Caralluma tuberculata N.E.Br Manifests Extraction Medium Reliant Disparity in Phytochemical and Pharmacological Analysis
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents and Solvents
2.2. Plant Collection and Identification
2.3. Extraction
2.4. Phytochemical Analysis
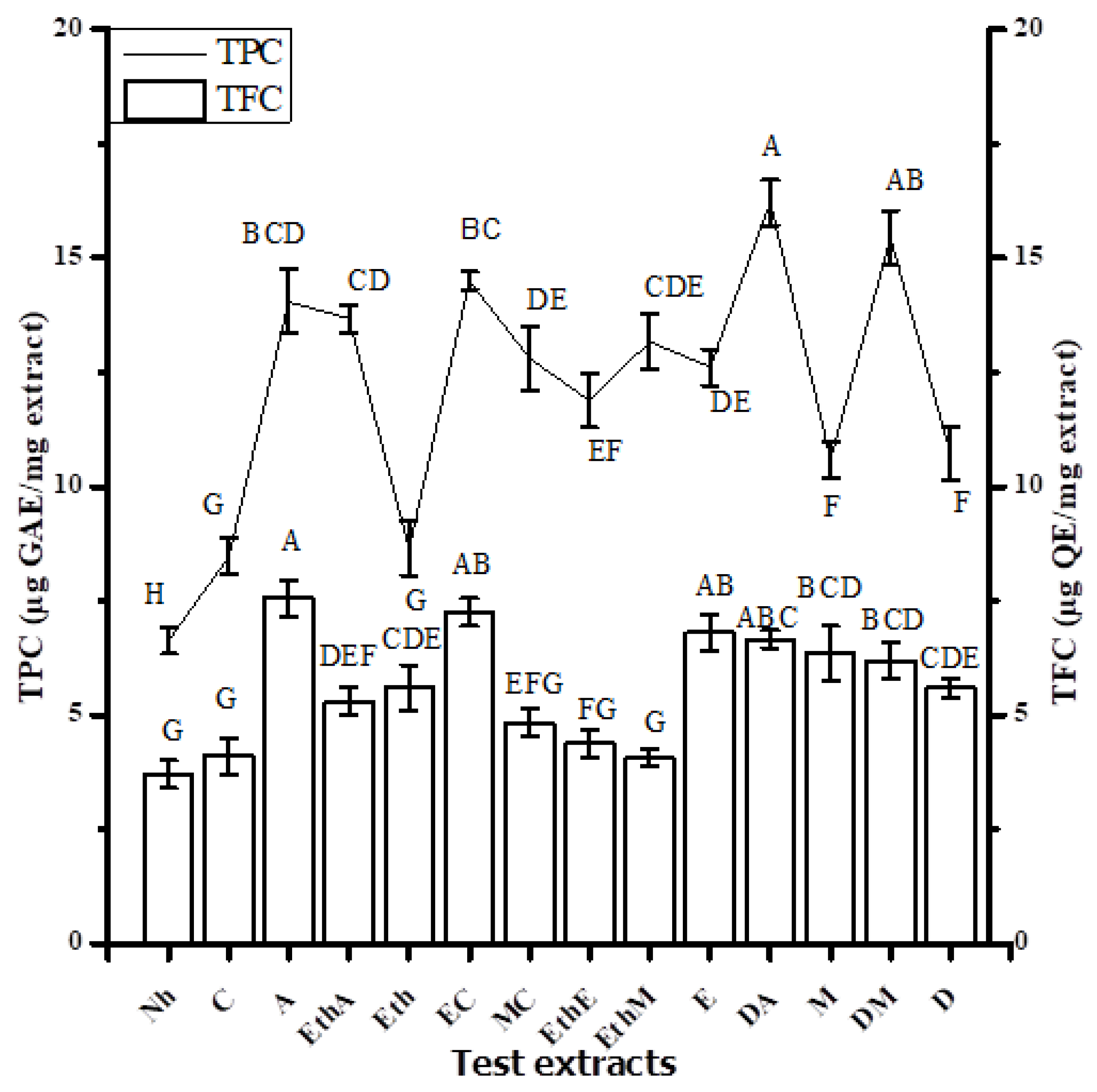
2.4.1. Total Phenolics Content Estimation (TPC)
2.4.2. Total Flavonoids Content Estimation (TFC)
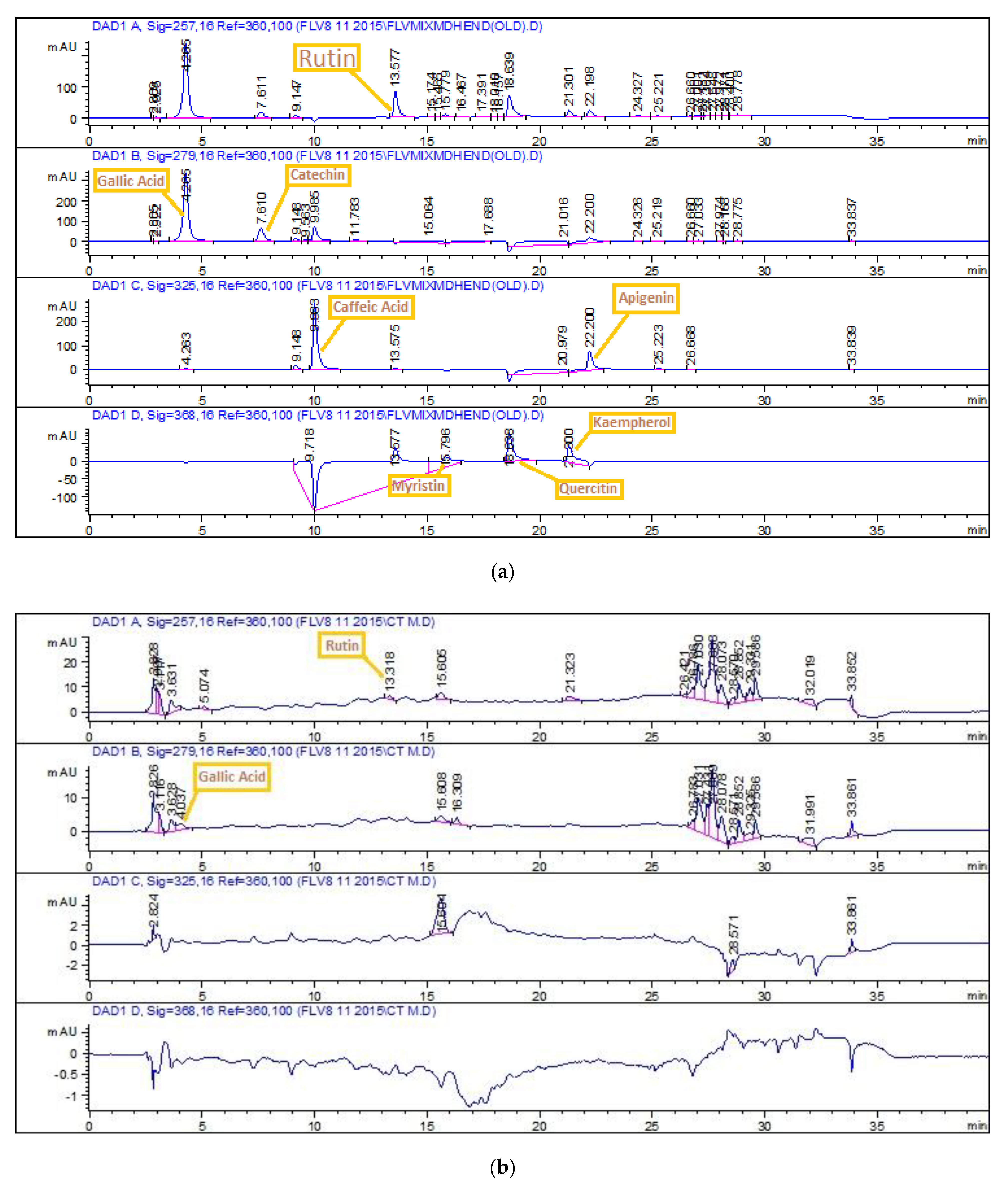
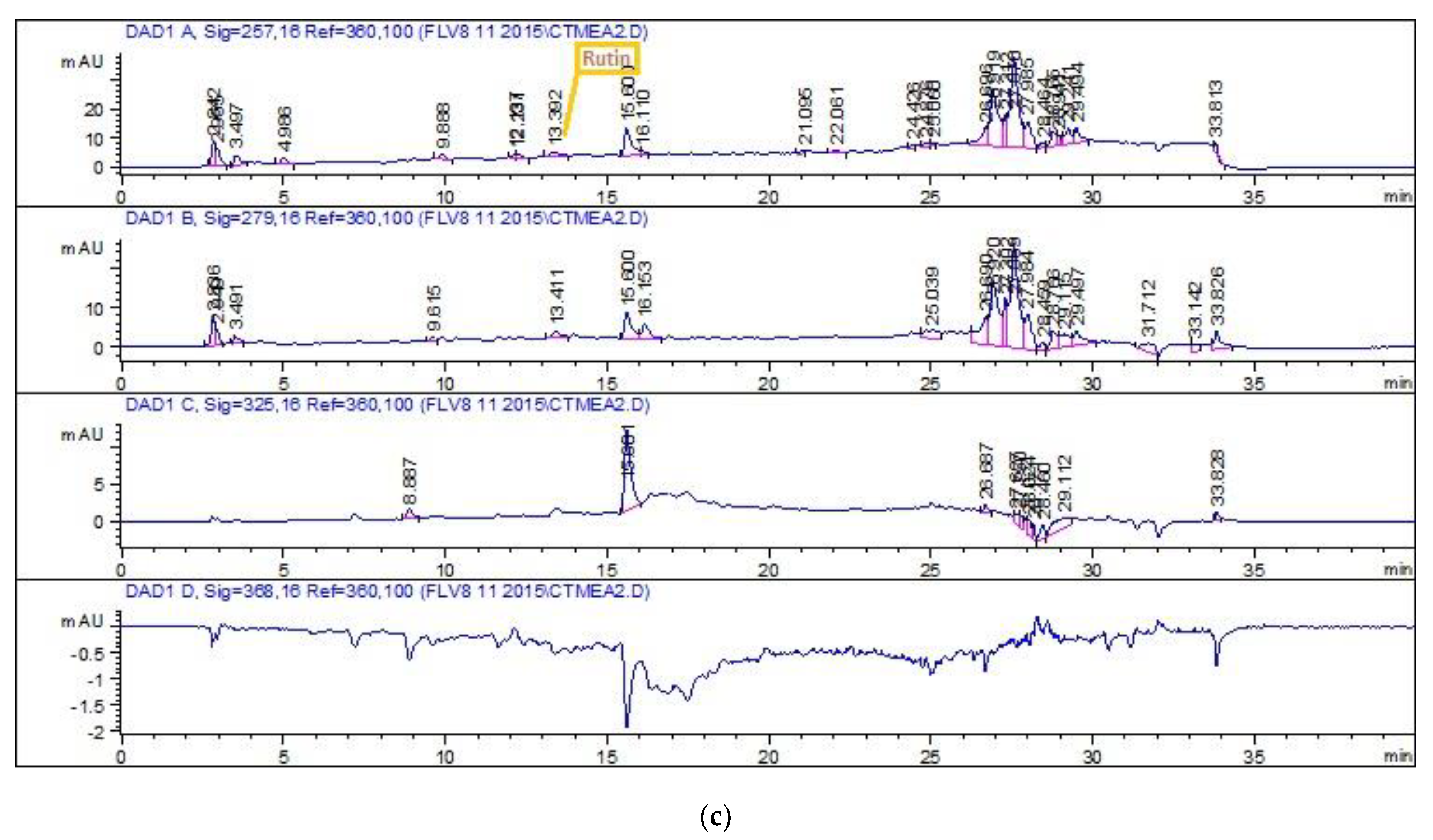
2.4.3. HPLC-DAD Quantitative Analysis
2.5. Biological Evaluation
2.5.1. Antioxidant Assays
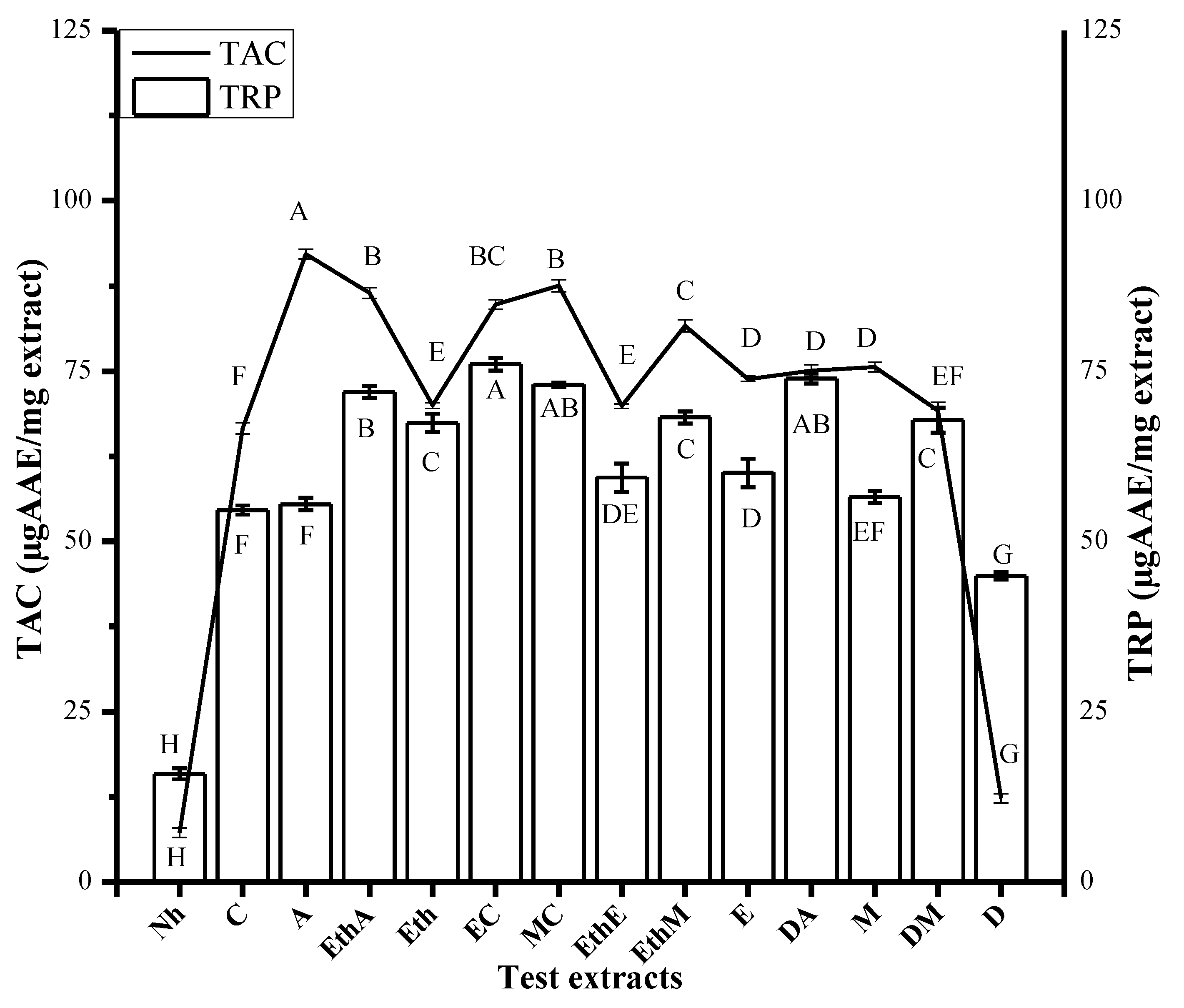
2.5.2. Total Antioxidant Capacity Determination
2.5.3. Total Reducing Power Estimation
2.5.4. Antimicrobial Assays
2.5.5. In Vitro Antileishmanial Potential Determination
2.5.6. Cytotoxicity Assays
Lethality Testing in Brine Shrimps
2.5.7. Cytotoxicity Assay Using THP-1 Human Leukemia Cell Line
2.5.8. Cytotoxicity Assay Using Isolated Human Lymphocytes
2.5.9. Protein Kinase Inhibition Assay
2.5.10. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Biological Evaluation
3.2.1. Antioxidant Assays
3.2.2. Antimicrobial Assays
3.2.3. Cytotoxicity Assays
3.2.4. Protein Kinase Inhibition Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahal, A.; Verma, A.K.; Kumar, A.; Tiwari, R.; Kapoor, S.; Chakraborty, S.; Dhama, K. Phytonutrients and nutraceuticals in vegetables and their multi-dimensional medicinal and health benefits for humans and their companion animals: A review. J. Biol. Sci. 2014, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional comparison of fresh, frozen and canned fruits and vegetables. Part 1. Vitamins C and B and phenolic compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Rehman, R.; Chaudhary, M.; Khawar, K.; Lu, G.; Mannan, A.; Zia, M. In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia 2014, 69, 341–349. [Google Scholar] [CrossRef]
- Bibi, Y.; Tabassum, S.; Zahara, K.; Bashir, T.; Haider, S. Ethnomedicinal and pharmacological properties of Caralluma tuberculata NE Brown-A review. Pure Appl. Biol. 2015, 4, 503. [Google Scholar]
- Adnan, M.; Jan, S.; Mussarat, S.; Tariq, A.; Begum, S.; Afroz, A.; Shinwari, Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus Caralluma R. Br. J. Pharm. Pharmacol. 2014, 66, 1351–1368. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, E.; Harraz, F.M.; Ghareib, S.A.; Elberry, A.A.; Gabr, S.; Suliaman, M.I. Antihyperglycaemic and hypolipidaemic effects of the methanolic extract of Caralluma tuberculata in streptozotocin-induced diabetic rats. Nat. Prod. Res. 2011, 25, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, E.A.; Abdallah, H.M.; Khedr, A.; Abdel-Naim, A.B.; Shehata, I.A. Antihyperglycemic activity of Caralluma tuberculata in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2013, 59, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Barker, J.; Barton, S.J.; Khan, G.-M.; Najm-us-Saqib, Q.; Hussain, M.; Ahmed, S.; Owen, C.; Carew, M.A. Novel acylated steroidal glycosides from Caralluma tuberculata induce caspase-dependent apoptosis in cancer cells. J. Ethnopharmacol. 2011, 137, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasir, B.; Baig, M.W.; Majid, M.; Ali, S.M.; Khan, M.Z.I.; Kazmi, S.T.B.; Haq, I.-u. Preclinical anticancer studies on the ethyl acetate leaf extracts of Datura stramonium and Datura inoxia. BMC Complementary Med. Ther. 2020, 20, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Fatima, H.; Qasim, M.; Gul, B. Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Complementary Altern. Med. 2017, 17, 1–16. [Google Scholar] [CrossRef]
- Fatima, H.; Khan, K.; Zia, M.; Ur-Rehman, T.; Mirza, B.; Haq, I.-u. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complementary Altern. Med. 2015, 15, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zahra, S.S.; Ahmed, M.; Qasim, M.; Gul, B.; Zia, M.; Mirza, B.; Haq, I.-u. Polarity based characterization of biologically active extracts of Ajuga bracteosa Wall. ex Benth. and RP-HPLC analysis. BMC Complementary Altern. Med. 2017, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Hsu, B.; Coupar, I.M.; Ng, K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem. 2006, 98, 317–328. [Google Scholar] [CrossRef]
- Rauf, A.; Jan, M.; Rehman, W.; Muhammad, N. Phytochemical, phytotoxic and antioxidant profile of Caralluma tuberculata NE Brown. Wudpecker J. Pharm. Pharmacol. 2013, 2, 21–25. [Google Scholar]
- Majid, M.; Khan, M.R.; Shah, N.A.; Haq, I.U.; Farooq, M.A.; Ullah, S.; Sharif, A.; Zahra, Z.; Younis, T.; Sajid, M. Studies on phytochemical, antioxidant, anti-inflammatory and analgesic activities of Euphorbia dracunculoides. BMC Complementary Altern. Med. 2015, 15, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Devasagayam, T.; Tilak, J.; Boloor, K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R. Free radicals and antioxidants in human health: Current status and future prospects. Japi 2004, 52, 4. [Google Scholar]
- Bibi, T.; Ahmad, M.; Tareen, R.B.; Tareen, N.M.; Jabeen, R.; Rehman, S.-U.; Sultana, S.; Zafar, M.; Yaseen, G. Ethnobotany of medicinal plants in district Mastung of Balochistan province-Pakistan. J. Ethnopharmacol. 2014, 157, 79–89. [Google Scholar] [CrossRef]
- Karamac, M.; Kosiñska, A.; Pegg, R.B. Comparison of radical-scavenging activities for selected phenolic acids. Pol. J. Food Nutr. Sci. 2005, 14, 165–170. [Google Scholar]
- Khan, I.; AbdElsalam, N.M.; Fouad, H.; Tariq, A.; Ullah, R.; Adnan, M. Application of ethnobotanical indices on the use of traditional medicines against common diseases. Evid.-Based Complementary Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Antioxidant properties of several commercial mushrooms. Food Chem. 2002, 77, 229–235. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Khan, M.; Khan, R.; Ahmed, M.; Muhammad, N.; Khan, M.; Khan, H.; Atlas, N.; Khan, F. Biological screening of methanolic crude extracts of Caralluma tuberculata. Int. J. Indig. Med. Plants 2013, 46, 2051–4263. [Google Scholar]
- Wang, H.; Gao, X.D.; Zhou, G.C.; Cai, L.; Yao, W.B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008, 106, 888–895. [Google Scholar] [CrossRef]
- Matsushita, M.; Janda, K.D. Histidine kinases as targets for new antimicrobial agents. Bioorganic Med. Chem. 2002, 10, 855–867. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Psichogiou, M.; Poulakou, G. Skin manifestations of Pseudomonas aeruginosa infections. Curr. Opin. Infect. Dis. 2021, 34, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Mahmood, A.; Tabassum, A. Ethnomedicinal Survy of Plants From District Sialkot, Pakistan. J. Appl. Pharm. 2011, 3, 212–220. [Google Scholar] [CrossRef]
- Ullah, M.O.; Haque, M.; Urmi, K.F.; Zulfiker, A.H.M.; Anita, E.S.; Begum, M.; Hamid, K. Anti–bacterial activity and brine shrimp lethality bioassay of methanolic extracts of fourteen different edible vegetables from Bangladesh. Asian Pac. J. Trop. Biomed. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; ul Hassan, S.S. Marine sponges as a drug treasure. Biomol. Ther. 2016, 24, 347. [Google Scholar] [CrossRef] [Green Version]
- Batista, B.G.; Chaves, M.A.d.; Reginatto, P.; Saraiva, O.J.; Fuentefria, A.M. Human fusariosis: An emerging infection that is difficult to treat. Rev. Soc. Bras. Med. Trop. 2020, 53. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Z.; Sardari, S. An investigation into the antifungal property of Fabaceae using bioinformatics tools. Avicenna J. Med. Biotechnol. 2010, 2, 93. [Google Scholar] [PubMed]
- Al-Musayeib, N.M.; Mothana, R.A.; Al-Massarani, S.; Matheeussen, A.; Cos, P.; Maes, L. Study of the in vitro antiplasmodial, antileishmanial and antitrypanosomal activities of medicinal plants from Saudi Arabia. Molecules 2012, 17, 11379–11390. [Google Scholar] [CrossRef] [Green Version]
- Al-Musayeib, N.M.; Mothana, R.A.; Matheeussen, A.; Cos, P.; Maes, L. In Vitro antiplasmodial, antileishmanial and antitrypanosomal activities of selected medicinal plants used in the traditional Arabian Peninsular region. BMC Complementary Altern. Med. 2012, 12, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, R.; Ibrar, M.; Shah, S.; Hameed, I. Phytotoxic, cytotoxic and insecticidal activities of Calendula arvensis L. L. J. Biotechnol. Pharm. Res. 2012, 3, 104–111. [Google Scholar]
- Khan, I.; Yasinzai, M.M.; Mehmood, Z.; Ilahi, I.; Khan, J.; Khalil, A.; Saqib, M.S.; Rahman, W.U. Comparative study of green fruit extract of Melia azedarach Linn. with its ripe fruit extract for antileishmanial, larvicidal, antioxidant and cytotoxic activity. Am. J. Phytomed. Clin. Ther. 2014, 2, 442–452. [Google Scholar]
- Al-Bekairi, A.; Qureshi, S.; Ahmed, M.; Qazi, N.; Khan, Z.; Shah, A. Effect of Caralluma tuberculata on the cytological and biochemical changes induced by cyclophosphamide in mice. Food Chem. Toxicol. 1992, 30, 719–722. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, S.; Song, Y.-r.; Kim, S.; Kim, H.-J.; Na, H.S.; Chung, J. Rutin induces autophagy in cancer cells. Int. J. Oral Biol. 2016, 41, 45–51. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Yu, R.; Kim, J.-S.; Kim, Y.-K.; Sung, M.-K. Antiproliferative crude soy saponin extract modulates the expression of IκBα, protein kinase C, and cyclooxygenase-2 in human colon cancer cells. Cancer Lett. 2004, 210, 1–6. [Google Scholar] [CrossRef]
- You, B.R.; Park, W.H. The effects of mitogen-activated protein kinase inhibitors or small interfering RNAs on gallic acid-induced HeLa cell death in relation to reactive oxygen species and glutathione. J. Agric. Food Chem. 2011, 59, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P. Protein kinases—The major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002, 1, 309–315. [Google Scholar] [CrossRef]
- Smyth, L.A.; Collins, I. Measuring and interpreting the selectivity of protein kinase inhibitors. J. Chem. Biol. 2009, 2, 131–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waters, B.; Saxena, G.; Wanggui, Y.; Kau, D.; Wrigley, S.; Stokes, R.; Davies, J. Identifying protein kinase inhibitors using an assay based on inhibition of aerial hyphae formation in Streptomyces. J. Antibiot. 2002, 55, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Time (min) | %B | Flow Rate (mL) | Max Pressure |
|---|---|---|---|
| 0 | 0 | 1 | 350 |
| 20 | 50 | 1 | 350 |
| 25 | 100 | 1 | 350 |
| 30 | 100 | 1 | 350 |
| 35 | 0 | 1 | 350 |
| 40 | 0 | 1 | 350 |
| S. No. | Solvent Extract Code | % Extract Yield |
|---|---|---|
| 1 | Nh | 0.86 |
| 2 | C | 6.76 |
| 3 | A | 10.02 |
| 4 | EthA | 7.12 |
| 5 | Eth | 4.44 |
| 6 | EC | 9.04 |
| 7 | MC | 10.34 |
| 8 | EthE | 10.2 |
| 9 | EthM | 8.70 |
| 10 | E | 12.10 |
| 11 | DA | 16.08 |
| 12 | M | 13.78 |
| 13 | DM | 9.70 |
| 14 | D | 25.16 |
| Extract Name | Polyphenols (µg/mg Extract) | |
|---|---|---|
| Rutin | Gallic Acid | |
| M | 0.51 | 0.35 |
| E | ---- | ---- |
| Eth | ---- | ---- |
| EthM | 0.58 | ---- |
| Antibacterial Assay | Antifungal Assay | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extract Names | Diameter of ZOI mm (Mean ± SD) (MIC: µg/mL) | Diameter of ZOI in mm (Mean ± SD) (MIC: µg/disc) | ||||||||
| P. aeruginosa | MIC | Mucor sp. | MIC | F. solani | MIC | A. Flavus | MIC | A. fumigatus | MIC | |
| Nh | 11 ± 1 cd | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| C | 9 ± 1 d | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| A | 12 ± 1 c | 200 | -- | -- | -- | -- | -- | -- | -- | -- |
| EthA | 15 ± 2 b | 66.66 | -- | -- | 10 ± 1 d | -- | -- | -- | -- | -- |
| Eth | 15 ± 1 b | 66.66 | -- | -- | 12 ± 1 c | 100 | 11 ± 1 bc | -- | 12 ± 1 c | 100 |
| EC | 9 ± 1 d | -- | -- | -- | 8 ± 1 e | -- | 9 ± 1 cd | -- | 8 ± 1 d | -- |
| MC | 12 ± 1 c | 200 | -- | -- | 16 ± 1 b | 100 | 8 ± 1 d | -- | -- | -- |
| EthE | 15 ± 1 b | 66.66 | -- | -- | 16 ± 1 b | 100 | -- | -- | -- | -- |
| EthM | 11 ± 1 cd | -- | 25 ± 2 b | 50 | 11 ± 1 cd | -- | -- | -- | -- | -- |
| E | 8 ± 1 d | -- | 12 ± 2 c | 100 | -- | -- | 10 ± 1 c | -- | 14 ± 2 b | 100 |
| DA | -- | -- | -- | -- | -- | -- | 10 ± 1 c | -- | 12 ± 1 c | 100 |
| M | -- | -- | -- | -- | 13 ± 1 c | 100 | 12 ± 1 b | 100 | 15 ± 2 b | 100 |
| DM | -- | -- | 15 ± 1 c | 100 | 10 ± 1 d | -- | 12 ± 1 b | 100 | 12 ± 1 c | 100 |
| D | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Cefixime | 19 ± 1 a | 1.11 | -- | |||||||
| Cipro ** | 19.5 ± 1 a | 3.33 | -- | -- | -- | -- | -- | -- | -- | -- |
| Clotri * | 35 ± 2 a | |||||||||
| DMSO | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Antileishmanial (µg/mL) | Brine Shrimp Cytotoxicity (µg/mL) | THP-1 Cytotoxicity(µg/mL) | Lymphocyte Cytotoxicity (µg/mL) | Protein Kinase Inhibition (µg/disc) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | % Mortality | LC50 | % Mortality | LD50 | % Inhibition | IC50 | % Inhibition | IC50 | Diameter (mm) at 100 µg/disc | |
| 200 | 200 | 200 | 200 | Clear Zone | Bald Zone | |||||
| Nh | 97.5 ± 1.1 | 120.8 ± 3.7 b | 0 | 0 | 16.4 ± 1.1 | >200 | 6.2 ± 1.1 | >200 | -- | -- |
| C | 82.5 ± 1.3 | 139.1 ± 4.2 cd | 100 | 50.09 ± 2.2 c | 21.3 ± 1.7 | >200 | 5.9 ± 1.3 | > 200 | -- | 14 ± 0.57 c |
| A | 80.3 ± 2 | 142 ± 5.7 d | 100 | 98.6 ± 3.4 e | 30 ± 2.0 | >200 | 6.6 ± 2.1 | >200 | -- | 17 ± 0.57 b |
| EthA | 83.4 ± 2.1 | 137.6 ± 6.1 cd | 100 | 48.75 ± 2.9 c | 19.6 ± 2.1 | >200 | 7.1 ± 1.5 | >200 | -- | 14 ± 0.57 c |
| Eth | 80.4 ± 1.8 | 142 ± 5.9 d | 100 | 29.94 ± 1.6 b | 21.7 ± 0.7 | >200 | 7.4 ± 1.1 | >200 | -- | 13 ± 1 c |
| EC | 83 ± 1.2 | 137.6 ± 3.9 cd | 100 | 50 ± 3.01 c | 24.2 ± 1.5 | >200 | 5.5 ± 0.9 | >200 | -- | 12 ± 1 cd |
| MC | 79 ± 2.2 | 143.5 ± 6.2 de | 100 | 74.22 ± 3.4 d | 26.8 ± 0.8 | >200 | 5.7 ± 1.1 | >200 | -- | 19 ± 1 b |
| EthE | 81 ± 1.0 | 140.5 ± 3.5 cd | 100 | 49.16 ± 2.8 c | 22.1 ± 1.5 | >200 | 6.7 ± 1.3 | >200 | -- | 10± 1 d |
| EthM | 91 ± 2.2 | 127.4 ± 6.3 bc | 100 | 49.15 ± 2.7 c | 27.5 ± 1.3 | >200 | 6.8 ± 1.6 | >200 | -- | 11 ± 0.57 cd |
| E | 85 ± 2.1 | 134.9 ± 6.0 c | 80 ± 5.77 | 102.24 ± 4.7 e | 31 ± 2.8 | >200 | 4.4 ± 0.83 | >200 | -- | 10 ± 1 d |
| DA | 82 ± 1.2 | 139.1 ± 3.5 cd | 100 | 48.01 ± 3.0 c | 80.3 ± 2.4 b | 118 ± 3.4 b | 4.6 ± 1.3 | >200 | -- | 8 ± 1 de |
| M | 80 ± 1.8 | 142 ± 4.3 d | 90 ± 5.77 | 51.34 ± 3.5 c | 27.8 ± 2.2 | >200 | 5.8 ± 2.1 | >200 | -- | 8 ± 1 de |
| DM | 78 ± 2.3 | 145.1 ± 6.3 e | 90 ± 5.77 | 50.32 ± 3.3 c | 31.7 ± 1.4 | >200 | 4.9 ± 1.2 | >200 | -- | 7 ± 0.57 e |
| D | 0 | 0 | 30 ± 10 | ≥200 | 64.5 ± 1.4 c | 140 ± 3.2 | 4.1 ± 0.66 | >200 | -- | -- |
| Amphoterecin B | 100 | 0.016 a | ||||||||
| Doxorubicin | 100 | 6.24 ± 0.7 a | ||||||||
| 5-Florouracil | 100 | 5.3 ± 0.68 | ||||||||
| Vincristine | 100 | 8.5 ± 0.92 | 74.49 ± 3.35 | 6.6 ± 0.2 | ||||||
| Surfactin | 29 ± 1 a | |||||||||
| DMSO | - | - | ||||||||
| 1% DMSO in PBS/sea water | - | - | - | -- | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, M.W.; Ahmed, M.; Akhtar, N.; Okla, M.K.; Nasir, B.; Haq, I.-U.; Al-Ghamdi, J.; Al-Qahtani, W.H.; AbdElgawad, H. Caralluma tuberculata N.E.Br Manifests Extraction Medium Reliant Disparity in Phytochemical and Pharmacological Analysis. Molecules 2021, 26, 7530. https://doi.org/10.3390/molecules26247530
Baig MW, Ahmed M, Akhtar N, Okla MK, Nasir B, Haq I-U, Al-Ghamdi J, Al-Qahtani WH, AbdElgawad H. Caralluma tuberculata N.E.Br Manifests Extraction Medium Reliant Disparity in Phytochemical and Pharmacological Analysis. Molecules. 2021; 26(24):7530. https://doi.org/10.3390/molecules26247530
Chicago/Turabian StyleBaig, Muhammad Waleed, Madiha Ahmed, Nosheen Akhtar, Mohammad K. Okla, Bakht Nasir, Ihsan-Ul Haq, Jihan Al-Ghamdi, Wahidah H. Al-Qahtani, and Hamada AbdElgawad. 2021. "Caralluma tuberculata N.E.Br Manifests Extraction Medium Reliant Disparity in Phytochemical and Pharmacological Analysis" Molecules 26, no. 24: 7530. https://doi.org/10.3390/molecules26247530
APA StyleBaig, M. W., Ahmed, M., Akhtar, N., Okla, M. K., Nasir, B., Haq, I.-U., Al-Ghamdi, J., Al-Qahtani, W. H., & AbdElgawad, H. (2021). Caralluma tuberculata N.E.Br Manifests Extraction Medium Reliant Disparity in Phytochemical and Pharmacological Analysis. Molecules, 26(24), 7530. https://doi.org/10.3390/molecules26247530







