Evaluation of Dimer of Epicatechin from an Endophytic Fungus Curvularia australiensis FC2AP on Acute Toxicity Levels, Anti-Inflammatory and Anti-Cervical Cancer Activity in Animal Models
Abstract
1. Introduction
2. Methodology
2.1. Curvularia australiensis Strain and Source
2.2. In Vitro Study: Anti-Angiogenic Analysis
2.3. In Vivo Studies: Animal Models
2.3.1. Acute Toxicity Study
Animal and Experimental Design
Samples and Analysis
2.3.2. Anti-Inflammatory Study
2.3.3. Anti-Cancer Study
2.4. Statistical Analysis
3. Results
3.1. Anti-Angiogenic Study by HET-CAM Analysis
3.2. Animal Studies
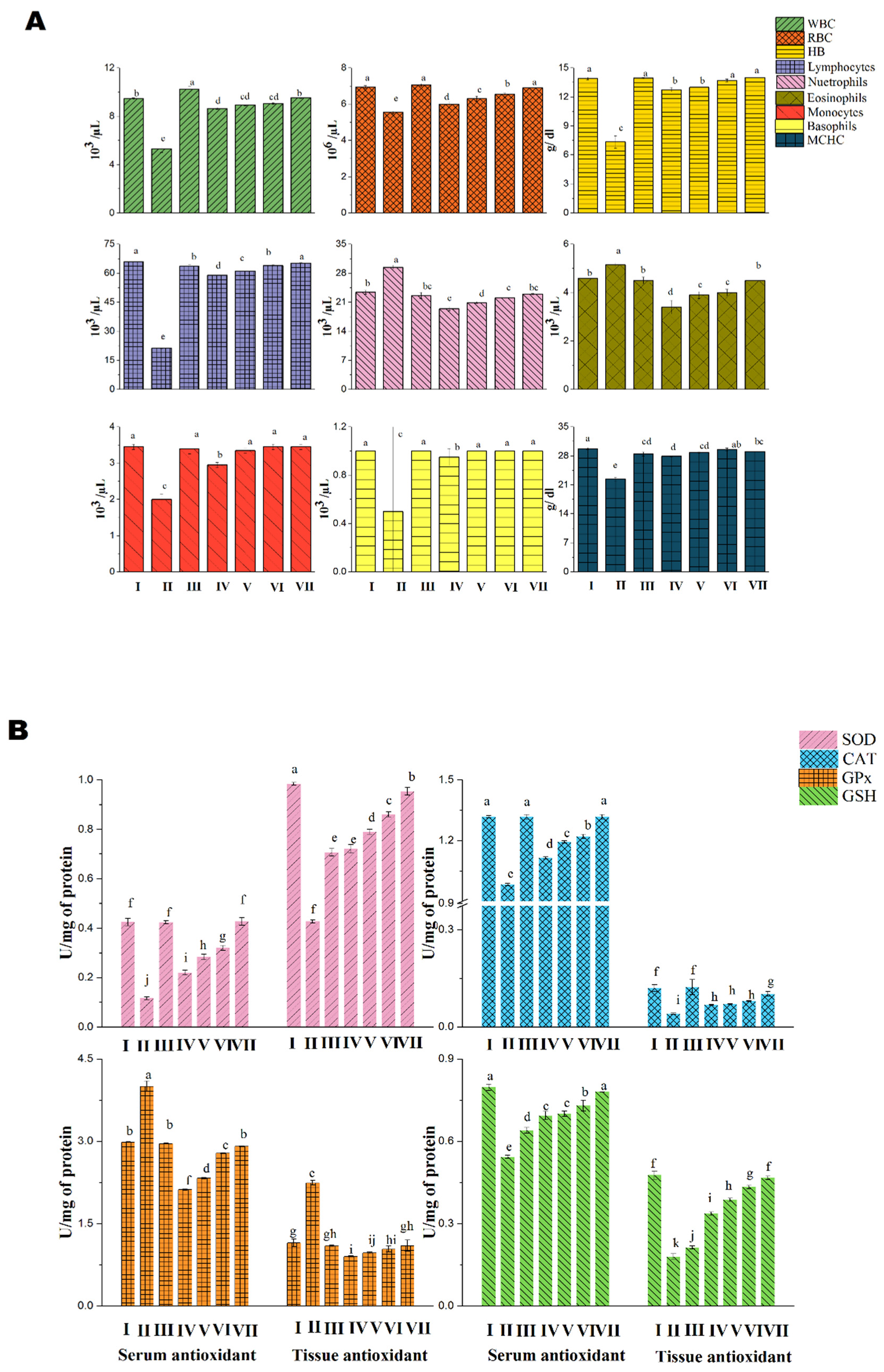
3.2.1. Acute Toxicity Analysis in Albino Mice
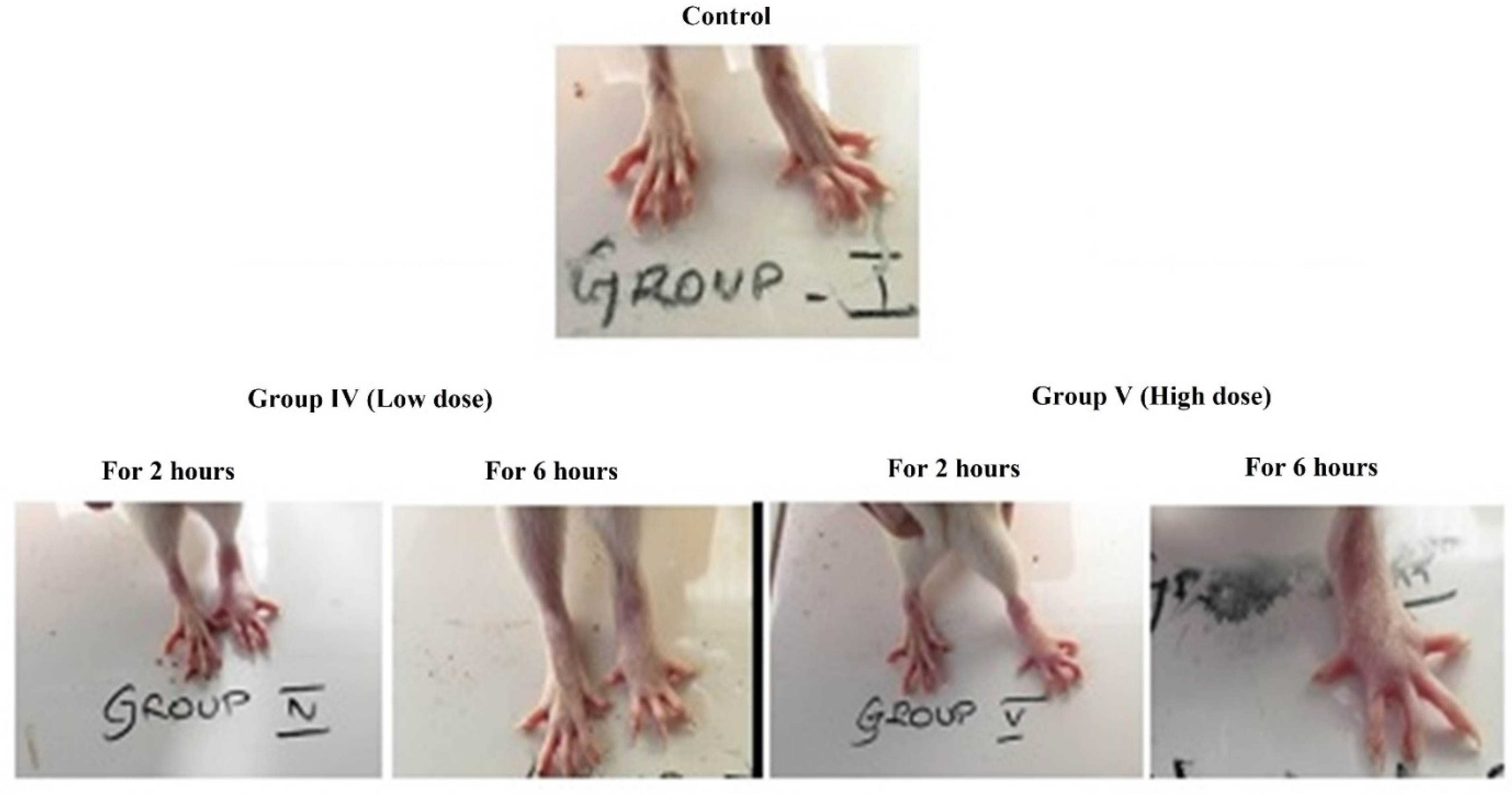
3.2.2. Anti-Inflammatory Study
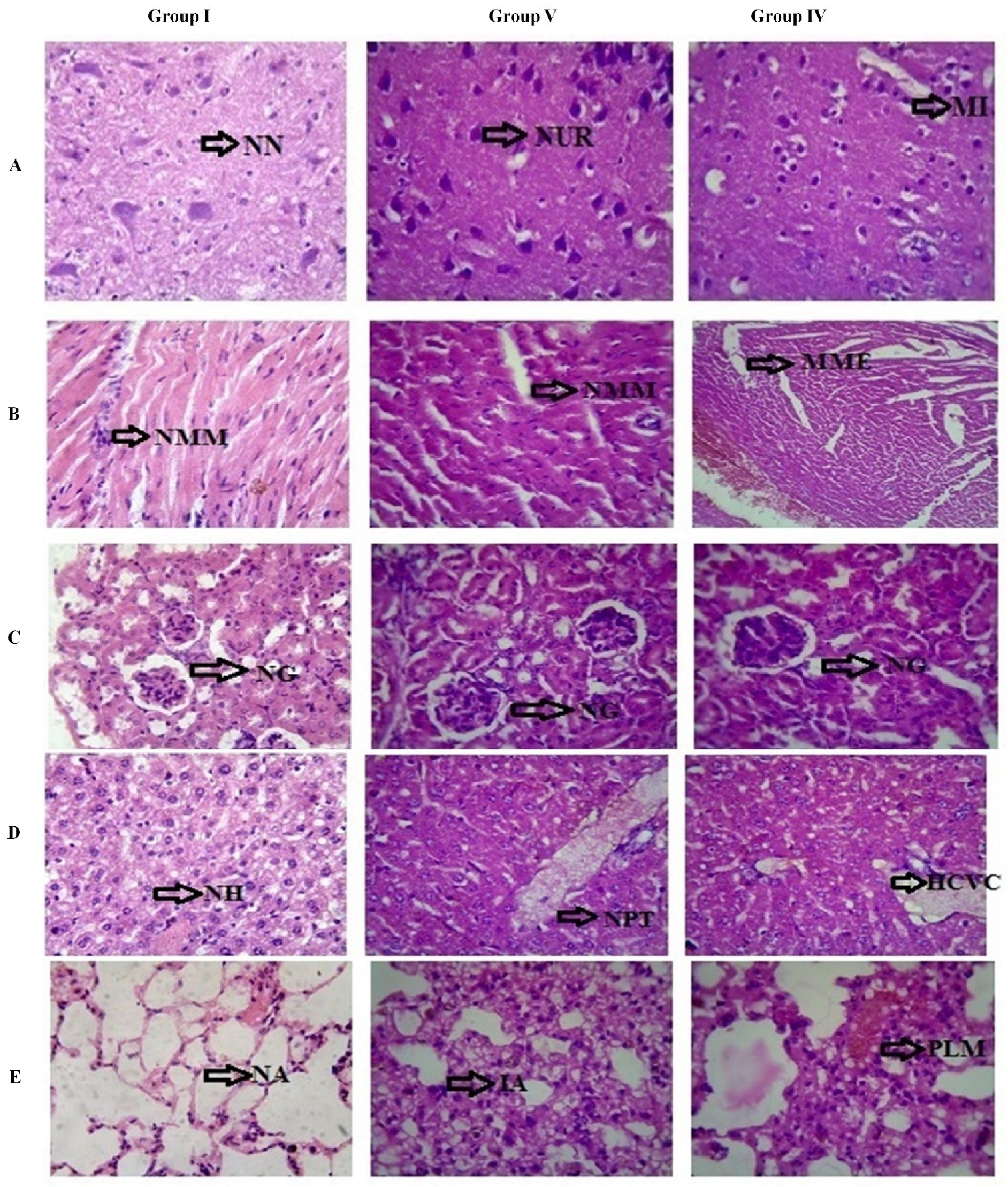
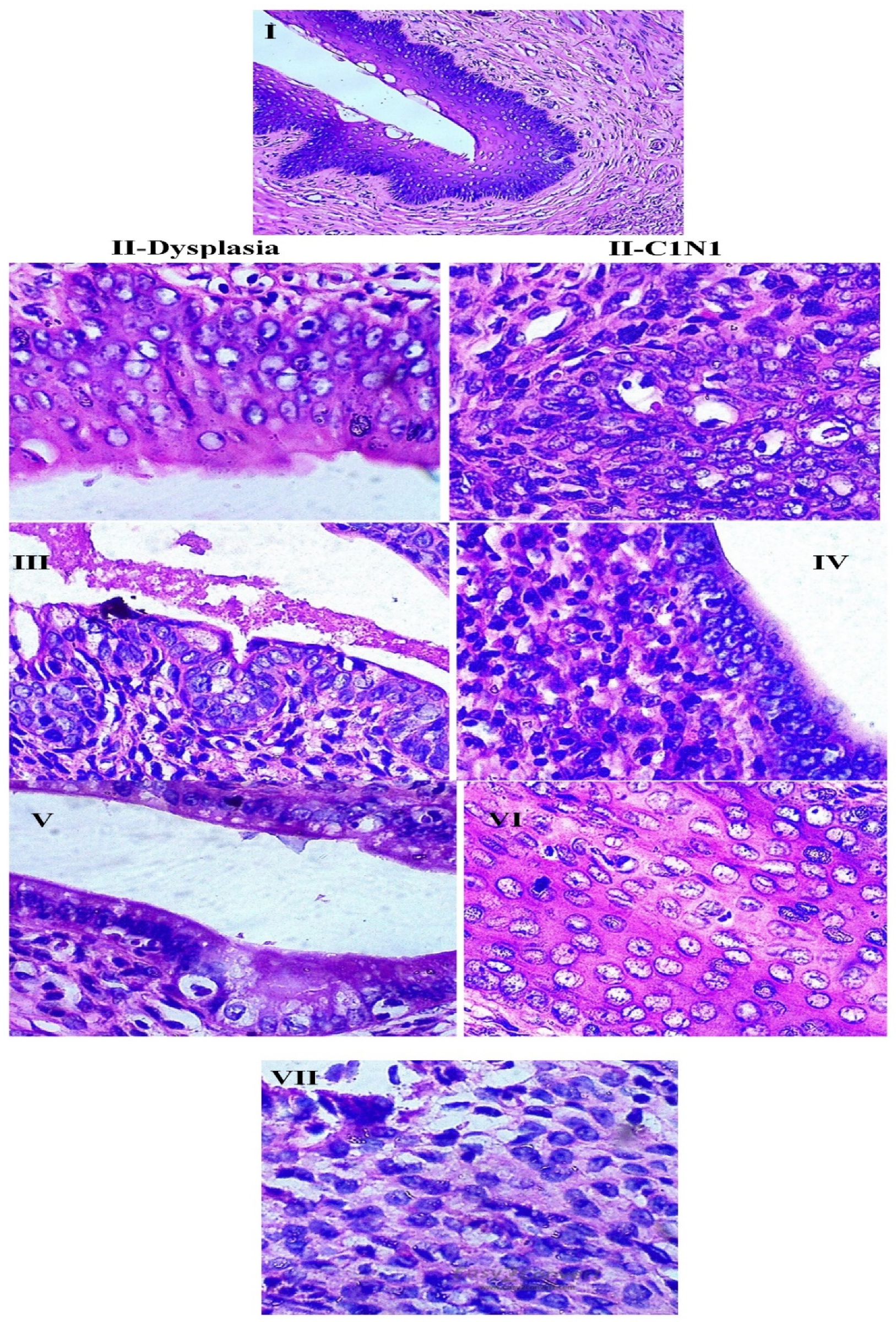
3.2.3. Anti-Cancer Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Franco, E.L.; Schlecht, N.F.; Saslow, D. The epidemiology of cervical cancer. Cancer 2003, 9, 348–359. [Google Scholar] [CrossRef]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef]
- Wright, T.C.; Stoler, M.H.; Behrens, C.M.; Sharma, A.; Zhang, G.; Wright, T.L. Primary cervical cancer screening with human papillomavirus: End of study results from the ATHENA study using HPV as the first-line screening test. Gynecol. Oncol. 2015, 136, 9–97. [Google Scholar] [CrossRef]
- He, Q.; Zeng, Q.; Shao, Y.; Zhou, H.; Li, T.; Song, F.; Liu, W. Anti-cervical cancer activity of secondary metabolites of endophytic fungi from Ginkgo biloba. Cancer Biomark. 2020, 28, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Zou, W. Endophytes: A rich source of functional metabolites. Natl. Prod. Rep. 2001, 18, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Girmay, S.; Martins da Silva, V.; Perry, B.; Hu, X.; Tan, G.T. The Role of Endophytic Fungi in the Anticancer Activity of Morinda citrifolia Linn. (Noni). Evid Based Complement. Alt. Med. 2015, 2015, 393960. [Google Scholar] [CrossRef]
- Huang, H.B.; Xiao, Z.E.; Feng, X.J.; Huang, C.H.; Zhu, X.; Ju, J.H.; Li, M.F.; Lin, Y.C.; Liu, L.; She, Z.G. Cytotoxic naphtho-g-pyrones from the Mangrove endophytic fungus Aspergillus tubingensis (GX1-5E). Helv. Chim. Acta 2011, 94, 1732–1740. [Google Scholar] [CrossRef]
- Liu, D.; Li, X.-M.; Meng, L.; Li, C.S.; Gao, S.S.; Shang, Z.; Proksch, P.; Huang, C.G.; Wang, B.G. Nigerapyrones A–H, α-pyrone derivatives from the marine mangrove-derived endophytic fungus Aspergillus niger MA-132. J. Nat. Prod. 2011, 74, 1787–1791. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Tincho, M.B.; Toghueo, R.M.K.; Morris, T.; Hiss, D.C.; Boyom, F.F.; Mandal, C. Cytotoxicity Potential of Endophytic Fungi Extracts from Terminalia catappa against Human Cervical Cancer Cells. J. Toxicol. 2020, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lu, J.L.; Liang, Y.R.; Li, Q.S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Lin, T.; Wang, G.; Zeng, D.; Chen, H. Cytotoxic metabolites from Botryotinia fuckelianaA-S-3: An endophytic fungus from Ajuga decumbens. Phytochem. Lett. 2015, 13 (Suppl. C), 206–211. [Google Scholar] [CrossRef]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Abedini, A.; Plesner, A.; Verchere, C.B.; Raleigh, D.P. The Flavanol (-)-epigallocatechin 3-gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP induced toxicity. Biochemistry 2010, 49, 8127–8133. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.; Saraiva, M.J.; Almeida, M.R. Epigallocatechin3-gallate as a potential therapeutic drug for TTR-related amyloidosis: ‘in vivo’ evidence from FAP mice models. PLoS ONE 2012, 7, e29933. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Lee, I.; Antwih, D.A.; Liu, J.; Hüttemann, M.; Zielske, S.P. Epicatechin Stimulates Mitochondrial Activity and Selectively Sensitizes Cancer Cells to Radiation. PLoS ONE 2014, 9, e88322. [Google Scholar] [CrossRef]
- Nagarajan, S.; Nagarajan, R.; Braunhut, S.J.; Bruno, F.; McIntosh, D.; Samuelson, L.; Kumar, J. Biocatalytically Oligomerized Epicatechin with Potent and Specific Anti-proliferative Activity for Human Breast Cancer Cells. Molecules 2008, 13, 2704–2716. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Daugelaite, J.; Shahidi, F. DNA scission and LDL cholesterol oxidation inhibition and antioxidant activities of Bael (Aegle marmelos) flower extracts. J. Trad. Complement. Med. 2018, 8, 428–435. [Google Scholar] [CrossRef]
- Mondol, M.A.; Farthouse, J.; Islam, M.T.; Schüffler, A.; Laatsch, H. Metabolites from the Endophytic Fungus Curvularia sp. M12 Act as Motility Inhibitors against Phytophthora capsici Zoospores. J. Nat. Prod. 2017, 80, 347–355. [Google Scholar] [CrossRef]
- Khiralla, A. Phytochemical Study, Cytotoxic and Antibacterial Potentialities of Endophytic Fungi from Medicinal Plants from Sudan; Other, English. ffNNT: 2015LORR0159ff. fftel-01752032f; Université de Lorraine: Metz, France, 2015. [Google Scholar]
- Mani, V.M.; Soundari, A.P.G.; Karthiyaini, D.; Preethi, K. Bioprospecting for endophytic fungi and their metabolites from medicinal tree Aegle marmelos in Western Ghats, India. Mycobiology 2015, 43, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.M.; Soundari, A.P.G.; Tamilarasi, S. Determination of in vitro cytotoxicity and anti-angiogenesis for a bioactive compound from Aspergillus terrus FC36AY1 isolated from Aegle marmelos around Western Ghats, India. In Medicinal Chemistry; InTech Open-Book: London, UK, 2018; pp. 13–28. [Google Scholar]
- Lewis, S.M.; Bain, B.J.; Bates, I. Dacie and Lewis Practical Haematology, 10th ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Hoffbrand, V.; Moss, P. Essential Haematology: Includes Free Desktop Edition, 6th ed.; John Wiley and Sons Ltd.: New York, NY, USA, 2011. [Google Scholar]
- Barrett, K.E.; Barman, S.M. Ganong’s Review of Medical Physiology, 25th ed.; McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Delwatta, S.L.; Gunatilake, M.; Baumans, V.; Seneviratne, M.D.; Dissanayaka, M.L.B.; Batagoda, S.S.; Udagedara, A.H.; Walpola, P.B. Reference values for selected hematological, biochemical and physiological parameters of Sprague-Dawley rats at the Animal House, Faculty of Medicine, University of Colombo, Sri Lanka. Anim. Model Exp. Med. 2018, 1, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.R.S.; Soliman, R.H.; Abdelhaleem Ali, A.M.; Tawfeik, M.H.; Abdelrahim, M.E.A. Effect of antiepileptic drugs on liver enzymes. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 14–19. [Google Scholar] [CrossRef]
- Khoo, L.W.; Foong Kow, A.S.; Maulidiani, M.; Lee, M.T.; Tan, C.P.; Shaari, K.; Tham, C.L.; Abas, F. Hematological, Biochemical, Histopathological and 1H-NMR Metabolomics Application in Acute Toxicity Evaluation of Clinacanthus nutans Water Leaf Extract. Molecules 2018, 23, 2172. [Google Scholar] [CrossRef]
- Avwioro, G.; Iyiola, S.; Aghoghovwia, B. Histological and biochemical markers of the liver of Wistar rats on subchronic oral administration of green tea. N. Am. J. Med. Sci. 2010, 2, 376–380. [Google Scholar] [CrossRef] [PubMed]
- He, W.Z.; Guo, G.F.; Yin, C.X.; Jiang, C.; Wang, F.; Qiu, H.J.; Chen, X.X.; Rong, R.M.; Zhang, B.; Xia, L.P. Gamma-glutamyl transpeptidase level is a novel adverse prognostic indicator in human metastatic colorectal cancer. Colorectal Dis. 2013, 15, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Hirchaud, F.; Hermetet, F.; Ablise, M.; Fauconnet, S.; Vuitton, D.A.; Pretet, J.L.; Mougin, C. Isoliquiriti genin induces caspase-dependent apoptosis via down regulation of hpv16 e6 expression in cervical cancer caski cells. Planta Med. 2013, 79, 1628–1635. [Google Scholar]
- Vasundra, D.P.; Suja, S. Antioxidant and chemotherapeutic potential of Curcuma amada rhizome extract on benzo(a)pyrene induced cervical carcinoma in Sprague Dawley rats. Asian J. Pharm. Clin. Res. 2017, 10, 235–242. [Google Scholar]
- Bosch, F.X.; Burchell, A.N.; Schiffman, M.; Giuliano, A.R.; De Sanjose, S.; Bruni, L.; Tortolero-Luna, G.; Kjaer, S.K.; Munoz, N. Epidemiology and natural history of human papillomavirus infections and type-specific implications in cervical neoplasia. Vaccine 2008, 26, K1–K16. [Google Scholar] [CrossRef]
- Di Domenico, F.; Foppoli, C.; Coccia, R.; Perluigi, M. Antioxidants in cervical cancer: Chemopreventive and chemotherapeutic effects of polyphenols. Biochim. Biophys. Acta 2012, 1822, 737–747. [Google Scholar] [CrossRef]
- Darr, D.; Fridovich, I. Free radicals in cutaneous biology. J. Investig. Dermatol. 1994, 102, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Krishna, P.B.; Krishna, G.; Eppakayala, L.; Prakasham, R.S.; Charya, M.A.S. Evaluation of the Angiosuppresive Activity of Prodigiosin Using the Chorioallantoic Membrane Assay. Int. J. Chem. Anal. Sci. 2014, 5, 31–36. [Google Scholar]
- Yildiz, C.; Cetin, A.; Demirci, F.; Polat, Z.A.; Kiyan, T.; Altun, A.; Cetin, M.; Yildiz, O.K.; Goze, I. Anti angiogenic effects of diltiazem, imatinib and bevacizumab in the CAM assay. Int. J. Sci. Res. Pub. 2013, 3, 1–8. [Google Scholar]
- Wang, Y.Q.; Miao, Z.H. Marine-derived angiogenesis inhibitors for cancer therapy. Mar. Drugs 2013, 11, 903–933. [Google Scholar] [CrossRef]
- Baliga, M.S.; Meleth, S.; Katiyar, S.K. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1cells in vitro and in vivo systems. Clin. Cancer Res. 2005, 11, 1918–1927. [Google Scholar] [CrossRef]
- Fassina, G.; Vene, R.; Morini, M.; Minghelli, S.; Benelli, R.; Noonan, D.M.; Albini, A. Mechanisms of inhibition of tumor angiogenesis and vascular tumor growth by epigallocatechin-3-gallate. Clin. Cancer Res. 2004, 10, 4865–4873. [Google Scholar] [CrossRef]
- Nihal, M.; Ahmad, N.; Mukhtar, H.; Wood, G.S. Anti-proliferative and proapoptotic effects of (−)-epigallocatechin-3-gallate on human melanoma: Possible implications for the chemoprevention of melanoma. Int. J. Cancer 2005, 114, 513–521. [Google Scholar] [CrossRef]
- Mukinda, J.T.; Syce, J.A. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J. Ethnopharmacol. 2007, 112, 138–144. [Google Scholar] [CrossRef]
- Ghosh, D.; Mondal, S.; Ramakrishna, K. Acute and sub-acute (30-day) toxicity studies of Aegialitis rotundifolia Roxb., leaves extract in Wistar rats: Safety assessment of a rare mangrove traditionally utilized as pain antidote. Clin. Phytosci. 2019, 5, 13. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Shaked, H.; Hofseth, L.J.; Chumanevich, A.; Chumanevich, A.A.; Wang, J.; Wang, Y.; Taniguchi, K.; Guma, M.; Shenouda, S.; Clevers, H.; et al. Chronic epithelial NF-kappa B activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc. Natl. Acad. Sci. USA 2012, 109, 14007–14012. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Syngle, A.; Krishan, P. Nitric oxide: Link between inflammation and Endothelial dysfunction in rheumatoid arthritis. Int. J. Angiol. 2017, 26, 165–169. [Google Scholar] [CrossRef] [PubMed]
- González-Gallego, J.; Sánchez-Campos, S.; Tuñón, M.J. Anti-inflammatory properties of dietary flavonoids. Nutr. Hosp. 2007, 22, 287–293. [Google Scholar] [PubMed]
- Bouhlali, E.T.; Hmidani, A.; Bourkhis, B.; Khouya, T.; Ramchoun, M.; Filali-Zegzouti, Y.; Alem, C. Phenolic profile and anti-inflammatory activity of four Moroccan date (Phoenix dactylifera L.) seed varieties. Heliyon 2020, 6, e03436. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Review: Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016. [Google Scholar] [CrossRef]
- Zhao, J.; Li, C.; Wang, W.; Zhao, C.; Luo, M.; Mu, F.; Fu, Y.; Zu, Y.; Yao, M. Hypocrea lixii, novel endophytic fungi producing anticancer agent cajanol, isolated from pigeon pea (Cajanus cajan [L.] Millsp.). J. Appl. Microbiol. 2013, 115, 102–113. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.Y.; Jia, M.; Ming, Q.L.; Yue, W.; Rahman, K.; Qin, L.P.; Han, T. Endophytic fungi with antitumor activities: Their occurrence and anticancer compounds. Crit. Rev. Microbiol. 2016, 42, 454–473. [Google Scholar] [CrossRef]
- Liang, Y.R.; Ye, Q.; Jin, J.; Liang, H.; Lu, J.L.; Du, Y.Y.; Dong, J.J. Chemical and instrumental assessment of green tea sensory preference. Int. J. Food Prop. 2008, 11, 258–272. [Google Scholar] [CrossRef]
- Shibuya, H.; Agusta, A.; Ohashi, K.; Maehara, S.; Simanjuntak, P. Biooxidation of (+)-catechin and (-)-epicatechin into 3,4-dihydroxyflavan derivatives by the endophytic fungus Diaporthe sp. isolated from a tea plant. Chem. Pharm. Bull. 2005, 53, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Yokozawa, T. Direct scavenging of nitric oxide and superoxide by green tea. Food Chem. Toxicol. 2002, 40, 1745–1750. [Google Scholar] [CrossRef]
- Ahn, W.S.; Huh, S.W.; Bae, S.M.; Lee, I.P.; Lee, J.M.; Namkoong, S.E.; Kim, C.K.; Sin, J.I. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G(1) arrest, and regulation of gene expression. DNA Cell Biol. 2003, 22, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sah, J.F.; Balasubramanian, S.; Eckert, R.L.; Rorke, E.A. Epigallocatechin-3-gallate inhibits epidermal growth factor receptor signaling pathway. Evidence for direct inhibition of ERK1/2 and AKT kinases. J. Biol. Chem. 2005, 279, 12755–12762. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signalling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Burns, A.C.; Kazi, A.; Dou, Q.P. Direct inhibition of the ubiquitin-proteasome pathway by ester bond-containing green tea polyphenols is associated with increased expression of sterol regulatory element-binding protein 2and LDL receptor. Biochim. Biophys. Acta 2004, 1682, 1–10. [Google Scholar] [CrossRef]
- Yokoyama, M.; Noguchi, M.; Nakao, Y.; Pater, A.; Iwasaka, T. The tea polyphenol, (−)-epigallocatechin gallate effects on growth, apoptosis, and telomerase activity in cervical cell lines. Gynecol. Oncol. 2004, 92, 197–204. [Google Scholar] [CrossRef]
- Noguchi, M.; Yokoyama, M.; Watanabe, S.; Uchiyama, M.; Nakao, Y.; Hara, K.; Iwasaka, T. Inhibitory effect of the tea polyphenol, (−)-epigallocatechin gallate, on growth of cervical adenocarcinoma cell lines. Cancer Lett. 2006, 234, 135–142. [Google Scholar] [CrossRef]
- Qiao, J.; Cao, L.; Xie, L.; Shi, X. Cell growth inhibition and gene expression regula-tion by (−)-epigallocatechin-3-gallate in human cervical cancer cells. Arch. Pharm. Res. 2009, 32, 1309–1315. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Ganguli, A.; Das, A.; Nag, D.; Chakrabarti, G. Epigallocatechin-3-gallate shows anti-proliferative activity in HeLa cells targeting tubulin-microtubule equilibrium. Chem. Biol. Interact. 2015, 242, 380–389. [Google Scholar] [CrossRef] [PubMed]
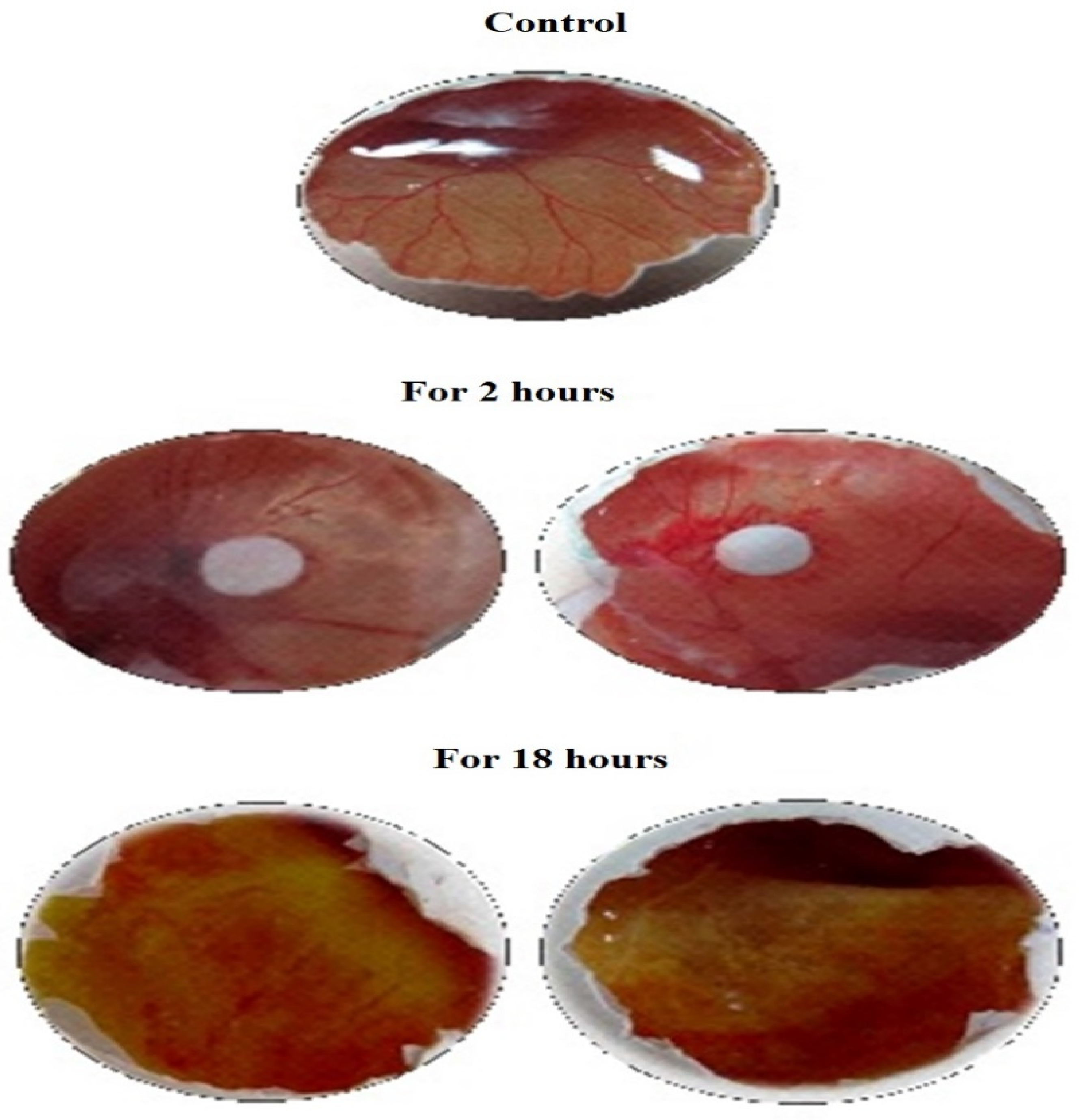



| Sample | For 2 h | For 18 h | ||||
|---|---|---|---|---|---|---|
| No. of Vessels in Untreated CAM | No. of Vessels in Treated CAM | Inhibition (%) | No. of Vessels in Untreated CAM | No. of Vessels in Treated CAM | Inhibition (%) | |
| Negative Control (0.9% NaCl) | 09 | 09 | 0 | 09 | 09 | 0 |
| Sample-1 | 12 | 04 | 66.6 | 12 | 01 | 91.67 |
| Sample-1 | 13 | 03 | 76.92 | 13 | 01 | 92.3 |
| Sample-1 | 11 | 03 | 72.72 | 11 | 01 | 90.9 |
| Positive Control (1N NaOH) | 08 | 02 | 75.0 | 08 | 0 | 100 |
| Acetone | 11 | 09 | 18.18 | 11 | 08 | 27.27 |
| S. No. | Biochemical Markers | Group I | Group V | Group IV |
|---|---|---|---|---|
| 1 | ALP (IU/L) | 118.02 ± 0.07 | 111.22 ± 0.98 | 178.45 ± 0.98 |
| 2 | ALT (IU/L) | 52.8 ± 0.41 | 51.18 ± 0.82 | 98.4 ± 0.82 |
| 3 | AST (IU/L) | 69.16 ± 0.49 | 70.24 ± 0.61 | 203.39 ± 0.52 |
| 4 | Bilirubin (mg/dL) | 0.95 ± 0.03 | 0.92 ± 0.30 | 6.17 ± 0.05 |
| 5 | Total protein (mg/dL) | 9.4 ± 0.36 | 9.5 ± 0.24 | 7.31 ± 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, V.M.; Soundari, A.J.P.G.; Balasubramanian, B.; Park, S.; Issara, U.; Preethi, K.; Liu, W.-C. Evaluation of Dimer of Epicatechin from an Endophytic Fungus Curvularia australiensis FC2AP on Acute Toxicity Levels, Anti-Inflammatory and Anti-Cervical Cancer Activity in Animal Models. Molecules 2021, 26, 654. https://doi.org/10.3390/molecules26030654
Mani VM, Soundari AJPG, Balasubramanian B, Park S, Issara U, Preethi K, Liu W-C. Evaluation of Dimer of Epicatechin from an Endophytic Fungus Curvularia australiensis FC2AP on Acute Toxicity Levels, Anti-Inflammatory and Anti-Cervical Cancer Activity in Animal Models. Molecules. 2021; 26(3):654. https://doi.org/10.3390/molecules26030654
Chicago/Turabian StyleMani, Vellingiri Manon, Arockiam Jeyasundar Parimala Gnana Soundari, Balamuralikrishnan Balasubramanian, Sungkwon Park, Utthapon Issara, Kathirvel Preethi, and Wen-Chao Liu. 2021. "Evaluation of Dimer of Epicatechin from an Endophytic Fungus Curvularia australiensis FC2AP on Acute Toxicity Levels, Anti-Inflammatory and Anti-Cervical Cancer Activity in Animal Models" Molecules 26, no. 3: 654. https://doi.org/10.3390/molecules26030654
APA StyleMani, V. M., Soundari, A. J. P. G., Balasubramanian, B., Park, S., Issara, U., Preethi, K., & Liu, W.-C. (2021). Evaluation of Dimer of Epicatechin from an Endophytic Fungus Curvularia australiensis FC2AP on Acute Toxicity Levels, Anti-Inflammatory and Anti-Cervical Cancer Activity in Animal Models. Molecules, 26(3), 654. https://doi.org/10.3390/molecules26030654









