998, 9, 347–350. [Google Scholar] [CrossRef]
- Geiger, M.-A.; Jagtap, A.P.; Kaushik, M.; Sun, H.; Stöppler, D.; Sigurdsson, S.T.; Corzilius, B.; Oschkinat, H. Efficiency of Water-Soluble Nitroxide Biradicals for Dynamic Nuclear Polarization in Rotating Solids at 9.4 T: bcTol-M and cyolyl-TOTAPOL as New Polarizing Agents. Chem. Eur. J. 2018, 24, 13485–13494. [Google Scholar] [CrossRef]
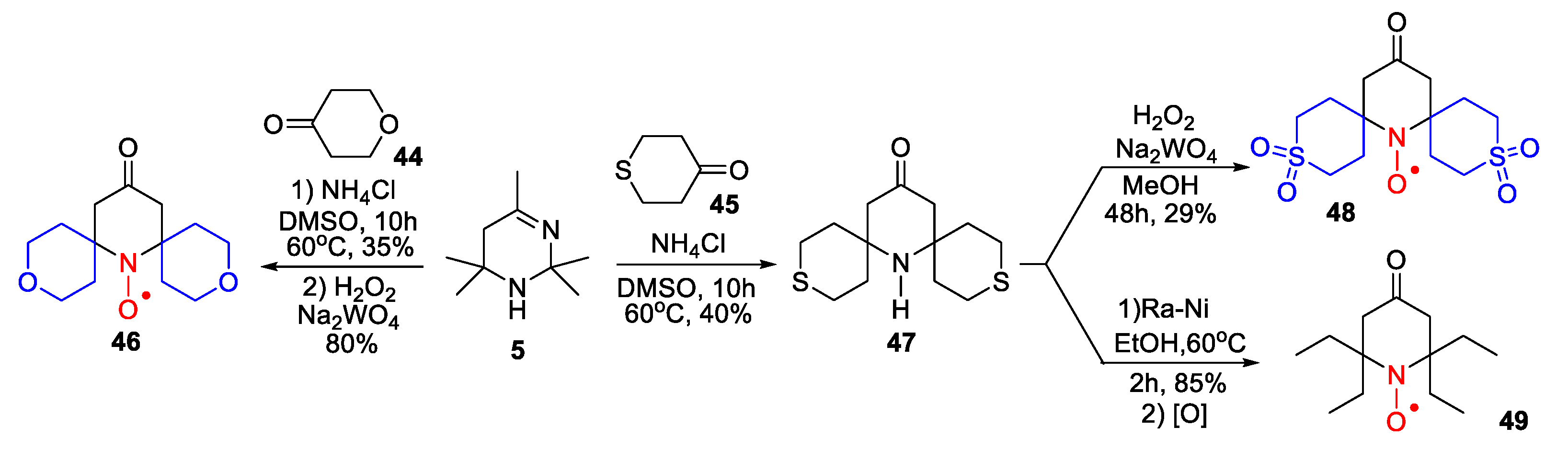
- Kinoshita, Y.; Yamada, K.-I.; Yamasaki, T.; Sadasue, H.; Sakai, K.; Utsumi, H. Development of novel nitroxyl radicals for controlling reactivity with ascorbic acid. Free Rad. Res. 2009, 43, 565–571. [Google Scholar] [CrossRef]
- Sakai, K.; Yamada, K.; Yamasaki, T.; Kinoshita, Y.; Mito, F.; Utsumi, H. Effective 2,6-substitution of piperidine nitroxyl radical by carbonyl compound. Tetrahedron 2010, 66, 2311–2315. [Google Scholar] [CrossRef]
- Yoshioka, T.; Higashida, S.; Murayama, K. Studies on Stable Free Radicals. VIII. The Synthesis and Oxidation of Hindered 4-Oxopiperidine Derivatives. Bull. Chem. Soc. Jpn. 1972, 45, 636–638. [Google Scholar] [CrossRef]
- Drahl, M.A.; Manpadi, M.; Williams, L.J. C-C Fragmentation: Origins and Recent Applications. Angew. Chem. Int. Ed. 2013, 52, 11222–11251. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T.; Mito, F.; Ito, Y.; Pandian, S.; Kinoshita, Y.; Nakano, K.; Murugesan, R.; Sakai, K.; Utsumi, H.; Yamada, K. Structure–Reactivity Relationship of Piperidine Nitroxide: Electrochemical, ESR and Computational Studies. J. Org. Chem. 2011, 76, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Rassat, A.; Rey, P. XXIII–Preparation d‘amino acides radicalaires et de leurs sels complexes. Bull. Chem. Soc. Fr. 1967, 3, 815–818. [Google Scholar]
- Dulog, L.; Wang, W. A Bisnitroxyl Dipeptide. Liebigs Ann. Chem. 1992, 301–303. [Google Scholar] [CrossRef]
- Crisma, M.; Deschamps, J.R.; George, C.; Flippen-Anderson, J.L.; Kaptein, B.; Broxterman, Q.B.; Moretto, A.; Oancea, S.; Jost, M.; Formaggio, F.; et al. A topographically and conformationally constrained, spin-labeled, α-amino acid: Crystallographic characterization in peptides. J. Peptide Res. 2005, 65, 564–579. [Google Scholar] [CrossRef]
- Sauvée, C.; Rosay, M.; Casano, G.; Aussenac, F.; Weber, R.T.; Ouari, O.; Tordo, P. Highly Efficient, Water-Soluble Polarizing Agents for Dynamic Nuclear Polarization at High Frequency. Angew. Chem. Int. Ed. 2013, 52, 10858–10861. [Google Scholar] [CrossRef]
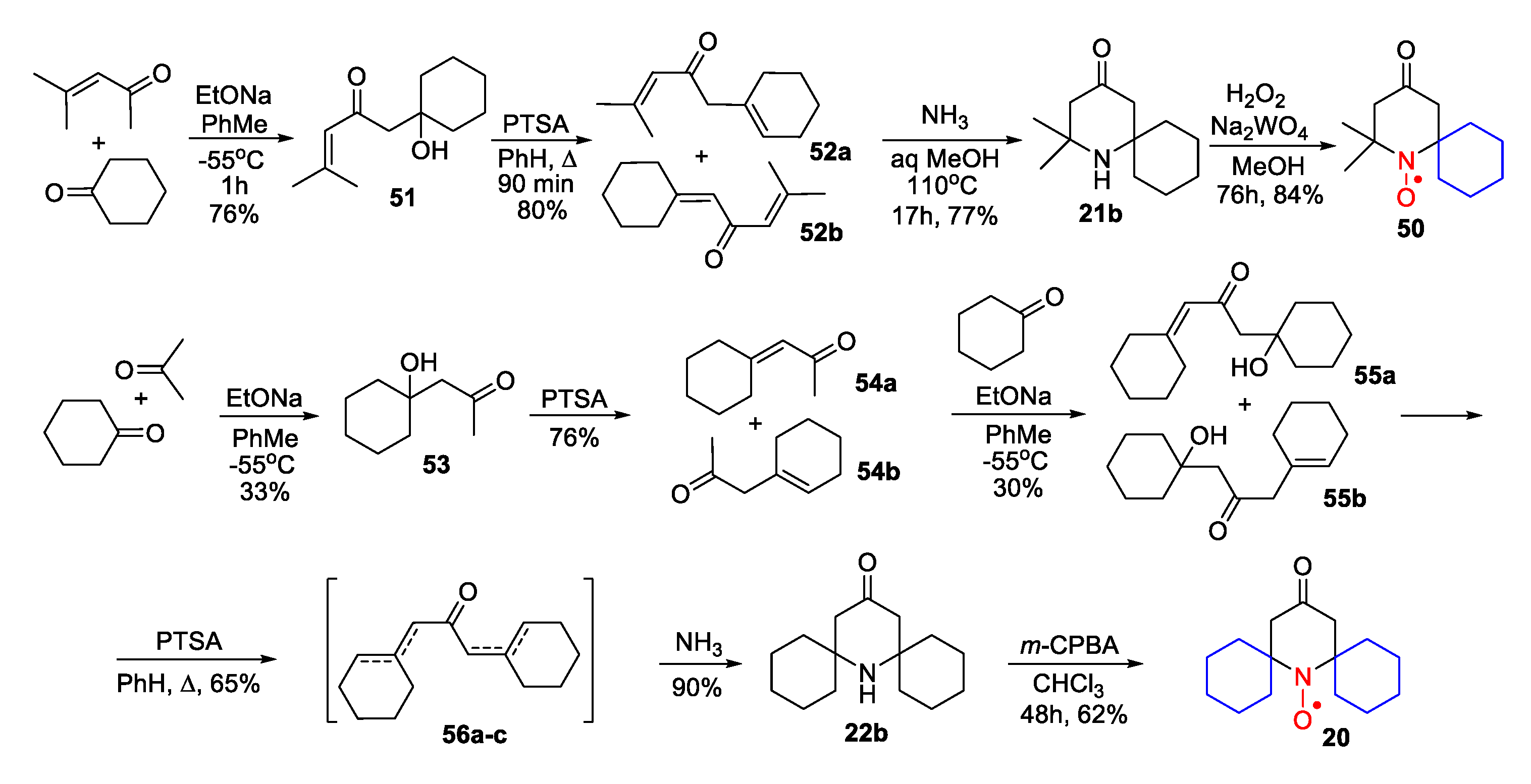
- Mikhailov, B.M.; Povarov, L.S.; Grigos, V.I.; Karakhanov, R.A. Reactions of dihydrosylvan with Schiff bases. Izv. Akad. Nauk SSSR Ser. Khim. 1964, 1693–1695, Chem. Abstr. 1964, 61, 92328. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Rozantsev, E.G.; Povarov, L.S.; Grigos, V.I. A new stable free radical: 4-methyl-2-spirocyclohexyl-3,4:3′,2′-tetrahydrofurano-1,2,3,4-tetrahydroquinolin-1-oxyl. Izv. Akad. Nauk SSSR Ser. Khim. 1964, 1725, Chem. Abstr. 1964, 61, 92327. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Rozantsev, E.G.; Povarov, L.S.; Grigos, V.I. Paramagnetic derivatives in the series of hydrogenated quinolines. Izv. Akad. Nauk SSSR Ser. Khim. 1965, 1102–1104, Chem. Abstr. 1965, 63, 46178. [Google Scholar] [CrossRef]
- Povarov, L.S.; Shapiro, A.B.; Rozantsev, E.G. Free nitroxyl radicals based on benzoquinolines. Izv. Akad. Nauk SSSR Ser. Khim. 1966, 339–341, Chem. Abstr. 1966, 64, 92646. [Google Scholar]
- Shibaeva, R.P.; Rozenberg, L.P.; Shapiro, A.B.; Povarov, L.S. Crystal and molecular structure of 4-(spirotetrahydro-2′-furyl)-2-spirocyclohexyl-1,2,3,4-tetrahydroquinoline and a stable nitroxyl radical formed during its catalytic oxidation. Zh. Strukt. Khim. 1981, 22, 140–145. [Google Scholar]
- Lobanova, T.V.; Kasaikina, O.T.; Povarov, L.S.; Shapiro, A.B.; Gagarina, A.B. 4-Methyl-2-spirocyclohexyl-2′,3′,3,4-tetrahydrofurano-1,2,3,4-tetrahydroquinoline N-oxyl as an indicator of peroxide radicals. Dokl. AN SSSR Phys. Chem. 1979, 245, 1154–1159. [Google Scholar]
- Ooishi, M.; Seino, M.; Imachi, R.; Ishida, T.; Nogami, T. Convenient synthesis and host–guest compounds of 9,9′(10H,10′H)-spirobiacridines. Tetrahedron Lett. 2002, 43, 5521–5524. [Google Scholar] [CrossRef]
- Yamamoto, K.; Higashibayashi, S. Synthesis of Three-Dimensional Butterfly Slit-Cyclobisazaanthracenes and Hydrazinobisanthenes through One-Step Cyclodimerization and Their Properties. Chem. Eur. J. 2016, 22, 663–671. [Google Scholar] [CrossRef]
- Ichihashi, K.; Kanetomo, T.; Enomoto, M.; Ishida, T. 2,7-Di-tert-butyl-9,9′(10H,10′H)-spirobiacridine-10,10′-dioxyl as a Ground Triplet Biradical: The Role of tert-Butylation. Tetrahedron Lett. 2020. [Google Scholar] [CrossRef]
- Yoshioka, T.; Mori, E.; Murayama, K. Studies on Stable Free Radicals. XIII. Synthesis and ESR Spectral Properties of Hindered Piperazine N-Oxyls. Bull. Chem. Soc. Jpn. 1972, 45, 1855–1860. [Google Scholar] [CrossRef]
- Sudo, R.; Ichihara, S. Reactions of Alicyclic Aminonitrile. Bull. Chem. Soc. Jpn. 1963, 36, 34–37. [Google Scholar] [CrossRef]
- Lai, J.T. Hindered amines. Novel synthesis of 1,3,3,5,5-pentasubstituted 2-piperazinones. J. Org. Chem. 1980, 45, 754–755. [Google Scholar] [CrossRef]
- Lai, J.T. Hindered Amines; III. Highly Regioselective Syntheses of 1,3,3,5,5-Pentasubstituted 2-Piperazinones and their Nitroxyl Radicals. Synthesis 1981, 40–42. [Google Scholar] [CrossRef]
- Lai, J.T. Hindered Amines. 3,3,5,5-Tetrasubstituted-2-oxomorpholines and Derivatives. Synthesis 1984, 122–123. [Google Scholar] [CrossRef]
- Lai, J.T. Hindered Amines. 4-Hydroxy-1,3,5,5-pentasubstituted-2-piperazinones. Synthesis 1984, 124–125. [Google Scholar] [CrossRef]
- Bargellini, G. Azione del cloroformio e idrato sodico sui fenoli. Gazz. Chim. Ital. 1906, 36, 329–335. [Google Scholar]
- Rychnovsky, S.D.; Beauchamp, T.; Vaidyanathan, R.; Kwan, T. Synthesis of Chiral Nitroxides and an Unusual Racemization Reaction. J. Org. Chem. 1998, 63, 6363–6374. [Google Scholar] [CrossRef] [PubMed]
- Soloshonok, V.A.; Sorochinsky, A.E. Practical Methods for the Synthesis of Symmetrically α,α-Disubstituted α-Amino Acids. Synthesis 2010, 2319–2344. [Google Scholar] [CrossRef]
- Ruider, S.A.; Müller, S.; Carreira, E.M. Ring Expansion of 3-Oxetanone-Derived Spirocycles: Facile Synthesis of Saturated Nitrogen Heterocycle. Angew. Chem. Int. Ed. 2013, 52, 11908–11911. [Google Scholar] [CrossRef]
- Jackl, M.K.; Gordon, C.P.; Copéret, C.; Bode, J.W. Spirocyclic Nitroxide Biradicals: Synthesis and Evaluation as Dynamic Nuclear Polarizing Agents. Helv. Chim. Acta 2020. accepted article. [Google Scholar] [CrossRef]
- Saito, F.; Trapp, N.; Bode, J.W. Iterative Assembly of Polycyclic Saturated Heterocycles from Monomeric Building Blocks. J. Am. Chem. Soc. 2019, 141, 5544–5554. [Google Scholar] [CrossRef]
- Luescher, M.U.; Bode, J.W. Catalytic Synthesis of N-Unprotected Piperazines, Morpholines, and Thiomorpholines from Aldehydes and SnAP Reagents. Angew. Chem. Int. Ed. 2015, 54, 10884–10888. [Google Scholar] [CrossRef]
- Sosnovski, G.; Cai, Z. A Study of the Favorskii Rearrangement with 3-Bromo-4-oxo-2,2,6,6-tetramethylpiperidine-1-oxyl. J. Org. Chem. 1995, 60, 3414–3418. [Google Scholar] [CrossRef]
- Wang, Y.; Paletta, J.T.; Berg, K.; Reinhart, E.; Rajca, S.; Rajca, A. Synthesis of Unnatural Amino Acids Functionalized with Sterically Shielded Pyrroline Nitroxides. Org. Lett. 2014, 16, 5298–5300. [Google Scholar] [CrossRef]
- Krumkacheva, O.A.; Timofeev, I.O.; Politanskaya, L.V.; Polienko, Y.F.; Tretyakov, E.V.; Rogozhnikova, O.Y.; Trukhin, D.V.; Tormyshev, V.M.; Chubarov, A.S.; Bagryanskaya, E.G.; et al. Triplet Fullerenes as Prospective Spin Labels for Nanoscale Distance Measurements by Pulsed Dipolar EPR Spectroscopy. Angew. Chem. Int. Ed. 2019, 58, 13271–13275. [Google Scholar] [CrossRef] [PubMed]
- Polienko, Y.F.; Snytnikova, O.A.; Yanshole, V.V.; Chernyak, E.I.; Morozov, S.V.; Grigor’ev, I.A.; Tsentalovich, Y.P. Effect of the spacer length and nitroxide sterical shielding upon photostability of spin-labeled kynurenines. J. Photochem. Photobiol. 2016, 322–323, 76–84. [Google Scholar] [CrossRef]
- Motherwell, W.B.; Roberts, J.S. A Convenient Nitroxide Radical Synthesis. J. Chem. Soc. Chem. Commun. 1972, 328–329. [Google Scholar] [CrossRef]
- Keana, J.W.F.; Lee, T.D.; Bernard, E.M. Side-chain substituted 2,2,5,5-tetramethylpyrrolidine-N-oxyl (proxyl) nitroxides. A new series of lipid spin labels showing improved properties for the study of biological membranes. J. Am. Chem. Soc. 1976, 98, 3052–3053. [Google Scholar] [CrossRef] [PubMed]
- Sar, C.P.; Jeko, J.; Hideg, K. Synthesis of 2-Alkenyl-1-pyrrolin-1-oxides and Polysubstituted Nitrones. Synthesis 2003, 1367–1372. [Google Scholar] [CrossRef]
- Uchida, Y.; Matsuoka, N.; Takahashi, H.; Shimono, S.; Ikuma, N.; Tamura, R. Synthesis, Crystal Structure, and Magnetic Properties of 4-(2-Methyl-1-azaspiro[4.5]deca-1-oxyl-2-yl)phenol. Heterocycles 2007, 74, 607–614. [Google Scholar] [CrossRef]
- Suzuki, K.; Mazhukin, D.G.; Takahashi, H.; Yoshiaki, U.; Tamura, R.; Grigor’ev, I.A. Synthesis and Stereochemistry of Novel Rigid Nitroxide Biradicals Based on Paramagnetic Pyrrolidine Core. Heterocycles 2009, 78, 3091–3099. [Google Scholar] [CrossRef]
- Takemoto, Y.; Zaytseva, E.; Suzuki, K.; Yoshioka, N.; Takanishi, Y.; Funahashi, M.; Uchida, Y.; Akita, T.; Park, J.; Sato, S.; et al. Unique Superparamagnetic-like Behavior Observed in Non-π-delocalized Nitroxide Diradical Compounds Showing Discotic Liquid Crystalline Phase. Chem. Eur. J. 2018, 24, 17293–17302. [Google Scholar] [CrossRef]
- Shiraishi, R.; Kaneko, T.; Usui, K.; Naganuma, T.; Iizuka, N.; Morishita, K.; Kobayashi, S.; Fuchi, Y.; Matsuoka, Y.; Hirai, G.; et al. Effects of Substituents on the Properties of Metal-Free MRI Contrast Agents. ACS Omega 2019, 4, 20715–20723. [Google Scholar] [CrossRef]
- Cicchi, S.; Hold, I.; Brandi, A. New synthesis of five-membered cyclic nitrones from tartaric acid. J. Org. Chem. 1993, 115, 5274–5275. [Google Scholar] [CrossRef]
- Khoroshunova, Y.V.; Morozov, D.A.; Taratayko, A.I.; Gladkikh, P.D.; Glazachev, Y.I.; Kirilyuk, I.A. Synthesis of 1-azaspiro[4.4]nonan-1-oxyls via intramolecular 1,3-dipolar cycloaddition. Beilstein J. Org. Chem. 2019, 15, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, V.A.; Volodarskii, L.B. Interaction of 5,5-dimethyl-2-phenacylpyrroline 1-oxide–an exocyclic β-oxonitrone–with nucleophilic reagents. Chem. Heterocycl. Compd. 1996, 32, 652–660. [Google Scholar] [CrossRef]
- Sevast’yanova, T.K.; Volodarskii, L.B. Preparation of stable iminoxyl radicals of 3-imidazolines. Bull. Acad. Sci. USSR Div. Chem. Sci. 1972, 21, 2276–2278. [Google Scholar] [CrossRef]
- Reznikov, V.A.; Volodarskii, L.B. Synthesis of bifunctional derivatives of nitroxyl radicals of imidazoline. Chem. Heterocycl. Compd. 1990, 26, 643–648. [Google Scholar] [CrossRef]
- Zubenko, D.; Tsentalnovich, Y.; Lebedeva, N.; Kirilyuk, I.; Roshchupkina, G.; Zhurko, I.; Reznikov, V.; Marque, S.R.A.; Bagryanskaya, E. Laser Flash Photolysis and CIDNP Studies of Steric Effects on Coupling Rate Constants of Imidazolidine Nitroxide with Carbon-Centered Radicals, Methyl Isobutyrate-2-yl and tert-Butyl Propionate-2-yl. J. Org. Chem. 2006, 71, 6044–6052. [Google Scholar] [CrossRef]
- Hintermaier, F.; Volodarsky, L.B.; Polborn, K.; Beck, W. New 2,5-Dihydroimidazole-1-oxyls with Functional Side Groups (N, O, S Donors). Liebigs Ann. 1995, 2189–2194. [Google Scholar] [CrossRef]
- Zaytseva, E.V.; Gatilov, Y.V.; Amitina, S.A.; Tamura, R.; Grigor’ev, I.A.; Mazhukin, D.G. Spirocyclic 2,5-Dihydro-1H-imidazole 1-Oxyl Radicals with a Mesogenic Substituent on C4. Synthesis and Crystal Structure. Russ. J. Org. Chem. 2014, 50, 72–77. [Google Scholar] [CrossRef]
- Zaytseva, E.V.; Shernyukov, A.V.; Genaev, A.M.; Tamura, R.; Grigor’ev, I.A.; Mazhukin, D.G. New spirocyclic nitroxides of 2,5-dihydroimidazole series flanked by two mesogenic fragments. Arkivoc 2014, vi, 10–24. [Google Scholar] [CrossRef]
- Zaytseva, E.V.; Shernyukov, A.V.; Amitina, S.A.; Tamura, R.; Grigor’ev, I.A.; Mazhukin, D.G. Synthesis of diastereomeric spirocyclic nitroxyl radicals of 3-imidazoline series with two mesogenic groups. Chem. Heterocycl. Compd. 2014, 50, 1113–1125. [Google Scholar] [CrossRef]
- Zaytseva, E.; Timofeev, I.; Krumkacheva, O.; Parkhomenko, D.; Mazhukin, D.; Sato, K.; Matsuoka, H.; Takui, T.; Bagryanskaya, E. EPR and DEER Characterization of New Mixed Weakly Coupled Nitroxide Triradicals for Molecular Three-Spin Qubits. Appl. Magn. Reson. 2019, 50, 967–976. [Google Scholar] [CrossRef]
- Zaytseva, E.V.; Gatilov, Y.V.; Mazhukin, D.G. The synthesis of new functionalized 1,3,5-triazine-based stable bi- and trinitroxides of the 2,5-dihydroimidazole series. Arkivoc 2018, V, 359–374. [Google Scholar] [CrossRef]
- Zaytseva, E.V. Spirocyclic mono- and Dinitroxides of 3-Imidazoline Type as Precursors of Paramagnetic Functional Materials. Ph.D. Thesis, Novosibirsk Institute of Organic Chemistry SB RAS, Novosibirsk, Russia, 2014. [Google Scholar]
- Kirilyuk, I.A.; Grigor’ev, I.A.; Volodarskii, L.B. Synthesis of 2H-imidazole 1-oxides and stable nitroxyl radicals based on them. Russ. Chem. Bull. 1991, 40, 1871–1879. [Google Scholar] [CrossRef]
- Bakunova, S.M.; Grigor’ev, I.A.; Kirilyuk, I.A.; Volodarsky, L.B. Reduction of α,α-dialkoxy-substituted nitroxides: The synthesis of α-alkoxynitrones and acetals of N-hydroxyamides. Russ. Chem. Bull. 1999, 48, 2136–2143. [Google Scholar] [CrossRef]
- Jones, R.C.F.; Martin, J.N. Nitrones. In Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; Padwa, A., Pearson, W.H., Eds.; Wiley: New York, NY, USA, 2003; Volume 59, pp. 1–81. [Google Scholar] [CrossRef]
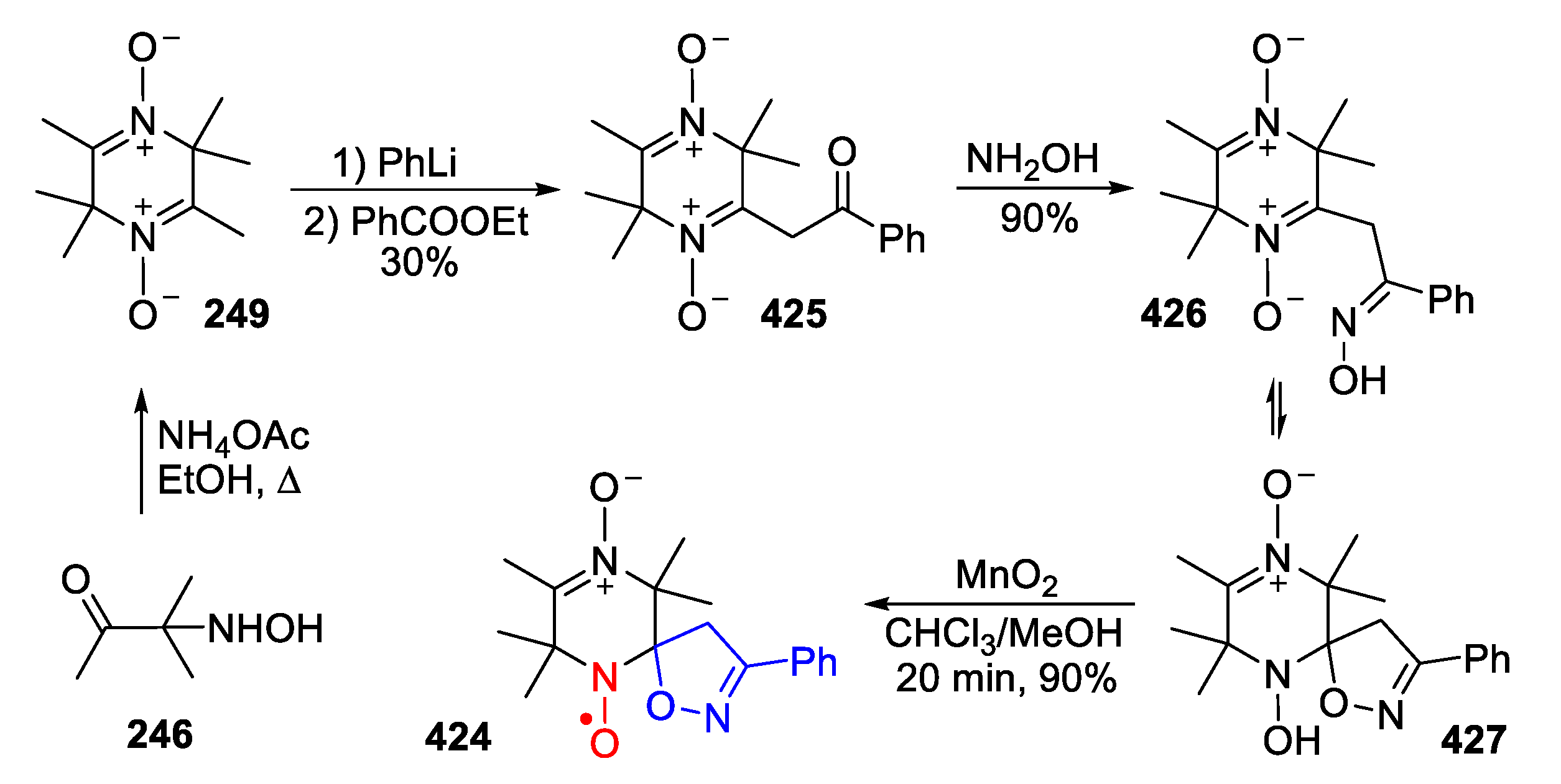
- Reznikov, V.A.; Volodarsky, L.B. Ammonium acetate as a catalyst of the condensation of sterically hindered functionalized hydroxylamines with ketones. Russ. Chem. Bull. 1997, 46, 1577–1581. [Google Scholar] [CrossRef]
- Martin, V.V.; Volodarskii, L.B. Synthesis and some reactions of sterically hindered 3-imidazoline 3-oxides. Chem. Heterocycl. Compd. 1979, 15, 92–98. [Google Scholar] [CrossRef]
- Grigor’ev, I.A.; Shchukin, G.I.; Volodarskii, L.B. Influence of radical center on oxidative properties of nitrone group in reaction of nitroxyl radicals of 3-imidazoline 3-oxide with hydrazine. Russ. Chem. Bull. 1983, 32, 1030–1035. [Google Scholar] [CrossRef]
- Grigor’ev, I.A.; Kirilyuk, I.A.; Starichenko, V.F.; Volodarskii, L.B. Oxidative alkoxylation of 4H-imidazole N-oxides as a new method of synthesis of stable nitroxyl radicals of the 2- and 3-imidazoline series with alkoxy groups at the α-carbon atom of the radical center. Russ. Chem. Bull. 1989, 38, 1488–1494. [Google Scholar] [CrossRef]
- Osiecki, J.H.; Ullman, E.F. Studies of Free Radicals. I. α-Nitronyl Nitroxides, a New Class of Stable Radicals. J. Am. Chem. Soc. 1968, 90, 1078–1079. [Google Scholar] [CrossRef]
- Ullman, E.F.; Osiecki, J.H.; Boocock, D.G.B.; Darcy, R. Stable free radicals. X. Nitronyl nitroxide monoradicals and biradicals as possible small molecule spin labels. J. Am. Chem. Soc. 1972, 94, 7049–7059. [Google Scholar] [CrossRef]
- Artiukhova, N.A.; Maryunina, K.Y.; Fokin, S.V.; Tretyakov, E.V.; Romanenko, G.V.; Polushkin, A.V.; Bogomyakov, A.S.; Sagdeev, R.Z.; Ovcharenko, V.I. Spirocyclic derivatives of nitronyl nitroxides in the design of heterospin CuII complexes manifesting spin transitions. Russ. Chem. Bull. 2013, 62, 2132–2140. [Google Scholar] [CrossRef]
- Kai, Y.; Knochel, P.; Kwiatkowski, S.; Dunitz, J.D.; Oth, J.F.M.; Seebach, D.; Kalinowski, H.-O. Structure, Synthesis, and Properties of Some Persubstituted 1,2-Dintroethanes-in Quest of Nitrocyclopropyl-Anion Derivatives. Helv. Chim. Acta 1982, 65, 137–161. [Google Scholar] [CrossRef]
- Tolstikov, S.E.; Artiukhova, N.A.; Romanenko, G.V.; Bogomyakov, A.S.; Zueva, E.M.; Barskaya, I.Y.; Fedin, M.V.; Maryunina, K.Y.; . Tretyakov, E.V.; Sagdeev, R.Z.; et al. Heterospin complex showing spin transition at room temperature. Polyhedron 2015, 100, 132–138. [Google Scholar] [CrossRef]
- Artiukhova, N.A.; Romanenko, G.V.; Bogomyakov, A.S.; Barskaya, I.Y.; Veber, S.L.; Fedin, M.V.; Maryunina, K.Y.; Inoue, K.; Ovcharenko, V.I. Cu(II) complex with nitronyl nitroxide whose paramagnetism is suppressed by temperature decrease and/or pressure increase. J. Mater. Chem. C 2016, 4, 11157–11163. [Google Scholar] [CrossRef]
- Artiukhova, N.; Romanenko, G.; Letyagin, G.; Bogomyakov, A.; Veber, S.; Minakova, O.; Petrova, M.; Morozov, V.; Ovcharenko, V. Spin Transition in the Cu(hfac)2 Complex with (4-Ethylpyridin-3-yl)-Substituted Nitronyl Nitroxide Caused by the “Asymmetric” Structural Rearrangement of Exchange Clusters in the Heterospin Molecule. Crystals 2019, 9, 285. [Google Scholar] [CrossRef]
- Reznikov, V.A.; Volodarsky, L.B. Synthesis and oxidation of 1-hydroxy-3(2)-imidazoline-derived enaminones. Russ. Chem. Bull. 1996, 45, 1699–1706. [Google Scholar] [CrossRef]
- Reznikov, V.A.; Volodarskii, L.B.; Spoyalov, A.P.; Dikanov, S.A. Monocyclic biradicals in the imidazolidine series. Russ. Chem. Bull. 1993, 42, 881–884. [Google Scholar] [CrossRef]
- Reznikov, V.A.; Volodarskii, L.B. Reaction of β-Oxonitrones—Imidazoline and Pyrroline Derivatives—with Nucleophilic Reagents. Chem. Heterocycl. Compd. 1991, 27, 722–728. [Google Scholar] [CrossRef]
- Kuzhelev, A.A.; Strizhakov, R.K.; Krumkacheva, O.A.; Polienko, Y.F.; Morozov, D.A.; Shevelev, G.Y.; Pyshnyi, D.V.; Kirilyuk, I.A.; Fedin, M.V.; Bagryanskaya, E.G. Room-Temperature Electron Spin Relaxation of Nitroxides Immobilized in Trehalose: Effect of Substituents Adjacent to NO-group. J. Magn. Reson. 2016, 266, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Taratayko, A.I.; Rybalova, T.V.; Reznikov, V.A. Synthesis of paramagnetic spiropyran derivatives. Chem. Heterocycl. Compd. 2018, 54, 981–983. [Google Scholar] [CrossRef]
- Toda, T.; Morimura, S.; Mori, E.; Horiuchi, H.; Murayama, K. Studies on Stable Free Radicals. VI. Synthesis of Substituted 4-Imidazolidinone-1-oxyls. Bull. Chem. Soc. Jpn. 1971, 44, 3445–3450. [Google Scholar] [CrossRef]
- Dervan, P.; Aldabbagh, F.; Zetterlund, P.B.; Yamada, B. Mechanism and Kinetics of the Imidazolidinone Nitroxide Mediated Free-Radical Polymerization of Styrene. J. Polym. Sci. A Polym. Chem. 2003, 41, 327–334. [Google Scholar] [CrossRef]
- Toda, T.; Morimura, S.; Murayama, K. Studies on Stable Free Radicals. VII. The Mechanism for Cyclization Reaction of α-Amino Nitriles with Carbonyl Compounds. Bull. Chem. Soc. Jpn. 1972, 45, 557–561. [Google Scholar] [CrossRef]
- Khalaj, A.; Bazaz, R.D.; Shekarchi, M. Synthesis of novel imidazolidinones. Monatsh. Chem. 1997, 128, 395–398. [Google Scholar] [CrossRef]
- Ranieri, K.; Conradi, M.; Chavant, P.-Y.; Blandin, V.; Barner-Kowollik, C.; Junkers, T. Enhanced Spin-capturing Polymerization and Radical Coupling Mediated by Cyclic Nitrones. Aust. J. Chem. 2012, 65, 1110–1116. [Google Scholar] [CrossRef]
- López, M.C.; Royal, G.; Philouze, C.; Chavant, P.Y.; Blandin, V. Imidazolidinone Nitroxides as Catalysts in the Aerobic Oxidation of Alcohols, en Route to Atroposelective Oxidative Desymmetrization. Eur. J. Org. Chem. 2014, 4884–4896. [Google Scholar] [CrossRef]
- Michon, P.; Rassat, A. Nitroxydes XLII: Signe des couplages a longue distance, electro-protons dans des nitroxydes oxazolidiniques. Etude conformationelle par RMN. Bull. Soc. Chim. Fr. 1971, 3561–3567. [Google Scholar]
- Smith, D.L.; Spencer, T.A. Synthesis and Photolysis of Doxyl Derivatives of 5β-Androstan-3-ones. J. Heterocycl. Chem. 1979, 16, 807–809. [Google Scholar] [CrossRef]
- Ciecierska-Tworek, Z.; Van, S.P.; Griffith, O.H. Electron-electron dipolar splitting anisotropy of a dinitroxide oriented in a crystalline matrix. J. Mol. Struct. 1973, 16, 139–148. [Google Scholar] [CrossRef]
- Michon, P.; Rassat, A. Nitroxides. LXIX. 1,4-Bis(4′,4′-dimethyloxazolidine-3′-oxyl)cyclohexane structure determination by electron spin resonance and nuclear magnetic resonance. J. Am. Chem. Soc. 1975, 97, 696–700. [Google Scholar] [CrossRef]
- Gleason, W.B. 1,4-Bis(4′,4′-dimethyloxazolidine-N-oxyl)cyclohexane. Acta. Crystallogr. B Struct. Sci. Cryst. Eng. Mater. 1973, 29, 2959–2960. [Google Scholar] [CrossRef]
- Ysacco, C.; Giorgi, M.; Tordo, P.; Bardelang, D.; Ouari, O. Dinitroxide biradical crystals with polar order. Can. J. Chem. 2015, 93, 920–924. [Google Scholar] [CrossRef]
- Gleason, W.B.; Barnett, R.E. Use of the Point Dipole Approximation for Nitroxide Biradicals. J. Am. Chem. Soc. 1976, 98, 2701–2705. [Google Scholar] [CrossRef]
- Metzner, E.K.; Libertini, L.J.; Calvin, M. Electron spin exchange in rigid biradicals. J. Am. Chem. Soc. 1977, 99, 4500–4502. [Google Scholar] [CrossRef][Green Version]
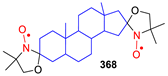
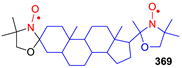
- Ramasseul, R.; Rassat, A. Nitroxides XLIX: Steroidal Nitroxides. Tetrahedron Lett. 1971, 12, 4623–4624. [Google Scholar] [CrossRef]
- Metzner, K.; Libertini, L. A new steroidal biradical. Tetrahedron Lett. 1978, 16, 81–84. [Google Scholar] [CrossRef]
- Kolocouris, N.; Foscolos, G.B.; Kolocouris, A.; Marakos, P.; Pouli, N.; Fytas, G.; Ikeda, S.; De Clercq, E. Synthesis and Antiviral Activity Evaluation of Some Aminoadamantane Derivatives. J. Med. Chem. 1994, 37, 2896–2902. [Google Scholar] [CrossRef]
- Kolocouris, N.; Kolocouris, A.; Foscolos, G.B.; Fytas, G.; Neyts, J.; Padalko, E.; Balzarini, J.; Snoeck, R.; Andrei, G.; De Clercq, E. Synthesis and Antiviral Activity Evaluation of Some New Aminoadamantane Derivatives. 2. J. Med. Chem. 1996, 39, 3307–3318. [Google Scholar] [CrossRef]
- Morat, C.; Rassat, A. Syntheses d’oxazolidines substituees par l’adamantane et de nitroxydes oxazolidines stables derives de l’adamantane. Tetrahedron Lett. 1979, 19, 4561–4564. [Google Scholar] [CrossRef]
- Michon, J.; Rassat, A. Diamine et biradical nitroxide di-spiranniques et leurs procedes de preparation. Fr. Demande 1973, 2135417. [Google Scholar]
- Michon, J.; Rassat, A. Nitroxides. LIX. Rotational correlation time determination of nitroxide biradical. Application to solvation studies. J. Am. Chem. Soc. 1974, 96, 335–337. [Google Scholar] [CrossRef]
- Michon, J.; Rassat, A. Nitroxides. 73. Electron Spin Resonance Study of Chiral Recognition by Cyclodextrin. J. Am. Chem. Soc. 1979, 101, 995–996. [Google Scholar] [CrossRef]
- Braslau, R.; Kuhn, H.; Burill, L.C.; Lanham, K.; Stenland, C.J. Synthesis of Several Novel Optically Active Nitroxyl Radicals. Tetrahedron Lett. 1996, 37, 7933–7936. [Google Scholar] [CrossRef]
- Braslau, R.; Burill, L.C.; Chaplinski, V.; Howden, R.; Para, P.W. Studies in the stereoselective trapping of prochiral carbon radicals by optically active camphoxyl nitroxides. Tetrahedron Assymetry 1997, 8, 3209–3212. [Google Scholar] [CrossRef]
- Banks, M.R.; Cadogan, J.I.G.; Gosney, I.; Grant, K.J.; Hodgson, P.K.G.; Thorburn, P. Synthesis of Enantiomerically Pure (5S)-4-Aza-2-oxa-6,6-dimethyl-7,10-methylene-5-spiro[4.5]-decan-3-one, a Novel Chiral Oxazolidin-2-one from (-)-Camphene for Use as a Recyclable Chiral Auxiliary in Asymmetric Transformations. Heterocycles 1994, 37, 199–206. [Google Scholar] [CrossRef]
- Balaban, A.T.; Negoiţă, N.; Baican, R. A New Stable Spiropyranic Aminyloxide (Nitroxide). Org. Magn. Reson. 1977, 9, 553–554. [Google Scholar] [CrossRef]
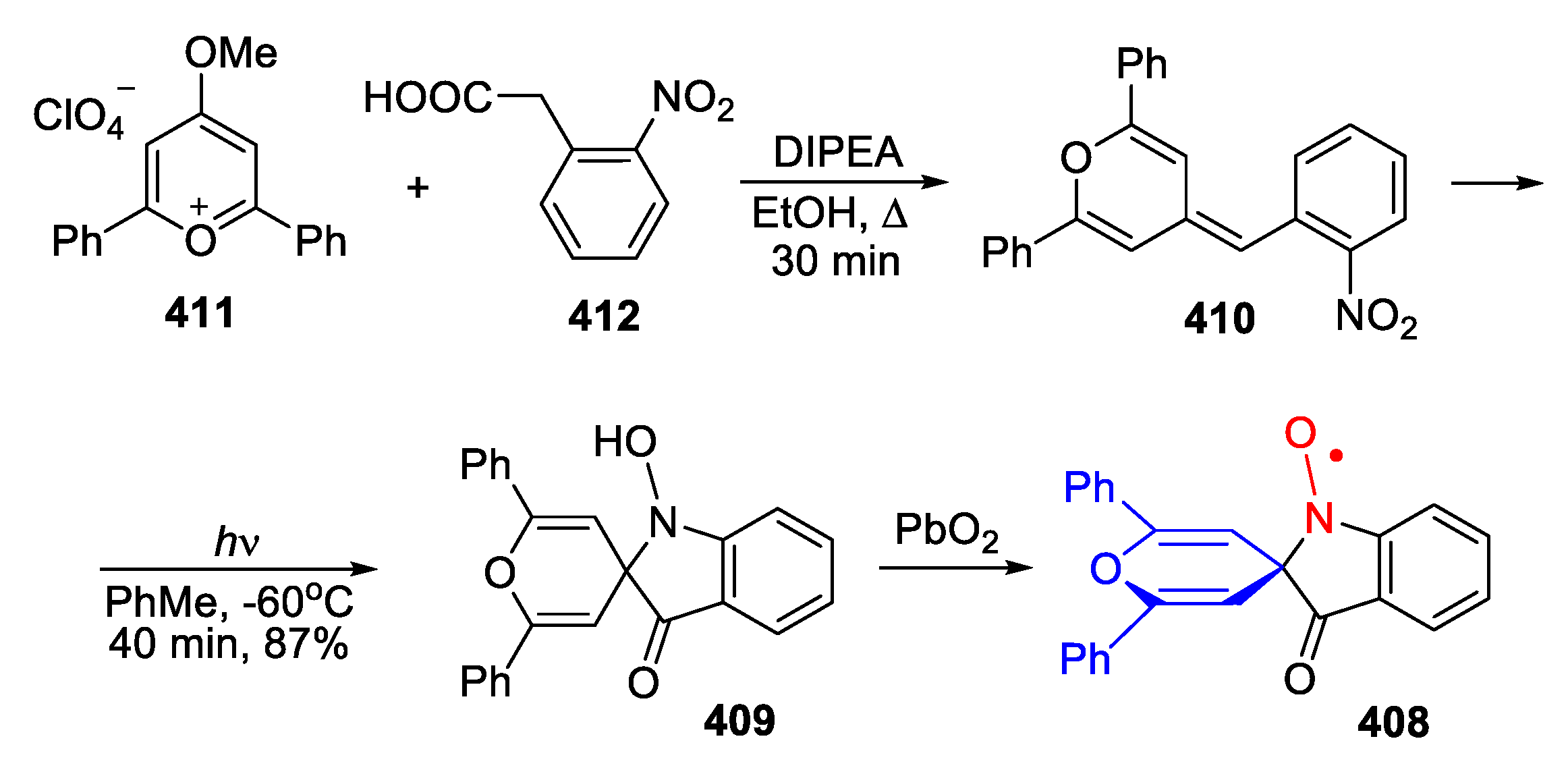
- Van Allan, J.A.; Faird, S.; Reynolds, G.A.; Chang, S.C. Photochemical conversion of 4-(o-nitrobenzylidene)-4H-pyrans to 1-hydroxy-3-oxospiro[indoline-2,4′-4′H-pyran] derivatives. J. Org. Chem. 1973, 38, 2834–2838. [Google Scholar] [CrossRef]
- Forrester, A.R.; Howie, R.A.; Jabbar, A.; Lewis, J.R.; Nizami, S.S.; Rozsa, Z.; Szendrei, K. Reductive Phenolic Coupling: A Novel Intramolecular Capture of a Nitro-Intermediate Generated Through Reduction of a Nitrobenzophenone. Tetrahedron Lett. 1992, 33, 4645–4648. [Google Scholar] [CrossRef]
- Shapiro, A.B.; Medzhidov, A.A.; Rozantsev, E.G. Syntheses of free nitroxyl radicals from hydrogenated quinolines, benzoxazines, and indoles. Zh. Org. Khim. (Russ.) 1966, 2, 1873–1877. [Google Scholar]
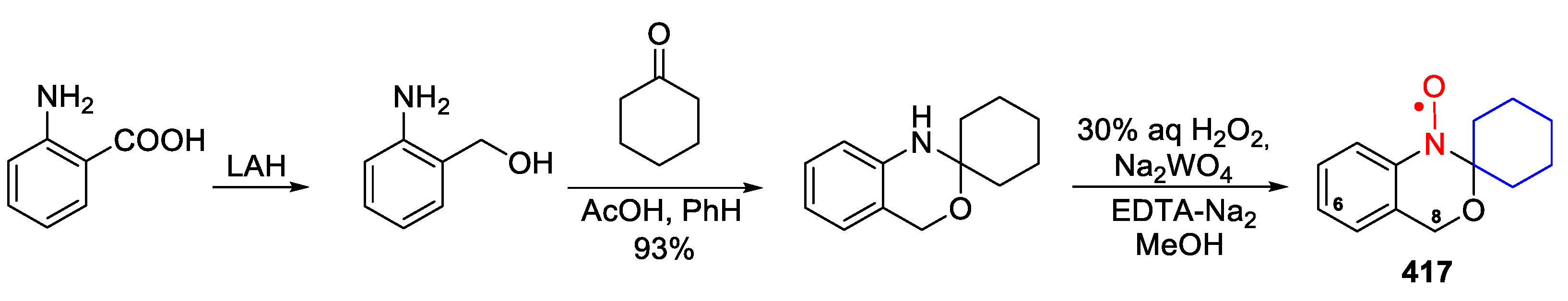
- Spagnol, G.; Rajca, A.; Rajca, S. Efficient Synthesis of Tricyclic Benzobisoxazines by Silica Gel Catalysis. J. Org. Chem. 2007, 72, 1867–1869. [Google Scholar] [CrossRef]
- Reznikov, V.A.; Volodarsky, L.B. Interaction of heterocyclic nitrones with organometallic reagents as a method for the synthesis of new types of nitroxides. Tetrahedron 1993, 49, 10669–10692. [Google Scholar] [CrossRef]
- Zheng, D.-G.; Li, C.-W.; Li, Y.-L. The Reactivity of Aldehyde (or Ketone) and α -Amino Acid Towards the Synthetic Reaction of [60]Fulleropyrrolidine Derivative. Synth. Commun. 1998, 28, 2007–2015. [Google Scholar] [CrossRef]
- Li, Y.-L.; Xu, J.-H.; Zheng, D.-G.; Yang, J.-K.; Pan, C.-Y.; Zhu, D.-B. Synthesis and Characterization of Stable Nitroxides Based on Fullerenes (C60, C70) and their Magnetic Study. Solid State Commun. 1997, 101, 123–128. [Google Scholar] [CrossRef]



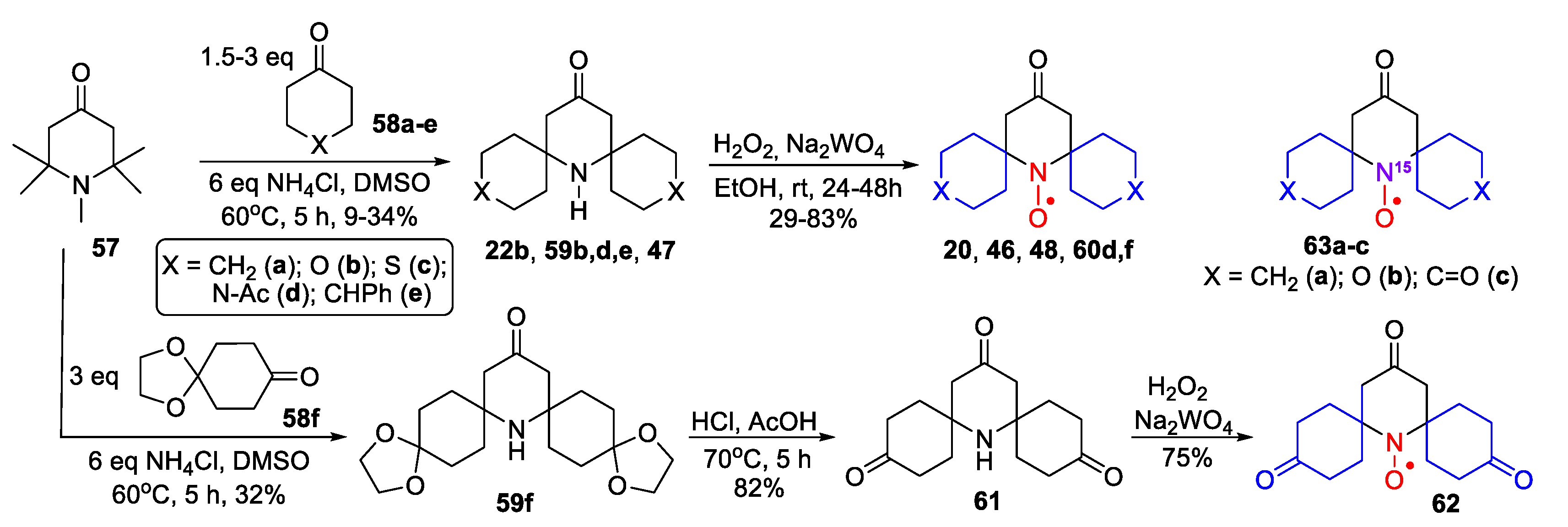
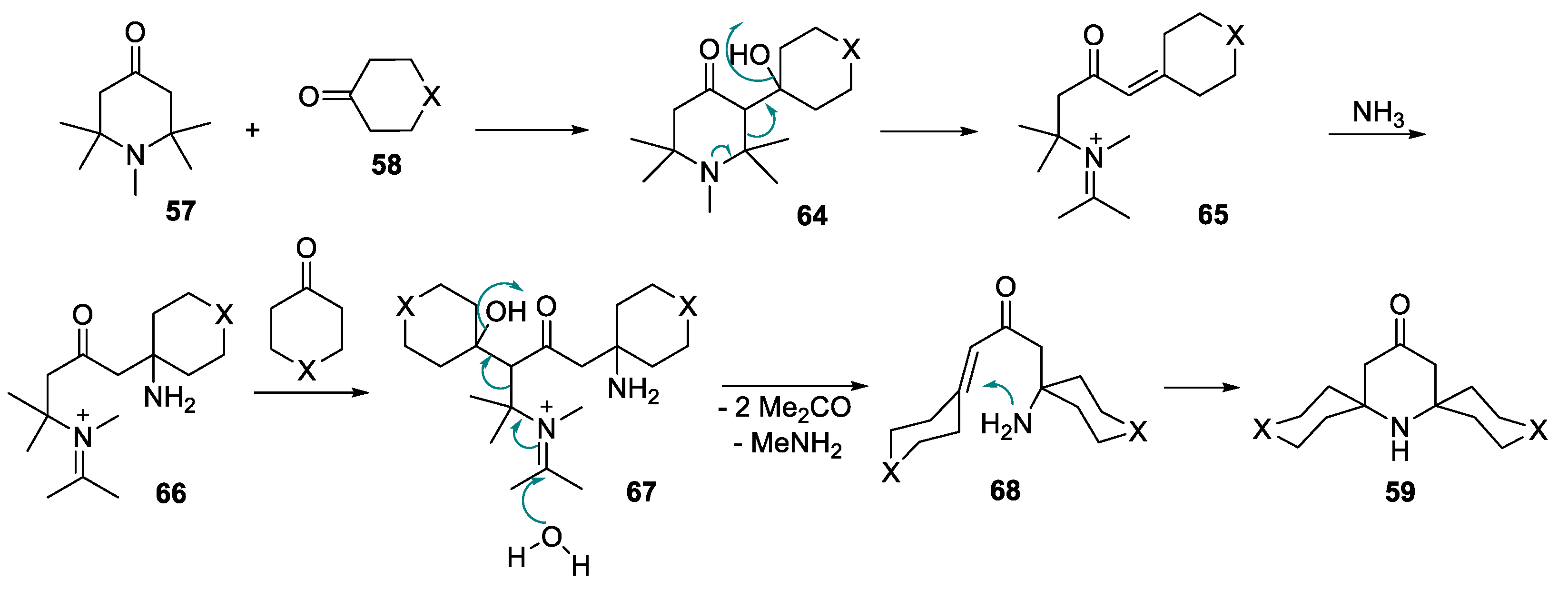

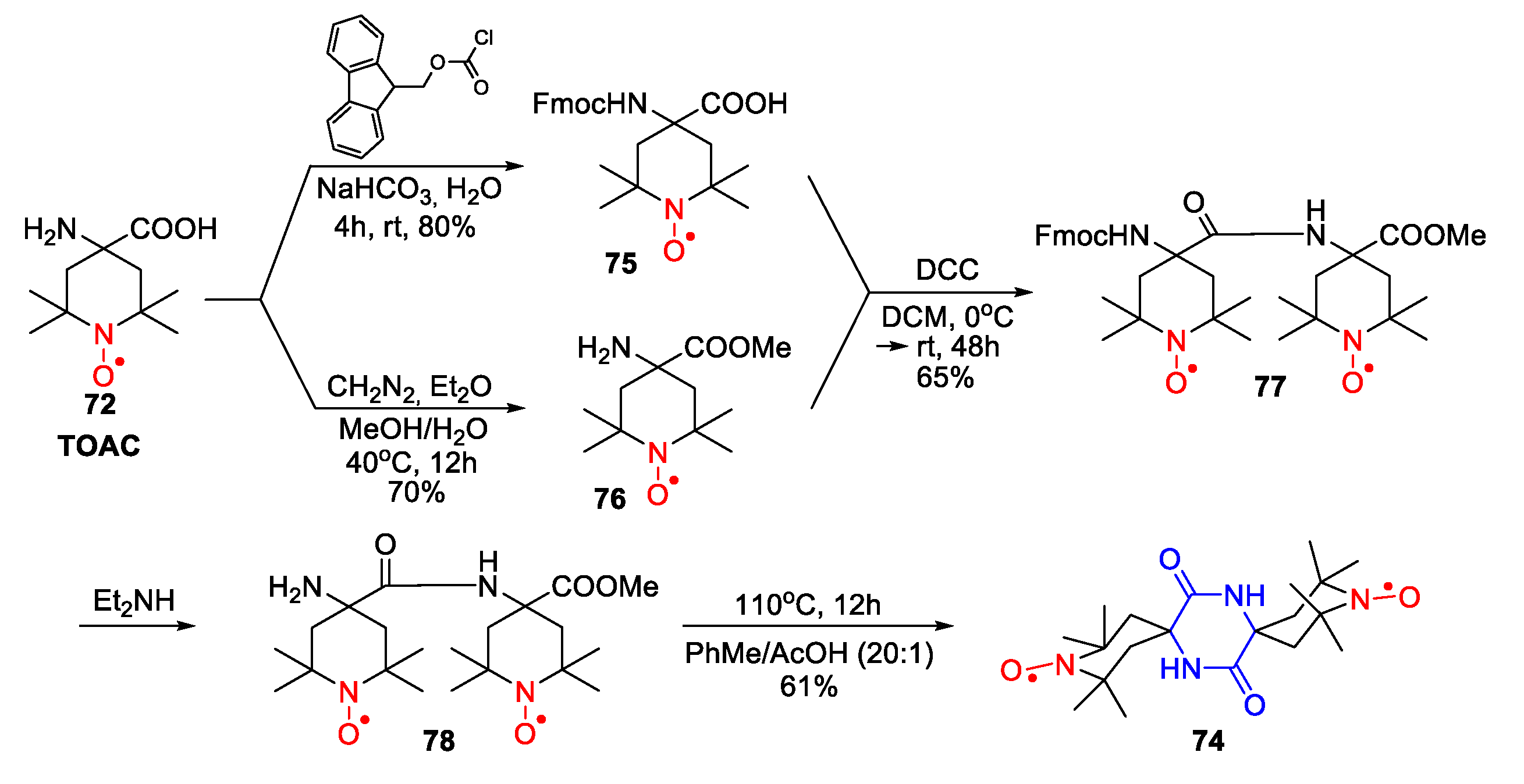













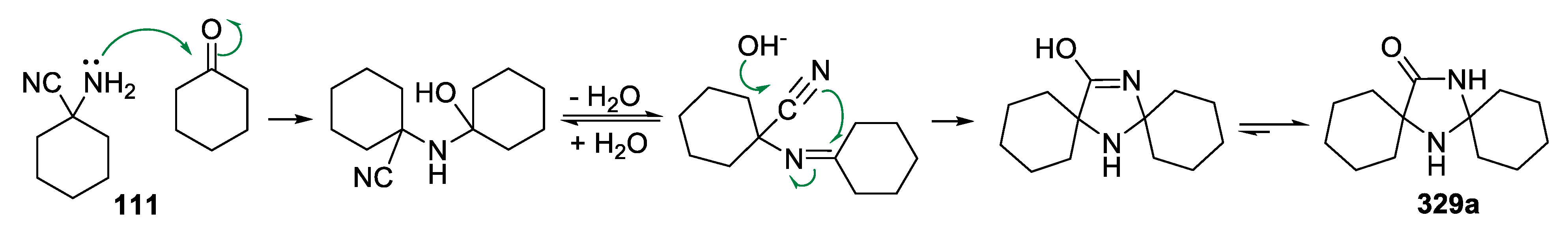








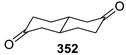
| Entry | Ketone | 6, % | 7, % | 8, % |
|---|---|---|---|---|
| 1 | 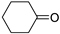 | 4 | 37 | 59 |
| 2 | 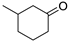 | 25 | 63 | 12 |
| 3 |  | 18 | 22 | 0 |
| 4 | 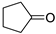 | 14 | 62 | 14 |
| 5 | 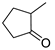 | 21 | 46 | 33 |
| 6 | 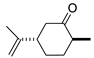 | 18 | 82 | 0 |
| 7 | 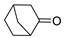 | 28 | 72 | 0 |
| 8 |  | 84 | 16 | 0 |
| 9 |  | 34 | 66 | 0 |
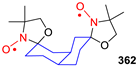
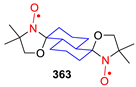
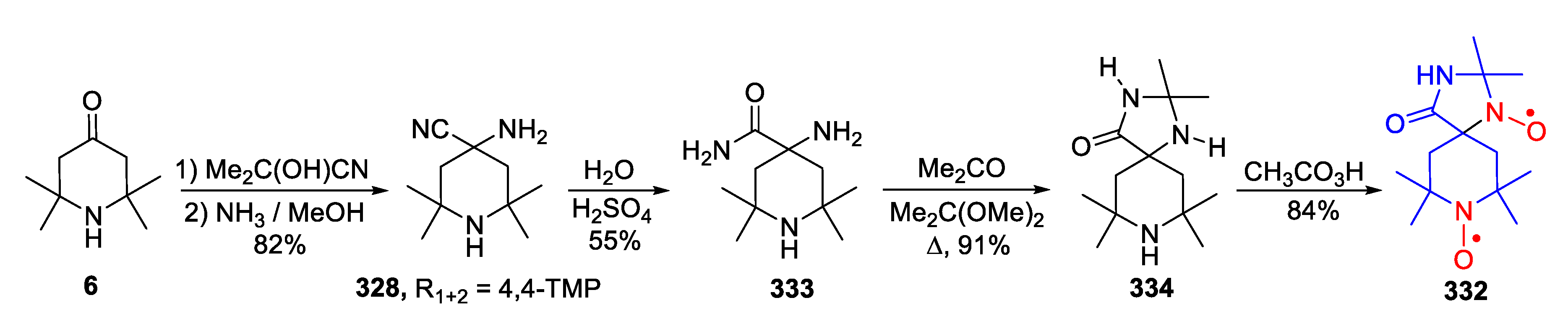
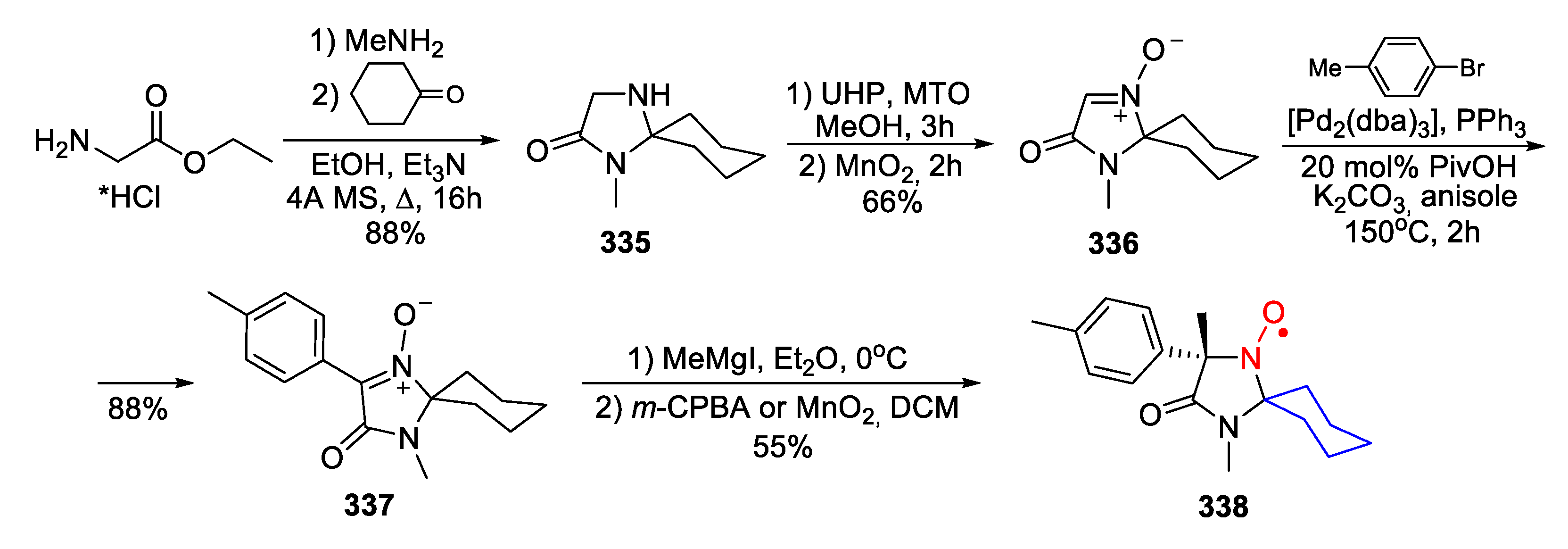
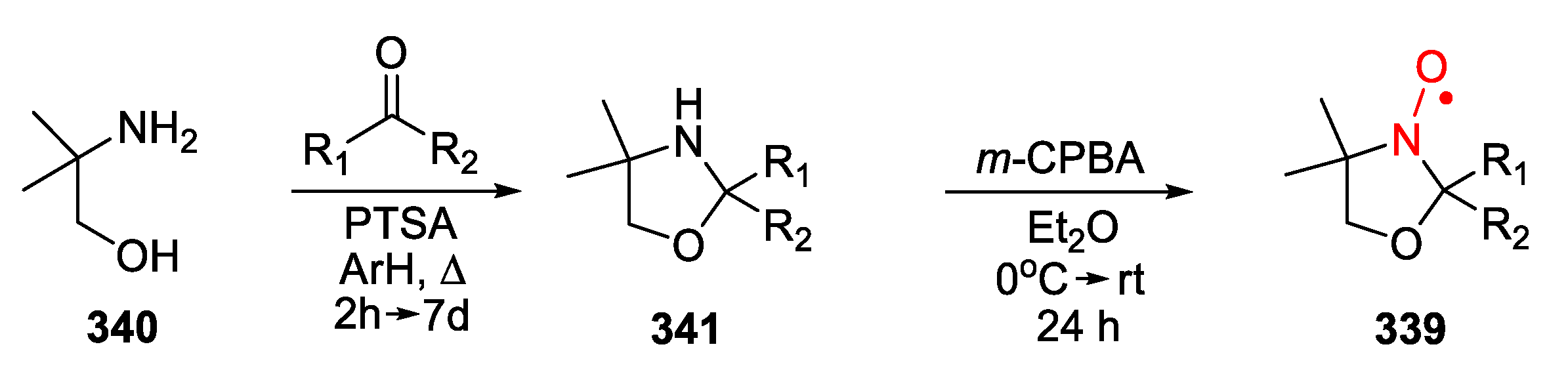
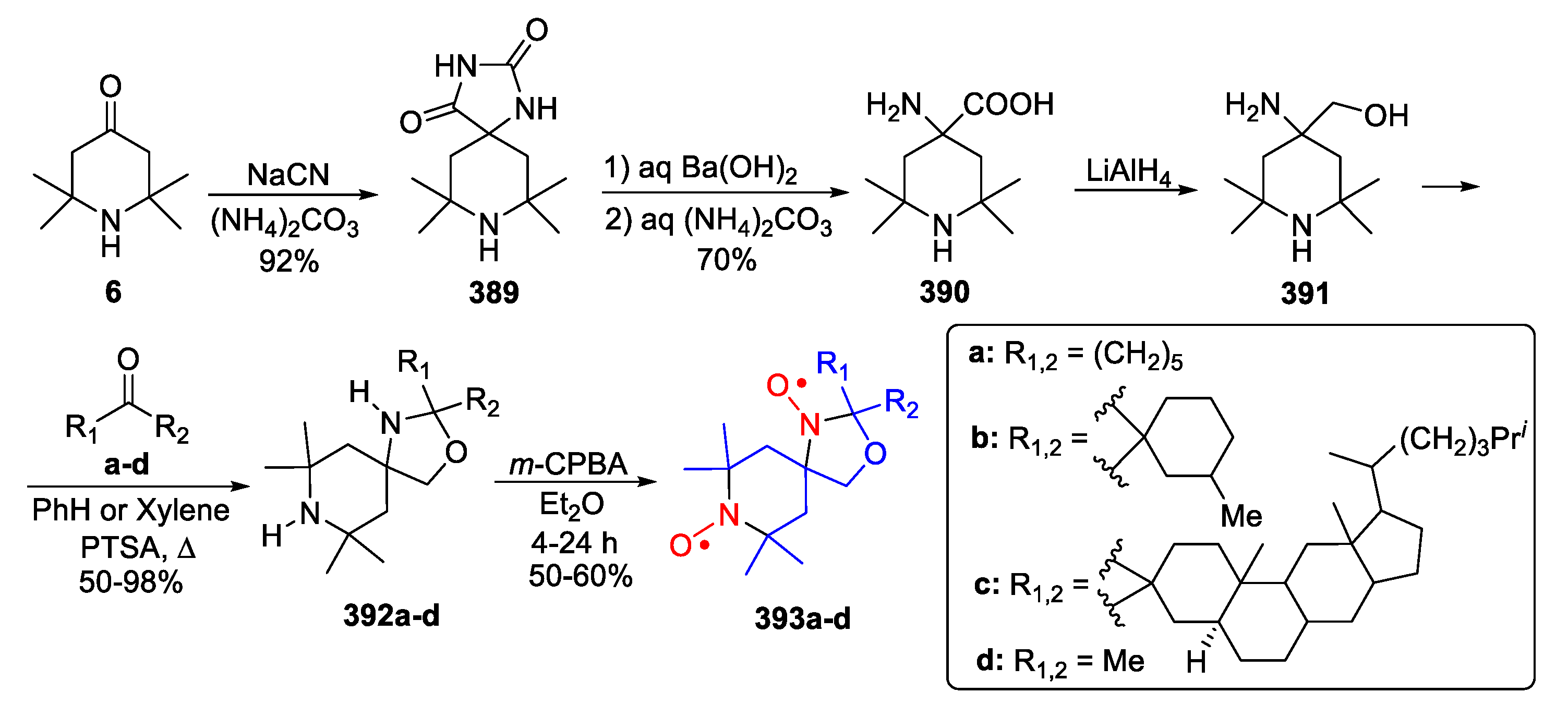
| 329, 330 | R1 | R2 | R3 | R4 | Yield of Amine 329, % | Yield of SNR 330, % |
|---|---|---|---|---|---|---|
| a | (CH2)5 | (CH2)5 | 86 | 78 | ||
| b | (CH2)5 | H | n-C3H7 | 76 | nonradical | |
| c | CH3 | CH3 | (CH2)5 | 90 | 89 | |
| d | (CH2)5 | (CH2)4 | 70 | ND | ||
| e | (CH2)5 | H | n–C11H23 | 24 | nonradical | |
| f | (CH2)5 | H | Ph | 86 | nonradical | |
| g | (CH2)5 | CH3 | 3-Pyridyl | 67 | ND | |
| h | (CH2)5 | 4,4-TMP | 40 | ND | ||
| i | (CH2)5 | 4,4-TEMPO | 34 | 16 | ||
| j | 4,4-TMP | H | CCl3 | 88 | nonradical | |
| k | 4,4-TMP | H | p-Cl-C6H4 | 69 | nonradical | |
| l | 4,4-TEMPO | H | n–C3H7 | 61 | nonradical | |
| m | 4,4-TEMPO | (CH2)5 | 45 | 81 | ||
| n | 4,4-TEMPO | H | Ph | 64 | nonradical | |
| o | 4,4-TEMPO | H | o-CH3-C6H4 | 88 | nonradical | |
| p | 4,4-TEMPO | H | p–CH3O-C6H4 | 81 | nonradical | |
| q | 4,4-TEMPO | 4,4-TEMPO | 24 | ND | ||
| r | H | Ph | (CH2)5 | 40 | nonradical | |
| s | CH3 | Ph | (CH2)5 | 40 | ND | |
| t | 2–CH3-Cyclohexyl | 2–CH3-Cyclohexyl | 36 | 67 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaytseva, E.V.; Mazhukin, D.G. Spirocyclic Nitroxides as Versatile Tools in Modern Natural Sciences: From Synthesis to Applications. Part I. Old and New Synthetic Approaches to Spirocyclic Nitroxyl Radicals. Molecules 2021, 26, 677. https://doi.org/10.3390/molecules26030677
Zaytseva EV, Mazhukin DG. Spirocyclic Nitroxides as Versatile Tools in Modern Natural Sciences: From Synthesis to Applications. Part I. Old and New Synthetic Approaches to Spirocyclic Nitroxyl Radicals. Molecules. 2021; 26(3):677. https://doi.org/10.3390/molecules26030677
Chicago/Turabian StyleZaytseva, Elena V., and Dmitrii G. Mazhukin. 2021. "Spirocyclic Nitroxides as Versatile Tools in Modern Natural Sciences: From Synthesis to Applications. Part I. Old and New Synthetic Approaches to Spirocyclic Nitroxyl Radicals" Molecules 26, no. 3: 677. https://doi.org/10.3390/molecules26030677
APA StyleZaytseva, E. V., & Mazhukin, D. G. (2021). Spirocyclic Nitroxides as Versatile Tools in Modern Natural Sciences: From Synthesis to Applications. Part I. Old and New Synthetic Approaches to Spirocyclic Nitroxyl Radicals. Molecules, 26(3), 677. https://doi.org/10.3390/molecules26030677