Protective Role of Quercetin in Carbon Tetrachloride Induced Toxicity in Rat Brain: Biochemical, Spectrophotometric Assays and Computational Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Brain Enzymes
2.5. Measurement of Brain Sodium
2.6. Measurement of Brain Urea
2.7. Lipid Peroxidation
2.8. Ascorbic Acid (AsA)
2.9. Reduced Glutathione (GSH)
2.10. In Silico Testing of Quercetin Toxicity and Molecular Docking
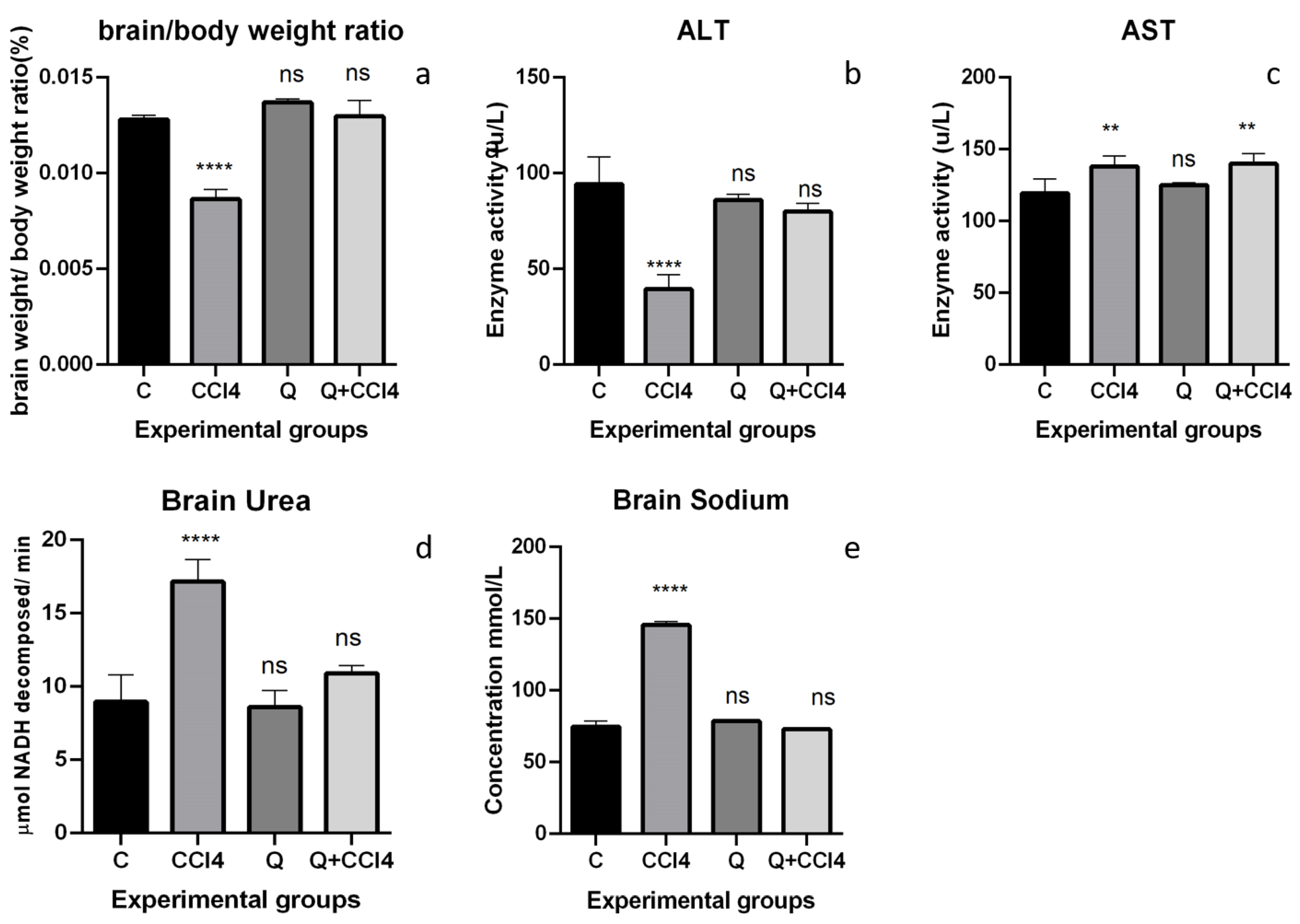
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usui, T.; Foster, S.S.; Petrini, J.H. Maintenance of the DNA-damage checkpoint requires DNA-damage-induced mediator protein oligomerization. Mol. Cell 2009, 33, 147–159. [Google Scholar] [CrossRef] [Green Version]
- Petković, J.; Žegura, B.; Stevanović, M.; Drnovšek, N.; Uskoković, D.; Novak, S.; Filipič, M. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology 2011, 5, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Chen, Q.-L.; Song, Y.-N.; Sun, Y.; Wei, B.; Li, X.-Y.; Hu, Y.-Y.; Liu, P.; Su, S.-B. Mechanisms of CCl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. J. Toxicol. Sci. 2016, 41, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Alkreathy, H.M.; Khan, R.A.; Khan, M.R.; Sahreen, S. CCl 4 induced genotoxicity and DNA oxidative damages in rats: Hepatoprotective effect of Sonchus arvensis. BMC Complementary Altern. Med. 2014, 14, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritesh, K.; Suganya, A.; Dileepkumar, H.; Rajashekar, Y.; Shivanandappa, T. A single acute hepatotoxic dose of CCl4 causes oxidative stress in the rat brain. Toxicol. Rep. 2015, 2, 891–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafez, M.M.; Al-Shabanah, O.A.; Al-Harbi, N.O.; Al-Harbi, M.M.; Al-Rejaie, S.S.; Alsurayea, S.M.; Sayed-Ahmed, M.M. Association between paraoxonases gene expression and oxidative stress in hepatotoxicity induced by CCl4. Oxidative Med. Cell. Longev. 2014, 2014, 893212. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.A.; Khan, M.R.; Sahreen, S. CCl 4-induced hepatotoxicity: Protective effect of rutin on p53, CYP2E1 and the antioxidative status in rat. BMC Complementary Altern. Med. 2012, 12, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [Green Version]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; De Risi, C. Research progress in the modification of quercetin leading to anticancer agents. Molecules 2017, 22, 1270. [Google Scholar] [CrossRef]
- Ma, J.-Q.; Li, Z.; Xie, W.-R.; Liu, C.-M.; Liu, S.-S. Quercetin protects mouse liver against CCl4-induced inflammation by the TLR2/4 and MAPK/NF-κB pathway. Int. Immunopharmacol. 2015, 28, 531–539. [Google Scholar] [CrossRef]
- Paulke, A.; Eckert, G.P.; Schubert-Zsilavecz, M.; Wurglics, M. Isoquercitrin provides better bioavailability than quercetin: Comparison of quercetin metabolites in body tissue and brain sections after six days administration of isoquercitrin and quercetin. Die Pharm.-Int. J. Pharm. Sci. 2012, 67, 991–996. [Google Scholar]
- Zargar, S.; Siddiqi, N.J.; Ansar, S.; Alsulaimani, M.S.; El Ansary, A.K. Therapeutic role of quercetin on oxidative damage induced by acrylamide in rat brain. Pharm. Biol. 2016, 54, 1763–1767. [Google Scholar] [CrossRef] [Green Version]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem.-Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [Green Version]
- Gulec, S.; Ozdol, C.; Vurgun, K.; Selcuk, T.; Turhan, S.; Duzen, V.; Temizhan, A.; Ozturk, S.; Ozdemir, A.O.; Erol, C. The effect of high-dose aspirin pre-treatment on the incidence of myonecrosis following elective coronary stenting. Atherosclerosis 2008, 197, 171–176. [Google Scholar] [CrossRef]
- Vedani, A.; Dobler, M.; Hu, Z.; Smieško, M. OpenVirtualToxLab—A platform for generating and exchanging in silico toxicity data. Toxicol. Lett. 2015, 232, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqi, N.J.; Zargar, S. Effect of quercetin on cadmium fluoride-induced alterations in hydroxyproline/collagen content in mice liver. Connect. Tissue Res. 2014, 55, 234–238. [Google Scholar] [CrossRef]
- Ansar, S.; Siddiqi, N.J.; Zargar, S.; Ganaie, M.A.; Abudawood, M. Hepatoprotective effect of Quercetin supplementation against Acrylamide-induced DNA damage in wistar rats. BMC Complementary Altern. Med. 2016, 16, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zargar, S.; Siddiqi, N.J.; Al Daihan, S.K.; Wani, T.A. Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochim. Pol. 2015, 62, 207–213. [Google Scholar] [CrossRef]
- Zargar, S.; Siddiqi, N.J.; Khan, T.H.; Elredah, I.E. Effect of cadmium fluoride and quercetin on in vivo activity of indoleamine 2,3-dioxygenase in mice liver and kidney. Fluoride 2014, 47, 31–42. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Utley, H.G.; Bernheim, F.; Hochstein, P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967, 118, 29–32. [Google Scholar] [CrossRef]
- Jagota, S.; Dani, H. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Masuda, Y. Learning toxicology from carbon tetrachloride-induced hepatotoxicity. Yakugaku Zasshi J. Pharm. Soc. Jpn. 2006, 126, 885–899. [Google Scholar] [CrossRef] [Green Version]
- Shah, G.H.; Patel, B.G.; Shah, G.B. Development of carbon tetrachloride-induced chronic hepatotoxicity model in rats and its application in evaluation of hepatoprotective activity of silymarin. Asian J. Pharm. Clin. Res. 2017, 10, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Cragg, B.; Rees, S. Increased body: Brain weight ratio in developing rats after low exposure to organic lead. Exp. Neurol. 1984, 86, 113–121. [Google Scholar] [CrossRef]
- Graefe, G.; Karlson, P. Kurzes Lehrbuch der Biochemie für Mediziner und Naturwissenschaftler; Geleitwort von A. Butenandt, 8., völlig neubearbeitete Auflage, Georg Thieme Verlag, Stuttgart, 1972. 405 Seiten, 82 Abb. in 105 Einzeldarstellungen, 21 Tabellen, 1 Falttafel, PVC-kartoniert DM 29, 80; Wiley Online Library: Hoboken, NJ, USA, 1973. [Google Scholar]
- Kelbich, P.; Radovnický, T.; Selke-Krulichová, I.; Lodin, J.; Matuchová, I.; Sameš, M.; Procházka, J.; Krejsek, J.; Hanuljaková, E.; Hejčl, A. Can Aspartate Aminotransferase in the Cerebrospinal Fluid Be a Reliable Predictive Parameter? Brain Sci. 2020, 10, 698. [Google Scholar] [CrossRef]
- Netopilová, M.; Haugvicová, R.; Kubová, H.; Dršata, J.; Mareš, P. Influence of convulsants on rat brain activities of alanine aminotransferase and aspartate aminotransferase. Neurochem. Res. 2001, 26, 1285–1291. [Google Scholar] [CrossRef]
- Trinh-Trang-Tan, M.-M.; Cartron, J.-P.; Bankir, L. Molecular basis for the dialysis disequilibrium syndrome: Altered aquaporin and urea transporter expression in the brain. Nephrol. Dial. Transplant. 2005, 20, 1984–1988. [Google Scholar] [CrossRef]
- Huang, B.S.; Leenen, F. Sympathoexcitatory and pressor responses to increased brain sodium and ouabain are mediated via brain ANG II. Am. J. Physiol. -Heart Circ. Physiol. 1996, 270, H275–H280. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Chisholm, R.; Tauskela, J.S.; Mealing, G.A.; Johnston, L.J.; Morris, C.E. Force spectroscopy measurements show that cortical neurons exposed to excitotoxic agonists stiffen before showing evidence of bleb damage. PLoS ONE 2013, 8, e73499. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bánhegyi, G.; Csala, M.; Braun, L.; Garzó, T.; Mandl, J. Ascorbate synthesis-dependent glutathione consumption in mouse liver. FEBS Lett. 1996, 381, 39–41. [Google Scholar] [CrossRef] [Green Version]
- Mårtensson, J.; Meister, A. Glutathione deficiency increases hepatic ascorbic acid synthesis in adult mice. Proc. Natl. Acad. Sci. USA 1992, 89, 11566–11568. [Google Scholar] [CrossRef] [Green Version]
- Braun, L.; Csala, M.; Poussu, A.; Garzó, T.; Mandl, J.; Bánhegyi, G. Glutathione depletion induces glycogenolysis dependent ascorbate synthesis in isolated murine hepatocytes. FEBS Lett. 1996, 388, 173–176. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, F.H.; Schmatz, R.; Cardoso, A.M.; Carvalho, F.B.; Baldissarelli, J.; de Oliveira, J.S.; Rosa, M.M.; Nunes, M.A.G.; Rubin, M.A.; da Cruz, I.B. Quercetin protects the impairment of memory and anxiogenic-like behavior in rats exposed to cadmium: Possible involvement of the acetylcholinesterase and Na+, K+-ATPase activities. Physiol. Behav. 2014, 135, 152–167. [Google Scholar] [CrossRef]
- You, J.-M.; Yun, S.-J.; Nam, K.N.; Kang, C.; Won, R.; Lee, E.H. Mechanism of glucocorticoid-induced oxidative stress in rat hippocampal slice cultures. Can. J. Physiol. Pharmacol. 2009, 87, 440–447. [Google Scholar] [CrossRef]
- Uno, H.; Eisele, S.; Sakai, A.; Shelton, S.; Baker, E.; Dejesus, O.; Holden, J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994, 28, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Barton, L.; BUTTERBACH-BAHL, K.; Kiese, R.; Murphy, D.V. Nitrous oxide fluxes from a grain–legume crop (narrow-leafed lupin) grown in a semiarid climate. Global Change Biol. 2011, 17, 1153–1166. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, Y.; Tai, R.; Zhang, N. Estrogen receptor β suppresses inflammation and the progression of prostate cancer. Mol. Med. Rep. 2019, 19, 3555–3563. [Google Scholar] [CrossRef]
- Ruotolo, R.; Calani, L.; Brighenti, F.; Crozier, A.; Ottonello, S.; Del Rio, D. Glucuronidation does not suppress the estrogenic activity of quercetin in yeast and human breast cancer cell model systems. Arch. Biochem. Biophys. 2014, 559, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kawai, Y.; Terao, J. Suppressive effect of quercetin on acute stress-induced hypothalamic-pituitary-adrenal axis response in Wistar rats. J. Nutr. Biochem. 2010, 21, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Gélinas, S.; Martinoli, M.G. Neuroprotective effect of estradiol and phytoestrogens on MPP+-induced cytotoxicity in neuronal PC12 cells. J. Neurosci. Res. 2002, 70, 90–96. [Google Scholar] [CrossRef] [PubMed]


| Treatment Groups | Control | CCL4 (1 mg/kg) | Q (100 mg/kg) | Q + CCL4 |
|---|---|---|---|---|
| Lipid Peroxidation (mmoles of malonaldehyde formed/hour/mg protein) | 1.98 ± 0.44 b,d | 8.33 ±0.82 a,c,d | 0.94 ± 0.15 b,c | 0.83 ± 0.35 a,b,c |
| Ascorbic acid (µg of ascorbic acid/µg protein) | 0.73 ± 0.15 b,d | 3.23 ± 1.82 a,c,d | 0.45 ± 0.06 b,d | 1.70 ± 0.14 a,b,c |
| Reduced glutathione (µg of GSH/mg protein) | 5.30 ± 0.80 b | 2.53 ± 1.47 a,c,d | 5.55 ± 3.47 b | 6.90 ± 0.41 b |
| Catalase mmol H2O2/min/mg protein | 3.05 ± 0.55 b,c | 0.26 ± 0.30 a,c,d | 2.30 ± 0.45 a,b,d | 2.43 ± 0.85 b,c |
| Superoxide dismutase Units/mg protein | 135.29 ± 44.79 b | 63.11± 22.48 a,d | 131.26 ± 7.29 d | 126.10± 25.73 b,c |
| Target | Binding Type | Binding Affinity (VirtualToxLab) |
|---|---|---|
| Androgens | moderate binding | 948 nM |
| Aryl hydrocarbon | negligible | >100 μM |
| CYP1A2 | negligible | >100 μM |
| CYP2C9 | negligible | >100 μM |
| CYP2D6 | negligible | >100 μM |
| CYP3A4 | negligible | >100 μM |
| Estrogen α, | weak binding | 3.54 μM |
| Estrogen β | moderate binding | 448 nM |
| Glucocorticoid | moderate binding | 574 nM |
| Herg k+ channel | weak binding | 4.87 μM |
| Liver x | weak binding | 72.7 μM |
| Mineralocorticoid | weak binding | 14.7 μM |
| Pparγ | weak binding | 4.53 μM |
| Progesterone | weak binding | 37.2 μM |
| Thyroid α, | weak binding | 15.3 μM |
| Thyroid β | weak binding | 5.85 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zargar, S.; Wani, T.A. Protective Role of Quercetin in Carbon Tetrachloride Induced Toxicity in Rat Brain: Biochemical, Spectrophotometric Assays and Computational Approach. Molecules 2021, 26, 7526. https://doi.org/10.3390/molecules26247526
Zargar S, Wani TA. Protective Role of Quercetin in Carbon Tetrachloride Induced Toxicity in Rat Brain: Biochemical, Spectrophotometric Assays and Computational Approach. Molecules. 2021; 26(24):7526. https://doi.org/10.3390/molecules26247526
Chicago/Turabian StyleZargar, Seema, and Tanveer A. Wani. 2021. "Protective Role of Quercetin in Carbon Tetrachloride Induced Toxicity in Rat Brain: Biochemical, Spectrophotometric Assays and Computational Approach" Molecules 26, no. 24: 7526. https://doi.org/10.3390/molecules26247526
APA StyleZargar, S., & Wani, T. A. (2021). Protective Role of Quercetin in Carbon Tetrachloride Induced Toxicity in Rat Brain: Biochemical, Spectrophotometric Assays and Computational Approach. Molecules, 26(24), 7526. https://doi.org/10.3390/molecules26247526






