Assessment of Platelet Reactivity and Inflammatory Markers in Coronary Artery Bypass Graft Patients Treated with Acetylsalicylic Acid with Flavonoid Supplementation
Abstract
1. Introduction
2. Results
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Subjects
4.2. Blood Collection and Laboratory Analyses
4.3. Determination of Platelet Aggregation and Platelet Counts
4.4. Determination of Interleukin-6, C-Reactive Protein, and Fibrinogen Levels
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gasparyan, A.Y.; Watson, T.; Lip, G.Y.H. The role of aspirin in cardiovascular prevention: Implications of aspirin resistance. J. Am. Coll. Cardiol. 2008, 51, 1829–1843. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Silva, R.F.; Silva, D.J.; de Lima, V.C. Aspirin resistance: Fact or fiction? Arq. Bras. Cardiol. 2010, 95, e91–e94. [Google Scholar] [CrossRef]
- Sibbing, D.; Byrne, R.A.; Bernlochner, I.; Kastrati, A. High platelet reactivity and clinical outcome—Fact and fiction. Thromb. Haemost. 2011, 106, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Amsallem, M.; Silberman, S.M.; Dilinger, J.G.; Sideris, G.; Voicu, S.; dit Sollier, C.B.; Drouet, L.; Henry, P. Predictors of high on aspirin platelet reactivity in high vascular patients treated with single or dual antiplatelet therapy. Am. J. Cardiol. 2015, 115, 1305–1310. [Google Scholar] [CrossRef]
- Nappi, J. Benefits and limitations of current antiplatelet therapies. Am. J. Health-Syst. Pharm. 2008, 65, S5–S10. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.; Nielsen, S.J.; Bergfeldt, L.; Ahlsson, A.; Friberg, L.; Björck, S.; Franzén, S.; Jeppsson, A. New-onset atrial fibrillation after coronary artery bypass grafting ang long-term outcome: A population-based nationwide study from the SWEDEHEART registry. J. Am. Heart Assoc. 2021, 10, 017966. [Google Scholar] [CrossRef] [PubMed]
- Filardo, G.; Damiano, R.J.; Ailawadi, G.; Thourani, V.H.; Pollock, B.D.; Sass, D.M.; Phan, T.K.; Nguyen, H.; Da Graca, B. Epidemiology of new-onset atrial fibrillation following coronary artery bypass graft surgery. Heart 2018, 104, 985–992. [Google Scholar] [CrossRef]
- Deshpande, S.; Wann, L.S. Aspirin in atrial fibrillation: The clot thickens. J. Am. Coll. Cardiol. 2016, 67, 2924–2926. [Google Scholar] [CrossRef]
- Kopjar, T.; Petricevic, M.; Gasparovic, H.; Svetina, L.; Milicic, D.; Biocina, B. Postoperative atrial fibrillation is associated with high on-aspirin platelet reactivity. Ann. Thorac. Surg. 2015, 100, 1704–1711. [Google Scholar] [CrossRef]
- Choundhury, A.; Chung, I.; Blann, A.D.; Lip, G.Y.H. Platelet surface CD62P and CD63, mean platelet volume, and soluble/platelet P-selectin as indixes of platelet function in atrial fibrillation: A comparison of healthy control subjects and disease control subjects in sinus rhythm. J. Am. Coll. Cardiol. 2007, 49, 1957–1964. [Google Scholar] [CrossRef]
- Connolly, S.J.; Pogue, J.; Hart, R.G.; Hohnloser, S.H.; Pfeffer, M.; Chrolavicius, S.; Yusuf, S. Effect of clopoidogrel added to aspirin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 360, 2066–2078. [Google Scholar] [CrossRef]
- Zaragozá, C.; Monserrat, J.; Mantecón, C.; Villaescusa, L.; Álvarez-Mon, M.Á.; Zaragozá, F.; Álvarez-Mon, M. Binding and antiplatelet activity of quercetin, rutin, diosmetin, and diosmin flavonoids. Biomed. Pharmacother. 2021, 141, 111867. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Huff, M.W. Antiatherogenic properties of flavonoids: Implications for cardiovascular health. Can. J. Cardiol. 2010, 26, 17A–21A. [Google Scholar] [CrossRef]
- Wright, B.; Spencer, J.P.E.; Lovegrove, J.A.; Gibbins, J.M. Insights into dietary flavonoid as molecular templates for the design of antiplatelet drugs. Cardiovasc. Res. 2013, 97, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Dietary supplements with antiplatelet activity: A solution for everone? Adv. Nutr. 2018, 9, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Vazhappilly, C.G.; Ansari, S.A.; Al-Jaleeli, R.; Al-Azawi, A.M.; Ramadan, W.S.; Menon, V.; Hodeify, R.; Siddiqui, S.S.; Merheb, M.; Matar, R.; et al. Role of flavonoids in thrombotic, cardiovascular, and inflammatory. Inflammopharmacology 2019, 27, 863–869. [Google Scholar] [CrossRef]
- Ciumărnean, L.; Milaciu, M.V.; Runcan, O.; Vesa, Ș.C.; Răchișan, A.L.; Negrean, V.; Perné, M.G.; Donca, V.I.; Alexescu, T.G.; Para, I. The effects of flavonoids in cardiovascular diseases. Molecules 2020, 25, 4320. [Google Scholar] [CrossRef]
- Sirlak, M.A.; Akar, A.R.; Eryilmaz, S.; Cetinkanat, E.K.; Ozcinar, E.; Kaya, B.; Elhan, A.H.; Ozyurda, U. Micronized purified flavonoid fraction in pretreating CABG patients. Tex. Heart Inst. J. 2010, 37, 172–177. [Google Scholar]
- Kumar, R.M.; Van Gompel, J.J.; Bower, R.; Rabinstein, A.A. Spontaneous intraventricular hemorrhage associated with prolonged diosmin therapy. Neurocrit. Care 2011, 14, 438–440. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. The metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef]
- Zaragozá, C.; Villaescusa, L.; Monserrat, J.; Zaragozá, F.; Álvarez-Mon, M. Potential therapeutic anti-inflammatory and immunomodulatory effects of dihydroflavones, flavones, and flavonols. Molecules 2020, 25, 1017. [Google Scholar] [CrossRef]
- Feldo, M.; Wójcik-Kosior, M.; Sowa, I.; Kocki, J.; Bogucki, J.; Zubilewicz, T.; Kęsik, J.; Bogucka-Kocka, A. Effect of diosmin administration in patients with chronic venous disorders on selected factors affecting angiogenesis. Molecules 2019, 24, 3316. [Google Scholar] [CrossRef] [PubMed]
- Casili, G.; Lanza, M.; Campolo, M.; Messina, S.; Scuderi, S.; Ardizzone, A.; Filippone, A.; Paterniti, I.; Cuzzocrea, S.; Esposito, E. Therapeutic potential of flavonoids in the treatment of chronic venous insufficiency. Vascul Pharmacol. 2021, 137, 106825. [Google Scholar] [CrossRef]
- Gonzales-Martinez, S.; Tabuena, N.O.; Baranera, M.M.; Marti-Sauri, I.; Moll, J.L.; García, M.Á.M.; Grau, N.B.; Zurdo, J.M.P. Inflammatory markers as predictors of postoperative adverse outcome in octogenarian surgical patients: An observational prospective study. Cirugía Española 2015, 93, 166–173. [Google Scholar] [CrossRef]
- Paruk, F.; Chausse, J.M. Monitoring the post surgery inflammatory host response. J. Emerg. Crit. Care Med. 2019, 3, 47. [Google Scholar] [CrossRef]
- Straatman, J.; Cuesta, M.A.; Tuynman, J.B.; Veenhof, A.A.; Bemelman, W.A.; van der Peet, D.L. C-reactive protein in predicting major postoperative complications are there differences in open and minimally invasive colorectoal surgery? Substudy from a randomized clinical trial. Surg. Endosc. 2018, 32, 2877–2885. [Google Scholar] [CrossRef]
- Mostowik, M.; Siniarski, A.; Gołębiowska-Wiatrak, R.; Nessler, J.; Gajos, G. Prolonged CRP increase after percutaneous coronary intervention is associated with high thrombin concentrations and low platelet’ response to clopidogrel in patients with stable angina. Adv. Clin. Exp. Med. 2015, 24, 979–985. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Badimon, J.J.; Bhatt, D.L.; Herbert, J.M.; Lüscher, T. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic disease. Vasc. Med. 2007, 12, 113–122. [Google Scholar] [CrossRef]
- Graff, J.; Harder, S.; Wahl, O.; Scheuermann, E.H.; Gossmann, J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: Effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin. Pharmacol. Ther. 2005, 78, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Muhlestein, J.B. Effect of antiplatelet therapy on inflammatory markers in atherothrombotic patients. Thromb. Haemost. 2010, 103, 71–82. [Google Scholar] [CrossRef]
- Kosiorowska, K.; Łukaszewski, M.; Jakubaszko, J.; Kościelska-Kasprzak, K.; Bielicki, G.; Gozdzik, W.; Jasinski, M. Platelets function assessment in patients qualified for cardiac surgery—Clinical problems and newer diagnostic possibilities. J. Cardiothorac. Surg. 2018, 13, 131. [Google Scholar] [CrossRef]
- Bojić, M.; Maleš, Ž.; Antolić, A.; Babić, I.; Tomičić, M. Antithrombotic activity of flavonoids and polyphenols rich plant species. Acta Pharm. 2019, 69, 483–495. [Google Scholar] [CrossRef]
- Nignpense, B.E.; Chinkwo, K.A.; Blanchard, C.L.; Santhakumar, A.B. Polyphenols: Modulators of platelet function and platelet microparticle generation? Int. J. Mol. Sci. 2019, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Bijak, M.; Dziedzic, A.; Saluk-Bijak, J.S. Flavonolignans reduce the response of blood platelet to collagen. Int. J. Biol. Macromol. 2018, 106, 878–884. [Google Scholar] [CrossRef]
- Lösche, W.; Boettel, J.; Kabisch, B.; Winning, J.; Claus, R.A.; Bauer, M. Do aspirin and other antiplatelet drugs reduce the mortality in critically III patients? Thrombosis 2012, 2012, 720254. [Google Scholar] [CrossRef]
- Jylhä, M.; Paavilainen, P.; Lehtimäki, T.; Goebeler, S.; Karhunen, P.J.; Hervonen, A.; Hurme, M. Interleukin-1 receptor antagonist, interleukin-6 and C-reactive protein as predictors of mortality in nonagenarians: The vitality 90+ study. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Henry, O.F.; Blacher, J.; Verdavaine, J.; Duviquet, M.; Safar, M.E. Alpha 1-acid glycoprotein is an independent predictor of in-hospital death in the elderly. Age Ageing 2003, 32, 37–42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rønning, B.; Wyller, T.B.; Seljeflot, I.; Jordhøy, M.S.; Skovlund, E.; Nesbakken, A.; Kristjansson, S.R. Frailty measeres, inflammatory biomarkers and postoperative complications in older surgical patients. Age Ageing 2010, 39, 758–761. [Google Scholar] [CrossRef]
- Schrör, K.; Rauch, B.H. Aspirin and lipid mediators in the cardiovascular system. Prostaglandins Other Lipid Mediat. 2015, 121 Pt A, 17–23. [Google Scholar] [CrossRef]
- Lordkipanidze, M.; Pharand, C.; Nguyen, T.A.; Schampaert, E.; Palisaitis, D.A.; Diodati, J.G. Comparison of four tests to assess inhibition of platelet function by clopidogrel in stable coronary artery disease patients. Eur. Heart J. 2008, 29, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.J.; Bhatt, D.L.; Zidar, F.; Vivekananthan, D.; Chew, D.P.; Ellis, S.G.; Plow, E.; Topol, E.J. Effect of clopidogrel pretreatment on inflammatory marker expression in patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 2004, 93, 679–684. [Google Scholar] [CrossRef] [PubMed]
| Values Either Mean ± SD or N/% | Diosmin N = 26 | Placebo N = 27 | p-Values |
|---|---|---|---|
| Age (years) | 61.9 ± 6.4 | 64.7 ± 6.9 | 0.18 |
| BMI (kg/m2) | 29.4 ± 3.1 | 30.5 ± 4.8 | 0.63 |
| GFR (mL/min/1.73 m2) | 87.8 ± 13.9 | 84.2 ± 14.1 | 0.46 |
| HGB (mmol/L) | 8.7 ± 0.67 | 8.9 ± 0.56 | 0.26 |
| CK-MB (U/L) | 21.4 ± 7.7 | 26.0 ± 9.7 | 0.03 |
| D-Dimers (ng/mL) | 552 ± 472 | 707 ± 760 | 0.31 |
| Gender, male | 22/84.6 | 23/85.2 | 0.95 |
| Hypertension | 21/80.8 | 19/70.4 | 0.37 |
| Diabetes | 7/26.9 | 3/11.1 | 0.14 |
| Hyperlipidemia | 7/26.9 | 3/11.1 | 0.14 |
| Cerebrovascular accidents | 2/7.7 | 0/0.0 | 0.14 |
| Atrial fibrillation | 4/15.4 | 6/22.2 | 0.52 |
| Lower extremity peripheral artery disease | 1/3.8 | 2/7.4 | 0.57 |
| Parameter | Diosmin before Surgery (Mean ± SD) | Diosmin after Surgery (Mean ± SD) | p-Value |
|---|---|---|---|
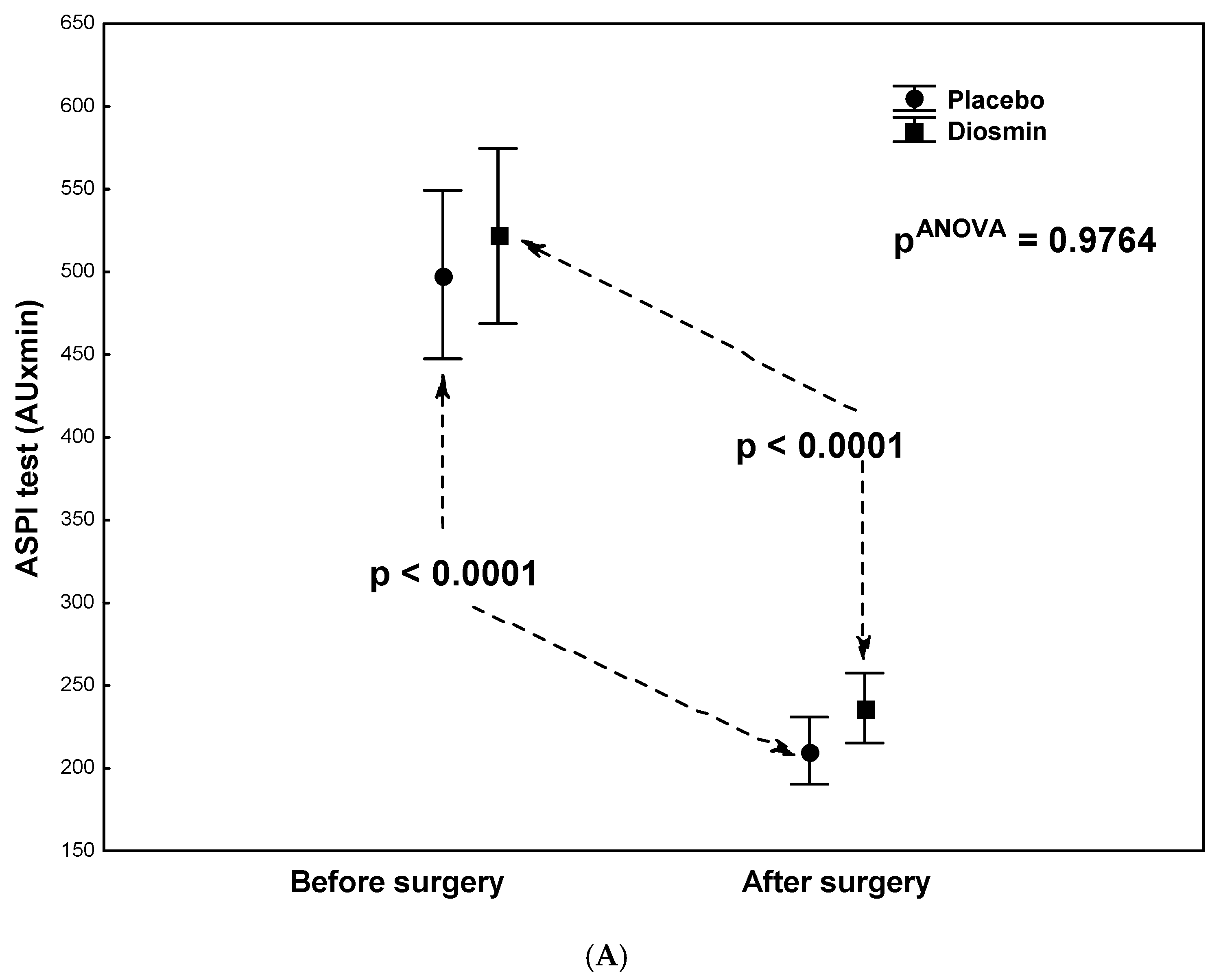
| ASPI test (AU × min) | 521 ± 255 | 236 ± 120 | <0.0001 |
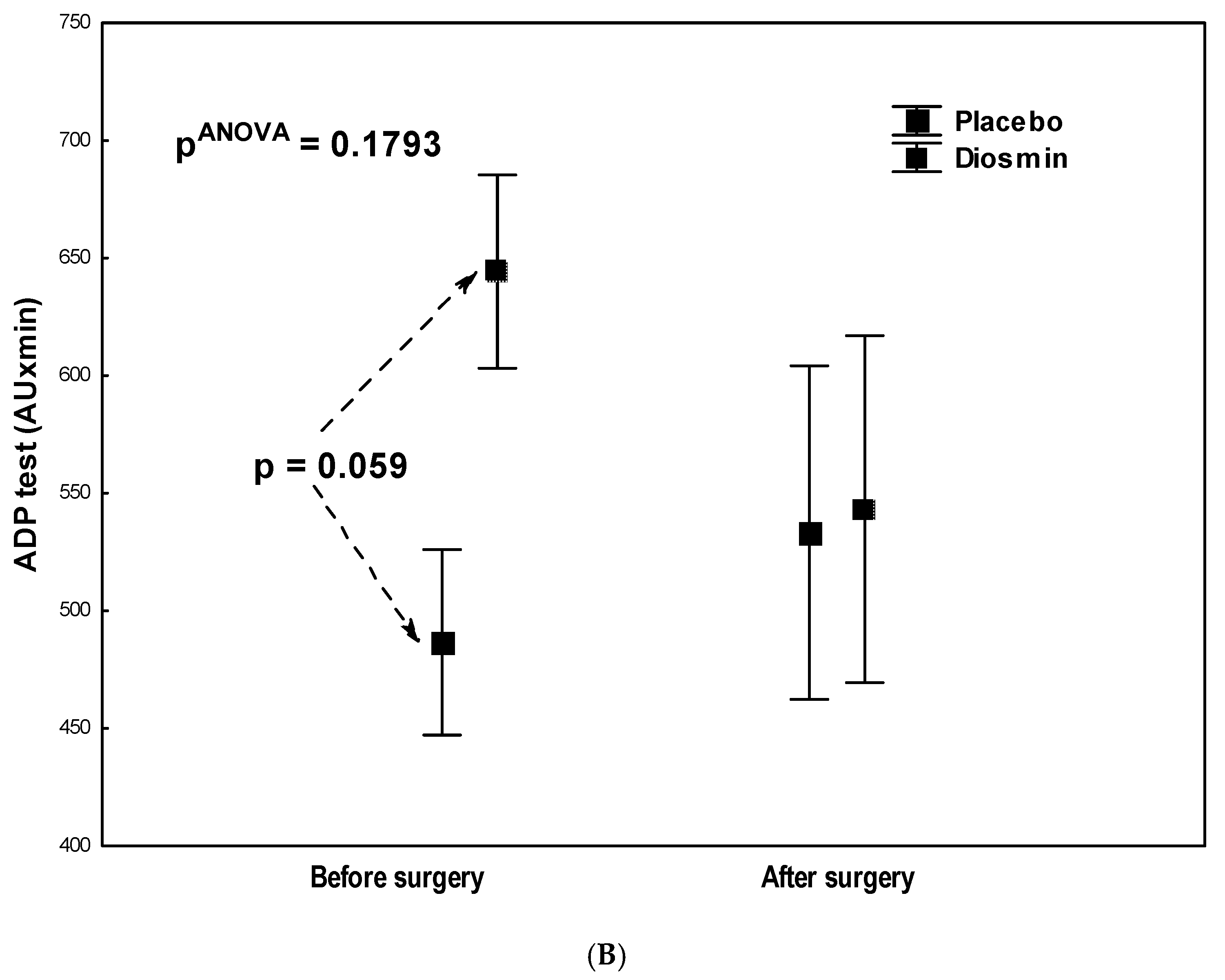
| ADP test (AU × min) | 644 ± 187 | 543 ± 368 | 0.1868 |
| PLT (G/L) | 240 ± 72 | 259 ± 82 * | 0.3709 |
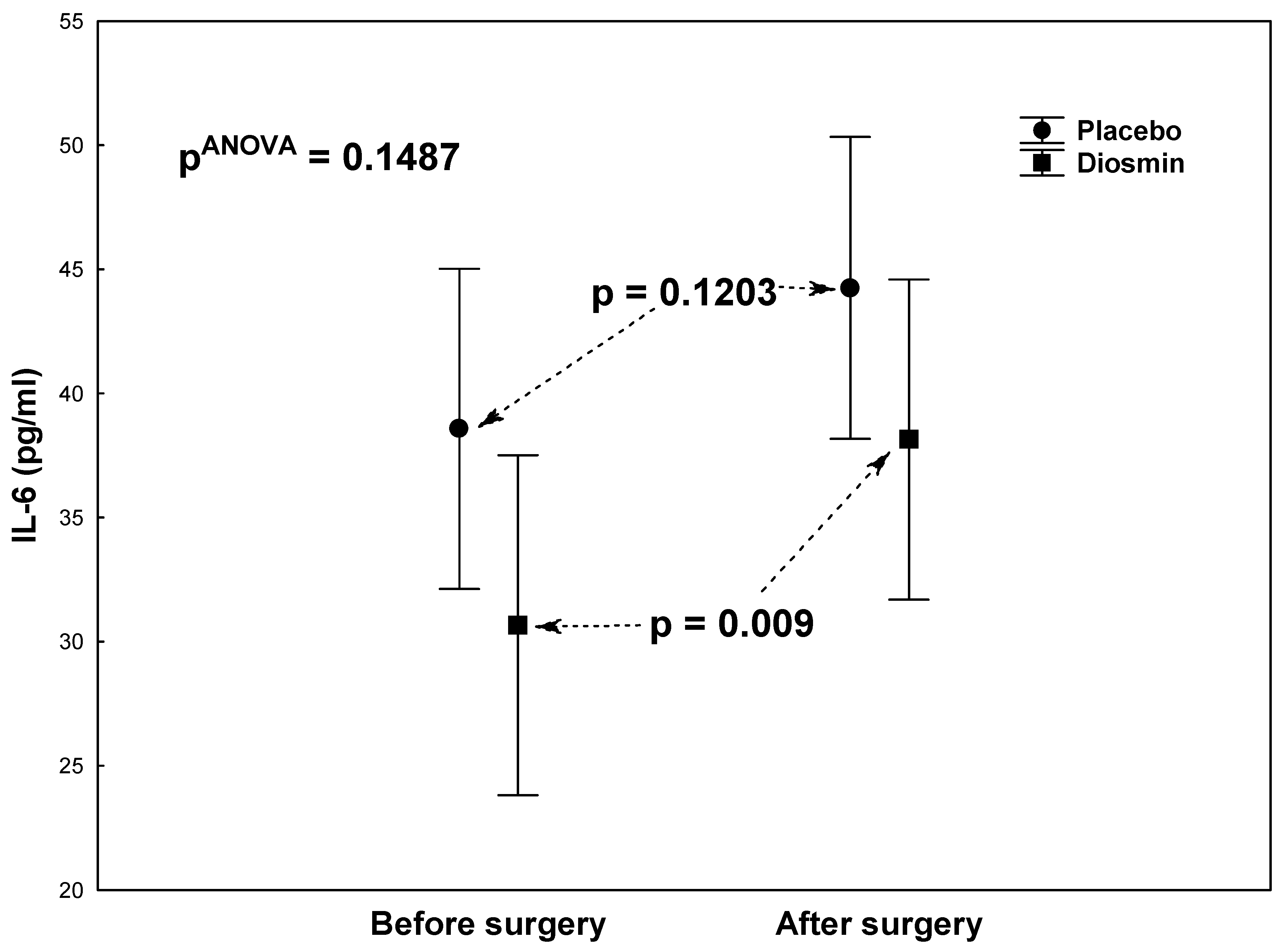
| IL-6 (pg/mL) | 30.7 ± 28.0 | 38.1 ± 25.4 | 0.0092 |
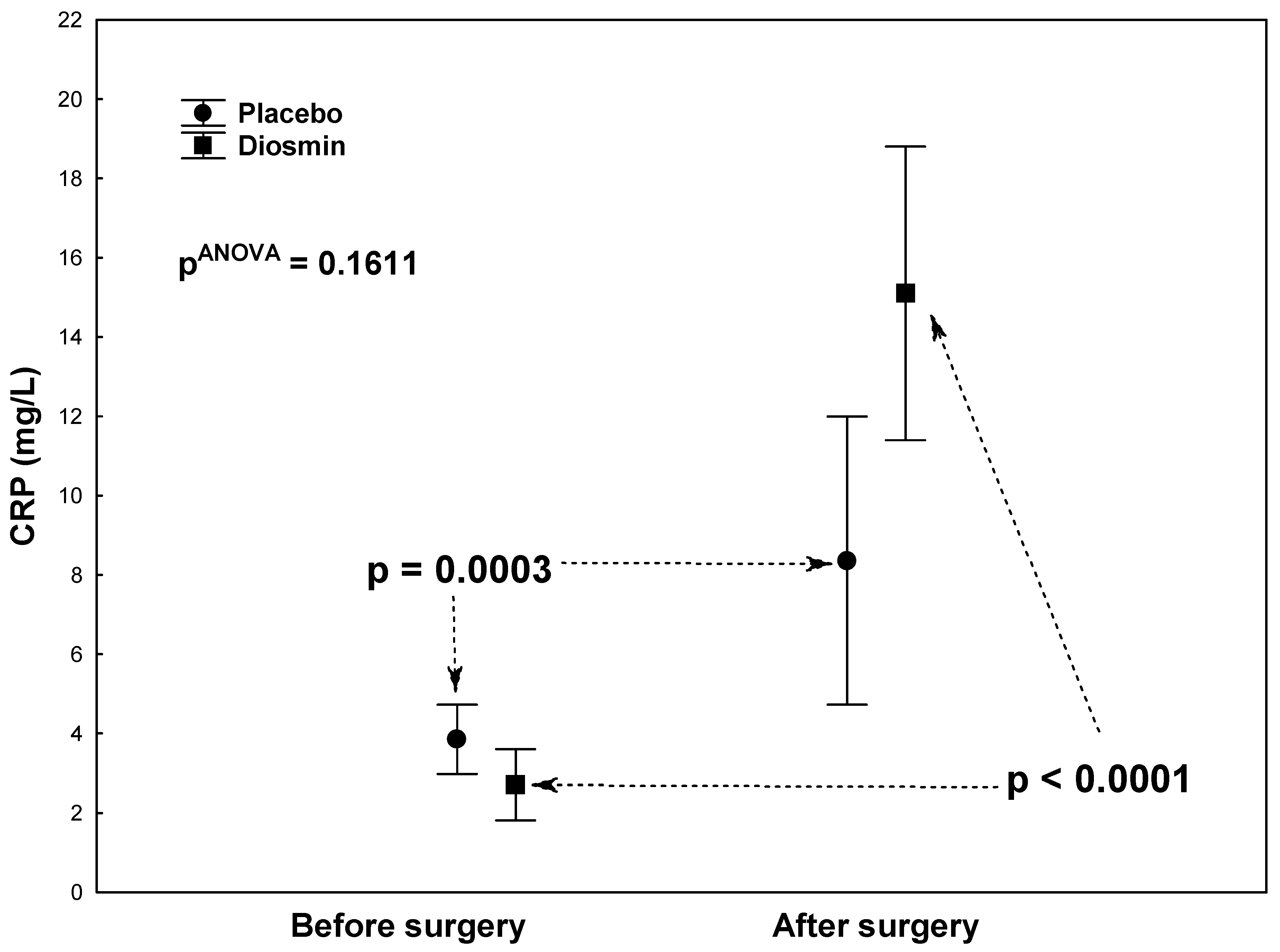
| CRP (mg/L) | 2.7 ± 3.8 | 15.1 ± 25.3 | <0.0001 |
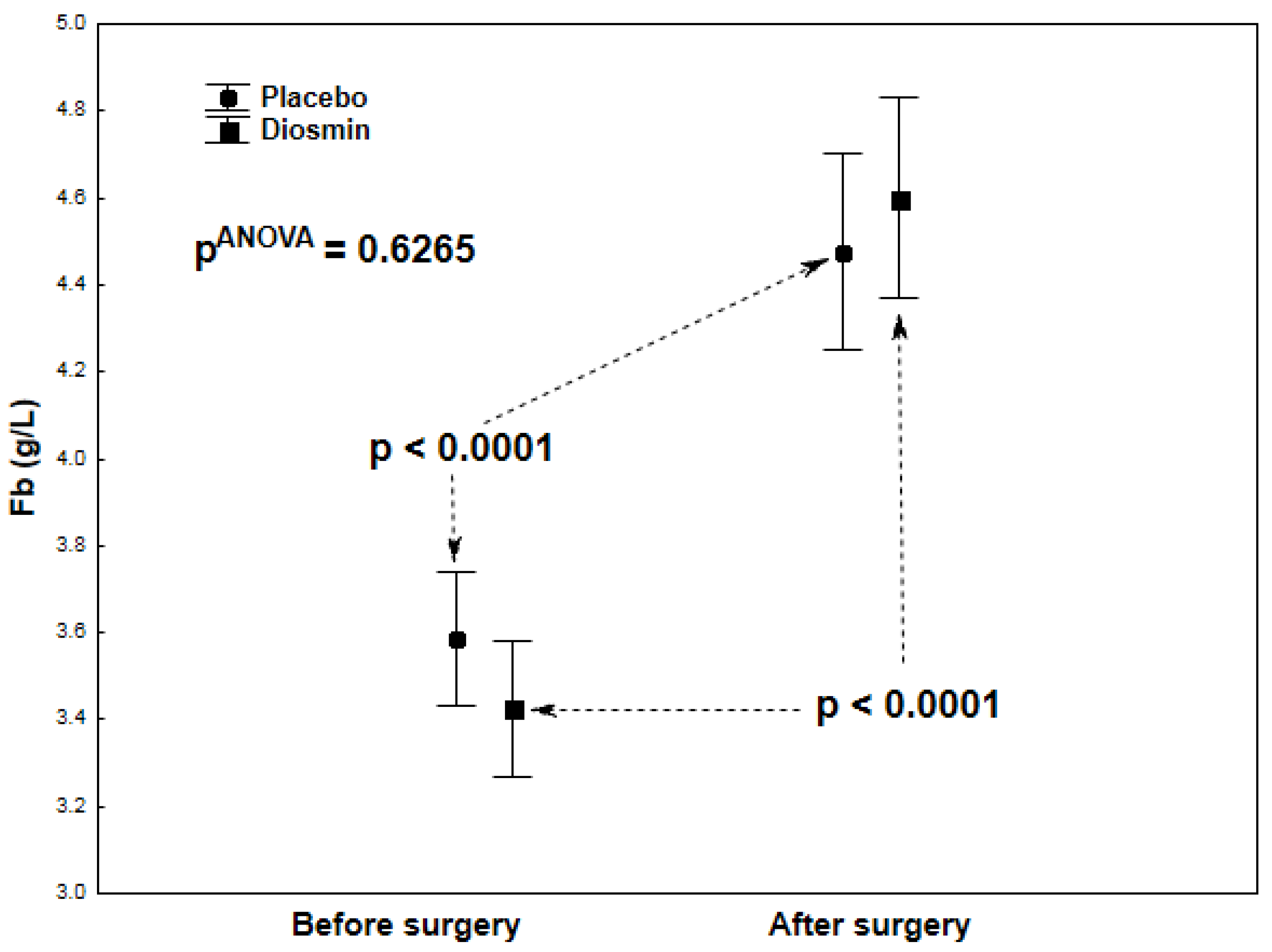
| Fb (g/L) | 3.43 ± 0.66 | 4.61 ± 1.49 | <0.0001 |
| Parameter | Placebo before Surgery (Mean ± SD) | Placebo after Surgery (Mean ± SD) | p-Value |
|---|---|---|---|
| ASPI test (AU × min) | 498 ± 263 | 210 ± 85 | <0.0001 |
| ADP test (AU × min) | 486 ± 212 | 533 ± 355 | 0.6184 |
| PLT (G/L) | 219 ± 64 | 255 ± 126 * | 0.1696 |
| IL-6 (pg/mL) | 38.6 ± 37.8 | 44.3 ± 36.2 | 0.1203 |
| CRP (mg/L) | 3.9 ± 5.2 | 8.4 ± 9.1 | 0.0003 |
| Fb (g/L) | 3.51 ± 0.83 | 4.44 ± 0.73 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siennicka, A.; Kłysz, M.; Adamska, M.; Chełstowski, K.; Biskupski, A.; Jastrzębska, M. Assessment of Platelet Reactivity and Inflammatory Markers in Coronary Artery Bypass Graft Patients Treated with Acetylsalicylic Acid with Flavonoid Supplementation. Molecules 2021, 26, 7486. https://doi.org/10.3390/molecules26247486
Siennicka A, Kłysz M, Adamska M, Chełstowski K, Biskupski A, Jastrzębska M. Assessment of Platelet Reactivity and Inflammatory Markers in Coronary Artery Bypass Graft Patients Treated with Acetylsalicylic Acid with Flavonoid Supplementation. Molecules. 2021; 26(24):7486. https://doi.org/10.3390/molecules26247486
Chicago/Turabian StyleSiennicka, Aldona, Magdalena Kłysz, Monika Adamska, Kornel Chełstowski, Andrzej Biskupski, and Maria Jastrzębska. 2021. "Assessment of Platelet Reactivity and Inflammatory Markers in Coronary Artery Bypass Graft Patients Treated with Acetylsalicylic Acid with Flavonoid Supplementation" Molecules 26, no. 24: 7486. https://doi.org/10.3390/molecules26247486
APA StyleSiennicka, A., Kłysz, M., Adamska, M., Chełstowski, K., Biskupski, A., & Jastrzębska, M. (2021). Assessment of Platelet Reactivity and Inflammatory Markers in Coronary Artery Bypass Graft Patients Treated with Acetylsalicylic Acid with Flavonoid Supplementation. Molecules, 26(24), 7486. https://doi.org/10.3390/molecules26247486






