Abstract
Novel Bi2W2O9 and Bi2Mo2O9 with irregular polyhedron structure were successfully synthesized by a hydrothermal method. Compared to ordinary Bi2WO6 and Bi2MoO6, the modified structure of Bi2W2O9 and Bi2Mo2O9 were observed, which led to an enhancement of photocatalytic performance. To investigate the possible mechanism of enhancing photocatalytic efficiency, the crystal structure, morphology, elemental composition, and optical properties of Bi2WO6, Bi2MO6, Bi2W2O9, and Bi2Mo2O9 were examined. UV-Vis diffuse reflectance spectroscopy revealed the visible-light absorption ability of Bi2WO6, Bi2MO6, Bi2W2O9, and Bi2Mo2O9. Photoluminescence (PL) and photocurrent indicated that Bi2W2O9 and Bi2Mo2O9 pose an enhanced ability of photogenerated electron–hole pairs separation. Radical trapping experiments revealed that photogenerated holes and superoxide radicals were the main active species. It can be conjectured that the promoted photocatalytic performance related to the modified structure, and a possible mechanism was discussed in detail.
1. Introduction
Semiconductor photocatalysis technology has received increasing attention as a green approach since it can be widely applied in the areas of carbon dioxide reduction, water splitting, and organic pollutants degradation [1,2,3,4]. It is no doubt that TiO2 is a popular photocatalyst. However, the band gap of TiO2 is so wide that it can only respond to ultraviolet (UV) light. Therefore, tremendous efforts have been made to develop new visible-light-driven (VLD) photocatalysts, such as Bi2WO6, Bi2MoO6, MoS2, g-C3N4, BiOBr, BiVO4, and so on [5,6,7,8].
Both Bi2WO6 and Bi2MoO6 are active members of the Aurivillius oxide family with a special layer structure [9,10,11,12]. Bi2WO6 and Bi2MoO6 (Bi2MO6, M = W/Mo) possess similar crystal structure. Bi2WO6 consists of WO6 layers and [Bi2O2]2+ layers, and Bi2MoO6 consists of [MoO2]2+ layers and [Bi2O2]2+ layers. Such a layered structure is favorable to the separation and transfer of photogenerated carriers [13]. Moreover, Bi2MO6 can absorb visible light and has good stability against photocorrosion. Thus, they have displayed potential photocatalytic performance for the decontamination of contaminants [14,15]. However, their practical application remains limited because of the high recombination rate of photogenerated electron–hole pairs in photocatalytic processes, and the visible-light use efficiency is still limited, which only responds to the light under 500 nm [16,17]. To solve these issues, composite materials with a heterojunction structure, doping of other ions, and loading of noble metal co-catalysts have been extensively investigated [18,19,20,21,22]. The results indicate that these methods essentially changed the structure of Bi2MO6 crystal to effectively inhibit the recombination of photogenerated electron–hole pairs under charge transmission, resulting in high photocatalytic performance. In this case, finding a kind of bismuthate with appropriate crystal structure is a potential approach to promote photocatalytic performance. Based on the special layer structure of Bi2MO6, a train of thought to modify the layer structure can be attempted.
The designation of Bi2M2O9 (M = W/Mo) was adopted because of the similar crystal structure between Bi2W2O9 and Bi2Mo2O9. There are similarities and differences between Bi2M2O9 and Bi2MO6. Both Bi2M2O9 and Bi2MO6 consist of (Bi2O2)2+ and (MxOy)2− (M = W/Mo) layers, while the difference is that the (MxOy)2− (M = W/Mo) layer is (M2O7)2− in Bi2M2O9 and (MO4)2− in Bi2MO6. Given this kind of difference, a sort of structure modification phenomenon can take place in Bi2M2O9 crystal, and certainly lead to chemical bond changes.
In this study, novel morphology Bi2M2O9 photocatalysts and ordinary Bi2MO6 were synthesized by a hydrothermal process. The modified structure of Bi2M2O9 can facilitate charge separation to promote photocatalytic performance. Moreover, the modification of structure and chemical bond changes were studied, and the relationship between modified structure and promoted photocatalytic performance was investigated. We hope to explore a potential strategy to obtain a highly efficient visible-light-driven photocatalyst.
2. Result and Discussion
2.1. XRD Analysis
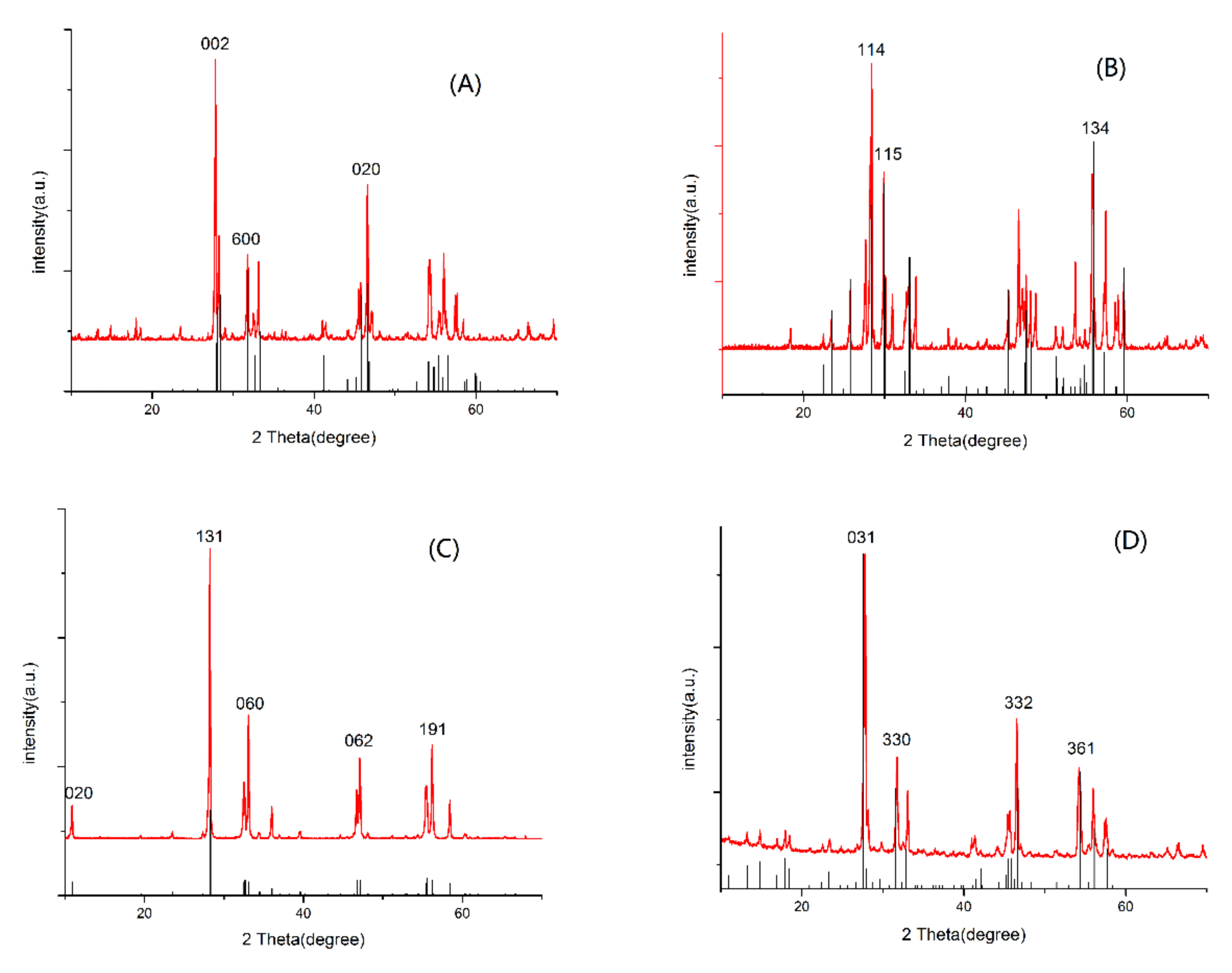
The typical diffraction patterns of the as-prepared samples can be observed in Figure 1, which indicates the successful synthesis of samples using the hydrothermal method. It also reveals the crystal style and major diffraction peaks of Bi2WO6, Bi2MO6, Bi2W2O9, and Bi2Mo2O9 in panel A to D, respectively. The major diffraction peaks at 2θ values of 27.5°, 33.4°, and 47.1° were indexed to (0 0 2), (6 0 0) and (0 2 0) of Bi2WO6 in Figure 1A, 2θ values of 27.7°, 29.9°, and 55.7° were indexed to (1 1 4), (1 1 5), and (1 3 4) of Bi2W2O9 in Figure 1B, 2θ values of 10.9°, 28.3°, 33.1°, 47.2°, and 56.2° were indexed to (0 2 0), (1 3 1), (0 6 0), (0 6 2), and (1 9 1) of Bi2MoO6 in Figure 1C, and 2θ values of 25.8°, 31.8°, 36.9°, and 54.3° were indexed to (031), (330), (332) and (361) of Bi2Mo2O9 in Figure 1D, respectively [23,24,25]. No signal for any crystalline phase of bismuth oxides were observed in the as-prepared photocatalysts.
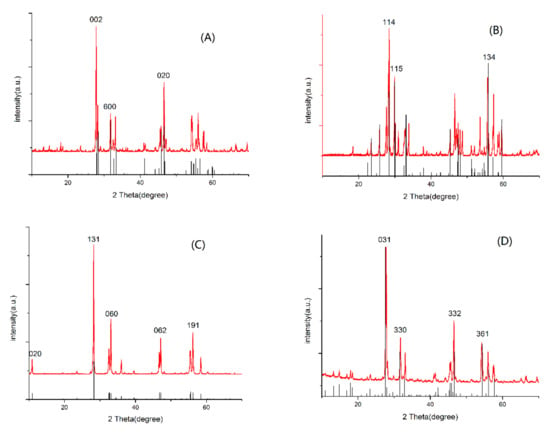
Figure 1.
X-ray diffraction patterns of prepared samples (A) Bi2WO6, (B) Bi2W2O9, (C) Bi2MoO6, and (D) Bi2Mo2O9.
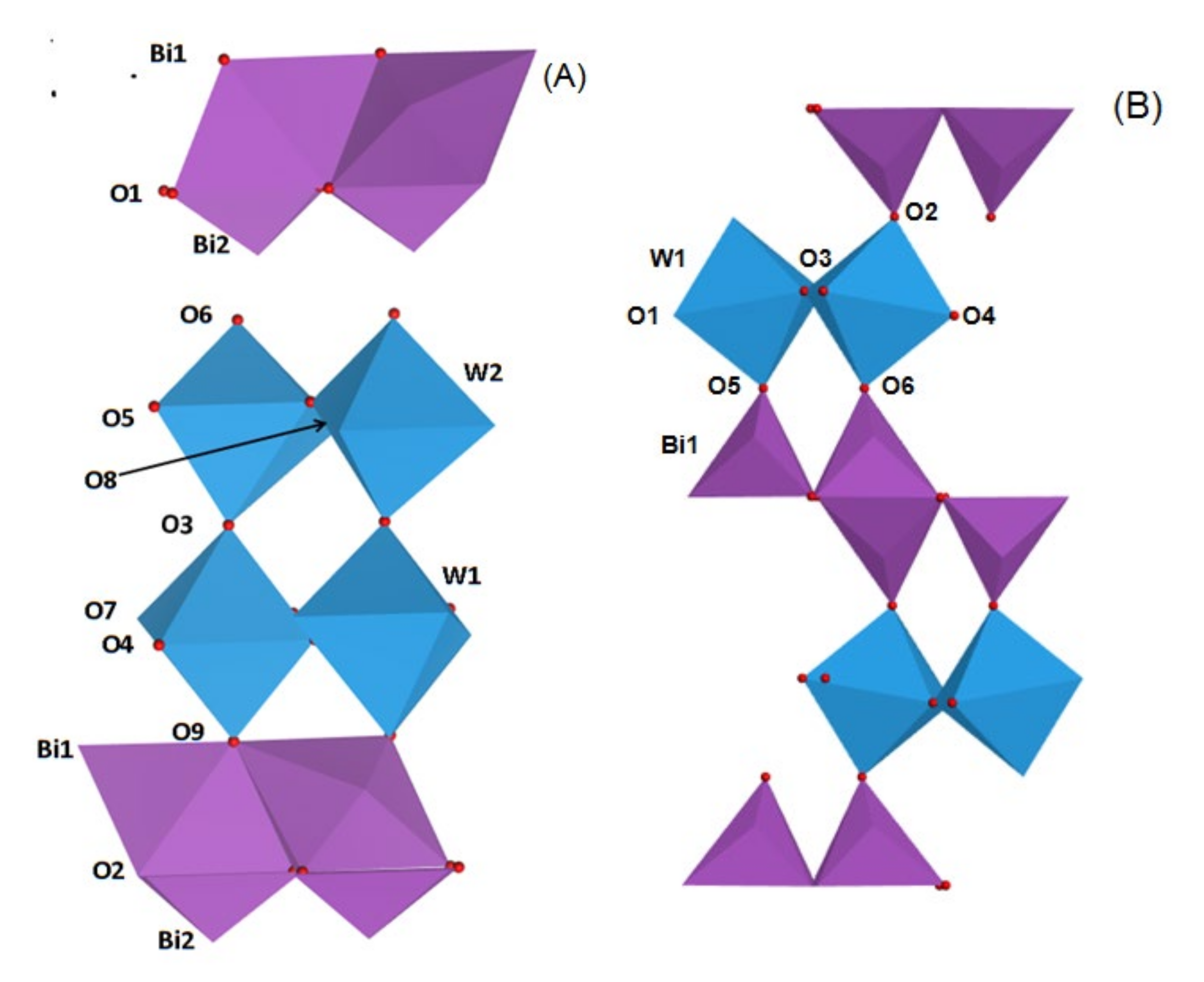
It was reported that the crystal structure of Bi2MO6 and Bi2M2O9 photocatalysts can be described as (Bi2O2)2+ layer and (MO4)2− or (M2O7)2− layer alternately connect to each other [26,27]. The polyhedron style model of Bi2WO6 and Bi2W2O9 were exhibited in Figure 2. As shown in Figure 2A, there is a double W-O layer between two (Bi2O2)2+ layers. The W atom and six surrounding oxygen atoms formed a WO6 octahedra, which connects with other similar octahedra by axial O3 in a lengthways direction and equatorial O4, O5, O7 and O8 oxygen atoms in a crosswise direction, forming double (W2O7)2− layers. Top and bottom oxygen atoms O6 and O9 are located close to (Bi2O2)2+ layers above and below WO6 octahedra respectively, which eventually formed Bi2W2O9 consisting of (W2O7)2− and (Bi2O2)2+ layers. In addition, as-prepared Bi2W2O9 structure can be regarded as a modification of the tetragonal structure. The formation principle of Bi2WO6 (Figure 2B) is similar to that of Bi2W2O9. The WO6 octahedra join another octahedra by axial O3 in a lengthways direction forming a single (WO4)2− layer, and equatorial O5, O6, and O2 no longer join other WO6 octahedra but join (Bi2O2)2+ instead. Bi2WO6 with a single (WO4)2− layer is formed in this way.
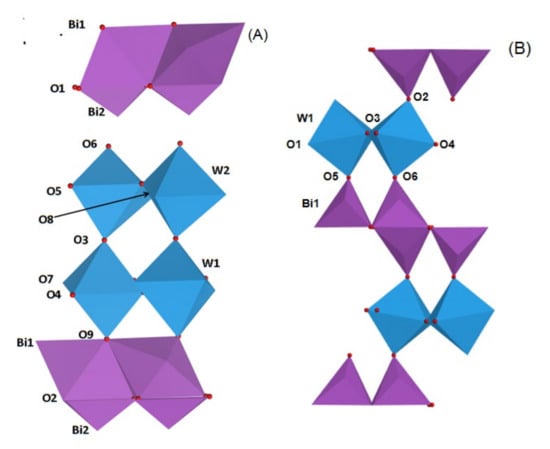
Figure 2.
Models of Bi2W2O9 (A) and Bi2WO6 (B) consist of (Bi2O2)2+ and (W2O7)2− or (WO4)2− layers.
As a result of the layer structure of Bi2WO6 and Bi2W2O9, some translational motions of layered crystal can be regarded as a Rigid Unit (RU) layer motion. There are two shared RU modes in Bi2W2O9, and the adjacent layers move parallel to the layer planes, which leads to modified compressional RU modes between adjacent layers in the perpendicular direction of the layer planes. As for Bi2WO6 structure, there is only one RU mode and the effect of compression between adjacent layers is weaker than that of Bi2W2O9. As a result, compression of Bi2W2O9 layer structure changes the chemical bond properties.
2.2. Morphology Characterization
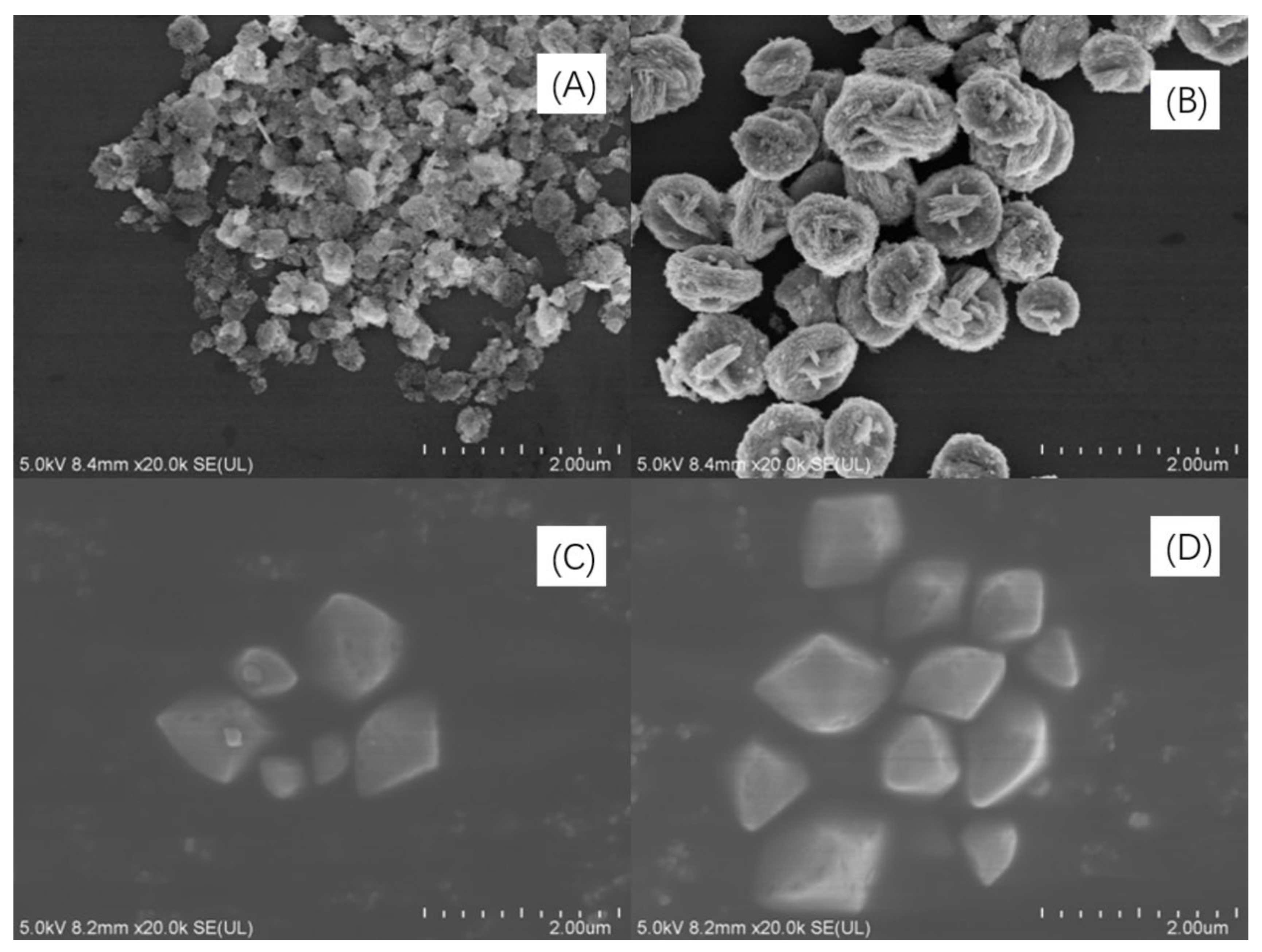
SEM images of Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 are illustrated in Figure 3 panels a to d, respectively. The morphology of Bi2WO6 in Figure 3A exhibits fastener-like nanoparticles with the radius of no more than 100 nm. The dimerization bismuthate Bi2W2O9 certainly kept a similar fastener-like morphology as that of Bi2WO6 (Figure 3B). However, it can be observed that a volume increase phenomenon exists in Bi2W2O9 crystal, and the average radius rose to about 400 nm at the same plotting scale compared to Bi2WO6. In terms of Bi2MoO6 and Bi2Mo2O9, Figure 3C,D represent the panoramic SEM images of Bi2MoO6 and Bi2Mo2O9. Both Bi2MoO6 and Bi2Mo2O9 are of irregular polyhedron morphology and the variation is that there is also an increase of radius in Bi2Mo2O9 compared to Bi2MoO6, but the increasing rate is less than that of Bi2W2O9 and Bi2WO6. Double RU of Bi2M2O9 increase of the distance of adjacent (Bi2O2)2+ layers may be the possible reason for the volume augment. The novel morphology observed in SEM images may be attributed to the introduction of AOT surfactant during the synthesis process.
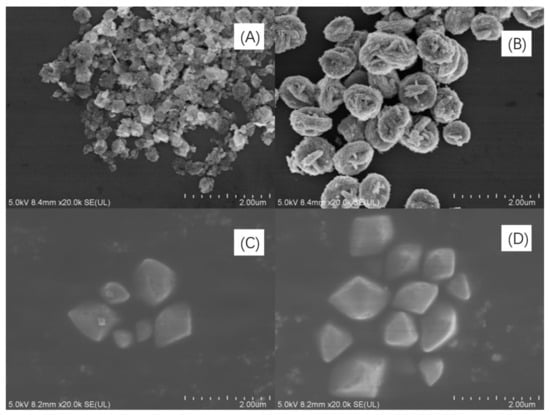
Figure 3.
SEM images of (A) Bi2WO6, (B) Bi2W2O9, (C) Bi2MoO6 and (D) Bi2Mo2O9.
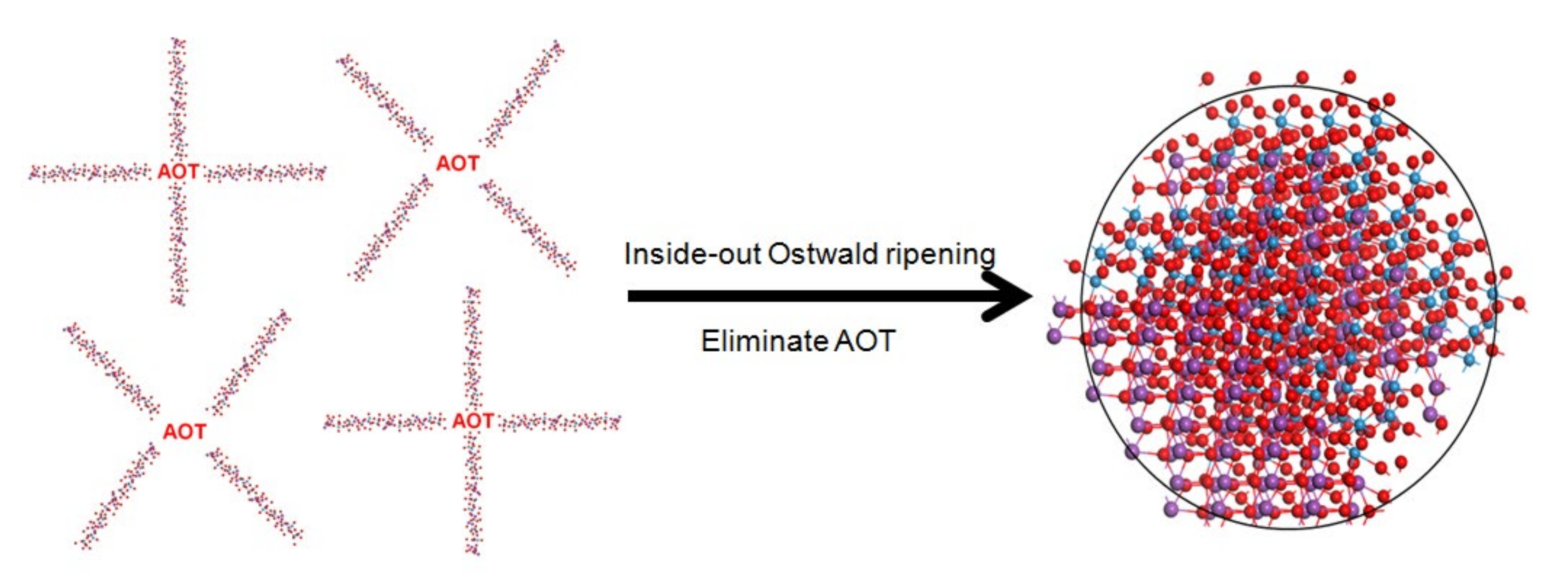
The possible synthesis mechanism is as follows. Raw materials have aggregated together to form some spheres during the hydrothermal process and finally the products have grown into clear-cut fastener-like or irregular polyhedron microspheres. At the beginning of the reaction, Bi3+ with (MxOy) 2− ions precipitated quickly under the driving force of low-solubility products of Bi2MxOy. Subsequently, AOT molecules are absorbed on the surface of nanoparticles through intermolecular interaction, and the newly formed nanoparticles are aggregated into loose microspheres, forming the Bi2MxOy-AOT composite systems. These Bi2MxOy-AOT composite systems connected with each other in various shapes. Finally, these amorphous nanoparticles underwent Ostwald ripening from the inside out as their surfaces come into contact with the surrounding solution. As a result, the internal nanoparticles tend to dissolve, which provides the driving force for spontaneous inside-out Ostwald ripening. This dissolution process could initiate at regions either near the surface or around the center of the microspheres. Redundant AOT was washed by the solution. Ostwald ripening occurred during the synthesis process of Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 presumably depending on the packing of primary nanoparticles and ripening characteristics of AOT. A simple schematic illustration for the formation of the process is given in Figure 4.
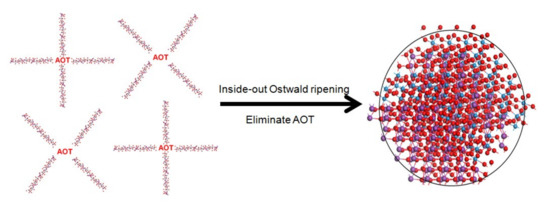
Figure 4.
Possible formation process of novel morphology samples with AOT surfactant.
To confirm the atomic composition of Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 samples conform to the theoretical value, EDX measurement was carried out and the data are listed in Table 1.

Table 1.
EDX data of Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9.
The EDX data show that although there is deviation compared to the experimental data with theoretical value, the deviation is within acceptable limits.
2.3. Chemical State Analysis
X-ray photoelectron spectroscopy (XPS) analysis is used to further investigate the chemical state and surface chemical composition of Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9, especially to understand the structure modification effect of Bi2W2O9 and Bi2Mo2O9 on the binding energy, which has a great influence on photocatalytic performance [28].
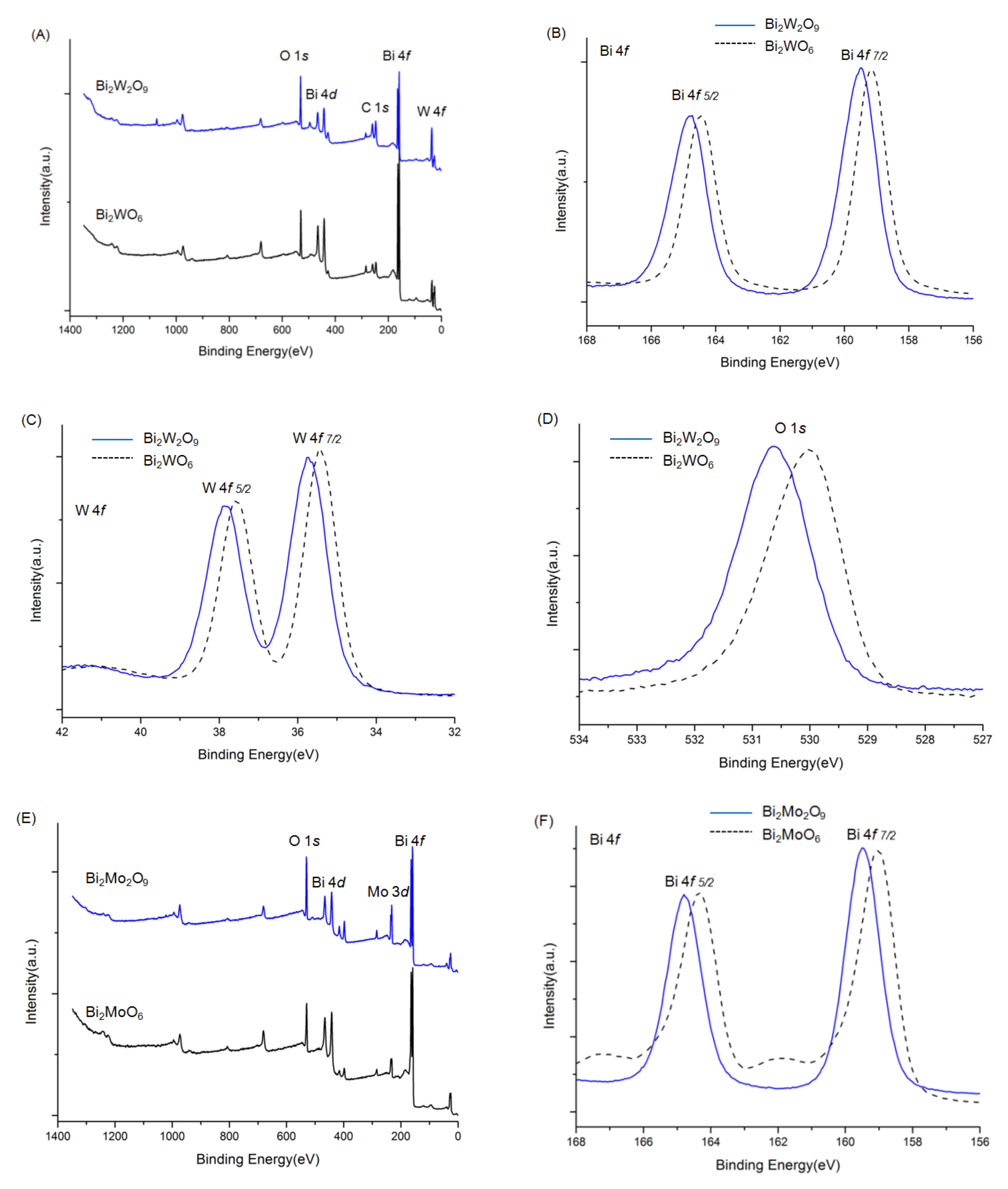
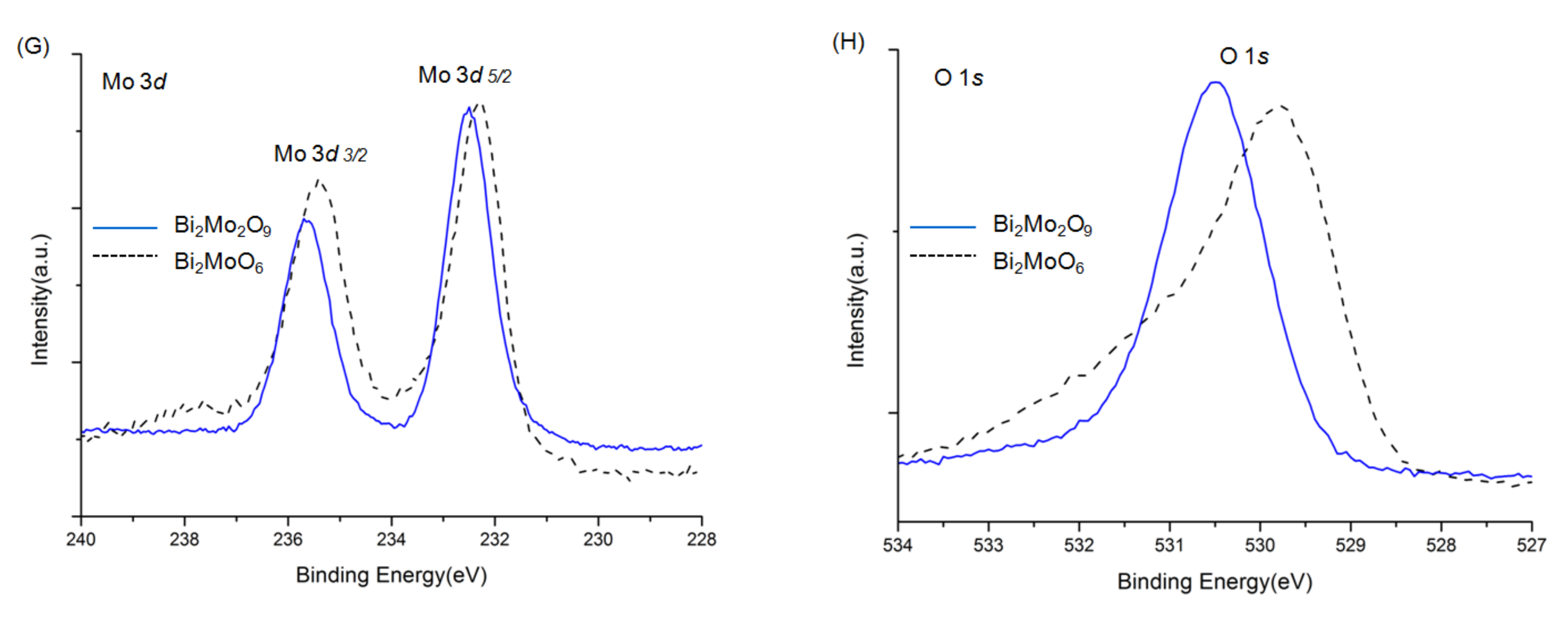
The overall XPS spectra of Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 are shown in Figure 5. The characteristic peaks of the Bi, W, Mo, and O elements were detected. Before the analysis, all peaks of the other elements were calibrated according to the deviation between the C 1s peak and the standard signal of C 1s at 284.8 eV [29]. No XPS characteristic peaks of N 1s were detected at around 400 eV, although raw material contained nitrogen, which indicated no nitrogen was doped in Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 samples. The signals of Bi2WO6 in Figure 5B were attributed to Bi 4f7/2 and Bi 4f5/2 states respectively at 159.2 and 164.5 eV [30]. The binding energy of W 4f7/2 and W 4f5/2 were observed at 35.4 and 37.6 eV (Figure 5C) that can be attributed to W6+ [31]. The binding energy peak in Figure 5D located at 530.0 eV corresponds to O 1s state in Bi2WO6 [32]. The same examined element spectra in Bi2W2O9 were exhibited together with those in Bi2WO6 in the same panels. It can be obviously observed that all Bi, W, and O have similar peak patterns, and the difference is that characteristic peaks of Bi2W2O9 shift towards higher binding energy, which illustrates that the chemical environment has changed, and a higher binding energy indicates the existence of the electron-drawing group. The same phenomenon can be observed in Bi2MoO6 and Bi2Mo2O9 in panels E to H, where Bi, Mo, and O elements in Bi2Mo2O9 pose a higher binding energy.
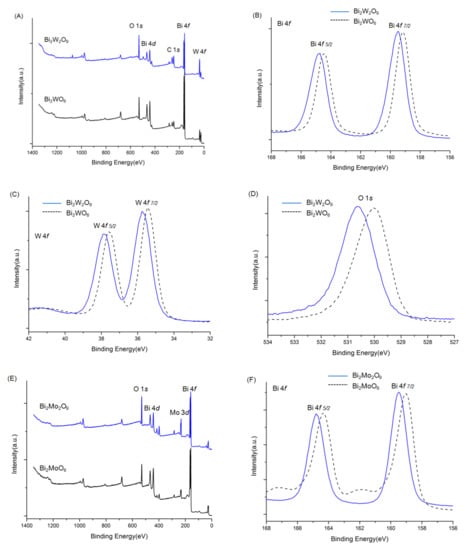
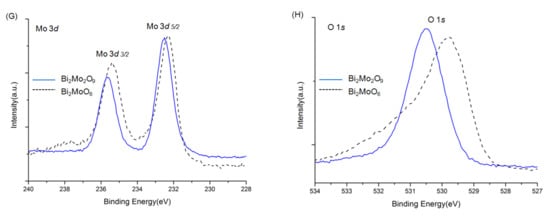
Figure 5.
XPS spectra of Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9: ((A): Bi2WO6 and Bi2W2O9) ((E): Bi2W2O9) overall spectra; (B,F) Bi 4f; (C) W f; (D,H) O 1s; (G) Mo 3d.
2.4. Optical Properties
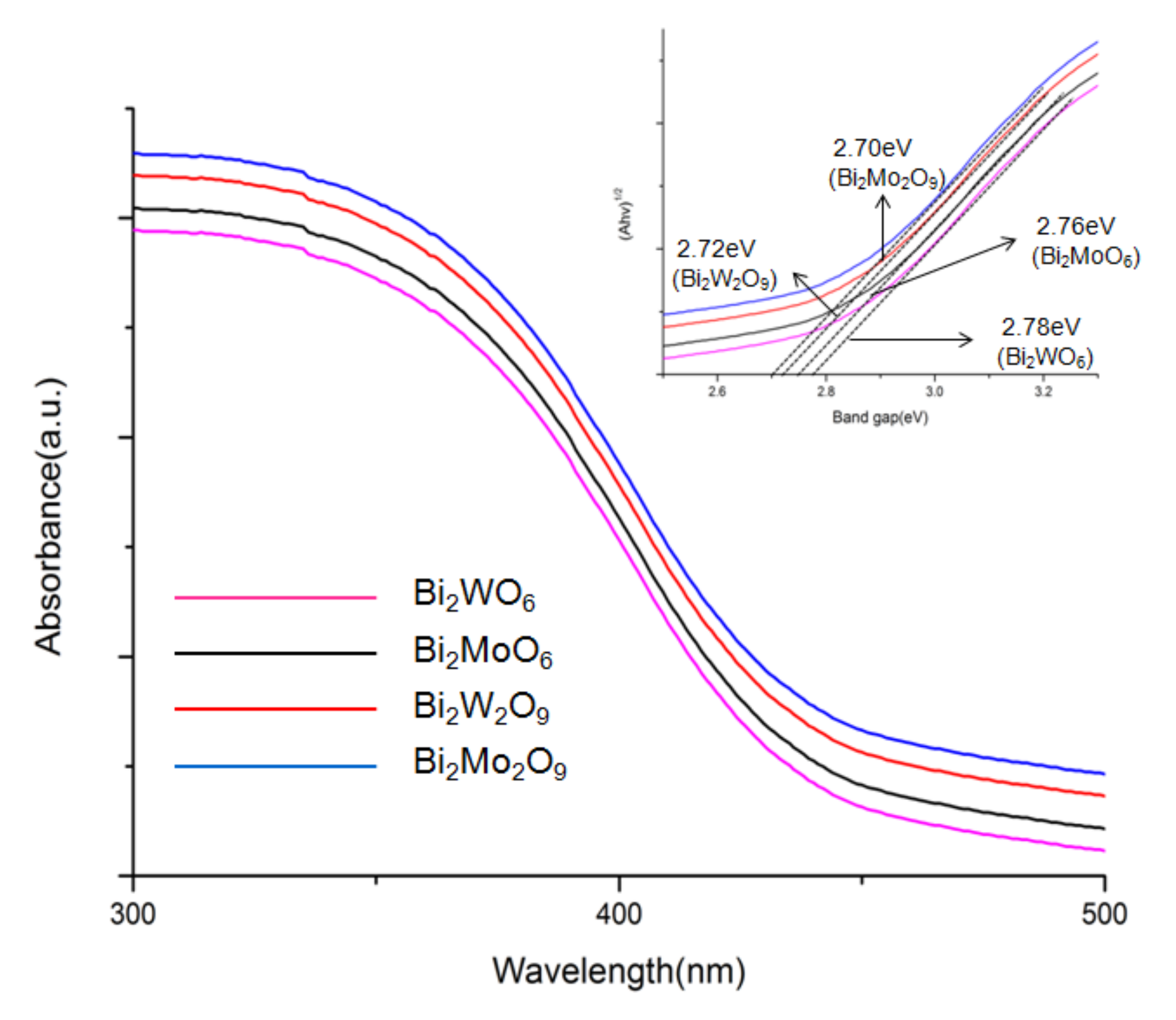
The optical properties of Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 were characterized through a UV-Vis diffuse reflectance spectrometer in the wavelength range of 250–800 nm [33]. Compared to Bi2WO6 and Bi2MoO6, it can be observed obviously that there is a red shift of the light absorption edge from Bi2W2O9 and Bi2Mo2O9 samples, respectively. The optical band gap of the as-prepared photocatalysts was calculated using the following Equation (1) [34].
in which h, α, A, ν, and Eg represent Planck’s constant with the unit of eV, a constant, the absorption coefficient near the absorption edge, light frequency, the absorption band gap energy, respectively, and n is equal to 1 or 4, depending on whether the optical transition type is direct or indirect. Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 have a direct band gap, and n is 1 herein. The inset shows the curve of (αhν)2 versus hν for the as-prepared samples. It can be observed that an evidential red shift exists in Bi2M2O9 samples compared to Bi2MO6 and Bi2Mo2O9 has the greatest absorption range (Figure 6). The band gap energy (Eg) of Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9 were computed to be 2.78, 2.76, 2.72, and 2.70 eV respectively and exhibit in the inset of Figure 6. The result indicated that Bi2M2O9 samples presented an enhanced absorbance ability compared to Bi2MO6.
Ahν = α(hν − Eg)n/2
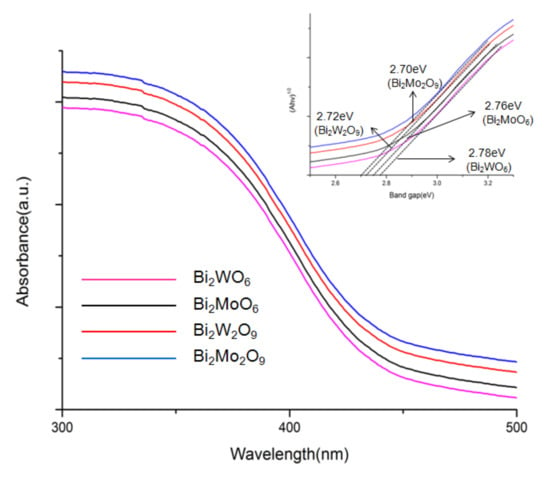
Figure 6.
DRS spectra of Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9. The inset shows the band gap energies.
2.5. Photocatalytic Properties
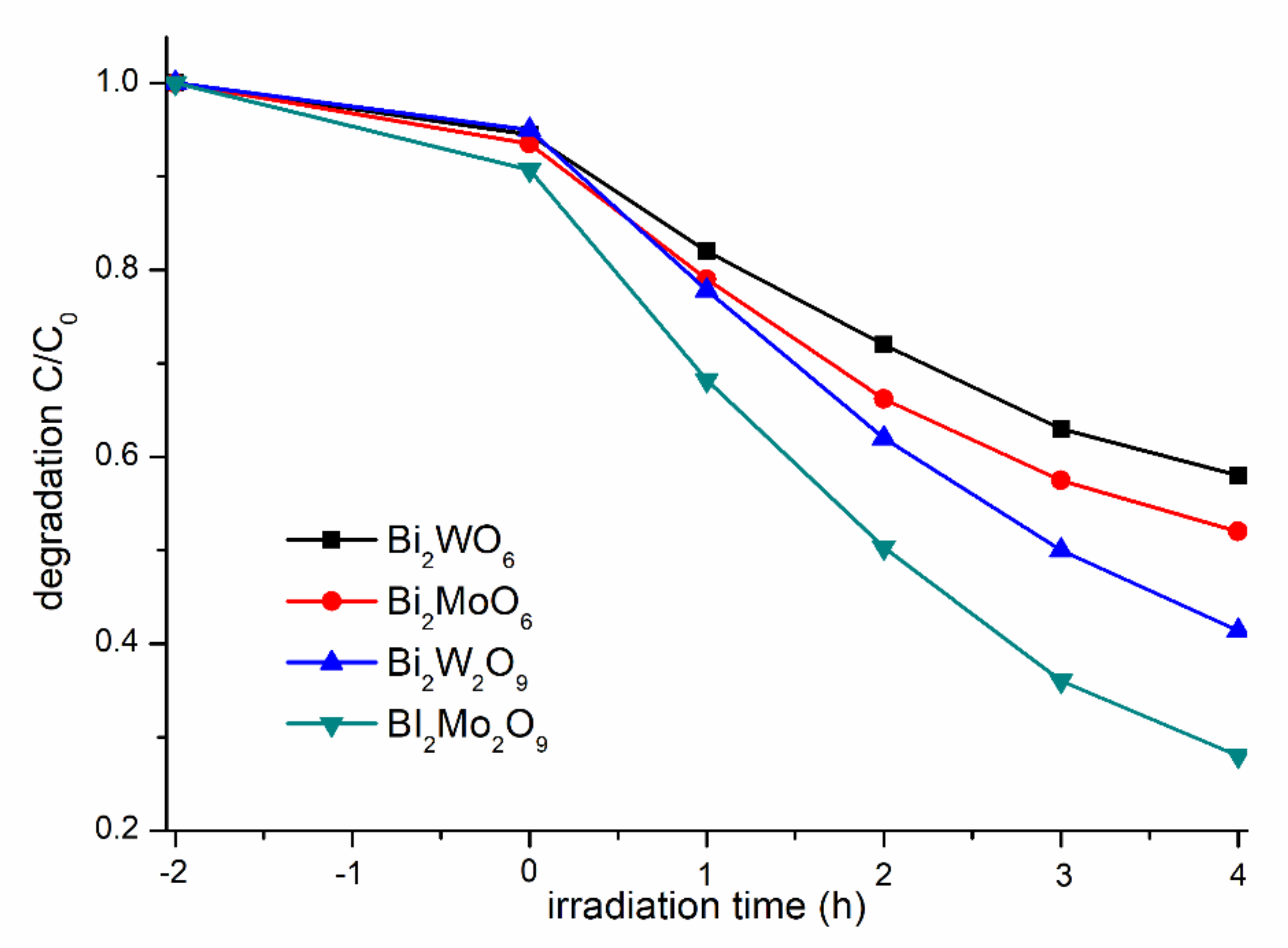
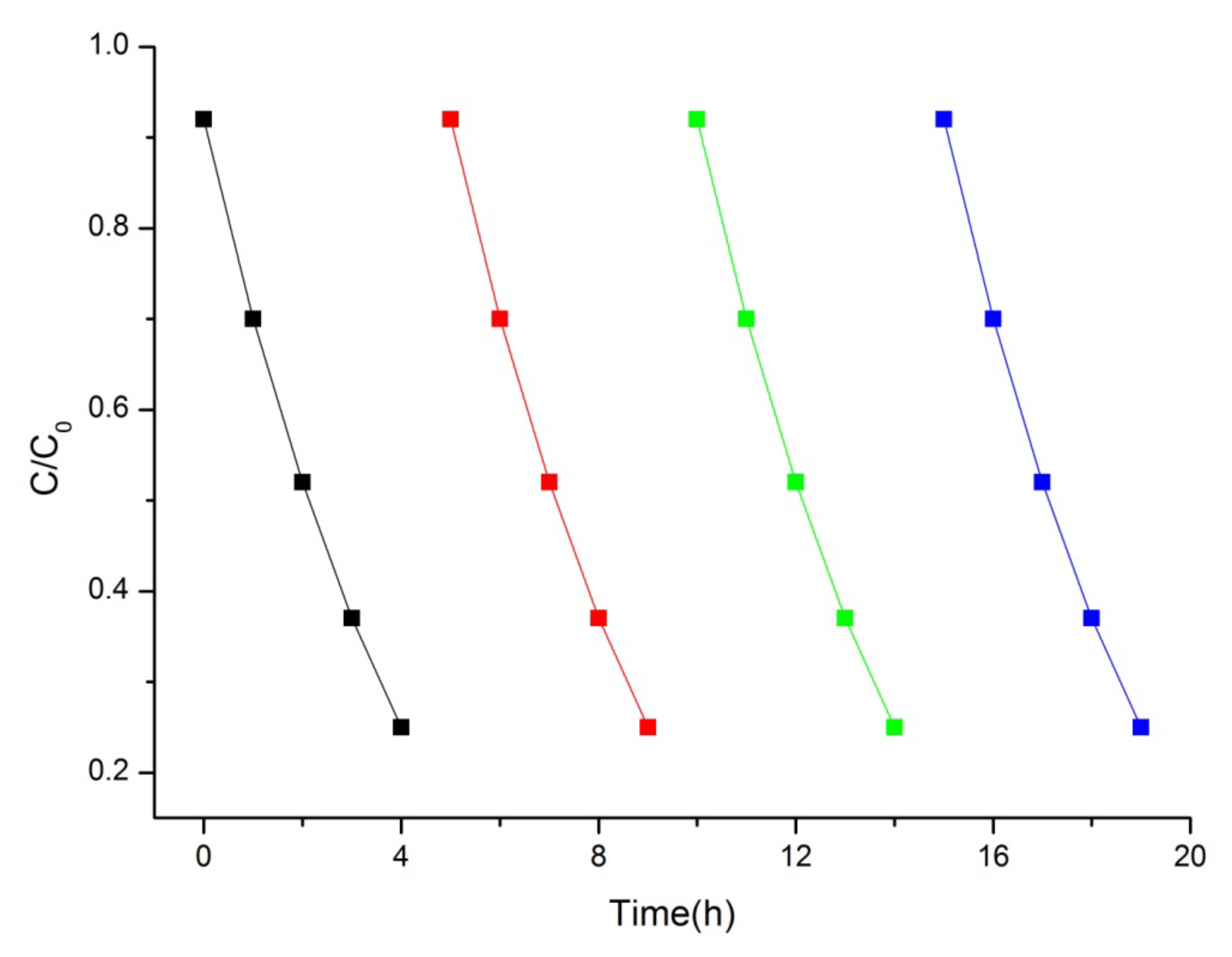
The photocatalytic performance of the as-synthesized photocatalysts were evaluated by examining the photodegradation of MB solution under visible-light irradiation (Figure 7). Generally, the different concentrations of MB adsorbed on the catalyst surface will have a great influence on the photocatalytic performance, so the adsorption ratio was collected when adsorption–desorption equilibrium was achieved before irradiation [35]. Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 samples presented a similar capacity for MB absorption, and it can be obviously observed that Bi2M2O9 samples exhibit a higher photocatalytic activity than that of Bi2MO6. Bi2Mo2O9 displayed the best photocatalytic activity among the four test samples, and the photodegradation rate reached up to 75% within 4 h.
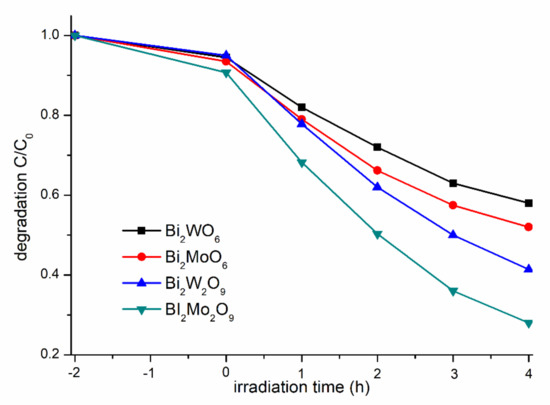
Figure 7.
Comparison of absorption and degradation rate of MB using Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9.
In our work, the cycling experiment for MB photocatalytic degradation was performed under visible-light irradiation to evaluate the stability of the best photocatalyst (Bi2Mo2O9). As shown in Figure 8, the photocatalytic performance of Bi2Mo2O9 did not display any significant reduction for MB degradation. This result confirms that Bi2M2O9 photocatalysts are not easily photo-corroded during the photodegradation of the pollutant molecules, which is important for their application.
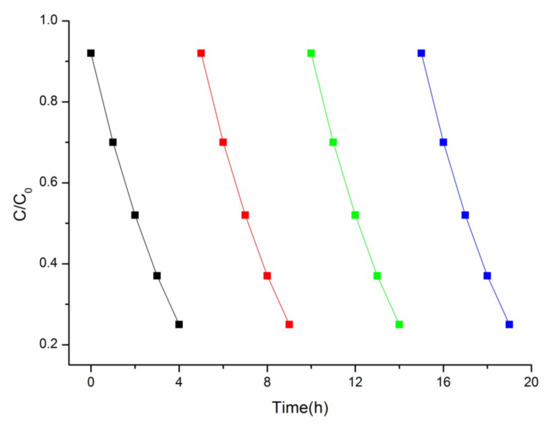
Figure 8.
Cyclic photocatalytic activity of MB by Bi2Mo2O9 photocatalyst.
2.6. Photocatalytic Mechanism
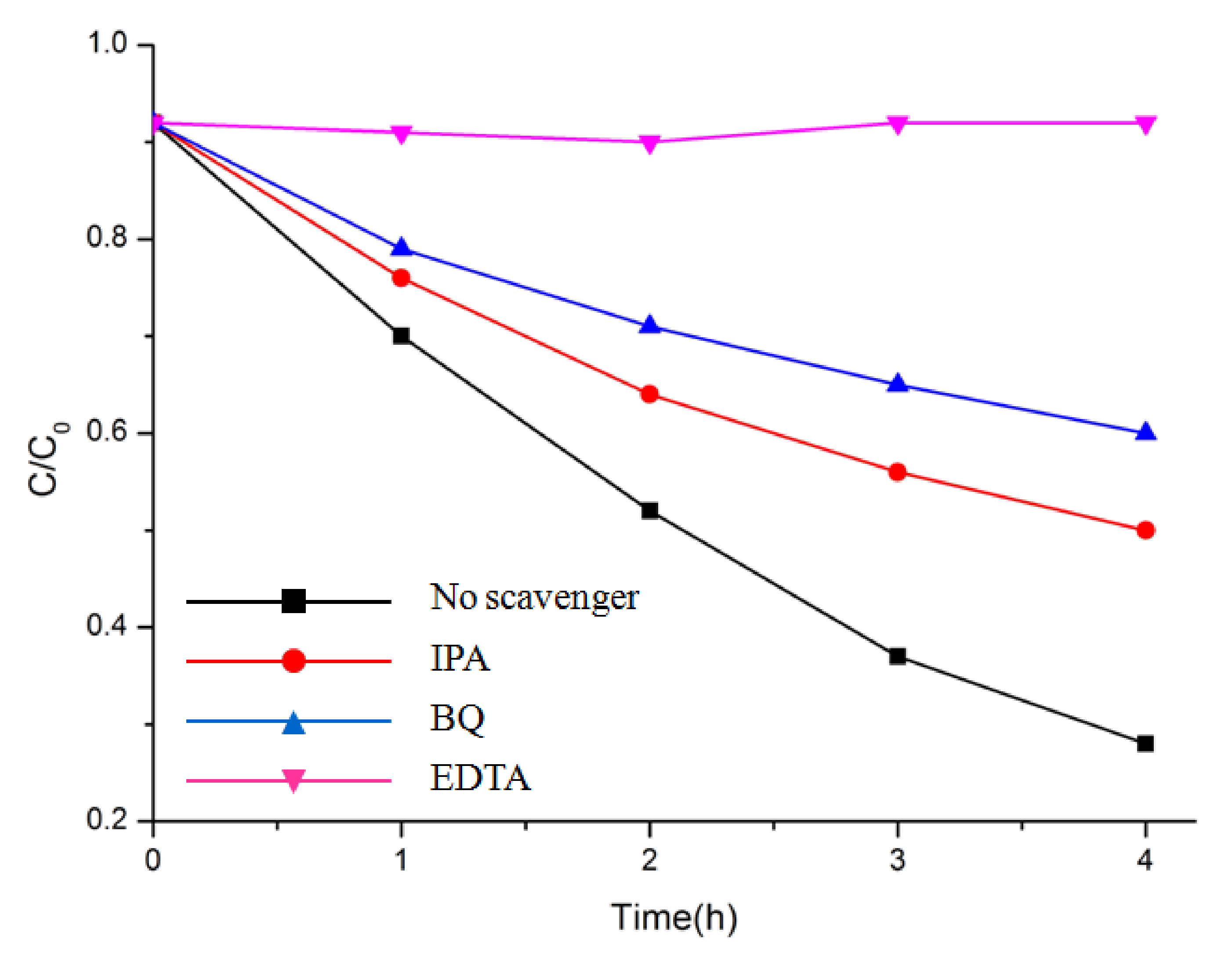
Overall, the photocatalytic activity of Bi2M2O9 was highly improved compared to ordinary Bi2MO6. As noted above, such enhancement may partially come from structure modification. To understand the structure modification effect on Bi2M2O9 and the charge behavior during the photocatalyst process, trapping experiments with different scavengers were performed with Bi2Mo2O9 (Figure 9). In this way, active species could be determined, including holes (h+), superoxide radicals (˙O2−) and hydroxyl radicals (˙OH) with effective oxidation and reduction potentials [36,37,38]. In the present study, isopropyl alcohol (IPA), ethylenediaminetetraacetic acid disodium salts (EDTA), and 1,4-benzoquinone (BQ) were used as scavengers of ˙OH, h+, and ˙O2−, respectively. Remarkably, MB degradation was halted as we added the scavenger EDTA (1 mM) for h+ to the reaction system. Meanwhile, the photodegradation rate of MB evidently declined with the addition of the scavenger BQ (1 mM) for ˙O2−. However, there was no clear reduction in the degradation rate of MB when the scavenger IPA (1 mM) for ˙OH was added. These results indicate that h+ and ˙O2− were the main reactive species, while ˙OH had little influence on the MB degradation process.
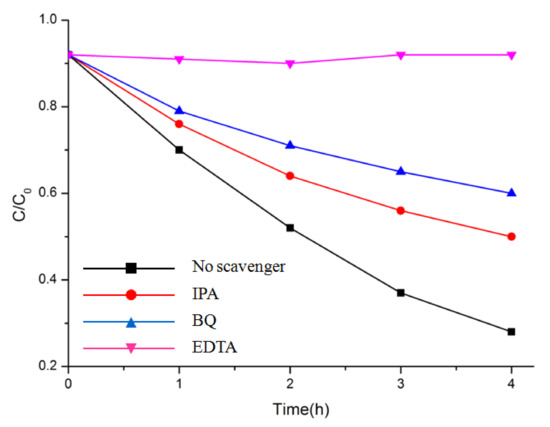
Figure 9.
Photocatalytic degradation of MB over Bi2Mo2O9 photocatalyst with the addition of scavengers EDTA, BQ, and IPA.
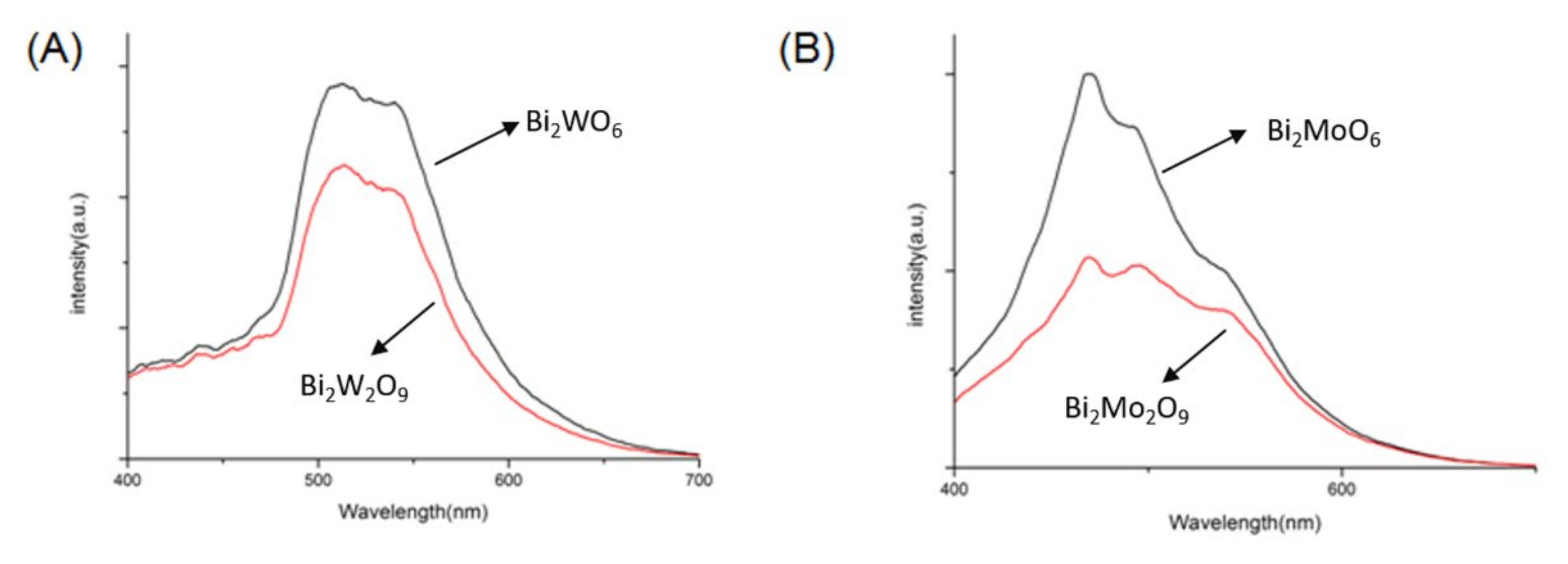
The electron–hole separation condition was tested by PL in the range of 400–700 nm. As shown in Figure 10, compared to Bi2MO6, the PL intensities of Bi2M2O9 were perceptibly weaker, and Bi2Mo2O9 has the lowest peak intensity. Usually, a lower PL intensity shows stronger photogenerated charge separation, which leads to excellent photocatalytic efficiency of the photocatalyst [39]. The experiment results reveal that Bi2M2O9 has an elevated ability of electron–hole separation.
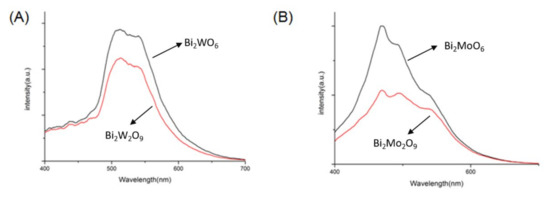
Figure 10.
Photoluminescence (PL) spectra of (A) Bi2WO6 and Bi2W2O9; (B) Bi2MoO6 and Bi2Mo2O9.
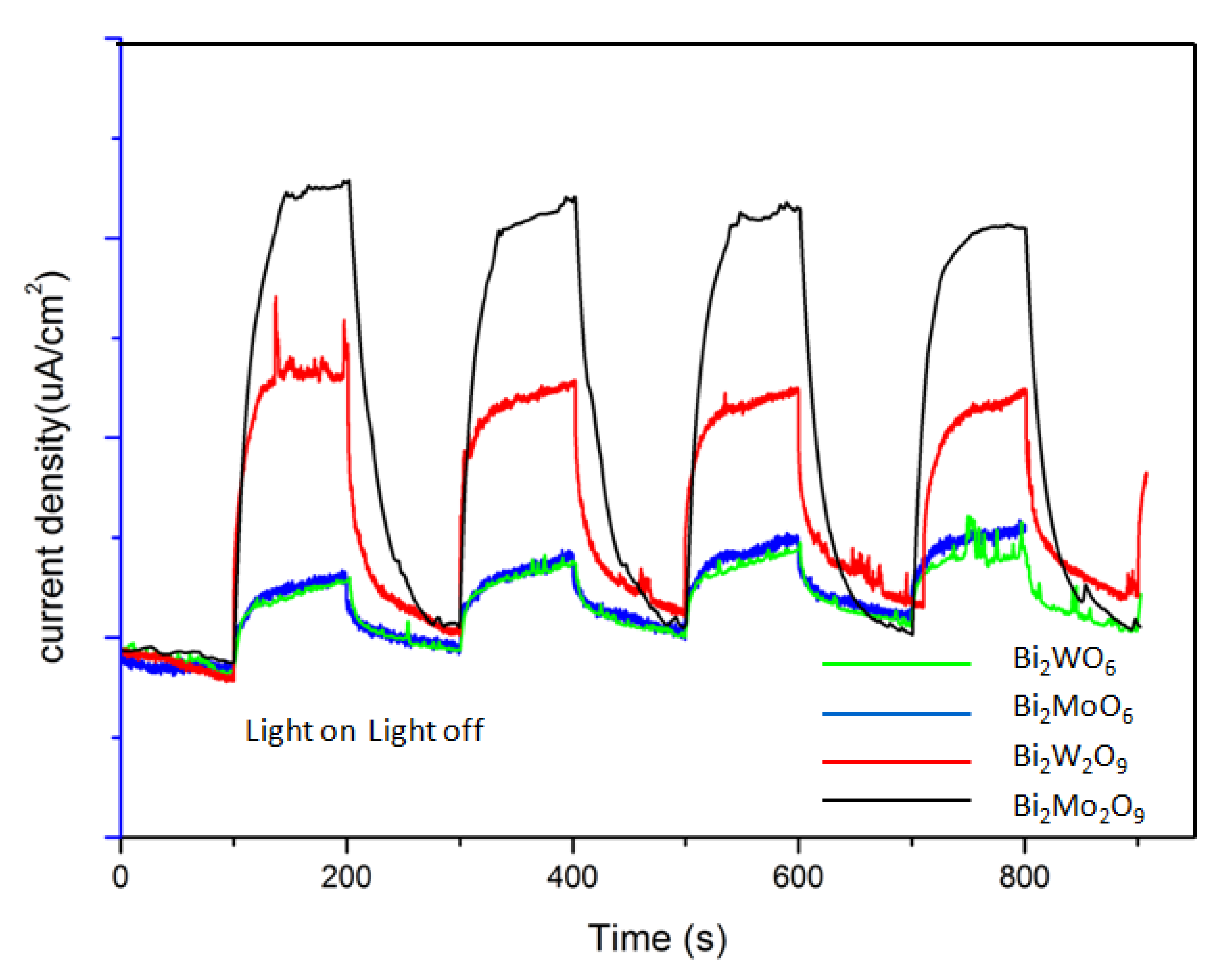
The photocurrent responses directly related to the generation and transfer of the photogenerated electrons and holes [40,41]. Figure 11 shows the photocurrent response of Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9 samples. Obviously, the current abruptly increased and decreased through on–off cycles under visible-light irradiation. Bi2M2O9 samples showed a significantly improved photocurrent response compared to that of the Bi2MO6 samples. This suggests that more efficient separation of the photogenerated charge carriers occurred in Bi2M2O9 samples. In addition, it should be noted that Bi2M2O9 samples hold obvious residual currents when the light source is switched off. This is probably attributed to the modified structure; it may lead to some remnant electron–hole pairs when the visible-light source is removed, which could release the trapped electrons or holes because of the self-thermal motion.
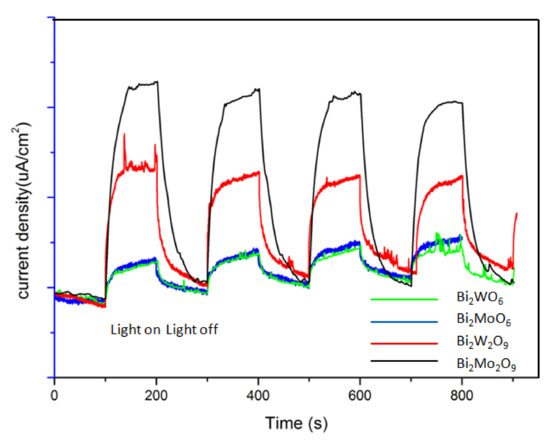
Figure 11.
Photocurrent responses of Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9 samples under visible-light irradiation.
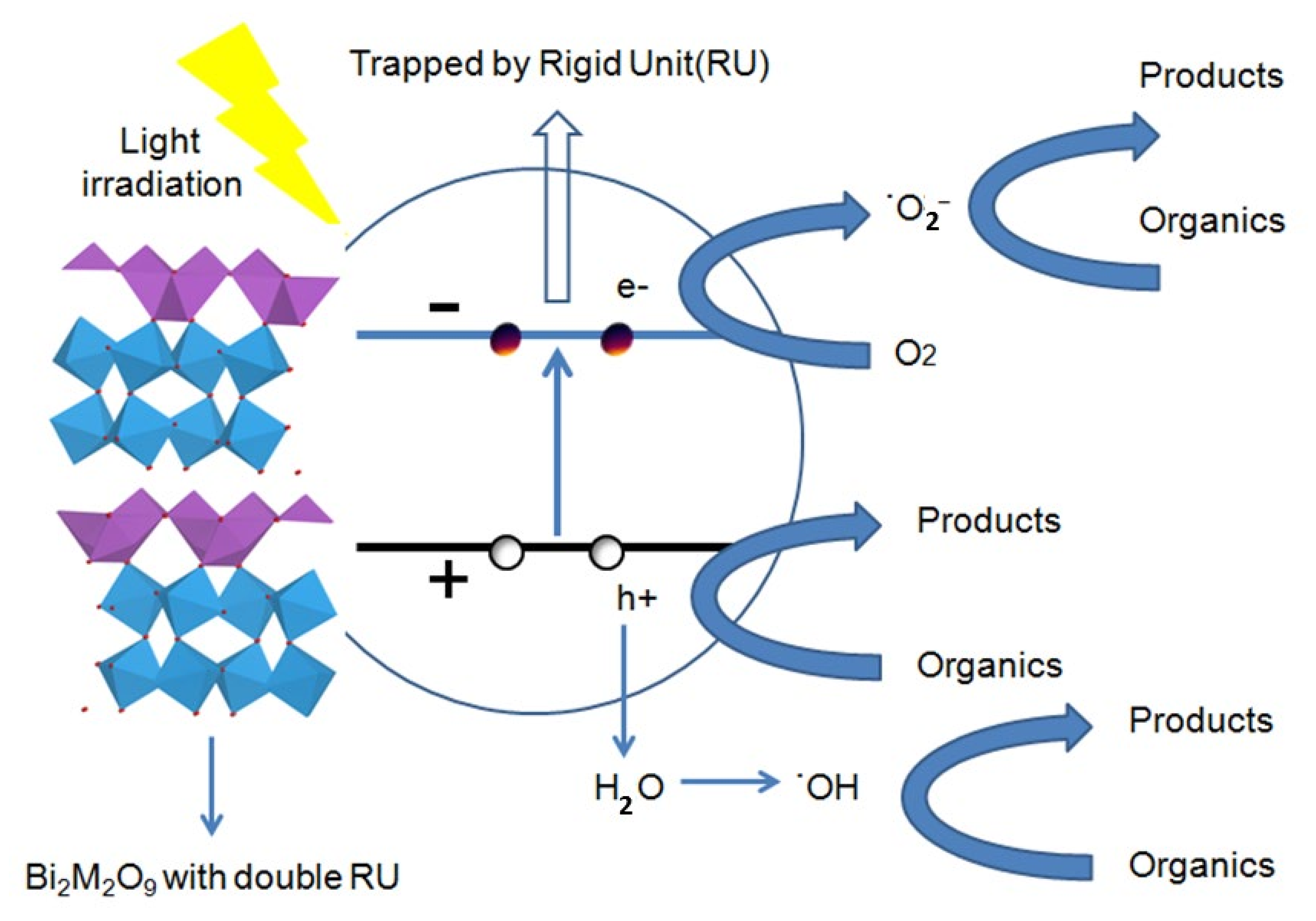
Based on the characterization methods above, a possible photocatalytic mechanism of the Bi2M2O9 photocatalyst under visible-light irradiation is therefore proposed, and the Bi2M2O9 photocatalyst has a stable double Rigid Unit (RU), which consists of two octahedral layers. Double RU mode can be regarded as a strong electron-drawing group, and Bi2M2O9 show a stable reduced bond distance of octahedral RU. The influence of modified structure is reflected in PL test and photocurrent measurement. The reduction of PL intensity and the enhancement of photocurrent express promoted photogenerated electron–hole pairs separation ability; the strong electron-drawing group traps the photogenerated electrons and leads to the increase of active species to promote the photocatalytic performance.
As shown in Figure 12, the photogenerated electrons could migrate to the conduction band (CB) from the valence band (VB) and the photogenerated holes formed in the valence band when the semiconductor was irradiated with visible light. The shortened bond distance of octahedral RU generates a strong electron-drawing efficiency that traps the electron firmly and reduces the recombination rate. Evidentially, the trapped electrons could easily transfer to the oxygen molecules (O2) adsorbed on the surface of the Bi2M2O9 catalysts. Subsequently, the released electrons react with O2 to form the active superoxide radical anion species (O2−). The electron capture and release process enhances the charge transfer and separation efficiency of photogenerated electrons and holes, which contributes to organic contaminant photodegradation by the h+ and ˙O2− species.
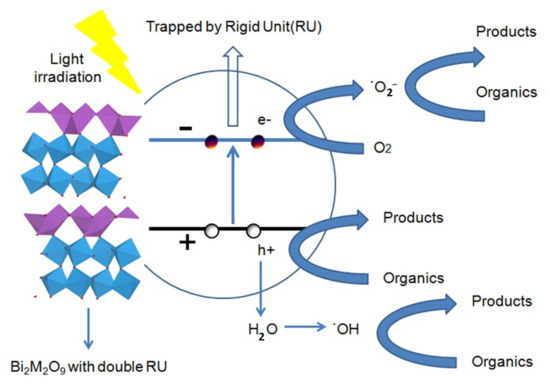
Figure 12.
Schematic illustration of the mechanism of Bi2M2O9 photocatalyst under visible-light irradiation.
Therefore, the above process indicates that an appropriate increase the number of (Bi2O2)2+ layers and (W2O7)2− layers to form a stable Rigid Unit (RU) mode could significantly improve photocatalytic activity.
3. Experimental
3.1. Synthesis of the Photocatalysts
All the reagents used in this study were of analytical purity (Sinopharm Chemical Reagents Co., Ltd., Shanghai, China) and used without any further purification. Bi2WO6, Bi2W2O9, Bi2MoO6 and Bi2Mo2O9 photocatalysts were prepared through a hydrothermal method and the general synthesis processes were as follows: proportionate amounts of sodium tungstate (Na2WO4·H2O) or sodium molybdate (Na2MoO4·H2O) were dissolved in Milli-Q water. Then proportional bismuth (III) nitrate pentahydrate (Bi(NO3)3·5H2O) was dissolved in nitric acid and anionic surfactant AOT was introduced into the solution sequence under stirring to form a transparent solution and ensure Bi:Mo and Bi:W keeps 2:1 during the preparation process of Bi2WO6 and Bi2MoO6, similarly, a value of the ratio of Bi and W or Mo stays at 1 when preparing Bi2W2O9 and Bi2Mo2O9. We adjust the pH value of the solution to ca. 7 using NaOH solution. The slurry was stirred for another 30 min and then poured into Teflon-lined stainless steel autoclaves. The sealed reactors were then heated at 180 °C for 12 h. Then, the products were cooled to room temperature naturally and collected via centrifugation and washed in Milli-Q water and ethanol several times to make sure that the residual impurities were all removed, and then the product was dried at 80 °C for 8 h.
3.2. Characterization of the Photocatalysts
The crystalline phases of the as-prepared samples were analyzed using X-ray diffraction (XRD) (D/MAX-RB, Rigaku, Tokyo, Japan). The diffraction patterns were recorded in the 2θ range from 10 to 70° with a Cu Kα source (λ = 1.5418 Å) running at 40 KV and 30 mA. The morphology images of the as-prepared samples were captured using scanning electron microscopy (SEM) equipped with an energy-dispersive X-ray spectrometer (EDX) on a SUPRA 55 SAP-PHIRE instrument operating at 20 KV. High-resolution transmission electron microscopy (HRTEM) images were acquired with a transmission electron microscopy (F-20, FEI, Hillsboro, OR, USA) at an accelerating voltage of 200 KV. The UV-Vis diffuse reflectance spectra of the as-prepared samples were examined at room temperature using a UV-Vis spectrophotometer (T9s; Persee, Beijing, China) equipped with an integrating sphere. BaSO4 was used as the blank reference. Photoluminescence (PL) spectra were recorded using a fluorescence spectrophotometer (F-4500; Hitachi, Tokyo, Japan) with a Xe lamp as the excitation light source. X-ray photoelectron spectroscopy (XPS) was examined on an X-ray photoelectron spectrometer (ESCALAB 250Xi, Thermo Scientific, Waltham, MA, USA) using an Al Kα radiation.
3.3. Photocatalytic Experiment
The photocatalytic activities of Bi2WO6, Bi2W2O9, Bi2MoO6, and Bi2Mo2O9 photocatalysts under visible light were assessed by degrading 10 mmol·L−1 methylene blue (MB). A 400 W Xe lamp with a UV-cut-off filter (λ > 420 nm) was used as a light source and set about 10 cm apart from the reactor. The experiments were as follows: 40 mg of the photocatalyst was dispersed in 40 mL of MB solution. It was then stirred for 120 min in the dark to achieve an adsorption–desorption equilibrium before light irradiation. During the irradiation, the reaction samples were collected at 60 min intervals and centrifuged to separate out the photocatalyst particles. The ratios (C/C0) of the MB were adopted to evaluate the degradation efficiency (i.e., C0 was the initial concentration, where C was the concentration at a certain time) by checking the absorbance spectrum at 664 nm for MB using a UV-Vis spectrophotometer (T9s; Persee, Beijing, China).
3.4. Measurement of Photocurrent
The measurement of the photocurrent was carried out with an electrochemical workstation (5060F, RST, Zhengzhou, China) in a standard three-electrode system including the samples, an Ag/AgCl electrode (saturated KCl), and a Pt filament used as the working electrode, reference electrode, and counter electrode, respectively. In addition, a pre-made 0.5 mol L−1 Na2SO4 aqueous solution was introduced as the electrolyte. A 100 W incandescent lamp with a 420 nm cut-off filter was used as the light source. The working electrode was manufactured as follows: 5 mg samples were appended to 2 mL of ethanol and Nafion mixture solution (v/v = 30:1), followed by spreading on the middle of an ITO glass in a rounded hole with a diameter of 6 mm.
4. Conclusions
In this study, ordinary Bi2WO6 and Bi2MoO6 samples were successfully synthesized; furthermore, a novel Bi2Mo2O9 fastener sphere and a Bi2W2O9 irregular polyhedron were prepared by a hydrothermal method with AOT introduced. The results revealed that structure-modified Bi2W2O9 and Bi2Mo2O9 exhibited enhanced photocatalytic performance. The Rigid Unit (RU) and modified bond structure influence the trapping–release process of electrons to promote the separation efficiency of photogenerated electron–hole pairs. Therefore, an appropriate increase of the layer number of (W2O7)2− or (Mo2O7)2− between (Bi2O2)2+ layers to form an RU strong electron-drawing group as a method of structure modification can be a potential strategy to improve visible-light photocatalytic activity by affecting the charge behavior.
Author Contributions
Conceptualization, F.W., X.L. and H.L.; methodology, G.Z.; validation, Y.C., H.L.; formal analysis, J.L.; investigation, L.Z., Q.L.; data curation, X.Z., Q.H.; writing—review and editing, F.W., X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Inspiration Fund of Shandong Agriculture and Engineering University (Grant No. BSQJ201811), Qingchuang Science and Technology Program of Shandong Province in China (grant no. 2019KJF029), and the National Natural Science Foundation of China (Grant No. 61801274).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the study are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Xu, C.; Anusuyadevi, P.R.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Asadzadeh-Khaneghah, S.; Habibi-Yangjeh, A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review. J. Clean. Prod. 2020, 276, 124319. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, Fabrication, and Mechanism of Nitrogen-Doped Graphene-Based Photocatalyst. Adv. Mater. 2021, 33, 2003521. [Google Scholar] [CrossRef]
- Liu, X.; Gu, S.; Zhao, Y.; Zhou, G.; Li, W. BiVO4, Bi2WO6 and Bi2MoO6 photocatalysis: A brief review. J. Mater. Sci. Technol. 2020, 56, 45–68. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Gu, S.; Li, H.; Wu, X.; Liu, X. Samarium and Nitrogen Co-Doped Bi2WO6 Photocatalysts: Synergistic Effect of Sm3+/Sm2+ Redox Centers and N-Doped Level for Enhancing Visible-Light Photocatalytic Activity. Chem.-Eur. J. 2016, 22, 12859–12867. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Z.; Jiang, J.; Li, H. Synthesis of Novel Ternary Dual Z-scheme AgBr/LaNiO3/g-C3N4 Composite with Boosted Visible-Light Photodegradation of Norfloxacin. Molecules 2020, 25, 3706. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Gu, S.; Wang, F.; Zhou, H. In-built Tb4+/Tb3+ redox centers in terbium-doped bismuth molybdate nanograss for enhanced photocatalytic activity. Catal. Sci. Technol. 2016, 6, 3510–3519. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.T.; et al. Liquid exfoliation of g-C3N4 nanosheets to construct 2D-2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity. Appl. Catal. B-Environ. 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Li, J.; Yin, Y.; Liu, E.; Ma, Y.; Wan, J.; Fan, J.; Hu, X. In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation. J. Hazard. Mater. 2017, 321, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Lu, J.; Yan, Y.; Zhang, C.; Qiu, Y.; Li, W. Highly efficient photocatalytic performance of BiI/Bi2WO6 for degradation of tetracycline hydrochloride in an aqueous phase. RSC Adv. 2020, 10, 12068–12077. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, D.; Wang, W.; Gao, P.; Bu, K.; Zhang, L.; Zhong, S.; Liu, B. Multiple heterojunction system of Bi2MoO6/WO3/Ag3PO4 with enhanced visible-light photocatalytic performance towards dye degradation. Adv. Powder Technol. 2019, 30, 1910–1919. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; Zeng, G.; Xue, W.; Chen, S.; Li, Z.; Deng, R.; Yang, Y.; Cheng, M. Facile construction of hierarchical flower-like Z-scheme AgBr/Bi2WO6 photocatalysts for effective removal of tetracycline: Degradation pathways and mechanism. Chem. Eng. J. 2019, 375, 121991. [Google Scholar] [CrossRef]
- Vesali-Kermani, E.; Habibi-Yangjeh, A.; Diarmand-Khalilabad, H.; Ghosh, S. Nitrogen photofixation ability of g-C3N4 nanosheets/Bi2MoO6 heterojunction photocatalyst under visible-light illumination. J. Colloid Interf. Sci. 2020, 563, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.; Xia, M.; An, H.; Bai, H.; Wei, J.; Yang, B.; Yang, G. Highly efficient charge transfer at 2D/2D layered P-La2Ti2O7/Bi2WO6 contact heterojunctions for upgraded visible-light-driven photocatalysis. Appl. Catal. B-Environ. 2020, 261, 118244. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B-Environ. 2019, 250, 313–324. [Google Scholar] [CrossRef]
- Zhen, Y.; Yang, C.; Shen, H.; Xue, W.; Gu, C.; Feng, J.; Zhang, Y.; Fu, F.; Liang, Y. Photocatalytic performance and mechanism insights of a S-scheme g-C3N4/Bi2MoO6 heterostructure in phenol degradation and hydrogen evolution reactions under visible light. Phys. Chem. Chem. Phys. 2020, 22, 26278–26288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, C.; Lv, K.; Lu, Y.; Li, Q.; Wu, X.; Li, Y.; Li, X.; Fan, J.; Li, M. SPR effect of bismuth enhanced visible photoreactivity of Bi2WO6 for NO abatement. Chin. J. Catal. 2019, 40, 755–764. [Google Scholar] [CrossRef]
- Ji, L.; Liu, B.; Qian, Y.; Yang, Q.; Gao, P. Enhanced visible-light-induced photocatalytic disinfection of Escherichia coli by ternary Bi2WO6/TiO2/reduced graphene oxide composite materials: Insight into the underlying mechanism. Adv. Powder Technol. 2020, 31, 128–138. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, K.; Ling, W.; Wei, X.; Wang, Y.; Wang, J. Investigation of the Kinetics and Reaction Mechanism for Photodegradation Tetracycline Antibiotics over Sulfur-Doped Bi2WO6-x/ZnIn2S4 Direct Z-Scheme Heterojunction. Nanomaterials 2021, 11, 2123. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, S.O.; la Cruz, A.M. Synthesis, characterization and visible-light photocatalytic properties of Bi2WO6 and Bi2W2O9 obtained by co-precipitation method. Appl. Catal. A Gen. 2010, 383, 128–133. [Google Scholar] [CrossRef]
- Wang, D.W.; Siame, B.; Zhang, S.Y.; Wang, G.; Ju, X.S.; Li, J.L.; Lu, Z.L.; Vardaxoglou, Y.; Whittow, W.; Cadman, D.; et al. Direct integration of cold sintered, temperature-stable Bi2Mo2O9-K2MoO4 ceramics on printed circuit boards for satellite navigation antennas. J. Eur. Ceram. Soc. 2020, 40, 4029–4034. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, T.; Zhao, X.; Zhu, Y. Controllable synthesis of Bi2MoO6 and effect of morphology and variation in local structure on photocatalytic activities. Appl. Catal. B Environ. 2010, 98, 138–146. [Google Scholar] [CrossRef]
- Huang, J.; Tan, G.; Ren, H.; Yang, W.; Xu, C.; Zhao, C.; Xia, A. Photoelectric Activity of a Bi2O3/Bi2WO6–x F2x Heterojunction Prepared by a Simple One-Step Microwave Hydrothermal Method. ACS Appl. Mater. Interfaces 2014, 6, 21041–21050. [Google Scholar] [CrossRef]
- Song, J.; Zhang, L.; Yang, J.; Huang, X.; Hu, J. Facile hydrothermal synthesis of Fe3+ doped Bi2Mo2O9 ultrathin nanosheet with improved photocatalytic performance. Ceram. Int. 2017, 43, 9214–9219. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, J.; Zhang, J.; Tang, Z.; Li, H.; Yuan, J. Z-scheme BiVO4/Ag/Ag2S composites with enhanced photocatalytic efficiency under visible light. RSC Adv. 2020, 10, 30245–30253. [Google Scholar] [CrossRef]
- He, G.; Zhang, J.; Hu, Y.; Bai, Z.; Wei, C. Dual-template synthesis of mesoporous TiO2 nanotubes with structure-enhanced functional photocatalytic performance. Appl. Catal. B-Environ. 2019, 250, 301–312. [Google Scholar] [CrossRef]
- Zhang, H.; He, J.; Zhai, C.; Zhu, M. 2D Bi2WO6/MoS2 as a new photo-activated carrier for boosting electrocatalytic methanol oxidation with visible light illumination. Chin. Chem. Lett. 2019, 30, 2338–2342. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Gu, S.; Li, H.; Wu, X.; Ren, C.; Liu, X. Facile fabrication of direct Z-scheme MoS2/Bi2WO6 heterojunction photocatalyst with superior photocatalytic performance under visible light irradiation. J. Photoch. Photobiol. A 2017, 335, 140–148. [Google Scholar] [CrossRef]
- Ren, J.; Wang, W.Z.; Sun, S.M.; Zhang, L.; Chang, J. Enhanced photocatalytic activity of Bi2WO6 loaded with Ag nanoparticles under visible light irradiation. Appl. Catal. B-Environ. 2009, 92, 50–55. [Google Scholar] [CrossRef]
- Zhang, M.; Du, H.; Ji, J.; Li, F.; Lin, Y.C.; Qin, C.; Zhang, Z.; Shen, Y. Highly Efficient Ag3PO4/g-C3N4 Z-Scheme Photocatalyst for its Enhanced Photocatalytic Performance in Degradation of Rhodamine B and Phenol. Molecules 2021, 26, 2062. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Guo, F.; Yan, Q.; Zhang, Z.; Li, D.; Wang, L.; Zhou, Y. Hydrothermal synthesis of type II ZnIn2S4/BiPO4 heterojunction photocatalyst with dandelion-like microflower structure for enhanced photocatalytic degradation of tetracycline under simulated solar light. J. Alloys Compd. 2019, 811, 151976. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S. MoS2/WO3 heterojunction with the intensified photocatalytic performance for decomposition of organic pollutants under the broad array of solar light. J. Clean. Prod. 2021, 324, 129290. [Google Scholar] [CrossRef]
- Feizpoor, S.; Habibi-Yangjeh, A.; Ahadzadeh, I.; Yubuta, K. Oxygen-rich TiO2 decorated with C-Dots: Highly efficient visible-light-responsive photocatalysts in degradations of different contaminants. Adv. Powder Technol. 2019, 30, 1183–1196. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, Y.; Xu, S.; Li, Y.; Lin, X.; Qin, Y. Rational design of 3D/2D In2O3 nanocube/ZnIn2S4 nanosheet heterojunction photocatalyst with large-area “high-speed channels” for photocatalytic oxidation of 2, 4-dichlorophenol under visible light. J. Hazard. Mater. 2020, 382, 121098. [Google Scholar] [CrossRef]
- Yu, B.; Meng, F.; Khan, M.W.; Qin, R.; Liu, X. Facile synthesis of AgNPs modified TiO2@ g-C3N4 heterojunction composites with enhanced photocatalytic activity under simulated sunlight. Mater. Res. Bull. 2020, 121, 110641. [Google Scholar] [CrossRef]
- Wang, F.; Gu, S.; Shang, R.; Jing, P.; Wang, Y.; Li, W. Fabrication of AgBr/La2Ti2O7 hierarchical heterojunctions: Boosted interfacial charge transfer and high efficiency visible-light photocatalytic activity. Sep. Purif. Technol. 2019, 229, 115798. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Gu, S.; Li, H.; Liu, X.; Wang, M. Fabrication of FeWO4@ZnWO4/ZnO heterojunction photocatalyst: Synergistic effect of ZnWO4/ZnO and FeWO4@ZnWO4/ZnO heterojunction structure on the enhancement of visible-light photocatalytic activity. ACS Sustain. Chem. Eng. 2016, 4, 6288–6298. [Google Scholar] [CrossRef]
- Sun, X.; Li, H.J.; Ou, N.; Lyu, B.; Gui, B.; Tian, S.; Qian, D.; Wang, X.; Yang, J. Visible-light driven TiO2 photocatalyst coated with graphene quantum dots of tunable nitrogen doping. Molecules 2019, 24, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).