Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals
Abstract
1. Introduction
2. Sources of Essential Oils
3. Demand for Essential Oils in Cosmetic Industries
4. Chemistry of Essential Oils
5. Classification of Essential Oils Fragrances
6. Aroma Profiles of Essential Oils and Their Individual Compounds
7. Safety of Essential Oils
8. Authenticity of Essential Oils
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability: Samples of essential oils are not available from the authors. |
References
- Coppen, J.J. Flavours and Fragrances of Plant Origin; FAO: Rome, Italy, 1995. [Google Scholar]
- Amberg, N.; Fogarassy, C. Green consumer behavior in the cosmetics market. Resources 2019, 8, 137. [Google Scholar] [CrossRef]
- Mitsui, T. New Cosmetic Science; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind. Crops Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Pouresmaeil, M.; Nojadeh, M.S.; Movafeghi, A.; Maggi, F. Exploring the bio-control efficacy of Artemisia fragrans essential oil on the perennial weed Convolvulus arvensis: Inhibitory effects on the photosynthetic machinery and induction of oxidative stress. Ind. Crops Prod. 2020, 155, 112785. [Google Scholar] [CrossRef]
- Zuzarte, M.; Salgueiro, L. Essential oils chemistry. In Bioactive Essential Oils and Cancer; Springer: Berlin, Germany, 2015; pp. 19–61. [Google Scholar]
- Burger, P.; Plainfossé, H.; Brochet, X.; Chemat, F.; Fernandez, X. Extraction of natural fragrance ingredients: History overview and future trends. Chem. Biodivers. 2019, 16, e1900424. [Google Scholar] [CrossRef]
- Loo, M. Integrative Medicine for Children; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Burnett, C.L.; Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J. Safety Assessment of Citrus-Derived Peel Oils as Used in Cosmetics. Int. J. Toxicol. 2019, 38, 33S–59S. [Google Scholar] [CrossRef]
- Pitman, V. Aromatherapy: A Practical Approach; Nelson Thornes: Cheltenham, UK, 2004. [Google Scholar]
- Hussain, H.; Al-Harrasi, A.; Green, I.R. Frankincense (Boswellia) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 431–440. [Google Scholar]
- Barbieri, C.; Borsotto, P. Essential Oils: Market and Legislation; El-Shemy, H., Ed.; Potential of Essential Oils, IntechOpen: London, UK, 2018; pp. 107–127. [Google Scholar]
- Dreger, M.; Wielgus, K. Application of essential oils as natural cosmetic preservatives. Herba Pol. 2013, 59, 142–156. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P.; Domagalska, B.W.; Młynarczyk, A. Essential oils and herbal extracts as antimicrobial agents in cosmetic emulsion. Indian J. Microbiol. 2013, 53, 232–237. [Google Scholar] [CrossRef]
- Manou, I.; Bouillard, L.; Devleeschouwer, M.; Barel, A. Evaluation of the preservative properties of Thymus vulgaris essential oil in topically applied formulations under a challenge test. J. Appl. Microbiol. 1998, 84, 368–376. [Google Scholar]
- Aumeeruddy-Elalfi, Z.; Lall, N.; Fibrich, B.; Van Staden, A.B.; Hosenally, M.; Mahomoodally, M.F. Selected essential oils inhibit key physiological enzymes and possess intracellular and extracellular antimelanogenic properties in vitro. J. Food Drug Anal. 2018, 26, 232–243. [Google Scholar] [CrossRef]
- Mishra, A.; Mishra, A.; Chattopadhyay, P. Assessment of in vitro sun protection factor of Calendula officinalis L.(asteraceae) essential oil formulation. J. Young Pharm. 2012, 4, 17–21. [Google Scholar] [CrossRef]
- Wongsukkasem, N.; Soynark, O.; Suthakitmanus, M.; Chongdiloet, E.; Chairattanapituk, C.; Vattanikitsiri, P.; Hongratanaworakit, T.; Tadtong, S. Antiacne-causing Bacteria, Antioxidant, Anti-Tyrosinase, Anti-Elastase and Anti-Collagenase Activities of Blend Essential Oil comprising Rose, Bergamot and Patchouli Oils. Nat. Prod. Commun. 2018, 13, 639–642. [Google Scholar] [CrossRef]
- Winkelman, W.J. Aromatherapy, botanicals, and essential oils in acne. Clin. Dermatol. 2018, 36, 299–305. [Google Scholar]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical Assessment of New Topical Cream Containing Two Essential Oils Combined with Tretinoin in the Treatment of Acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233. [Google Scholar] [CrossRef]
- Cao, H.; Yang, G.; Wang, Y.; Liu, J.P.; Smith, C.A.; Luo, H.; Liu, Y. Complementary therapies for acne vulgaris. Cochrane Database Syst. Rev. 2015, 1, CD009436. [Google Scholar] [CrossRef]
- Cavanaugh, J.L. Examining the Differential Effects of Natural and Synthetic Aromas of Lavender and Peppermint on Cognition, Mood, and Subjective Workload. Ph.D. Thesis, University of Colorado, Denver, CO, USA, 2013. [Google Scholar]
- Olujimi, O.; Fatoki, O.S.; Odendaal, J.P.; Okonkwo, J. Endocrine disrupting chemicals (phenol and phthalates) in the South African environment: A need for more monitoring. Water SA 2010, 36, 5. [Google Scholar] [CrossRef]
- Carson, P.A. Hazardous Chemicals Handbook; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Ahuja, K.; Singh, S. Essential Oils Market Size by Application (Orange oil, Lemon oil, Eucalyptus oil, Clove oil, Peppermint oil, Jasmine oil, Rosemary oil, Cornmint oil, Citronella oil, Geranium, Spearmint oil, Lavender oil, Tea tree oil and others) by Application (Food & beverage, Aromatherapy, Cosmetics & Toiletries, Pharmaceuticals, Cleaning & Home care, Animal Feed, Fragrances and Others) Industry Analysis Report, Regional Outlook, Growth Potential, Competitive Market Share & Forecast, 2019–2026. Global Market Insights, Inc. 2019. Available online: https://www.gminsights.com/industry-analysis/essential-oil-market (accessed on 11 December 2020).
- Butnariu, M.; Sarac, I. Essential oils from plants. J. Biotechnol. Biomed. Sci. 2018, 1, 35. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential oils and their single compounds in cosmetics—A critical review. Cosmetics 2018, 5, 11. [Google Scholar]
- De Silva, T. Development of essential oil industries in developing countries In A Manual on the Essential Oil Industry; United Nations Ndustrlal Development Organization: Vienna, Austria, 1995; Chapter 1. [Google Scholar]
- Burger, P.; Landreau, A.; Watson, M.; Janci, L.; Cassisa, V.; Kempf, M.; Azoulay, S.; Fernandez, X. Vetiver Essential Oil in Cosmetics: What Is New? Medicines 2017, 4, 41. [Google Scholar] [CrossRef]
- Babita, S.; Sellam, P.; Jayoti, M.; Puja, R. Floral essential oils: Importance and uses for mankind. HortFlora Res. Spectr. 2014, 3, 7–13. [Google Scholar]
- Franz, C.; Novak, J. Sources of essential oils. In Handbook of Essential Oils and Science, Technology and Applications; Hüsnü Can Baser, K., Buchbauer, G., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 39–81. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Başer, K.H.C.; Demirci, F. Chemistry of essential oils. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Berger, R.G., Ed.; Springer: New York, NY, USA, 2007; pp. 43–86. [Google Scholar]
- Sawamura, M. Citrus Essential Oils: Flavor and Fragrance; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Adlard, E. Handbook of Essential Oils. Science, Technology and Applications; Springer: Berlin, Germany, 2010. [Google Scholar]
- Ridder, M. Essential Oils Market Worldwide-Statistics & Facts. 2020. Available online: https://www.statista.com/topics/5174/essential-oils/ (accessed on 23 December 2020).
- Sadgrove, N.; Jones, G. A contemporary introduction to essential oils: Chemistry, bioactivity and prospects for Australian agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar]
- Grosch, W. Gas Chromatography—Olfactometry of Aroma Compounds. In Flavours and Fragrances; Springer: Berlin, Germany, 2007; pp. 363–378. [Google Scholar]
- Kusuma, H.S.; Mahfud, M. Chemical Composition of Essential Oil of Indonesia Sandalwood Extracted by Microwave-Assisted Hydrodistillation. AIP Conf. Proc. 2016, 1755, 050001-1–050001-6. [Google Scholar]
- Noudogbessi, J.; Yedomonhan, H.; Alitonou, G.; Chalard, P.; Figueredo, G.; Adjalian, E.; Avlessi, F.; Chalchat, J.; Sohounhloué, D. Physical characteristics and chemical compositions of the essential oils extracted from different parts of Siphonochilusaethiopicus (Schweinf.) BL Burtt (Zingiberaceae) harvested in Benin. J. Chem. Pharm. Res. 2012, 4, 4845–4851. [Google Scholar]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of terpenoids: Monoterpenes, sesquiterpenes and diterpenes. Annu. Plant Rev. 2018, 40, 258–303. [Google Scholar]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Maisanaba, S.; Puerto, M.; Prieto, A.I.; Gutierrez-Praena, D.; Jos, A.; Camean, A.M. In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: A review. Food Chem. Toxicol. 2015, 81, 9–27. [Google Scholar]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar]
- Sell, C.S. The Chemistry of Fragrances: From Perfumer to Consumer; Royal Society of Chemistry: London, UK, 2006. [Google Scholar]
- Lahlou, M. Essential oils and fragrance compounds: Bioactivity and mechanisms of action. Flavour Fragr. J. 2004, 19, 159–165. [Google Scholar] [CrossRef]
- Laska, M.; Teubner, P. Olfactory discrimination ability of human subjects for ten pairs of enantiomers. Chem. Senses 1999, 24, 161–170. [Google Scholar]
- Salgueiro, L.; Martins, A.; Correia, H. Raw materials: The importance of quality and safety. A review. Flavour Fragr. J. 2010, 25, 253–271. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T. Geographical impact on essential oil composition of endemic Kundmannia anatolica Hub.-Mor. (Apiaceae). Afr. J. Tradit. Complementary Altern. Med. 2017, 14, 131–137. [Google Scholar] [CrossRef]
- De Groot, A.C.; Schmidt, E. Essential oils, part IV: Contact allergy. Dermatitis 2016, 27, 170–175. [Google Scholar] [CrossRef]
- Masango, P. Towards Understanding Steam Distillation of Essential Oils by Differential Quantification of Principal Components Using Capillary Gas Chromatography. Ph.D. Thesis, University of Surrey, Guildford, UK, 2001. [Google Scholar]
- Irshad, M.; Subhani, M.A.; Ali, S.; Hussain, A. Biological Importance of Essential Oils, Essential Oils—Oils of Nature, Hany A. El-Shemy. 2020. Available online: https://www.intechopen.com/books/essential-oils-oils-of-nature/biological-importance-of-essential-oils (accessed on 15 December 2020).
- Vankar, P.S. Essential oils and fragrances from natural sources. Resonance 2004, 9, 30–41. [Google Scholar] [CrossRef]
- Simon, J. Essential oils and culinary herbs, Advances in new crops. In Proceedings of the First National Symposium ’New Crops: Research, Development, Economics’, Indianapolis, IN, USA, 3–26 October 1988; Timber Press: Portland, OR, USA, 1990; pp. 472–483. [Google Scholar]
- Da Silva-Santos, A.; Antunes, A.; D’avila, L.; Bizzo, H.; Souza-Santos, L. The use of essential oils and terpenics/terpenoids in cosmetics and perfumery: A study identifies the major uses of essential oils and terpenics/terpenoids through granted patents. Perfum. Flavor. 2005, 30, 50–55. [Google Scholar]
- ISO 3525: 2008(E). Oil of Amyris Balsamifera L. 2008. Available online: https://www.sis.se/api/document/preview/910039/ (accessed on 15 December 2020).
- Lawless, J. The Encyclopedia of Essential Oils: The Complete Guide to the Use of Aromatic oils in Aromatherapy, Herbalism, Health, and Well Being; Conari Press: San Francisco, CA, USA, 2013. [Google Scholar]
- Maia, J.G.S.; Andrade, E.H.A.; Couto, H.A.R.; Da Silva, A.C.M.; Marx, F.; Henke, C. Plant sources of Amazon rosewood oil. Quim. Nova 2007, 30, 1906–1910. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Mourão, R.H.V. Amazon Rosewood (Aniba rosaeodora Ducke) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 193–201. [Google Scholar]
- Lavabre, M. Aromatherapy Workbook; Inner Traditions/Bear & Co: Windsor County, VT, USA, 1996. [Google Scholar]
- Van Vuuren, S.F.; Kamatou, G.P.; Viljoen, A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010, 76, 686–691. [Google Scholar] [CrossRef]
- ISO 8896: 2016(E). Essential Oil of Caraway (Carum carvi L.) 2016. Available online: https://www.sis.se/api/document/preview/920364/ (accessed on 15 December 2020).
- Battaglia, S. Essential oil Monograph: Atlas Cedarwood. 2019. Available online: http://www.salvatorebattaglia.com.au/images/pdf/A4_Monograph_Atlas_Cedarwood.pdf (accessed on 15 December 2020).
- Jain, S.; Arora, P.; Popli, H. A comprehensive review on Citrus aurantifolia essential oil: Its phytochemistry and pharmacological aspects. Braz. J. Nat. Sci. 2020, 3, 354. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, R.-Z.; Qu, R.-F.; Li, Z.-Y. Comprehensive two-dimensional gas chromatography–time-of-flight mass spectrometry for the analysis of volatile components in Neroli essential oil. Mendeleev Commun. 2012, 1, 45–46. [Google Scholar] [CrossRef]
- Aguilar-Hernández, M.G.; Sánchez-Bravo, P.; Hernández, F.; Carbonell-Barrachina, Á.A.; Pastor-Pérez, J.J.; Legua, P. Determination of the Volatile Profile of Lemon Peel Oils as Affected by Rootstock. Foods 2020, 9, 241. [Google Scholar]
- ISO 3140:2019 (E). Essential Oil of Sweet Orange Expressed (Citrus sinensis L.). 2019. Available online: https://www.sis.se/api/document/preview/80011724/ (accessed on 15 December 2020).
- Arctander, S. Perfume and flavor materials of natural origin. Perfum. Flavor Mater. Nat. Origin. 1960, 370. [Google Scholar]
- Soares, B.V.; Morais, S.M.; dos Santos Fontenelle, R.O.; Queiroz, V.A.; Vila-Nova, N.S.; Pereira, C.M.; Brito, E.S.; Neto, M.A.; Brito, E.H.; Cavalcante, C.S.; et al. Antifungal activity, toxicity and chemical composition of the essential oil of Coriandrum sativum L. fruits. Molecules 2012, 17, 8439–8448. [Google Scholar] [PubMed]
- Groom, N.F. The Perfume Handbook; Groom, N., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1992; pp. 81–89. [Google Scholar]
- Mahmood, Z.; Ahmed, I.; Saeed, M.U.Q.; Sheikh, M.A. Investigation of physico-chemical composition and antimicrobial activity of essential oil extracted from lignin-containing Cupressus sempervirens. BioResources 2013, 8, 1625–1633. [Google Scholar] [CrossRef]
- Rao, B.R.R.; Rajput, D.K.; Patel, R.P.; Purnanand, S. Essential oil yield and chemical composition changes during leaf ontogeny of palmarosa (Cymbopogon martinii var. motia). Nat. Prod. Commun. 2010, 5, 1947–1950. [Google Scholar] [CrossRef]
- Rokbeni, N.; M’rabet, Y.; Dziri, S.; Chaabane, H.; Jemli, M.; Fernandez, X.; Boulila, A. Variation of the chemical composition and antimicrobial activity of the essential oils of natural populations of Tunisian Daucus carota L.(Apiaceae). Chem. Biodivers. 2013, 10, 2278–2290. [Google Scholar] [CrossRef]
- ISO 4733: 2004(E). Essential Oil of Cardamom. 2004. Available online: https://www.sis.se/api/document/preview/904961/ (accessed on 15 December 2020).
- Kladar, N.V.; Anačkov, G.T.; Rat, M.M.; Srđenović, B.U.; Grujić, N.N.; Šefer, E.I.; Božin, B.N. Biochemical characterization of Helichrysum italicum (Roth) G. Don subsp. italicum (Asteraceae) from Montenegro: Phytochemical screening, chemotaxonomy, and antioxidant properties. Chem. Biodivers. 2015, 12, 419–431. [Google Scholar]
- Judžentienė, A. Hyssop (Hyssopus officinalis L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 471–479. [Google Scholar]
- Majewska, E.; Kozłowska, M.; Kowalska, D.; Gruczyńska, E. Characterization of the essential oil from cone-berries of Juniperus communis L. (Cupressaceae). Herba Pol. 2017, 63, 48–55. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and biological activities of Laurel tree (Laurus nobilis). Nat. Prod. Commun. 2017, 12, 743–757. [Google Scholar] [CrossRef]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Singh, O.; Khanam, Z.; Misra, N.; Srivastava, M.K. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn Rev. 2011, 5, 82. [Google Scholar] [CrossRef] [PubMed]
- ISO 856:2006, Oil of Peppermint (Mentha x piperita L.). 2006. Available online: https://www.sis.se/api/document/preview/907351/ (accessed on 13 December 2020).
- Periasamy, G.; Karim, A.; Gibrelibanos, M.; Gebremedhin, G. Nutmeg (Myristica fragrans Houtt.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 607–616. [Google Scholar]
- ISO 21389:2004(E), Oil of Gum Turpentine (Pinus Massoniana Lamb.). 2004. Available online: https://cdn.standards.iteh.ai/samples/35861/a65f5ee52cc5413197cbc3e49c675fda/ISO-21389-2004.pdf (accessed on 18 December 2020).
- Van Beek, T.A.; Joulain, D. The essential oil of patchouli, Pogostemon cablin: A review. Flavour Fragr. J. 2018, 33, 6–51. [Google Scholar] [CrossRef]
- Atanasova, T.; Kakalova, M.; Stefanof, L.; Petkova, M.; Stoyanova, A.; Damyanova, S.; Desyk, M. Chemical composition of essential oil from Rosa Damascena mill., growing in new region of Bulgaria. Ukr. Food J. 2016, 5, 492–498. [Google Scholar]
- Jalali-Heravi, M.; Parastar, H.; Sereshti, H. Development of a method for analysis of Iranian damask rose oil: Combination of gas chromatography–mass spectrometry with Chemometric techniques. Anal. Chim. Acta 2008, 623, 11–21. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, A.; Yadav, A.K. Volatile constituents of essential oil and rose water of damask rose (Rosa damascena Mill.) cultivars from North Indian hills. Nat. Prod. Res. 2011, 25, 1577–1584. [Google Scholar] [CrossRef]
- Tomi, K.; Kitao, M.; Konishi, N.; Murakami, H.; Matsumura, Y.; Hayashi, T. Enantioselective GC–MS analysis of volatile components from rosemary (Rosmarinus officinalis L.) essential oils and hydrosols. Biosci. Biotechnol. Biochem. 2016, 80, 840–847. [Google Scholar] [CrossRef]
- Pešić, P.Ž.; Banković, V.M. Investigation on the essential oil of cultivated Salvia sclarea L. Flavour Fragr. J. 2003, 18, 228–230. [Google Scholar]
- Joy, P.P.; Thomas, J.; Mathew, S.; Skaria, B.P. Tropical aromatic and medicinal plants. Aromat. Med. Plants Res. Stn. Odakkali Asamannoor PO Kerala India 1998, 83–85. [Google Scholar]
- Howes, M.-J.R.; Simmonds, M.S.; Kite, G.C. Evaluation of the quality of sandalwood essential oils by gas chromatography–mass spectrometry. J. Chromatogr. 2004, 1028, 307–312. [Google Scholar] [CrossRef]
- Nurdjannah, N.; Bermawie, N.C. Cloves. Indonesian Agency for Agriculture Research and Development (IAARD). Indonesia 2012, 197–215. [Google Scholar]
- Chomchalow, N. The Utilization of Vetiver as Medicinal and Aromatic Plants with Special Reference to Thailand; Tech. Bull. No. 2001/1; PRVN/ORDPB: Bangkok, Thailand., 2001. [Google Scholar]
- David, A.; Wang, F.; Sun, X.; Li, H.; Lin, J.; Li, P.; Deng, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Vetiveria zizanioides (L.) Nash Essential Oil Extracted by Carbon Dioxide Expanded Ethanol. Molecules 2019, 24, 1897. [Google Scholar] [CrossRef] [PubMed]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile composition of essential oils from different aromatic herbs grown in mediterranean regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- D’Acampora Zellner, B.; Lo Presti, M.; Barata, L.E.S.; Dugo, P.; Dugo, G.; Mondello, L. Evaluation of leaf-derived extracts as an environmentally sustainable source of essential oils by using gas chromatography− mass spectrometry and enantioselective gas chromatography− olfactometry. Anal. Chem. 2006, 78, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Prakash, M.; Bhat, K.K. Aroma characterization of coriander (Coriandrum sativum L.) oil samples. Eur. Food Res. Technol. 2007, 225, 367–374. [Google Scholar] [CrossRef]
- Mortimer, S.; Reeder, M. Botanicals in dermatology: Essential oils, botanical allergens, and current regulatory practices. Dermatitis 2016, 27, 317–324. [Google Scholar]
- Tisserand, R.; Young, R. Essential oil profiles. In Essential Oil Safety A Guide for Health Care Professionals, 2nd ed.; Elsevier: London, UK, 2014; pp. 187–482. [Google Scholar]
- Vostinaru, O.; Heghes, S.C.; Filip, L. Safety Profile of Essential Oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Bouchiha, H.; Rouabhi, R.; Bouchama, K.; Djebar-Berrebbah, H.; Djebar, M. Potential toxicity of essential oil extracted from medicinal plant (Mentha piperita) on an alternative cellular model paramecium sp. Rev. Kasmera 2015, 43, 114–130. [Google Scholar]
- Buckle, J. Essential Oil Toxicity and Contraindications. In Clinical Aromatherapy (Third Edition) Essential Oils in Healthcare; Churchill Livingstone: London, UK, 2016; pp. 73–94. [Google Scholar]
- Balick, R. A peek into safe use of essential oils. Pharm. Today 2019, 25, 22–23. [Google Scholar] [CrossRef]
- Van Oosten, E.J.; Schuttelaar, M.L.A.; Coenraads, P.J. Clinical relevance of positive patch test reactions to the 26 EU-labelled fragrances. Contact Dermat. 2009, 61, 217–223. [Google Scholar] [CrossRef]
- SCCS, Reference: Scientific Committee on Consumer Safety (SCCS). Opinion on Fragrance Allergens in Cosmetic Products. 2012. Available online: https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_102.pdf (accessed on 26 December 2020).
- Es, I.; Khaneghah, A.M.; Akbariirad, H. Global regulation of essential oils. In Essential Oils in Food Processing: Chemistry, Safety and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 327–338. [Google Scholar]
- SCCNFP. The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers, Fragrance Allergy in Consumers. A Review of the Problem. Analysis of the Need for Appropriate Consumer Information and Identification of Consumer Allergens. 1999. Available online: https://ec.europa.eu/health/ph_risk/committees/sccp/documents/out98_en.pdf (accessed on 22 December 2020).
- Lis-Balchin, M. Aromatherapy Science: A Guide for Healthcare Professionals; Pharmaceutical Press: London, UK, 2006. [Google Scholar]
- Warshaw, E.M.; Zug, K.A.; Belsito, D.V.; Fowler, J.F., Jr.; DeKoven, J.G.; Sasseville, D.; Maibach, H.I.; Mathias, C.T.; DeLeo, V.A.; Taylor, J.S. Positive patch-test reactions to essential oils in consecutive patients from North America and Central Europe. Dermatitis 2017, 28, 246–252. [Google Scholar] [CrossRef]
- Do, T.K.T.; Hadji-Minaglou, F.; Antoniotti, S.; Fernandez, X. Authenticity of essential oils. TrAC Trends Anal. Chem. 2015, 66, 146–157. [Google Scholar]
- Kubeczka, K.-H. History and Sources of Essential Oil Research. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–39. [Google Scholar]
- Chin, S.T.; Nolvachai, Y.; Marriott, P.J. Enantiomeric Separation in Comprehensive Two-Dimensional Gas Chromatography with Accurate Mass Analysis. Chirality 2014, 26, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R. Regular monoterpenes and sesquiterpenes (essential oils). Nat. Prod. 2013, 10, 2973–3008. [Google Scholar]
- Fiorini, D.; Scortichini, S.; Bonacucina, G.; Greco, N.G.; Mazzara, E.; Petrelli, R.; Torresi, J.; Maggi, F.; Cespi, M. Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design. Ind. Crops Prod. 2020, 154, 112688. [Google Scholar]
- Mani, B.K.; Murthy, V.; Boland, M.; Yee, K. Analysis of constituents in different Fractions collected during distillation of Cardamom oil for flavour and fragrance applications. J. Appl. Pharm. Sci. 2017, 7, 177–183. [Google Scholar] [CrossRef][Green Version]
- Clery, R. High-impact odorants in essential oils. Flavour Fragr. J. 2010, 25, 117–120. [Google Scholar] [CrossRef]
- Benzo, M.; Gilardoni, G.; Gandini, C.; Caccialanza, G.; Finzi, P.V.; Vidari, G.; Abdo, S.; Layedra, P. Determination of the threshold odor concentration of main odorants in essential oils using gas chromatography–olfactometry incremental dilution technique. J. Chromatogr. 2007, 1150, 131–135. [Google Scholar]
- Breme, K.; Tournayre, P.; Fernandez, X.; Meierhenrich, U.J.; Brevard, H.; Joulain, D.; Berdague, J.L. Identification of odor impact compounds of Tagetes minuta L. essential oil: Comparison of two GC-olfactometry methods. J. Agric. Food Chem. 2009, 57, 8572–8580. [Google Scholar]
- Eyres, G.; Marriott, P.J.; Dufour, J.-P. The combination of gas chromatography–olfactometry and multidimensional gas chromatography for the characterisation of essential oils. J. Chromatogr. 2007, 1150, 70–77. [Google Scholar] [CrossRef]
- Da, S. Rauber, C.; Guterres, S.S.; Schapoval, E.E. LC determination of citral in Cymbopogon citratus volatile oil. J. Pharm. Biomed. Anal. 2005, 37, 597–601. [Google Scholar]
- Lockwood, G. Techniques for gas chromatography of volatile terpenoids from a range of matrices. J. Chromatogr. 2001, 936, 23–31. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Dugo, L.; Stancanelli, R.; Dugo, G. LC-MS for the identification of oxygen heterocyclic compounds in citrus essential oils. J. Pharm. Biomed. Anal. 2000, 24, 147–154. [Google Scholar] [CrossRef]
- König, W.A.; Hochmuth, D.H. Enantioselective gas chromatography in flavor and fragrance analysis: Strategies for the identification of known and unknown plant volatiles. J. Chromatogr. Sci. 2004, 42, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Mosandl, A. Authenticity assessment: A permanent challenge in food flavor and essential oil analysis. J. Chromatogr. Sci. 2004, 42, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Orlandini, G.; van Leeuwen, K.A.; Anesin, G.; Bertelli, D.; Paolini, M.; Benvenuti, S.; Camin, F. Gas chromatography combined with mass spectrometry, flame ionization detection and elemental analyzer/isotope ratio mass spectrometry for characterizing and detecting the authenticity of commercial essential oils of Rosa damascena Mill. Rapid Commun. Mass Spectrom. 2013, 27, 591–602. [Google Scholar] [CrossRef] [PubMed]
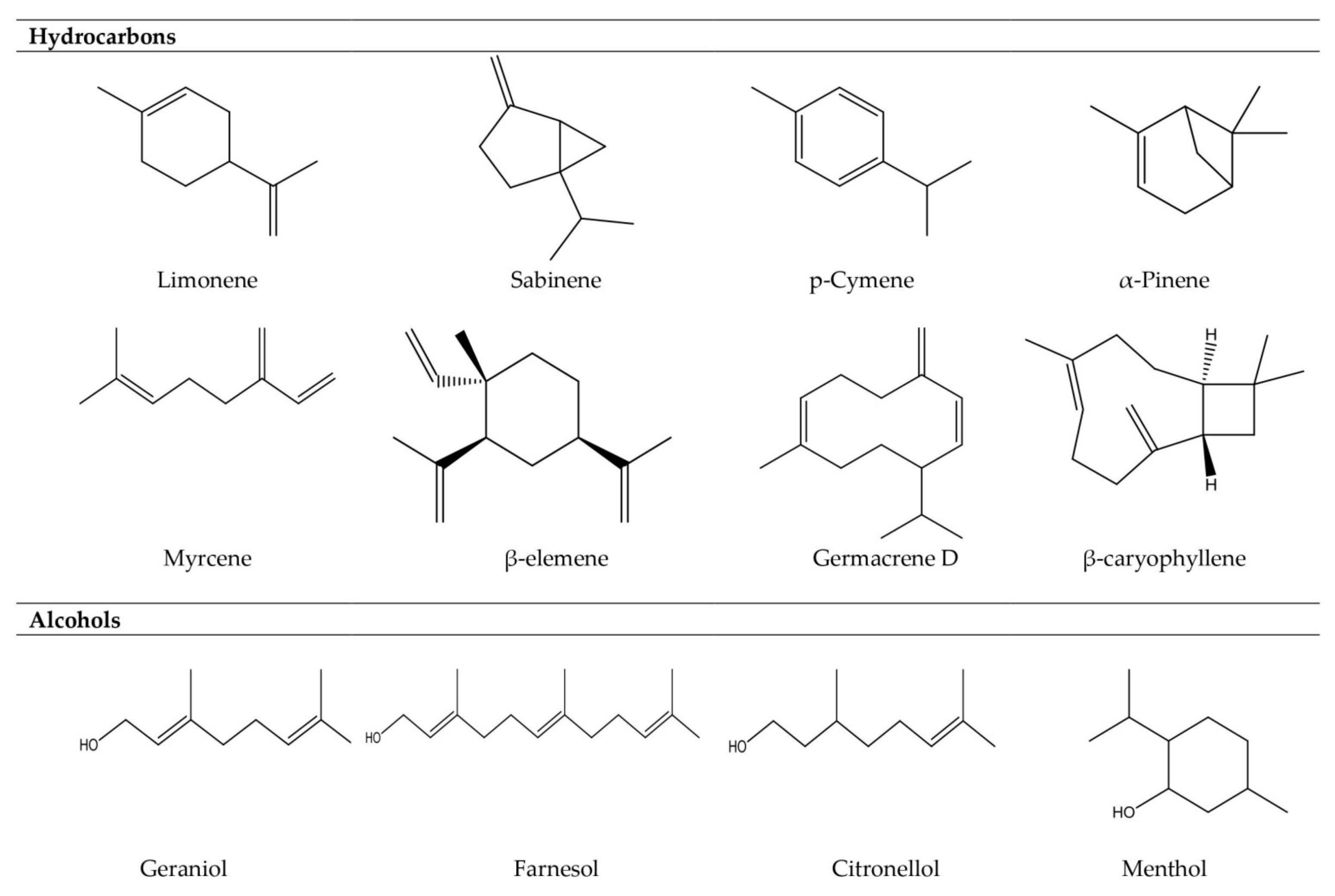
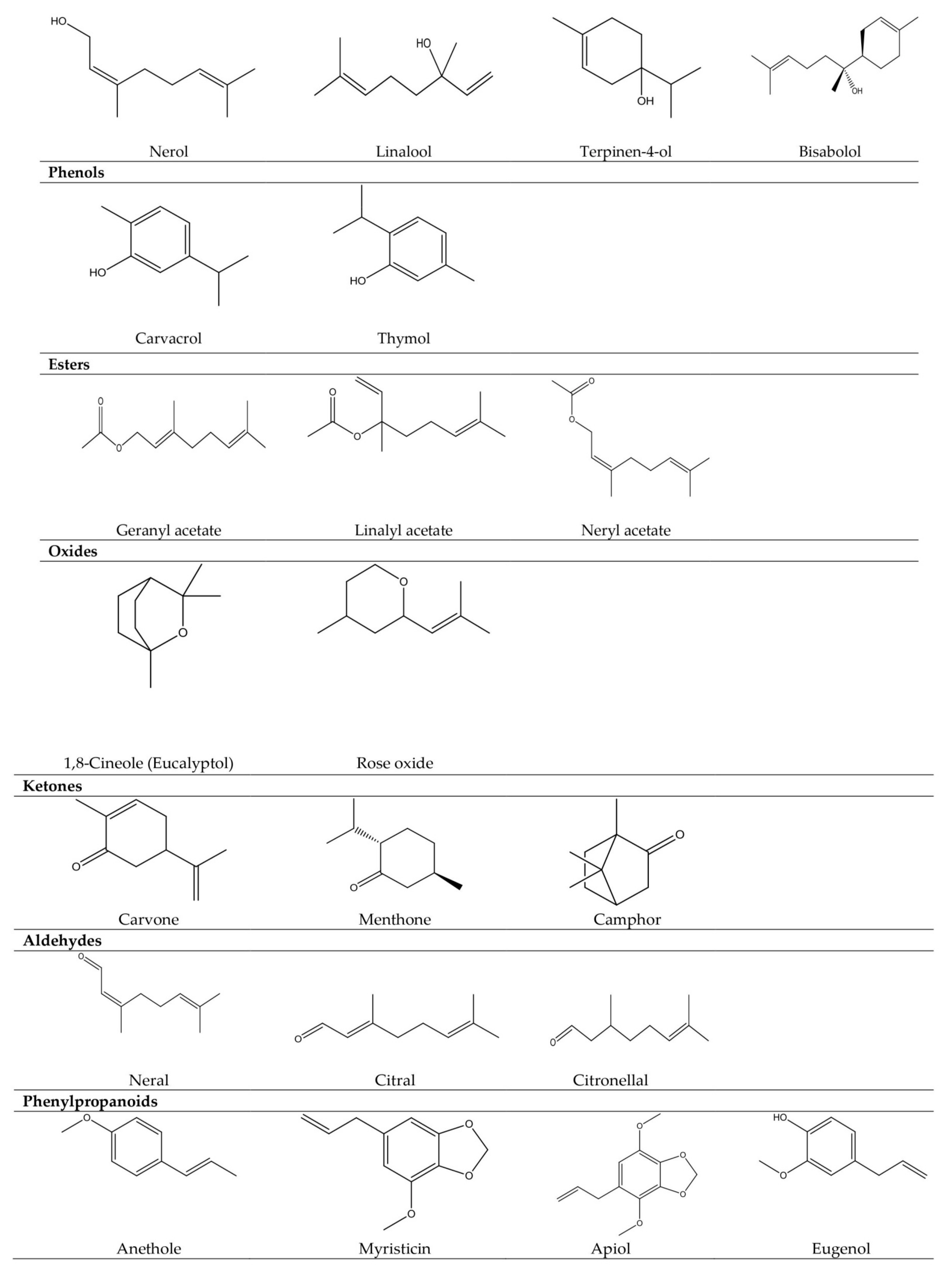
| Type of Vascular Plants | Plant Families | Examples of Essential Oil-Bearing Plants |
|---|---|---|
| 1. Gymnosperms | Cupressaceae | Cedar leaf, cedarwood, juniper |
| Pinaceae | Fir and pine | |
| 2. Angiosperms | ||
| Monocots | Acoraceae | Calamus |
| Poaceae | Vetiver and aromatic grass | |
| Zingiberaceae | Ginger and cardamom | |
| Dicots | Apiaceae | Coriander and fennel |
| Asteraceae | Tarragon, chamomile and wormwood | |
| Geraniaceae | Geranium | |
| Illiciaceae | Star anise | |
| Lamiaceae | Lavender, patchouli, mint, oregano | |
| Lauraceae | Litsea, cinnamon, camphor, sassafras | |
| Myristicaceae | Mace and nutmeg | |
| Myrtaceae | Allspice, myrtle and clove | |
| Oleaceae | Jasmine | |
| Rosaceae | Rose | |
| Santalaceae | Sandalwood |
| Type of Perfume | Fragrance/Essential Oil | Alcohol |
|---|---|---|
| Eau de parfum | 8–15% | 80–90% |
| Splash colognes | 1–3% | 80% |
| Eau de cologne | 3–5% | 70% |
| Eau de toilette | 4–8% | 80–90% |
| Plant Species from Which Essential Oils are Derived | Common Name | Part(s) Used | Principal Componentsa | Color Range of Oils | Aroma Description | References |
|---|---|---|---|---|---|---|
| 1. Amyris balsamifera L. | Balsam torchwood | Wood | Elemol, 10,γ-epi-eudesmol, γ-eudesmol, valerianol, α-eudesmol, 7-α-epi-eudesmol, β-eudesmol, drimenol | Pale yellow to amber yellow | Characteristic, woody | [60] |
| 2. Angelica archangelica | Angelica | Roots and rhizomes | Phellandrene, pinene, limonene, linalool and borneol; rich in coumarins including osthol, Angelicin, bergapten and imperatorin | Colorless or pale yellow | Rich herbaceous-earthy body note | [61] |
| Seed | Colorless | Fresher, spicy top note | ||||
| 3. Aniba rosaeodora Ducke | Rosewood | Wood/leaves | α-Pinene, β-pinene, limonene, 1,8-cineole, trans-linalool oxide (furanoid), cis-linalool oxide (furanoid), linalool, trans-linalool oxide (pyranoid), α-terpineol, α-copaene, β-selinene, α-selinene, spathulenol, caryophyllene oxide, benzyl benzoate | Pale-yellow | Floral, sweet, woody, and citric | [62,63] |
| 4. Boswellia sacra Flueck. | Frankincense | Gum | α-Pinene (2.0–64.7%), α-thujene (0.3–52.4%), β-pinene (0.3–13.1%), myrcene (1.1–22.4%), sabinene (0.5–7.0%), limonene (1.3–20.4%), p-cymene (2.7–16.9%) and β-caryophyllene (0.1–10.5%) | Clear or yellow | Balsamic, camphor-like, spicy, woody, slightly lemony | [64,65] |
| 5. Cananga odorata Hook. F. and Thoms) | Ylang ylang | Flowers | Prenyl acetate, p-cresyl methyl ether, methyl benzoate, linalool, benzyl acetate, geraniol, geranyl acetate, (E)-cinnamyl acetate, β-caryophyllene, germacrene-D, (E,E)-α-farnesene, (E,E)-farnesol, benzyl benzoate, (E,E)-farnesyl acetate and benzyl salicylate | Pale yellow to dark yellow | Characteristic, floral, recalling jasmine | ISO3063:2004(E) |
| 6. Carum carvi L. | Caraway | Ripe fruit | Myrcene, limonene, cis-dihyrocarvone, trans-carveol, cis-carveol, carvone | Colorless to pale yellow | Fresh, herbaceous, spicy | [66] |
| 7. Cedrus atlantica (Endl.) Manetti ex Carrière | Cedarwood | Wood/sawdust | Himachalene, α-himachalene, β-himachalene, cis-bisabolene, himachalol, allo-himachalol, α-atlantone, ƴ-atlantone, himachalene oxide | Yellowish to orange-yellow or deep amber-colored | Camphoraceous–cresylic top note with a sweet, tenacious woody undertone, reminiscent of cassie and mimosa | [67] |
| 8. Cinnamomum verum J. Presl | Cinnamon | Bark | (E)-Cinnamaldehyde, eugenol, β-caryophyllene, β-phellandrene, linalool | Reddish-brown | Characteristically spicy burning | [54,64] |
| 9. Citrus aurantiifolia (Christm.) Swingle | Lime | Peel | Limonene, β-pinene, γ-terpinene, citral | Colorless to greenish-yellow | Mild citrus, floral | [10,68] |
| 10. Citrus aurantium var. amara L. | Neroli/bitter orange | Flowers | Linalool, β-pinene, α-terpineol, limonene, sabinene, nerol, nerolidol, linalyl acetate and α-pinene | Pale yellow to coffee brown | Sweet, fresh and floral odor | [28,69] |
| 11. Citrus bergamia Risso | Bergamot | Rind of fruit | Linalyl acetate, limonene, α-terpinene, β-pinene, γ-terpinene, geraniol, linalool, lactones, bergaptene, β-bisabolene | Light green-yellow | Light, delicate citrus-like scent with slightly floral/sweet/balsamic character (top note) | [11] |
| 12. Citrus limon (L.) Osbeck | Lemon | Peel | Limonene, β-pinene, γ-terpinene, sabinene, α-pinene, geranial, neral, α-thujene, β-bisabolene, terpinolene, trans-α-bergamotene, α-terpineol, α-terpinene, neryl acetate, linalool, p-cymene, citronellal, trans-caryophyllene, terpineol-4, nerol, camphene, nonanal, geraniol, octyl aldehyde, α-phellandrene, and cis-sabinene-hydrate | Colorless to pale yellow | Fresh lemon peel | [10,70] |
| 13. Citrus reticulata Blanco | Tangerine | Peel | Limonene, γ-terpinene, terpinolene, myrcene, α-pinene, p-cymene, α-thujene | Dark orange to reddish-orange or brownish-orange | Orange-like | [10,54] |
| 14. Citrus sinensis (L.) Osbeck | Sweet orange | Peel | Limonene, α-pinene, β-pinene, sabinene, myrcene, geranial, n-octanal, n-decanal, n-nonanal, neral, β-sinensal, valencene, linalool | Yellow to reddish-yellow | Characteristic, orange peel odor | [71] |
| 15. Citrus paradisi Macfad. | Grapefruit | Peel of fruit | Limonene, citronellal, citral, sinensal, geranyl acetate, paradisol, geraniol, ketones, lactones, coumarins such as auraptene, limettin and sesquiterpenes such as cadinene | Yellow or greenish | Fresh, sharp, citrus aroma (top note) | [11] |
| 16. Commiphora myrrha (Nees) Engl. | Myrrh | Gum | Furanoeudesma-1,3-diene, curzerene, β-elemene, lindestrene, furanodiene | Yellow to reddish-brown | Pleasant balsamic, camphor-like, musty, incense-like | [54,64] |
| 17. Coriandrum sativum L. | Coriander | Fruit | Borneol, 2E-decenal, camphor, dodecanal, n-decanal, neryl acetate, geraniol, linalool | Colorless to pale yellow | Floral, balsamic undertone and peppery-woody suave top-note | [72,73] |
| 18. Cupressus sempervivens L. | Cypress | Leaves | α-Pinene, Δ-carene, limonene, sesquiterpene, α-terpinene, sabinene, carvone | Yellowish | Pleasingly smoky, woody smell, and amber-like | [74,75] |
| 19. Cymbopogon citratus (DC.) Stapf | Lemongrass | Grass leaf | Citral, myrcene, dipentene, linalool, geraniol, nerol, citronellol, and farnesol, sesquiterpenes, methyl heptenone, esters, acids and others | Yellow or pale sherry-colored | Intense sweet lemony, reminiscent of lemon drops (top note) | [11] |
| 20. Cymbopogon martini (Roxb.) W. Watson | Palmarosa | Leaves | Myrcene, linalool, β-caryophyllene, α-terpineol, geranyl acetate, geranyl isobutyrate, geraniol, (E,Z) farnesol, geranyl hexanoate | Yellow | Fresh rose-like | [64,76] |
| 21. Daucus carota L. | Carrot seed | Seeds | β-Bisabolene, elemicin, geranyl acetate, and sabinene | Yellowish-brown color | Soft, sweet earthy grounding aroma | [77] |
| 22. Elettaria cardamomum (L.) Maton | Cardamom | Fruits | α-Pinene, sabinene, myrcene, limonene, 1,8-cineole, linalool, linalyl acetate, terpinen-4-ol, α-terpineol, terpinyl acetate, trans-nerolidol | Almost colorless to pale yellow | Characteristic, spicy, cineolic | [78] |
| 23. Eucalyptus globulus Labill. | Eucalyptus | Leaves | 1,8-Cineole, α-pinene, limonene, aromadendrene, p-cymene, globulol | Yellow to red | Fresh balsamic camphor-like | [54,64] |
| 24. Feniculum vulgare Mill. | Fennel | Seeds | Phenols, trans-anethole, methyl chavicol, α-pinene, α-thujene, γ-terpinene, limonene, myrcene, phellandrene, fenchone, 1,8-cineole, fenchol, acids, lactones and coumarins | Yellowish | Strong, anisey, camphoric | [11,64] |
| 25. Helichrysum italicum (Roth) G. Don | Immortelle | Aerial parts | Neryl acetate, g-curcumene, neryl propionate and ar-curcumene | Pale yellow to red | Strong, honey-like aroma | [28,79] |
| 26. Hyssopus officinalis L. | Hyssop | Aerial parts | Isopinocamphone, pinocamphone, β-pinene | Light-yellow | Herbaceous, camphor-like odors with warm and spicy undertones | [80] |
| 27. Illicium verum Hook.f. | Star anise | Fruit | Trans-anethole (80–90%) | Pale yellow | Warm, spicy, extremely sweet, licorice-like scent | [61] |
| 28. Jasminum officinale L. | Jasmine | Flowers | Benzyl acetate, linalyl acetate, benzyl benzoate, methyl jasmonate, methyl anthranilate, linalool, nerol, geraniol, benzyl alcohol, farnesol, terpineol, phytols, eugenol, cis-jasmone; acids, aldehydes and others | Dark, orange-brown | Highly intense, rich, sweet, floral odor (base note) | [11] |
| 29. Juniper communis L. | Juniper | Ripe berries | α-Pinene (20–50%), sabinene (<20%), β-pinene (1–12%), β-myrcene 1–35%, α-phellandrene (<1%), limonene 2–12%, terpinen-4-ol (0.5–10%), bornyl acetate (<2%), and β-caryophyllene (< 7%) | Colorless or pale yellow-green | Fresh terebinth or turpentine-like/conifer-like aroma | [74,81] |
| 30. Laurus nobilis L. | Laurel | Leaves | 1,8-Cineole, sabinene, α-terpinyl acetate, linalool, eugenol, methyl eugenol, α-pinene | Yellow | Aromatic, spicy | [82] |
| 31. Lavandula angustifolia Mill. | Lavender | Flowering tops | Linalyl acetate (25–47%), linalool (max. 45%), terpinen-4-ol (max. 8%), camphor (max. 1.5%), limonene (max. 1%) and 1,8-cineole (max. 3%) | Colorless to pale yellow | Sweet floral aroma | [28] |
| 32. Litsea cubeba (Lour.) Pers. | Litsea | Fruits | Citral (neral and geranial) (78.7–87.4%), d-limonene (0.7–5.3%) | Pale yellow | An intense lemon-like, spicy aroma | [83] |
| 33. Matricaria chamomilla L. | German chamomile | Flowers and flower heads | (E)-β-Farnesene (4.9–8.1%), terpene alcohol (farnesol), chamazulene (2.3–10.9%), α-bisabolol (4.8–11.3%), and α-bisabolol oxides A (25.5–28.7%) and α-bisabolol oxides B (12.2–30.9%) | Blue | Warm, fruity scent Sweet herbaceous odor (middle note) | [84] |
| 34. Melaleuca alternifolia (Maiden and Betche) Cheel | Tea tree | Leaves | Terpinen-4-ol (30–48%), γ-terpinene (10–28%), 1,8-cineole (traces-15%), α-terpinene (5–13%), α-terpineol (1.5–8%), p-cymene (0.5–8%), α-pinene (1–6%), terpinolene (1.5–5%), sabinene (traces-3.5%), aromadendrene (traces-3%), δ-cadinene (traces-3%), viridiflorene (traces-3%), limonene (0.5–1.5%), globulol (traces-1%), viridiflorol (traces-1%) [ISO 4730 (2004)] | Colorless to pale yellow | Intensive aromatic fresh camphoraceous odor | [28] |
| 35. Melaleuca leucadendra (L.) L. | Cajeput | Fresh leaves and twigs | 1,8-Cineole (14–65%), terpenes (45%), alcohols (5%), esters | Pale yellow-green | Camphorous, highly penetrating odor with a slightly fruity note (top note) | [11] |
| 36. Mentha x piperita L. | Peppermint | Aerial parts | Menthol, menthone, isomenthone, menthofuran, neomenthol, pulegone, menthyl acetate, 3-octanal, 1,8-cineole, limonene, trans-sabinene hydrate, β-caryophyllene | Almost colorless to pale greenish yellow | Characteristic of mint, sweet, menthol-like | [85] |
| 37. Myristica fragrans Houtt. | Nutmeg | Seeds | Myristicin, α-pinene, sabinene, limonene, elemicin, eugenol, safrol and β-pinene | Pale yellow to nearly colorless | Spicy, sweet, woody | [86] |
| 38. Ocimum basilicum L. | Basil | Flowering herb | Linalool, citronellol, geraniol, terpinen-4-ol, α-terpineol, methyl chavicol, eugenol, ethyl eugenol, limonene, camphene, α-pinene, β-pinene, γ-terpinene, p-cymene, cis-ocimene, 1,8-cineole, linalyl acetate, fenchyl acetate, methyl cinnamate, β-caryophyllene | Colorless or pale yellow | Light sweet, spicy odor reminiscent of anise or clove (top note) | [11] |
| 39. Pelargonium graveolens L’Hér. | Geranium | Aerial parts | Citronellol, geraniol, linalool, citronellyl formate, geranyl formate, citral, guaiazulene, β-caryophyllene, cis-rose oxide, phellandrene, limonene, α-pinene, menthone | Pale or olive green | Sweet rose-like odor with a hint of mint or “greenness” (middle note) | [11] |
| 40. Pimenta dioica (L.) Merr. | Allspice | Leaf | Mainly eugenol, less in the fruit (60–80%) than in the leaves (up to 96%), also methyl eugenol, cineole, phellandrene and caryophyllene, among others | Yellowish-red or brownish liquid | Powerful sweet, spicy scent, similar to cloves | [61] |
| Berry | Pale yellow liquid | Sweet, warm balsamic, spicy body note (middle note) and fresh, clean top note | ||||
| 41. Pimpinella anisum L. | Aniseed | Seeds | Trans-anethole (75–90%) | Colorless to pale yellow | Warm, spicy-sweet characteristic scent | [61] |
| 42. Pinus massoniana Lamb. | Turpentine | Gum resin | α-Pinene, camphene, β-pinene, δ-3-carene, myrcene, limonene, p-cymene, longifolene, β-caryophyllene, caryophyllene oxide | Colorless | Characteristic of gum turpentine | [87] |
| 43. Piper nigrum L. | Black pepper | Unripe berries (peppercorns) | Sesquiterpenes (20–30%) and monoterpenes (70–80%) such as bisabolene, β-caryophyllene, thujene, pinene, camphene, sabinene, terpinene, myrcene, limonene, phellandrene, small amounts of ketone, phenols, alcohols and others | Light to pale olive | Dry, woody, warm, spicy, oriental (base note) | [11] |
| 44. Pogostemon cablin (Blanco) Benth. | Patchouli | Leaves | α-Pinene (0.01–0.3%), β-pinene (0.02–1%), Limonene (0.01–0.3%), δ-elemene (0.01–1.9%), β-patchoulene (0.03–12%), β-elemene (0.18–1.9%), cycloseychellene (0.02–0.8%), (E)-β-caryophyllene (0.75–6.8%), α-guaiene (2.9–23%), seychellene (2.3–13%), α-humulene (0.05–2%), α-patchoulene (1.2-13%), pogostone (0.1–27.7%), patchoulol (11–72%), pogostol (0.2–6.2%), α-bulnesene (2.9–23%), aciphyllene (0.7–4.2%), norpatchoulenol (0.11–4.0%), caryophyllene oxide (0.0–4.6%), germacrene D (0.0–0.2%) | Yellow to reddish-brown | Earthy, woody and camphoraceous | [88] |
| 45. Rosa damascena Mill. | Rose | Flowers | Citronellol, geraniol, nerol, nonadecane, heneicosane | Yellow to yellow-green | Sweet, floral, rosaceous | [89,90,91] |
| 46. Rosmarinus officinalis L. | Rosemary | Leaves | Eucalyptol and α-pinene, camphor, bornyl acetate, camphene, β-pinene, β-myrcene, limonene and borneol | Colorless to pale yellow | Strong, warm, woody, balsamic aroma | [28,92] |
| 47. Salvia sclarea L. | Clary sage | Inflorescences | Linalool, linalyl acetate | Yellow | Characteristic herbaceous odor | [93,94] |
| 48. Santalum album L. | Sandalwood | Heartwood | 90% Santalol content (cis-α-santalol and cis-β-santalol) (ISO 3518:2002) | Yellow to light brown | Sweet woody | [95] |
| 49. Syzygium aromaticum (L.) Merrill et. Perry | Clove | Bud | Eugenol (70–95%), eugenol acetate (up to 20%) and β-caryophyllene (12–17%) | Colorless or pale yellow | Clove aroma | [96] |
| 50. Vetiveria zizanioides (Linn.) Nash | Vetiver | Roots | α-Vetivone (8.4–13.3%), khusimol (0.6–8.9%), β-vetivone (2.2–3.7%), khusian-2-ol (1.8–2.3%) and khusimone (1.2–2.3%) | Pale-yellow to dark brown, olive or amber | Deep, smoky, earthy, and woody with a sweet persistent undertone | [30,97,98] |
| Essential Oil Compounds | Odor Description | Reference |
|---|---|---|
| Anethole | Pleasant odor of anise oil | [28] |
| Bisabolol | Sweet floral odor | [28] |
| Bornyl acetate | Woody, camphor, mentholic, spicy | [99] |
| Carvone | Minty herbaceous | [100] |
| Citral | Lemony | [28] |
| Citronellol | Strong floral, rose, sweet like | [101] |
| Cuminal | Spicy harsh | [101] |
| Decanal | Sweet, aldehydic, orange, waxy, citrus rind | [99] |
| Farnesol | Flowery, weak-citrus odor | [28] |
| Geraniol | Fresh, sweet, rose-like | [101] |
| Geranyl acetate | Pleasant, floral rose, herbal | [101] |
| Germacrene D | Woody, spice | [99] |
| Limonene | Strong odor of orange | [28] |
| Linalool | Floral, grassy, pleasant, citrus | [101] |
| Linalyl acetate | Floral, sweet citrus | [100] |
| Menthol | Sweet minty, cooling and fresh scent | [28] |
| Myrcene | Pleasant floral | [101] |
| Myristicin | Spice, warm, balsam, woody | [99] |
| Neral | Citric, green | [100] |
| p-Cymene | Fresh, citrus, terpene, woody, spice | [99] |
| Sabinene | Woody, terpene, citrus, pine, spice | [99] |
| Terpineol | Sweet, lilac odor | [101] |
| Terpinolene | Fresh, woody, sweet, pine, citrus | [99] |
| α-Pinene | Fresh, camphor, sweet, pine, earthy, woody | [99] |
| β-Phellandrene | Mint, turpentine | [99] |
| β-Pinene | Woody, turpentine | [101] |
| γ-Terpinene | Woody, terpene, lemon, lime, tropical, herbal | [99] |
| Amylcinnamal | Geraniol |
| Amylcinnamyl alcohol | Farnesol |
| Anisyl alcohol | Hexyl cinnamaldehyde |
| Benzyl alcohol | Hydroxy-citronellal |
| Benzyl benzoate | Hydroxy-methylpentylcyclohexenecarboxaldehyde |
| Benzyl cinnamate | Isoeugenol |
| Benzyl salicylate | D-Limonene |
| Cinnamyl alcohol | Linalool |
| Cinnamal | Methyl heptin carbonate |
| Citral | 3-Methyl-4-(2,6,6-tri-methyl-2-cyclohexen-1-yl)-3-buten-2-one |
| Citronellol | Oakmoss and treemoss extract |
| Coumarin | treemoss extract |
| Eugenol | 2-(4-tert-Butylbenzyl) propionaldehyde |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. https://doi.org/10.3390/molecules26030666
Sharmeen JB, Mahomoodally FM, Zengin G, Maggi F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules. 2021; 26(3):666. https://doi.org/10.3390/molecules26030666
Chicago/Turabian StyleSharmeen, Jugreet B., Fawzi M. Mahomoodally, Gokhan Zengin, and Filippo Maggi. 2021. "Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals" Molecules 26, no. 3: 666. https://doi.org/10.3390/molecules26030666
APA StyleSharmeen, J. B., Mahomoodally, F. M., Zengin, G., & Maggi, F. (2021). Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules, 26(3), 666. https://doi.org/10.3390/molecules26030666









