Encapsulation of Metronidazole in Biocompatible Macrocycles and Structural Characterization of Its Nano Spray-Dried Nanostructured Composite
Abstract
:1. Introduction
2. Results and Discussion
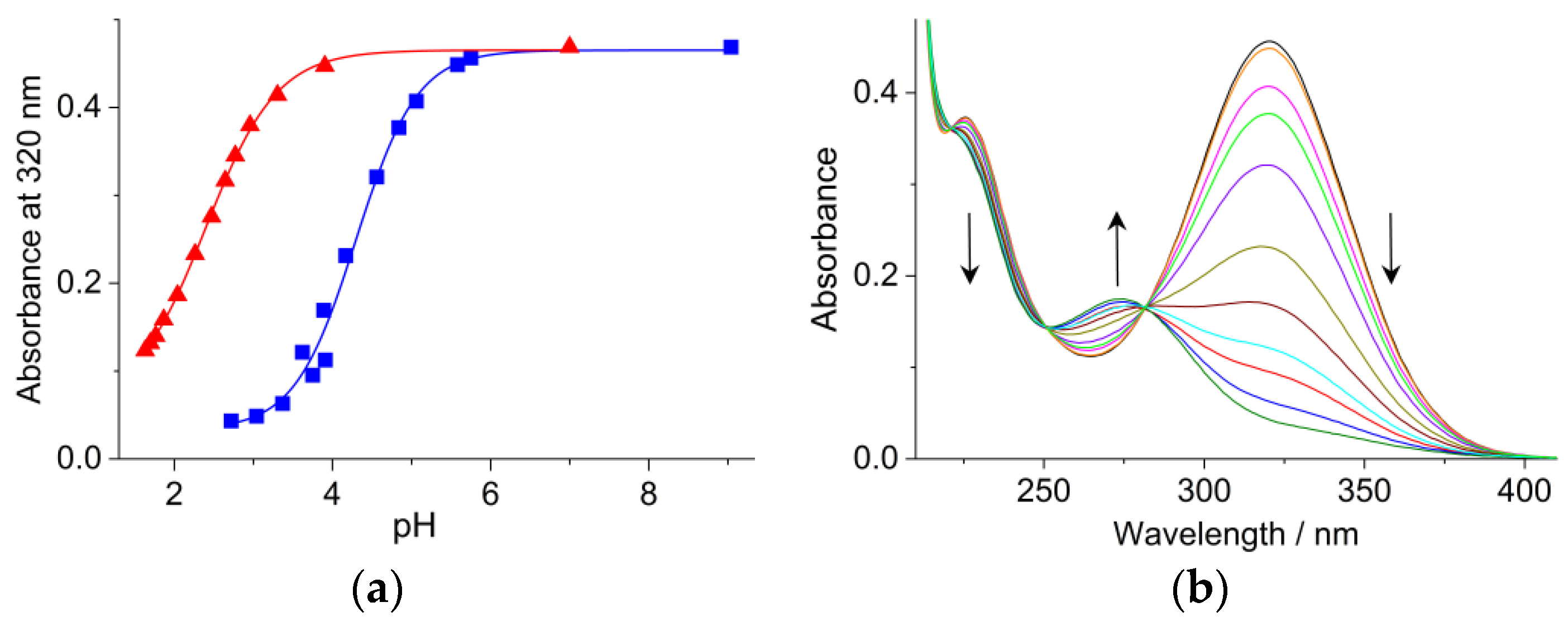
2.1. Effect of CB7 on the pH Dependence of the Absorption Spectra
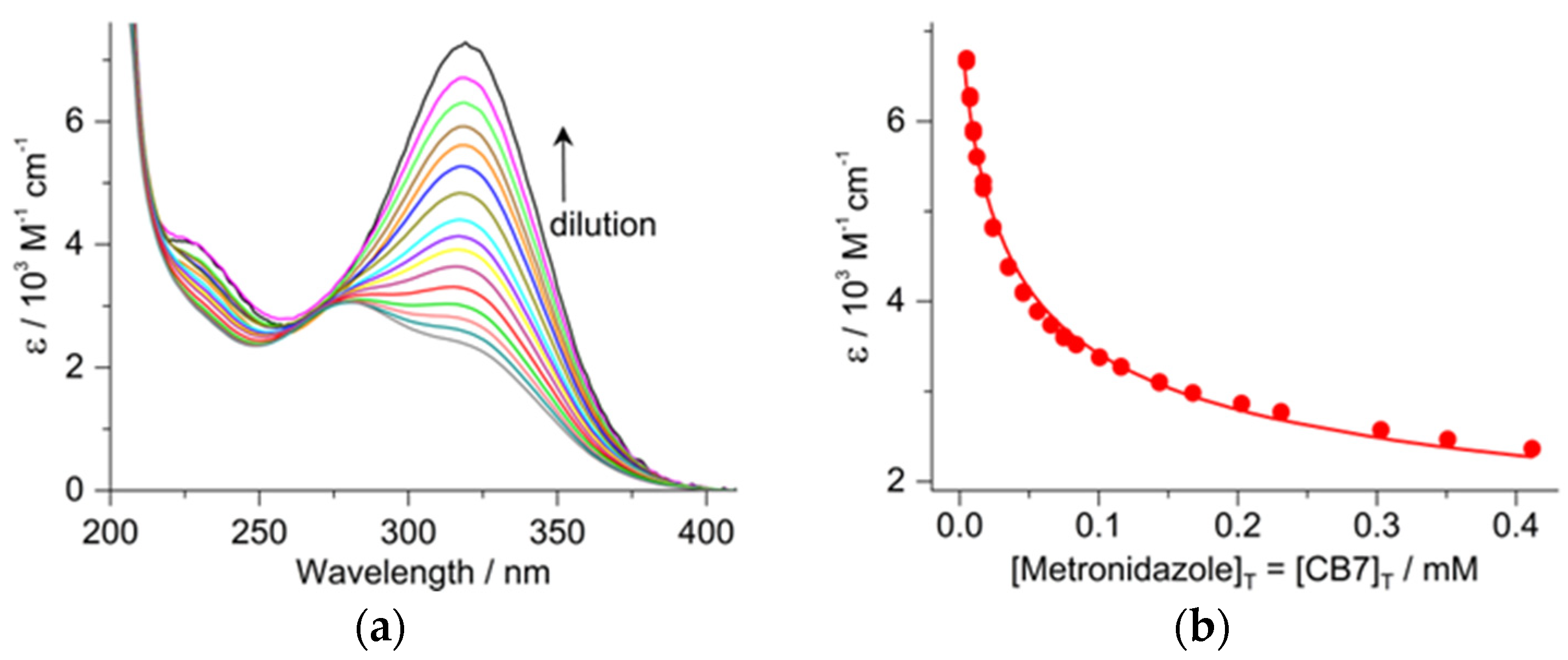
2.2. Determination of the Binding Constant of Inclusion in CB7
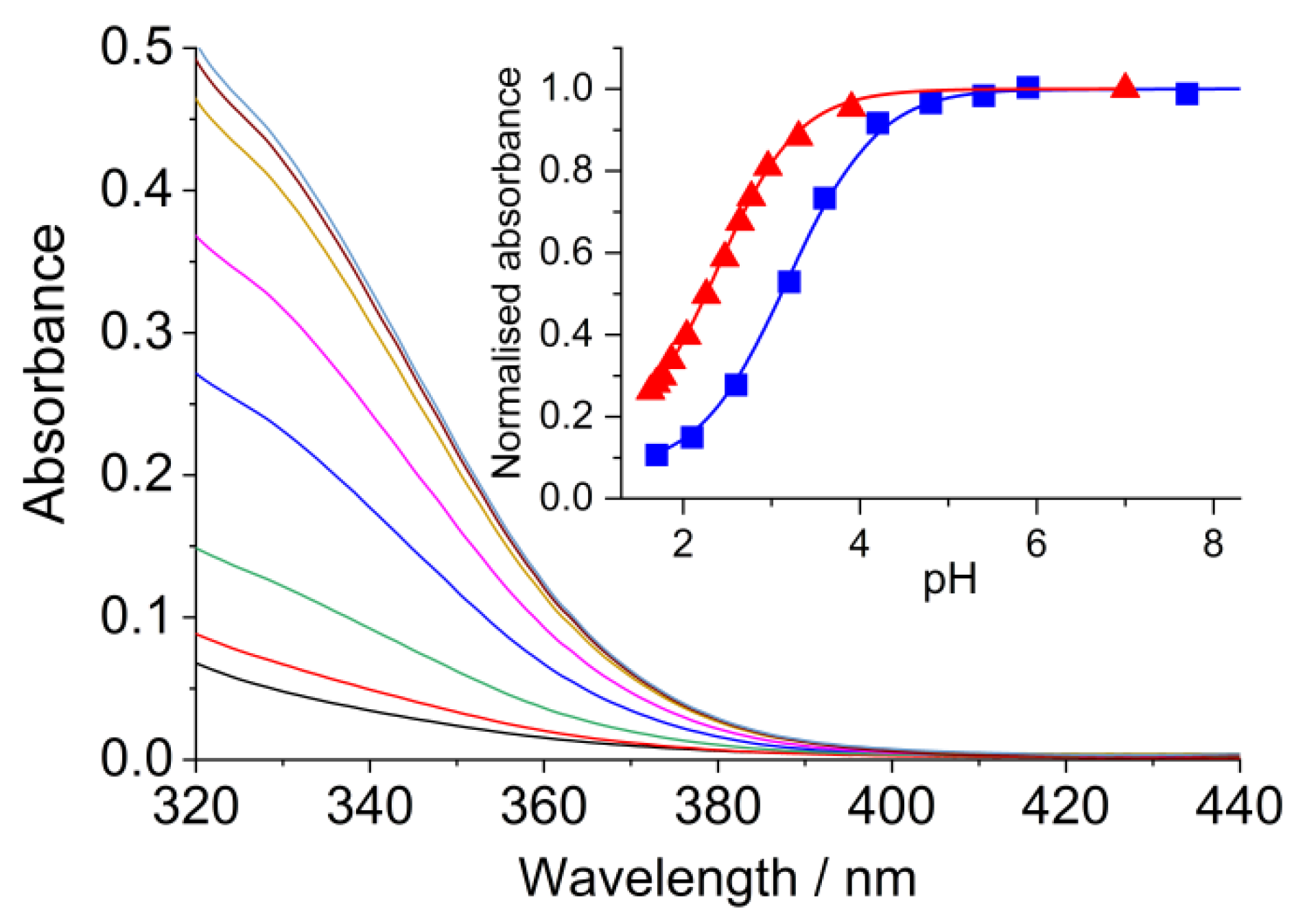
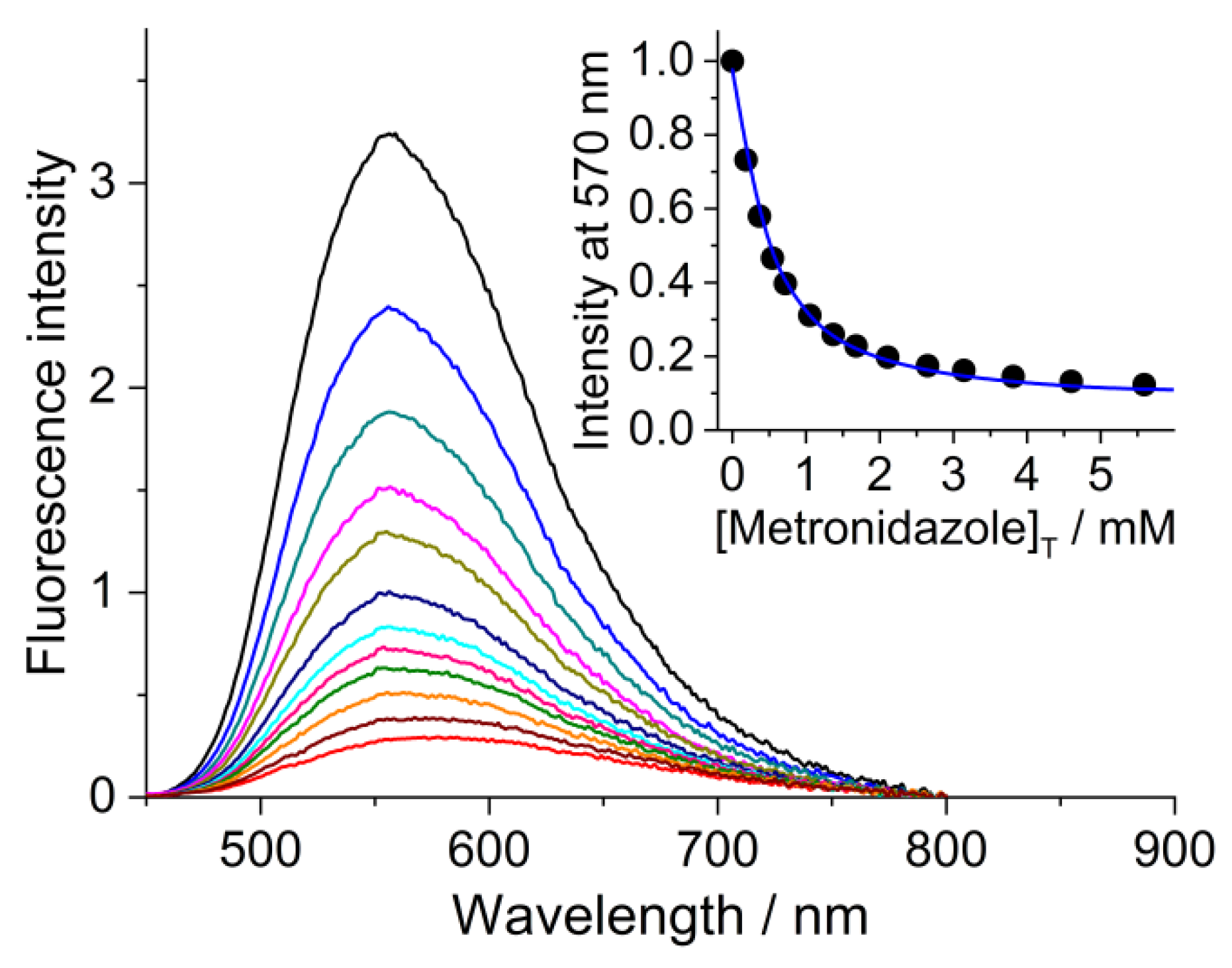
2.3. Association with SCX4
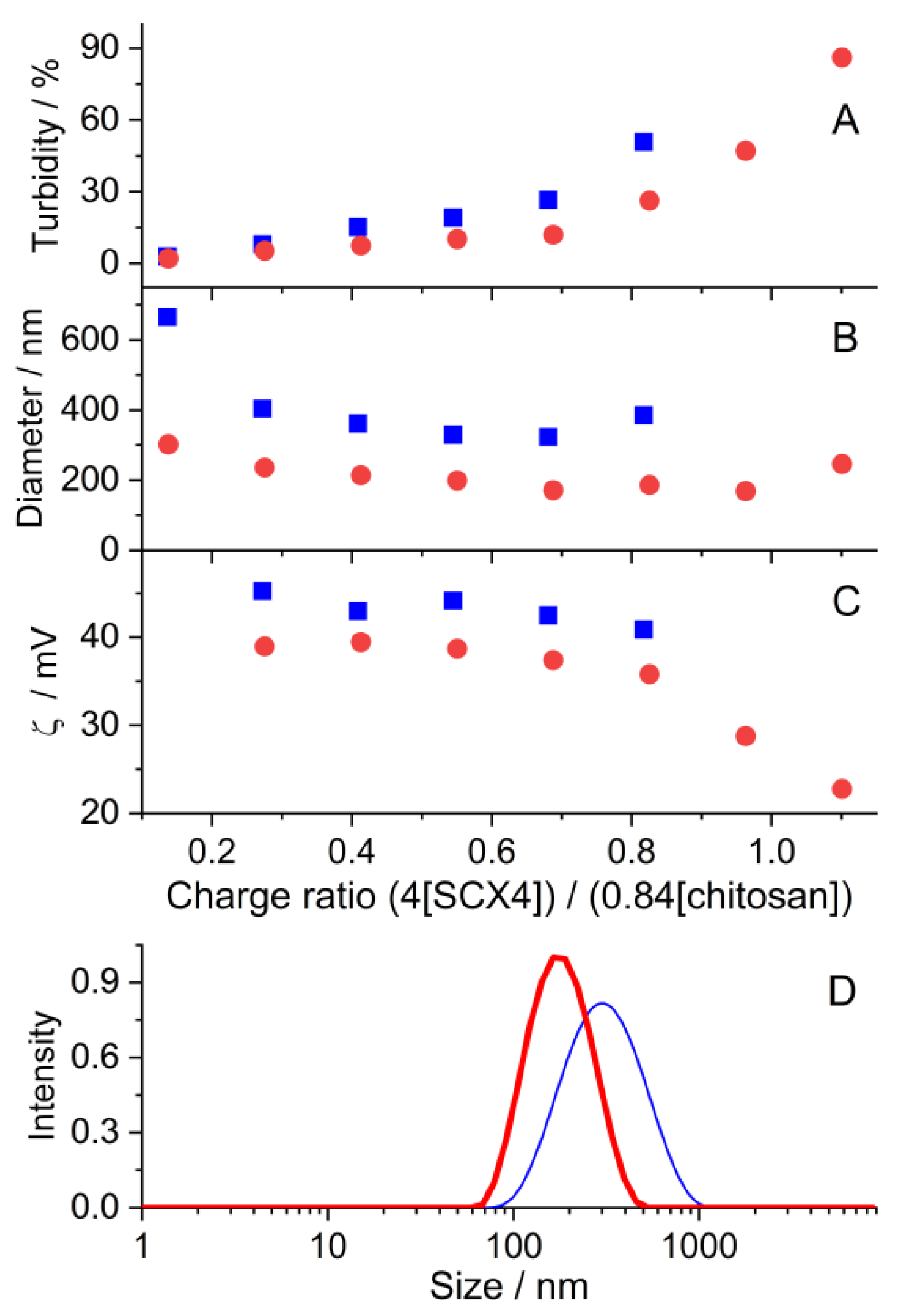
2.4. Nanoparticle Formation of Chitosan Induced by the MetH+-SCX4 Complex
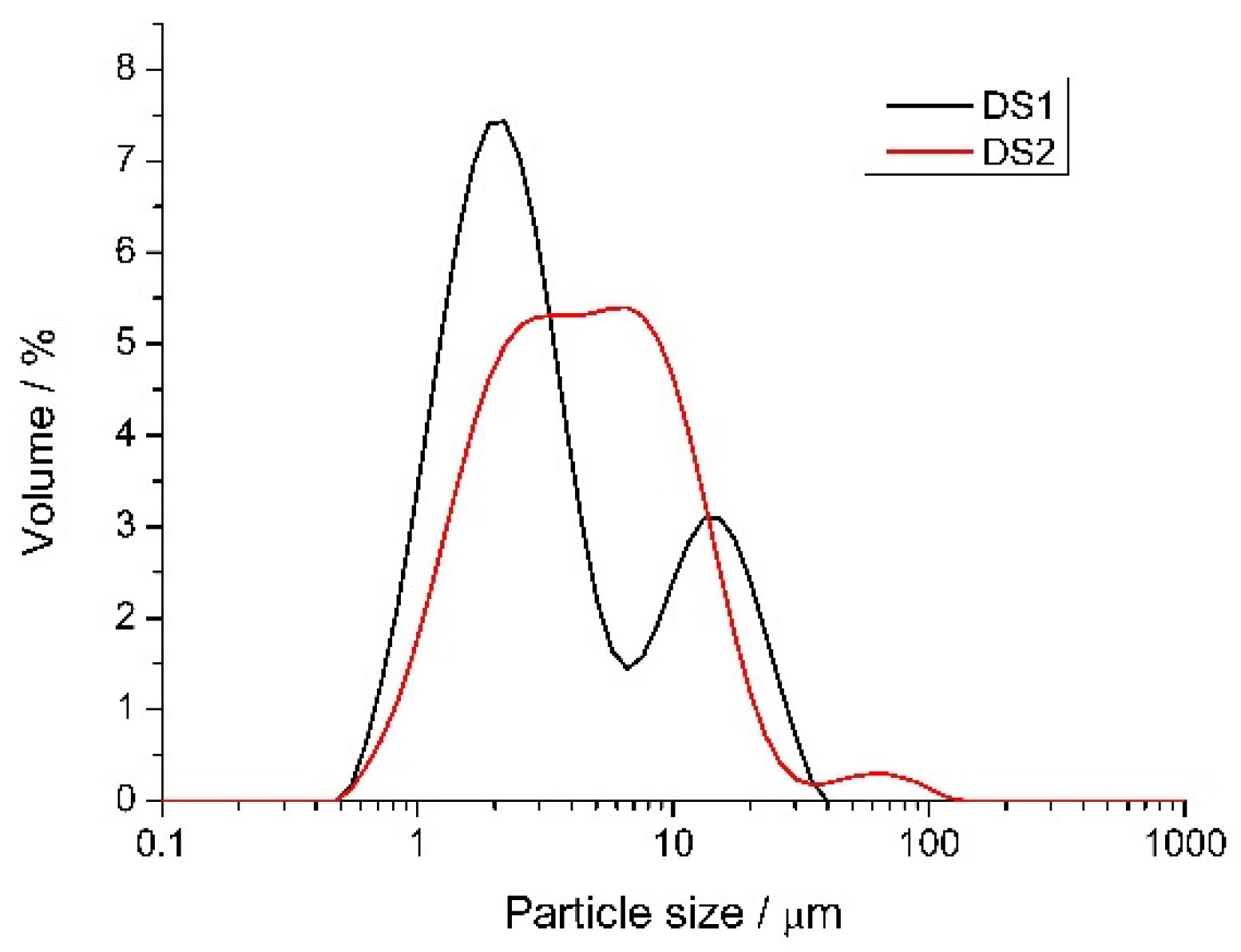
2.5. The Effect of Spray Drying the Nanoparticle Solutions
3. Materials and Methods
3.1. Nano Spray Drying Experiment
- -
- S1: 0.750 m/m% HPMC polymer was dissolved in a suspension of 0.352 m/m% Met, 0.030 m/m% SCX4 and 0.034 m/m% chitosan prepared in 0.01 M HCl (CMet,i. = 30.19 m/m%, where CMet,i. is the Met concentration in the nanosuspension).
- -
- S2: 0.750 m/m% HPMC polymer was dissolved in solutions of 0.352 m/m% Met prepared in 0.01 M HCl, (CMet,i. = 31.94 m/m%).
- -
- S3: 0.750 m/m% HPMC was dissolved in 0.01 M HCl solution.
- -
- S4: 0.100 m/m% Met and 0.100 m/m% HPMC were dissolved in 0.01 M HCl solution (CMet,i. = 50.00 m/m%).
3.2. Active Ingredient Content
3.3. Particle Size and Distribution
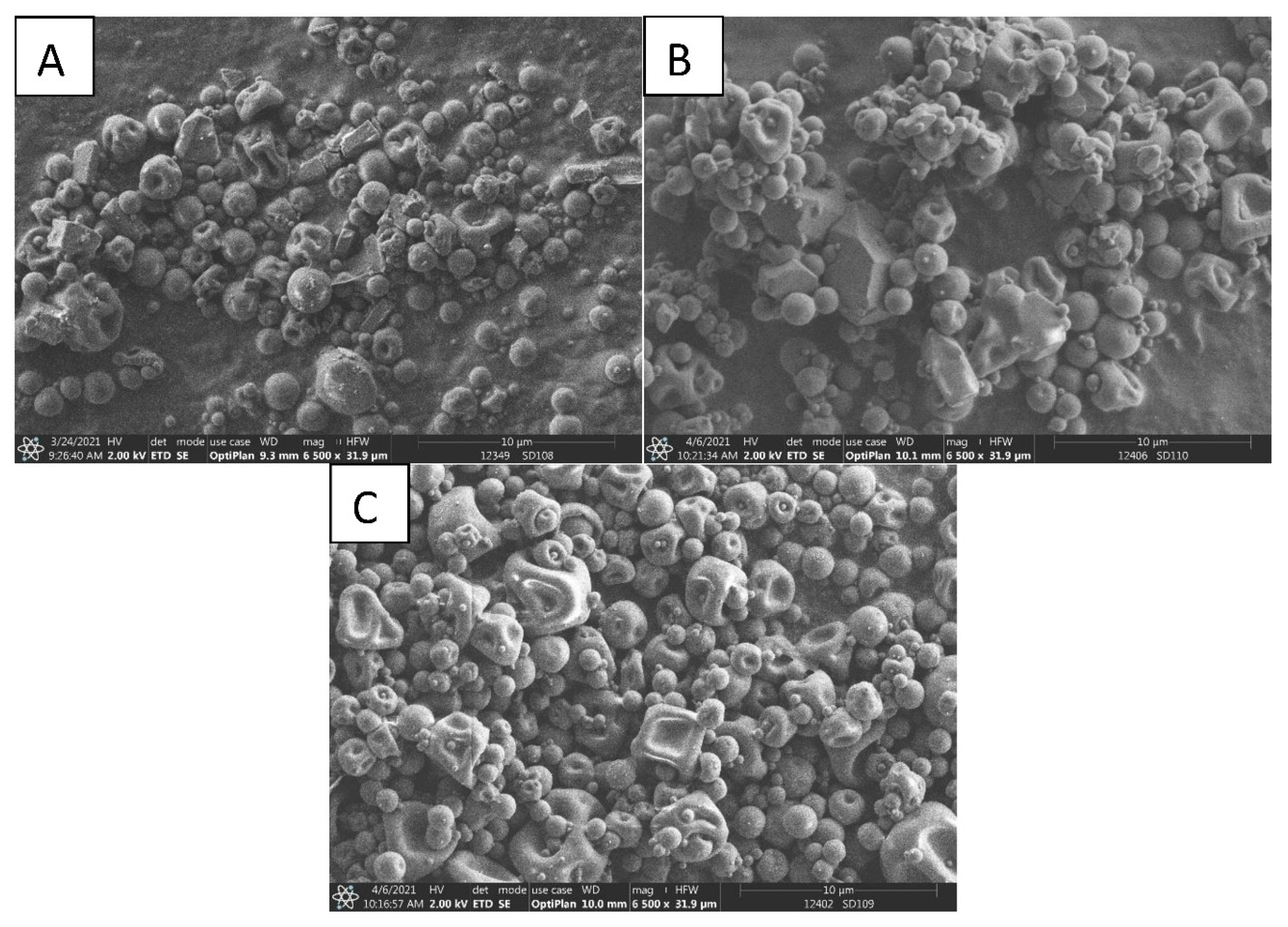
3.4. Morphology
3.5. X-ray Diffraction and FTIR Measurements
3.6. Thermal Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gu, A.; Wheate, N.J. Macrocycles as drug-enhancing excipients in pharmaceutical formulations. J. Incl. Phenom. Macrocycl. Chem. 2021, 100, 55–69. [Google Scholar] [CrossRef]
- Noruzi, E.B.; Molaparast, M.; Zarei, M.; Shaabani, B.; Kariminezhad, Z.; Ebadi, B.; Shafiei-Irannejad, V.; Rahimi, M.; Pietrasik, J. Para-sulfonatocalix[n]arene-based biomaterials: Recent progress in pharmaceutical and biological applications. Eur. J. Med. Chem. 2020, 190, 112121. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Fundamentals and applications of cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Fourmentin, S., Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–55. [Google Scholar]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef] [PubMed]
- Gangemi, C.M.A.; Puglisi, R.; Pappalardo, A.; Trusso Sfrazzetto, G. Supramolecular complexes for nanomedicine. Bioorg. Med. Chem. Lett. 2018, 28, 3290–3301. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, H.; Zheng, B.; Huang, F. Combating antibiotic resistance: Current strategies for the discovery of novel antibacterial materials based on macrocycle supramolecular chemistry. Giant 2021, 7, 100066. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Liu, Y.-H.; Liu, Y. Cyclodextrin-based multistimuli-responsive supramolecular assemblies and their biological functions. Adv. Mater. 2020, 32, 1806158. [Google Scholar] [CrossRef]
- Braegelman, A.S.; Webber, M.J. Integrating stimuli-responsive properties in host-guest supramolecular drug delivery systems. Theranostics 2019, 9, 3017–3040. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, M.; Jonkheijm, P. Stimuli-responsive cucurbit[n]uril-mediated host-guest complexes on surfaces. Isr. J. Chem. 2018, 58, 314–325. [Google Scholar] [CrossRef] [Green Version]
- Hernández Ceruelos, A.; Romero-Quezada, L.C.; Ruvalcaba Ledezma, J.C.; López Contreras, L. Therapeutic uses of metronidazole and its side effects: An update. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 397–401. [Google Scholar]
- Perioli, L.; Ambrogi, V.; Rubini, D.; Giovagnoli, S.; Ricci, M.; Blasi, P.; Rossi, C. Novel mucoadhesive buccal formulation containing metronidazole for the treatment of periodontal disease. J. Control. Release 2004, 95, 521–533. [Google Scholar] [CrossRef]
- Lahiani-Skiba, M.; Bounoure, F.; Shawky-Tous, S.; Arnaud, P.; Skiba, M. Optimization of entrapment of metronidazole in amphiphilic β-cyclodextrin nanospheres. J. Pharm. Biomed. 2006, 41, 1017–1021. [Google Scholar] [CrossRef]
- Pawar, A.P.; Gadhe, A.R.; Venkatachalam, P.; Sher, P.; Mahadik, K.R. Effect of core and surface cross-linking on the entrapment of metronidazole in pectin beads. Acta Pharm. 2008, 58, 78–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stjern, L.; Voittonen, S.; Weldemichel, R.; Thuresson, S.; Agnes, M.; Benkovics, G.; Fenyvesi, É.; Malanga, M.; Yannakopoulou, K.; Feiler, A.; et al. Cyclodextrin-mesoporous silica particle composites for controlled antibiotic release. A proof of concept toward colon targeting. Int. J. Pharm. 2017, 531, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Sreeharsha, N.; Rajpoot, K.; Tekade, M.; Kalyane, D.; Nair, A.B.; Venugopala, K.N.; Tekade, R.K. Development of metronidazole loaded chitosan nanoparticles using QbD approach—A novel and potential antibacterial formulation. Pharmaceutics 2020, 12, 920. [Google Scholar] [CrossRef]
- Binesh, N.; Farhadian, N.; Mohammadzadeh, A. Enhanced antibacterial activity of uniform and stable chitosan nanoparticles containing metronidazole against anaerobic bacterium of Bacteroides fragilis. Colloids Surf. B Biointerfaces 2021, 202, 111691. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Arthur, R.A.; Waeiss, R.A.; Kamocki, K.; Gregson, K.S.; Gregory, R.L. Biodegradable nanofibrous drug delivery systems: Effects of metronidazole and ciprofloxacin on periodontopathogens and commensal oral bacteria. Clin. Oral Investig. 2014, 18, 2151–2158. [Google Scholar] [CrossRef] [Green Version]
- Celebioglu, A.; Uyar, T. Metronidazole/hydroxypropyl-β-cyclodextrin inclusion complex nanofibrous webs as fast-dissolving oral drug delivery system. Int. J. Pharm. 2019, 572, 118828. [Google Scholar] [CrossRef] [PubMed]
- Mirzaeei, S.; Mansurian, M.; Asare-Addo, K.; Nokhodchi, A. Metronidazole- and amoxicillin-loaded PLGA and PCL nanofibers as potential drug delivery systems for the treatment of periodontitis: In Vitro and in vivo evaluations. Biomedicines 2021, 9, 975. [Google Scholar] [CrossRef] [PubMed]
- Mirankó, M.; Trif, L.; Tóth, J.; Feczkó, T. Nanostructured micronized solid dispersion of crystalline-amorphous metronidazole embedded in amorphous polymer matrix prepared by nano spray drying. Adv. Powder Technol. 2021, 32, 2621–2633. [Google Scholar] [CrossRef]
- Bielas, R.; Maksym, P.; Tarnacka, M.; Minecka, A.; Jurkiewicz, K.; Talik, A.; Geppert-Rybczyńska, M.; Grelska, J.; Mielańczyk, Ł.; Bernat, R.; et al. Synthetic strategy matters: The study of a different kind of PVP as micellar vehicles of metronidazole. J. Mol. Liq. 2021, 332, 115789. [Google Scholar] [CrossRef]
- Harangozó, J.G.; Wintgens, V.; Miskolczy, Z.; Amiel, C.; Biczók, L. Nanoparticle formation of chitosan induced by 4-sulfonatocalixarenes: Utilization for alkaloid encapsulation. Colloid. Polym. Sci. 2016, 294, 1807–1814. [Google Scholar] [CrossRef] [Green Version]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef] [Green Version]
- Tolls, J. Sorption of veterinary pharmaceuticals in soils: A review. Environ. Sci. Technol. 2001, 35, 3397–3406. [Google Scholar] [CrossRef]
- Ghosh, I.; Nau, W.M. The strategic use of supramolecular pKa shifts to enhance the bioavailability of drugs. Adv. Drug Deliv. Rev. 2012, 64, 764–783. [Google Scholar] [CrossRef] [PubMed]
- Macartney, D.H. Cucurbit[n]uril host-guest complexes of acids, photoacids, and super photoacids. Isr. J. Chem. 2018, 58, 230–243. [Google Scholar] [CrossRef]
- Khurana, R.; Barooah, N.; Bhasikuttan, A.C.; Mohanty, J. Modulation in the acidity constant of acridine dye with cucurbiturils: Stimuli-responsive pKa tuning and dye relocation into live cells. Org. Biomol. Chem. 2017, 15, 8448–8457. [Google Scholar] [CrossRef] [PubMed]
- Miskolczy, Z.; Megyesi, M.; Toke, O.; Biczók, L. Change of the kinetics of inclusion in cucurbit[7]uril upon hydrogenation and methylation of palmatine. Phys. Chem. Chem. Phys. 2019, 21, 4912–4919. [Google Scholar] [CrossRef] [Green Version]
- Miskolczy, Z.; Biczók, L. Photochromism in cucurbit[8]uril cavity: Inhibition of hydrolysis and modification of the rate of merocyanine-spiropyran transformations. J. Phys. Chem. B 2011, 115, 12577–12583. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Jia, X. Selective binding and highly sensitive fluorescent sensor of palmatine and dehydrocorydaline alkaloids by cucurbit[7]uril. Org. Biomol. Chem. 2009, 7, 2699–2703. [Google Scholar] [CrossRef]
- Miskolczy, Z.; Biczók, L. Inclusion complex formation of ionic liquids with 4-sulfonatocalixarenes studied by competitive binding of berberine alkaloid fluorescent probe. Chem. Phys. Lett. 2009, 477, 80–84. [Google Scholar] [CrossRef]
- Zhang, S.; Grimm, L.; Miskolczy, Z.; Biczók, L.; Biedermann, F.; Nau, W.M. Binding affinities of cucurbit[n]urils towards cations. Chem. Commun. 2019, 55, 14131–14134. [Google Scholar] [CrossRef] [PubMed]
- Montes-Navajas, P.; González-Béjar, M.; Scaiano, J.C.; García, H. Cucurbituril complexes cross the cell membrane. Photochem. Photobiol. Sci. 2009, 8, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Suga, K.; Ohzono, T.; Negishi, M.; Deuchi, K.; Morita, Y. Effect of various cations on the acidity of p-sulfonatocalixarenes. Supramol. Sci. 1998, 5, 9–14. [Google Scholar] [CrossRef]
- Megyesi, M.; Biczók, L. Considerable fluorescence enhancement upon supramolecular complex formation between berberine and p-sulfonated calixarenes. Chem. Phys. Lett. 2006, 424, 71–76. [Google Scholar] [CrossRef]
- Metronidazole. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m53670.html (accessed on 15 November 2021).
- Yang, W.; Manek, R.; Kolling, W.M.; Brits, M.; Liebenberg, W.; De Villiers, M.M. Physicochemical characterization of hydrated 4-sulphonato-calix[n]arenes: Thermal, structural, and sorption properties. Supramol. Chem. 2005, 17, 485–496. [Google Scholar] [CrossRef]
- Blundell, D.J. On the interpretation of multiple melting, peaks in poly(ether ether ketone). Polymer 1987, 28, 2248–2251. [Google Scholar] [CrossRef]
- Perera, G.; Barthelmes, J.; Bernkop-Schnürch, A. Novel pectin–4-aminothiophenole conjugate microparticles for colon-specific drug delivery. J. Control. Release 2010, 145, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Odani, H.; Nakajima, A. Determination of degree of deacetylation of chitosan by 1H NMR spectroscopy. Polym. Bull. 1991, 26, 87–94. [Google Scholar] [CrossRef]
- Arpagaus, C.; John, P.; Collenberg, A.; Rütti, D. Nanocapsules formation by nano spray drying. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017; pp. 346–401. [Google Scholar]
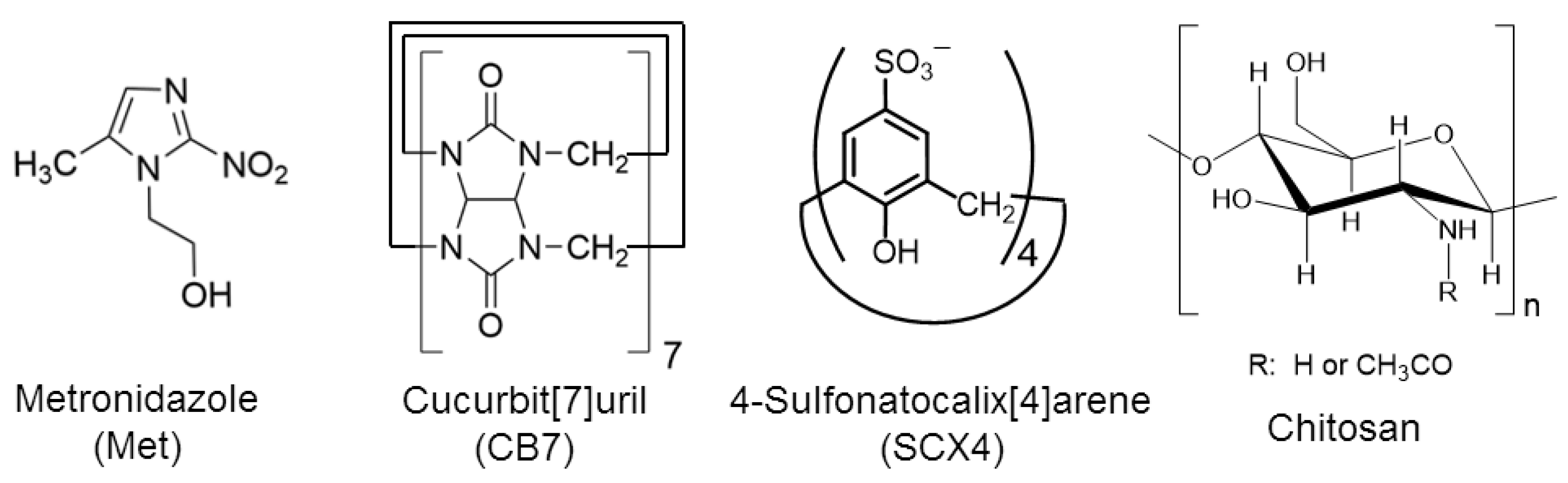
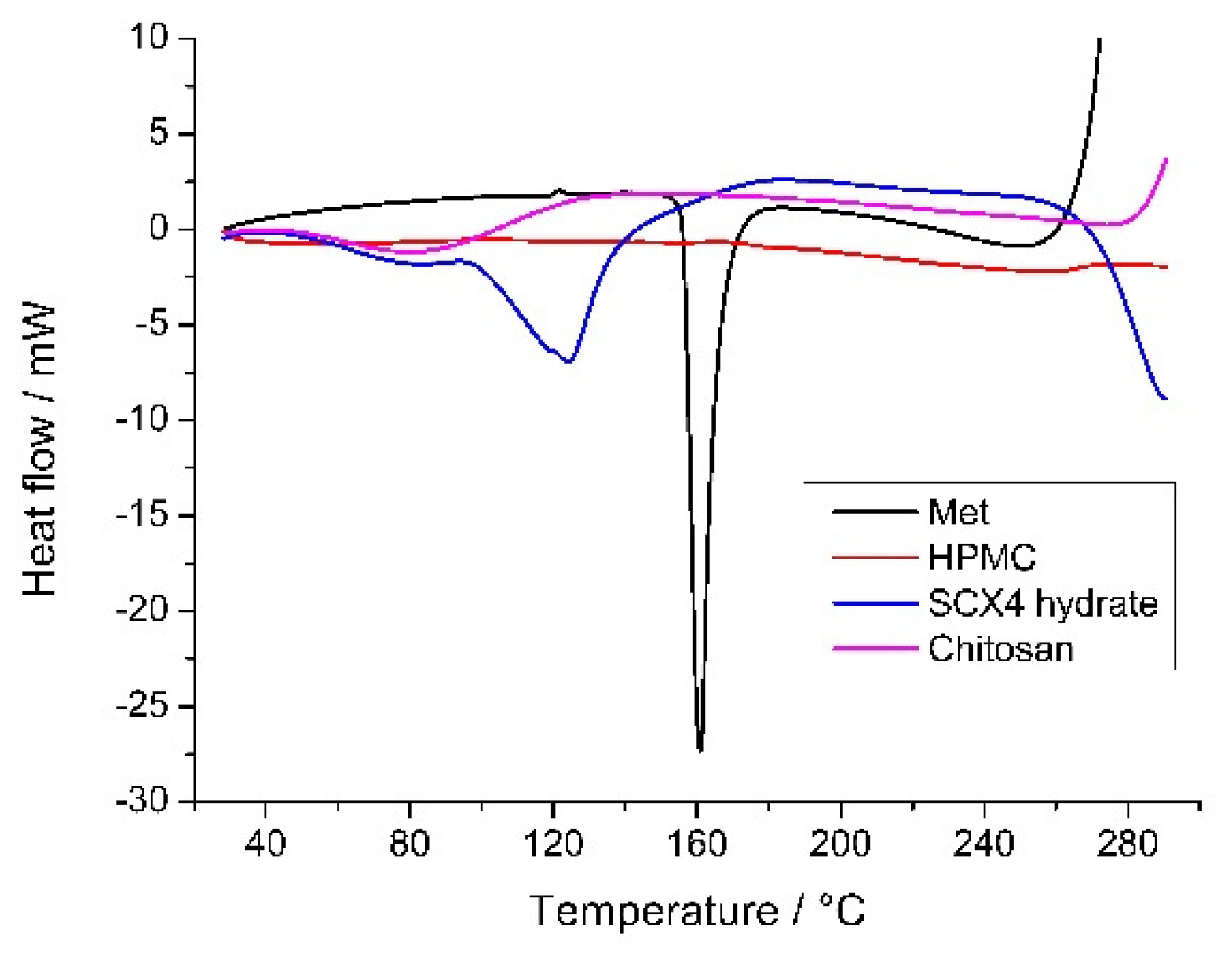
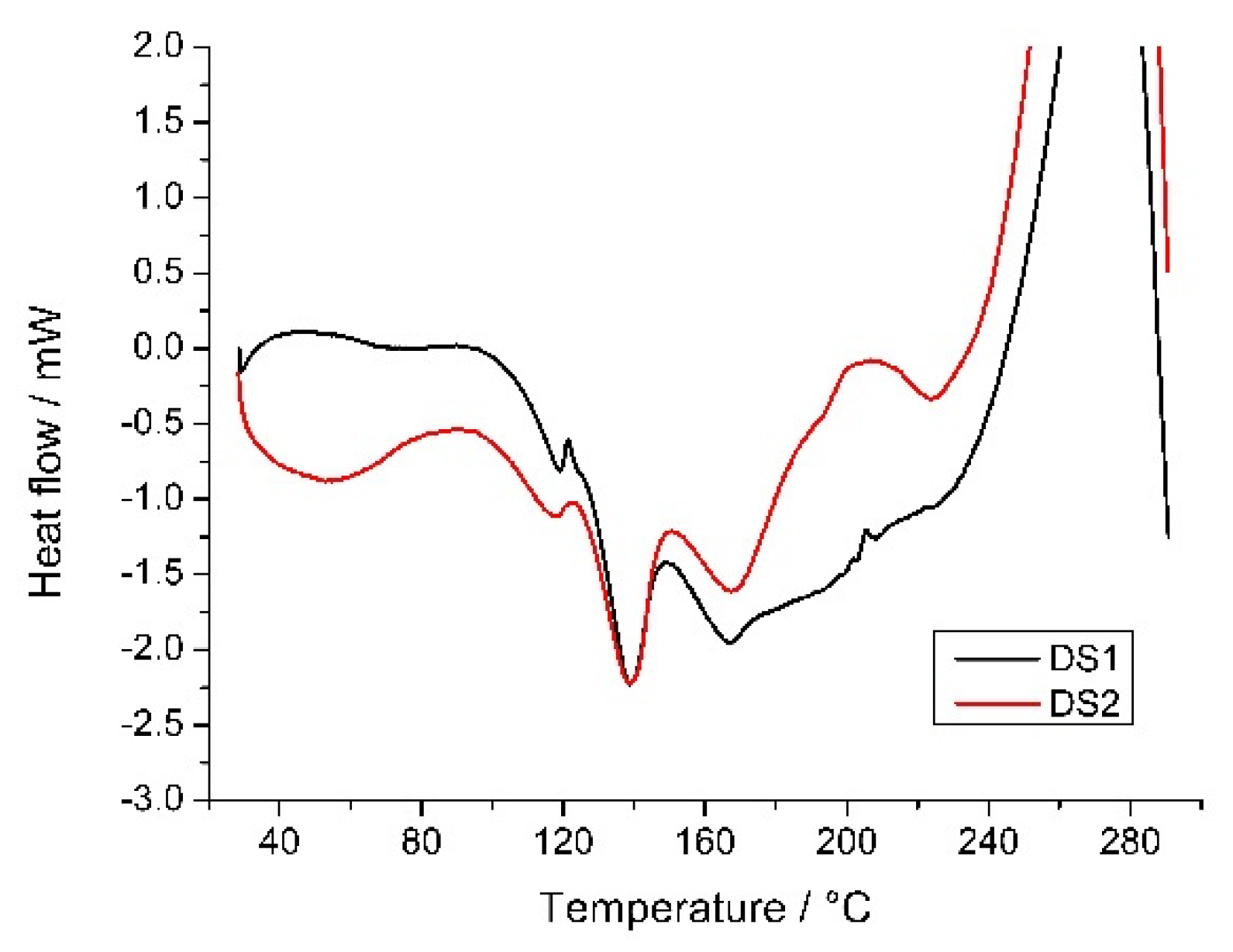
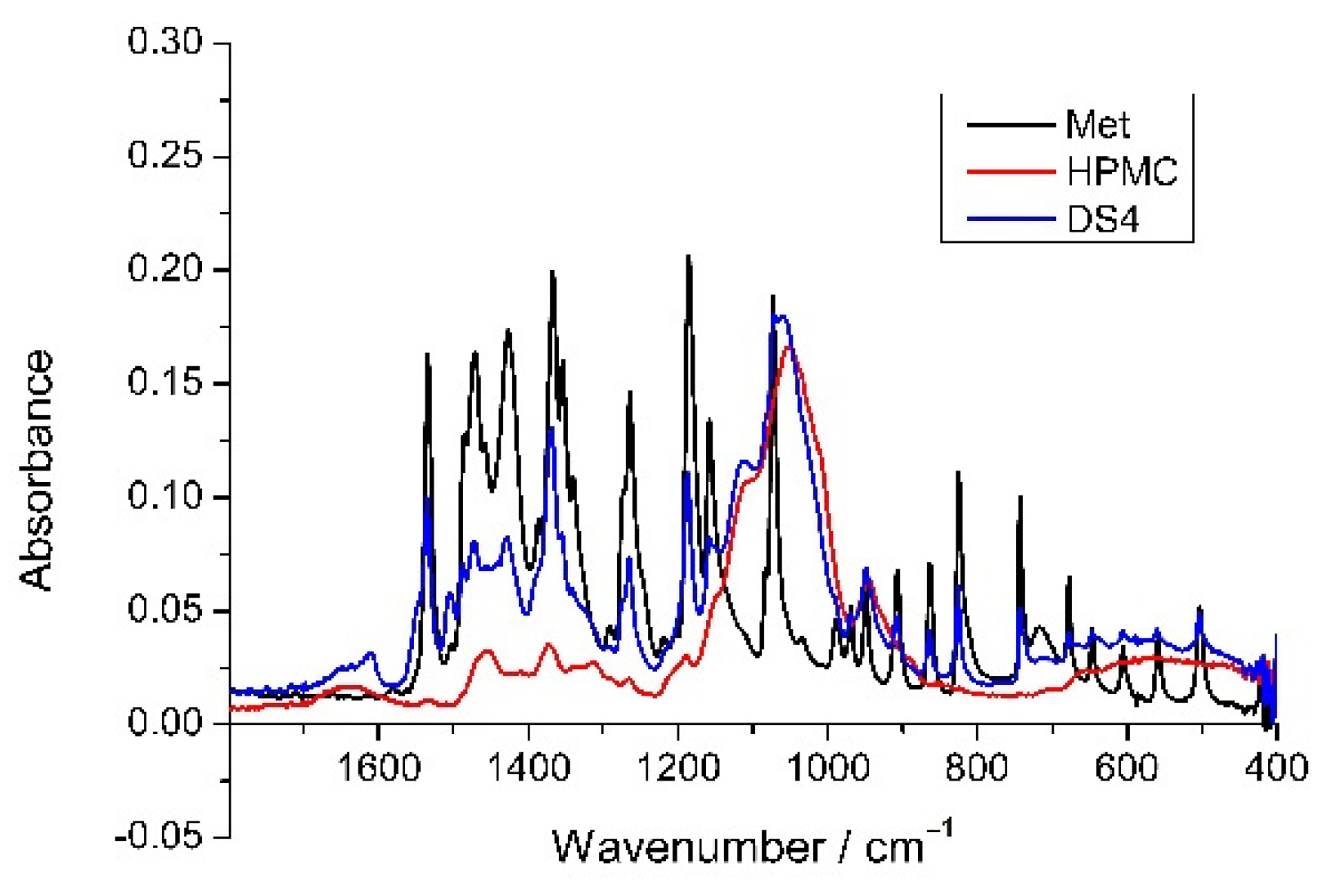
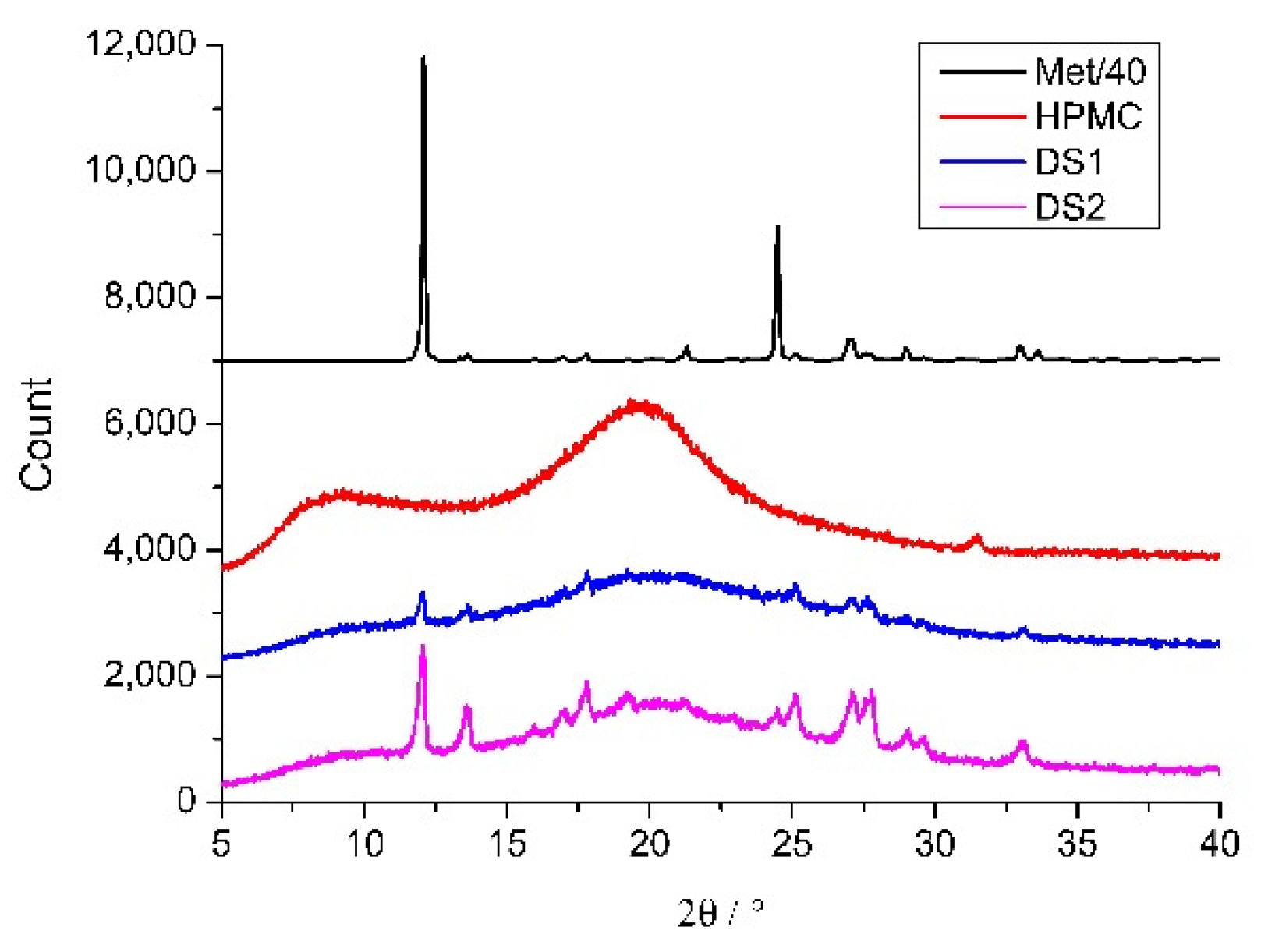
| Solutions | Dried Sample | Yield of Drying | (Met)/m/m% | Size/μm | ||||
|---|---|---|---|---|---|---|---|---|
| In Solution | In Dried Sample | D(4,3) | d10 | d50 | d90 | |||
| Met 0.352 m/m% SCX4 0.030 m/m% Chitosan 0.034 m/m% HPMC 0.750 m/m% HCl 0.01 M | DS1 | 60.2 | 30.2 | 30.5 | 6.0 | 1.2 | 2.8 | 16.8 |
| Met 0.352 m/m% HPMC 0.750 m/m% HCl 0.01 M | DS2 | 46.5 | 31.9 | 31.7 | 7.5 | 1.5 | 4.7 | 14.5 |
| HPMC 0.750 m/m% HCl 0.01 M | DS3 | 52.5 | - | - | 2.9 | 1.4 | 2.6 | 4.7 |
| Sample | Peak1 (°C) | Peak 2 (°C) |
|---|---|---|
| Met | - | 161.0 |
| SCX4 | 124.3 | - |
| DS1 | 138.6 | 166.2 |
| DS2 | 138.9 | 168.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirankó, M.; Megyesi, M.; Miskolczy, Z.; Tóth, J.; Feczkó, T.; Biczók, L. Encapsulation of Metronidazole in Biocompatible Macrocycles and Structural Characterization of Its Nano Spray-Dried Nanostructured Composite. Molecules 2021, 26, 7335. https://doi.org/10.3390/molecules26237335
Mirankó M, Megyesi M, Miskolczy Z, Tóth J, Feczkó T, Biczók L. Encapsulation of Metronidazole in Biocompatible Macrocycles and Structural Characterization of Its Nano Spray-Dried Nanostructured Composite. Molecules. 2021; 26(23):7335. https://doi.org/10.3390/molecules26237335
Chicago/Turabian StyleMirankó, Mirella, Mónika Megyesi, Zsombor Miskolczy, Judit Tóth, Tivadar Feczkó, and László Biczók. 2021. "Encapsulation of Metronidazole in Biocompatible Macrocycles and Structural Characterization of Its Nano Spray-Dried Nanostructured Composite" Molecules 26, no. 23: 7335. https://doi.org/10.3390/molecules26237335
APA StyleMirankó, M., Megyesi, M., Miskolczy, Z., Tóth, J., Feczkó, T., & Biczók, L. (2021). Encapsulation of Metronidazole in Biocompatible Macrocycles and Structural Characterization of Its Nano Spray-Dried Nanostructured Composite. Molecules, 26(23), 7335. https://doi.org/10.3390/molecules26237335









