Residues R1075, D1090, R1095, and C1130 Are Critical in ADAMTS13 TSP8-Spacer Interaction Predicted by Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Results
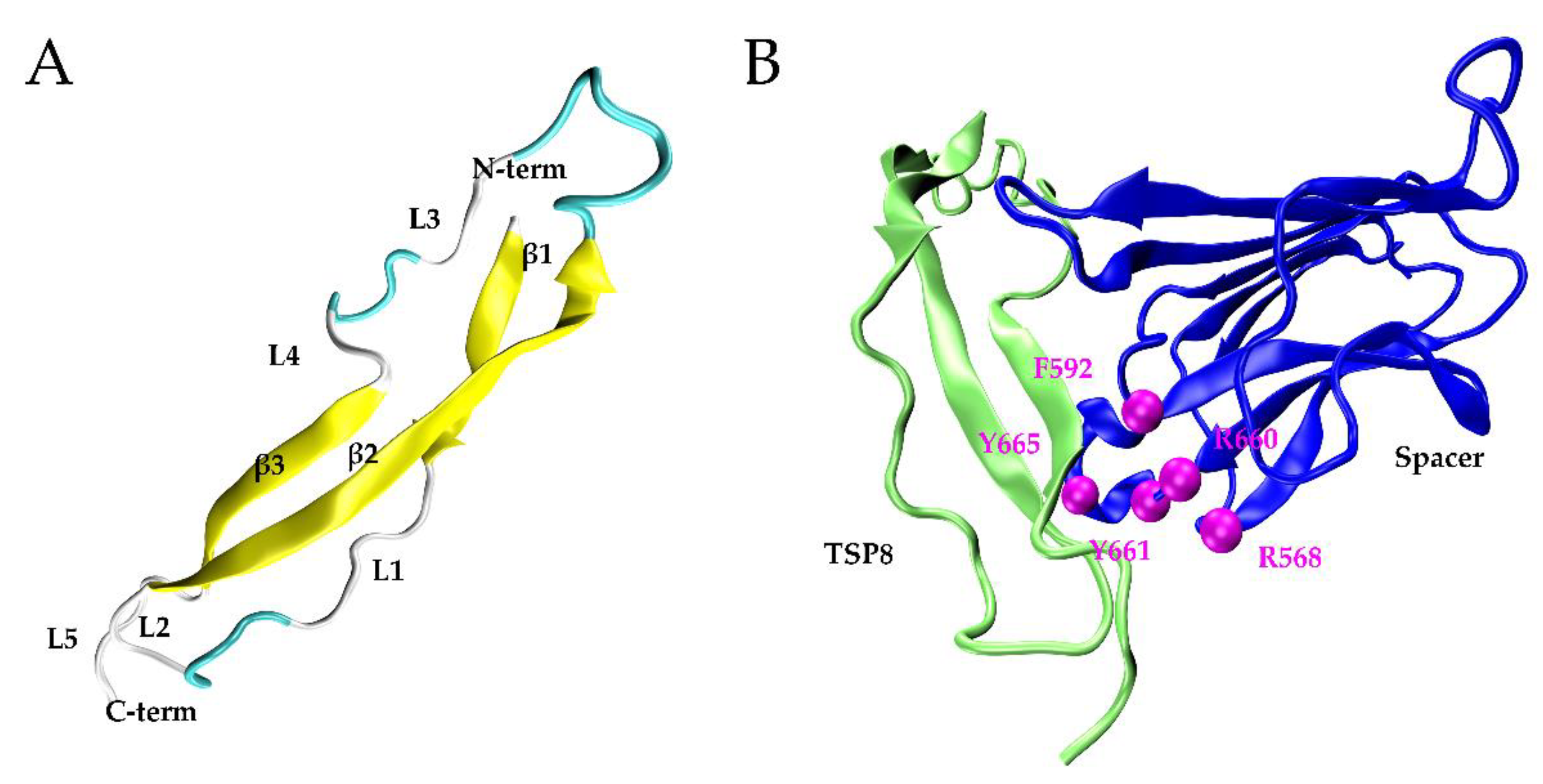
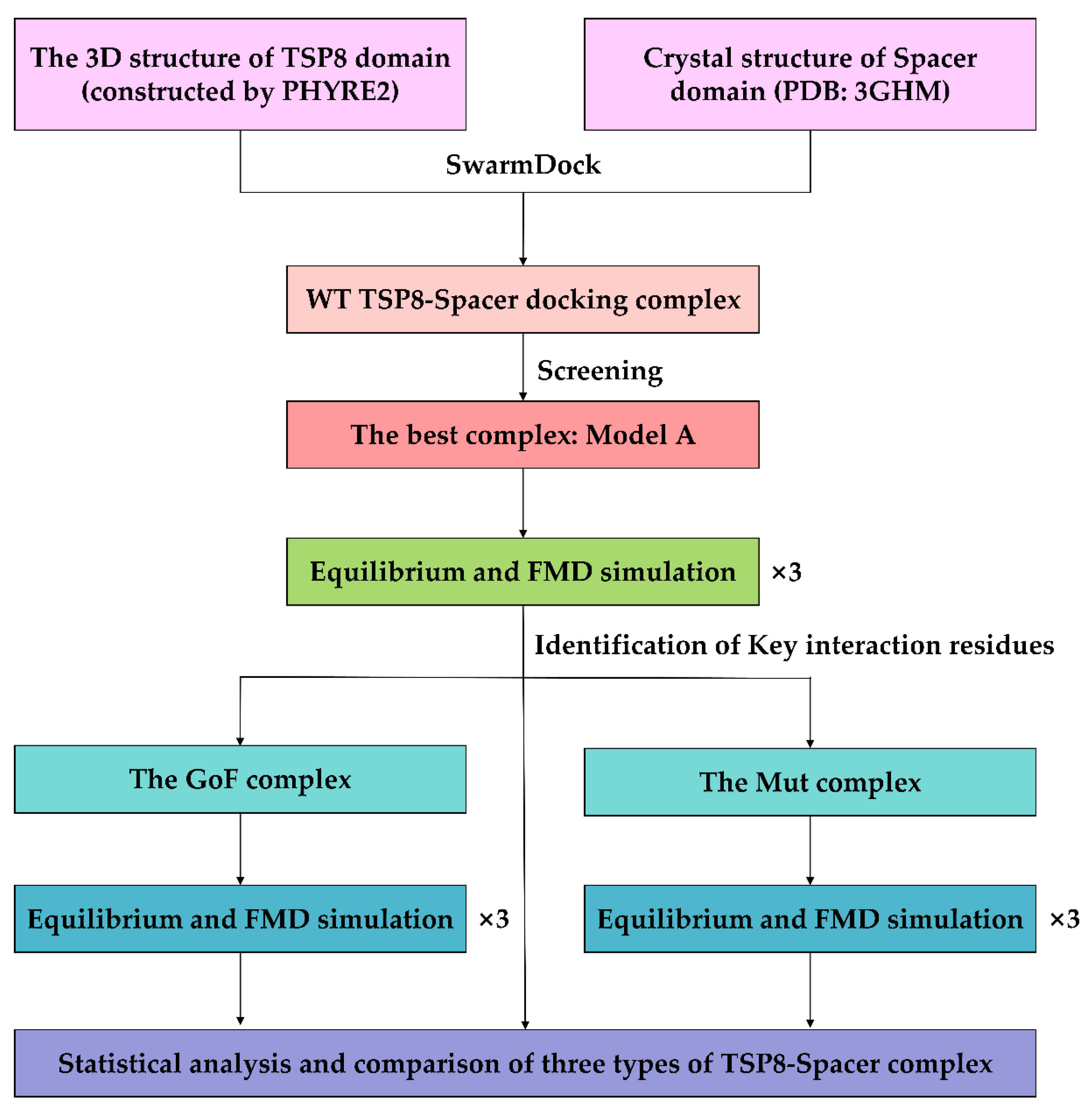
2.1. The Optimal TSP8-Spacer Docking Complex
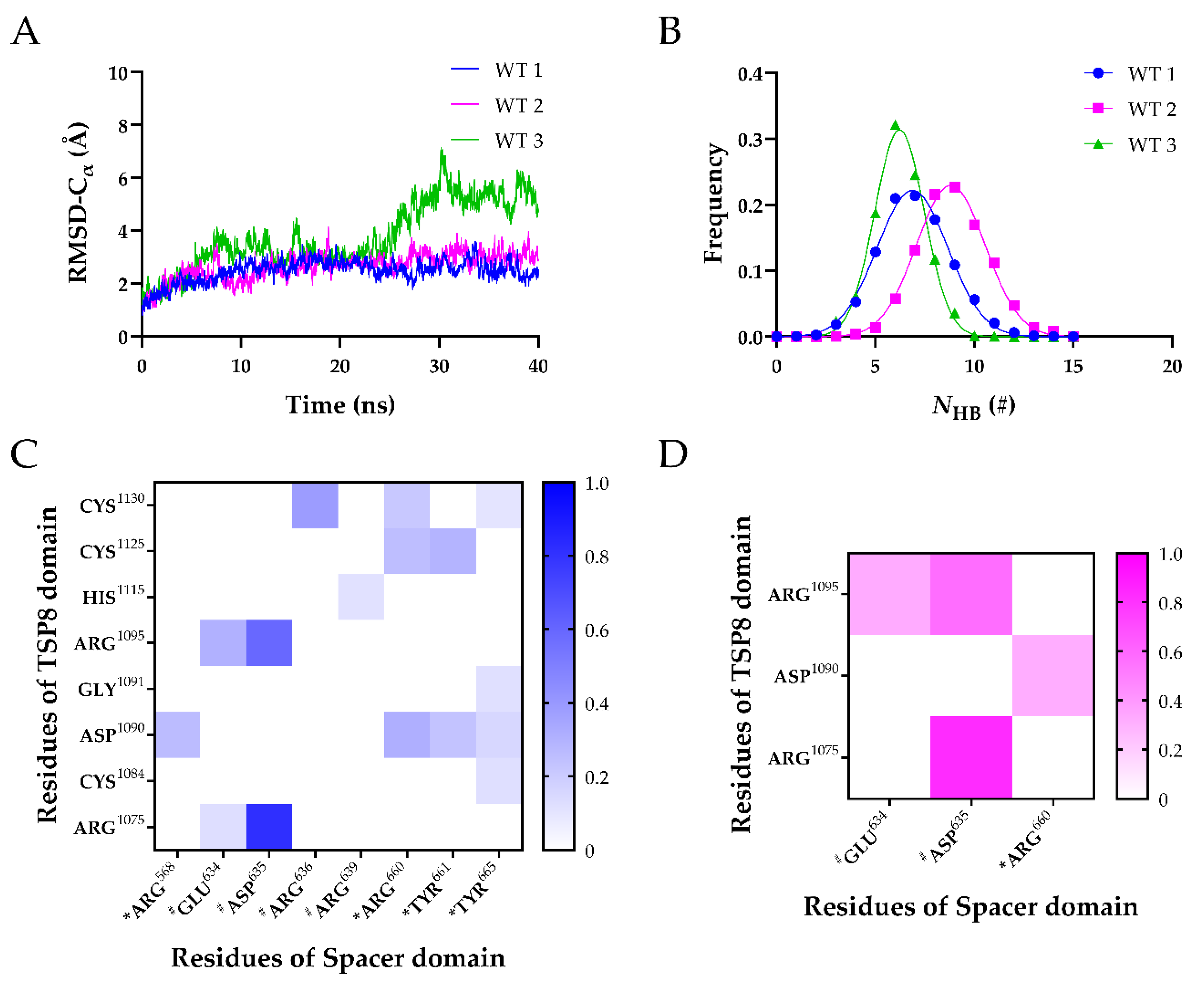
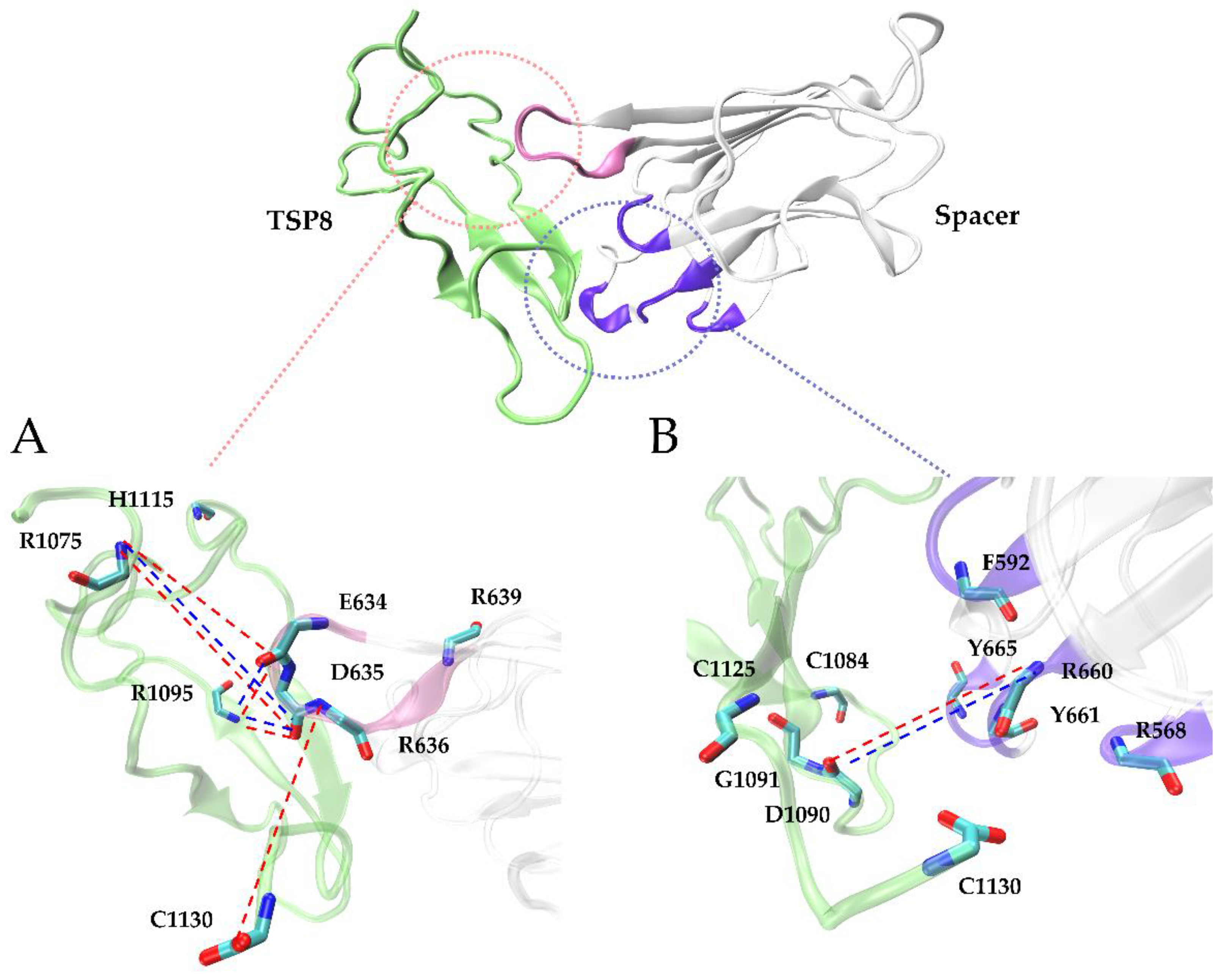
2.2. Arg1075, Asp1090, Arg1095, and Cys1130 Were the Key Residues on the TSP8 Domain Binding to the Spacer Domain
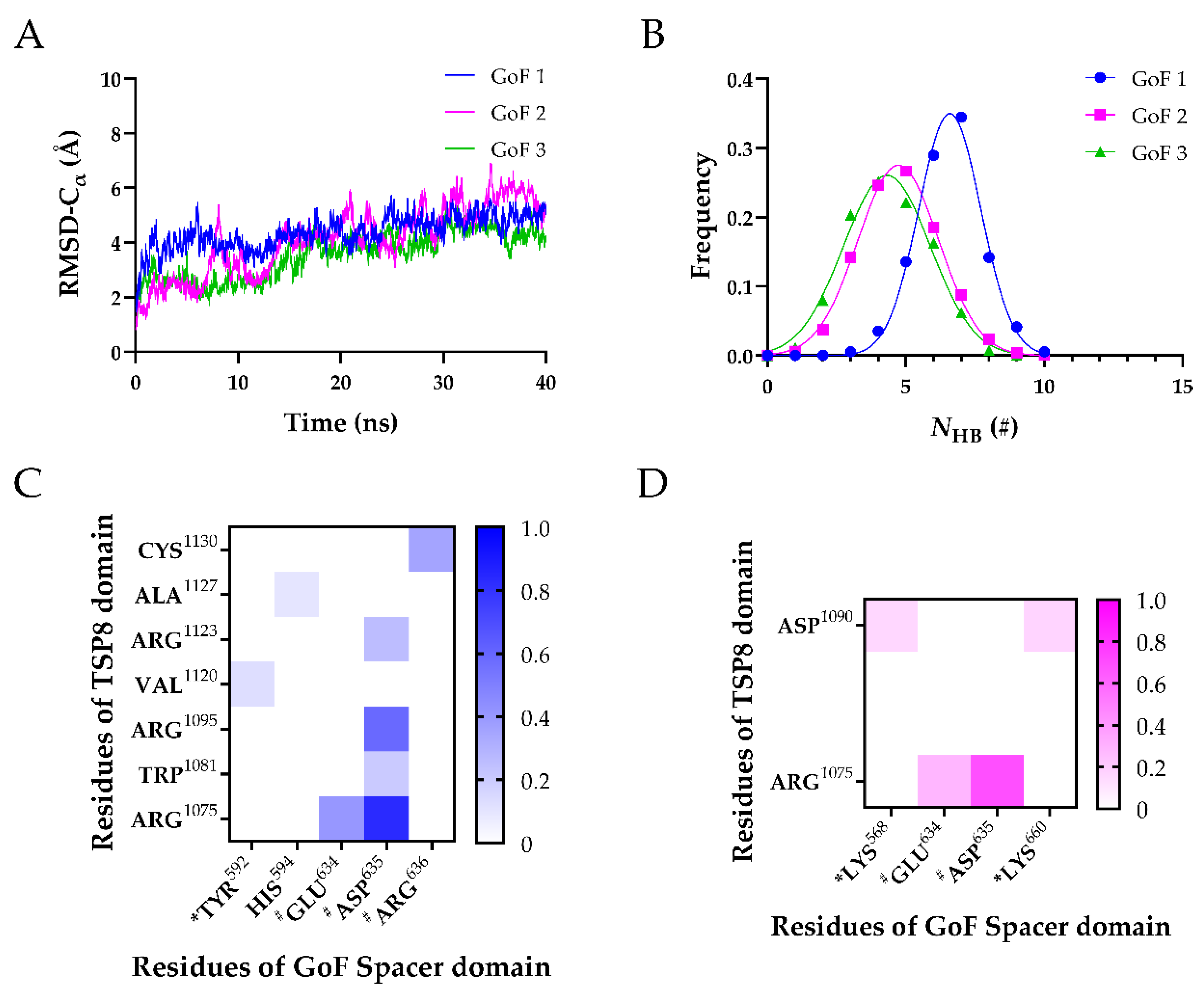
2.3. GoF Mutations Attenuated the Binding of Exosite-3 but Enhanced the Binding of Exosite-4 to the TSP8 Domain
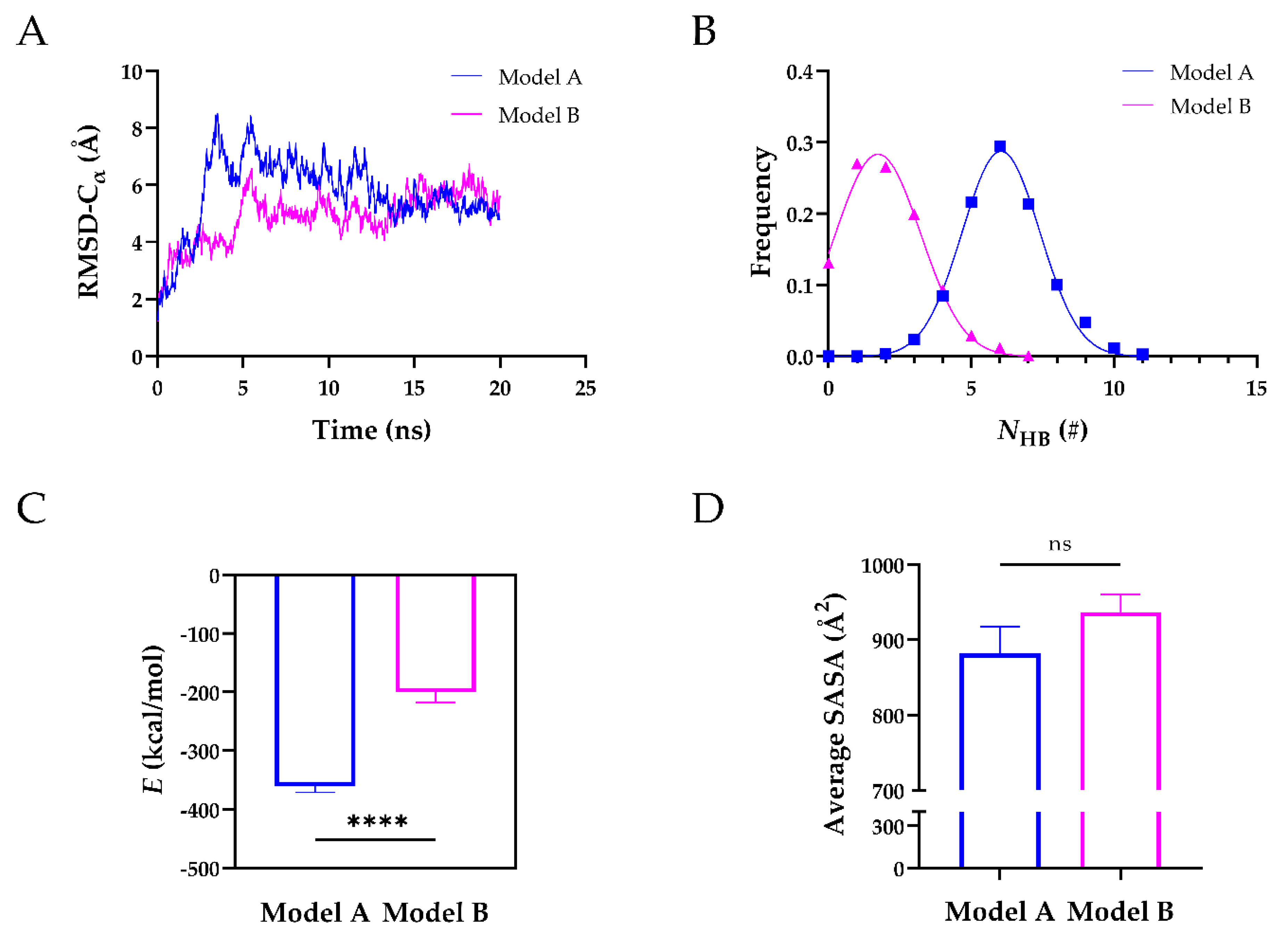
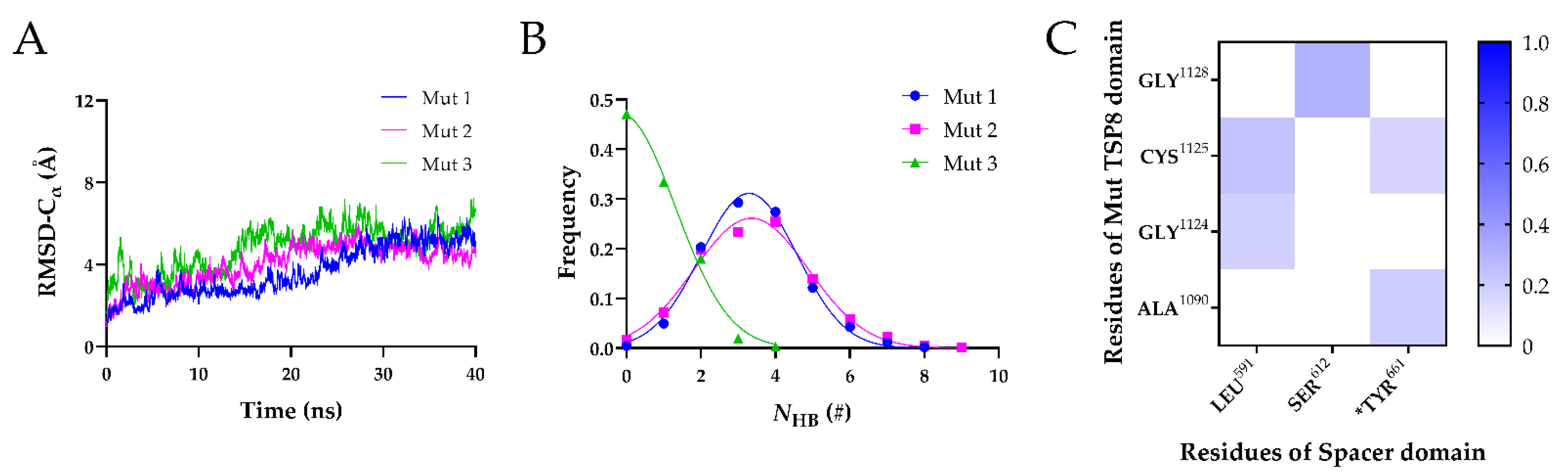
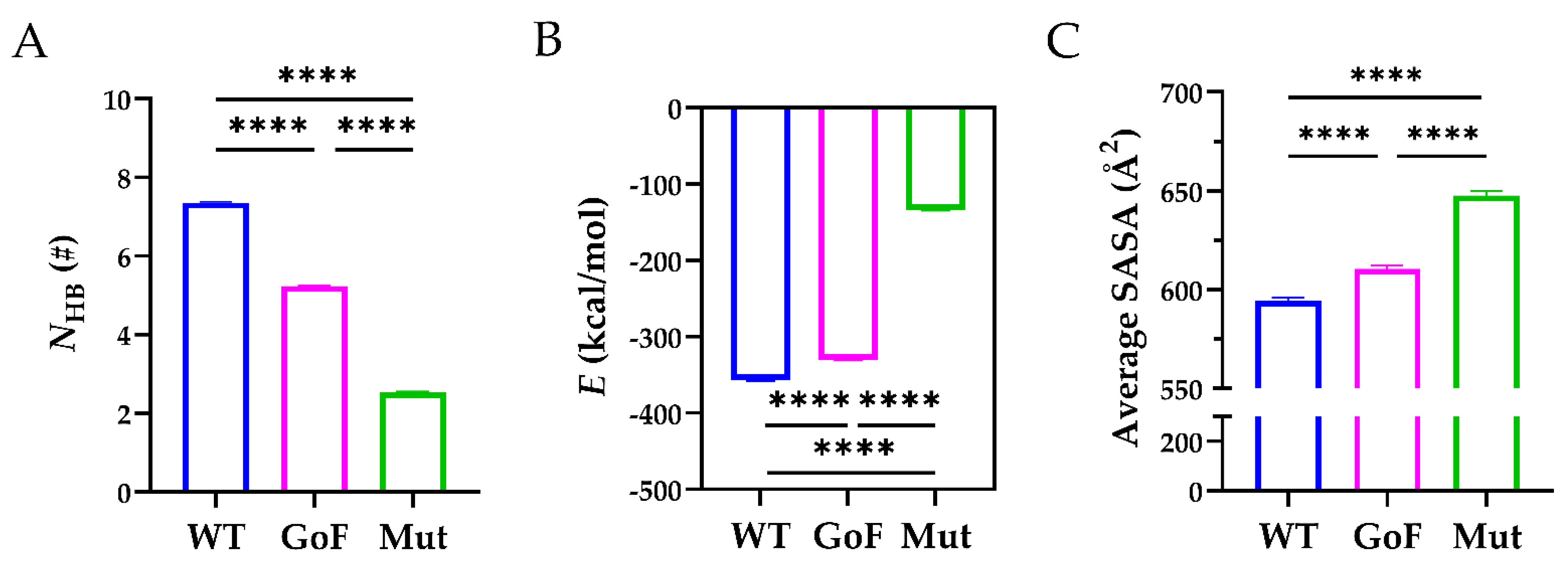
2.4. The TSP8 Mutations Reduced the Stability of the Complex
3. Discussion
4. Materials and Methods
4.1. Construction of the WT TSP8-Spacer Complex
4.2. Construction of the Mutated TSP8-Spacer Complexes
4.3. Molecular Dynamics Simulation
4.4. Data Analysis
4.4.1. The Selection of Key Residues
4.4.2. Structural Stability Analysis
4.4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ercig, B.; Wichapong, K.; Reutelingsperger, C.P.M.; Vanhoorelbeke, K.; Voorberg, J.; Nicolaes, G.A. Insights into 3D Structure of ADAMTS13: A Stepping Stone towards Novel Therapeutic Treatment of Thrombotic Thrombocytopenic Purpura. Thromb. Haemost. 2018, 118, 28–41. [Google Scholar] [CrossRef]
- Zanardelli, S.; Crawley, J.T.B.; Chion, C.K.N.C.K.; Lam, J.K.; Preston, R.; Lane, D.A. ADAMTS13 Substrate Recognition of von Willebrand Factor A2 Domain. J. Biol. Chem. 2006, 281, 1555–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akiyama, M.; Takeda, S.; Kokame, K.; Takagi, J.; Miyata, T. Crystal structures of the noncatalytic domains of ADAMTS13 reveal multiple discontinuous exosites for von Willebrand factor. Proc. Natl. Acad. Sci. USA 2009, 106, 19274–19279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, B.S.; Coppo, P.; Veyradier, A. An update on pathogenesis and diagnosis of thrombotic thrombocytopenic purpura. Expert Rev. Hematol. 2019, 12, 383–395. [Google Scholar] [CrossRef] [PubMed]
- South, K.; Luken, B.; Crawley, J.; Phillips, R.; Thomas, M.; Collins, R.F.; Deforche, L.; Vanhoorelbeke, K.; Lane, D.A. Conformational activation of ADAMTS13. Proc. Natl. Acad. Sci. USA 2014, 111, 18578–18583. [Google Scholar] [CrossRef] [Green Version]
- South, K.; Freitas, M.O.; Lane, D.A. A model for the conformational activation of the structurally quiescent metalloprotease ADAMTS13 by von willebrand factor. J. Biol. Chem. 2017, 292, 5760–5769. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Muia, J.; Gupta, G.; Westfield, L.A.; Vanhoorelbeke, K.; Tolia, N.H.; Sadler, J.E. Exploring the “minimal” structure of a functional ADAMTS13 by mutagenesis and small-angle X-ray scattering. Blood 2019, 133, 1909–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muia, J.; Zhu, J.; Gupta, G.; Haberichter, S.L.; Friedman, K.D.; Feys, H.; Deforche, L.; Vanhoorelbeke, K.; Westfield, L.A.; Roth, R.; et al. Allosteric activation of ADAMTS13 by von Willebrand factor. Proc. Natl. Acad. Sci. USA 2014, 111, 18584–18589. [Google Scholar] [CrossRef] [Green Version]
- Crawley, J.T.B.; de Groot, R.; Xiang, Y.; Luken, B.M.; Lane, D.A. Unraveling the scissile bond: How ADAMTS13 recognizes and cleaves von Willebrand factor. Blood 2011, 118, 3212–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petri, A.; Kim, H.J.; Xu, Y.; De Groot, R.; Li, C.; Vandenbulcke, A.; Vanhoorelbeke, K.; Emsley, J.; Crawley, J.T.B. Crystal structure and substrate-induced activation of ADAMTS13. Nat. Commun. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- South, K.; Freitas, M.O.; Lane, D.A. Conformational quiescence of ADAMTS-13 prevents proteolytic promiscuity. J. Thromb. Haemost. 2016, 14, 2011–2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, C.; Xiao, J.; Gong, L.; Skipwith, C.G.; Jin, S.Y.; Kwaan, H.C.; Zheng, X.L. Gain-of-function ADAMTS13 variants that are resistant to autoantibodies against ADAMTS13 in patients with acquired thrombotic thrombocytopenic purpura. Blood 2012, 119, 3836–3843. [Google Scholar] [CrossRef] [Green Version]
- Pos, W.; Sorvillo, N.; Fijnheer, R.; Feys, H.B.; Kaijen, P.H.P.; Vidarsson, G.; Voorberg, J. Residues Arg568 and Phe592 contribute to an antigenic surface for anti-ADAMTS13 antibodies in the spacer domain. Haematologica 2011, 96, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Pos, W.; Crawley, J.T.B.; Fijnheer, R.; Voorberg, J.; Lane, D.A.; Luken, B.M. An autoantibody epitope comprising residues R660, Y661, and Y665 in the ADAMTS13 spacer domain identifies a binding site for the A2 domain of VWF. Blood 2010, 115, 1640–1649. [Google Scholar] [CrossRef]
- Kim, H.J.; Xu, Y.; Petri, A.; Vanhoorelbeke, K.; Crawley, J.T.B.; Emsley, J. Crystal structure of ADAMTS13 CUB domains reveals their role in global latency. Sci. Adv. 2021, 7, eabg4403. [Google Scholar] [CrossRef]
- Yang, J.; Wu, Z.; Xie, X.; Liu, G.; Fang, Y.; Wu, J.; Lin, J. Characterization of the interactions of ADAMTS13 CUB1 domain to WT- and GOF-Spacer domain by molecular dynamics simulation. J. Mol. Graph. Model. 2021, 109, 108029. [Google Scholar] [CrossRef] [PubMed]
- Banno, F.; Chauhan, A.K.; Kokame, K.; Yang, J.; Miyata, S.; Wagner, D.D.; Miyata, T. The distal carboxyl-terminal domains of ADAMTS13 are required for regulation of in vivo thrombus formation. Blood 2009, 113, 5323–5329. [Google Scholar] [CrossRef]
- Moal, I.H.; Chaleil, R.A.G.; Bates, P.A. Flexible Protein-Protein Docking with SwarmDock. Methods Mol. Biol. 2018, 1764, 413–428. [Google Scholar] [CrossRef]
- Fang, X.; Fang, Y.; Liu, L.; Liu, G.; Wu, J. Mapping paratope on antithrombotic antibody 6B4 to epitope on platelet glycoprotein Ibalpha via molecular dynamic simulations. PLoS ONE 2012, 7, e42263. [Google Scholar] [CrossRef] [PubMed]
- Velásquez Pereira, L.C.; Roose, E.; Graça, N.A.G.; Sinkovits, G.; Kangro, K.; Joly, B.S.; Tellier, E.; Kaplanski, G.; Falter, T.; Von Auer, C.; et al. Immunogenic hotspots in the spacer domain of ADAMTS13 in immune-mediated thrombotic thrombocytopenic purpura. J. Thromb Haemost. 2021, 19, 478–488. [Google Scholar] [CrossRef]
- Casina, V.; Hanby, H.; Lyalenko, A.; Zheng, X.L. Identification of a Novel Exosite (Glu634-Arg639) in the Spacer Domain of ADAMTS13 Required for Recognition of Von Willebrand Factor. Blood 2012, 120, 2214. [Google Scholar] [CrossRef]
- Kremer Hovinga, J.A.; Heeb, S.R.; Skowronska, M.; Schaller, M. Pathophysiology of thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. J. Thromb. Haemost. 2018, 16, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Bell, W.R.; Braine, H.G.; Ness, P.M.; Kickler, T.S. Improved Survival in Thrombotic Thrombocytopenic Purpura–Hemolytic Uremic Syndrome. N. Engl. J. Med. 1991, 325, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Knoebl, P.; Kentouche, K.; Rice, L.; Windyga, J.; Schneppenheim, R.; Hovinga, J.A.K.; Kajiwara, M.; Fujimura, Y.; Maggiore, C.; et al. Recombinant ADAMTS-13: First-in-human pharmacokinetics and safety in congenital thrombotic thrombocytopenic purpura. Blood 2017, 130, 2055–2063. [Google Scholar] [CrossRef]
- South, K.; Saleh, O.; Lemarchand, E.; Coutts, G.; Smith, C.J.; Schiessl, I.; Allan, S.M. Robust Thrombolytic and Anti-Inflammatory Action of a Constitutively Active ADAMTS13 Variant in Murine Stroke Models. Blood 2021. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, W.; Fang, J.; Shi, X.; Wu, J.; Fang, Y.; Lin, J. AFM Imaging Reveals Multiple Conformational States of ADAMTS13. J. Biol. Eng. 2019, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J.E. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [Green Version]
- Darden, T.; York, D.M.; Pedersen, L.G. Particle mesh Ewald: An N⋅log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Sonne, J.; Jensen, M.; Hansen, F.Y.; Hemmingsen, L.; Peters, G.H. Reparameterization of All-Atom Dipalmitoylphosphatidylcholine Lipid Parameters Enables Simulation of Fluid Bilayers at Zero Tension. Biophys. J. 2007, 92, 4157–4167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; McCammon, J.A. Introduction to Molecular Dynamics: Theory and Applications in Biomolecular Modeling. In Computational Modeling of Biological Systems: From Molecules to Pathways; Dokholyan, N.V., Ed.; Springer: Boston, MA, USA, 2012; pp. 3–30. [Google Scholar]
- Jiang, X.; Sun, X.; Lin, J.; Ling, Y.; Fang, Y.; Wu, J. MD Simulations on a Well-Built Docking Model Reveal Fine Mechanical Stability and Force-Dependent Dissociation of Mac-1/GPIbα Complex. Front. Mol. Biosci. 2021, 8, 638396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, Z.; Fang, Y.; Wu, J. Prediction of Catch-Slip Bond Transition of Kindlin2/β3 Integrin via Steered Molecular Dynamics Simulation. J. Chem. Inf. Model. 2020, 60, 5132–5141. [Google Scholar] [CrossRef] [PubMed]
| Model | H-Bond | Salt Bridge | ||
|---|---|---|---|---|
| TSP8 | Spacer | TSP8 | Spacer | |
| Model A | VAL1078 | GLU634 | ARG1075 | GLU634 |
| GLY1089 | *ARG568 | ASP1090 | *ARG568 | |
| ASP1090 | *ARG660 | ARG1095 | ASP635 | |
| ARG1094 | *TYR665 | - | - | |
| ARG1095 | ASP635 | - | - | |
| LYS1118 | HIS588 | - | - | |
| ARG1123 | ARG636 | - | - | |
| Model B | CYS1072 | *ARG568 | - | - |
| TYR1074 | *ARG660 | - | - | |
| GLN1103 | ARG636 | - | - | |
| ALA1104 | *PHE592 | - | - | |
| PRO1109 | *ARG660 | - | - | |
| Model | TSP8 | Spacer | Survival Ratio | |
|---|---|---|---|---|
| H-Bond | Salt Bridge | |||
| Model A | ARG1095 | ASP635 | 0.971 | 0.988 |
| ASP1090 | *TYR661 | 0.228 | - | |
| ASP1090 | *ARG660 | 0.637 | 0.587 | |
| ASP1090 | *ARG568 | 0.587 | 0.381 | |
| LYS1118 | ASP672 | 0.180 | - | |
| SER1085 | *TYR665 | 0.165 | - | |
| ARG1075 | GLU634 | 0.808 | 0.706 | |
| Model B | ASP1111 | *ARG568 | 0.212 | - |
| THR1098 | ARG636 | 0.188 | - | |
| ARG1096 | ASP635 | 0.508 | 0.218 | |
| Ranking | TSP8 | Spacer | Survival Ratio | Average | ||
|---|---|---|---|---|---|---|
| WT 1 | WT 2 | WT 3 | (Mean ± SEM) | |||
| 1 | ARG1075 | #ASP635 | 0.652 | 0.911 | 0.883 | 0.815 ± 0.067 |
| 2 | ARG1095 | #ASP635 | 0.843 | 0.930 | 0.000 | 0.591 ± 0.242 |
| 3 | CYS1130 | #ARG636 | 0.051 | 0.423 | 0.681 | 0.385 ± 0.149 |
| 4 | ASP1090 | *ARG660 | 0.000 | 0.938 | 0.000 | 0.313 ± 0.255 |
| 5 | ARG1095 | #GLU634 | 0.000 | 0.000 | 0.915 | 0.305 ± 0.249 |
| 6 | CYS1125 | *TYR661 | 0.718 | 0.161 | 0.000 | 0.293 ± 0.178 |
| 7 | ASP1090 | *ARG568 | 0.446 | 0.336 | 0.000 | 0.261 ± 0.109 |
| 8 | CYS1125 | *ARG660 | 0.000 | 0.754 | 0.000 | 0.251 ± 0.205 |
| 9 | ASP1090 | *TYR661 | 0.000 | 0.703 | 0.000 | 0.234 ± 0.191 |
| 10 | CYS1130 | *ARG660 | 0.000 | 0.000 | 0.646 | 0.215 ± 0.176 |
| 11 | ASP1090 | *TYR665 | 0.466 | 0.000 | 0.000 | 0.155 ± 0.127 |
| 12 | ARG1075 | #GLU634 | 0.285 | 0.097 | 0.000 | 0.127 ± 0.068 |
| 13 | CYS1084 | *TYR665 | 0.365 | 0.000 | 0.000 | 0.122 ± 0.099 |
| 14 | GLY1091 | *TYR665 | 0.353 | 0.000 | 0.000 | 0.118 ± 0.096 |
| 15 | HIS1115 | #ARG639 | 0.350 | 0.000 | 0.000 | 0.117 ± 0.095 |
| 16 | CYS1130 | *TYR665 | 0.000 | 0.314 | 0.000 | 0.105 ± 0.085 |
| Ranking | TSP8 | Spacer | Survival Ratio | Average | ||
|---|---|---|---|---|---|---|
| WT 1 | WT 2 | WT 3 | (Mean ± SEM) | |||
| 1 | ARG1075 | #ASP635 | 0.560 | 0.985 | 0.943 | 0.829 ± 0.110 |
| 2 | ARG1095 | #ASP635 | 0.716 | 0.993 | 0.000 | 0.569 ± 0.241 |
| 3 | ARG1095 | #GLU634 | 0.000 | 0.000 | 0.989 | 0.330 ± 0.269 |
| 4 | ASP1090 | *ARG660 | 0.000 | 0.960 | 0.000 | 0.320 ± 0.261 |
| Ranking | TSP8 | Spacer | Survival Ratio | Average | ||
|---|---|---|---|---|---|---|
| GoF 1 | GoF 2 | GoF 3 | (Mean ± SEM) | |||
| 1 | ARG1075 | #ASP635 | 0.992 | 0.893 | 0.621 | 0.835 ± 0.091 |
| 2 | ARG1095 | #ASP635 | 0.844 | 0.000 | 0.910 | 0.585 ± 0.239 |
| 3 | ARG1075 | #GLU634 | 0.668 | 0.414 | 0.137 | 0.406 ± 0.125 |
| 4 | CYS1130 | #ARG636 | 0.987 | 0.079 | 0.000 | 0.356 ± 0.259 |
| 5 | ARG1123 | #ASP635 | 0.000 | 0.000 | 0.771 | 0.257 ± 0.210 |
| 6 | TRP1081 | #ASP635 | 0.618 | 0.000 | 0.000 | 0.206 ± 0.168 |
| 7 | VAL1120 | *TYR592 | 0.000 | 0.000 | 0.386 | 0.129 ± 0.105 |
| 8 | ALA1127 | HSD594 | 0.000 | 0.304 | 0.000 | 0.101 ± 0.083 |
| Ranking | TSP8 | Spacer | Survival Ratio | Average | ||
|---|---|---|---|---|---|---|
| GoF 1 | GoF 2 | GoF 3 | (Mean ± SEM) | |||
| 1 | ARG1075 | #ASP635 | 0.666 | 0.950 | 0.450 | 0.689 ± 0.118 |
| 2 | ARG1075 | #GLU634 | 0.000 | 0.000 | 0.858 | 0.286 ± 0.233 |
| 3 | ASP1090 | *LYS660 | 0.000 | 0.500 | 0.000 | 0.167 ± 0.136 |
| 4 | ASP1090 | *LYS568 | 0.000 | 0.467 | 0.000 | 0.156 ± 0.127 |
| Ranking | TSP8 | Spacer | Survival Ratio | Average | ||
|---|---|---|---|---|---|---|
| Mut 1 | Mut 2 | Mut 3 | (Mean ± SEM) | |||
| 1 | GLY1128 | SER612 | 0.900 | 0.000 | 0.000 | 0.300 ± 0.212 |
| 2 | CYS1125 | LEU591 | 0.000 | 0.718 | 0.000 | 0.239 ± 0.169 |
| 3 | ALA1090 | *TYR661 | 0.101 | 0.413 | 0.068 | 0.194 ± 0.078 |
| 4 | GLY1124 | LEU591 | 0.546 | 0.000 | 0.000 | 0.182 ± 0.129 |
| 5 | CYS1125 | *TYR661 | 0.492 | 0.000 | 0.000 | 0.164 ± 0.116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Yang, J.; Xie, X.; Liu, G.; Fang, Y.; Wu, J.; Lin, J. Residues R1075, D1090, R1095, and C1130 Are Critical in ADAMTS13 TSP8-Spacer Interaction Predicted by Molecular Dynamics Simulation. Molecules 2021, 26, 7525. https://doi.org/10.3390/molecules26247525
Wu Z, Yang J, Xie X, Liu G, Fang Y, Wu J, Lin J. Residues R1075, D1090, R1095, and C1130 Are Critical in ADAMTS13 TSP8-Spacer Interaction Predicted by Molecular Dynamics Simulation. Molecules. 2021; 26(24):7525. https://doi.org/10.3390/molecules26247525
Chicago/Turabian StyleWu, Zhiwei, Junxian Yang, Xubin Xie, Guangjian Liu, Ying Fang, Jianhua Wu, and Jiangguo Lin. 2021. "Residues R1075, D1090, R1095, and C1130 Are Critical in ADAMTS13 TSP8-Spacer Interaction Predicted by Molecular Dynamics Simulation" Molecules 26, no. 24: 7525. https://doi.org/10.3390/molecules26247525
APA StyleWu, Z., Yang, J., Xie, X., Liu, G., Fang, Y., Wu, J., & Lin, J. (2021). Residues R1075, D1090, R1095, and C1130 Are Critical in ADAMTS13 TSP8-Spacer Interaction Predicted by Molecular Dynamics Simulation. Molecules, 26(24), 7525. https://doi.org/10.3390/molecules26247525






