New Photochromic α-Methylchalcones Are Highly Photostable, Even under Singlet Oxygen Conditions: Breaking the α-Methyl Michael-System Reactivity by Reversible Peroxybiradical Formation
Abstract
1. Introduction
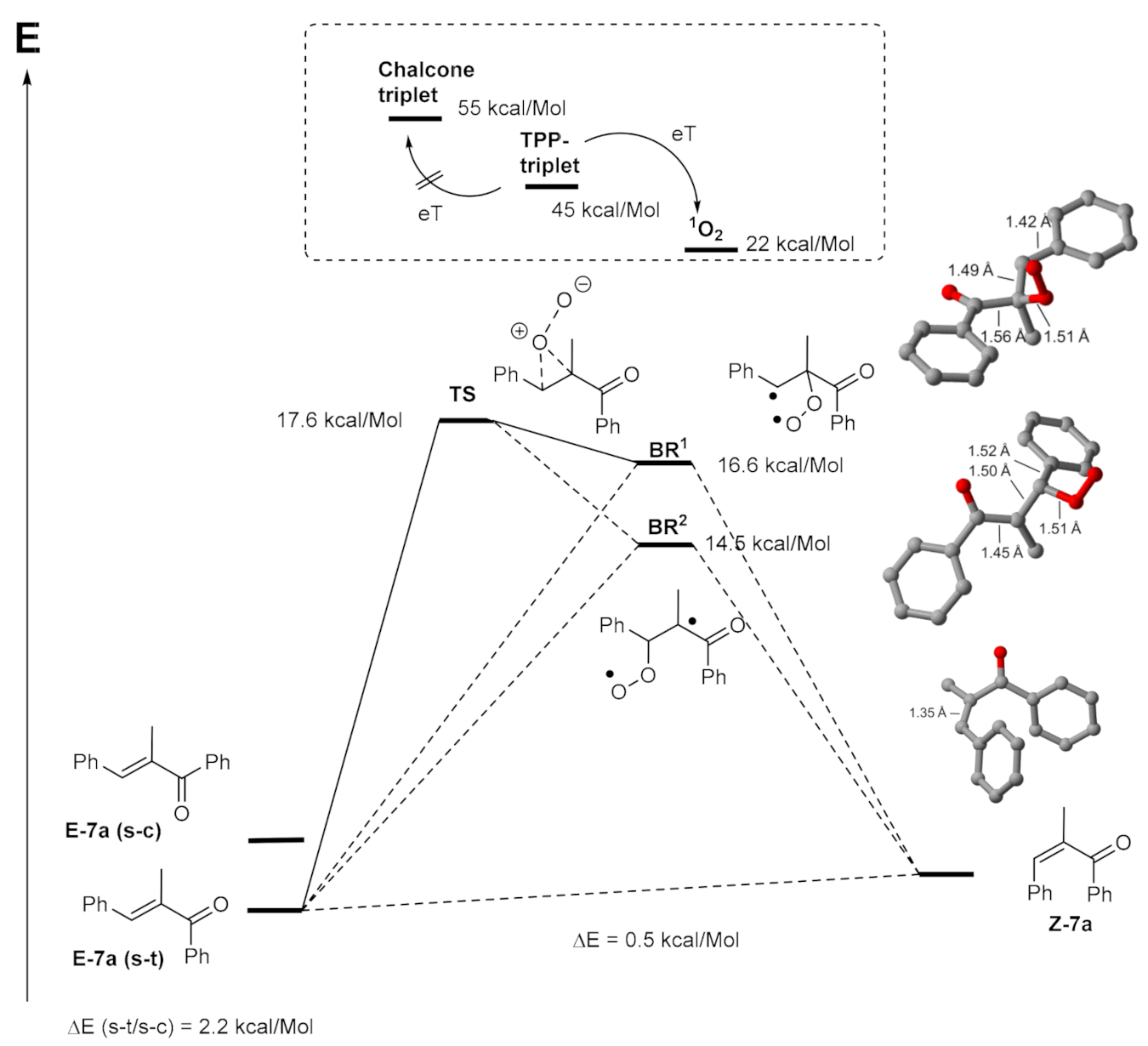
2. Results and Discussion
3. Conclusions
4. Experimental Section
5. Computational Part
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Greer, A. Christopher Foote´s discovery of the role of singlet oxygen [1O2 (1∆g)] in photosensitized oxidation reactions. Acc. Chem. Res. 2006, 39, 797–804. [Google Scholar]
- Schmidt, R. Photosensitized Generation of Singlet Oxygen. Photochem. Photobiol. 2007, 82, 1161–1177. [Google Scholar]
- Kanofyky, J.R. Photosensitization. In Singlet Oxygen: Application in Biosciences and Nanosciences; Flors, C., Nonell, S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; Volume 1, pp. 93–106. [Google Scholar]
- Schweitzer, C.; Schmidt, R. Physical Mechanisms of Generation and Deactivation of Singlet Oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar]
- Ghogara, A.A.; Greer, A. Using Singlet Oxygen to Synthesize Natural Products and Drugs. Chem. Rev. 2016, 116, 9994–10034. [Google Scholar]
- Griesbeck, A.G.; Kleczka, M.; de Kiff, A.; Vollmer, M.; Eske, A.; Sillner, S. Singlet oxygen and natural substrates: Functional polyunsaturated models for the photooxidative degradation of carotenoids. Pure Appl. Chem. 2015, 87, 639–647. [Google Scholar]
- Prein, M.; Adam, W. The Schenck ene reaction: Diastereoselective oxyfunctionalization with singlet oxygen in synthetic applications. Angew. Chem. Int. Ed. 1996, 35, 477–494. [Google Scholar]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar]
- Iesce, M.; Cermola, F.; Temussi, F. Photooxygenation of Heterocycles. Curr. Org. Chem. 2005, 9, 109–139. [Google Scholar]
- Clennan, E.L.; Pace, A. Advances in singlet oxygen chemistry. Tetrahedron 2005, 61, 6665–6691. [Google Scholar]
- Tzirakis, M.D.; Alberti, M.N. On the mechanism of the [4+2] cycloaddition of 1O2 with 1,3-dienes: Identifying the putative biradical/dipolar intermediate by employing the gem-diphenylcyclopropyl-carbinyl radical clock as a mechanistic probe. Arkivoc 2015, 2015, 83–100. [Google Scholar]
- Bartlett, P.D.; Schaap, A.P. Stereospecific Formation of 1,2-Dioxetanes from cis- and trans-Diethoxy-ethylenes by Singlet Oxygen. J. Am. Chem. Soc. 1970, 92, 3223–3225. [Google Scholar]
- Bayer, P.; Perez-Ruiz, R.; Jacobi von Wangelin, A. Stereoselective Photooxidations by the Schenck Ene Reaction. ChemPhotoChem 2018, 2, 559–570. [Google Scholar]
- Boix-Garriga, E.; Rodríguez-Amigo, B.; Planas, O.; Nonell, S. Properties of Singlet Oxygen. In Singlet Oxygen: Application in Biosciences and Nanosciences; Flors, C., Nonell, S., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; Volume 1, pp. 23–46. [Google Scholar]
- Kearns, D.R. Physical and chemical properties of singlet molecular oxygen. Chem. Rev. 1971, 71, 395–427. [Google Scholar]
- Kearns, D.R.; Merkel, P.M. Radiationless decay of singlet molecular oxygen in solution. An experimental and theoretical study of electronic-to-vibrational energy transfer. J. Am. Chem. Soc. 1972, 94, 7244–7253. [Google Scholar]
- Gorman, A.A.; Rodgers, M.A.J. Singlet molecular oxygen. Chem. Soc. Rev. 1981, 10, 205–231. [Google Scholar]
- Griesbeck, A.G.; Schlundt, V.; Neudörfl, J.M. Functionalized polar 1,2,4-trioxanes as bulding blocks by singlet oxygenation of 4-hydroxy tiglic acid using the deuterium isotope trick. RSC Adv. 2013, 3, 7265–7270. [Google Scholar]
- Alberti, M.N.; Orfanopoulos, M. Recent Mechanistic Insights in the Singlet Oxygen Ene Reaction. Chem. Eur. J. 2010, 16, 9414–9421. [Google Scholar]
- Adam, W.; Bottke, N.; Krebs, O. The new skew and the established cis and gem regioselectivities in the ene reaction of trisubstituted olefins: Comparison of the singlet oxygen, triazolinedione, and nitrosoarene enophiles. J. Am. Chem. Soc. 2000, 122, 6791–6792. [Google Scholar]
- Orfanopoulos, M.; Stratakis, M.; Elemes, Y. Geminal selectivity of singlet oxygen ene reactions—The nonbonding large group effect. J. Am. Chem. Soc. 1990, 112, 6417–6419. [Google Scholar]
- Griesbeck, A.G.; Goldfuss, B.; Jäger, C.; Brüllingen, E.; Lippold, T.; Kleczka, M. Strong Asymmetry in the Perepoxide Bifurcation Mechanism: The Large-Group Effect in the Singlet Oxygen Ene Reaction with Allylic Alcohols. ChemPhotoChem 2017, 1, 213–221. [Google Scholar]
- Garavelli, M.; Bernardi, F.; Olivucci, M.; Robb, M.A. DFT Study of the Reactions between Singlet-Oxygen and a Carotenoid Model. J. Am. Chem. Soc. 1998, 120, 10210–10222. [Google Scholar]
- Iesce, M.R.; Cremola, F. Photooxygenation, [2+2] and [4+2]. In CRC Handbook of Organic Photochemistry and Photobiology; Griesbeck, A.A., Oelgemöller, M., Ghetti, F., Eds.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2012; pp. 727–764. [Google Scholar]
- Petrou, A.L.; Petrou, P.L.; Ntanos, T.; Liapis, A. A possible role for singlet oxygen in the degradation of various antioxidants. A meta-analysis and review of literature data. Antioxidants 2018, 7, 35. [Google Scholar] [CrossRef]
- Islamian, J.P.; Mehrali, H. Lycopene as a carotenoid provides radioprotectant and antioxidant effects by quenching radiation-induced free radical singlet oxygen: An overview. Cell J. 2015, 16, 386–391. [Google Scholar]
- Gaonkar, S.L.; Vignesh, U.N. Synthesis and pharmacological properties of chalcones: A review. Res. Chem. Intermed. 2017, 43, 6043–6077. [Google Scholar]
- Von Kostanecki, S.; Tambor, J. Über die sechs isomeren Monooxybenzalacetophenone (Monooxychalkone). Ber. Dtsch. Chem. Ges. 1899, 32, 1921–1926. [Google Scholar]
- Mahapatra, D.K.; Asati, V.; Bharti, K.S. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur. J. Med. Chem. 2015, 92, 839–865. [Google Scholar]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar]
- Vever-Bizet, C.; Dellinger, M.; Brault, D.; Rougee, M.; Bensasson, R.V. Singlet molecular oxygen quenching by saturated and unsaturated fatty-acids and by cholesterol. Photochem. Photobiol. 1989, 50, 321–325. [Google Scholar]
- Chawla, H.M.; Chakrabarty, K. Dye-sensitized Photo-oxygenation of Chalcones. J. Chem. Soc. Perkin Trans. I 1984, 132, 1511–1513. [Google Scholar]
- Ren, B.-Z.; Ablise, M.; Yang, X.-C.; Liao, B.; Yang, Z. Synthesis and biological evaluation of α-methyl-chalcone for anti-cervical cancer activity. Med. Chem. Res. 2017, 26, 1871–1883. [Google Scholar]
- Wei, Y.; Tang, J.; Cong, X.; Zeng, X. Practical metal-free synthesis of chalcone derivatives via a tandem cross-dehydrogenative-coupling/elimination reaction. Green Chem. 2013, 15, 3165–3169. [Google Scholar]
- Lei, T.; Zhou, C.; Huang, M.-Y.; Zhao, L.-M.; Yang, B.; Ye, C.; Xiao, H.; Meng, Q.-Y.; Ramamurthy, V.; Tung, C.-H.; et al. General and efficient intermolecular [2+2] photodimerization of chalcones and cinnamic acid derivatives in solution through visible-light catalysis. Angew. Chem. Int. Ed. 2017, 56, 15407–15410. [Google Scholar]
- Seely, R. The energetics of electron-transfer reactions of chlorophyll and other compounds. Photochem. Photobiol. 1978, 27, 639–654. [Google Scholar]
- Kleczka, M. Application of the Large-Group-Non-Bonding Effect in the Synthesis of Hydroperoxides via Ene Reaction. Ph.D. Thesis, University of Cologne, Cologne, Germany, 2016. [Google Scholar]
- Gollnick, K.; Griesbeck, A.G. [4+2]-Cycloadditionen of Singlet Oxygen to Conjugated Acyclic Hexadienes: Evidence of Singlet Oxygen Induced cis to trans Isomerization. Tetrahedron Lett. 1983, 24, 3303–3306. [Google Scholar]
- Eske, A.; Goldfuss, B.; Griesbeck, A.G.; de Kiff, A.; Kleczka, M.; Leven, M.; Neudörfl, J.M.; Vollmer, M. Ene-Diene Transmissive Cycloaddition Reactions with Singlet Oxygen: The Vinylogous Gem Effect and its Use for Polyoxyfunctionalization of Dienes. J. Org. Chem. 2014, 79, 1818–1829. [Google Scholar]
- Ducki, S.; Forrest, R.; Hadfield, J.A.; Kendall, A.; Lawrence, N.J.; McGown, A.T.; Rennison, D. Potent Antimitotic and Cell Growth Inibitory Properties of Substituted Chalcones. Bioorg. Med. Chem. Lett. 1998, 8, 1051–1056. [Google Scholar]
- Singleton, D.A.; Hang, C.; Szymanski, M.J.; Meyer, M.P.; Leach, A.G.; Kuwata, K.T.; Chen, J.S.; Greer, A.; Foote, C.S.; Houk, K.N. Mechanism of Ene Reactions of Singlet Oxygen. A Two-Step No-Intermediate Mechanism. J. Am. Chem. Soc. 2003, 125, 1319–1328. [Google Scholar]
- Harvey, J.N. Spin-forbidden Reactions: Computational Insight into Mechanism and Kinetics. WIREs Comput. Mol. Sci. 2014, 4, 1–14. [Google Scholar]
- Harvey, J.N.; Aschi, M.; Schwarz, H.; Koch, W. The Singlet and Triplet States of Phenyl Cation. A Hybrid Approach for Locating Minimum Energy Crossing Points Between Non-interacting Potential Energy Surfaces. Theor. Chem. Acc. 1998, 99, 95–99. [Google Scholar]
- Clennan, E.L.; Noe, L.J.; Szneier, E.; Wen, T. Hydrazines: New Charge-Transfer Physical Quenchers of Singlet Oxygen. J. Am. Chem. Soc. 1990, 112, 5080–5085. [Google Scholar]
- Öngel, B.; Brüllingen, E.; Griesbeck, A.G. Photochromic α-Methyl Chalcones: Photoswitching and Halochromic Behaviour. ChemPhotoChem. manuscript in preparation.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar]
- Legault, C.Y. CYLview, 1.0b; Université de Sherbrooke: Sherbrooke, QC, Canada, 2009; Available online: http://www.cylview.org (accessed on 21 November 2020).
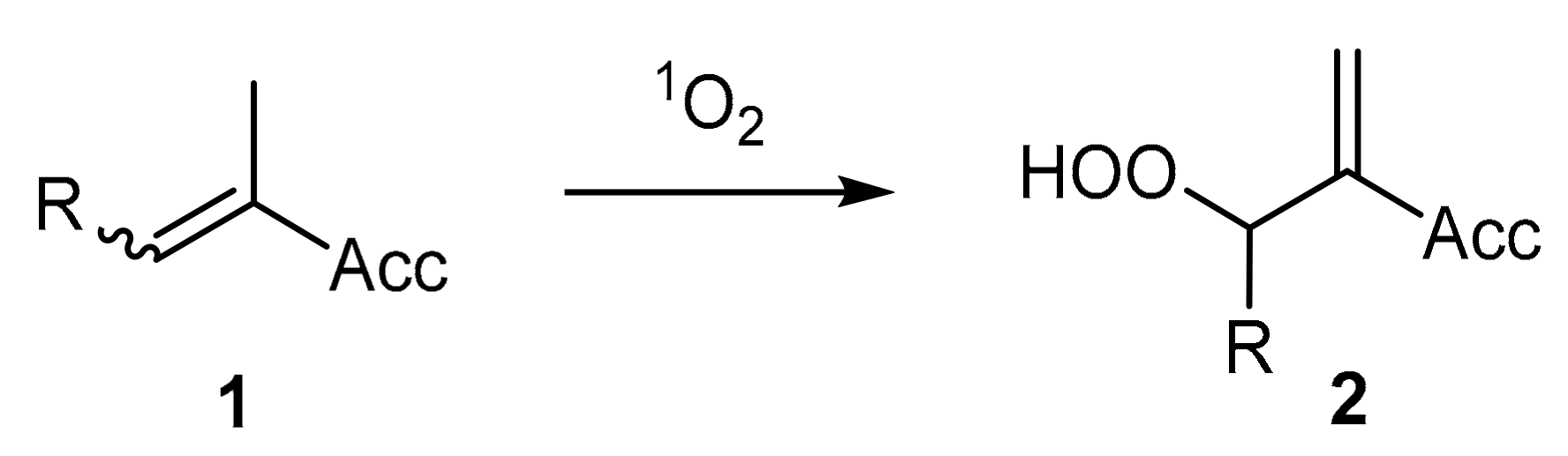
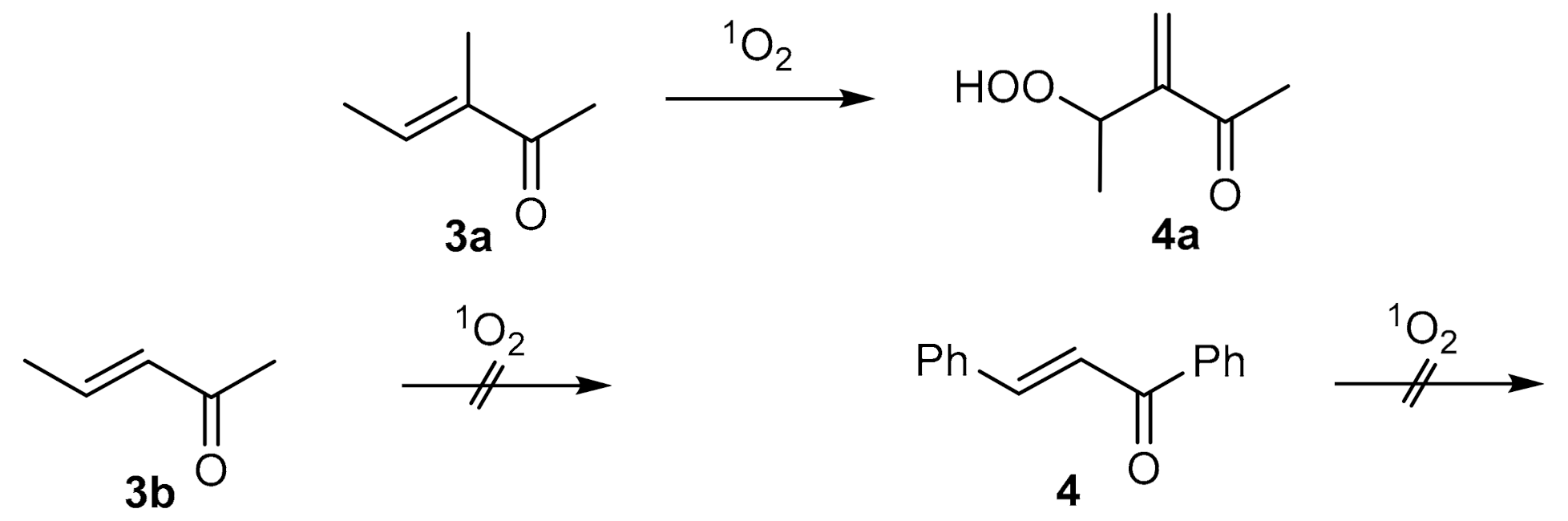
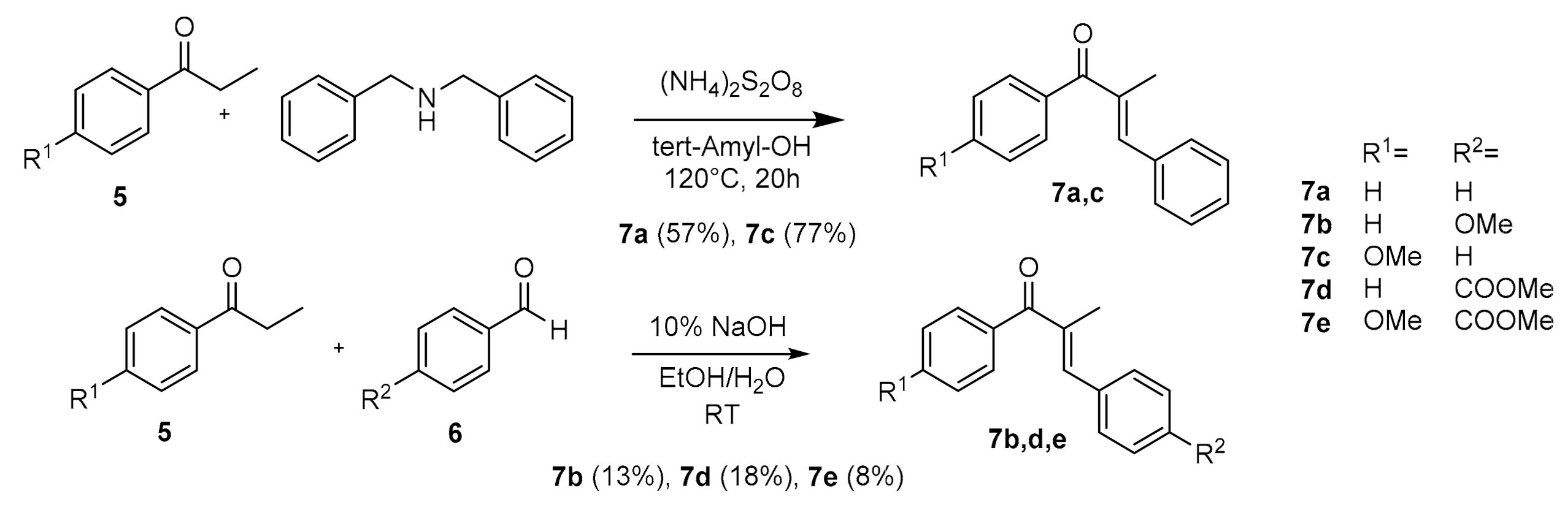
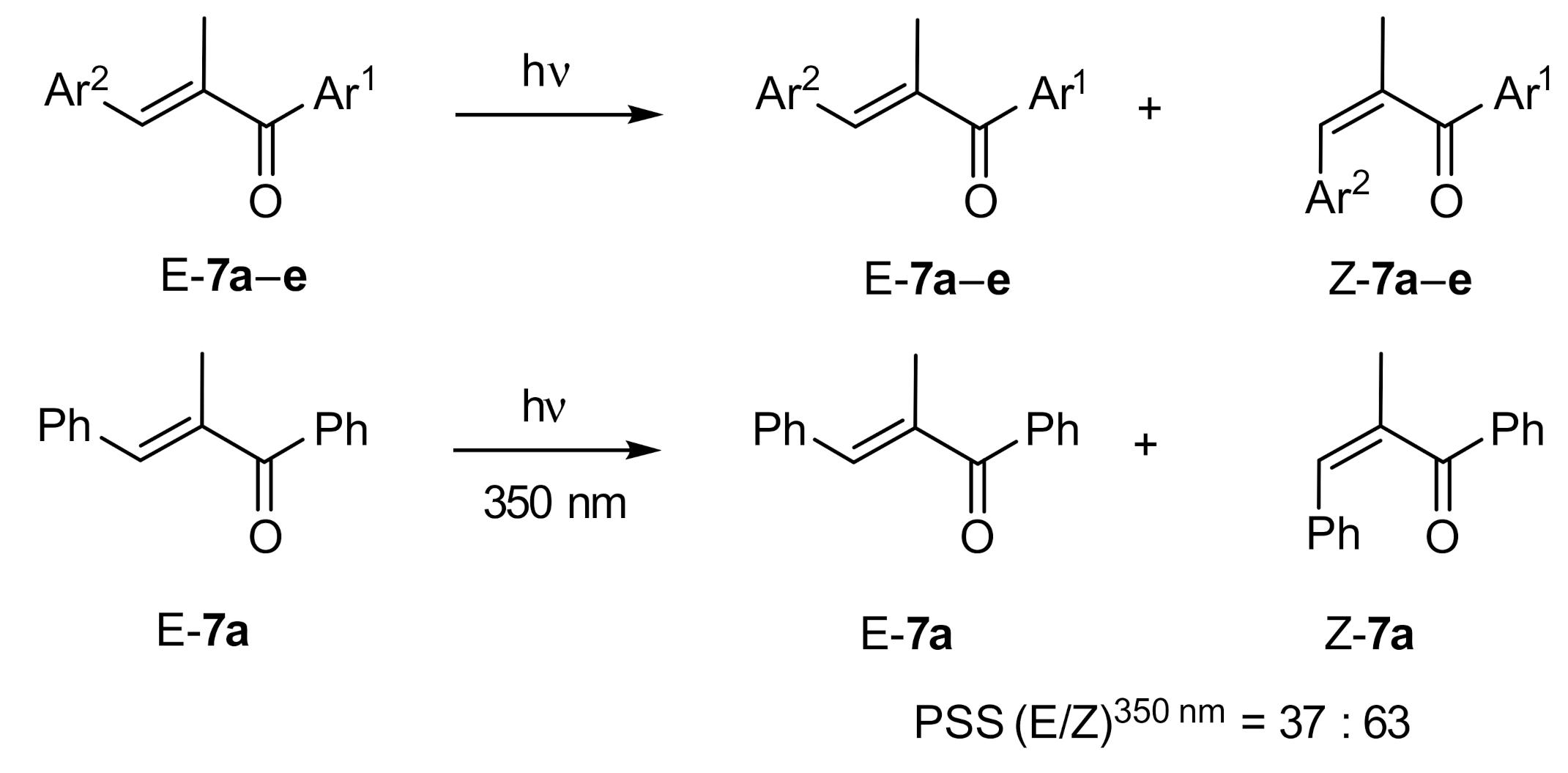
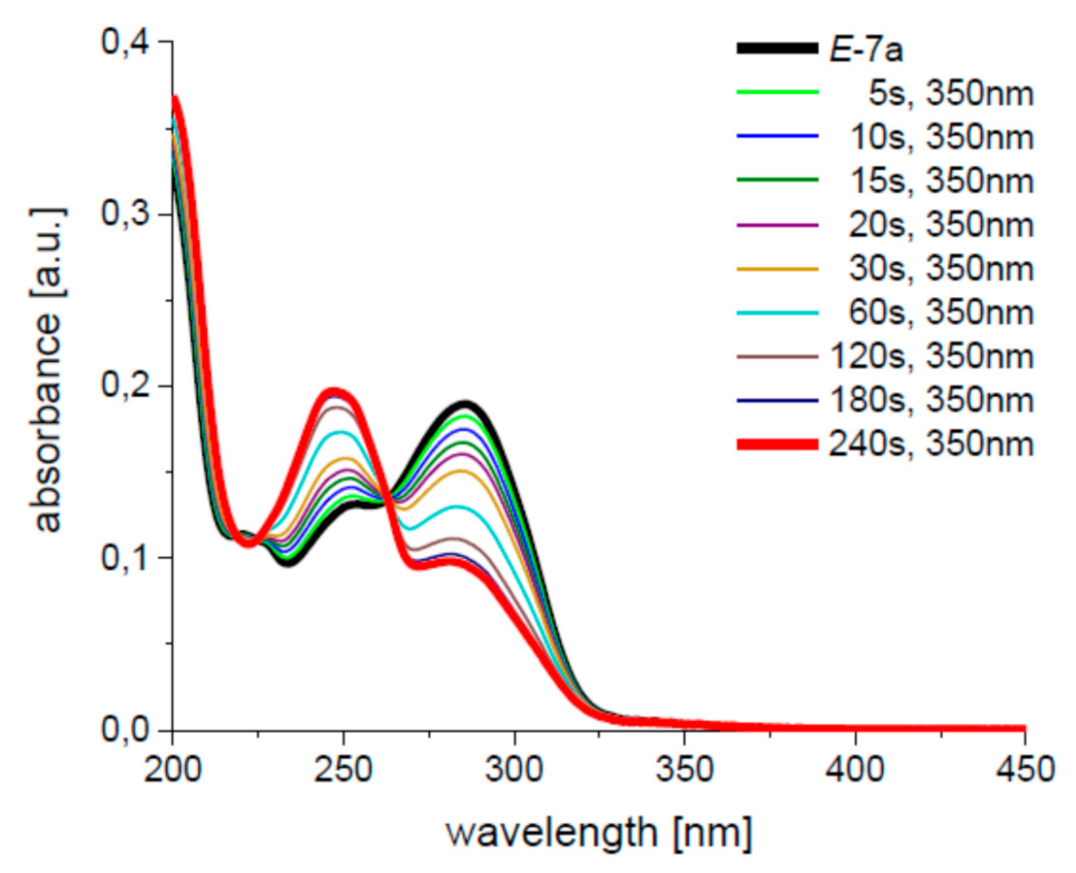
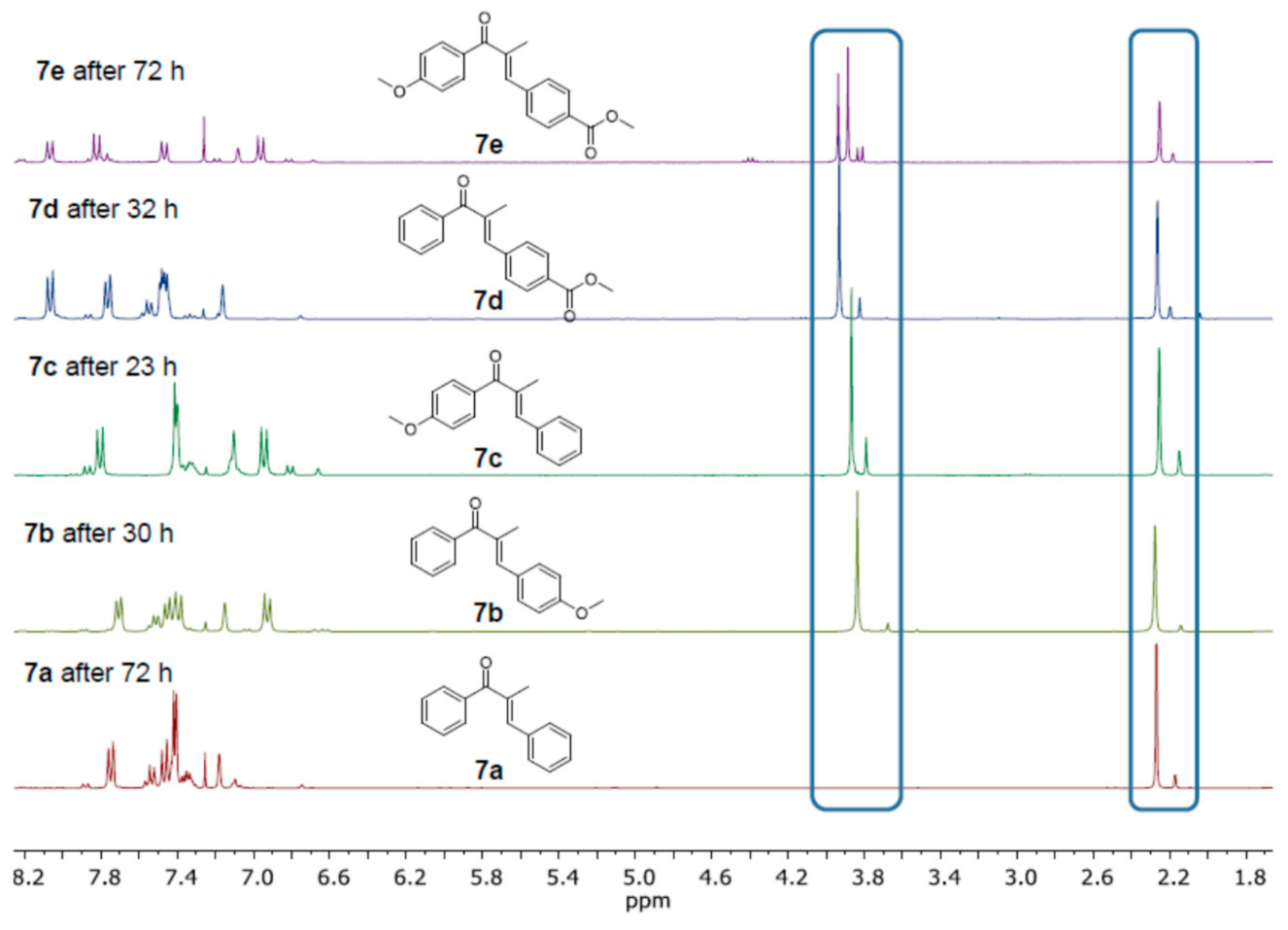
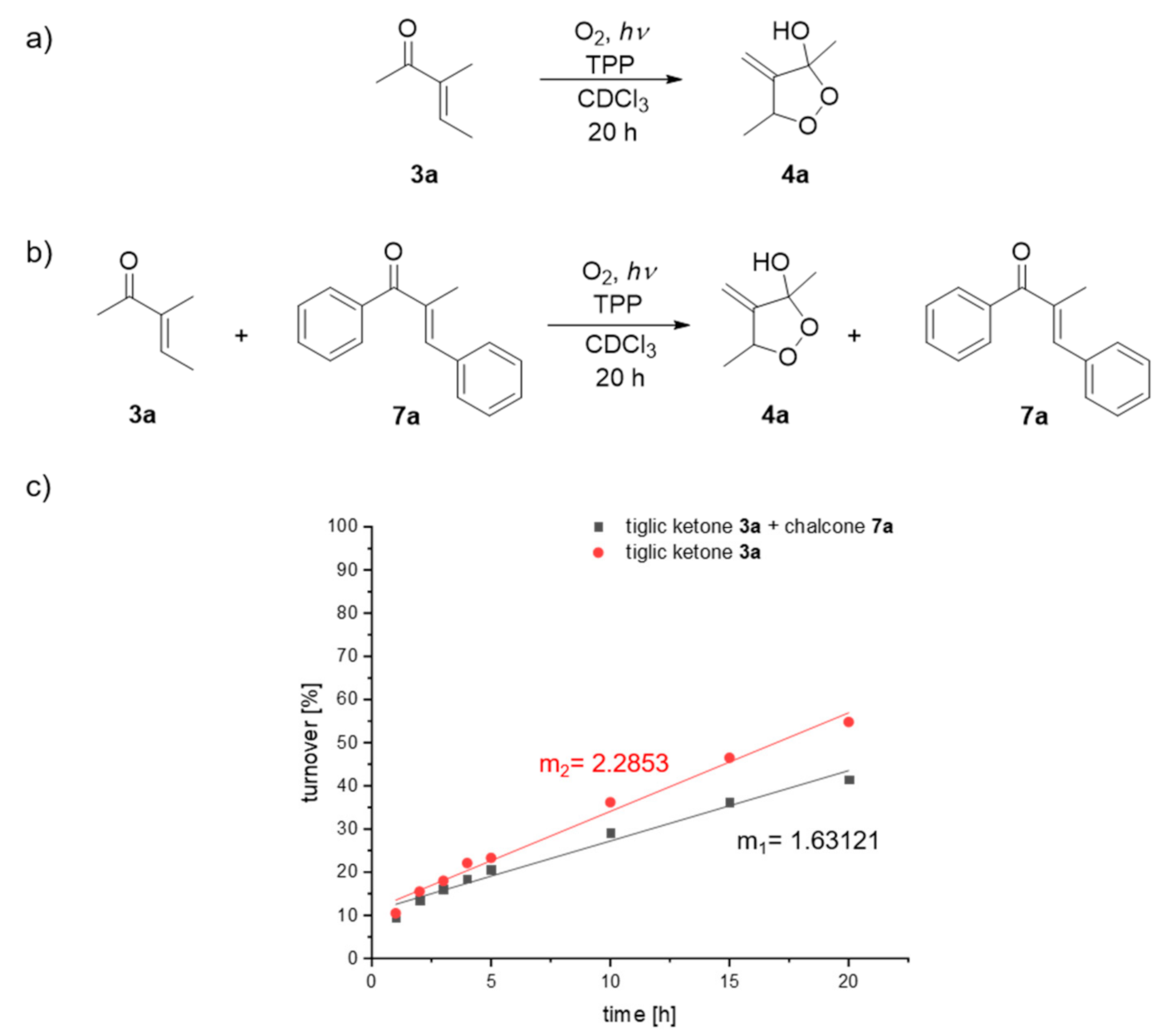
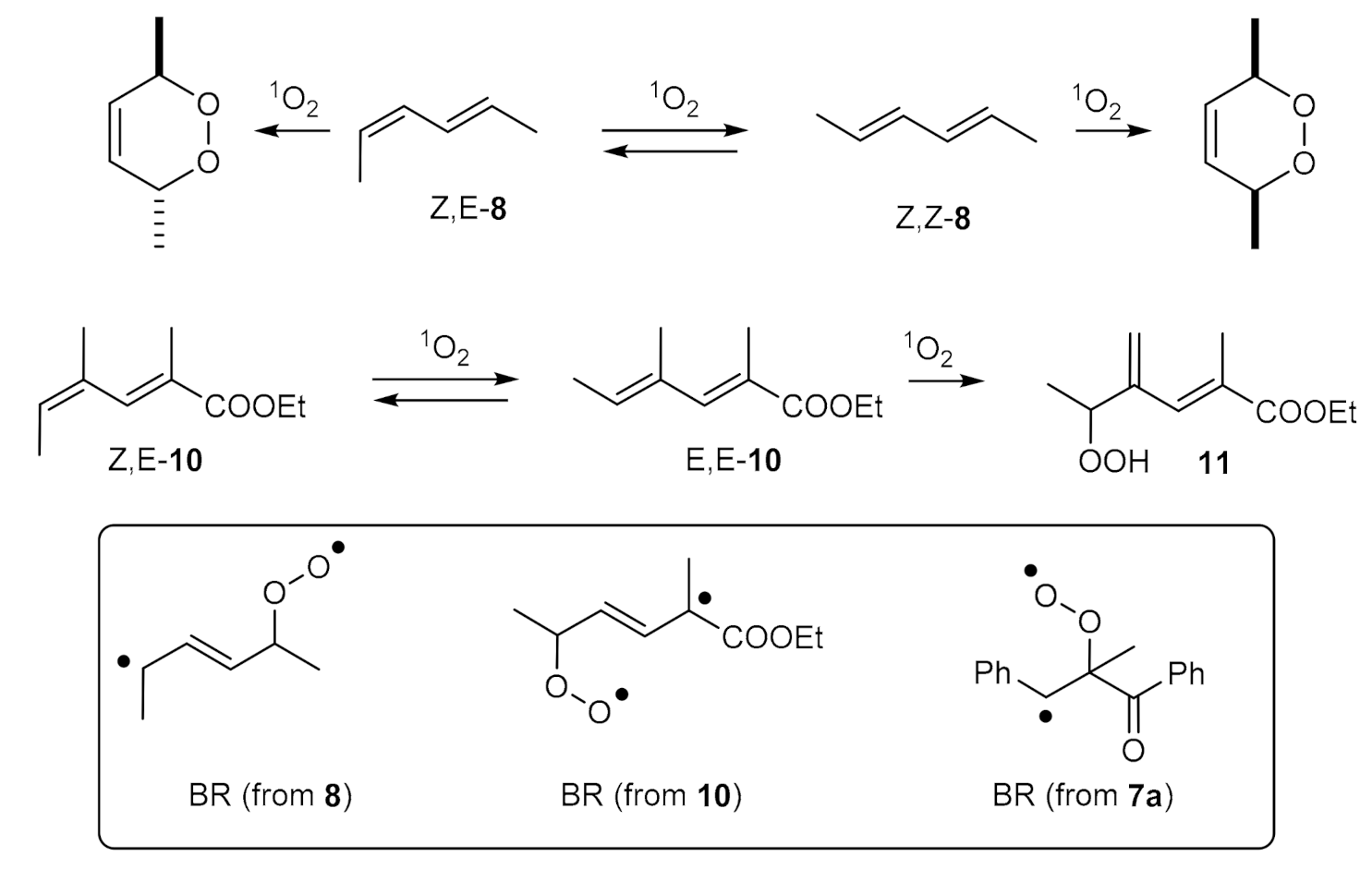
| Compound: | E/Z (350 nm) | E/Z (1O2-induced) |
|---|---|---|
| 7a | 37:63 | 91:9 |
| 7b | 44:56 | 89:11 |
| 7c | 27:73 | 90:10 |
| 7d | 29:71 | 90:10 |
| 7e | 26:74 | 89:11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griesbeck, A.G.; Öngel, B.; Brüllingen, E.; Renner, M. New Photochromic α-Methylchalcones Are Highly Photostable, Even under Singlet Oxygen Conditions: Breaking the α-Methyl Michael-System Reactivity by Reversible Peroxybiradical Formation. Molecules 2021, 26, 642. https://doi.org/10.3390/molecules26030642
Griesbeck AG, Öngel B, Brüllingen E, Renner M. New Photochromic α-Methylchalcones Are Highly Photostable, Even under Singlet Oxygen Conditions: Breaking the α-Methyl Michael-System Reactivity by Reversible Peroxybiradical Formation. Molecules. 2021; 26(3):642. https://doi.org/10.3390/molecules26030642
Chicago/Turabian StyleGriesbeck, Axel G., Banu Öngel, Eric Brüllingen, and Melissa Renner. 2021. "New Photochromic α-Methylchalcones Are Highly Photostable, Even under Singlet Oxygen Conditions: Breaking the α-Methyl Michael-System Reactivity by Reversible Peroxybiradical Formation" Molecules 26, no. 3: 642. https://doi.org/10.3390/molecules26030642
APA StyleGriesbeck, A. G., Öngel, B., Brüllingen, E., & Renner, M. (2021). New Photochromic α-Methylchalcones Are Highly Photostable, Even under Singlet Oxygen Conditions: Breaking the α-Methyl Michael-System Reactivity by Reversible Peroxybiradical Formation. Molecules, 26(3), 642. https://doi.org/10.3390/molecules26030642






