Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review
Abstract
1. Introduction
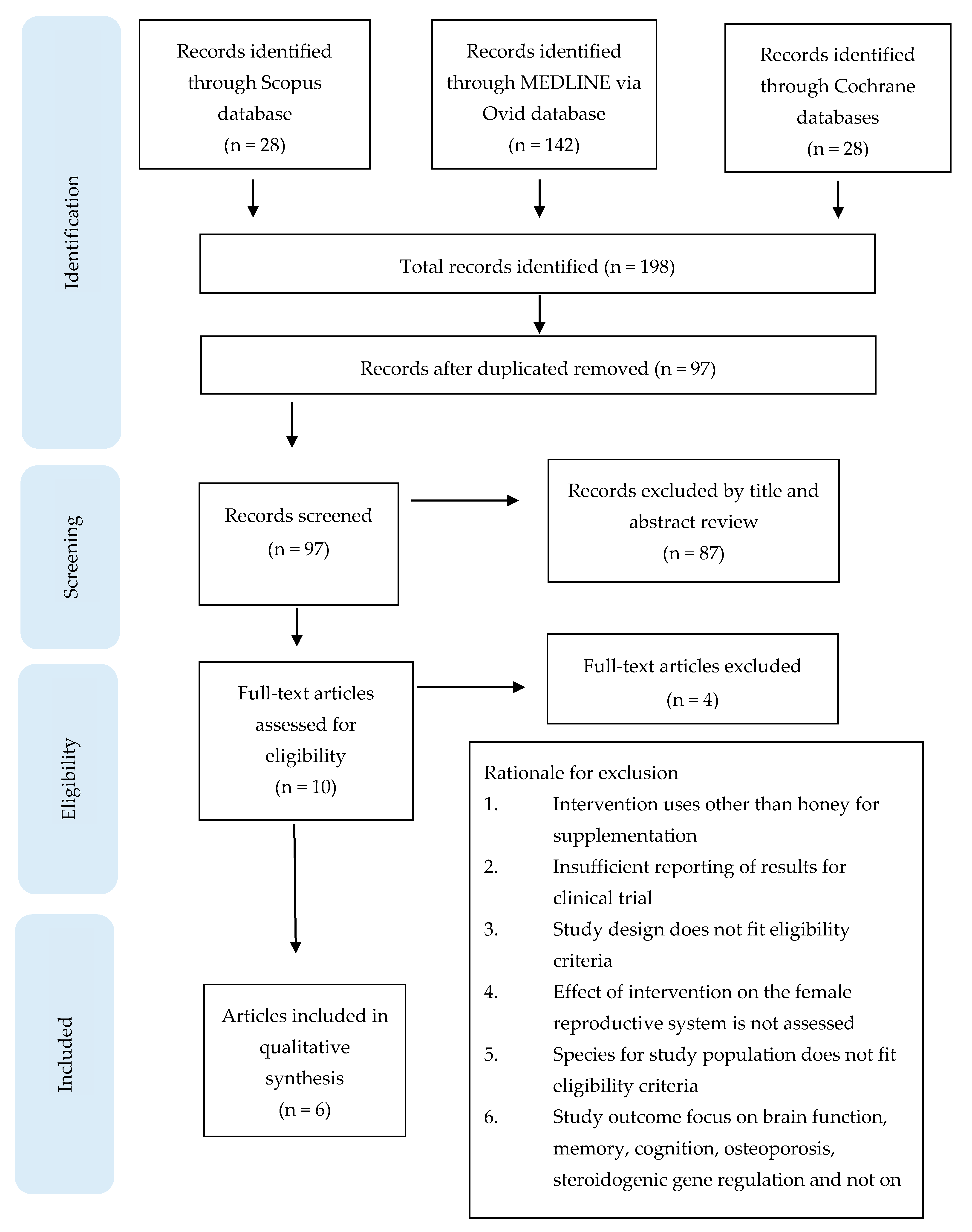
2. Evidence Acquisition
2.1. Data Sources and Search Strategy
2.2. Data Study Exclusion and Inclusion Criteria
2.3. Data Extraction and Management
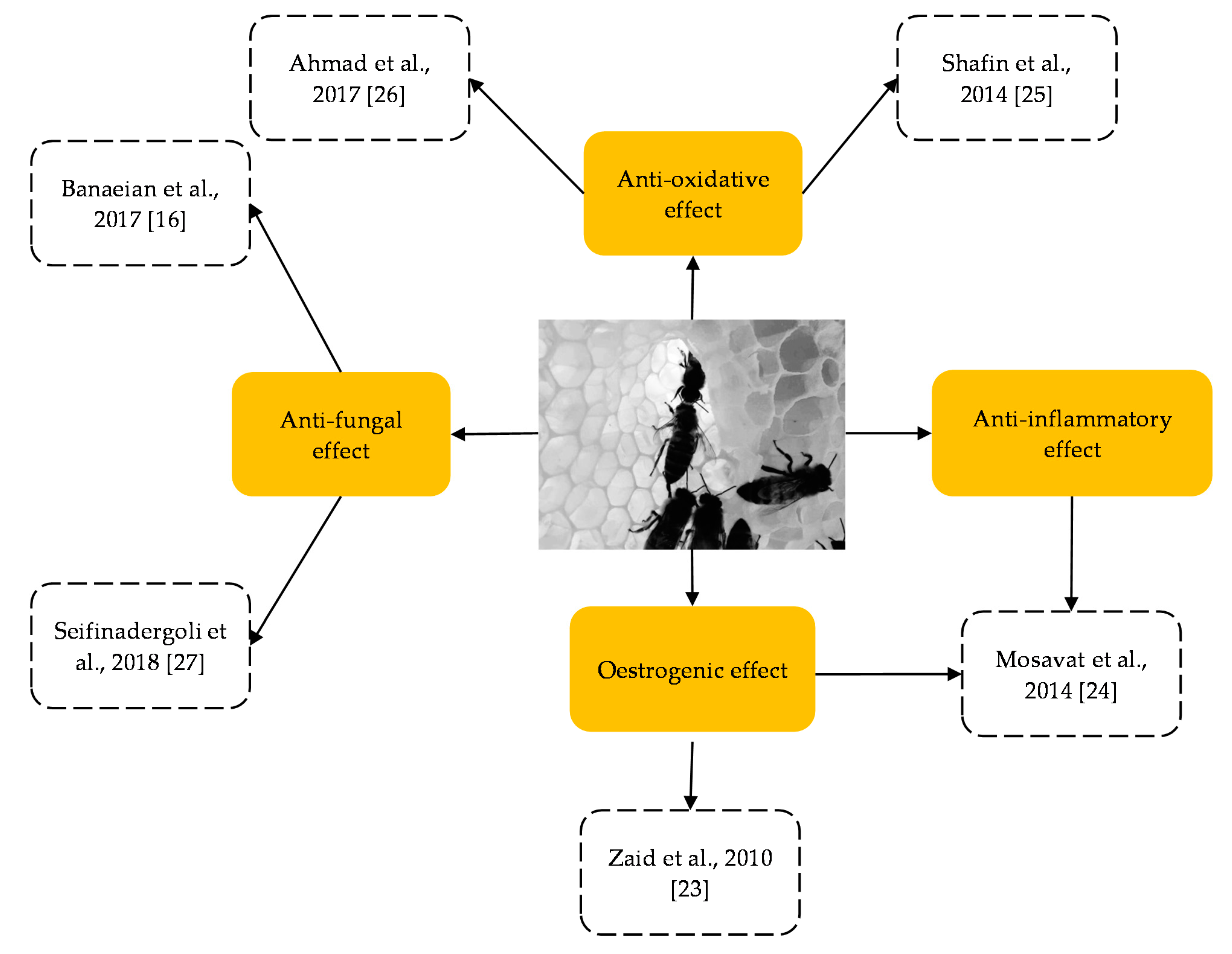
3. Principal Findings
| Type of HS (No. of HS Group) | Dosage of HS | Route of HS | Duration of HS | Target Female Reproductive Organ/Hormone of Study | References |
|---|---|---|---|---|---|
| Tualang honey (3) | 0.2 g/kg bwt, 1.0 g/kg bwt and 2.0g/kg bwt | Oral route | 2 weeks | Uterus (relative weight and thickness) and vagina (relative weight and thickness); oestrogen and progesterone levels | [23] |
| Tualang honey (1) | 1 g/kg bwt/day | Oral route | 8 weeks | Uterus (relative weight); oestrogen and progesterone levels | [24] |
| Tualang honey (1) | 20 g/day | Oral route | 16 weeks | None. The study measured blood GSH (reduced glutathione) to GSSG (oxidised glutathione) ratio, CAT, SOD, GPx, 4-HNE and total protein | [25] |
| Tualang honey (2) | 0.75 g/kg bwt and 1.5 g/kg bwt | Oral route | 5 h | None. The study measured TPC, antioxidant stress activity and oxidative stress markers (MDA and ROS) | [26] |
| a Chaharmahal and Bakhtiari region, Iran honey (1) | 5 g of 70% honey cream (with applicator) | Topical route | 7 nights | Reproductive tract. The study measured symptoms of VVC: inflammation, discharge, irritation and the level of satisfaction of treatment | [16] |
| b Sabalan Mountain, Iran honey (1) | 5 g of 50% honey gel (with applicator) | Topical route | 8 nights | Reproductive tract. The study measured symptoms of VVC: vaginal discharge, itching, dyspareunia, burning, urinary incontinence and culture of vaginal sample to confirm the reduction in Candida albicans colony number infection | [27] |
| Sample/Subject | Sample Size (n) Per HS Group; Total Number of Groups Given HS in Study; Number of Control Individuals | Age of Sample/Subject | Effect of HS on the Female Reproductive System | Study Sample/Subject is Menopause, OVX or Normal Rats | Biological Effect of Honey on the Female Reproductive System | References |
|---|---|---|---|---|---|---|
| Sprague Dawley rats | 7/group; 3 groups; 14 | 12 week old | HS prevented the ovariectomised rats from uterine atrophy and vaginal epithelium atrophy. The oestradiol and progesterone levels were significantly decreased in the HS group. The testosterone level was increased in the low-HS group (0.2 g/kg bwt). Effects on other than the female reproductive system: promoted bone density and suppressed body weight increase | OVX rats | Oestrogenic effect | [23] |
| Sprague Dawley rats | 12/group; 3 groups; 24 | 9 week old | HS effectively maintained relative uterine weight and attenuated the adverse effects of jumping exercise on the female reproductive hormone (the increase in the oestradiol levels was not significant, whereas the increase in the progesterone levels was significant) and stress hormones (cortisol decreased but not significant). No effect on relative ovary weight. | Non-menopausal, Non-OVX (Normal rats) | Oestrogenic effect and anti-inflammatory | [24] |
| Sample/Subject | Sample Size (n) Per HS Group; Total Number of Groups Given HS in Study; Total Sample Size (N) for Study | Age of Sample/Subject | Effect of HS on the Female Reproductive System | Study Sample/Subject is Menopause, OVX or Non-Indicated | Biological Effect of Honey on the Female Reproductive System | References |
|---|---|---|---|---|---|---|
| Human (postmenopausal) | 39/group; 1 group; 78 | 45–60 years old | HS resulted in significant elevation of CAT and GPx activity and reduction in the 4-HNE levels. SOD activity and GSH/GSSG ratio were insignificant. | Menopause subjects | Anti-oxidant | [25] |
| Human (athletes) | 10/group; 1 group; 20 | 18–25 years old | HS significantly lowered the MDA levels. The changes in the TPC, FRAP and ROS levels were insignificant. | Non-indicated | Anti-oxidant | [26] |
| Human (VVC patient) | 44/group; 1 group; 80 | 21–59 years old | HS (in cream formulation) showed a significant decrease in discharge, inflammation and itching. However, the magnitude was less than control (clotrimazole cream). HS showed a lower rate of recurrence (in women with more frequent VVC history) during follow-up. | Non-indicated | Anti-fungal | [16] |
| Human (VVC patient) | 53/group; 1 group; 106 | 18–45 years old | HS (in gel formulation) has significantly reduced the problems associated with VVC (symptoms of itching, burning, dyspareunia, urinary problems and vaginal discharge), and culture plate showed no colonies of Candida albicans. Results of HS was comparable or having nearly equal effect to a standard treatment; clotrimazole cream group. | Non-indicated | Anti-fungal | [27] |
4. Discussion
4.1. Role of Honey in Suppressing Oestrogen Deficiency-Induced OS
4.2. Role of Honey in Suppressing OS-Induced Inflammation Stress
4.3. Role of Honey as an Anti-Fungal Agent
4.4. Is Honey Suitable for Patients with Hyperglycaemia?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Bansal, V.; Medhi, B.; Pandhi, P. Honey—A remedy rediscovered and its therapeutic utility. Kathmandu Univ. Med. J. 2005, 3, 305–309. [Google Scholar]
- Lipsey, R.G.; Carlaw, K.I.; Bekar, C.T. Historical Record on the Control of Family Size. In Economic Transformations: General Purpose Technologies and Long-Term Economic Growth; OUP Oxford: Oxford, UK, 2005; pp. 335–340. ISBN 9780191558092. [Google Scholar]
- Middleberg, M.I. Promoting Reproductive Security in Developing Countries Women’s Health Issues, Illustrated ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; ISBN 9780306479359. [Google Scholar]
- Haydak, M.H.; Palmer, L.S.; Tanquary, M.C. The role of honey in the prevention and cure ofnutritional anemia in rats. J. Pediatr. 1942, 21, 763–768. [Google Scholar] [CrossRef]
- Jeffrey, A.E.; Echazarreta, C.M. Medical uses of honey. Rev. Bioméd. 1996, 7, 43–49. [Google Scholar]
- Cooper, R.A.; Fehily, A.M.; Pickering, J.E.; Erusalimsky, J.D.; Elwood, P.C. Honey, Health and Longevity. Curr. Aging Sci. 2010, 3, 239–241. [Google Scholar] [CrossRef]
- Abdulrhman, M. Honey as a Sole Treatment of Type 2 Diabetes Mellitus. Endocrinol. Metab. Syndr. 2016, 5, 232. [Google Scholar] [CrossRef]
- Eteraf-Oskouei, T.; Najafi, M. Traditional and Modern Uses of Natural Honey in Human Diseases: A Review. Iran. J. Basic Med. Sci. 2013, 16, 731–742. [Google Scholar]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxidative Med. Cell. Longev. 2017, 2017, 1–21. [Google Scholar] [CrossRef]
- Ball, D.W. The Chemical Composition of Honey. J. Chem. Educ. 2007, 84, 1643. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical composition of honey. In Bee Products-Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer: Cham, Swizerland, 2017; pp. 43–82. [Google Scholar] [CrossRef]
- Bogdanov, S.; Haldimann, M.; Luginbühl, W.; Gallmann, P. Minerals in honey: Environmental, geographical and botanical aspects. J. Apic. Res. 2007, 46, 269–275. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Belushi, S.; Al-Amri, A.; Al-Hadhrami, A.; Al-Rusheidi, M.; Al-Alawi, A. Quality evaluation of Omani honey. Food Chem. 2018, 262, 162–167. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. TrAC Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Poljsak, B.; Milisav, I. Aging, oxidative stress and antioxidants. In Oxidative Stress Chronic Degenerative Diseases—A Role for Antioxidants; Morales-Gonzalez, J.A., Ed.; IntechOpen: Rijeka, Croatia, 2013; pp. 331–353. [Google Scholar] [CrossRef]
- Banaeian, S.; Sereshti, M.; Rafieian-Kopaei, M.; Farahbod, F.; Kheiri, S. Comparison of vaginal ointment of honey and clotrimazole for treatment of vulvovaginal candidiasis: A random clinical trial. J. Med. Mycol. 2017, 27, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Soelaiman, I.-N.; Shuid, A.N. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. A Review on the Protective Effects of Honey against Metabolic Syndrome. Nutrition 2018, 10, 1009. [Google Scholar] [CrossRef]
- Abdelmonem, A.M.; Rasheed, S.M.; Mohamed, A.S. Bee-honey and yogurt: A novel mixture for treating patients with vulvovaginal candidiasis during pregnancy. Arch. Gynecol. Obstet. 2012, 286, 109–114. [Google Scholar] [CrossRef]
- Amiri-Farahani, L.; Hasanpoor-Azghdy, S.B.; Kasraei, H.; Heidari, T. Comparison of the effect of honey and mefenamic acid on the severity of pain in women with primary dysmenorrhea. Arch. Gynecol. Obstet. 2017, 296, 277–283. [Google Scholar] [CrossRef]
- Abdelhafiz, A.; Abdelmoneim, J.; Abdlerahman, M.; Omar, A.; Aly, D. An In Vitro Model for the Use of Egyptian Bee Honey and Royal Jelly in Cases of Premature Rupture of the Fetal Membranes (PROM). Int. J. Fertil. Steril. 2013, 7 (Suppl. 1), 43. [Google Scholar]
- Zaid, S.S.M.; Sulaiman, S.A.; Sirajudeen, K.N.M.; Othman, N.H. The effects of tualang honey on female reproductive organs, tibia bone and hormonal profile in ovariectomised rats—Animal model for menopause. BMC Complement. Altern. Med. 2010, 10, 82. [Google Scholar] [CrossRef]
- Mosavat, M.; Ooi, F.K.; Mohamed, M. Stress Hormone and Reproductive System in Response to Honey Supplementation Combined with Different Jumping Exercise Intensities in Female Rats. BioMed Res. Int. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Shafin, N.; Othman, Z.; Zakaria, R.; Hussain, N.H.N. Tualang Honey Supplementation Reduces Blood Oxidative Stress Levels/Activities in Postmenopausal Women. ISRN Oxidative Med. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Ahmad, N.S.; Aziz, A.A.; Kong, K.W.; Hamid, M.S.A.; Cheong, J.P.G.; Hamzah, S.H. Dose–Response Effect of Tualang Honey on Postprandial Antioxidant Activity and Oxidative Stress in Female Athletes: A Pilot Study. J. Altern. Complement. Med. 2017, 23, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Seifinadergoli, Z.; Nahidi, F.; Safaiyan, A.; Javadzadeh, Y.; Eteraf-Oskouei, T. Comparison of the efficacy of honey gel and clotrimazole cream in the treatment of vaginal candidiasis symptoms: A randomized clinical trial. Electron. Phys. 2018, 10, 6904–6911. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M. Actions of gonadotrophins on the uterus. J. Reprod. Fertil. 2001, 121, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, H.N.; Sales, K.J.; Catalano, R.D.; Norman, J.E. Inflammatory pathways in female reproductive health and disease. Reproduction 2009, 138, 903–919. [Google Scholar] [CrossRef]
- Sherwood, L. Human Physiology: From Cells to Systems, 9th ed.; Cengage learning: Boston, MA, USA, 2015. [Google Scholar]
- Agarwal, A.; Gupta, S.; Sharma, R. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, T.; Adakama, M.E.; Li, S.; Kim, T.; Terasaka, E.; Li, D.; Lawson, M.A. Reactive Oxygen Species Link Gonadotropin-Releasing Hormone Receptor Signaling Cascades in the Gonadotrope. Front. Endocrinol. 2017, 8, 286. [Google Scholar] [CrossRef]
- Li, S.; Herrera, G.G.; Tam, K.K.; Lizarraga, J.S.; Beedle, M.-T.; Winuthayanon, W. Estrogen Action in the Epithelial Cells of the Mouse Vagina Regulates Neutrophil Infiltration and Vaginal Tissue Integrity. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Vaughan, J.E.; Walsh, S.W. Activation of NF-κB in Placentas of Women with Preeclampsia. Hypertens. Pregnancy 2012, 31, 243–251. [Google Scholar] [CrossRef]
- Uddin, S.; Rahman, M.; Jakaria; Rahman, S.; Hossain, S.; Islam, A.; Ahmed, M.; Mathew, B.; Omar, U.M.; Barreto, G.E.; et al. Estrogen Signaling in Alzheimer’s Disease: Molecular Insights and Therapeutic Targets for Alzheimer’s Dementia. Mol. Neurobiol. 2020, 57, 2654–2670. [Google Scholar] [CrossRef]
- Ltaif, M.; Gargouri, M.; Magné, C.; El Feki, A.; Soussi, A. Protective effects of Avena sativa against oxidative stress-induced kidney damage resulting from an estrogen deficiency in ovariectomized Swiss mice model. J. Food Biochem. 2020, 44, e13205. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Cremers, N.A.; Leeming, J.P.; Van Der Werf, E.T. Sweet Relief: Determining the Antimicrobial Activity of Medical Grade Honey against Vaginal Isolates of Candida albicans. J. Fungi 2019, 5, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, M.; Zhang, N.; Wang, G. Effectiveness of honey dressing in the treatment of diabetic foot ulcers: A systematic review and meta-analysis. Complement. Ther. Clin. Pr. 2019, 34, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Shehu, A.; Ismail, S.; Rohin, M.; Harun, A.; Aziz, A.; Haque, M. Antifungal Properties of Malaysian Tualang Honey and Stingless Bee Propolis against Candida Albicans and Cryptococcus Neoformans. J. Appl. Pharm. Sci. 2016, 6, 044–050. [Google Scholar] [CrossRef]
- Yaghoobi, R.; Kazerouni, A.; Kazerouni, O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-bacterial, Anti-inflammatory Anti-oxidant and Anti-viral Agent: A Review. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Dantas, A.D.S.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative Stress Responses in the Human Fungal Pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef]
- Mohan, A.; Quek, S.Y.; Gutierrez-Maddox, N.; Gao, Y.; Shu, Q. Effect of honey in improving the gut microbial balance. Food Qual. Saf. 2017, 1, 107–115. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef]
- Pajor, M.; Worobo, R.W.; Milewski, S.; Szweda, P. The Antimicrobial Potential of Bacteria Isolated from Honey Samples Produced in the Apiaries Located in Pomeranian Voivodeship in Northern Poland. Int. J. Environ. Res. Public Health 2018, 15, 2002. [Google Scholar] [CrossRef]
- Sharma, D. Classification and Properties of Biosurfactants. Biosurf. Food 2016, 2, 21–42. [Google Scholar] [CrossRef]
- Akgul, T.; Karakan, T. The role of probiotics in women with recurrent urinary tract infections. Turk. J. Urol. 2018, 44, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Brannon, J.R.; Dunigan, T.L.; Beebout, C.J.; Ross, T.; Wiebe, M.A.; Reynolds, W.S.; Hadjifrangiskou, M. Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S. Honey—A Novel Antidiabetic Agent. Int. J. Biol. Sci. 2012, 8, 913–934. [Google Scholar] [CrossRef]
- Bahrami, M.; Ataie-Jafari, A.; Hosseini, S.; Foruzanfar, M.H.; Rahmani, M.; Pajouhi, M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009, 60, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Öztaşan, N.; Altinkaynak, K.; Akçay, F.; Göçer, F.; Dane, Ş. Effects of mad honey on blood glucose and lipid levels in rats with streptozocin-induced diabetes. Turk. J Vet. Anim. Sci. 2005, 29, 1093–1096. [Google Scholar]
- Erejuwa, O.O. The Use of Honey in Diabetes Mellitus: Is It Beneficial or Detrimental? Int. J. Endocrinol. Metab. 2012, 10, 444–445. [Google Scholar] [CrossRef]
- Samanta, A.; Burden, A.C.; Jones, A.R. Plasma Glucose Responses to Glucose, Sucrose, and Honey in Patients with Diabetes Mellitus: An Analysis of Glycaemic and Peak Incremental Indices. Diabet. Med. 1985, 2, 371–373. [Google Scholar] [CrossRef]
- Al-Waili, N. Intrapulmonary administration of natural honey solution, hyperosmolar dextrose or hypoosmolar distill water to normal individuals and to patients with type-2 diabetes mellitus or hypertension: Their effects on blood glucose level, plasma insulin and C-peptide, blood pressure and peaked expiratory flow rate. Eur. J. Med. Res. 2003, 8, 295–303. [Google Scholar]
- Agrawal, O.; Pachauri, A.; Yadav, H.; Urmila, J.; Goswamy, H.; Chapperwal, A.; Bisen, P.; Prasad, G.B.K.S. Subjects with Impaired Glucose Tolerance Exhibit a High Degree of Tolerance to Honey. J. Med. Food 2007, 10, 473–478. [Google Scholar] [CrossRef]
- Dolan, L.C.; Potter, S.M.; Burdock, G.A. Evidence-Based Review on the Effect of Normal Dietary Consumption of Fructose on Blood Lipids and Body Weight of Overweight and Obese Individuals. Crit. Rev. Food Sci. Nutr. 2010, 50, 889–918. [Google Scholar] [CrossRef]
- Abdulrhman, M.; El-Hefnawy, M.; Hussein, R.; El-Goud, A.A. The glycemic and peak incremental indices of honey, sucrose and glucose in patients with type 1 diabetes mellitus: Effects on C-peptide level—A pilot study. Acta Diabetol. 2009, 48, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M.; El, M.; Ali, R.; Abou, A. Honey and Type 1 Diabetes Mellitus. In Type 1 Diabetes—Complications, Pathogenesis, and Alternative Treatments; Liu, C.P., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 423–436. [Google Scholar]
- Abdulrhman, M.M.; El-Hefnawy, M.H.; Aly, R.H.; Shatla, R.H.; Mamdouh, R.M.; Mahmoud, D.M.; Mohamed, W.S. Metabolic Effects of Honey in Type 1 Diabetes Mellitus: A Randomized Crossover Pilot Study. J. Med. Food 2013, 16, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes. Oxidative Med. Cell. Longev. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Gleason, J.A.; Dansinger, M.L. Dietary Fructose and Glucose Differentially Affect Lipid and Glucose Homeostasis. J. Nutr. 2009, 139, 1257S–1262S. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, S.M.; Gurtu, S. Glibenclamide or Metformin Combined with Honey Improves Glycemic Control in Streptozotocin-Induced Diabetic Rats. Int. J. Biol. Sci. 2011, 7, 244–252. [Google Scholar] [CrossRef]
- Ramli, N.Z.; Chin, K.-Y.; Zarkasi, K.A.; Ahmad, F. The Beneficial Effects of Stingless Bee Honey from Heterotrigona itama against Metabolic Changes in Rats Fed with High-Carbohydrate and High-Fat Diet. Int. J. Environ. Res. Public Health 2019, 16, 4987. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.M.R.; Abasalti, Z.; Esmaeili, H.; Kazemi-Bajestani, S.M.R.; Aghasizadeh, R.; Saloom, K.Y.; Ferns, G.A.A. Natural Honey and Cardiovascular Risk Factors; Effects on Blood Glucose, Cholesterol, Triacylglycerole, CRP, and Body Weight Compared with Sucrose. Sci. World J. 2008, 8, 463–469. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The Protective Effects of Estrogen on the Cardiovascular System. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef]
- Nordin, A.; Sainik, N.Q.A.V.; Chowdhury, S.R.; Bin Saim, A.; Idrus, R.B.H. Physicochemical properties of stingless bee honey from around the globe: A comprehensive review. J. Food Compos. Anal. 2018, 73, 91–102. [Google Scholar] [CrossRef]
- Ali, A.M. Bee Honey as a Potentially Effective Treatment for Depression: A Review of Clinical and Preclinical Findings. JOJ Nurs. Health Care 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R.; Aziz, C.B.A.; Othman, Z. Tualang Honey Attenuates Noise Stress-Induced Memory Deficits in Aged Rats. Oxidative Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Biological Activity | Active Compounds (AC) and Physico-Chemical Attributes (PCA) of Honey | Biological Effect on Female Reproductive Disorder |
|---|---|---|
| Oestrogenic | AC: Flavonoids and phenolic acids [24] |
|
| Anti-inflammatory and antioxidant | AC: Phenols, flavonoids, ascorbic acid, α-tocopherol, carotenoid compounds, enzymes, Maillard reaction products between reducing sugars and amino acids [67] | |
| AC: Choline and acetylcholine [68] |
| |
| Anti-fungal | PCA: hypertonicity (due to high sugar content), high acidity [37,38], hygroscopicity and hyperosmolarity properties [40] AC: propolis, chrysin, pinobanksin, galangin, quercetin, luteolin and kaempferol [51,52], glucose oxidase [53], oligosaccharides [56] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, N.H.; Ibrahim, S.F.; Jaffar, F.H.F.; Mokhtar, M.H.; Chin, K.Y.; Osman, K. Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review. Molecules 2021, 26, 649. https://doi.org/10.3390/molecules26030649
Ismail NH, Ibrahim SF, Jaffar FHF, Mokhtar MH, Chin KY, Osman K. Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review. Molecules. 2021; 26(3):649. https://doi.org/10.3390/molecules26030649
Chicago/Turabian StyleIsmail, Nur Hilwani, Siti Fatimah Ibrahim, Farah Hanan Fathihah Jaffar, Mohd Helmy Mokhtar, Kok Yong Chin, and Khairul Osman. 2021. "Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review" Molecules 26, no. 3: 649. https://doi.org/10.3390/molecules26030649
APA StyleIsmail, N. H., Ibrahim, S. F., Jaffar, F. H. F., Mokhtar, M. H., Chin, K. Y., & Osman, K. (2021). Augmentation of the Female Reproductive System Using Honey: A Mini Systematic Review. Molecules, 26(3), 649. https://doi.org/10.3390/molecules26030649







