Nano-Structured Lipid Carrier-Based Oral Glutathione Formulation Mediates Renoprotection against Cyclophosphamide-Induced Nephrotoxicity, and Improves Oral Bioavailability of Glutathione Confirmed through RP-HPLC Micellar Liquid Chromatography
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterizations of GSH-Loaded Nanostructured Lipid Carriers
2.1.1. Particle Morphology
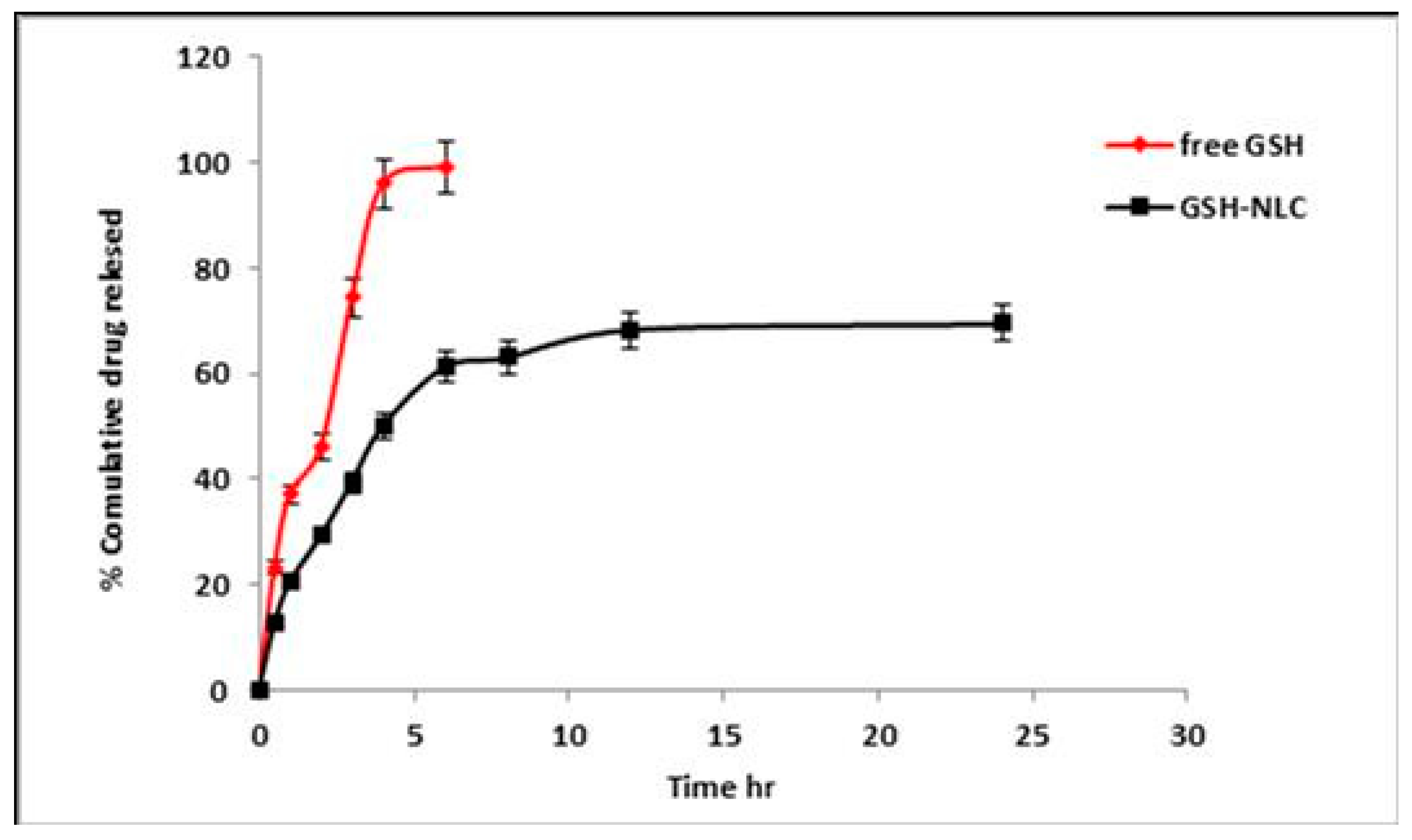
2.1.2. In Vitro Release Profile of the Glutathione from NLC Formulation
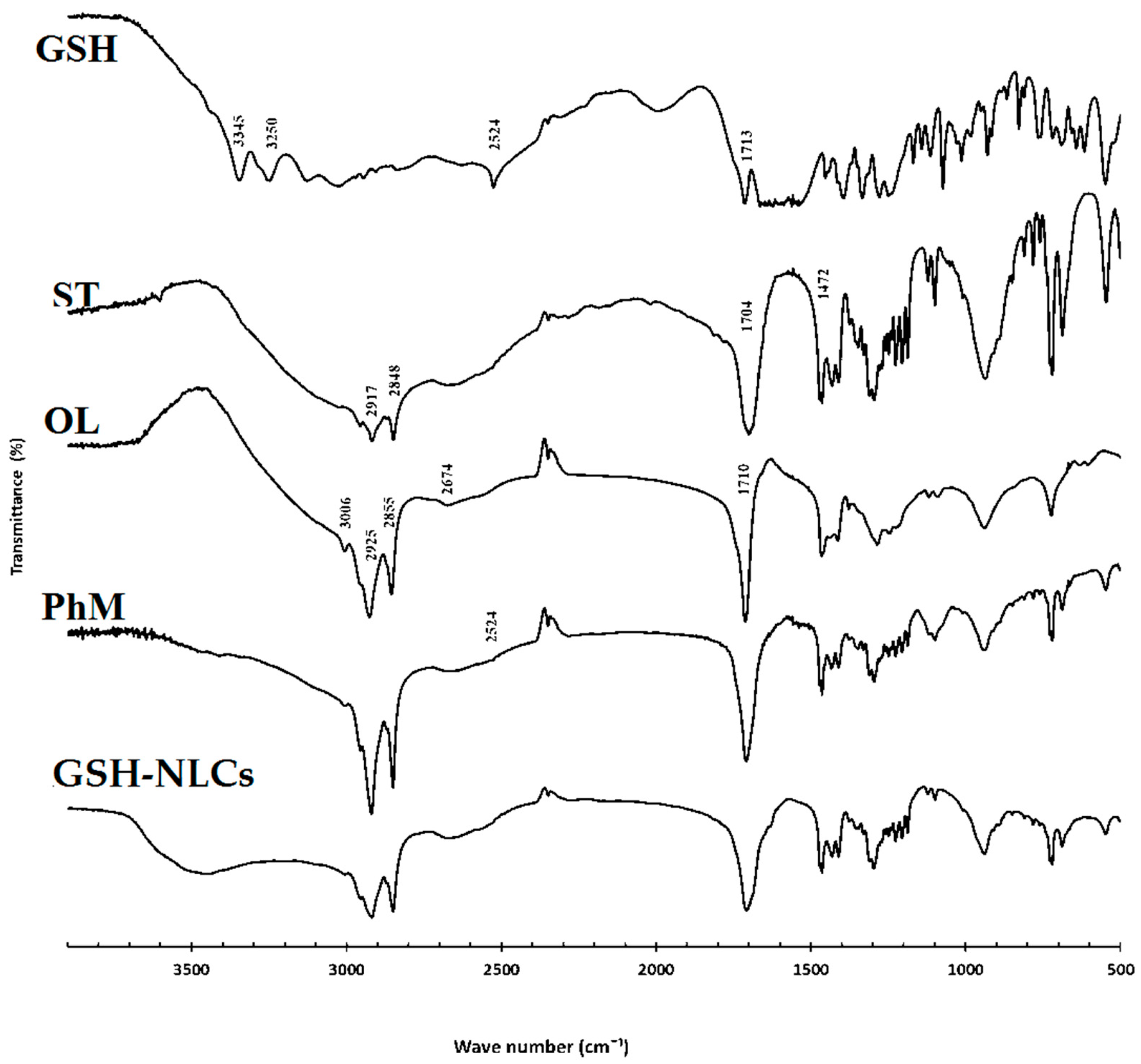
2.1.3. Fourier Transform-Infrared (FT-IR) Spectroscopy
2.2. Stability Studies
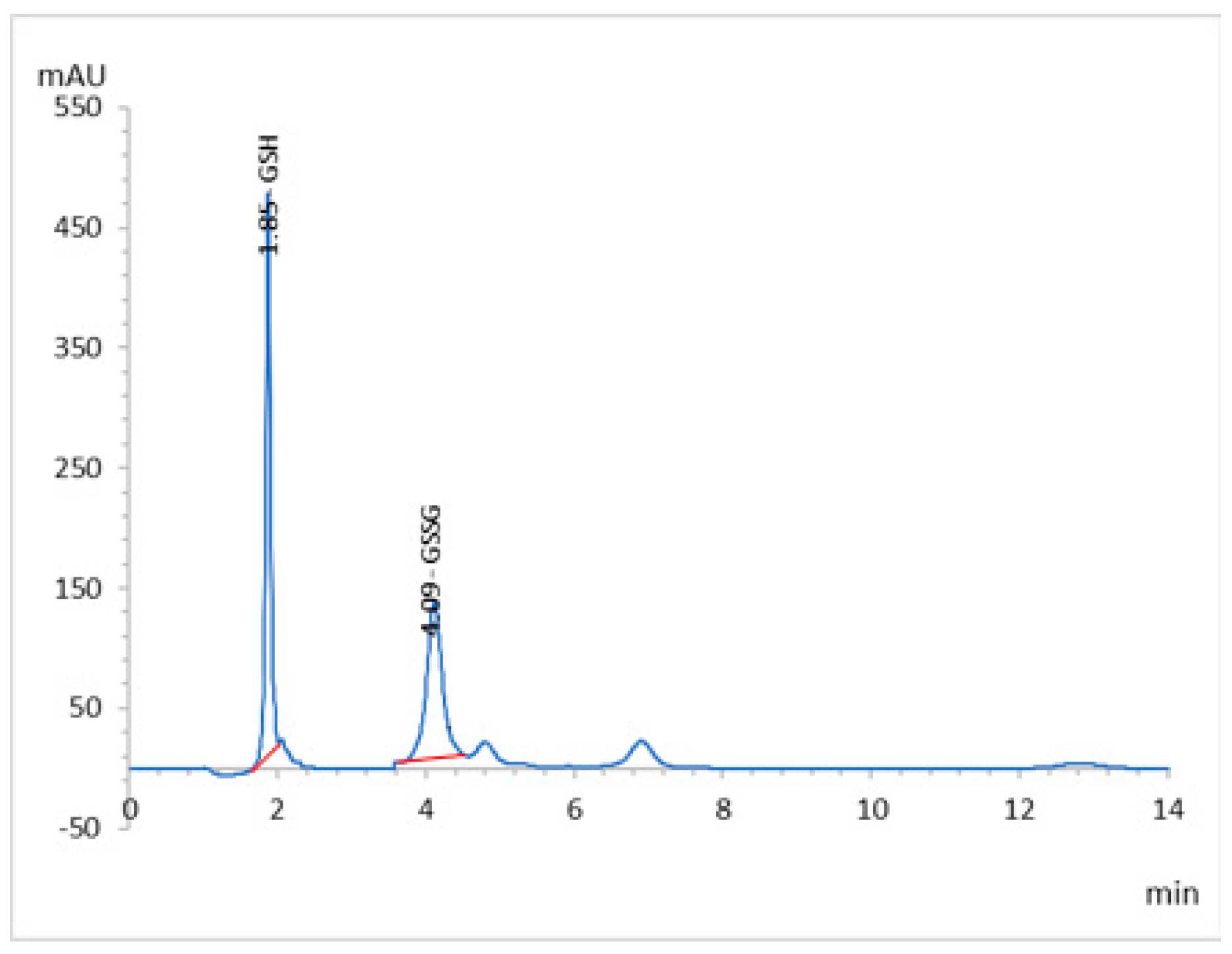
2.3. HPLC Analyses and Validation
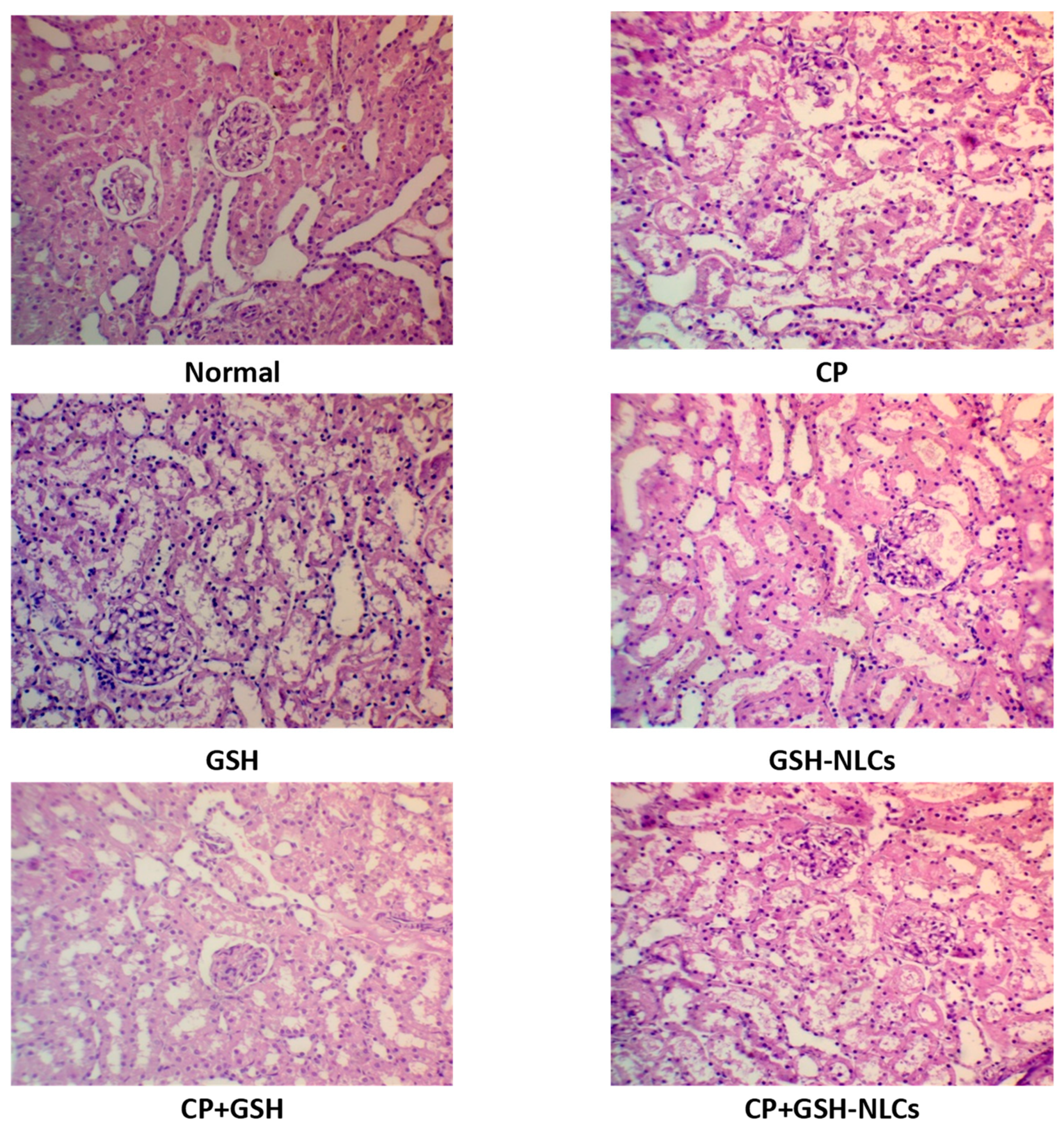
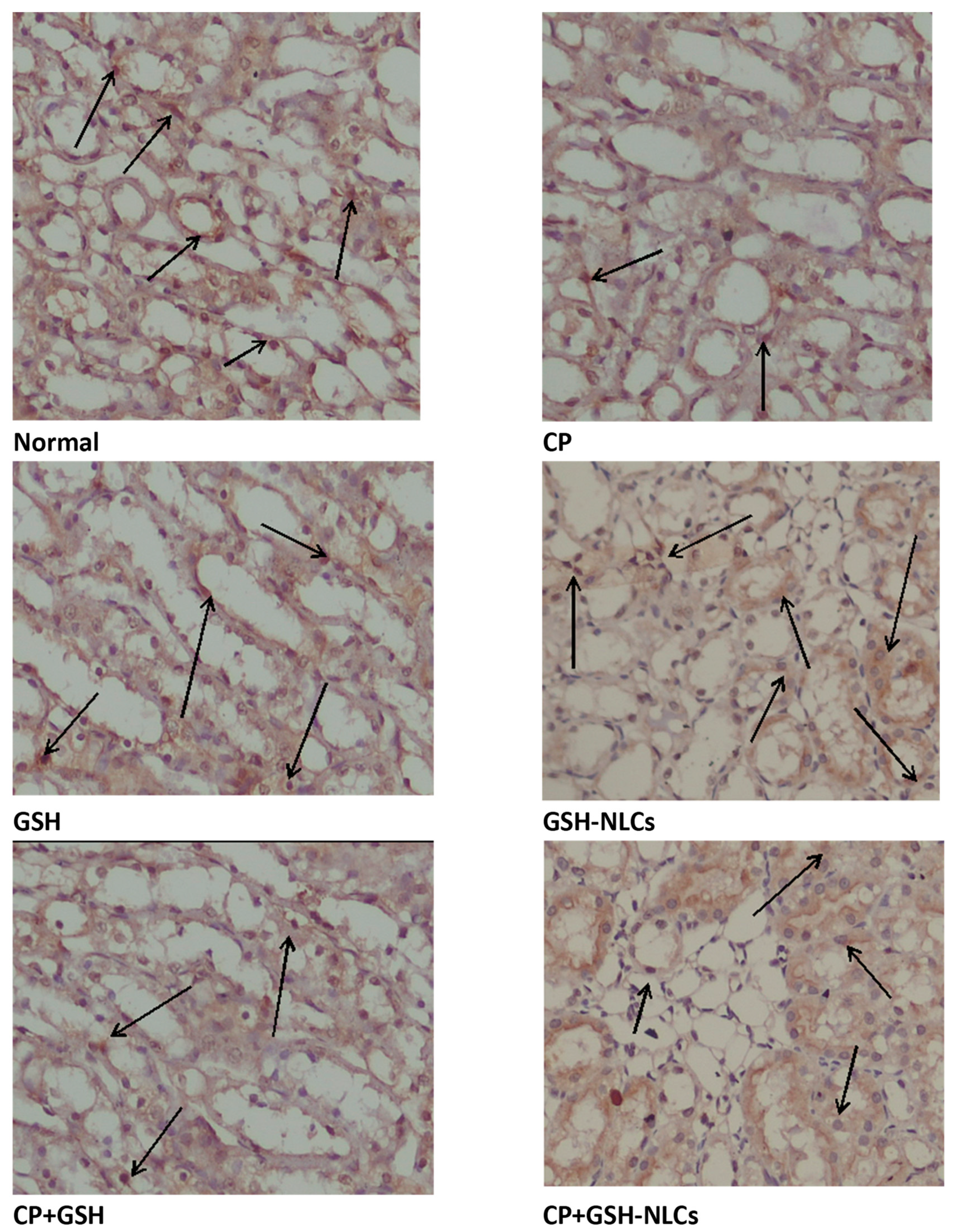
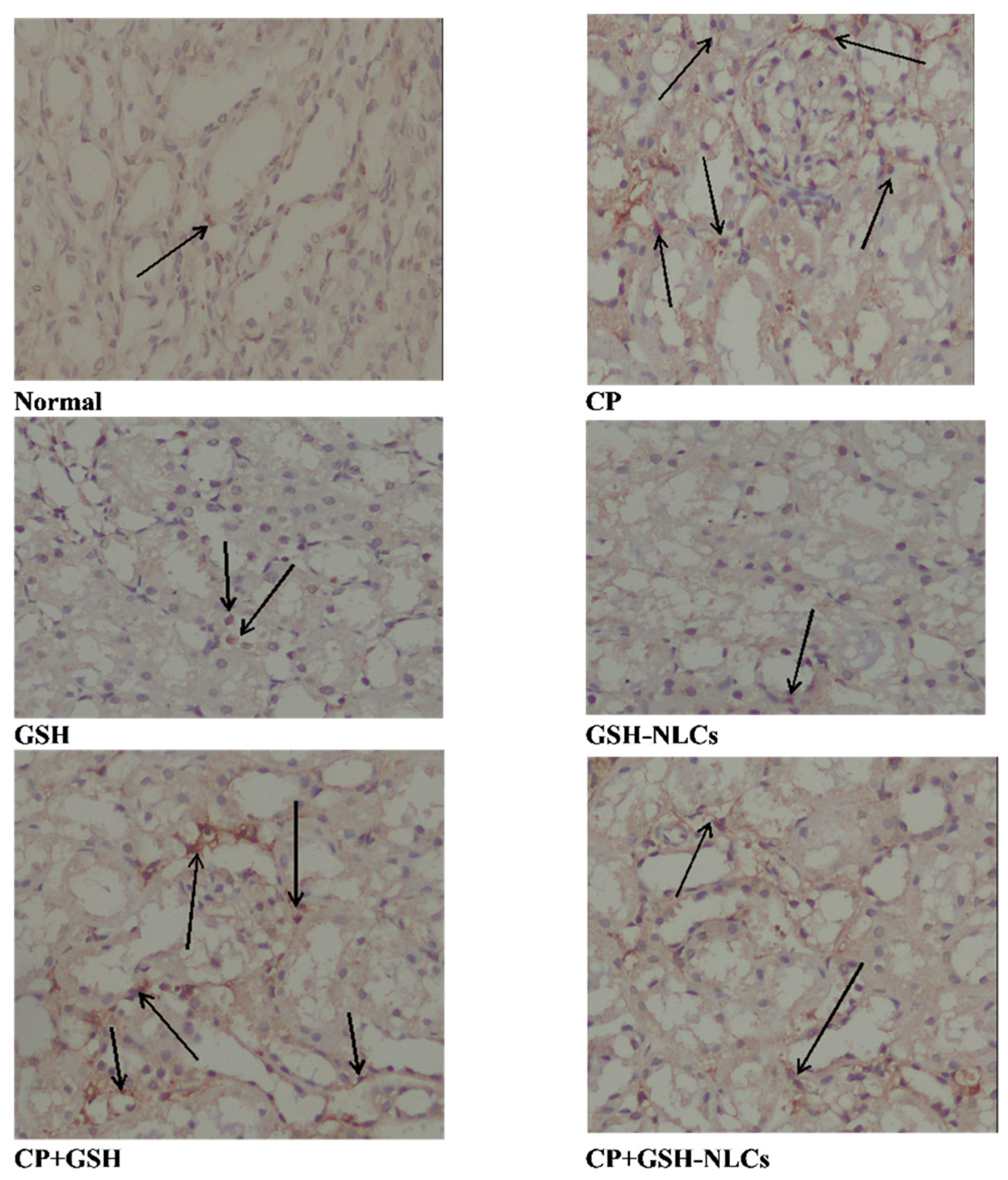
2.4. Histopathological Examinations
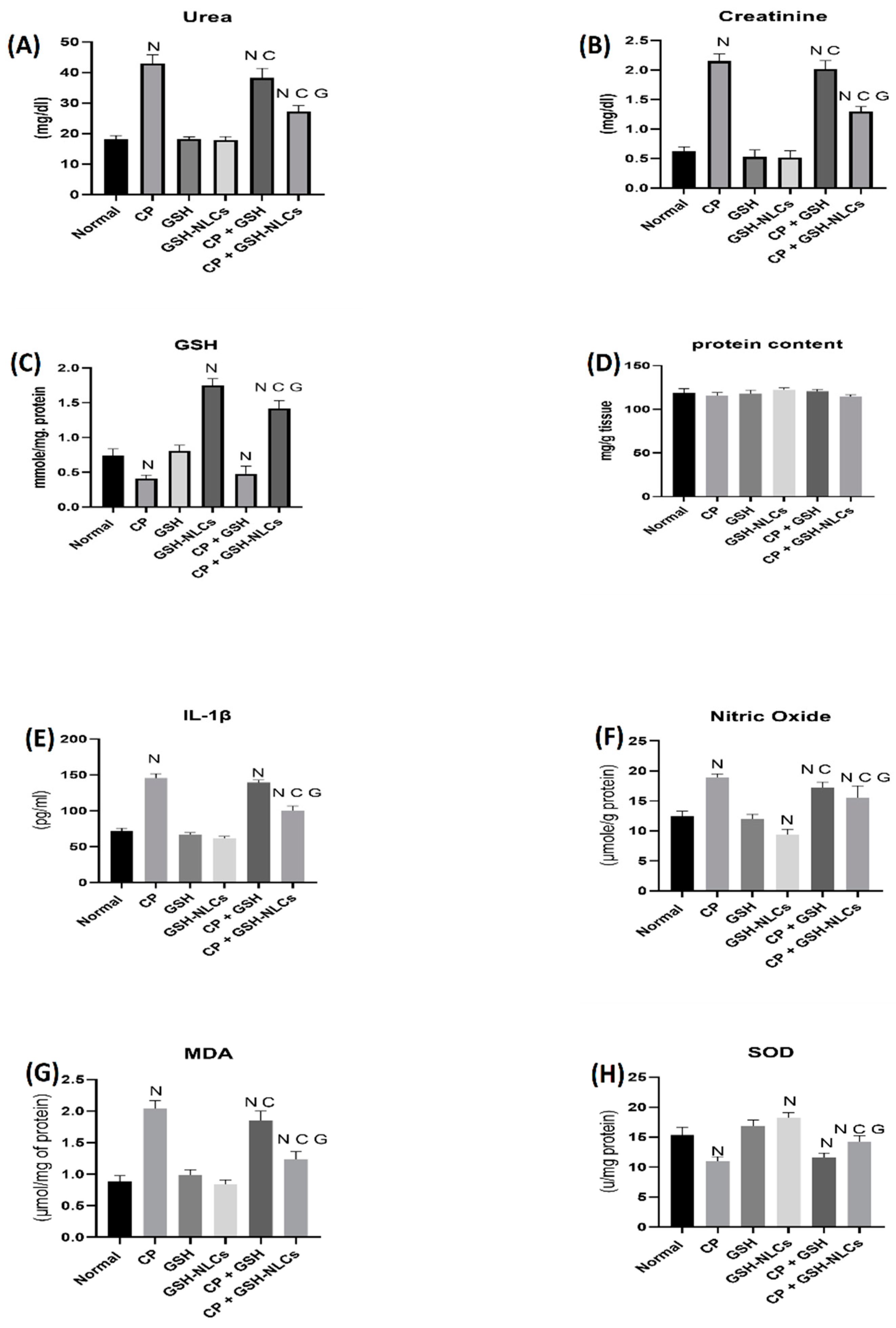
2.5. Renal Functions Tests
2.6. Effects on Nitric Oxide
2.7. Effects on GSH Concentrations
2.8. Effects on IL-1β
2.9. Effects on MDA
2.10. Effects on SOD
2.11. Effects on Bcl-2 Protein
2.12. Effects on NF-KB
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Glutathione-Loaded Nanostructured Lipid Carriers (GSH-NLCs)
4.3. RP-HPLC: Analytical Procedure
4.4. Characterization of Glutathione-Loaded Nanostructured Lipid Carriers (GSH-NLCs)
4.4.1. Encapsulation Efficiency and Loading Capacity
4.4.2. Particle Size, and Zeta Potential Measurements
4.4.3. Particles Morphology
4.4.4. In Vitro Drug Release Studies
4.4.5. Fourier Transform Infrared (FT-IR) Spectroscopy
4.4.6. Stability Studies
4.5. Animal Study
4.5.1. Experimental Designs
4.5.2. Histopathological and Immunohistochemical Examinations
Determination of Total Proteins
Determination of Renal Function Tests
Determination of Nitric Oxide Concentrations (NO)
Assessments of Superoxide Dismutase (SOD)
Assessment of Inflammatory Marker (IL-Iβ)
Malondialdehyde (MDA) Determination
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Coughlin, S.S.; Ekwueme, D.U. Breast cancer as a global health concern. Cancer Epidemiol. 2009, 33, 315–318. [Google Scholar] [CrossRef]
- Lehmann, F.; Wennerberg, J. Evolution of nitrogen-based alkylating anticancer agents. Processes 2021, 9, 377. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Sulaiman, G.M.; Anwar, S.S.; Tawfeeq, A.T.; Khan, R.A.; Mohammed, S.A.A.; Al-Omar, M.S.; Alsharidah, M.; Rugaie, O.A.; Al-Amiery, A.A. Quercetin against MCF7 and CAL51 breast cancer cell lines: Apoptosis, gene expression and cytotoxicity of nano-quercetin. Nanomedicine 2021, 16, 1937–1961. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, Y.; Song, W.; Zhang, L. Therapeutic potential of nitrogen mustard based hybrid molecules. Front. Pharmacol. 2018, 9, 1453. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Mohammed, S.A.A.; Khan, R.A.; Yusuf, M.; Singh, V.; Mohammed, H.A.; Al-Omar, M.S.; Abdellatif, A.A.H.; Naz, M.; Khadri, H. Self-Generating nano-emulsification techniques for alternatively-routed, bioavailability enhanced delivery, especially for anti-cancers, anti-diabetics, and miscellaneous drugs of natural, and synthetic origins. J. Drug Deliv. Sci. Technol. 2020, 58, 101808. [Google Scholar] [CrossRef]
- Carr, C.; Ng, J.; Wigmore, T. The side effects of chemotherapeutic agents. Curr. Anaesth. Crit. Care 2008, 19, 70–79. [Google Scholar] [CrossRef]
- Conklin, K.A. Dietary antioxidants during cancer chemotherapy: Impact on chemotherapeutic effectiveness and development of side effects. Nutr. Cancer 2000, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Beyer, I.; Cao, H.; Persson, J.; Song, H.; Richter, M.; Feng, Q.; Yumul, R.; van Rensburg, R.; Li, Z.; Berenson, R. Coadministration of epithelial junction opener JO-1 improves the efficacy and safety of chemotherapeutic drugs. Clin. Cancer Res. 2012, 18, 3340–3351. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-based drug delivery system: A patient-friendly chemotherapy for oncology. Dose-Response 2020, 18, 1559325820936161. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Zak, J.K. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef]
- Steinberg, A.D. Cyclophosphamide: Should It Be Used Daily, Monthly, or Never? N. Engl. J. Med. 1984, 310, 458–459. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Gustafson, D.L.; Page, R.L. Cancer chemotherapy. MacEwen’s Small Anim. Clin. Oncol. 2013, 9, 157–179. [Google Scholar]
- Teske, E.; van Straten, G.; van Noort, R.; Rutteman, G.R. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: New results with an old protocol. J. Vet. Intern. Med. 2002, 16, 179–186. [Google Scholar] [CrossRef]
- Lin, J.; Kouznetsova, V.; Tsigelny, I. Molecular Mechanisms of Feline Cancers. OBM Genet. 2021, 5, 722. [Google Scholar] [CrossRef]
- Fraiser, L.H.; Kanekal, S.; Kehrer, J.P. Cyclophosphamide toxicity. Drugs 1991, 42, 781–795. [Google Scholar] [CrossRef]
- Abraham, P.; Isaac, B. The effects of oral glutamine on cyclophosphamide-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2011, 30, 616–623. [Google Scholar] [CrossRef]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Sci. Pollut. Res. 2018, 25, 20968–20984. [Google Scholar] [CrossRef]
- Mahipal, P.; Pawar, R.S. Nephroprotective effect of Murraya koenigii on cyclophosphamide induced nephrotoxicity in rats. Asian Pac. J. Trop. Med. 2017, 10, 808–812. [Google Scholar] [CrossRef]
- Horvath, J.J.; Witmer, C.M.; Witz, G. Nephrotoxicity of the 1: 1 acrolein-glutathione adduct in the rat. Toxicol. Appl. Pharmacol. 1992, 117, 200–207. [Google Scholar] [CrossRef]
- Ohno, Y.; Ormstad, K. Formation, toxicity and inactivation of acrolein during biotransformation of cyclophosphamide as studied in freshly isolated cells from rat liver and kidney. Arch. Toxicol. 1985, 57, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, N.; Shuaib, M.; Garg, V.K.; Sharma, A. A review on renal protective agents for cyclophosphamide induced nephrotoxicity. World J. Pharm. Pharm. Sci. 2014, 3, 737–747. [Google Scholar]
- Pizzorno, J. Glutathione! Integr. Med. A Clin. J. 2014, 13, 8. [Google Scholar]
- Abdellatif, A.A.H.; Mohammed, H.A.; Khan, R.A.; Singh, V.; Bouazzaoui, A.; Yusuf, M.; Akhtar, N.; Khan, M.; Al-Subaiyel, A.; Mohammed, S.A.A. Nano-scale delivery: A comprehensive review of nano-structured devices, preparative techniques, site-specificity designs, biomedical applications, commercial products, and references to safety, cellular uptake, and organ toxicity. Nanotechnol. Rev. 2021, 10, 1493–1559. [Google Scholar] [CrossRef]
- Kumar, S.; Randhawa, J.K. High melting lipid based approach for drug delivery: Solid lipid nanoparticles. Mater. Sci. Eng. C 2013, 33, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Fathi, H.A.; Allam, A.; Elsabahy, M.; Fetih, G.; El-Badry, M. Nanostructured lipid carriers for improved oral delivery and prolonged antihyperlipidemic effect of simvastatin. Colloids Surf. B Biointerfaces 2018, 162, 236–245. [Google Scholar] [CrossRef]
- Huguet-Casquero, A.; Moreno-Sastre, M.; López-Méndez, T.B.; Gainza, E.; Pedraz, J.L. Encapsulation of oleuropein in nanostructured lipid carriers: Biocompatibility and antioxidant efficacy in lung epithelial cells. Pharmaceutics 2020, 12, 429. [Google Scholar] [CrossRef]
- Bertoni, S.; Albertini, B.; Facchini, C.; Prata, C.; Passerini, N. Glutathione-loaded solid lipid microparticles as innovative delivery system for oral antioxidant therapy. Pharmaceutics 2019, 11, 364. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, X.; Wang, B.; Lv, M.; Zhu, Y.; Gao, J. Fabrication and characterization of novel shape-stabilized stearic acid composite phase change materials with tannic-acid-templated mesoporous silica nanoparticles for thermal energy storage. RSC Adv. 2017, 7, 15625–15631. [Google Scholar] [CrossRef] [Green Version]
- Worsfold, P.; Townshend, A.; Poole, C.F.; Miró, M. Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 008101984X. [Google Scholar]
- Deng, J.; Wu, Z.; Zhao, Z.; Wu, C.; Yuan, M.; Su, Z.; Wang, Y.; Wang, Z. Berberine-loaded nanostructured lipid carriers enhance the treatment of ulcerative colitis. Int. J. Nanomed. 2020, 15, 3937. [Google Scholar] [CrossRef]
- Hu, F.-Q.; Jiang, S.-P.; Du, Y.-Z.; Yuan, H.; Ye, Y.-Q.; Zeng, S. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf. B Biointerfaces 2005, 45, 167–173. [Google Scholar] [CrossRef]
- Shahzadi, I.; Fürst, A.; Knoll, P.; Bernkop-Schnürch, A. Nanostructured Lipid Carriers (NLCs) for Oral Peptide Drug Delivery: About the Impact of Surface Decoration. Pharmaceutics 2021, 13, 1312. [Google Scholar] [CrossRef]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef]
- El-Shabrawy, M.; Mishriki, A.; Attia, H.; Emad Aboulhoda, B.; Emam, M.; Wanas, H. Protective effect of tolvaptan against cyclophosphamide-induced nephrotoxicity in rat models. Pharmacol. Res. Perspect. 2020, 8, e00659. [Google Scholar] [CrossRef] [PubMed]
- Salama, R.M.; Nasr, M.M.; Abdelhakeem, J.I.; Roshdy, O.K.; ElGamal, M.A. Alogliptin attenuates cyclophosphamide-induced nephrotoxicity: A novel therapeutic approach through modulating MAP3K/JNK/SMAD3 signaling cascade. Drug Chem. Toxicol. 2020, 18, 1814319. [Google Scholar] [CrossRef]
- Lassé, M.; Stampfli, A.R.; Orban, T.; Bothara, R.K.; Gerrard, J.A.; Fairbanks, A.J.; Pattinson, N.R.; Dobson, R.C.J. Reaction dynamics and residue identification of haemoglobin modification by acrolein, a lipid-peroxidation by-product. Biochim. Biophys. Acta-Gen. Subj. 2021, 1865, 130013. [Google Scholar] [CrossRef]
- Jaganjac, M.; Sredoja Tisma, V.; Zarkovic, N. Short Overview of Some Assays for the Measurement of Antioxidant Activity of Natural Products and Their Relevance in Dermatology. Molecules 2021, 26, 5301. [Google Scholar] [CrossRef]
- Ghezzi, P. Role of glutathione in immunity and inflammation in the lung. Int. J. Gen. Med. 2011, 4, 105. [Google Scholar] [CrossRef] [Green Version]
- Mehanna, J.; Haddad, F.G.; Eid, R.; Lambertini, M.; Kourie, H.R. Triple-negative breast cancer: Current perspective on the evolving therapeutic landscape. Int. J. Women’s Health 2019, 11, 431–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankiewicz, A.; Skrzydlewska, E. Protection against cyclophosphamide-induced renal oxidative stress by amifostine: The role of antioxidative mechanisms. Toxicol. Mech. Methods 2003, 13, 301–308. [Google Scholar] [CrossRef]
- Sheth, V.G.; Navik, U.; Maremanda, K.P.; Jena, G. Effect of diethyldithiocarbamate in cyclophosphamide-induced nephrotoxicity: Immunohistochemical study of superoxide dismutase 1 in rat. Indian J. Pharmacol. 2018, 50, 4. [Google Scholar] [PubMed]
- Baharmi, S.; Kalantari, H.; Kalantar, M.; Goudarzi, M.; Mansouri, E.; Kalantar, H. Pretreatment with Gallic Acid Mitigates Cyclophosphamide Induced Inflammation and Oxidative Stress in Mice. Curr. Mol. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Sobeh, M.; El-Maadawy, W.H.; Mohammed, H.S.; Khalil, H.; Botros, S.; Wink, M. Chemical profiling of polyphenolics in Eucalyptus globulus and evaluation of its hepato–renal protective potential against cyclophosphamide induced toxicity in mice. Antioxidants 2019, 8, 415. [Google Scholar] [CrossRef] [Green Version]
- Junita, D.; Prasetyo, A.A.; Muniroh, M.; Kristina, T.N.; Mahati, E. The effect of glutathione as adjuvant therapy on levels of TNF-α and IL-10 in wistar rat peritonitis model. Ann. Med. Surg. 2021, 66, 102406. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, P.; Kulurkar, P.; Singh, D.; Kumar, D.; Patial, V. Iridoid glycosides fraction from Picrorhiza kurroa attenuates cyclophosphamide-induced renal toxicity and peripheral neuropathy via PPAR-γ mediated inhibition of inflammation and apoptosis. Phytomedicine 2017, 36, 108–117. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, X.; Tang, Y.; Han, T. Laminaria japonica fucoidan ameliorates cyclophosphamide-induced liver and kidney injury possibly by regulating Nrf2/HO-1 and TLR4/NF-κB signaling pathways. J. Sci. Food Agric. 2021. [Google Scholar] [CrossRef]
- Rodrigues, W.F.; Miguel, C.B.; Napimoga, M.H.; Oliveira, C.J.F.; Lazo-Chica, J.E. Establishing standards for studying renal function in mice through measurements of body size-adjusted creatinine and urea levels. Biomed. Res. Int. 2014, 2014, 872827. [Google Scholar] [CrossRef]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 325–337. [Google Scholar] [CrossRef]
- Basile, D.P.; Liapis, H.; Hammerman, M.R. Expression of bcl-2 and bax in regenerating rat renal tubules following ischemic injury. Am. J. Physiol. Physiol. 1997, 272, F640–F647. [Google Scholar] [CrossRef]
- Gobe, G.; Zhang, X.-J.; Willgoss, D.A.; Schoch, E.; Hogg, N.A.; Endre, Z.H. Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J. Am. Soc. Nephrol. 2000, 11, 454–467. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Nesterova, A.; Jambal, P.; Audesirk, G.; Kern, M.; Cabell, L.; Eves, E.; Rosner, M.R.; Boxer, L.M.; Reusch, J.E. Oxidative stress-mediated down-regulation of bcl-2 promoter in hippocampal neurons. J. Neurochem. 2003, 84, 982–996. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, W.; Qiu, E. Protection of oxidative stress induced apoptosis in osteosarcoma cells by dihydromyricetin through down-regulation of caspase activation and up-regulation of BcL-2. Saudi J. Biol. Sci. 2017, 24, 837–842. [Google Scholar] [CrossRef]

| Formulation | Oleic Acid (mg) | Stearic Acid (mg) | Tween® 80 (mg) | EE% | LD% |
|---|---|---|---|---|---|
| F1 | 100 | 200 | 200 | 52 ± 1.4 | 1.5 ± 0.08 |
| F2 | 200 | 100 | 200 | 55 ± 1.3 | 2.3 ± 0.05 |
| F3 | 100 | 100 | 200 | 49 ± 0.9 | 3.2 ± 0.09 |
| F4 | 200 | 200 | 200 | 59 ± 1.2 | 4.1 ± 0.03 |
| F5 | 300 | 100 | 200 | 62 ± 0.87 | 5.7 ± 1.1 |
| F6 | 100 | 300 | 200 | 65 ± 1.5 | 5.9 ± 0.9 |
| F7 | 300 | 300 | 200 | 79.8 ± 1.9 | 6.78 ± 0.05 |
| Characterization Property | Value |
|---|---|
| Particle size, nm | 452.4 ± 33.19 |
| Polydispersity index | 0.500 ± 0.12 |
| Zeta potential, mV | −38.5 ± 1.4 |
| Encapsulation efficiency, % | 79.8 ± 1.9 |
| Loading capacity, % | 6.78 ± 0.05 |
| Storage Temperature | 4 °C | At Room Temperature (25 °C) |
|---|---|---|
| Size (nm) | 580.2 ± 10.25 | 598.9 ± 12.12 |
| PDI | 0.519 ± 0.14 | 0.580 ± 0.08 |
| Zeta potential (mV) | −32.6 ± 0.09 | −30.6 ± 0.34 |
| EE% | 75.87 ± 1.3 | 73.2 ± 1.5 |
| Parameter | Value |
|---|---|
| λmax | 210 nm |
| Linearity range (µgmL−1) | 5.0–25.0 |
| LOD (µgmL−1) | 0.439 |
| LOQ (µgmL−1) | 1.33 |
| R2 | 0.9998 |
| Regression Equation | A* = bx** + a |
| Slope (×106) ± SD | 585.03 ± 0.022 |
| Intercept (×106) ± SD | 77.83 ± 0.047 |
| HPLC Parameter | GSH | Acceptable Limits |
|---|---|---|
| Asymmetry factor | 0.933 | >1.5 |
| Theoretical plates/m | 3911 | <200 |
| Tailing factor | 1.23 | >2.0 |
| HETP (cm) | 0.0038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.M.; Mohammed, H.A.; Faris, T.M.; Hassan, A.S.; Mohamed, H.B.; El Dosoky, M.I.; Aboubakr, E.M. Nano-Structured Lipid Carrier-Based Oral Glutathione Formulation Mediates Renoprotection against Cyclophosphamide-Induced Nephrotoxicity, and Improves Oral Bioavailability of Glutathione Confirmed through RP-HPLC Micellar Liquid Chromatography. Molecules 2021, 26, 7491. https://doi.org/10.3390/molecules26247491
Ahmad AM, Mohammed HA, Faris TM, Hassan AS, Mohamed HB, El Dosoky MI, Aboubakr EM. Nano-Structured Lipid Carrier-Based Oral Glutathione Formulation Mediates Renoprotection against Cyclophosphamide-Induced Nephrotoxicity, and Improves Oral Bioavailability of Glutathione Confirmed through RP-HPLC Micellar Liquid Chromatography. Molecules. 2021; 26(24):7491. https://doi.org/10.3390/molecules26247491
Chicago/Turabian StyleAhmad, Adel M., Hamdoon A. Mohammed, Tarek M. Faris, Abeer S. Hassan, Hebatallah B. Mohamed, Mahmoud I. El Dosoky, and Esam M. Aboubakr. 2021. "Nano-Structured Lipid Carrier-Based Oral Glutathione Formulation Mediates Renoprotection against Cyclophosphamide-Induced Nephrotoxicity, and Improves Oral Bioavailability of Glutathione Confirmed through RP-HPLC Micellar Liquid Chromatography" Molecules 26, no. 24: 7491. https://doi.org/10.3390/molecules26247491
APA StyleAhmad, A. M., Mohammed, H. A., Faris, T. M., Hassan, A. S., Mohamed, H. B., El Dosoky, M. I., & Aboubakr, E. M. (2021). Nano-Structured Lipid Carrier-Based Oral Glutathione Formulation Mediates Renoprotection against Cyclophosphamide-Induced Nephrotoxicity, and Improves Oral Bioavailability of Glutathione Confirmed through RP-HPLC Micellar Liquid Chromatography. Molecules, 26(24), 7491. https://doi.org/10.3390/molecules26247491







