Retrieval of High Added Value Natural Bioactive Coumarins from Mandarin Juice-Making Industrial Byproduct
Abstract
:1. Introduction
2. Results
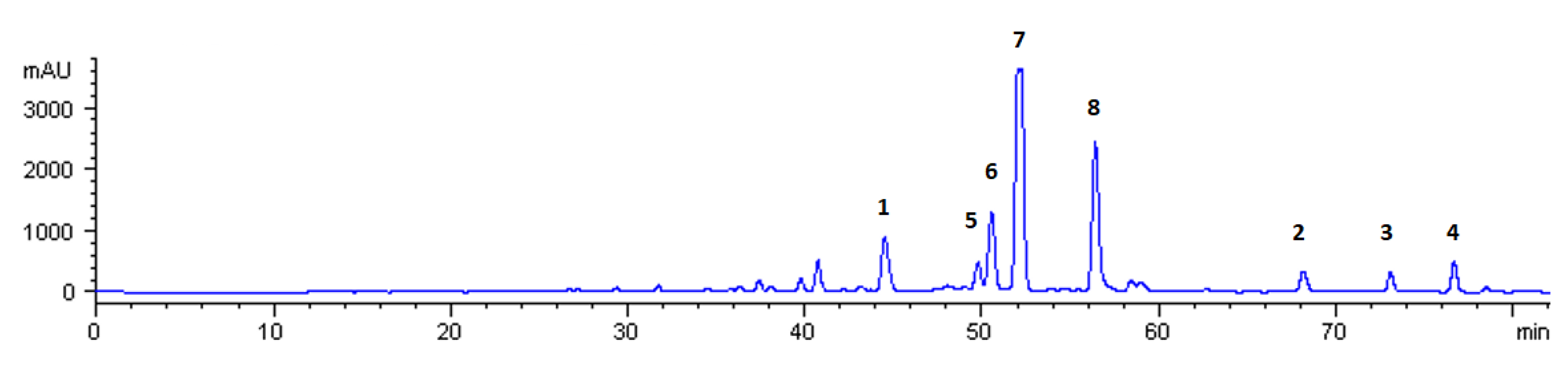
2.1. Isolation of Bioactive Molecules from Mandarin CPEO
2.2. Identification of the Isolated Compounds
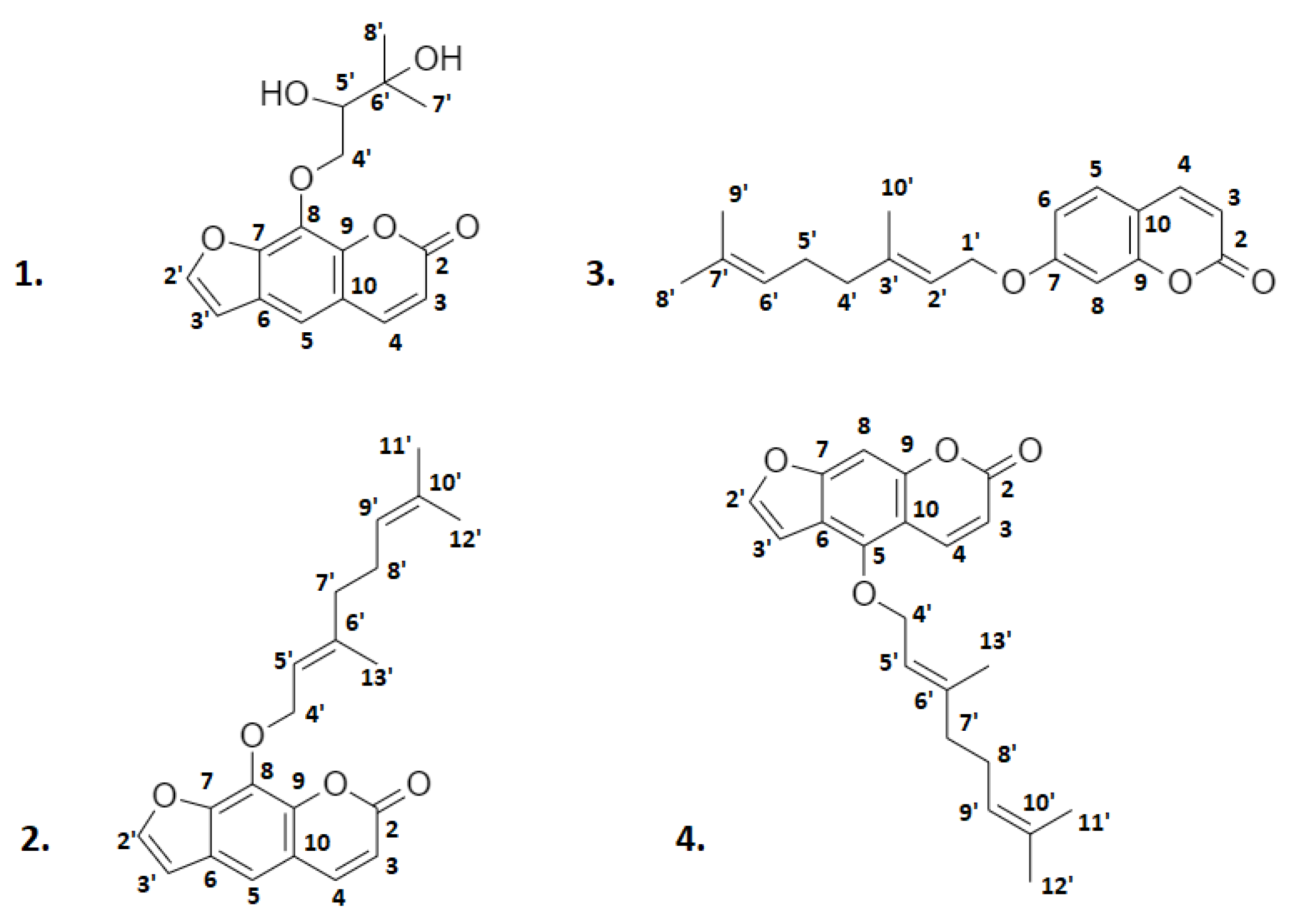
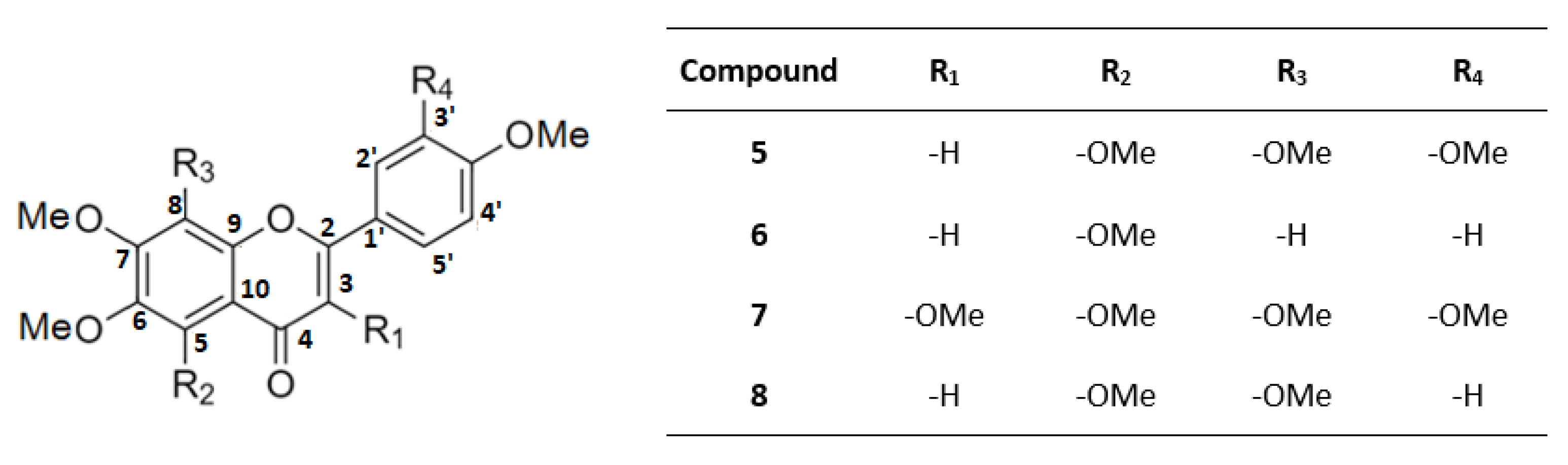
3. Discussion
4. Materials and Methods
4.1. Mandarin CPEO
4.2. Solvents and Standards
4.3. Retrieval of Natural Coumarins
4.3.1. Isolation of Non-Volatiles Fraction
4.3.2. Retrieval of Natural Coumarins Using Preparative HPLC
4.3.3. Analytical HPLC
4.3.4. NMR Spectroscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Önder, A. Anticancer activity of natural coumarins for biological targets. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; Volume 64, pp. 85–109. [Google Scholar]
- Kostova, I.; Raleva, S.; Genova, P.; Argirova, R. Structure-Activity Relationships of Synthetic Coumarins as HIV-1 Inhibitors. Bioinorg. Chem. Appl. 2006, 2006, 068274. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as Antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- Souri, E.; Farsam, H.; Sarkheil, P.; Ebadi, F. Antioxidant Activity of Some Furanocoumarins Isolated from Heracleum Persicum. Pharm. Biol. 2004, 42, 396–399. [Google Scholar] [CrossRef]
- Piao, X.L.; Park, I.H.; Baek, S.H.; Kim, H.Y.; Park, M.K.; Park, J.H. Antioxidative Activity of Furanocoumarins Isolated from Angelicae Dahuricae. J. Ethnopharmacol. 2004, 93, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Payá, M.; Halliwell, B.; Hoult, J.R.S. Interactions of a Series of Coumarins with Reactive Oxygen Species. Biochem. Pharmacol. 1992, 44, 205–214. [Google Scholar] [CrossRef]
- Jung, J.-C.; Jung, Y.-J.; Park, O.-S. A Convenient one-pot synthesis of 4-hydroxycoumarin, 4-hydroxythiocoumarin, and 4-hydroxyquinolin-2 (1H)-one. Synth. Commun. 2001, 31, 1195–1200. [Google Scholar] [CrossRef]
- Lee, B.W.; Ha, T.K.Q.; Cho, H.M.; An, J.-P.; Kim, S.K.; Kim, C.-S.; Kim, E.; Oh, W.K. Antiviral Activity of Furanocoumarins Isolated from Angelica Dahurica against Influenza a Viruses H1N1 and H9N2. J. Ethnopharmacol. 2020, 259, 112945. [Google Scholar] [CrossRef]
- Liu, L.; Hu, Y.; Shen, Y.-F.; Wang, G.-X.; Zhu, B. Evaluation on Antiviral Activity of Coumarin Derivatives against Spring Viraemia of Carp Virus in Epithelioma Papulosum Cyprini Cells. Antiviral Res. 2017, 144, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic Potential of Coumarins as Antiviral Agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Al-Haiza, M.; Mostafa, M.; El-Kady, M. Synthesis and Biological Evaluation of Some New Coumarin Derivatives. Molecules 2003, 8, 275–286. [Google Scholar] [CrossRef]
- Musicki, B.; Periers, A.-M.; Laurin, P.; Ferroud, D.; Benedetti, Y.; Lachaud, S.; Chatreaux, F.; Haesslein, J.-L.; Iltis, A.; Pierre, C.; et al. Improved Antibacterial Activities of Coumarin Antibiotics Bearing 5′,5′-Dialkylnoviose: Biological Activity of RU79115. Bioorg. Med. Chem. Lett. 2000, 10, 1695–1699. [Google Scholar] [CrossRef]
- Fylaktakidou, K.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolaides, D. Natural and Synthetic Coumarin Derivatives with Anti-Inflammatory/Antioxidant Activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A. Studies on Coumarins and Coumarin-Related Compounds to Determine Their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Fais, A.; Corda, M.; Era, B.; Fadda, M.B.; Matos, M.J.; Quezada, E.; Santana, L.; Picciau, C.; Podda, G.; Delogu, G. Tyrosinase Inhibitor Activity of Coumarin-Resveratrol Hybrids. Molecules 2009, 14, 2514–2520. [Google Scholar] [CrossRef]
- Matos, M.; Vazquez-Rodriguez, S.; Santana, L.; Uriarte, E.; Fuentes-Edfuf, C.; Santos, Y.; Muñoz-Crego, A. Synthesis and Structure-Activity Relationships of Novel Amino/Nitro Substituted 3-Arylcoumarins as Antibacterial Agents. Molecules 2013, 18, 1394–1404. [Google Scholar] [CrossRef]
- Wang, H.; Lu, X.; Yao, H.; Feng, J.; Liu, R. Research Progress on Application of Coumarin and Its Derivatives. Chem. Ind. Times 2009, 23, 40–43. [Google Scholar]
- Matos, M.J.; Vazquez-Rodriguez, S.; Fonseca, A.; Uriarte, E.; Santana, L.; Borges, F. Heterocyclic Antioxidants in Nature: Coumarins. Curr. Org. Chem. 2017, 21, 311–324. [Google Scholar] [CrossRef]
- Francisco, C.S.; Francisco, C.S.; Constantino, A.F.; Neto, Á.C.; Lacerda, V. Synthetic Methods Applied in the Preparation of Coumarin-Based Compounds. Curr. Org. Chem. 2020, 23, 2722–2750. [Google Scholar] [CrossRef]
- Kirsch, G.; Abdelwahab, A.; Chaimbault, P. Natural and Synthetic Coumarins with Effects on Inflammation. Molecules 2016, 21, 1322. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Buckle, J. Basic Plant Taxonomy, Basic Essential Oil Chemistry, Extraction, Biosynthesis, and Analysis. In Clinical Aromatherapy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 37–72. [Google Scholar]
- Dugrand-Judek, A.; Olry, A.; Hehn, A.; Costantino, G.; Ollitrault, P.; Froelicher, Y.; Bourgaud, F. The Distribution of Coumarins and Furanocoumarins in Citrus Species Closely Matches Citrus Phylogeny and Reflects the Organization of Biosynthetic Pathways. PLoS ONE 2015, 10, e0142757. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An Important Class of Phytochemicals. In Phytochemicals-Isolation, Characterisation and Role in Human Health; Rao, A.V., Rao, L.G., Eds.; InTech: London, UK, 2015. [Google Scholar]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and Antioxidant Activities of Coumarins from the Roots of Ferulago Campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Weng, K.-G.; Yuan, Y.-L. Synthesis and Evaluation of Coumarin Derivatives against Human Lung Cancer Cell Lines. Braz. J. Med. Biol. Res. 2017, 50, e6455. [Google Scholar] [CrossRef]
- Kasumbwe, K.; Venugopala, K.N.; Mohanlall, V.; Odhav, B. Synthetic Mono/Di-Halogenated Coumarin Derivatives and Their Anticancer Properties. Anticancer Agents Med. Chem. 2017, 17, 276–285. [Google Scholar] [CrossRef]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A Review of Coumarin Derivatives in Pharmacotherapy of Breast Cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wu, X.; Tu, X.; Wang, G.-X. Synthesis and Biological Evaluation of Coumarin Derivatives Containing Imidazole Skeleton as Potential Antibacterial Agents. Eur. J. Med. Chem. 2018, 143, 958–969. [Google Scholar] [CrossRef]
- Hung, W.-L.; Suh, J.H.; Wang, Y. Chemistry and Health Effects of Furanocoumarins in Grapefruit. J. Food Drug Anal. 2017, 25, 71–83. [Google Scholar] [CrossRef]
- Río, J.A.D.; Díaz, L.; García-Bernal, D.; Blanquer, M.; Ortuño, A.; Correal, E.; Moraleda, J.M. Furanocoumarins. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, pp. 145–195. [Google Scholar]
- Calka, O.; Akdeniz, N.; Metin, A.; Behcet, L. Phototoxic Dermatitis Due to Chenopodium Album in a Mother and Son. Contact Dermatitis 2005, 53, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Polyphenols and Polyphenol-Derived Compounds From Plants and Contact Dermatitis. In Polyphenols: Prevention and Treatment of Human Disease; Elsevier: Amsterdam, The Netherlands, 2018; pp. 349–384. [Google Scholar]
- Conforti, F.; Marrelli, M.; Menichini, F.; Bonesi, M.; Statti, G.; Provenzano, E.; Menichini, F. Natural and Synthetic Furanocoumarins as Treatment for Vitiligo and Psoriasis. Curr. Drug Ther. 2009, 4, 38–58. [Google Scholar] [CrossRef]
- Koenigs, L.L.; Trager, W.F. Mechanism-Based Inactivation of P450 2A6 by Furanocoumarins. Biochemistry 1998, 37, 10047–10061. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef]
- Miyazawa, M.; Tsukamoto, T.; Anzai, J.; Ishikawa, Y. Insecticidal Effect of Phthalides and Furanocoumarins from Angelica Acutiloba against Drosophila Melanogaster. J. Agric. Food Chem. 2004, 52, 4401–4405. [Google Scholar] [CrossRef]
- Pavela, R.; Vrchotová, N. Insecticidal Effect of Furanocoumarins from Fruits of Angelica Archangelica L. against Larvae Spodoptera Littoralis Boisd. Ind. Crops Prod. 2013, 43, 33–39. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, Y.; Zhu, H.; Mu, Y.; Yu, J.; Li, J.; Wang, X. Preparative Separation of Six Coumarins from the Pummelo (Citrus Maxima (Burm.) Merr. Cv. Shatian Yu) Peel by High-Speed Countercurrent Chromatography. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 991–996. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO) 2021. Available online: http://www.fao.org/faostat/en/#data (accessed on 15 January 2021).
- Garcia-Castello, E.; Lora-Garcia, J.; Garcia-Garrido, J.; Rodriguez-Lopez, A.D. Energetic Comparison for Leaching Waste Liquid from Citric Juice Production Using Both Reverse Osmosis and Multiple-Effect Evaporation. Desalination 2006, 191, 178–185. [Google Scholar] [CrossRef]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-Products from Different Citrus Processes as a Source of Customized Functional Fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Boukroufa, M.; Boutekedjiret, C.; Petigny, L.; Rakotomanomana, N.; Chemat, F. Bio-Refinery of Orange Peels Waste: A New Concept Based on Integrated Green and Solvent Free Extraction Processes Using Ultrasound and Microwave Techniques to Obtain Essential Oil, Polyphenols and Pectin. Ultrason. Sonochem. 2015, 24, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cacho, P.R.; Rouseff, R. Processing and Storage Effects on Orange Juice Aroma: A Review. J. Agric. Food Chem. 2008, 56, 9785–9796. [Google Scholar] [CrossRef]
- Kapsaski-Kanelli, V.N.; Evergetis, E.; Michaelakis, A.; Papachristos, D.P.; Myrtsi, E.D.; Koulocheri, S.D.; Haroutounian, S.A. “Gold” Pressed Essential Oil: An Essay on the Volatile Fragment from Citrus Juice Industry By-Products Chemistry and Bioactivity. BioMed Res. Int. 2017, 2017, 2761461. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M. “Christodoulou Bros SA,” Industry of Fruit Juices. Argolida, 21055, Agia Triada. Nafplio, Greece. Personal communication, 2021. [Google Scholar]
- Dugo, G.; Mondello, L. (Eds.) Citrus Oils: Composition, Advanced Analytical Techniques, Contaminants, and Biological Activity; Medicinal and aromatic plants-industrial profiles; CRC Press: Boca Raton, FL, USA, 2011; p. 561. [Google Scholar]
- Russo, M.; Rigano, F.; Arigò, A.; Dugo, P.; Mondello, L. Coumarins, Psoralens and Polymethoxyflavones in Cold-Pressed Citrus Essential Oils: A Review. J. Essent. Oil Res. 2021, 33, 221–239. [Google Scholar] [CrossRef]
- Ramírez-Pelayo, C.; Martínez-Quiñones, J.; Gil, J.; Durango, D. Coumarins from the Peel of Citrus Grown in Colombia: Composition, Elicitation and Antifungal Activity. Heliyon 2019, 5, e01937. [Google Scholar] [CrossRef]
- Stanley, W.L.; Jurd, L. Citrus Coumarins. J. Agric. Food Chem. 1971, 19, 1106–1110. [Google Scholar] [CrossRef]
- Benkiki, N.; Benkhaled, M.; Kabouche, Z.; Bruneau, C. Heraclenol and Isopimpinellin: Two Rare Furocoumarins from Ruta montana. In Biodiversity; Şener, B., Ed.; Springer: Boston, MA, USA, 2002; pp. 303–307. [Google Scholar]
- Curini, M.; Cravotto, G.; Epifano, F.; Giannone, G. Chemistry and Biological Activity of Natural and Synthetic Prenyloxycoumarins. Curr. Med. Chem. 2006, 13, 199–222. [Google Scholar] [CrossRef]
- García-Argáez, A.; Ramírez Apan, T.; Delgado, H.; Velázquez, G.; Martínez-Vázquez, M. Anti-Inflammatory Activity of Coumarins from Decatropis Bicolor on TPA Ear Mice Model. Planta Med. 2000, 66, 279–281. [Google Scholar] [CrossRef]
- Filho, C.L.D.M.; Marinho, E.M.; Silva, L.P.D.; Marinho, M.M.; Marinho, E.S. Quantum Study of Geometric Properties of The AntiHIV Coumarin Heraclenol: A Study of Density Functional Theory (Dft). Int. J. Eng. Technol. Res. Manag. 2018, 2, 27–37. [Google Scholar] [CrossRef]
- Chidambaram, S.K.; Ali, D.; Alarifi, S.; Radhakrishnan, S.; Akbar, I. In Silico Molecular Docking: Evaluation of Coumarin Based Derivatives against SARS-CoV-2. J. Infect. Public Health 2020, 13, 1671–1677. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Nové, M.; Spengler, G.; Kúsz, N.; Hohmann, J.; Csupor, D. Antiproliferative and Cytotoxic Activities of Furocoumarins of Ducrosia Anethifolia. Pharm. Biol. 2018, 56, 658–664. [Google Scholar] [CrossRef]
- Razavi, S.M.; Zahri, S.; Nazemiyeh, H.; Zarrini, G.; Mohammadi, S.; Abolghassemi-Fakhri, M.-A. A Furanocoumarin from Prangos Uloptera Roots, Biological Effects. Nat. Prod. Res. 2009, 23, 1522–1527. [Google Scholar] [CrossRef]
- Razavi, S.M. Plant Coumarins as Allelopathic Agents. Int. J. Biol. Chem. 2010, 5, 86–90. [Google Scholar] [CrossRef]
- Row, E.C.; Brown, S.A.; Stachulski, A.V.; Lennard, M.S. Synthesis of 8-Geranyloxypsoralen Analogues and Their Evaluation as Inhibitors of CYP3A4. Bioorg. Med. Chem. 2006, 14, 3865–3871. [Google Scholar] [CrossRef]
- Marumoto, S.; Miyazawa, M. Structure–Activity Relationships for Naturally Occurring Coumarins as β-Secretase Inhibitor. Bioorg. Med. Chem. 2012, 20, 784–788. [Google Scholar] [CrossRef]
- Takahashi, N.; Senda, M.; Lin, S.; Goto, T.; Yano, M.; Sasaki, T.; Murakami, S.; Kawada, T. Auraptene Regulates Gene Expression Involved in Lipid Metabolism through PPARα Activation in Diabetic Obese Mice. Mol. Nutr. Food Res. 2011, 55, 1791–1797. [Google Scholar] [CrossRef]
- Napolitano, H.B.; Silva, M.; Ellena, J.; Rodrigues, B.D.G.; Almeida, A.L.C.; Vieira, P.C.; Oliva, G.; Thiemann, O.H. Aurapten, a Coumarin with Growth Inhibition against Leishmania Major Promastigotes. Braz. J. Med. Biol. Res. 2004, 37, 1847–1852. [Google Scholar] [CrossRef]
- Yan, H.; Ma, Z.; Peng, S.; Deng, X. Anti-Inflammatory Effect of Auraptene Extracted from Trifoliate Orange (Poncirus Trifoliate) on LPS-Stimulated RAW 264.7 Cells. Inflammation 2013, 36, 1525–1532. [Google Scholar] [CrossRef]
- Nishimoto, S.; Muranaka, A.; Nishi, K.; Kadota, A.; Sugahara, T. Immunomodulatory Effects of Citrus Fruit Auraptene in Vitro and in Vivo. J. Funct. Foods 2012, 4, 883–890. [Google Scholar] [CrossRef]
- Tayarani-Najaran, Z.; Tayarani-Najaran, N.; Eghbali, S. A Review of Auraptene as an Anticancer Agent. Front. Pharmacol. 2021, 12, 698352. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Sahebkar, A. Auraptene and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature; Gupta, S.C., Prasad, S., Aggarwal, B.B., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Basel, Switzerland, 2016; Volume 929, pp. 399–407. [Google Scholar]
- Joghataee, S.; Mohammad-Zadeh, M.; Amin, B.; Jafari, F.; Tondar, M.; Gholami, O. Auraptene Has Neuroprotective and Memory Enhancing Effects in a Rat Model of Alzheimer’s Disease. Neurol. Asia 2020, 25, 353–360. [Google Scholar]
- Chung, Y.C.; Kim, Y.B.; Kim, B.S.; Hyun, C.-G. Anti-Melanogenic Effects of Bergamottin via Mitogen-Activated Protein Kinases and Protein Kinase B Signaling Pathways. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, J.H.; Sethi, G.; Kim, C.; Baek, S.H.; Nam, D.; Chung, W.-S.; Kim, S.-H.; Shim, B.S.; Ahn, K.S. Bergamottin, a Natural Furanocoumarin Obtained from Grapefruit Juice Induces Chemosensitization and Apoptosis through the Inhibition of STAT3 Signaling Pathway in Tumor Cells. Cancer Lett. 2014, 354, 153–163. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, C.; Cao, Y.; Wang, Y.; Duan, W.; Xie, L.; Sun, C.; Li, X. Characterization and Purification of Bergamottin from Citrus Grandis (L.) Osbeck Cv. Yongjiazaoxiangyou and Its Antiproliferative Activity and Effect on Glucose Consumption in HepG2 Cells. Molecules 2017, 22, 1227. [Google Scholar] [CrossRef]
- Ko, J.-H.; Arfuso, F.; Sethi, G.; Ahn, K. Pharmacological Utilization of Bergamottin, Derived from Grapefruits, in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2018, 19, 4048. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Aktar, S.; Davis, M.; Hefter Feuss, E.; Roman-Holba, S.; Wen, K.; Gahn, C.; Caruso, F. The Grapefruit Effect: Interaction between Cytochrome P450 and Coumarin Food Components, Bergamottin, Fraxidin and Osthole. X-Ray Crystal Structure and DFT Studies. Molecules 2020, 25, 3158. [Google Scholar] [CrossRef]
- Olguín-Reyes, S.; Camacho-Carranza, R.; Hernández-Ojeda, S.; Elinos-Baez, M.; Espinosa-Aguirre, J.J. Bergamottin Is a Competitive Inhibitor of CYP1A1 and Is Antimutagenic in the Ames Test. Food Chem. Toxicol. 2012, 50, 3094–3099. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Montemayor, N.E.; García, A.; Elizondo-Treviño, E.; Garza-González, E.; Alvarez, L.; del Rayo Camacho-Corona, M. Chemical Composition of Hexane Extract of Citrus Aurantifolia and Anti-Mycobacterium Tuberculosis Activity of Some of Its Constituents. Molecules 2012, 17, 11173–11184. [Google Scholar] [CrossRef] [PubMed]
- Berg, L. Separation of alpha-phellandrene from D-limonene by azeotropic distillation. U.S. Patent 5,698,080, 22 November 1996. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myrtsi, E.D.; Angelis, A.; Koulocheri, S.D.; Mitakou, S.; Haroutounian, S.A. Retrieval of High Added Value Natural Bioactive Coumarins from Mandarin Juice-Making Industrial Byproduct. Molecules 2021, 26, 7527. https://doi.org/10.3390/molecules26247527
Myrtsi ED, Angelis A, Koulocheri SD, Mitakou S, Haroutounian SA. Retrieval of High Added Value Natural Bioactive Coumarins from Mandarin Juice-Making Industrial Byproduct. Molecules. 2021; 26(24):7527. https://doi.org/10.3390/molecules26247527
Chicago/Turabian StyleMyrtsi, Eleni D., Apostolis Angelis, Sofia D. Koulocheri, Sofia Mitakou, and Serkos A. Haroutounian. 2021. "Retrieval of High Added Value Natural Bioactive Coumarins from Mandarin Juice-Making Industrial Byproduct" Molecules 26, no. 24: 7527. https://doi.org/10.3390/molecules26247527
APA StyleMyrtsi, E. D., Angelis, A., Koulocheri, S. D., Mitakou, S., & Haroutounian, S. A. (2021). Retrieval of High Added Value Natural Bioactive Coumarins from Mandarin Juice-Making Industrial Byproduct. Molecules, 26(24), 7527. https://doi.org/10.3390/molecules26247527






