3. Materials and Methods
NMR spectra were recorded on Bruker Advance spectrometers (300 MHz, 400 MHz, 500 MHz) and are reported as δ values in ppm relative to CDCl3 (calibrated to 7.26 ppm in 1H NMR and 77.16 ppm in 13C NMR, unless otherwise indicated). Splitting patterns are abbreviated as follows: singlet (s), doublet (d), triplet (t), quartet (q), multiplet (m), broad (br), and combinations thereof. Column chromatography was conducted on silica gel 60 (240–400 mesh) purchased from Sillicycle. Thin-layer chromatography (TLC) was performed using pre-coated, glass-backed plates (silica gel 60 PF254, 0.25 mm) and visualized using a combination of UV and potassium permanganate staining. HPLC analyses were carried out using an Agilent 1200 HPLC system equipped with an Agilent Quadrupole 6130 ESI-MS detector. Mobile phase was prepared with 0.1% TFA.
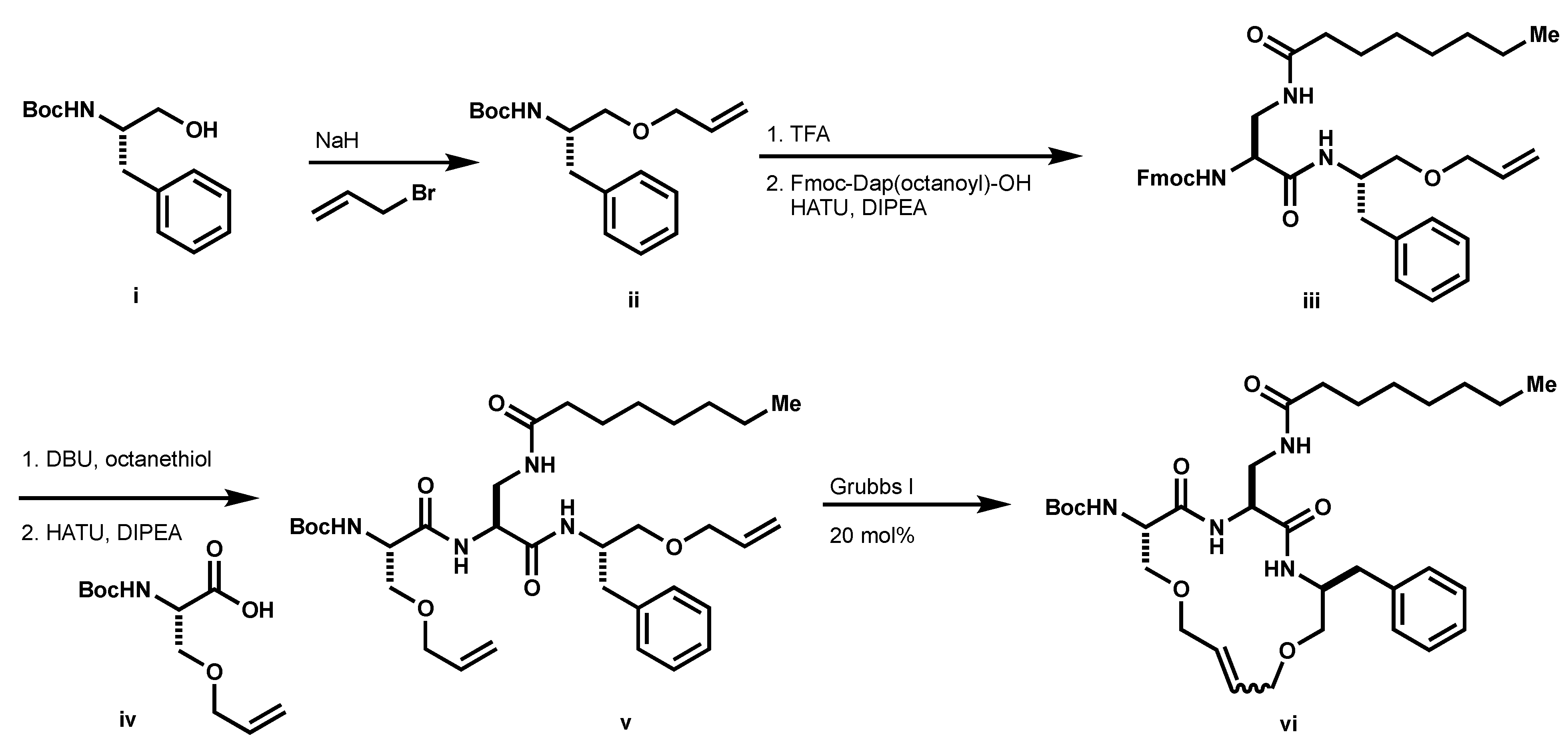
3.1. Preparation of Macrocycles 9A and 9B
tert-butyl (S)-(1-(allyloxy)-3-phenylpropan-2-yl)carbamate (
ii, Scheme 3): To a solution of
i (1.0 g, 3.98 mmol) in DMF (22 mL) at 0 °C, NaH was added (60% in mineral oil, 365 mg, 9.12 mmol) followed by allyl bromide (0.77 mL, 9.72 mmol). The resulting mixture was stirred at room temperature for 4 h. The reaction was quenched with saturated aqueous NH
4Cl and extracted with EtOAc (×2). The organics were dried (MgSO
4), filtered, and concentrated under reduced pressure. The resultant oil was purified by column chromatography using 9:1 hexanes: EtOAc as eluents to afford
ii (526 mg, 45%) as a clear oil.
1H NMR (500 MHz, CDCl
3) δ 7.28 (q,
J = 4.9 Hz, 2H), 7.21 (d,
J = 7.4 Hz, 3H), 5.91 (m, 1H), 5.27 (q,
J = 6.3 Hz, 1H), 5.19 (q,
J = 4.0 Hz, 1H), 4.86 (s, 1H), 3.96 (m, 2H), 3.34 (m, 2H), 2.87 (m, 2H), 1.42 (s, 9H). HPLC/MS MH
+ 192.1 (-Boc).
(9H-fluoren-9-yl) methyl((S)-1-(((S)-1-(allyloxy)-3-phenylpropan-2-yl)amino)-3-octanamido-1-oxopropan-2-yl)carbamate (iii): To a solution of ii (1 eq.) in CH2Cl2 (0.4 M) was added TFA (10% v/v). The resulting solution was stirred at room temperature until completion as determine by LCMS. The reaction mixture was concentrated under reduced pressure and the resultant TFA salt was used without further purification. The resultant residue (1.1 eq.), Fmoc-Dap(octanoyl)-OH (1 eq.) and HATU (1.1 eq.) was suspended in DMF (0.3 M). To the suspension DIPEA was added (2.5 eq.) and the resulting solution was stirred at room temperature until completion, as determined by LCMS. Upon completion, the reaction mixture was diluted with EtOAc and washed with saturated aqueous NH4Cl (×2), water (×3), saturated aqueous NaHCO3 (×1), and brine (×1). The organics were dried over MgSO4, filtered, and concentrated under reduced pressure to afford iii (178 mg, 95%). 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 7.5 Hz, 2H), 7.60 (d, J = 7.2 Hz, 2H), 7.40 (t, J = 7.5 Hz, 2H), 7.32 (m, 2H), 7.25 (d, J = 14.7 Hz, 2H), 7.19 (q, J = 3.5 Hz, 3H), 6.97 (d, J = 7.8 Hz, 1H), 6.41 (d, J = 4.3 Hz, 1H), 6.12 (s, 1H), 5.84 (m, 1H), 5.22 (d, J = 17.1 Hz, 1H), 5.12 (d, J = 10.3 Hz, 1H), 4.37 (t, J = 8.9 Hz, 1H), 4.31 (m, 2H), 4.21 (t, J = 7.1 Hz, 2H), 3.94 (s, 2H), 3.69 (d, J = 11.6 Hz, 1H), 3.49 (t, J = 7.0 Hz, 1H), 3.37 (s, 2H), 2.87 (m, 2H), 2.13 (t, J = 7.2 Hz, 2H), 1.74 (s, 1H), 1.60 (s, 2H), 1.26 (t, J = 5.2 Hz, 8H), 0.85 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 175.37, 169.47, 143.74, 141.29 (d, J = 2.4 Hz), 137.81, 134.40, 129.30, 128.44, 127.80, 127.15 (d, J = 2.7 Hz), 126.53, 125.20, 120.03, 117.20, 72.14, 69.90, 67.52, 56.73, 50.35, 47.07, 42.15, 37.54, 36.51, 31.67, 29.24, 29.01, 25.66, 22.62, 14.06. HPLC/MS MH+ 626.1.
Boc-Ser(O-allyl)-OMe: To a solution of Boc-Ser-OMe (1.28 g, 5.85 mmol) in THF (11.7 mL) a solution of allyl ethyl carbonate (1.54 mL, 11.7 mmol), allyl palladium chloride dimer (43 mg, 0.12 mmol) and PPh3 (138 mg, 0.53 mmol) was added dropwise to THF (5.85 mL). The resulting reaction mixture was refluxed under argon overnight. The reaction mixture was concentrated under a reduced pressure and the resultant residue was purified by column chromatography using 9:1 hexanes: EtOAc as eluents to afford the desired product (1.24 g, 82%) as a clear oil. 1H NMR (500 MHz, CDCl3) δ 5.82 (m, 1H), 5.37 (d, J = 8.1 Hz, 1H), 5.23 (m, 1H), 5.17 (d, J = 10.4 Hz, 1H), 4.42 (t, J = 4.4 Hz, 1H), 3.97 (t, J = 5.7 Hz, 2H), 3.84 (q, J = 4.1 Hz, 1H), 3.75 (s, 9H), 3.64 (q, J = 4.2 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 171.23, 155.51, 134.06, 117.40, 79.99, 72.24, 69.94, 53.99, 52.48, 28.32. HPLC/MS MH+ 1601.1 (-Boc).
Boc-Ser(O-allyl)-OH (iv): To a solution of Boc-Ser(O-allyl)-OMe (1.24 g, 4.78 mmol) in a mixture of THF/MeOH/H2O (1:1:1, 75 mL) LiOH•H2O (502 mg, 11.96 mmol) was added and the reaction was stirred at room temperature until completion as determined by TLC. THF and MeOH were removed under reduced pressure and the resulting solution was partitioned between 1 N HCl (70 mL) and DCM (150 mL). The aqueous layer was extracted with DCM (×2) and the combined organics were dried over MgSO4, filtered, and concentrated under reduced pressure to afford the desired product, iv (1.1 g) as a colorless oil, which was used without further purification. 1H NMR (500 MHz, CDCl3) δ 5.85 (m, 1H), 5.40 (d, J = 7.9 Hz, 1H), 5.25 (m, 1H), 5.19 (q, J = 3.9 Hz, 1H), 4.45 (t, J = 3.9 Hz, 1H), 4.01 (d, J = 5.7 Hz, 2H), 3.90 (d, J = 4.5 Hz, 1H), 3.67 (q, J = 4.4 Hz, 1H).
tert-butyl ((6S,9S,12S)-6-benzyl-9-(octanamidomethyl)-8,11-dioxo-4,14-dioxa-7,10-diazaheptadeca-1,16-dien-12-yl)carbamate (v): To a solution of the Fmoc-protected amine iv (1 eq.) and octanethiol (10 eq.) in THF (0.1 M) DBU (1.1 eq.) was added. The resultant solution was stirred at room temperature for 15 min and then concentrated under reduced pressure. The resultant residue was purified by column chromatography. The resultant residue (1.1 eq.), iv (1 eq.) and HATU (1.1 eq.) was suspended in DMF (0.3 M). To the suspension was added DIPEA (2.5 eq.) and the resulting solution was stirred at room temperature until completion, as determined by LCMS. Upon completion, the reaction mixture was diluted with EtOAc and washed with saturated aqueous NH4Cl (×2), water (×3), saturated aqueous NaHCO3 (×1), and brine (×1). The organics were dried over MgSO4, filtered, and concentrated under reduced pressure to afford v (195 mg). 1H NMR (500 MHz, CDCl3) δ 7.98 (d, J = 4.7 Hz, 1H), 7.28 (t, J = 3.8 Hz, 2H), 7.20 (q, J = 3.4 Hz, 3H), 7.08 (d, J = 8.6 Hz, 1H), 6.01 (s, 1H), 5.87 (m, 2H), 5.42 (d, J = 4.9 Hz, 1H), 5.28 (m, 1H), 5.24 (m, 1H), 5.19 (dd, J = 1.5, 10.5 Hz, 1H), 5.16 (dd, J = 1.5, 10.5 Hz, 1H), 4.40 (m, 1H), 4.35 (t, J = 3.8 Hz, 1H), 4.23 (t, J = 4.9 Hz, 1H), 3.98 (m, 4H), 3.78 (q, J = 4.8 Hz, 1H), 3.65 (q, J = 5.7 Hz, 1H), 3.58 (q, J = 4.9 Hz, 1H), 3.46 (m, 1H), 3.38 (m, 2H), 2.94 (q, J = 7.0 Hz, 1H), 2.77 (q, J = 7.2 Hz, 1H), 2.01 (q, J = 4.9 Hz, 1H), 1.64 (s, 6H), 1.56 (s, 1H), 1.46 (s, 9H), 1.26 (s, 8H), 0.87 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 175.55, 170.73, 134.72, 133.96, 129.31, 129.16, 128.37, 126.38, 117.78, 117.02, 72.29, 72.26, 72.16, 70.33, 69.43, 55.64, 54.95, 50.17, 41.78, 37.50, 36.29, 31.66, 29.22, 28.98, 28.34, 25.57, 22.61, 14.05. HPLC/MS MH+ 631.1.
tert-butyl ((3S,6S,9S)-3-benzyl-6-(octanamidomethyl)-5,8-dioxo-1,11-dioxa-4,7-diazacyclopentadec-13-en-9-yl)carbamate (vi): The diene, v (158 mg, 0.25 mmol) was dissolved in CHCl3 (0.01 M) and sparged with argon for 10 min, and then a solution of Grubbs I (20 mol %) in CHCl3 (0.02 M) was added. The resultant solution was stirred under argon until completion as determined by LCMS. Column purification afforded vi (134 mg) as a mixture of alkene isomers. 1H NMR (500 MHz, CDCl3) δ 7.25 (m, 2H), 7.18 (m, 3H), 5.83 (m, 1H), 5.49 (dd, J = 8.5, 32.0 Hz, 1H), 5.20 (m, 1H), 4.33 (m, 2H), 4.12 (m, 1H), 3.97 (m, 2H), 3.85 (q, J = 5.7 Hz, 1H), 3.77 (m, 1H), 3.69 (q, J = 5.1 Hz, 1H), 3.57 (m, 2H), 3.44 (m, 1H), 3.31 (m, 2H), 2.91 (m, 1H), 2.76 (m, 1H), 2.25 (s, 1H), 2.13 (q, J = 7.2 Hz, 1H), 2.04 (m, 1H), 1.90 (d, J = 12.7 Hz, 3H), 1.82 (s, 5H), 1.71 (s, 2H), 1.56 (m, 2H), 1.50 (s, 1H), 1.42 (m, 11H), 1.23 (s, 13H), 0.85 (t, J = 4.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 176.40, 175.77, 175.58, 175.52, 171.65, 170.67, 170.57, 169.48, 169.24, 169.00, 168.77, 155.24, 155.18, 138.20, 129.37, 129.31, 129.15, 128.43, 128.42, 128.38, 128.34, 126.47, 126.44, 117.74, 72.23, 72.13, 71.19, 70.31, 70.22, 69.23, 69.46, 69.34, 69.02, 68.69, 67.29, 67.18, 56.44, 54.34, 50.95, 41.74, 37.45, 36.37, 36.32, 35.54, 35.06, 31.68, 31.66, 29.30, 29.27, 29.01, 28.33, 26.95, 26.86, 26.34, 26.32, 26.13, 22.61, 14.06. HPLC/MS MH+ 603.1.
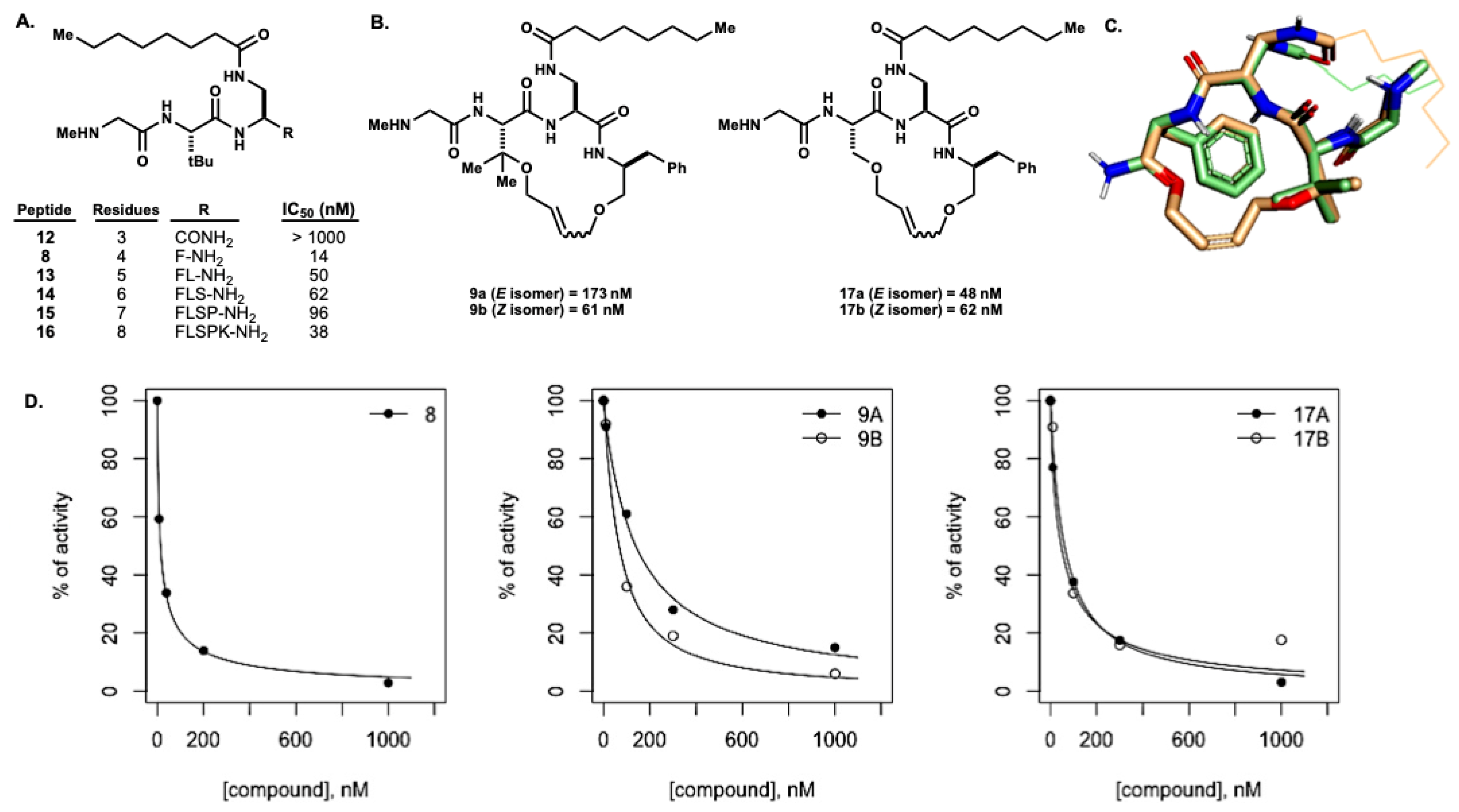
N-(((3S,6S,9S)-9-(2-aminoacetamido)-3-benzyl-5,8-dioxo-1,11-dioxa-4,7-diazacyclopentadec-13-en-6-yl)methyl)octanamide (9): To a solution of ii (1 eq.) in CH2Cl2 (0.4 M) was added TFA (10% v/v). The resulting solution was stirred at room temperature until completion as determine by LCMS. The reaction mixture was concentrated under reduced pressure and the resultant TFA salt was used without further purification. The resultant residue (1.1 eq.), Boc-Gly-OH (1 eq.) and HATU (1.1 eq.) was suspended in DMF (0.3 M). DIPEA (2.5 eq.) was added to the suspension and the resulting solution was stirred at room temperature until completion, as determined by LCMS. Upon completion, the reaction mixture was diluted with EtOAc and washed with saturated aqueous NH4Cl (×2), water (×3), saturated aqueous NaHCO3 (×1), and brine (x1). The organics were dried over MgSO4, filtered, and concentrated under reduced pressure to afford Boc-vi (178 mg, 95%). To a solution of Boc-vi. (1 eq.) in CH2Cl2 (0.4 M), TFA (10% v/v) was added. The resulting solution was stirred at room temperature until completion, as determined by LCMSvto afford 9 (50 mg) which was purified by preparative HPLC to separate the alkene isomers. HRMS m/z: [M + H]+ Calculated for C32H52N5O9 602.3918; Found 602.3871. Z-9: 1H NMR (500 MHz, MeOD) δ 8.35 (d, J = 7.3 Hz, 1H), 7.26 (d, J = 6.6 Hz, 3H), 7.18 (m, 2H), 5.79 (m, 2H), 4.46 (d, J = 2.3 Hz, 1H), 4.38 (m, 1H), 4.17 (d, J = 14.1 Hz, 1H), 4.03 (m, 3H), 3.83 (dd, J = 3.2, 9.8 Hz, 1H), 3.76 (dd, J = 8.1, 14.3 Hz, 1H), 3.54 (dd, J = 5.2, 10.5 Hz, 1H), 3.48 (q, J = 5.2 Hz, 1H), 3.35 (dd, J = 2.2, 10.5 Hz, 1H), 3.19 (dd, J = 2.4, 14.2 Hz, 1H), 2.92 (dd, J = 6.5, 13.0 Hz, 1H), 2.78 (dd, J = 8.7, 13.4 Hz, 1H), 2.20 (m, 2H), 1.93 (m, 2H), 1.85 (m, 2H), 1.75 (s, 1H), 1.59 (t, J = 6.7 Hz, 2H), 1.42 (d, J = 12.2 Hz, 2H), 1.31 (m, 11H), 0.88 (t, J = 6.7 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 172.09, 170.08, 166.86, 138.15, 130.16, 129.01, 128.04, 127.50, 126.12, 69.47, 69.22, 69.02, 66.63, 57.29, 57.19, 55.21, 52.59, 41.30, 41.25, 40.60, 36.47, 35.67, 34.96, 34.47, 31.48, 29.01, 28.76, 26.41, 26.32, 25.82 (d, J = 2.9 Hz, 1C), 25.75, 25.65, 22.30. E-9: 1H NMR (500 MHz, DMSO) δ 7.35 (d, J = 8.4 Hz, 1H), 7.28 (t, J = 7.4 Hz, 2H), 7.24 (t, J = 4.1 Hz, 2H), 7.20 (m, 1H), 5.87 (m, 2H), 4.57 (t, J = 5.4 Hz, 1H), 4.35 (m, 2H), 4.10 (dd, J = 4.7, 12.2 Hz, 2H), 4.01 (dd, J = 5.7, 11.4 Hz, 1H), 3.94 (dd, J = 6.3, 12.7 Hz, 1H), 3.77 (m, 2H), 3.72 (s, 2H), 3.47 (m, 2H), 3.37 (dq, J = 3.2, 9.5 Hz, 2H), 2.92 (q, J = 6.5 Hz, 1H), 2.82 (q, J = 7.4 Hz, 1H), 2.19 (m, 2H), 1.89 (m, 1H), 1.59 (m, 2H), 1.42 (m, 1H), 1.31 (s, 9H), 0.89 (t, J = 6.9 Hz, 3H). HPLC/MS MH+ 560.3.
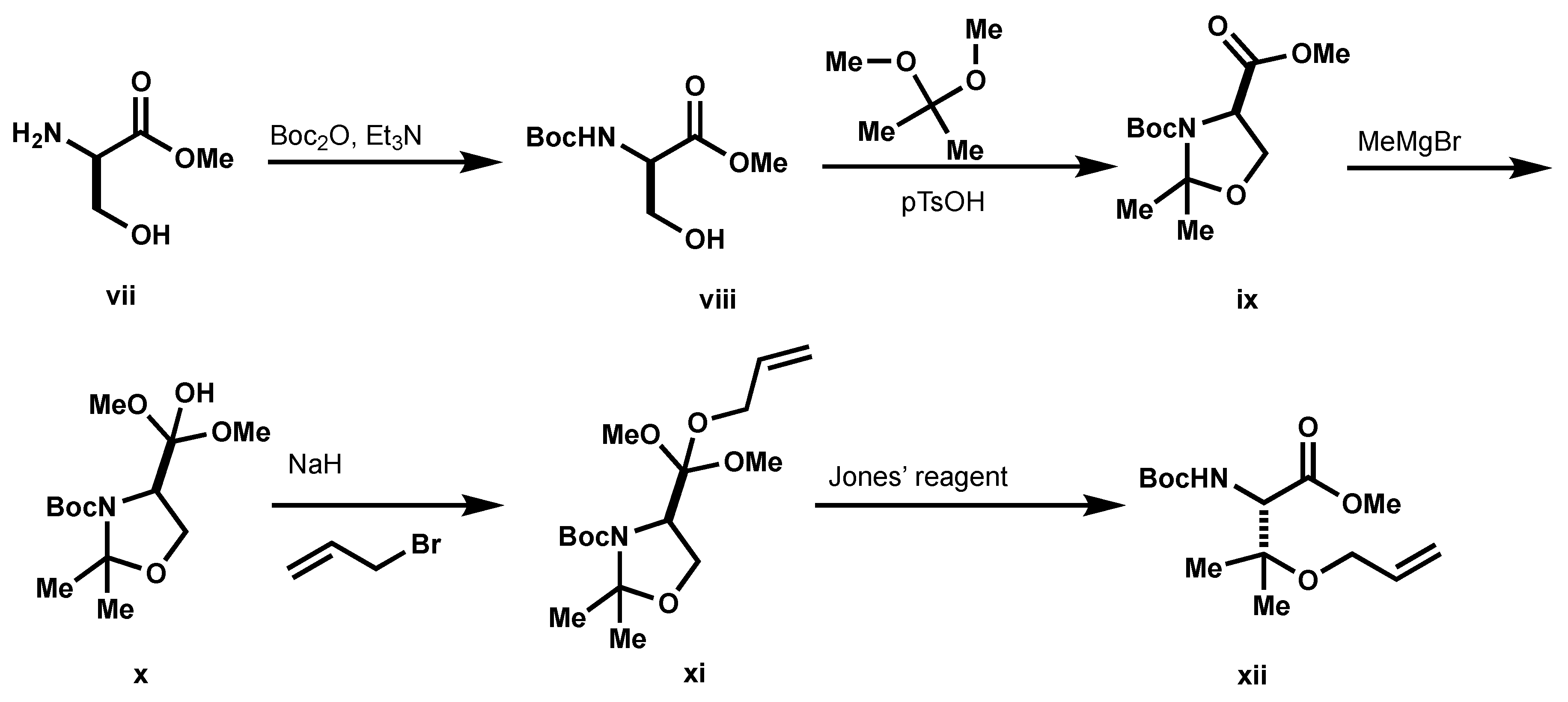
3.2. Preparation of Macrocycles 17A and 17B
Boc-D-Ser-OMe (
viii,
Scheme 4): To a solution of
vii (1.0 g, 8.39 mmol) and Et
3N (2.51 mL, 18.04 mmol) in THF (22 mL) at 0 °C, a solution of Boc
2O was added (1.81 g, 8.31 mmol) in THF (7 mL) dropwise over 20 min. The resulting solution was warmed to room temperature, stirred at that temperature overnight, and then stirred at 50 °C for 3 h. The solvent was removed under reduced pressure and the resultant residue was taken up in ether (15 mL) and saturated aqueous NaHCO
3 (20 mL). The aqueous phase was extracted with ether (×3). The organics were combined, dried over MgSO
4, filtered, and concentrated under a reduced pressure to afford
viii (1.61 g, 87%) as a colorless oil which was used without further purification.
1H NMR (500 MHz, CDCl
3) δ 5.46 (s, 1H), 4.38 (s, 1H), 3.95 (m, 1H), 3.89 (m, 1H), 3.77 (s, 3H), 2.46 (t,
J = 6.2 Hz, 1H), 1.44 (s, 9H).
3-(tert-butyl) 4-methyl (R)-2,2-dimethyloxazolidine-3,4-dicarboxylate (ix): To a solution of viii (1.67 g, 7.33 mmol) in CH2Cl2 (9 mL) at 0 °C, 2,2-dimethoxypropane (4.50 mL, 36.66 mmol) and pTsOH (140 mg, 0.73 mmol) were added. The resulting mixture was warmed to room temperature and then stirred at room temperature overnight. The reaction mixture was then poured into saturated aqueous NaHCO3 (10 mL) and extracted with ether (×3). The organics were combined and washed with NaHCO3 and brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography using 1:1 hexanes: EtOAc as eluents to afford ix (1.63 g, 86%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 4.48 (dd, J = 2.4, 6.8 Hz, 0.4H), 4.37 (dd, J = 3.0, 6.8 Hz, 0.6H), 4.13 (m, 1H), 4.03 (m, 1H), 3.75 (s, 3H), 1.66 (s, 1.89H), 1.63 (s, 1.42H), 1.52 (s, 1.77H), 1.49 (s, 5.11H), 1.40 (s, 5.29H).
tert-butyl(R)-4-(2-hydroxypropan-2-yl)-2,2-dimethyloxazolidine-3-carboxylate (x): To a solution of ix (1.63 g, 6.29 mmol) in THF (50 mL) at −20 °C MeMgBr (1.95 M in Et2O, 10.8 mL, 21.13 mmol) was added dropwise. The resulting mixture was stirred at 0 °C for 4 h. Saturated, aqueous NH4Cl was added to the reaction mixture to quench the reaction, and then extracted with EtOAc (×3). The organics were combined, washed with brine (×2), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography using 3:1 hexanes:EtOAc as eluents to afford x (982 mg, 60%). 1H NMR (500 MHz, CDCl3) δ 4.00 (m, 2H), 3.79 (s, 1H), 1.59 (s, 3H), 1.50 (s, 12H), 1.17 (d, J = 7.0 Hz, 6H). HPLC/MS MH+ 186.2 (-Boc + Na).
tert-butyl (R)-4-(2-(allyloxy)propan-2-yl)-2,2-dimethyloxazolidine-3-carboxylate (xi): To a solution of x (289 mg, 1.12 mmol) in DMF (5.2 mL) at 0 °C, NaH (60% in mineral oil, 90 mg, 2.24 mmol) was added, followed by allyl bromide (0.11 mL, 1.25 mmol). The resulting solution was warmed to room temperature, stirred for 1 h, and cooled back to 0 °C. The reaction was quenched with the addition of saturated aqueous NH4Cl and organics were extracted with EtOAc (×3). The organics were combined, washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The resultant residue was purified by column chromatography using 3:1 hexanes: EtOAc to afford xi (89 mg). 1H NMR (500 MHz, CDCl3) δ 5.88 (m, 1H), 5.25 (m,1H), 5.09 (d, 1H), 4.20 (d, J = 7.7 Hz, 1H), 3.94 (m, 2H), 3.87 (m, 1H), 1.60 (s, 3H), 1.49 (s, 3H), 1.48 (s, 9H), 1.22 (s, 3H), 1.17 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 145.18, 136.00, 115.46, 80.16, 62.87, 29.71, 28.35. HPLC/MS MH+ 200.2 (-Boc).
(S)-3-(allyloxy)-2-((tert-butoxycarbonyl)amino)-3-methylbutanoic acid (xii): To a solution of xi (80 mg, 0.27 mmol) in acetone (3 mL), Jones’ reagent (2.5 M in H2O, 0.16 mL, 0.40 mmol) was added at 0 °C. The resulting mixture was warmed to room temperature, and then stirred at this temperature overnight. To the reaction mixture, celite (100 mg) and isopropanol (0.5 mL) were added, and the resulting precipitate was filtered through off through a plug of celite. The filtrate was adjusted to pH 9 with aqueous NaHCO3, and then concentrated under reduced pressure. The aqueous layer was washed with ether (×2) and acidified to pH 3 with citric acid. The resulting solution was extracted with EtOAc (×3) and the combined extracts were washed with brine (×2), dried over MgSO4, filtered, and concentrated under reduced pressure to afford xii (50 mg, 68%), which was used without further purification. 1H NMR (500 MHz, CDCl3) δ 5.89 (m, J = 5.5 Hz, 1H), 5.30 (q, J = 6.2 Hz, 1H), 5.20 (q, J = 3.8 Hz, 1H), 4.38 (s, 1H), 4.04 (m, 2H), 1.44 (s, 9H), 1.35 (s, 3H), 1.24 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 172.36, 56.00, 133.90, 117.54, 80.37, 78.67, 63.60, 28.26, 22.64, 21.11.
N-(((3S,6S,9S)-9-(2-aminoacetamido)-3-benzyl-10,10-dimethyl-5,8-dioxo-1,11-dioxa-4,7-diazacyclopentadec-13-en-6-yl)methyl)octanamide (17): Macrocycles 17 were synthesized in the same manner as 9 with the modified residue substituted in where necessary. Z-17: 1H NMR (500 MHz, MeOD) δ 8.06 (q, J = 3.7 Hz, 1H), 7.64 (m, 1H), 7.54 (t, J = 4.0 Hz, 1H), 7.27 (t, J = 7.3 Hz, 2H), 7.23 (t, J = 4.1 Hz, 2H), 7.19 (m, 1H), 5.79 (m, 2H), 4.56 (s, 1H), 4.49 (m, 1H), 4.02 (m, 2H), 3.94 (t, J = 5.3 Hz, 2H), 3.90 (t, J = 3.8 Hz, 1H), 3.74 (q, J = 13.5 Hz, 2H), 3.62 (m, 1H), 3.45 (q, J = 4.2 Hz, 1H), 3.36 (q, J = 4.6 Hz, 1H), 3.34 (s, 1H), 2.97 (q, J = 7.1 Hz, 1H), 2.82 (q, J = 7.0 Hz, 1H), 2.14 (t, J = 7.6 Hz, 2H), 1.57 (m, 2H), 1.43 (s, 2H), 1.29 (m, 9H), 1.27 (s, 3H), 1.24 (s, 3H), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 175.69, 170.13, 169.79, 165.86, 138.42, 131.02, 128.93, 128.09, 127.22, 126.12, 77.02, 69.39, 67.88, 60.51, 60.33, 54.35, 53.18, 41.01, 40.15, 35.83, 35.70, 31.51, 29.00, 28.80, 25.47, 22.62, 22.31, 18.77, 13.02. E-17: 1H NMR (500 MHz, MeOD) δ 7.25 (m, 5H), 5.82 (m, 2H), 4.50 (s, 1H), 4.41 (t, J = 5.4 Hz, 1H), 4.24 (q, J = 5.6 Hz, 1H), 4.17 (q, J = 6.3 Hz, 1H), 4.05 (m, 2H), 3.96 (q, J = 5.5 Hz, 1H), 3.73 (d, J = 4.3 Hz, 2H), 3.52 (t, J = 4.7 Hz, 2H), 3.43 (q, J = 4.4 Hz, 1H), 3.34 (q, J = 4.2 Hz, 1H), 2.97 (m, 1H), 2.78 (q, J = 7.5 Hz, 1H), 2.18 (m, 2H), 1.60 (t, J = 7.2 Hz, 2H), 1.36 (s, 3H), 1.30 (m, 11H), 0.89 (t, J = 6.9 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 176.54, 169.93, 138.18, 131.29, 129.02, 128.82, 128.13, 126.14, 77.43, 68.71, 65.98, 60.13, 58.53, 55.14, 51.81, 40.56, 36.52, 35.57, 31.49, 29.04, 28.84, 25.54, 22.47, 22.33, 21.13, 13.03. HPLC/MS MH+ 588.3.
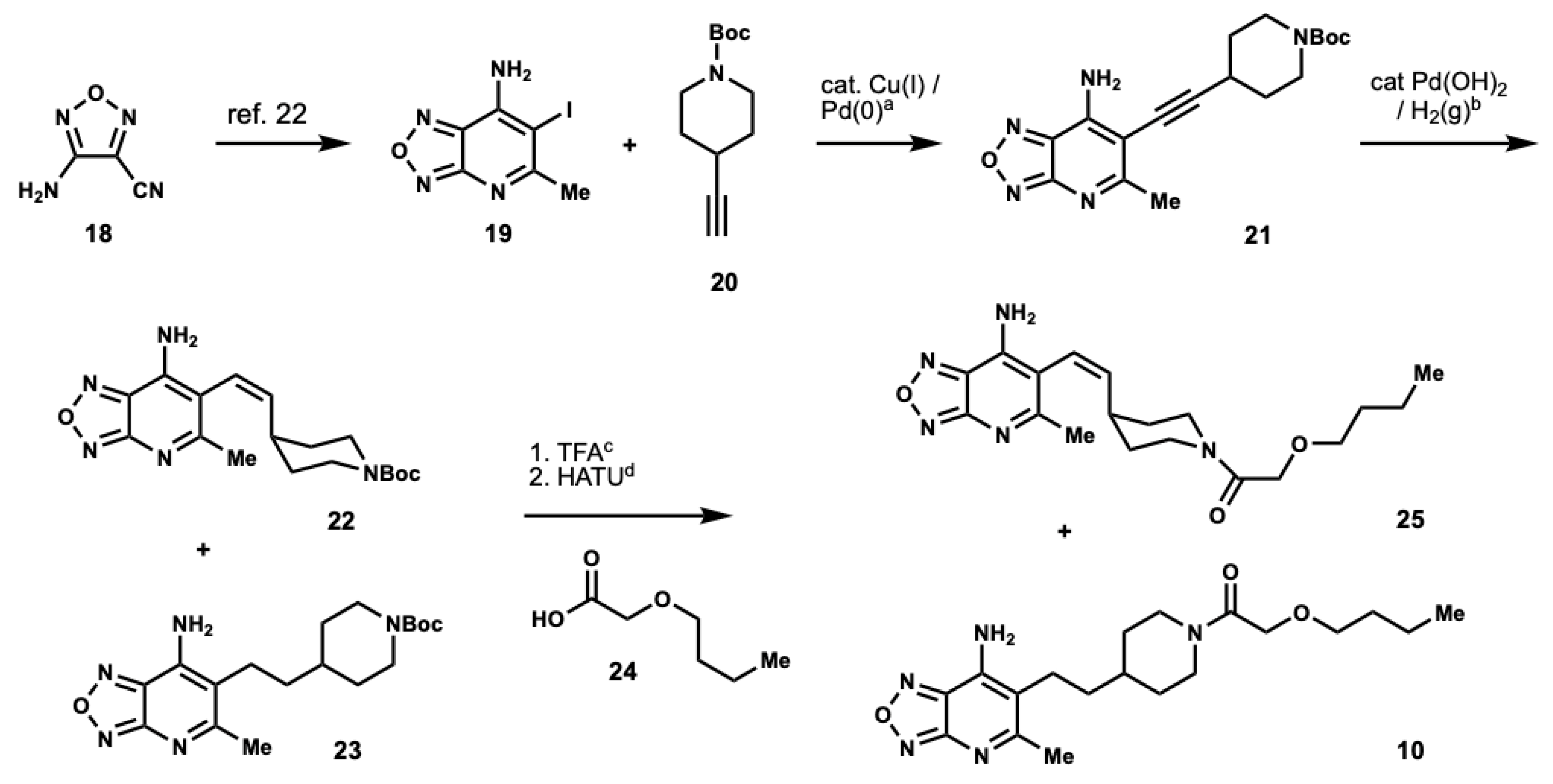
3.3. Preparation of Heterocyclic Analog 10
tert-butyl 4-((7-amino-5-methyl-[1,2,5]oxadiazolo[3,4-b]pyridin-6-yl)ethynyl)piperi-dine-1-carboxylate (
21):
18 and
19 were prepared using methods described in the literature [
22,
32]. A solution of
19 (1.35 g, 4.89 mmol),
20 (1.45 g, 7.15 mmol), (PPh
3)
2PdCl
2 (687 mg, 0.98 mmol), and CuI (93 mg, 0.49 mmol) in Et
3N (20 mL) was degassed with argon for 15 min and then heated at 80 °C for 24 h. The mixture was then stirred at room temperature for 2 days, diluted with EtOAc and filtered through a pad of celite. The filter cake was rinsed with EtOAc. The organics were washed with brine (×2), dried over MgSO
4, filtered, and concentrated under a reduced pressure. The resultant residue was purified via column chromatography using hexanes:EtOAc as eluents to afford
21 (889 mg, 51%).
1H NMR (500 MHz, DMSO) δ 3.63 (m, 2H), 3.15 (s, 2H), 2.94 (m, 1H), 2.53 (s, 3H), 1.83 (m, 2H), 1.61 (m, 2H), 1.38 (s, 9H). HPLC/MS MH
+ 358.3.
tert-butyl (Z)-4-(2-(7-amino-5-methyl-[1,2,5]oxadiazolo[3,4-b]pyridin-6-yl)vinyl)piperidine-1-carboxylate (23): To a solution of 21 (250 mg, 0.70 mmol) in EtOH (7 mL) under argon Pd(OH)2 was added (20% on carbon, 150 mg, 0.21 mmol). Argon was removed and reaction was conducted under an H2 atmosphere and stirred at room temperature. Upon completion, as determined by LCMS, the reaction mixture was diluted with EtOH and filtered through a pad of celite to remove the catalyst. The filter cake was rinsed with EtOH and the filtrate was concentrated under reduced pressure to afford 22 and 23 (276 mg crude) which were identified by HPLC/MS analysis. The crude mixture was used in the next step without further purification.
(Z)-1-(4-(2-(7-amino-5-methyl-[1,2,5]oxadiazolo[3,4-b]pyridin-6-yl)vinyl)piperidin-1-yl)-2-butoxyethan-1-one (25) and 1-(4-(2-(7-amino-5-methyl-[1,2,5]oxadiazolo[3,4-b]pyridin-6-yl)ethyl)piperidin-1-yl)-2-butoxyethan-1-one (10): To the crude mixture of 22 and 23 (252 mg, 0.70 mmol) in CH2Cl2 (16 mL) TFA was added (10% v/v, 1.61 mL, 21.0 mmol). The resulting mixture was stirred at room temperature until completion as determined by LCMS. Upon completion, the reaction mixture was concentrated under a reduced pressure to afford the TFA salts, which were immediately suspended with (0.70 mmol), n-butoxyacetic acid (0.10 mL, 0.77 mmol), and HATU (293 mg, 0.77 mmol) in DMF (3 mL). DIPEA (0.37 mL, 2.10 mmol) was added to the suspension, and the resulting mixture was stirred at room temperature until completion, as determined by LCMS. Upon completion, the reaction was diluted with EtOAc and washed with saturated, aqueous NH4Cl (×2), water (×2); saturated, aqueous NaHCO3 (×2); and brine (×2). The organics were dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography using hexanes:EtOAc as eluents to afford 25 (300 mg) and 10 (10 mg) as yellow residues. 25: 1H NMR (500 MHz, MeOD) δ 7.89 (s, 2H), 6.15 (d, J = 10.9 Hz, 1H), 5.87 (dd, J = 9.7, 10.7 Hz, 1H), 4.39 (d, J = 13.0 Hz, 1H), 4.12 (q, J = 18.1 Hz, 2H), 3.88 (s, 1H), 3.85 (d, J = 12.8 Hz, 1H), 3.51 (t, J = 6.6 Hz, 1H), 3.46 (q, J = 6.7 Hz, 2H), 2.98 (s, 1H), 2.94 (d, J = 12.0 Hz, 1H), 2.85 (d, J = 0.6 Hz, 1H), 2.80 (s, 1H), 2.58 (t, J = 11.7 Hz, 1H), 2.44 (s, 3H), 2.26 (m, 1H), 1.57 (m, 4H), 1.38 (m, 5H), 0.92 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 169.65, 168.58, 158.30, 141.64, 140.78, 139.02, 120.46, 109.81, 78.08, 71.12, 70.88, 69.36, 44.37, 41.23, 37.48, 36.17, 31.33, 31.25, 23.91, 18.89, 12.79. HRMS m/z: [M + H]+ Calculated for C19H28N5O3 374.2192; Found 374.2159. 10: 1H NMR (500 MHz, CDCl3) δ 5.13 (br s, 2H), 4.62 (d, J = 13.2 Hz, 1H), 4.13 (d, J = 12.3 Hz, 2H), 4.00 (d, J = 13.6 Hz, 1H), 3.49 (q, J = 7.1 Hz, 2H), 3.03 (t, J = 12.0 Hz, 1H), 2.62 (s, 3H), 2.59 (d, J = 8.5 Hz, 1H), 1.87 (t, J = 12.8 Hz, 2H), 1.66 (m, 1H), 1.58 (m, 2H), 1.49 (m, 2H), 1.37 (m, 2H), 1.24 (s, 2H), 1.21 (s, 1H), 1.20 (s, 1H), 0.91 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 170.01, 167.86, 157.97, 139.23, 138.21, 114.14, 71.29, 70.72, 45.33, 42.13, 36.51, 34.10, 32.81, 31.67, 29.71, 25.37, 25.00, 24.02, 19.28, 13.88. HRMS m/z: [M + H]+ Calculated for C19H30N5O3 376.2349; Found 376.2315.
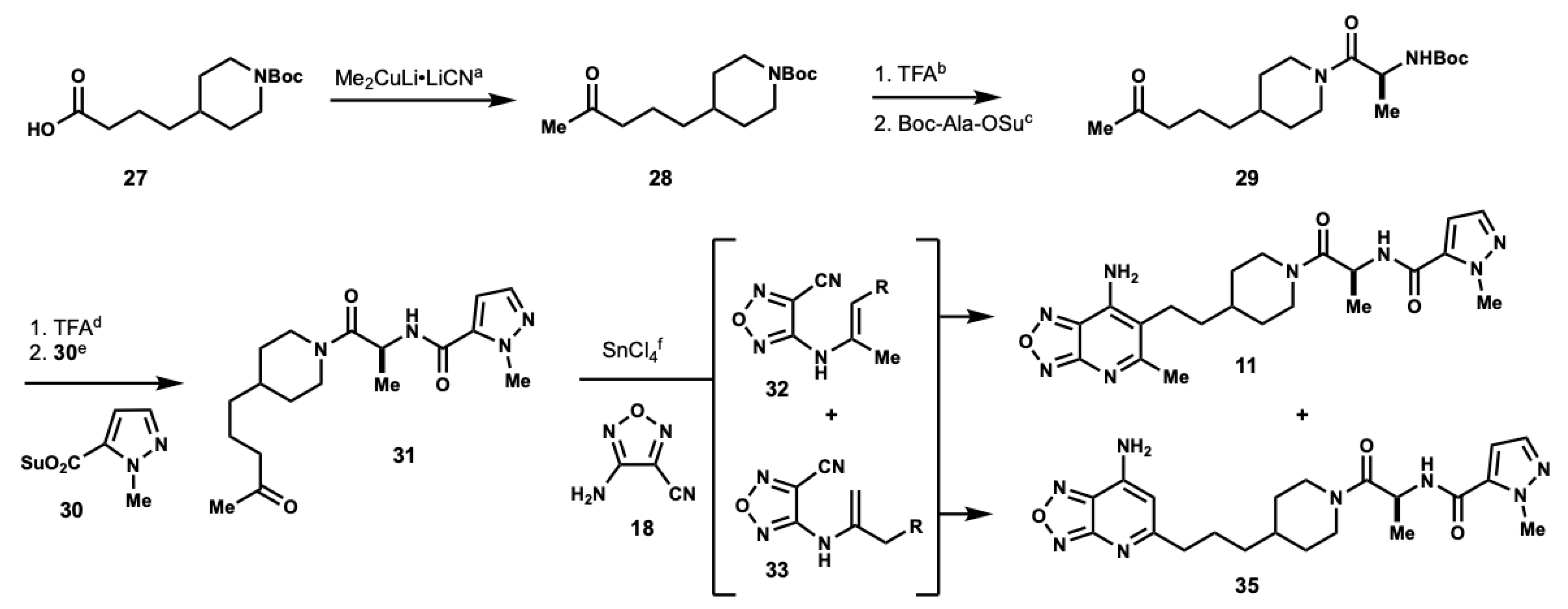
3.4. Preparation of Heterocyclic Analog 11
tert-butyl 4-(4-oxopentyl)piperidine-1-carboxylate (28): To a flask containing CuCN (1.65 g, 18.42 mmol), dry Et2O (30 mL) was added. The resulting suspension was cooled to 0 °C and MeLi (1.6 M in Et2O, 23 mL, 36.85 mmol) was added dropwise. After addition, the resulting mixture was stirred at 0 °C for 5 min before the dropwise addition of a solution of 27 (1.00 g, 3.68 mmol) in Et2O (18.4 mL). The resulting reaction mixture was warmed to room temperature over an hour and then stirred at room temperature overnight. The reaction was quenched with saturated aqueous NH4Cl, and then extracted with CH2Cl2 (×2). The organics were dried over MgSO4, filtered, and concentrated under reduced pressure to afford 28 (703 mg, 71%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 4.05 (d, J = 13.2 Hz, 2H), 2.65 (dt, J = 2.6, 12.8 Hz, 2H), 2.40 (t, J = 7.4 Hz, 2H), 2.12 (s, 3H), 1.63 (m, 2H), 1.58 (m, 2H), 1.44 (s, 9H), 1.36 (m, 1H), 1.21 (m, 2H), 1.05 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 208.99, 154.90, 79.20, 43.99, 43.82, 35.98, 35.91, 32.07, 29.93, 28.48, 20.88. HPLC/MS MH+ 170.2 (-Boc).
tert-butyl (S)-(1-oxo-1-(4-(4-oxopentyl)piperidin-1-yl)propan-2-yl)carbamate (29): To a solution of 28 (200 mg, 0.74 mmol) in CH2Cl2 (10 mL) at 0 °C TFA was added (10% v/v, 1.14 mL, 14.85 mmol). The resulting solution was stirred at 0 °C until completion as determined by TLC. The reaction mixture was concentrated under reduced pressure and the resultant residue was immediately used. To a solution of the TFA salt (188 mg, 0.74 mmol) and Boc-Ala-OSu (203 mg, 0.71 mmol) in MeCN (3 mL) DIPEA was added (0.39 mL, 2.22 mmol) at 0 °C. The resulting mixture was stirred at 0 °C for 2 h, diluted with EtOAc and washed with saturated aqueous NH4Cl and H2O (×3). The organics were dried over MgSO4, filtered, and concentrated under reduced pressure. Residue was purified by column chromatography using 1:1 hexanes: EtOAc as eluents to afford 29 (122 mg, 50%) as a colorless oil. 1H NMR (500 MHz, CDCl3) δ 5.59 (s, 1H), 4.56 (m, 2H), 3.83 (t, J = 12.4 Hz, 1H), 3.00 (m, 1H), 2.57 (m, 1H), 2.42 (t, J = 6.7 Hz, 2H), 2.13 (s, 3H), 1.77 (m, 2H), 1.59 (t, J = 3.8 Hz, 2H), 1.49 (m, 2H), 1.43 (d, J = 4.0 Hz, 9H), 1.28 (dd, J = 6.9, 8.5 Hz, 1H), 1.10 (m, 3H).
(S)-1-methyl-N-(1-oxo-1-(4-(4-oxopentyl)piperidin-1-yl)propan-2-yl)-1H-pyrazole-5-carboxamide (31): To a solution of 29 (122 mg, 0.36 mmol) in CH2Cl2 (5 mL) at 0 °C, TFA was added (10% v/v, 0.55 mL, 7.16 mmol). The resulting mixture was stirred at 0 °C until completion as determined by TLC. The reaction mixture was concentrated under a reduced pressure and the resultant residue was used immediately without purification. The deprotected material (0.36 mmol) and 30 (80 mg, 0.36 mmol) were suspended in MeCN (1.5 mL) at 0 °C. DIPEA was added (0.19 mL, 1.08 mmol) to the suspension, and the resulting reaction mixture was stirred at 0 °C for 2 h and diluted with EtOAc. Organics were washed with saturated, aqueous NH4Cl and H2O (×3), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography using 1:1 hexanes: EtOAc as eluents to afford 31 (90 mg, 72%). (Rotational isomers present.) 1H NMR (500 MHz, CDCl3) δ 7.44 (d, J = 1.5 Hz, 1H), 7.28 (s, 1H), 6.60 (q, J = 2.6 Hz, 1H), 4.97 (q, J = 6.6 Hz, 1H), 4.56 (t, J = 12.9 Hz, 1H), 4.18 (s, 3H), 3.87 (m, 1H), 3.06 (m, 1H), 2.62 (m, 1H), 2.43 (q, J = 7.1 Hz, 2H), 2.13 (d, J = 3.7 Hz, 3H), 1.81 (m, 2H), 1.58 (m, 2H), 1.52 (m, 1H), 1.40 (q, J = 5.5 Hz, 3H), 1.24 (t, J = 18.2 Hz, 2H), 1.11 (m, 2H). 13C NMR (126 MHz, MeOD) δ 208.73, 170.13, 169.94, 158.81, 137.51, 106.76, 60.41, 45.90, 45.52, 45.41, 43.64 (d, J = 5.3 Hz, 1C), 42.85, 42.51, 39.26, 36.06, 35.89, 35.69, 35.62, 32.66, 32.47, 31.71 (d, J = 6.5 Hz, 1C), 29.98, 20.74, 19.42, 18.91, 14.21.
(S)-N-(1-(4-(2-(7-amino-5-methyl-[1,2,5]oxadiazolo[3,4-b]pyridin-6-yl)ethyl)pip-eridin-1-yl)-1-oxopropan-2-yl)-1-methyl-1H-pyrazole-5-carboxamide (11) and (S)-N-(1-(4-(3-(7-amino-[1,2,5]oxadiazolo[3,4-b]pyridin-5-yl)propyl)pip-eridin-1-yl)-1-oxopropan-2-yl)-1-methyl-1H-pyrazole-5-carboxamide (35): To a suspension of 31 (90 mg, 0.26 mmol) and 18 (28 mg, 0.26 mmol) in toluene (0.2M) tin(IV) chloride was added (2 eq). The resulting mixture was stirred at room temperature for 30 min, and then refluxed for 18 h. The reaction was cooled to room temperature and concentrated under reduced pressure. The resultant residue was dissolved in CH2Cl2 and washed with saturated aqueous NaHCO3 (×1). The aqueous layer was extracted with CH2Cl2 (×3). The organics were combined, washed with brine (×1), dried over MgSO4, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography using 5% MeOH in CH2Cl2 as eluents to afford 11 and 35. HRMS m/z: [M + H]+ Calculated for C21H29N5O6 441.2363; Found 441.2313. 11: 1H NMR (500 MHz, MeOD) δ 7.43 (d, J = 2.0 Hz, 1H), 6.83 (s, 1H), 4.97 (m, 1H), 4.47 (m, 1H), 4.06 (d, J = 5.6 Hz, 1H), 4.01 (d, J = 10.5 Hz, 1H), 3.12 (m, 1H), 2.65 (m, 1H), 2.46 (q, J = 7.0 Hz, 2H), 2.10 (d, J = 3.6 Hz, 3H), 1.78 (m, 2H), 1.55 (m, 3H), 1.36 (dd, J = 7.1, 13.9 Hz, 3H), 1.24 (m, 3H), 1.07 (m, 2H). 13C NMR (126 MHz, MeOD) δ 210.31, 170.8, 164.8, 137.12, 107.16, 45.69, 45.36, 45.22, 42.74, 42.55, 42.19, 37.73, 35.64, 35.43, 35.35, 35.23, 32.35, 32.21, 31.53, 31.43, 28.29, 20.32, 16.46, 15.98. 35: 1H NMR (500 MHz, MeOD) δ 7.42 (q, J = 2.4 Hz, 1H), 6.83 (m, 1H), 6.29 (d, J = 4.5 Hz, 1H), 4.97 (m, 1H), 4.46 (m, 1H), 4.05 (s, 3H), 4.02 (s, 1H), 3.13 (m, 1H), 2.67 (m, 3H), 2.53 (d, J = 9.5 Hz, 1H), 1.88 (q, J = 12.7 Hz, 1H), 1.75 (m, 3H), 1.59 (s, 1H), 1.46 (q, J = 8.0 Hz, 1H), 1.35 (dd, J = 7.1, 13.0 Hz, 3H), 1.25 (s, 2H), 1.08 (m, 2H). 13C NMR (126 MHz, MeOD) δ 174.48, 159.60, 137.10, 107.15, 100.26, 45.23, 42.56, 38.87, 37.72, 35.53, 35.38, 32.20, 31.53, 31.43, 25.78, 23.05, 16.44, 15.95.
3.5. In Vitro Assay
pFastBac1-mouseGOAT and pGEX-GST-proGhrelin8His plasmid encoding for mouse proghrelin, fused to GST with TEV cleavage site to release proghrelin moiety, was a kind gift from the Brown and Goldstein laboratory [
18]. The BL21Gold
E. coli chemically competent cells were transformed with the pGEX-GST-proGhrelin8His plasmid and selected on agar plates with ampicillin. A few colonies were used to inoculate 50 mL LB, and cultures were grown overnight at 37 °C. The next morning, 10 mL of overnight culture was used to inoculate 1L LB. A total of 4 flasks with 1L LB each were inoculated with 10 mL of the overnight culture, and bacterial cultures were grown at 37 °C until they reached OD
600 ~ 0.6. The cultures were chilled on ice for 30 min and then 0.25 mM of IPTG was added to each culture. The cultures were then moved back to a shaker set to 18 °C and incubated overnight. Cells were harvested and resuspended in 200 mL buffer containing 25 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA and 0.5 mg/mL lysozyme. The cell suspension was sonicated, and cell debris was removed by centrifugation. The soluble fraction was passed through glutathione resin by gravity. The resin was washed with 75 mL of lysis buffer without lysozyme. The GST-proGhrelin8His protein was eluted with lysis buffer without lysozyme, supplemented with 15 mM reduced glutathione, GSH. About 50 mg of target protein, as evaluated by SDS-PAGE, was eluted. The eluate was supplemented with 1 mM DTT and about 7.5 mg of recombinant GST-TEV protease (see below) was added. The solution was incubated at 16 °C overnight to allow complete release of proghrelin8His from GST. Prior to Ni-NTA chromatography, 5 mM CaCl
2 was added, and the solution was spun down at 4000×
g for 10 min. After elution from Ni-NTA resin, protein solution was dialyzed against buffer containing 10 mM Tris-HCl pH 8.5, 50 mM NaCl, 10% glycerol and 0.01% CHAPS using 3 kDa cutoff membrane with 3 buffer exchanges. The protein was quantified by absorbance at A
280 and qualified by SDS-PAGE.
Generation of GST-TEV protease. The pMHT vector [
34] (gift from Dr. Arbing, UCLA) was linearized by PCR reaction with primers outside of MBP gene: forw- 5′ gcgaccatcctccaaaatcgggagaaagcttgtttaaggggccg 3′ and rev- 5′ caataacctagtataggggacatggttaatttctcctctttaatg 3′. The resulting linear plasmid was used to clone GST gene in-frame at 5′ of TEV gene by in vitro Gibson assembly. The GST gene was prepared by PCR amplification with primers: form 5′ atgtcccctatactaggttattg and rev 5′ cgattttggaggatggtcgc 3′ from pGEX-GST-proGhrelin8His plasmid as a template. The final bacterial expression vector, pGST-TEV was used for expression and purification of GST-TEV fusion protein. BL21Gold chemically competent
E. coli cells were transformed with pGST-TEV plasmid and entire transformation mixture was used to inoculate 50 mL LB supplemented with kanamycin. Next day, 10 mL of the overnight culture was used to inoculate 1 L of LB. Four flasks with 1 L LB each were inoculated with 10 mL of the overnight culture, and bacterial cultures were grown at 37 °C till OD600 ~ 0.8. was reached. After adding 1 mM IPTG, culture was allowed to grow for 4 h, and cells were harvested by centrifugation. The cell pellet was resuspended in 200 mL lysis buffer containing 25 mM Tris-HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 5% glycerol, 0.5 mg/mL lysozyme and 1 mM DTT. Cell suspension after freeze–thaw cycle was sonicated and cellular debris was removed by centrifugation. The soluble fraction was bound to glutathione resin by gravity. The resin was washed with 50 mL washing buffer (lysis buffer without lysozyme and DTT) and fusion GST-TEV protein was eluted with 25 mL washing buffer supplemented with 15 mM reduced glutathione (GSH). The elution was fractionated into 5 fractions and after SDS-PAGE/Coomassie analysis, fractions containing most of the GST-TEV protein were combined and dialyzed against buffer containing 25 mM Tris-HCl pH 8.0, 150 mM NaCl and 50% glycerol using 3 kDa cutoff membrane with 3 buffer exchanges. The total yield was roughly 8 mg per 1 L bacterial culture.
The baculovirus expression of mouse GOAT. DH10Bac E. coli strain (ThermoFisher, Waltham, MA, USA) was transformed with pFastBac1-mouseGOAT plasmid, and Bacmid DNA from 12 white colonies was analyzed by DNA sequencing and PCR reaction. All clones were transfected to sf9 cells and level of mGOAT expression was compared between clones from cells producing P2. The clone with the highest mGOAT expression level was used to produce P3. For membrane isolation, sf9 cells were seeded at 2–4 × 106 /mL in total 1 L volume of sf-900 serum-free medium (ThermoFisher) and infected with P3 virus. At day 2 post-infection, cells were harvested and resuspended in 40 mL of buffer containing 50 mM NaPi pH 7.2, 150 mM NaCl, 1 mM EDTA, 100 μM bis (4-nitrophenyl) phosphate, 2.5 μg/mL aprotinin, 10 μg/mL leupeptin, 10 μg/mL pepstatin A. Cell suspension was briefly sonicated and cell debris was removed by centrifugation at 3000× g for 10 min. Membrane fraction was collected from supernatant by centrifugation at 100,000× g for 1 h. Membrane pellet was resuspended in storage buffer (50 mM NaPi pH 7.2, 150 mM NaCl and 10% glycerol) and kept at −80 °C. Acyltransferase assay: The assay conditions included per 50 μL reaction: 50 μg of total sf9 membranes, various (for modality experiment) or fixed (for IC50) concentrations of recombinant proghrelin8His peptide and various concentrations of tested compound (for IC50), 100 μM palmitoyl CoA, 50 mM HEPES pH 7.0 and 1 μM [3H] octanoyl CoA (~5.5 dpm/fmol—American Radioactive Chemicals). After incubation of the reaction mixture at 37C for 10 min, tubes were placed on ice and 10 μL of 1M HCl was added to each tube. Following the addition of 740 μL of cold quench buffer (50 mM NaPi pH 7.4, 10 mM Imidazole, 150 mM NaCl, 100 μM bis (4-nitrophenyl) phosphate, 1 mM phenylmethylsulfonyl fluoride and 0.1% Triton), 0.2 mL of 50% Ni-NTA slurry was added to each reaction. Tubes were incubated at 4 °C for 1 h with rotation to capture proGhrl8His. After washing Ni resin with 40 mM Imidazole, all bound proghrelin8His was eluted with 250 mM Imidazole, and an amount of octanylated proghrelin was assessed with scintillation counting.
3.6. INS1-Cellular Assay
Generation of Recombinant Retrovirus for mGOAT Expression. The mouse GOAT cDNA with C-terminal HA tag was amplified from pcDNA3.1-mouseGOAT-HA vector (gift from Brown and Goldstein lab [
2]) using the following primers: mGOAT_attb1 (forward primer) 5′-gggg
acaagtttgtacaaaaaagcaggctaccatggattggctccagctc—3′(
attb1 recombination site is in italics, 5′ of mGOAT coding sequence is in
bold, and Kozak coding sequence is
underlined) and mGOAT_attb2 (reverse primer) 5′-gggg
accactttgtacaagaaagctgggtctaagcgtaatctggaacatc -3′ (attb2 recombination site is in
italics, 3′ of mGAOT-HA coding sequence is in
bold, and stop codon is
underlined). The PCR product was cloned into donor vector pDONR221 (ThermoFisher) with BP clonase according to manufacturer’s instructions. Positive clones were verified by DNA sequencing with M13F and M13R primers. The resulting entry plasmid pDONR221-mGOAT-HA was used to transfer mGOAT-HA cDNA to destination vector pBabe-puro (Addgene cat# 51070 [
35]) using LR clonase according to manufacturer’s instructions. Positive clones were verified by DNA sequencing with pBABE-5 and pBABE-3 primers. The resulting plasmid, pBabe-puro-mGOAT-HA, was used to generate retrovirus. The retrovirus was packed in 293T PhoE (Phoenix-ECO AT0CC
® CRL-3214
™) cells and used to infect INS-1 cells. The stable INS/GOAT cells were selecting on 1 μg/mL puromycin and GOAT expression was confirmed with immunoblot of membrane fraction isolated from antibiotic resistant culture using anti-HA antibody.
Generation of Lentivirus for Expression of Ghrelin. The cDNA for mouse preproghrelin was subcloned from pcDNA3.1-preproghrelin (gift from Brown and Goldstein lab [
18]) into pULTRA vector (Addgene cat# 24129 [
36]) with
XbaI and
BamHI restriction enzymes. Using of these restriction sites for cloning will result in the creation of bi-cistronic expression of ghrelin along with EGFP to facilitate identification of positive cells by fluorescence. The above restriction sites were engineered via PCR reaction using the following primers: ghrl_pultra_forw 5′-taccgagctc
tctagaatgctgtcttcaggc-3′ (5′ of preproghrelin coding sequence is in
bold;
XbaI site is in
italics) and ghrl_pultra_rev 5′-agcggccgc
ggatccttacttgtcagctggc -3′ (3′ of preproghrelin coding sequence is in
bold, stop codon is
underlined, and
BamHI site is
italics). The recombinant lentivirus encoding preproghrelin cDNA was packed in Lenti-X 293T cells (Takara cat# 632180) by co-transfection of pUltra-EGFP-mouse-preproghrelin, with packaging encoding plasmid pCMV ΔR8.2 (Addgene cat# 12263) and envelope encoding plasmid pCMV-VSV g (Addgene cat# 8454). The INS/GOAT cells were infected with recombinant lentivirus to generate INS/GOAT/GHRL cell line. After few passages, the population of cells with puromycin resistance and ~90% fluorescence was saved for cell-based assay.
Cellular assay. INS/GOAT/GHRL cell line was routinely cultured in RPMI medium supplemented with 10% FBS, 1% Pen/Strep, 10 mM HEPES pH 7.2 and 50 μM 2-mercaptoethanol. Day 0: One 10 cm dish at full confluency was used to seed one 96-well plate. Day 1: Growth media was removed and cells were washed with PBS prior to adding fresh growth media as above but without 2-mercaptoethanol. The serial dilutions of tested compound at 50× concentration were prepared in vehicle containing growth media without 2-mercaptoethnol supplemented with 6% DMSO and were added to cells in duplicates. Day 2: 10 μL of growth media were removed from each well and amount of secreted acyl-ghrelin was measured with ELISA (Cayman cat# 10006307).