The Application of Nitric Oxide for Ocular Hypertension Treatment
Abstract
1. Introduction
2. IOP Regulation in Physiological and Pathological Conditions
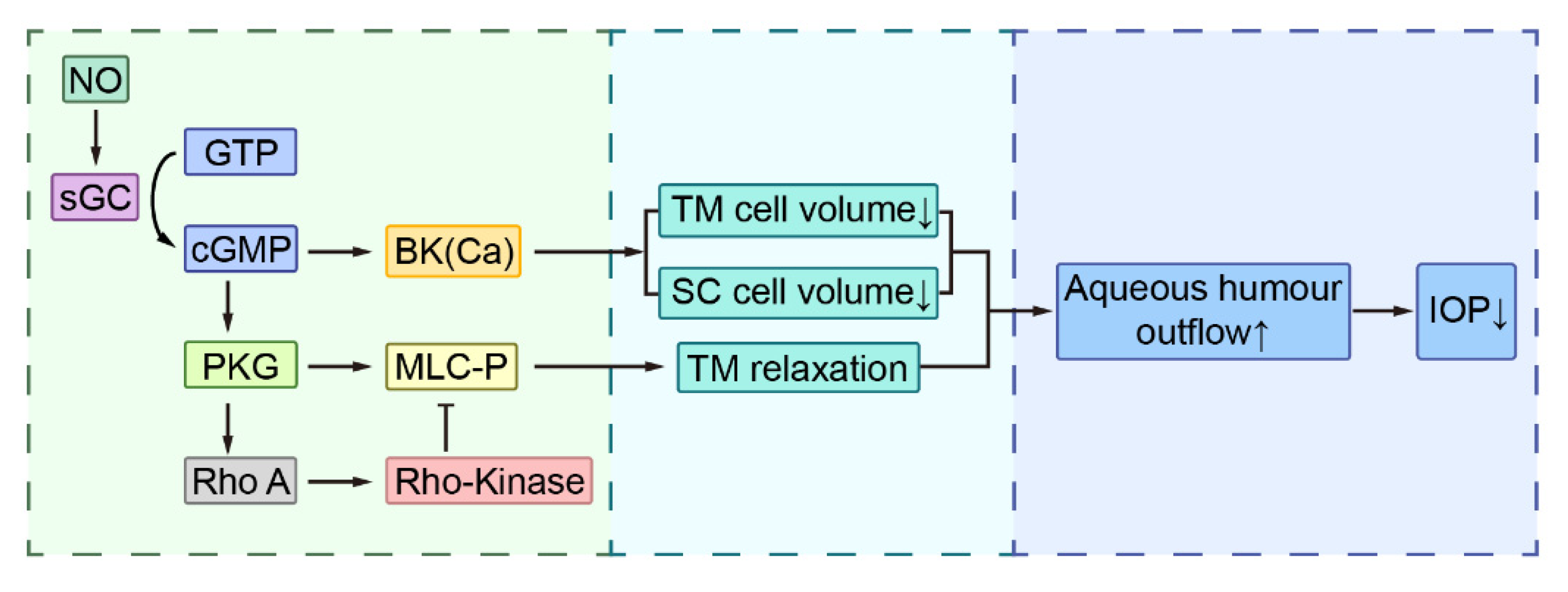
3. NO and IOP Regulation
4. Nitric Oxide-Donating Drugs for IOP Lowering
4.1. Nitric Oxide-Donating Prostaglandins Analogues
4.2. Nitric Oxide-Donating Carbonic Anhydrase Inhibitor
4.3. Nano-Material Based NO Donors
4.4. Other Kinds of NO Donors
5. Challenges Associated with NO Donors
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Heijl, A.; Hyman, L.; Bengtsson, B.; Dong, L.; Yang, Z. Predictors of Long-term Progression in the Early Manifest Glaucoma Trial. Ophthalmol. 2007, 114, 1965–1972. [Google Scholar] [CrossRef]
- Stamer, W.D.; Acott, T.S. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Aliancy, J.; Stamer, W.D.; Wirostko, B. A Review of Nitric Oxide for the Treatment of Glaucomatous Disease. Ophthalmol. Ther. 2017, 6, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Impagnatiello, F.; Bastia, E.; Almirante, N.; Brambilla, S.; Duquesroix, B.; Kothe, A.C.; Bergamini, M.V.W. Prostaglandin analogues and nitric oxide contribution in the treatment of ocular hypertension and glaucoma. Br. J. Pharmacol. 2019, 176, 1079–1089. [Google Scholar] [CrossRef]
- Shahidullah, M.; Yap, M.; To, C.-H. Cyclic GMP, sodium nitroprusside and sodium azide reduce aqueous humour formation in the isolated arterially perfused pig eye. Br. J. Pharmacol. 2005, 145, 84–92. [Google Scholar] [CrossRef]
- Shahidullah, M.; Delamere, N. NO donors inhibit Na,K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br. J. Pharmacol. 2006, 148, 871–880. [Google Scholar] [CrossRef]
- Cavet, M.E.; Vittitow, J.L.; Impagnatiello, F.; Ongini, E.; Bastia, E. Nitric Oxide (NO): An Emerging Target for the Treatment of Glaucoma. Investig. Opthalmology Vis. Sci. 2014, 55, 5005–5015. [Google Scholar] [CrossRef]
- Wiederholt, M.; Thieme, H.; Stumpff, F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog. Retin. Eye Res. 2000, 19, 271–295. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, X.; Song, M.; Wu, J.-H.; Sun, X. Aqueous Humor Outflow Physiology in NOS3 Knockout Mice. Investig. Opthalmology Vis. Sci. 2015, 56, 4891–4898. [Google Scholar] [CrossRef]
- Winkler, N.; Fautsch, M.P. Effects of Prostaglandin Analogues on Aqueous Humor Outflow Pathways. J. Ocul. Pharmacol. Ther. 2014, 30, 102–109. [Google Scholar] [CrossRef]
- Alm, A.; Nilsson, S.F. Uveoscleral outflow—A review. Exp. Eye Res. 2009, 88, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Orzalesi, N.; Rossetti, L.; Invernizzi, T.; Bottoli, A.; Autelitano, A. Effect of timolol, latanoprost, and dorzolamide on circadian IOP in glaucoma or ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2566–2573. [Google Scholar]
- Lorenz, K.; Pfeiffer, N. Efficacy and safety of tafluprost 0.0015% and timolol maleate 0.5% fixed combination in patients with ocular hypertension or open-angle glaucoma. Expert Opin. Pharmacother. 2014, 15, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Kanno, M.; Araie, M.; Koibuchi, H.; Masuda, K. Effects of topical nipradilol, a beta blocking agent with alpha blocking and nitroglycerin-like activities, on intraocular pressure and aqueous dynamics in humans. Br. J. Ophthalmol. 2000, 84, 293–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanno, M.; Araie, M.; Tomita, K.; Sawanobori, K. Effects of topical nipradilol, a beta-blocking agent with alpha-blocking and nitroglycerin-like activities, on aqueous humor dynamics and fundus circulation. Investig. Ophthalmol. Vis. Sci. 1998, 39, 736–743. [Google Scholar]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Mincione, F.; Starnotti, M.; Masini, E.; Bacciottini, L.; Scrivanti, C.; Casini, A.; Vullo, D.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Design of thioureido sulfonamides with potent isozyme II and XII inhibitory properties and intraocular pressure lowering activity in a rabbit model of glaucoma. Bioorganic Med. Chem. Lett. 2005, 15, 3821–3827. [Google Scholar] [CrossRef]
- Steele, R.M.; Benedini, F.; Biondi, S.; Borghi, V.; Carzaniga, L.; Impagnatiello, F.; Miglietta, D.; Chong, W.K.; Rajapakse, R.; Cecchi, A.; et al. Nitric oxide-donating carbonic anhydrase inhibitors for the treatment of open-angle glaucoma. Bioorganic Med. Chem. Lett. 2009, 19, 6565–6570. [Google Scholar] [CrossRef]
- Jia, F.; Li, L.; Fang, Y.; Song, M.; Man, J.; Jin, Q.; Lei, Y.; Ji, J. Macromolecular Platform with Super-Cation Enhanced Trans-Cornea Infiltration for Noninvasive Nitric Oxide Delivery in Ocular Therapy. ACS Nano 2020, 14, 16929–16938. [Google Scholar] [CrossRef]
- Hu, C.; Sun, J.; Zhang, Y.; Chen, J.; Lei, Y.; Sun, X.; Deng, Y. Local Delivery and Sustained-Release of Nitric Oxide Donor Loaded in Mesoporous Silica Particles for Efficient Treatment of Primary Open-Angle Glaucoma. Adv. Heal. Mater. 2018, 7, e1801047. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Song, M.; Li, L.; Niu, L.; Chen, Y.; Han, B.; Sun, X.; Yang, Z.; Lei, Y.; Chen, X. Endogenous dual stimuli-activated NO generation in the conventional outflow pathway for precision glaucoma therapy. Biomaterials 2021, 277, 121074. [Google Scholar] [CrossRef] [PubMed]
- Chandrawati, R.; Chang, J.Y.; Reina-Torres, E.; Jumeaux, C.; Sherwood, J.M.; Stamer, W.D.; Zelikin, A.N.; Overby, D.R.; Stevens, M.M. Localized and Controlled Delivery of Nitric Oxide to the Conventional Outflow Pathway via Enzyme Biocatalysis: Toward Therapy for Glaucoma. Adv. Mater. 2017, 29, 1604932. [Google Scholar] [CrossRef] [PubMed]
- Heijl, A.; Bengtsson, B.; Hyman, L.; Leske, M.C. Natural History of Open-Angle Glaucoma. Ophthalmology 2009, 116, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Tamm, E.R. The trabecular meshwork outflow pathways: Structural and functional aspects. Exp. Eye Res. 2009, 88, 648–655. [Google Scholar] [CrossRef]
- Andrés-Guerrero, V.; Feijoo, J.G.; Konstas, A.G. Targeting Schlemm’s Canal in the Medical Therapy of Glaucoma: Current and Future Considerations. Adv. Ther. 2017, 34, 1049–1069. [Google Scholar] [CrossRef]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Ster, A.M.; Popp, R.A.; Petrisor, F.M.; Stan, C.; Pop, V.I. The Role of Oxidative Stress and Vascular Insufficiency in Primary Open Angle Glaucoma. Clujul Med. 2014, 87, 143. [Google Scholar]
- Nettesheim, A.; Dixon, A.; Shim, M.S.; Coyne, A.; Walsh, M.; Liton, P.B. Autophagy in the Aging and Experimental Ocular Hypertensive Mouse Model. Investig. Opthalmol. Vis. Sci. 2020, 61, 31. [Google Scholar] [CrossRef]
- Lei, Y.; Stamer, W.D.; Wu, J.; Sun, X. Cell Senescence Reduced the Mechanotransduction Sensitivity of Porcine Angular Aqueous Plexus Cells to Elevation of Pressure. Investig. Opthalmol. Vis. Sci. 2014, 55, 2324–2328. [Google Scholar] [CrossRef]
- Lei, Y.; Stamer, W.D.; Wu, J.-H.; Sun, X. Oxidative Stress Impact on Barrier Function of Porcine Angular Aqueous Plexus Cell Monolayers. Investig. Opthalmol. Vis. Sci. 2013, 54, 4827–4835. [Google Scholar] [CrossRef]
- Niu, L.; Li, L.; Xing, C.; Luo, B.; Hu, C.; Song, M.; Niu, J.; Ruan, Y.; Sun, X.; Lei, Y. Airborne particulate matter (PM2.5) triggers cornea inflammation and pyroptosis via NLRP3 activation. Ecotoxicol. Environ. Saf. 2021, 207, 111306. [Google Scholar] [CrossRef]
- Saccà, S.C.; Pascotto, A.; Camicione, P.; Capris, P.; Izzotti, A. Oxidative DNA damage in the human trabecular mesh-work: Clinical correlation in patients with primary open-angle glaucoma. Arch. Ophthalmol. 2005, 123, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Overby, D.R.; Read, A.T.; Stamer, W.D.; Ethier, C.R. A new method for selection of angular aqueous plexus cells from porcine eyes: A model for Schlemm’s canal endothelium. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5744–5750. [Google Scholar] [CrossRef] [PubMed]
- Liton, P.B.; Lin, Y.; Luna, C.; Li, G.; Gonzalez, P.; Epstein, D.L. Cultured Porcine Trabecular Meshwork Cells Display Altered Lysosomal Function When Subjected to Chronic Oxidative Stress. Investig. Opthalmol. Vis. Sci. 2008, 49, 3961–3969. [Google Scholar] [CrossRef]
- Hirt, J.; Porter, K.; Dixon, A.; McKinnon, S.; Liton, P.B. Contribution of autophagy to ocular hypertension and neurodegeneration in the DBA/2J spontaneous glaucoma mouse model. Cell Death Discov. 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Last, J.A.; Pan, T.; Ding, Y.; Reilly, C.M.; Keller, K.; Acott, T.S.; Fautsch, M.P.; Murphy, C.J.; Russell, P. Elastic Modulus Determination of Normal and Glaucomatous Human Trabecular Meshwork. Investig. Opthalmol. Vis. Sci. 2011, 52, 2147–2152. [Google Scholar] [CrossRef]
- Camras, L.J.; Stamer, W.D.; Epstein, D.; Gonzalez, P.; Yuan, F. Differential Effects of Trabecular Meshwork Stiffness on Outflow Facility in Normal Human and Porcine Eyes. Investig. Opthalmol. Vis. Sci. 2012, 53, 5242–5250. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Johnstone, M.A.; Xin, C.; Song, S.; Padilla, S.; Vranka, J.A.; Acott, T.S.; Zhou, K.; Schwaner, S.A.; Wang, R.; et al. Estimating Human Trabecular Meshwork Stiffness by Numerical Modeling and Advanced OCT Imaging. Investig. Opthalmol. Vis. Sci. 2017, 58, 4809–4817. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Z. Zellforsch. Mikrosk. Anat. 2011, 347, 279–290. [Google Scholar] [CrossRef]
- Ethier, C.R.; Read, A.T.; Chan, D.W.-H. Effects of Latrunculin-B on Outflow Facility and Trabecular Meshwork Structure in Human Eyes. Investig. Opthalmol. Vis. Sci. 2006, 47, 1991–1998. [Google Scholar] [CrossRef]
- Tian, B.; Gabelt, B.T.; A Peterson, J.; A Kiland, J.; Kaufman, P.L. H-7 increases trabecular facility and facility after ciliary muscle disinsertion in monkeys. Investig. Ophthalmol. Vis. Sci. 1999, 40, 239–242. [Google Scholar]
- McKee, C.T.; Wood, J.A.; Shah, N.M.; Fischer, M.E.; Reilly, C.M.; Murphy, C.J.; Russell, P. The effect of biophysical attributes of the ocular trabecular meshwork associated with glaucoma on the cell response to therapeutic agents. Biomaterials 2011, 32, 2417–2423. [Google Scholar] [CrossRef]
- Wareham, L.K.; Buys, E.S.; Sappington, R.M. The nitric oxide-guanylate cyclase pathway and glaucoma. Nitric Oxide 2018, 77, 75–87. [Google Scholar] [CrossRef]
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric oxide and its derivatives in the cancer battlefield. Nitric Oxide 2019, 93, 102–114. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nat. Cell Biol. 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Palmer, R.M.; Moncada, S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989, 158, 348–352. [Google Scholar] [CrossRef]
- A Nathanson, J.; McKee, M. Identification of an extensive system of nitric oxide-producing cells in the ciliary muscle and outflow pathway of the human eye. Investig. Ophthalmol. Vis. Sci. 1995, 36, 1765–1773. [Google Scholar]
- Becquet, F.; Courtois, Y.; Goureau, O. Nitric oxide in the eye: Multifaceted roles and diverse outcomes. Surv. Ophthalmol. 1997, 42, 71–82. [Google Scholar] [CrossRef]
- Chang, J.Y.; Stamer, W.D.; Bertrand, J.; Read, A.T.; Marando, C.; Ethier, C.R.; Overby, D.R. Role of nitric oxide in murine conventional outflow physiology. Am. J. Physiol. Physiol. 2015, 309, C205–C214. [Google Scholar] [CrossRef] [PubMed]
- Knowles, R.G.; Palacios, M.; Palmer, R.M.; Moncada, S. Formation of nitric oxide from L-arginine in the central nervous system: A transduction mechanism for stimulation of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 1989, 86, 5159–5162. [Google Scholar] [CrossRef]
- Mergia, E.; Russwurm, M.; Zoidl, G.; Koesling, D. Major occurrence of the new alpha2beta1 isoform of NO-sensitive guanylyl cyclase in brain. Cell Signal. 2003, 15, 189–195. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D. cGMP-Dependent Protein Kinases and cGMP Phosphodiesterases in Nitric Oxide and cGMP Action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- Fernández-Durango, R.; Fernández-Martínez, A.; Feijoo, J.G.; Castillo, A.; De La Casa, J.M.; Bueno, B.G.; Perez-Nievas, B.G.; Fernández-Cruz, A.; Leza, J.C. Expression of Nitrotyrosine and Oxidative Consequences in the Trabecular Meshwork of Patients with Primary Open-Angle Glaucoma. Investig. Opthalmol. Vis. Sci. 2008, 49, 2506–2511. [Google Scholar] [CrossRef]
- Sierra, A.; Navascués, J.; Cuadros, M.A.; Calvente, R.; Martín-Oliva, D.; Ferrer-Martín, R.M.; Martín-Estebané, M.; Car-rasco, M.C.; Marín-Teva, J.L. Expression of inducible nitric oxide synthase (iNOS) in microglia of the developing quail retina. PLoS ONE 2014, 9, e106048. [Google Scholar]
- Kang, J.H.; Willett, W.C.; Rosner, B.A.; Buys, E.; Wiggs, J.L.; Pasquale, L.R. Association of Dietary Nitrate Intake With Primary Open-Angle Glaucoma: A Prospective Analysis From the Nurses’ Health Study and Health Professionals Follow-up Study. JAMA Ophthalmol. 2016, 134, 294–303. [Google Scholar] [CrossRef]
- Hara, M.R.; Agrawal, N.; Kim, S.F.; Cascio, M.B.; Fujimuro, M.; Ozeki, Y.; Takahashi, M.; Cheah, J.H.; Tankou, S.K.; Hester, L.D.; et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005, 7, 665–674. [Google Scholar] [CrossRef]
- Handy, D.E.; Loscalzo, J. Nitric oxide and posttranslational modification of the vascular proteome: S-nitrosation of reactive thiols. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Buys, E.S.; Potter, L.R.; Pasquale, L.R.; Ksander, B.R. Regulation of intraocular pressure by soluble and membrane guanylate cyclases and their role in glaucoma. Front. Mol. Neurosci. 2014, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Goureau, O.; Lepoivre, M.; Becquet, F.; Courtois, Y. Differential regulation of inducible nitric oxide synthase by fibroblast growth factors and transforming growth factor beta in bovine retinal pigmented epithelial cells: Inverse correlation with cellular proliferation. Proc. Natl. Acad. Sci. USA 1993, 90, 4276–4280. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, R.; Rokicki, W.; Żaba, M.; Wyględowska-Promieńska, D.; Kabiesz, A.; Reichman-Warmusz, E.; Brzozowa, M.; Majewski, W. Inducible and Endothelial Nitric Synthetase Expression and Nitrotyrosine Accumulation in Iris Vasculature of Patients with Primary Open-Angle Glaucoma: A Pilot Study. Med. Sci. Monit. 2015, 21, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Garhöfer, G.; Schmetterer, L. Nitric oxide: A drug target for glaucoma revisited. Drug Discov. Today 2019, 24, 1614–1620. [Google Scholar] [CrossRef]
- Ellis, D.Z. Guanylate cyclase activators, cell volume changes and IOP reduction. Cell. Physiol. Biochem. 2011, 28, 1145–1154. [Google Scholar] [CrossRef]
- Nathanson, J.A. Direct application of a guanylate cyclase activator lowers intraocular pressure. Eur. J. Pharmacol. 1988, 147, 155–156. [Google Scholar] [CrossRef]
- Behar-Cohen, F.F.; Goureau, O.; D’Hermies, F.; Courtois, Y. Decreased intraocular pressure induced by nitric oxide donors is correlated to nitrite production in the rabbit eye. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1711–1715. [Google Scholar]
- Krauss, A.H.; Impagnatiello, F.; Toris, C.B.; Gale, D.C.; Prasanna, G.; Borghi, V.; Chiroli, V.; Chong, W.; Carreiro, S.T.; Ongini, E. Ocular hypotensive activity of BOL-303259-X, a nitric oxide donating Prostaglandin F2α agonist, in preclinical models. Exp. Eye Res. 2011, 93, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Dismuke, W.M.; Mbadugha, C.C.; Ellis, D.Z. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am. J. Physiol. Physiol. 2008, 294, C1378–C1386. [Google Scholar] [CrossRef]
- Dismuke, W.M.; Sharif, N.A.; Ellis, D.Z. Human Trabecular Meshwork Cell Volume Decrease by NO-Independent Soluble Guanylate Cyclase Activators YC-1 and BAY-58-2667 Involves the BKCaIon Channel. Investig. Opthalmol. Vis. Sci. 2009, 50, 3353–3359. [Google Scholar] [CrossRef][Green Version]
- Heyne, G.W.; Kiland, J.A.; Kaufman, P.L.; Gabelt, B.T. Effect of Nitric Oxide on Anterior Segment Physiology in Monkeys. Investig. Opthalmol. Vis. Sci. 2013, 54, 5103–5110. [Google Scholar] [CrossRef]
- Kotikoski, H.; Alajuuma, P.; Moilanen, E.; Salmenperä, P.; Oksala, O.; Laippala, P.; Vapaatalo, H. Comparison of Nitric Oxide Donors in Lowering Intraocular Pressure in Rabbits: Role of Cyclic GMP. J. Ocul. Pharmacol. Ther. 2002, 18, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Korenfeld, M.S.; Becker, B. Atrial natriuretic peptides. Effects on intraocular pressure, cGMP, and aqueous flow. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2385–2392. [Google Scholar]
- Becker, B. Topical 8-bromo-cyclic GMP lowers intraocular pressure in rabbits. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1647–1649. [Google Scholar]
- Samuelsson-Almén, M.; Nilsson, S.F.; Mäepea, O.; Bill, A. Effects of atrial natriuretic factor (ANF) on intraocular pressure and aqueous humor flow in the cynomolgus monkey. Exp. Eye Res. 1991, 53, 253–260. [Google Scholar] [CrossRef]
- Muenster, S.; Lieb, W.S.; Fabry, G.; Allen, K.N.; Kamat, S.S.; Guy, A.H.; Dordea, A.C.; Teixeira, L.; Tainsh, R.E.; Yu, B.; et al. The Ability of Nitric Oxide to Lower Intraocular Pressure Is Dependent on Guanylyl Cyclase. Investig. Opthalmol. Vis. Sci. 2017, 58, 4826–4835. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Navarro, I.D.; Kessler, M.M.; Bernier, S.G.; Perl, N.R.; Sarno, R.; Masferrer, J.; Hannig, G.; Stamer, W.D. The Soluble Guanylate Cyclase Stimulator IWP-953 Increases Conventional Outflow Facility in Mouse Eyes. Investig. Opthalmol. Vis. Sci. 2016, 57, 1317–1326. [Google Scholar] [CrossRef]
- Ellis, D.Z.; Dismuke, W.M.; Chokshi, B.M. Characterization of Soluble Guanylate Cyclase in NO-Induced Increases in Aqueous Humor Outflow Facility and in the Trabecular Meshwork. Investig. Opthalmol. Vis. Sci. 2009, 50, 1808–1813. [Google Scholar] [CrossRef]
- Ellis, D.Z.; Sharif, N.A.; Dismuke, W.M. Endogenous regulation of human Schlemm’s canal cell volume by nitric oxide signaling. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5817–5824. [Google Scholar] [CrossRef]
- Mao, Y.-J.; Wu, J.-B.; Yang, Z.-Q.; Zhang, Y.-H.; Huang, Z.-J. Nitric oxide donating anti-glaucoma drugs: Advances and prospects. Chin. J. Nat. Med. 2020, 18, 275–283. [Google Scholar] [CrossRef]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Marrion, N.V.; Peters, J.A.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; Sharman, J.L.; et al. THE Concise Guide to Pharmacology 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 2017, 174 (Suppl. 1), S17–S129. [Google Scholar] [CrossRef]
- A Garcia, G.; Ngai, P.; Mosaed, S.; Lin, K.Y. Critical evaluation of latanoprostene bunod in the treatment of glaucoma. Clin. Ophthalmol. 2016, ume 10, 2035–2050. [Google Scholar] [CrossRef]
- Cavet, M.E.; DeCory, H.H. The Role of Nitric Oxide in the Intraocular Pressure Lowering Efficacy of Latanoprostene Bunod: Review of Nonclinical Studies. J. Ocul. Pharmacol. Ther. 2018, 34, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Gulati, V.; Fan, S.; Zhao, M.; Maslonka, M.A.; Gangahar, C.; Toris, C.B. Diurnal and Nocturnal Variations in Aqueous Humor Dynamics of Patients with Ocular Hypertension Undergoing Medical Therapy. Arch. Ophthalmol. 2012, 130, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Impagnatiello, F.; Toris, C.B.; Batugo, M.; Prasanna, G.; Borghi, V.; Bastia, E.; Ongini, E.; Krauss, A.H.P. Intraocular Pressure–Lowering Activity of NCX 470, a Novel Nitric Oxide–Donating Bimatoprost in Preclinical Models. Investig. Opthalmol. Vis. Sci. 2015, 56, 6558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blangetti, M.; Rolando, B.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Durante, M.; Masini, E.; Almirante, N.; Bastia, E.; Impagnatiello, F.; et al. New furoxan derivatives for the treatment of ocular hypertension. Bioorganic Med. Chem. Lett. 2017, 27, 479–483. [Google Scholar] [CrossRef]
- Cavet, M.E.; Vollmer, T.R.; Harrington, K.L.; VanDerMeid, K.R.; Richardson, M.E. Regulation of Endothelin-1–Induced Trabecular Meshwork Cell Contractility by Latanoprostene Bunod. Investig. Opthalmol. Vis. Sci. 2015, 56, 4108–4116. [Google Scholar] [CrossRef]
- Costa, V.P.; Harris, A.; Anderson, D.; Stodtmeister, R.; Cremasco, F.; Kergoat, H.; Lovasik, J.; Stalmans, I.; Zeitz, O.; Lanzl, I.; et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014, 92, e252–e266. [Google Scholar] [CrossRef]
- Araie, M.; Sforzolini, B.S.; Vittitow, J.; Weinreb, R.N. Evaluation of the Effect of Latanoprostene Bunod Ophthalmic Solution, 0.024% in Lowering Intraocular Pressure over 24 h in Healthy Japanese Subjects. Adv. Ther. 2015, 32, 1128–1139. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Ong, T.; Sforzolini, B.S.; Vittitow, J.L.; Singh, K.; Kaufman, P.L. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: The VOYAGER study. Br. J. Ophthalmol. 2015, 99, 738–745. [Google Scholar] [CrossRef]
- Liu, J.H.; Slight, J.R.; Vittitow, J.L.; Sforzolini, B.S.; Weinreb, R.N. Efficacy of Latanoprostene Bunod 0.024% Compared with Timolol 0.5% in Lowering Intraocular Pressure Over 24 Hours. Am. J. Ophthalmol. 2016, 169, 249–257. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Scassellati Sforzolini, B.; Vittitow, J.; Liebmann, J. Latanoprostene Bunod 0.024% versus Timolol Male-ate 0.5% in Subjects with Open-Angle Glaucoma or Ocular Hypertension: The APOLLO Study. Ophthalmology 2016, 123, 965–973. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Martin, K.R.; Peace, J.; Sforzolini, B.S.; Vittitow, J.L.; Weinreb, R.N. Comparison of Latanoprostene Bunod 0.024% and Timolol Maleate 0.5% in Open-Angle Glaucoma or Ocular Hypertension: The LUNAR Study. Am. J. Ophthalmol. 2016, 168, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Becker, B. The Mechanism of the Fall in Intraocular Pressure Induced by the CARBONIC Anhydrase Inhibitor, Diamox*. Am. J. Ophthalmol. 1955, 39, 177–184. [Google Scholar] [CrossRef]
- Sugrue, M.F. Pharmacological and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog. Retin. Eye Res. 2000, 19, 87–112. [Google Scholar] [CrossRef]
- Maren, T.H.; Jankowska, L.; Sanyal, G.; Edelhauser, H.F. The transcorneal permeability of sulfonamide carbonic anhydrase inhibitors and their effect on aqueous humor secretion. Exp. Eye Res. 1983, 36, 457–479. [Google Scholar] [CrossRef]
- Mincione, F.; Scozzafava, A.; Supuran, C.T. The development of topically acting carbonic anhydrase inhibitors as an-tiglaucoma agents. Curr. Pharm. Des. 2008, 14, 649–654. [Google Scholar]
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future perspective in topical glaucoma therapeutics. Int. J. Pharm. 2002, 248, 1–14. [Google Scholar] [CrossRef]
- Schwartzenberg, G.W.T.; E Trope, G. Anorexia, depression and dementia induced by dorzolamide eyedrops (Trusopt). Can. J. Ophthalmol. 1999, 34, 93–94. [Google Scholar]
- Carlsen, J.; Durcan, J.; Zabriskie, N.; Swartz, M.; Crandall, A. Nephrolithiasis with dorzolamide. Arch. Ophthalmol. 1999, 117, 1087–1088. [Google Scholar] [CrossRef]
- Mitsuyama, S.; Abe, F.; Higuchi, T. Allergic contact dermatitis due to dorzolamide eyedrops. Contact Dermat. 2021, 84, 58–59. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Lancaster, J.R., Jr.; Freeman, B.A.; Schechter, A.N. Nitric oxide’s reactions with hemoglobin: A view through the SNO-storm. Nat. Med. 2003, 9, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Reighard, K.P.; Saavedra, J.E.; Schoenfisch, M.H. O2-Protected diazeniumdiolate-modified silica nanoparticles for extended nitric oxide release from dental composites. Biomater. Sci. 2013, 1, 456–459. [Google Scholar] [CrossRef]
- Riccio, D.A.; Schoenfisch, M.H. Nitric oxide release: Part I. Macromolecular scaffolds. Chem. Soc. Rev. 2012, 41, 3731–3741. [Google Scholar] [CrossRef]
- Blecher, K.; Martinez, L.R.; Tuckman-Vernon, C.; Nacharaju, P.; Schairer, D.; Chouake, J.; Friedman, J.M.; Alfieri, A.; Guha, C.; Nosanchuk, J.D.; et al. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1364–1371. [Google Scholar] [CrossRef]
- Han, G.; Nguyen, L.N.; Macherla, C.; Chi, Y.; Friedman, J.M.; Nosanchuk, J.D.; Martinez, L.R. Nitric Oxide–Releasing Nanoparticles Accelerate Wound Healing by Promoting Fibroblast Migration and Collagen Deposition. Am. J. Pathol. 2012, 180, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Shin, J.H.; Stasko, N.A.; Johnson, C.B.; Wespe, D.A.; Holmuhamedov, E.; Schoenfisch, M.H. Bactericidal Efficacy of Nitric Oxide-Releasing Silica Nanoparticles. ACS Nano 2008, 2, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Cabrales, P.; Han, G.; Nacharaju, P.; Friedman, A.J.; Friedman, J.M. Reversal of hemoglobin-induced vasoconstriction with sustained release of nitric oxide. Am. J. Physiol. Circ. Physiol. 2011, 300, H49–H56. [Google Scholar] [CrossRef] [PubMed]
- Nachuraju, P.; Friedman, A.J.; Friedman, J.M.; Cabrales, P. Exogenous nitric oxide prevents cardiovascular collapse during hemorrhagic shock. Resuscitation 2011, 82, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Nacharaju, P.; Tuckman-Vernon, C.; Maier, K.E.; Chouake, J.; Friedman, A.; Cabrales, P.; Friedman, J.M. A nanoparticle delivery vehicle for S-nitroso-N-acetyl cysteine: Sustained vascular response. Nitric Oxide 2012, 27, 150–160. [Google Scholar] [CrossRef]
- Johnson, T.A.; Stasko, N.A.; Matthews, J.L.; Cascio, W.E.; Holmuhamedov, E.; Johnson, C.B.; Schoenfisch, M.H. Reduced ischemia/reperfusion injury via glutathione-initiated nitric oxide-releasing dendrimers. Nitric Oxide 2010, 22, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Tar, M.; Kuppam, D.S.; Friedman, A.; Melman, A.; Friedman, J.; Davies, K.P. Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J. Sex. Med. 2010, 7, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.; Dong, Z.; Su, L.; Boyer, C.; George, J.; Davis, T.P.; Wang, J. The Use of Nanoparticles to Deliver Nitric Oxide to Hepatic Stellate Cells for Treating Liver Fibrosis and Portal Hypertension. Small 2015, 11, 2291–2304. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Ignarro, L.J. Nitric oxide and atherosclerosis. Nitric Oxide 2001, 5, 88–97. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Stamler, J.S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA 2002, 99, 8306–8311. [Google Scholar] [CrossRef] [PubMed]
- Sydow, K.; Daiber, A.; Oelze, M.; Chen, Z.; August, M.; Wendt, M.; Ullrich, V.; Mülsch, A.; Schulz, E.; Keaney, J.F., Jr.; et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitro-glycerin tolerance and cross-tolerance. J. Clin. Investig. 2004, 113, 482–489. [Google Scholar] [CrossRef]
- Knott, A.B.; Bossy-Wetzel, E. Nitric Oxide in Health and Disease of the Nervous System. Antioxid. Redox Signal. 2009, 11, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, Y.; Song, M.; Deng, Y.; Sun, X.; Lei, Y. Prolonged use of nitric oxide donor sodium nitroprusside induces ocular hypertension in mice. Exp. Eye Res. 2021, 202, 108280. [Google Scholar] [CrossRef] [PubMed]
| NO-Donating Drugs | Underlying Mechanisms Involving IOP Lowering | |
|---|---|---|
| NO-donating Prostaglandins | LBN [81,82] | ECM digestion, sGC stimulation TM relaxation |
| NCX 470 [84] | PGF2a and NO/cGMP pathways activation | |
| NO donating CAIs | NCX 274, NCX 278 [19] | carbonic anhydrase type-II isozyme inhibition, NO/sGC/cGMP pathway stimulation |
| Nano-materials based NO donors | PEG-PAspTETA-SNO [20] SNP@MSNs delivery [21] | concentrated endogenous GSH-triggered NO release NO-cGMP pathway stimulation |
| Other kinds of NO donors | Furoxan derivatives [85] β-gal-NONOate [23] | NO-cGMP pathway stimulation NO release via β-gal-NONOate enzymatic biocatalysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Song, M.; Li, L.; Sun, X.; Lei, Y. The Application of Nitric Oxide for Ocular Hypertension Treatment. Molecules 2021, 26, 7306. https://doi.org/10.3390/molecules26237306
Han B, Song M, Li L, Sun X, Lei Y. The Application of Nitric Oxide for Ocular Hypertension Treatment. Molecules. 2021; 26(23):7306. https://doi.org/10.3390/molecules26237306
Chicago/Turabian StyleHan, Binze, Maomao Song, Liping Li, Xinghuai Sun, and Yuan Lei. 2021. "The Application of Nitric Oxide for Ocular Hypertension Treatment" Molecules 26, no. 23: 7306. https://doi.org/10.3390/molecules26237306
APA StyleHan, B., Song, M., Li, L., Sun, X., & Lei, Y. (2021). The Application of Nitric Oxide for Ocular Hypertension Treatment. Molecules, 26(23), 7306. https://doi.org/10.3390/molecules26237306





