Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications
Abstract
1. Introduction
2. NO Production, Concentration, and Activity
2.1. NO Production, Concentration, and Activity
2.2. NO Mediated Biofilm Formation and Dispersal
2.3. Bacterial Species, Strains, Growth Conditions, and Stage of Biofilm Development Influence Bacterial Response to NO and the Effectiveness of NO Treatment
2.4. Combinations of NO and Antibiotic Treatments
3. Gaseous NO and Other Low Molecular Weight (LMW) NO Donors
3.1. Gaseous NO
3.2. Metal Nitrosyl Complexes
3.3. S-Nitrosothiols
3.4. N-Diazeniumdiolates
3.5. Furoxans
3.6. Photo Responsive/Photoactivated Ruthenium Compounds
3.7. Hybrid-NO Donors
4. Macromolecular NO Donor Scaffolds
General Properties of Macromolecular NO Donor Scaffolds That Can Influence Their Activity
5. Natural Polymer-Based NO-Releasing Scaffolds
5.1. Types of Natural Polymer-Based NO-Releasing Scaffolds
5.2. NO-Releasing Chitosan Oligosaccharides (COS/NO)
5.3. Positive Charge of COS and Association of COS/NO with Bacterial Membranes or Biofilms the Main Driver of Antimicrobial Activity
5.4. Chitosan Gels
5.5. Chitosan-Graft Dendrimers
5.6. NO-Releasing Alginate Scaffolds and Hydrogels
5.7. NO-Releasing Cyclodextrins
6. NO Delivery via Inorganic and Polymeric Nanoparticles and Nanocarriers
6.1. NO-Releasing Silica Nanoparticles
Physical and Surface Properties of NP Affect Their Association with Bacteria and the Activity of Their NO-Releasing NO-NP Counterparts
6.2. NO-Releasing Polymeric Nanoparticles
6.2.1. POEGMA Containing NO-Releasing NPs
6.2.2. PGLA-Based NO-Releasing Nanoparticles
6.2.3. Antibiotic Conjugated or Surface Charge Switchable NO-NPs with Bacteria and Biofilm Targeting Properties
6.2.4. NO-Releasing Materials and Photodynamic and Photothermal Therapy for Antimicrobial Treatment
6.2.5. NO-Releasing Dendrimers and Hyperbranched Polymers
6.3. NO-Releasing Gel, Polymers, and Coatings
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| Ag NPs | Silver nanoparticles |
| C3D | Cephalosporin-linked diazeniumdiolate NO-donor prodrugs |
| CD | Cyclodextrins |
| Ce6 | Chlorin e6 |
| CF | Cystic fibrosis |
| COS-NO | Chitosan oligosaccharides |
| CS/NO | NO-releasing chitosan |
| ED | 1,2 -epoxy-9-decene |
| EPS | Extracellular polysaccharides |
| GSH | Gluthathione |
| GSNO | S-Nitrosoglutathione |
| ICG | Indocyanine green |
| IONPs | Iron oxide NPs |
| ISMN | Isosorbide mononitrate |
| LMW | Low molecular weight |
| MDR | Multidrug resistant |
| MPs | Microparticles |
| MW | Molecular weight |
| NIR | Near infrared |
| NO | Nitric oxide |
| NONOate | Diazeniumdiolates |
| NO-NPs | NO-releasing nanoparticles |
| NOS | NO synthase |
| NPs | Nanoparticles |
| PAMAM | Poly(amidoamine) |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PGLA | Poly(lactic-co-glycolic acid) |
| PGMA | Poly(glycidyl methacrylate) |
| PKA | Poly kanamycin |
| PO | Propylene oxide |
| POEGMA | Poly (oligoethylene glycol methacrylate) |
| Poly(HEMA) | poly(hydroxyethyl methacrylate) |
| Poly(SMBA) | poly(sulfobetaine methacrylate) |
| PPI | Poly(propylene imine) |
| PTFE | Polytetrafluoroethylene |
| PTT | Photothermal therapy |
| PU | Polyurethane |
| PVBA | Poly (vinylbenzaldehyde) |
| QS | Quorum sensing |
| RNI | Reactive nitrogen intermediates |
| RNS | Reactive nitrogen species |
| ROI | Reactive oxygen intermediates |
| ROS | Reactive oxygen species |
| RSNO | S-nitrosothiols |
| SNAC | S-nitroso-N-acetylcysteine |
| SNAP | S-nitroso-N-acetylpenicillamine |
| SNP | Sodium nitroprusside |
| SWF | Simulated wound fluid |
References
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020, 96, fiaa031. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- De Carvalho, C.C. Marine Biofilms: A Successful Microbial Strategy with Economic Implications. Front. Mar. Sci. 2018, 5, 126. [Google Scholar] [CrossRef]
- Barraud, N.; Hassett, D.J.; Hwang, S.H.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef] [PubMed]
- Van Sorge, N.M.; Beasley, F.C.; Gusarov, I.; Gonzalez, D.J.; von Köckritz-Blickwede, M.; Anik, S.; Borkowski, A.W.; Dorrestein, P.C.; Nudler, E.; Nizet, V. Methicillin-resistant Staphylococcus aureus bacterial nitric-oxide synthase affects antibiotic sensitivity and skin abscess development. J. Biol. Chem. 2013, 288, 6417–6426. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.M.; Tentolouris, C.; Papageorgiou, N.; Stefanadis, C. The role of nitric oxide on endothelial function. Curr. Vasc. Pharm. 2012, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.B.; Punihaole, D.; Levine, T.B. Characterization of the Role of Nitric Oxide and Its Clinical Applications. Cardiology 2012, 122, 55–68. [Google Scholar] [CrossRef]
- Cai, Y.M.; Zhang, Y.D.; Yang, L. NO donors and NO delivery methods for controlling biofilms in chronic lung infections. Appl. Microbiol. Biotechnol. 2021, 105, 3931–3954. [Google Scholar] [CrossRef]
- Grasemann, H.; Ratjen, F. Nitric Oxide and L-Arginine Deficiency in Cystic Fibrosis. Curr. Pharm. Des. 2012, 18, 726–736. [Google Scholar] [CrossRef]
- Rouillard, K.R.; Hill, D.B.; Schoenfisch, M.H. Antibiofilm and mucolytic action of nitric oxide delivered via gas or macromolecular donor using in vitro and ex vivo models. J. Cyst. Fibros. 2020, 19, 1004–1010. [Google Scholar] [CrossRef]
- Choi, M.; Hasan, N.; Cao, J.; Lee, J.; Hlaing, S.P.; Yoo, J.W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromol. 2020, 142, 680–692. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Eiserich, J.P.; Patel, R.P.; O’Donnell, V.B. Pathophysiology of nitric oxide and related species: Free radical reactions and modification of biomolecules. Mol. Asp. Med. 1998, 19, 221–357. [Google Scholar] [CrossRef]
- Stamler Jonathan, S.; Singel David, J.; Loscalzo, J. Biochemistry of Nitric Oxide and Its Redox-Activated Forms. Science 1992, 258, 1898–1902. [Google Scholar] [CrossRef]
- Wink David, A.; Kasprzak Kazimierz, S.; Maragos Chris, M.; Elespuru Rosalie, K.; Misra, M.; Dunams Tambra, M.; Cebula Thomas, A.; Koch Walter, H.; Andrews, A.W.; Allen Jane, S.; et al. DNA Deaminating Ability and Genotoxicity of Nitric Oxide and its Progenitors. Science 1991, 254, 1001–1003. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.K.; Ka Kit Ho, K.; Kumar, N. Chapter 7-Nitric Oxide Donors as Antimicrobial Agents. In Nitric Oxide Donors; Seabra, A.B., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 169–189. [Google Scholar]
- Ridnour, L.; Thomas, D.; Mancardi, D.; Espey, M.; Miranda, K.; Paolocci, N.; Feelisch, M.; Fukuto, J.; Wink, D. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol. Chem. 2004, 385, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 2000, 97, 8841. [Google Scholar] [CrossRef]
- Li, G.; Yu, S.; Xue, W.; Ma, D.; Zhang, W. Chitosan-graft-PAMAM loading nitric oxide for efficient antibacterial application. Chem. Eng. J. 2018, 347, 923–931. [Google Scholar] [CrossRef]
- Shen, Z.Q.; He, K.W.; Ding, Z.L.; Zhang, M.D.; Yu, Y.Q.; Hu, J.M. Visible-Light-Triggered Self-Reporting Release of Nitric Oxide (NO) for Bacterial Biofilm Dispersal. Macromolecules 2019, 52, 7668–7677. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Jung, K.; Kutty, S.K.; Agustina, S.; Adnan, N.N.M.; Basuki, J.S.; Kumar, N.; Davis, T.P.; Barraud, N.; Boyer, C. Nanoparticle (Star Polymer) Delivery of Nitric Oxide Effectively Negates Pseudomonas aeruginosa Biofilm Formation. Biomacromolecules 2014, 15, 2583–2589. [Google Scholar] [CrossRef]
- Hou, Z.; Wu, Y.; Xu, C.; Reghu, S.; Shang, Z.F.; Chen, J.J.; Pranantyo, D.; Marimuth, K.; De Pratim, P.; Ng, O.T.; et al. Precisely Structured Nitric-Oxide-Releasing Copolymer Brush Defeats Broad-Spectrum Catheter-Associated Biofilm Infections In Vivo. Acs Cent. Sci. 2020, 6, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Yepuri, N.R.; Barraud, N.; Mohammadi, N.S.; Kardak, B.G.; Kjelleberg, S.; Rice, S.A.; Kelso, M.J. Synthesis of cephalosporin-3 ‘-diazeniumdiolates: Biofilm dispersing NO-donor prodrugs activated by beta-lactamase. Chem. Commun. 2013, 49, 4791–4793. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Wu, J.B.; An, H.W.; Zhu, K.; Peng, B.; Cai, J.Q.; Zhang, Y.H.; Li, L.L.; Wang, H.; Huang, Z.J. Identification of New Nitric Oxide-Donating Peptides with Dual Biofilm Eradication and Antibacterial Activities for Intervention of Device-Related Infections. J. Med. Chem. 2020, 63, 9127–9135. [Google Scholar] [CrossRef]
- Crane, B.R.; Sudhamsu, J.; Patel, B.A. Bacterial nitric oxide synthases. Annu. Rev. Biochem. 2010, 79, 445–470. [Google Scholar] [CrossRef]
- Sudhamsu, J.; Crane, B.R. Bacterial nitric oxide synthases: What are they good for? Trends Microbiol. 2009, 17, 212–218. [Google Scholar] [CrossRef]
- Hutfless, E.H.; Chaudhari, S.S.; Thomas, V.C. Chapter Five-Emerging Roles of Nitric Oxide Synthase in Bacterial Physiology. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 72, pp. 147–191. [Google Scholar]
- Vázquez-Torres, A.; Bäumler, A.J. Nitrate, nitrite and nitric oxide reductases: From the last universal common ancestor to modern bacterial pathogens. Curr. Opin. Microbiol. 2016, 29, 1–8. [Google Scholar] [CrossRef]
- Mihu, M.R.; Cabral, V.; Pattabhi, R.; Tar, M.T.; Davies, K.P.; Friedman, A.J.; Martinez, L.R.; Nosanchuk, J.D. Sustained Nitric Oxide-Releasing Nanoparticles Interfere with Methicillin-Resistant Staphylococcus aureus Adhesion and Biofilm Formation in a Rat Central Venous Catheter Model. Antimicrob. Agents Chemother. 2017, 61, e02020-16. [Google Scholar] [CrossRef]
- Howlin, R.P.; Cathie, K.; Hall-Stoodley, L.; Cornelius, V.; Duignan, C.; Allan, R.N.; Fernandez, B.O.; Barraud, N.; Bruce, K.D.; Jefferies, J.; et al. Low-Dose Nitric Oxide as Targeted Anti-biofilm Adjunctive Therapy to Treat Chronic Pseudomonas aeruginosa Infection in Cystic Fibrosis. Mol. Ther. 2017, 25, 2104–2116. [Google Scholar] [CrossRef]
- Arora, D.P.; Hossain, S.; Xu, Y.; Boon, E.M. Nitric Oxide Regulation of Bacterial Biofilms. Biochemistry 2015, 54, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Deja, M.; Busch, T.; Bachmann, S.; Riskowski, K.; Campean, V.; Wiedmann, B.; Schwabe, M.; Hell, B.; Pfeilschifter, J.; Falke, K.J.; et al. Reduced nitric oxide in sinus epithelium of patients with radiologic maxillary sinusitis and sepsis. Am. J. Respir. Crit. Care Med. 2003, 168, 281–286. [Google Scholar] [CrossRef]
- Hausladen, A.; Privalle, C.T.; Keng, T.; DeAngelo, J.; Stamler, J.S. Nitrosative Stress: Activation of the Transcription Factor OxyR. Cell 1996, 86, 719–729. [Google Scholar] [CrossRef]
- Vallance, P.; Charles, I. Nitric oxide as an antimicrobial agent: Does NO always mean NO? Gut 1998, 42, 313. [Google Scholar] [CrossRef][Green Version]
- Kuang, S.-f.; Feng, D.-y.; Chen, Z.-g.; Liang, Z.-z.; Xiang, J.-j.; Li, H.; Peng, X.-x.; Zhang, T.; Bean Heather, D. Inactivation of Nitrite-Dependent Nitric Oxide Biosynthesis Is Responsible for Overlapped Antibiotic Resistance between Naturally and Artificially Evolved Pseudomonas aeruginosa. mSystems 2021, 6, e00732-21. [Google Scholar] [CrossRef]
- Ehrt, S.; Schnappinger, D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell. Microbiol. 2009, 11, 1170–1178. [Google Scholar] [CrossRef]
- Kerr, A.; Wei, X.-Q.; Andrew, P.; Mitchell, T. Nitric oxide exerts distinct effects in local and systemic infections with Streptococcus pneumoniae. Microb. Pathog. 2004, 36, 303–310. [Google Scholar] [CrossRef]
- Reighard, K.P.; Schoenfisch, M.H. Antibacterial Action of Nitric Oxide-Releasing Chitosan Oligosaccharides against Pseudomonas aeruginosa under Aerobic and Anaerobic Conditions. Antimicrob. Agents Chemother. 2015, 59, 6506–6513. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Teles, F.; Gong, W.D.; Dua, S.A.; Martin, L.; Schoenfisch, M.H. Antibacterial action of nitric oxide-releasing hyperbranched polymers against ex vivo dental biofilms. Dent. Mater. 2020, 36, 635–644. [Google Scholar] [CrossRef]
- Barnes, R.J.; Bandi, R.R.; Wong, W.S.; Barraud, N.; McDougald, D.; Fane, A.; Kjelleberg, S.; Rice, S.A. Optimal dosing regimen of nitric oxide donor compounds for the reduction of Pseudomonas aeruginosa biofilm and isolates from wastewater membranes. Biofouling 2013, 29, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Oh, H.S.; Ng, Y.C.B.; Tang, P.Y.P.; Barraud, N.; Rice, S.A. Nitric Oxide-Mediated Induction of Dispersal in Pseudomonas aeruginosa Biofilms Is Inhibited by Flavohemoglobin Production and Is Enhanced by Imidazole. Antimicrob. Agents Chemother. 2018, 62, e01832-17. [Google Scholar] [CrossRef]
- Hasan, S.; Albayaty, Y.N.S.; Thierry, B.; Prestidge, C.A.; Thomas, N. Mechanistic studies of the antibiofilm activity and synergy with antibiotics of isosorbide mononitrate. Eur. J. Pharm. Sci. 2018, 115, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wu, J.F.; Colletta, A.; Meyerhoff, M.E.; Xi, C.W. Efficient Eradication of Mature Pseudomonas aeruginosa Biofilm via Controlled Delivery of Nitric Oxide Combined with Antimicrobial Peptide and Antibiotics. Front. Microbiol. 2016, 7, 1260. [Google Scholar] [CrossRef] [PubMed]
- Zemke, A.C.; Madison, C.J.; Kasturiarachi, N.; Pearce, L.L.; Peterson, J. Antimicrobial Synergism Toward Pseudomonas aeruginosaby Gallium(III) and Inorganic Nitrite. Front. Microbiol. 2020, 11, 2113. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, K.R.; Novak, O.P.; Pistiolis, A.M.; Yang, L.; Ahonen, M.J.R.; McDonald, R.A.; Schoenfisch, M.H. Exogenous Nitric Oxide Improves Antibiotic Susceptibility in Resistant Bacteria. ACS Infect. Dis. 2021, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ouadrhiri, Y.; Scorneaux, B.; Sibille, Y.; Tulkens, P.M. Mechanism of the intracellular killing and modulation of antibiotic susceptibility of Listeria monocytogenes in THP-1 macrophages activated by gamma interferon. Antimicrob. Agents Chemother. 1999, 43, 1242–1251. [Google Scholar] [CrossRef]
- Huang, D.N.; Wang, J.; Jia, F.; Fang, Y.; Gao, Q.; Gao, Y.F.; Li, H.Y.; Ren, K.F.; Ji, J. Nitric oxide pretreatment enhances ofloxacin susceptibility of biofilm concomitant with exopolysaccharide depletion. Colloid Interface Sci. Commun. 2021, 41, 100371. [Google Scholar] [CrossRef]
- Allan, R.N.; Morgan, S.; Brito-Mutunayagam, S.; Skipp, P.; Feelisch, M.; Hayes, S.M.; Hellier, W.; Clarke, S.C.; Stoodley, P.; Burgess, A.; et al. Low Concentrations of Nitric Oxide Modulate Streptococcus pneumoniae Biofilm Metabolism and Antibiotic Tolerance. Antimicrob. Agents Chemother. 2016, 60, 2456–2466. [Google Scholar] [CrossRef]
- Nandanwar, N.; Gibson, J.E.; Neely, M.N. Growth medium and nitric oxide alter Mycobacterium abscessus morphotype and virulence. Microbiol. Res. 2021, 253, 126887. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef] [PubMed]
- McCollister, B.D.; Hoffman, M.; Husain, M.; Vázquez-Torres, A. Nitric oxide protects bacteria from aminoglycosides by blocking the energy-dependent phases of drug uptake. Antimicrob. Agents Chemother. 2011, 55, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.K.; Selvanayagam, R.; Ho, K.K.K.; Chen, R.X.; Kutty, S.K.; Rice, S.A.; Kumar, N.; Barraud, N.; Duong, H.T.T.; Boyer, C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016, 7, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.T.; Zhang, M.Y.; Shen, Z.Q.; Zhang, M.D.; Zheng, B.; Cheng, S.; Hu, J.M. Photoresponsive Vesicles Enabling Sequential Release of Nitric Oxide (NO) and Gentamicin for Efficient Biofilm Eradication. Macromol. Rapid Commun. 2021, 42, e2000759. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, S.B.; Traeger, L.; Nguyen, H.K.; Rouillard, K.R.; Fischbach, A.; Zadek, F.; Ichinose, F.; Schoenfisch, M.H.; Carroll, R.W.; Bloch, D.B.; et al. Antimicrobial effects of nitric oxide in murine models of Klebsiella pneumonia. Redox. Biol. 2021, 39, 101826. [Google Scholar] [CrossRef]
- Michaelsen, V.S.; Vp Ribeiro, R.; Wang, A.; Price, C.; Wannberg, B.; Zhang, Y.; Pires, L.; del Sorbo, L.; Ramadan, K.; Gomes, B.; et al. Gaseous Nitric Oxide (gNO) as a Potential Antimicrobial Therapy during Ex Vivo Lung Perfusion: An Efficacy and Safety Study. J. Heart Lung Transplant. 2020, 39, S46. [Google Scholar] [CrossRef]
- Bogdanovski, K.; Chau, T.; Robinson, C.J.; MacDonald, S.D.; Peterson, A.M.; Mashek, C.M.; Wallin, W.A.; Rimkus, M.; Montgomery, F.; Lucas da Silva, J.; et al. Antibacterial activity of high-dose nitric oxide against pulmonary Mycobacterium abscessus disease. Access Microbiol. 2020, 2, acmi000154. [Google Scholar] [CrossRef]
- Bentur, L.; Gur, M.; Ashkenazi, M.; Livnat-Levanon, G.; Mizrahi, M.; Tal, A.; Ghaffari, A.; Geffen, Y.; Aviram, M.; Efrati, O. Pilot study to test inhaled nitric oxide in cystic fibrosis patients with refractory Mycobacterium abscessus lung infection. J. Cyst. Fibros. 2020, 19, 225–231. [Google Scholar] [CrossRef]
- Deppisch, C.; Herrmann, G.; Graepler-Mainka, U.; Wirtz, H.; Heyder, S.; Engel, C.; Marschal, M.; Miller, C.C.; Riethmüller, J. Gaseous nitric oxide to treat antibiotic resistant bacterial and fungal lung infections in patients with cystic fibrosis: A phase I clinical study. Infection 2016, 44, 513–520. [Google Scholar] [CrossRef]
- Bartley, B.L.; Gardner, K.J.; Spina, S.; Hurley, B.P.; Campeau, D.; Berra, L.; Yonker, L.M.; Carroll, R.W. High-Dose Inhaled Nitric Oxide as Adjunct Therapy in Cystic Fibrosis Targeting Burkholderia multivorans. Case Rep. Pediatri. 2020, 2020, 1536714. [Google Scholar] [CrossRef]
- Ghaffari, A.; Miller, C.C.; McMullin, B.; Ghahary, A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 2006, 14, 21–29. [Google Scholar] [CrossRef]
- Barraud, N.; Storey, M.V.; Moore, Z.P.; Webb, J.S.; Rice, S.A.; Kjelleberg, S. Nitric oxide-mediated dispersal in single- and multi-species biofilms of clinically and industrially relevant microorganisms. Microb. Biotechnol. 2009, 2, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.N.; Li, H.N.; Tao, X.; Xie, Y.X.; Yang, L.; Mao, Z.W.; Xia, W. Light-Triggered Nitric Oxide Release by a Photosensitizer to Combat Bacterial Biofilm Infections. Chem. A Eur. J. 2021, 27, 5453–5460. [Google Scholar] [CrossRef] [PubMed]
- Boce, M.; Tasse, M.; Mallet-Ladeira, S.; Pillet, F.; Da Silva, C.; Vicendo, P.; Lacroix, P.G.; Malfant, I.; Rols, M.P. Effect of trans(NO, OH)- RuFT(CI)(OH)NO (PF6) ruthenium nitrosyl complex on methicillin-resistant Staphylococcus epidermidis. Sci. Rep. 2019, 9, 4867. [Google Scholar] [CrossRef] [PubMed]
- Cariello, A.J.; Bispo, P.J.M.; de Souza, G.F.P.; Pignatari, A.C.C.; de Oliveira, M.G.; Hofling-Lima, A.L. Bactericidal effect of S-nitrosothiols against clinical isolates from keratitis. Clin. Ophthalmol. 2012, 6, 1907–1914. [Google Scholar] [CrossRef]
- Barnes, R.J.; Low, J.H.; Bandi, R.R.; Tay, M.; Chua, F.; Aung, T.; Fane, A.G.; Kjelleberg, S.; Rice, S.A. Nitric oxide treatment for the control of reverse osmosis membrane biofouling. Appl. Environ. Microbiol. 2015, 81, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Marvasi, M.; Chen, C.; Carrazana, M.; Durie, I.A.; Teplitski, M. Systematic analysis of the ability of Nitric Oxide donors to dislodge biofilms formed by Salmonella enterica and Escherichia coli O157:H7. AMB Express 2014, 4, 42. [Google Scholar] [CrossRef]
- Poh, W.H.; Barraud, N.; Guglielmo, S.; Lazzarato, L.; Rolando, B.; Fruttero, R.; Rice, S.A. Furoxan Nitric Oxide Donors Disperse Pseudomonas aeruginosa Biofilms, Accelerate Growth, and Repress Pyoverdine Production. ACS Chem. Biol. 2017, 12, 2097–2106. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Bolognese, F.; Rolando, B.; Guglielmo, S.; Lazzarato, L.; Fruttero, R. Anti-Pseudomonas activity of 3-nitro-4-phenylfuroxan. Microbiology 2018, 164, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Allan, R.N.; Kelso, M.J.; Rineh, A.; Yepuri, N.R.; Feelisch, M.; Soren, O.; Brito-Mutunayagam, S.; Salib, R.J.; Stoodley, P.; Clarke, S.C.; et al. Cephalosporin-NO-donor prodrug PYRRO-C3D shows beta-lactam-mediated activity against Streptococcus pneumoniae biofilms. Nitric Oxide Biol. Chem. 2017, 65, 43–49. [Google Scholar] [CrossRef]
- Barraud, N.; Kardak, B.G.; Yepuri, N.R.; Howlin, R.P.; Webb, J.S.; Faust, S.N.; Kjelleberg, S.; Rice, S.A.; Kelso, M.J. Cephalosporin-3 ‘-diazeniumdiolates: Targeted NO-Donor Prodrugs for Dispersing Bacterial Biofilms. Angew. Chem. Int. Ed. 2012, 51, 9057–9060. [Google Scholar] [CrossRef]
- Collins, S.A.; Kelso, M.J.; Rineh, A.; Yepuri, N.R.; Coles, J.; Jackson, C.L.; Halladay, G.D.; Walker, W.T.; Webb, J.S.; Hall-Stoodley, L.; et al. Cephalosporin-3 ‘-Diazeniumdiolate NO Donor Prodrug PYRRO-C3D Enhances Azithromycin Susceptibility of Nontypeable Haemophilus influenzae Biofilms. Antimicrob. Agents Chemother. 2017, 61, e02086-16. [Google Scholar] [CrossRef]
- Rineh, A.; Soren, O.; McEwan, T.; Ravikumar, V.; Poh, W.H.; Azamifar, F.; Naimi-Jamal, M.R.; Cheung, C.Y.; Elliott, A.G.; Zuegg, J.; et al. Discovery of Cephalosporin-3 ‘-Diazeniumdiolates That Show Dual Antibacterial and Antibiofilm Effects against Pseudomonas aeruginosa Clinical Cystic Fibrosis Isolates and Efficacy in a Murine Respiratory Infection Model. Acs Infect. Dis. 2020, 6, 1460–1479. [Google Scholar] [CrossRef] [PubMed]
- Soren, O.; Rineh, A.; Silva, D.G.; Cai, Y.M.; Howlin, R.P.; Allan, R.N.; Feelisch, M.; Davies, J.C.; Connett, G.J.; Faust, S.N.; et al. Cephalosporin nitric oxide-donor prodrug DEA-C3D disperses biofilms formed by clinical cystic fibrosis isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2020, 75, 117–125. [Google Scholar] [CrossRef]
- Kutty, S.K.; Barraud, N.; Pham, A.; Iskander, G.; Rice, S.A.; Black, D.S.; Kumar, N. Design, Synthesis, and Evaluation of Firnbrolide-Nitric Oxide Donor Hybrids as Antimicrobial Agents. J. Med. Chem. 2013, 56, 9517–9529. [Google Scholar] [CrossRef] [PubMed]
- Pepke-Zaba, J.; Higenbottam, T.W.; Dinh-Xuan, A.T.; Stone, D.; Wallwork, J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 1991, 338, 1173–1174. [Google Scholar] [CrossRef]
- Frostell, C.; Fratacci, M.D.; Wain, J.C.; Jones, R.; Zapol, W.M. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 1991, 83, 2038–2047. [Google Scholar] [CrossRef]
- Ghaffari, A.; Jalili, R.; Ghaffari, M.; Miller, C.; Ghahary, A. Efficacy of gaseous nitric oxide in the treatment of skin and soft tissue infections. Wound Repair Regen 2007, 15, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Sulemankhil, I.; Ganopolsky, J.G.; Dieni, C.A.; Dan, A.F.; Jones, M.L.; Prakash, S. Prevention and Treatment of Virulent Bacterial Biofilms with an Enzymatic Nitric Oxide-Releasing Dressing. Antimicrob. Agents Chemother. 2012, 56, 6095–6103. [Google Scholar] [CrossRef]
- Waite, R.D.; Stewart, J.E.; Stephen, A.S.; Allaker, R.P. Activity of a nitric oxide-generating wound treatment system against wound pathogen biofilms. Int. J. Antimicrob. Agents 2018, 52, 338–343. [Google Scholar] [CrossRef]
- Hall, J.R.; Rouillard, K.R.; Suchyta, D.J.; Brown, M.D.; Ahonen, M.J.R.; Schoenfisch, M.H. Mode of Nitric Oxide Delivery Affects Antibacterial Action. ACS Biomater. Sci. Eng. 2020, 6, 433–441. [Google Scholar] [CrossRef]
- Chau, T.; Blade, K.; Da Silva, J.; Ghaffari, A.; Zelazny, A.; Olivier, K. High Efficacy of High-dose Nitric Oxide and its Synergistic Effect with Antibiotics against Mycobacterium Abscessus. Eur. Respir. J. 2019, 54, OA4950. [Google Scholar] [CrossRef]
- Salguero, K.L.; Cummings, J.J. Inhaled nitric oxide and methemoglobin in full-term infants with persistent pulmonary hypertension of the newborn. Pulm. Pharm. Ther. 2002, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Young, J.D.; Dyar, O.; Xiong, L.; Howell, S. Methaemoglobin production in normal adults inhaling low concentrations of nitric oxide. Intensive Care Med. 1994, 20, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA. INOmax (Nitric Oxide). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/20845_INOmax.cfm (accessed on 17 December 2021).
- Miller, C.; McMullin, B.; Ghaffari, A.; Stenzler, A.; Pick, N.; Roscoe, D.; Ghahary, A.; Road, J.; Av-Gay, Y. Gaseous nitric oxide bactericidal activity retained during intermittent high-dose short duration exposure. Nitric Oxide 2009, 20, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Schulz, V.; Gross, R.; Pasch, T.; Busse, J.; Loeschcke, G. Cyanide toxicity of sodium nitroprusside in therapeutic use with and without sodium thiosulphate. Klin. Wochenschr. 1982, 60, 1393–1400. [Google Scholar] [CrossRef]
- Seabra, A.B.; Fitzpatrick, A.; Paul, J.; De Oliveira, M.G.; Weller, R. Topically applied S-nitrosothiol-containing hydrogels as experimental and pharmacological nitric oxide donors in human skin. Br. J. Derm. 2004, 151, 977–983. [Google Scholar] [CrossRef]
- Hornyák, I.; Pankotai, E.; Kiss, L.; Lacza, Z. Current developments in the therapeutic potential of S-nitrosoglutathione, an endogenous NO-donor molecule. Curr. Pharm. Biotechnol. 2011, 12, 1368–1374. [Google Scholar] [CrossRef]
- Ganzarolli de Oliveira, M. S-Nitrosothiols as Platforms for Topical Nitric Oxide Delivery. Basic Clin. Pharmacol. Toxicol. 2016, 119, 49–56. [Google Scholar] [CrossRef]
- Lu, Y.; Shah, A.; Hunter, R.A.; Soto, R.J.; Schoenfisch, M.H. S-Nitrosothiol-modified nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Acta Biomater. 2015, 12, 62–69. [Google Scholar] [CrossRef]
- De Souza, G.F.P.; Denadai, J.P.; Picheth, G.F.; de Oliveira, M.G. Long-term decomposition of aqueous S-nitrosoglutathione and S-nitroso-N-acetylcysteine: Influence of concentration, temperature, pH and light. Nitric Oxide 2019, 84, 30–37. [Google Scholar] [CrossRef]
- Zhelyaskov, V.R.; Gee, K.R.; Godwin, D.W. Control of NO concentration in solutions of nitrosothiol compounds by light. Photochem. Photobiol. 1998, 67, 282–288. [Google Scholar] [CrossRef]
- Kumari, S.; Sammut, I.A.; Giles, G.I. The design of nitric oxide donor drugs: S-nitrosothiol tDodSNO is a superior photoactivated donor in comparison to GSNO and SNAP. Eur. J. Pharmacol. 2014, 737, 168–176. [Google Scholar] [CrossRef]
- Riccio, D.A.; Coneski, P.N.; Nichols, S.P.; Broadnax, A.D.; Schoenfisch, M.H. Photoinitiated nitric oxide-releasing tertiary S-nitrosothiol-modified xerogels. ACS Appl. Mater. Interfaces 2012, 4, 796–804. [Google Scholar] [CrossRef]
- Hrabie, J.A.; Keefer, L.K. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem. Rev. 2002, 102, 1135–1154. [Google Scholar] [CrossRef]
- Keefer, L.K. Fifty years of diazeniumdiolate research. From laboratory curiosity to broad-spectrum biomedical advances. ACS Chem. Biol. 2011, 6, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, M.J.R.; Dorrier, J.M.; Schoenfisch, M.H. Antibiofilm Efficacy of Nitric Oxide-Releasing Alginates against Cystic Fibrosis Bacterial Pathogens. ACS Infect. Dis. 2019, 5, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Worley, B.V.; Schoenfisch, M.H. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against Streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [Google Scholar] [CrossRef]
- Worley, B.V.; Schilly, K.M.; Schoenfisch, M.H. Anti-Biofilm Efficacy of Dual-Action Nitric Oxide-Releasing Alkyl Chain Modified Poly(amidoamine) Dendrimers. Mol. Pharm. 2015, 12, 1573–1583. [Google Scholar] [CrossRef]
- Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Nitric Oxide-Releasing Quaternary Ammonium-Modified Poly(amidoamine) Dendrimers as Dual Action Antibacterial Agents. Bioconjugate Chem. 2014, 25, 918–927. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, B.; Li, C.; Schoenfisch, M.H. Structurally Diverse Nitric Oxide-Releasing Poly(propylene Imine) Dendrimers. Chem. Mater. 2011, 23, 4227–4233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hetrick, E.M.; Shin, J.H.; Stasko, N.A.; Johnson, C.B.; Wespe, D.A.; Holmuhamedov, E.; Schoenfisch, M.H. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano 2008, 2, 235–246. [Google Scholar] [CrossRef]
- Adnan, N.N.M.; Sadrearhami, Z.; Bagheri, A.; Nguyen, T.K.; Wong, E.H.H.; Ho, K.K.K.; Lim, M.; Kumar, N.; Boyer, C. Exploiting the Versatility of Polydopamine-Coated Nanoparticles to Deliver Nitric Oxide and Combat Bacterial Biofilm. Macromol. Rapid Commun. 2018, 39, e1800159. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Rice, S.A.; Barraud, N. Nitric Oxide and Iron Signaling Cues Have Opposing Effects on Biofilm Development in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2019, 85, e02175-18. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.M.; Webb, J.S. Optimization of nitric oxide donors for investigating biofilm dispersal response in Pseudomonas aeruginosaclinical isolates. Appl. Microbiol. Biotechnol. 2020, 104, 8859–8869. [Google Scholar] [CrossRef]
- Gasco, A.; Fruttero, R.; Sorba, G.; Stilo, A.D.; Calvino, R. NO donors: Focus on furoxan derivatives. Pure Appl. Chem. 2004, 76, 973–981. [Google Scholar] [CrossRef]
- Sorba, G.; Medana, C.; Fruttero, R.; Cena, C.; Di Stilo, A.; Galli, U.; Gasco, A. Water soluble furoxan derivatives as NO prodrugs. J. Med. Chem. 1997, 40, 463–469. [Google Scholar] [CrossRef]
- Guo, M.; Xiang, H.-J.; Wang, Y.; Zhang, Q.-L.; An, L.; Yang, S.-P.; Ma, Y.; Wang, Y.; Liu, J.-G. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem. Commun. 2017, 53, 3253–3256. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.J.; Mascharak, P.K. Photoactive ruthenium nitrosyls: Effects of light and potential application as NO donors. Coord. Chem. Rev. 2008, 252, 2093–2114. [Google Scholar] [CrossRef]
- Taunk, A.; Chen, R.; Iskander, G.; Ho, K.K.K.; Black, D.S.; Willcox, M.D.P.; Kumar, N. Dual-Action Biomaterial Surfaces with Quorum Sensing Inhibitor and Nitric Oxide To Reduce Bacterial Colonization. ACS Biomater. Sci. Eng. 2018, 4, 4174–4182. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Schoenfisch, M.H. Nitric oxide-releasing chitosan oligosaccharides as antibacterial agents. Biomaterials 2014, 35, 1716–1724. [Google Scholar] [CrossRef]
- Reighard, K.P.; Hill, D.B.; Dixon, G.A.; Worley, B.V.; Schoenfisch, M.H. Disruption and eradication of P. aeruginosa biofilms using nitric oxide-releasing chitosan oligosaccharides. Biofouling 2015, 31, 775–787. [Google Scholar] [CrossRef]
- Tang, R.G.; Jiang, F.G.; Wen, J.C.; Deng, Y.; Sun, Y.Y. Managing bacterial biofilms with chitosan-based polymeric nitric oxides: Inactivation of biofilm bacteria and synergistic effects with antibiotics. J. Bioact. Compat. Polym. 2016, 31, 393–410. [Google Scholar] [CrossRef]
- Hasan, S.; Thomas, N.; Thierry, B.; Prestidge, C.A. Controlled and Localized Nitric Oxide Precursor Delivery From Chitosan Gels to Staphylococcus aureus Biofilms. J. Pharm. Sci. 2017, 106, 3556–3563. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, J.; Pant, J.; Goudie, M.J.; Mani, S.; Handa, H. Antimicrobial and Physicochemical Characterization of Biodegradable, Nitric Oxide-Releasing Nanocellulose–Chitosan Packaging Membranes. J. Agric. Food Chem. 2016, 64, 5260–5266. [Google Scholar] [CrossRef]
- Kim, J.O.; Noh, J.-K.; Thapa, R.K.; Hasan, N.; Choi, M.; Kim, J.H.; Lee, J.-H.; Ku, S.K.; Yoo, J.-W. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int. J. Biol. Macromol. 2015, 79, 217–225. [Google Scholar] [CrossRef]
- Liu, S.X.; Cai, X.; Xue, W.; Ma, D.; Zhang, W. Chitosan derivatives co-delivering nitric oxide and methicillin for the effective therapy to the methicillin-resistant S. aureus infection. Carbohydr. Polym. 2020, 234, 115928. [Google Scholar] [CrossRef]
- Ahonen, M.J.R.; Suchyta, D.J.; Zhu, H.Y.; Schoenfisch, M.H. Nitric Oxide-Releasing Alginates. Biomacromolecules 2018, 19, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Rouillard, K.R.; Markovetz, M.R.; Bacudio, L.G.; Hill, D.B.; Schoenfisch, M.H. Pseudomonas aeruginosa Biofilm Eradication via Nitric Oxide-Releasing Cyclodextrins. ACS Infect. Dis. 2020, 6, 1940–1950. [Google Scholar] [CrossRef]
- Jin, H.; Yang, L.; Ahonen, M.J.R.; Schoenfisch, M.H. Nitric Oxide-Releasing Cyclodextrins. J. Am. Chem Soc. 2018, 140, 14178–14184. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Slomberg, D.L.; Rao, K.S.; Schoenfisch, M.H. Influence of Scaffold Size on Bactericidal Activity of Nitric Oxide-Releasing Silica Nanoparticles. ACS Nano 2011, 5, 7235–7244. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Worley, B.V.; Slomberg, D.L.; Schoenfisch, M.H. Dual Action Antimicrobials: Nitric Oxide Release from Quaternary Ammonium-Functionalized Silica Nanoparticles. Biomacromolecules 2012, 13, 3334–3342. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of Size and Shape on Biofilm Eradication for Nitric Oxide-Releasing Silica Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329. [Google Scholar] [CrossRef]
- Backlund, C.J.; Worley, B.V.; Sergesketter, A.R.; Schoenfisch, M.H. Kinetic-dependent Killing of Oral Pathogens with Nitric Oxide. J. Dent. Res. 2015, 94, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Han, G.; Chacko, M.; Mihu, M.R.; Jacobson, M.; Gialanella, P.; Friedman, A.J.; Nosanchuk, J.D.; Friedman, J.M. Antimicrobial and Healing Efficacy of Sustained Release Nitric Oxide Nanoparticles Against Staphylococcus Aureus Skin Infection. J. Investig. Dermatol. 2009, 129, 2463–2469. [Google Scholar] [CrossRef]
- Sadrearhami, Z.; Yeow, J.; Nguyen, T.K.; Ho, K.K.K.; Kumar, N.; Boyer, C. Biofilm dispersal using nitric oxide loaded nanoparticles fabricated by photo-PISA: Influence of morphology. Chem. Commun. 2017, 53, 12894–12897. [Google Scholar] [CrossRef]
- Hasan, S.; Thomas, N.; Thierry, B.; Prestidge, C.A. Biodegradable nitric oxide precursor-loaded micro- and nanoparticles for the treatment of Staphylococcus aureus biofilms. J. Mater. Chem. B 2017, 5, 1005–1014. [Google Scholar] [CrossRef]
- Hasan, N.; Cao, J.; Lee, J.; Naeem, M.; Hlaing, S.P.; Kim, J.; Jung, Y.; Lee, B.L.; Yoo, J.W. PEI/NONOates-doped PLGA nanoparticles for eradicating methicillin-resistant Staphylococcus aureus biofilm in diabetic wounds via binding to the biofilm matrix. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109741. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Dong, D.; Li, X.; Li, Z.; Li, S.; Wang, J. Effects of isosorbide mononitrate loaded nanoparticles conjugated with anti-Staphylococcus aureus α-toxin on Staphylococcus aureus biofilms. Exp. Ther. Med. 2020, 19, 1267–1274. [Google Scholar] [CrossRef]
- Liu, T.W.; Wei, J.J.; Fu, G.D.; Zhang, P.; Zhang, Z.D.; Guo, D.S.; Yang, X.L. Surface charge switchable nanoparticles capable of controlled nitric oxide release for the treatment of acidity-associated bacterial infections. Polym. Chem. 2021, 12, 1023–1029. [Google Scholar] [CrossRef]
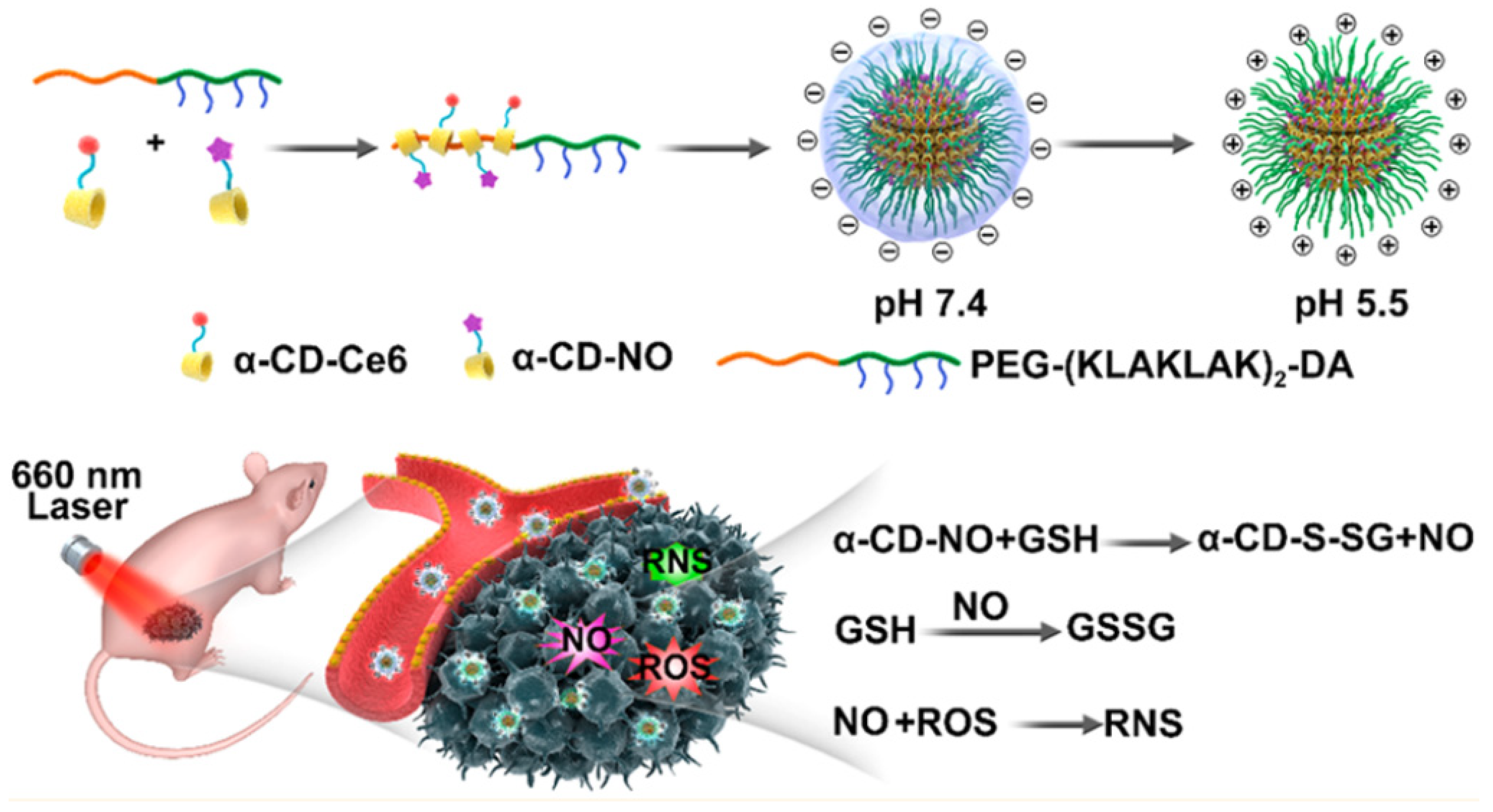
- Hu, D.F.; Deng, Y.Y.; Jia, F.; Jin, Q.; Ji, J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano 2020, 14, 347–359. [Google Scholar] [CrossRef] [PubMed]
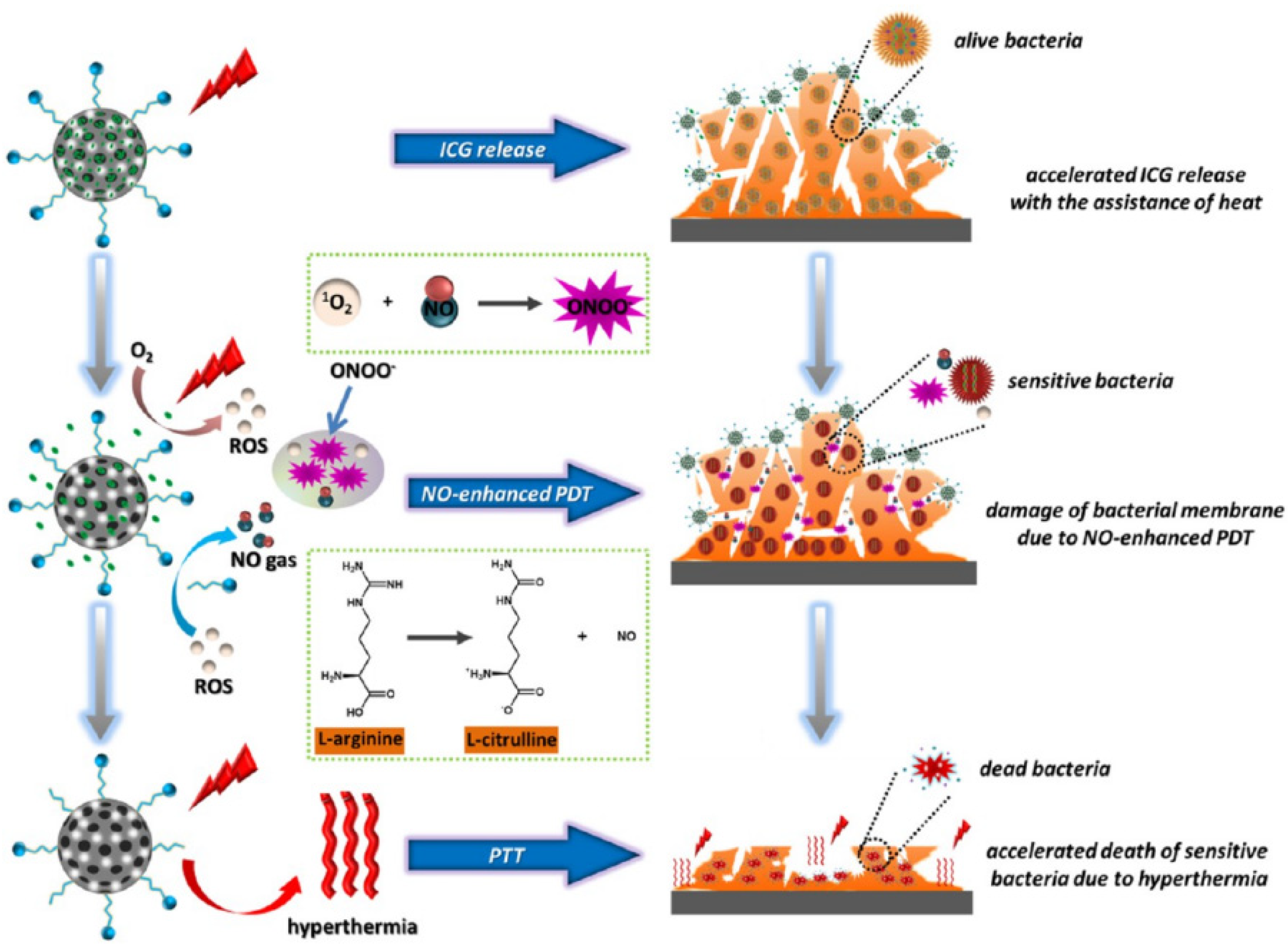
- Yuan, Z.; Lin, C.; He, Y.; Tao, B.; Chen, M.; Zhang, J.; Liu, P.; Cai, K. Near-Infrared Light-Triggered Nitric-Oxide-Enhanced Photodynamic Therapy and Low-Temperature Photothermal Therapy for Biofilm Elimination. ACS Nano 2020, 14, 3546–3562. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Y.; Ye, W.; Tian, L.M.; Niu, S.C.; Ming, W.H.; Zhao, J.; Ren, L.Q. Near-infrared light triggered photodynamic and nitric oxide synergistic antibacterial nanocomposite membrane. Chem. Eng. J. 2021, 417, 128049. [Google Scholar] [CrossRef]
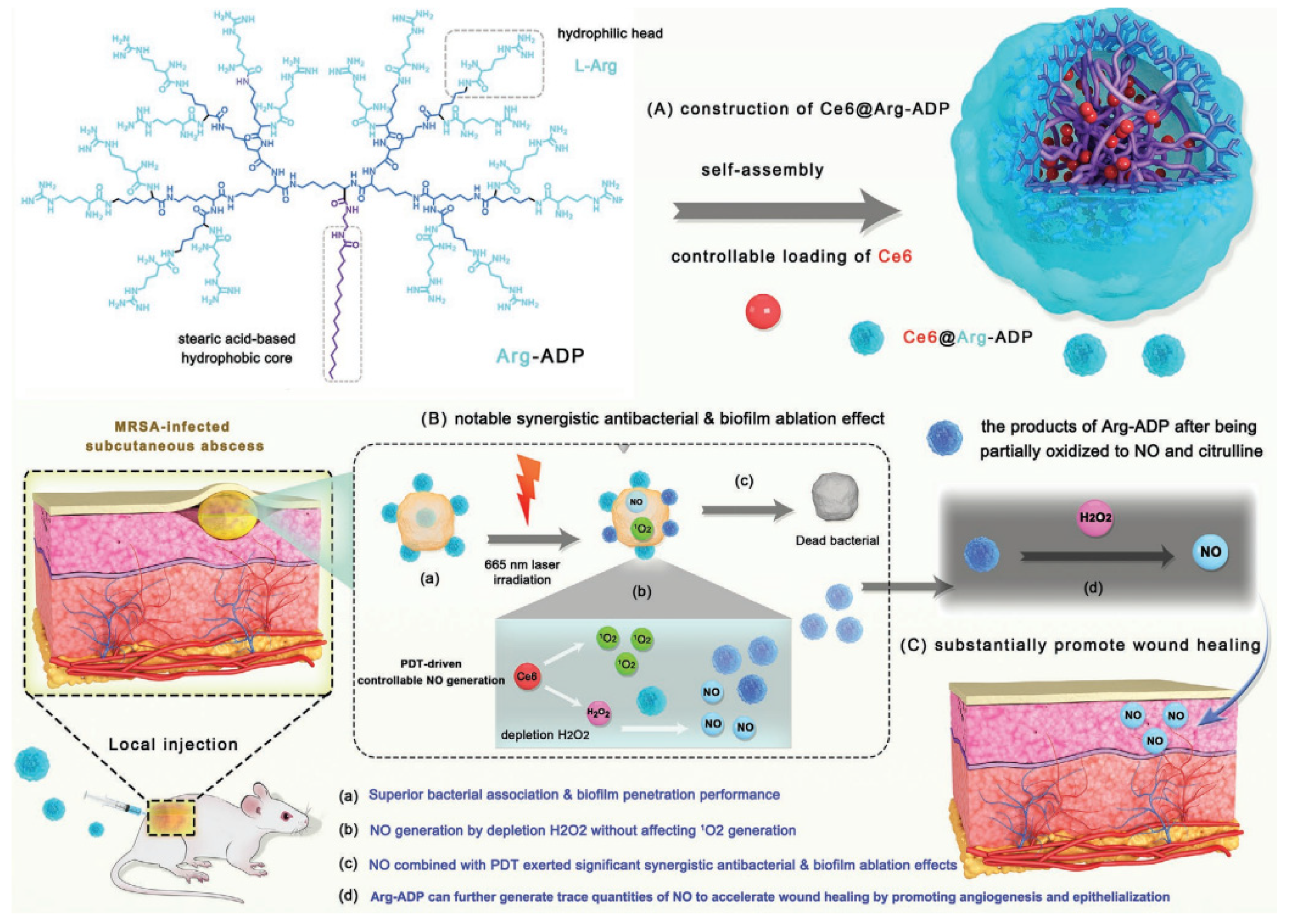
- Zhu, J.W.; Tian, J.; Yang, C.; Chen, J.P.; Wu, L.H.; Fan, M.N.; Cai, X.J. L-Arg-Rich Amphiphilic Dendritic Peptide as a Versatile NO Donor for NO/Photodynamic Synergistic Treatment of Bacterial Infections and Promoting Wound Healing. Small 2021, 17, 2101495. [Google Scholar] [CrossRef]
- Lu, Y.; Slomberg, D.L.; Shah, A.; Schoenfisch, M.H. Nitric Oxide-Releasing Amphiphilic Poly(amidoamine) (PAMAM) Dendrimers as Antibacterial Agents. Biomacromolecules 2013, 14, 3589–3598. [Google Scholar] [CrossRef]
- Backlund, C.J.; Sergesketter, A.R.; Offenbacher, S.; Schoenfisch, M.H. Antibacterial Efficacy of Exogenous Nitric Oxide on Periodontal Pathogens. J. Dent. Res. 2014, 93, 1089–1094. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Suchyta, D.J.; Schoenfisch, M.H. Antibacterial Activity of Nitric Oxide-Releasing Hyperbranched Polyamidoamines. Bioconjug. Chem. 2018, 29, 35–43. [Google Scholar] [CrossRef]
- Storm, W.L.; Youn, J.; Reighard, K.P.; Worley, B.V.; Lodaya, H.M.; Shin, J.H.; Schoenfisch, M.H. Superhydrophobic nitric oxide-releasing xerogels. Acta Biomater. 2014, 10, 3442–3448. [Google Scholar] [CrossRef]
- Craven, M.; Kasper, S.H.; Canfield, M.J.; Diaz-Morales, R.R.; Hrabie, J.A.; Cady, N.C.; Strickland, A.D. Nitric oxide-releasing polyacrylonitrile disperses biofilms formed by wound-relevant pathogenic bacteria. J. Appl. Microbiol. 2016, 120, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Fleming, G.; Aveyard, J.; Fothergill, J.L.; McBride, F.; Raval, R.; D’Sa, R.A. Nitric Oxide Releasing Polymeric Coatings for the Prevention of Biofilm Formation. Polymers 2017, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Wo, Y.Q.; Li, Z.; Colletta, A.; Wu, J.F.; Xi, C.W.; Matzger, A.J.; Brisbois, E.J.; Bartlett, R.H.; Meyerhoff, M.E. Study of crystal formation and nitric oxide (NO) release mechanism from S-nitroso-N-acetylpenicillamine (SNAP)-doped CarboSil polymer composites for potential antimicrobial applications. Compos. Part B Eng. 2017, 121, 23–33. [Google Scholar] [CrossRef]
- Singha, P.; Pant, J.; Goudie, M.J.; Workman, C.D.; Handa, H. Enhanced antibacterial efficacy of nitric oxide releasing thermoplastic polyurethanes with antifouling hydrophilic topcoats. Biomater. Sci. 2017, 5, 1246–1255. [Google Scholar] [CrossRef]
- Mondal, A.; Devine, R.; Estes, L.; Manuel, J.; Singha, P.; Mancha, J.; Palmer, M.; Handa, H. Highly hydrophobic polytetrafluoroethylene particle immobilization via polydopamine anchor layer on nitric oxide releasing polymer for biomedical applications. J. Colloid Interface Sci. 2021, 585, 716–728. [Google Scholar] [CrossRef]
- Homeyer, K.H.; Goudie, M.J.; Singha, P.; Handa, H. Liquid-Infused Nitric-Oxide-Releasing Silicone Foley Urinary Catheters for Prevention of Catheter-Associated Urinary Tract Infections. Acs Biomater. Sci. Eng. 2019, 5, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Colletta, A.; Wu, J.; Wo, Y.; Kappler, M.; Chen, H.; Xi, C.; Meyerhoff, M.E. S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device to Prevent Catheter-Associated Urinary Tract Infections. ACS Biomater. Sci. Eng. 2015, 1, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Sadrearhami, Z.; Shafiee, F.N.; Ho, K.K.K.; Kumar, N.; Krasowska, M.; Blencowe, A.; Wong, E.H.H.; Boyer, C. Antibiofilm Nitric Oxide-Releasing Polydopamine Coatings. ACS Appl. Mater. Interfaces 2019, 11, 7320–7329. [Google Scholar] [CrossRef]
- Li, M.; Aveyard, J.; Fleming, G.; Curran, J.M.; McBride, F.; Raval, R.; D’Sa, R.A. Nitric Oxide Releasing Titanium Surfaces for Antimicrobial Bone-Integrating Orthopedic Implants. ACS Appl. Mater. Interfaces 2020, 12, 22433–22443. [Google Scholar] [CrossRef] [PubMed]
- Sadrearhami, Z.; Namivandi-Zangeneh, R.; Price, E.; Krasowska, M.; Al-Bataineh, S.A.; Whittle, J.; Wong, E.H.H.; Blencowe, A.; Boyer, C. S-Nitrosothiol Plasma-Modified Surfaces for the Prevention of Bacterial Biofilm Formation. Acs Biomater. Sci. Eng. 2019, 5, 5881–5887. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Batista, P.S.P.; de Morais, A.M.M.B.; Pintado, M.M.E.; de Morais, R.M.S.C. Alginate: Pharmaceutical and Medical Applications. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 649–691. [Google Scholar]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Antibacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Feura, E.S.; Ahonen, M.J.R.; Schoenfisch, M.H. Nitric Oxide-Releasing Macromolecular Scaffolds for Antibacterial Applications. Adv. Healthc. Mater. 2018, 7, e1800155. [Google Scholar] [CrossRef] [PubMed]
- Reighard, K.P.; Ehre, C.; Rushton, Z.L.; Ahonen, M.J.R.; Hill, D.B.; Schoenfisch, M.H. Role of Nitric Oxide-Releasing Chitosan Oligosaccharides on Mucus Viscoelasticity. ACS Biomater. Sci. Eng. 2017, 3, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.S.; Lee, H.H.; Sanchez, D.A.; Friedman, A.J.; Tar, M.T.; Davies, K.P.; Nosanchuk, J.D.; Martinez, L.R. Sustained Nitric Oxide-Releasing Nanoparticles Induce Cell Death in Candida albicans Yeast and Hyphal Cells, Preventing Biofilm Formation In Vitro and in a Rodent Central Venous Catheter Model. Antimicrob. Agents Chemother. 2016, 60, 2185–2194. [Google Scholar] [CrossRef]
- Rong, F.; Tang, Y.Z.; Wang, T.J.; Feng, T.; Song, J.; Li, P.; Huang, W. Nitric Oxide-Releasing Polymeric Materials for Antimicrobial Applications: A Review. Antioxidants 2019, 8, 556. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Blecher, K.; Schairer, D.; Tuckman-Vernon, C.; Nacharaju, P.; Sanchez, D.; Gialanella, P.; Martinez, L.R.; Friedman, J.M.; Nosanchuk, J.D. Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S-nitrosoglutathione. Nitric Oxide 2011, 25, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, W.; Zheng, X.; Chang, S.; Liu, C.; Cheng, Q.; Zhu, S. Synergistic in vitro effects of indocyanine green and ethylenediamine tetraacetate-mediated antimicrobial photodynamic therapy combined with antibiotics for resistant bacterial biofilms in diabetic foot infection. Photodiagnosis Photodyn. Ther. 2019, 25, 300–308. [Google Scholar] [CrossRef]
- Biel, M.A.; Sievert, C.; Usacheva, M.; Teichert, M.; Balcom, J. Antimicrobial photodynamic therapy treatment of chronic recurrent sinusitis biofilms. Int. Forum Allergy Rhinol. 2011, 1, 329–334. [Google Scholar] [CrossRef]
- Biel, M.A.; Pedigo, L.; Gibbs, A.; Loebel, N. Photodynamic therapy of antibiotic-resistant biofilms in a maxillary sinus model. Int. Forum Allergy Rhinol. 2013, 3, 468–473. [Google Scholar] [CrossRef]
- Caminade, A.-M.; Yan, D.; Smith, D.K. Dendrimers and hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 3870–3873. [Google Scholar] [CrossRef]
- Liu, T.; Li, G.W.; Wu, X.D.; Chen, S.H.; Zhang, S.Y.; Han, H.; Zhang, H.B.; Luo, X.N.; Cai, X.; Ma, D. Beta-Cyclodextrin-graft-poly(amidoamine) dendrons as the nitric oxide deliver system for the chronic rhinosinusitis therapy. Drug Deliv. 2021, 28, 306–318. [Google Scholar] [CrossRef]
- Stasko, N.A.; Schoenfisch, M.H. Dendrimers as a scaffold for nitric oxide release. J. Am. Chem Soc. 2006, 128, 8265–8271. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Schoenfisch, M.H. Nitric Oxide-Releasing Hyperbranched Polyaminoglycosides for Antibacterial Therapy. ACS Appl. Bio Mater. 2018, 1, 1066–1073. [Google Scholar] [CrossRef]
- Sun, B.; Slomberg, D.L.; Chudasama, S.L.; Lu, Y.; Schoenfisch, M.H. Nitric oxide-releasing dendrimers as antibacterial agents. Biomacromolecules 2012, 13, 3343–3354. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.I.; Reins, R.Y.; McDermott, A.M.; Trautner, B.W.; Cai, C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol. Biosyst. 2009, 5, 1148–1156. [Google Scholar] [CrossRef]
- Wang, B.; Navath, R.S.; Menjoge, A.R.; Balakrishnan, B.; Bellair, R.; Dai, H.; Romero, R.; Kannan, S.; Kannan, R.M. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int. J. Pharm. 2010, 395, 298–308. [Google Scholar] [CrossRef]
- Devine, R.; Singha, P.; Handa, H. Versatile biomimetic medical device surface: Hydrophobin coated, nitric oxide-releasing polymer for antimicrobial and hemocompatible applications. Biomater. Sci. 2019, 7, 3438–3449. [Google Scholar] [CrossRef]
- Clarke, M.L.; Wang, J.; Chen, Z. Conformational Changes of Fibrinogen after Adsorption. J. Phys. Chem. B 2005, 109, 22027–22035. [Google Scholar] [CrossRef]
- Wo, Y.; Brisbois, E.J.; Bartlett, R.H.; Meyerhoff, M.E. Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: Just say yes to nitric oxide (NO). Biomater. Sci. 2016, 4, 1161–1183. [Google Scholar] [CrossRef] [PubMed]
- Michl, T.D.; Coad, B.R.; Doran, M.; Osiecki, M.; Kafshgari, M.H.; Voelcker, N.H.; Hüsler, A.; Vasilev, K.; Griesser, H.J. Nitric oxide releasing plasma polymer coating with bacteriostatic properties and no cytotoxic side effects. Chem. Commun. 2015, 51, 7058–7060. [Google Scholar] [CrossRef]
- Marxer, S.M.; Rothrock, A.R.; Nablo, B.J.; Robbins, M.E.; Schoenfisch, M.H. Preparation of nitric oxide (NO)-releasing sol-gels for biomaterial applications. Chem. Mater. 2003, 15, 4193–4199. [Google Scholar] [CrossRef]
- Privett, B.J.; Nutz, S.T.; Schoenfisch, M.H. Efficacy of surface-generated nitric oxide against Candida albicans adhesion and biofilm formation. Biofouling 2010, 26, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Nablo, B.J.; Schoenfisch, M.H. Poly(vinyl chloride)-Coated Sol−Gels for Studying the Effects of Nitric Oxide Release on Bacterial Adhesion. Biomacromolecules 2004, 5, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
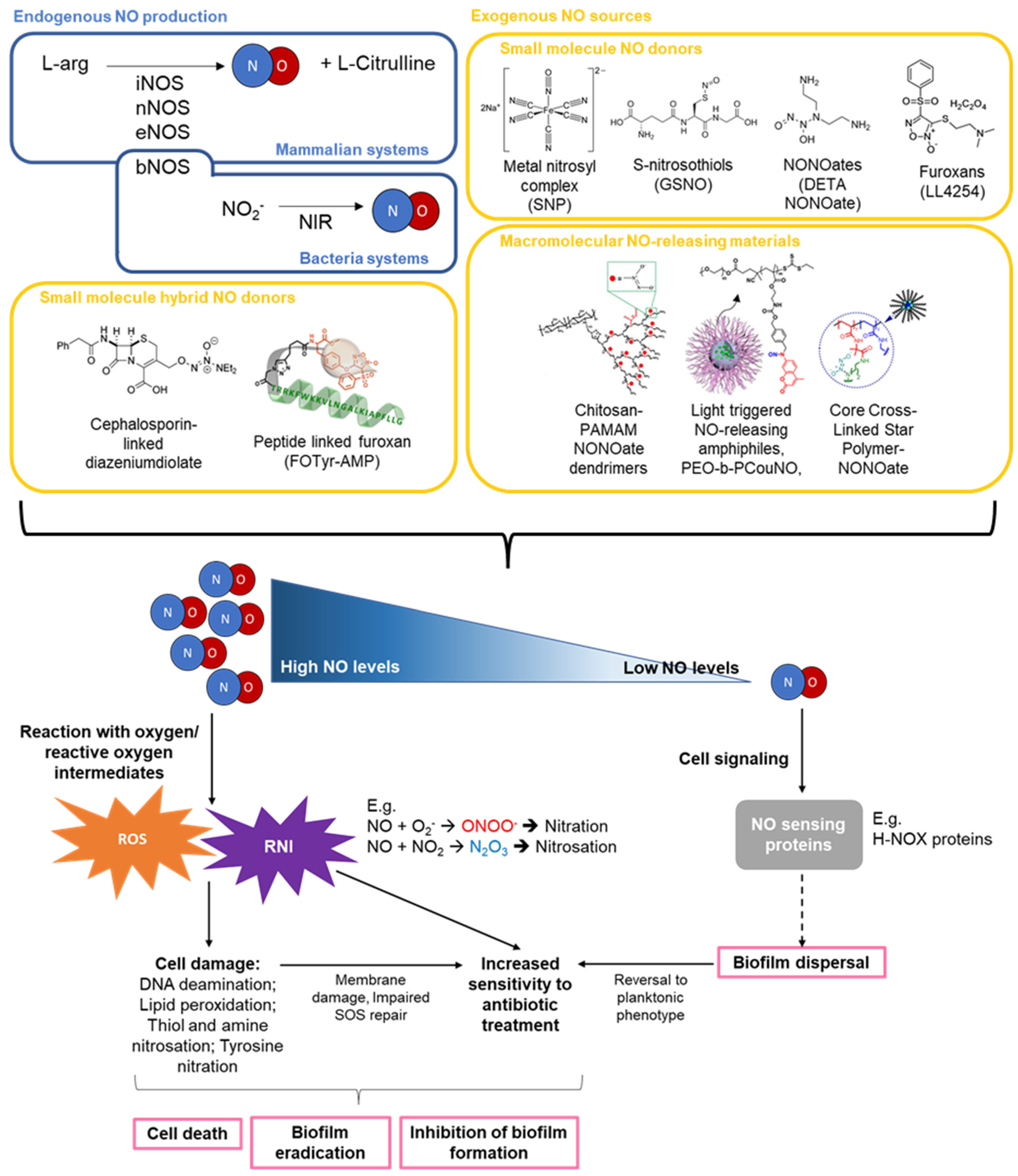
| Targeted Delivery of NO to Infection Site | Spontaneous NO Release | |
|---|---|---|
| High NO concentration |
|
|
| Low NO concentration |
|
| NO Donor | Concentration of NO Donor/NO | Stains/Test Conditions | Activity | Ref. |
|---|---|---|---|---|
| Gaseous NO | Continuous 200 ppm NO or intermittent 300 ppm NO | In vitro P. aeruginosa, S. aureus, clinical strains In vivo K. pneumoniae, MDR Klebsiella and S. aureus |
| [56,57] |
| Intermittent 160–240 ppm NO | Clinical trials and case studies M. abscessus, E. coli, P. aeruginosa, antibiotic-resistant P. aeruginosa, antibiotic-resistant B. multivoran, S. aureus, and Group B Streptococcus |
| [32,58,59,60,61,62] | |
| Metal nitrosyl complexes (e.g., Sodium nitroprusside (SNP)) | 25 µM–100 mM SNP (≈25 nM to 100 µM NO); 5–80 µM SNP (12 h treatment time) | In vitro P. aeruginosa, S. marcescens, V. cholerae, E. coli, F. nucleatum, B. licheniformis, S. epidermidis, C. albicans, and isolates from MBR and RO membrane |
| [6,42,63] |
| Photoresponsive ruthenium compounds | µM | In vitro S. epidermidis, P. aeruginosa |
| [64,65] |
| S-nitrosothiols (RSNO) (e.g., GSNO, SNAC) | nM–mM | In vitro P. aeruginosa, S. marcescens, V. cholerae, and Keratitis-causing isolates |
| [6,63,66] |
| N-diazeniumdiolates (NONOates) | 10 pM–80 µM (Varying treatment duration and dosing regimens) | In vitro Isolates from MBR and RO membrane, S. enterica, E. coli O157:H7, P. aeruginosa, and CF isolates |
| [42,67,68] |
| Furoxans | 5–500 µM | In vitro P. aeruginosa |
| [69,70] |
| Antimicrobial-NO donor hybrid donors | nM–µM | In vitro P. aeruginosa, S. pneumoniae, Haemophilus influenzae (NTHi), clinical CF isolates of P. aeruginosa, S. aureus, and E. coli In vivo S. aureus |
| [25,26,71,72,73,74,75] |
| QS inhibitor—NO hybrid donor | 150 µM | In vitro P. aeruginosa |
| [76] |
| Macromolecular NO-Releasing Material | Concentration of NO/NO Donor Used | Stains/Test Conditions | Activity | Ref. |
|---|---|---|---|---|
| NO-releasing chitosan oligosaccharide (COS/NO) | 0.12–3.1 µmol NO/mL | In vitro Mucoid/non-mucoid/clinical P. aeruginosa, E. coli, and S. aureus |
| [40,113,114,115] |
| NO-releasing chitosan gels | Variable depending on design and NO donors used. NO concentrations of ~ nmol NO/mg film or initial NO flux of ~ nmol cm−2 min−1 | In vitro S. aureus, P. aeruginosa, MRSA, L. monocytogenes and E. faecalis In vivo MRSA |
| [13,116,117,118] |
| NO-releasing chitosan-dendrimer (CS-PAMAM/NO) | 1–2.5 mg/mL chitosan dendrimer (~1.5 µmol NO/mg) | In vitro E. coli, S. aureus and MRSA In vivo MRSA |
| [21,119] |
| NO-releasing alginate | ~ µmol NO/mL for NONOate conjugated alginate | In vitro P. aeruginosa, S. aureus, B. cepacia complex, MRSA, S. mutans, and E. coli |
| [99,120] |
| NO-releasing cyclodextrins (NO/CD) | 100–2000 µg/mL NO/CD (~ nmol NO/mL) | In vitro P. aeruginosa |
| [121,122] |
| NO-releasing silica NPs (NO-NPs) | ~ µg/mL to mg/mL NO-NPs with varying NO release kinetics and flux | In vitro P. aeruginosa, E. coli, S. aureus, S. epidermidis, S. aureus, A. actinomycetemcomitans, P. gingivalis, and S. mutans |
| [104,123,124,125,126,127] |
| NO-releasing silane-based hydrogel nanoparticle platform | Steady state NO in nM range | In vitro MRSA and MSSA S. aureus In vivo MRSA |
| [31,128] |
| NO-releasing P(OEGMA) containing polymeric nanoparticles | Variable, dependent on design (see activity for more details) | In vitro P. aeruginosa | Gentamicin-NONOate NPs block copolymer NP
| [54] |
Spherical (S-NO) and worm-like NO-NPs (W-NO)
| [129] | |||
NO releasing polydopamine (PDA)-coated iron oxide NPs
| [105] | |||
Core cross-Linked star polymers
| [23] | |||
| NO-releasing polymeric nanoparticles, microparticles, and liposomes | NPs and MPs used in mg/mL range | In vitro S. aureus, MRSA |
| [130,131] |
| [132,133] | |||
| Photo-activated NO-releasing polymeric materials | Variable, dependent on design (see activity for more details) | In vitro P. aeruginosa, S. aureus, MRSA, E. coli In vivo S. aureus, MRSA | Self-assembled micellar NPs with hydrophobic antibiotic in core
| [22] |
Surface charge switchable, GSH activated α-CD-Ce6-NO-DA
| [134] | |||
Phototherapeutic nanoplatform AI-MPDA
| [135] | |||
Electrospun nanocomposite membrane (UCNP@PCN@LA-PVDF)
| [136] | |||
PDT-driven NO controllable generation system (Ce6@Arg-ADP)
| [137] | |||
| NO-releasing dendrimers | Variable, dependent on design. ~0.69 to 1 µmol NO/mg dendrimer released over 2–4 h in PBS, pH 7.4, 37 °C with max. flux of 2400–15,000 ppb/mg | In vitro P. aeruginosa, S. mutans, S. aureus, S. sanguinis, A. acetinomycetemcomitans, and P. gingivali |
| [100,102,138,139] |
| NO-releasing hyperbranched dendrimers | NO storage and NO release ~µmol/mg with half-life ranging from 28 to 80 min depending on design and modifications | In vitro P. gingivalis, A. acetinomycetemcomitans, S. mutansm, S. viscosus, and ex vivo multispecies subgingival biofilms |
| [41,140] |
| NO-releasing xerogels and polymer coatings | Variable, dependent on pH, coating, and media (see activity for more details) | In vitro P. aeruginosa | Super-hydrophobic NO-releasing xerogels with fluorinated silane/silica composite topcoat
| [141] |
NO-releasing (poly)acrylonitrile (PAN/NO) polymer
| [142] | |||
NO-releasing coatings on PET and silicone elastomer
| [143] | |||
| SNAP-containing Carbosil 2080A polymer (Carbosil-SNAP) with different top coats | In vitro P. aeruginosa, P. mirabilis, S. aureus, E. coli |
| [144,145,146] | |
| SNAP-impregnated silicone catheters | NO release ~0.04 nmol/cm2/mL over 60 days or ~ >0.07 nmol/min/cm2 over a month | In vitro P. aeruginosa, P. mirabilis, S. aureus, S. epidermidis |
| [147,148] |
| Other NO-releasing surfaces | NO flux in µM range (PBS, pH 7.4, 37 °C) | In vitro P. aeruginosaS. aureus | NO-releasing polydopamine (PDA) coating with PEG grafted onto PDA
| [149] |
NO-releasing titanium surfaces
| [150] | |||
Thiol-functionalized coatings
| [151] | |||
| NO release sustained over 15 days at levels >1 nmol/cm2/min and a maximum flux of ~ 3 nmol/cm2/min within <15 min | In vitro S. aureus, S. epidermis, E. faecalis, P. aeruginosa, K. pneumoniae, A. baumannii, and E. coli and relevant MDR isolates, In vivo (Murine subcutaneous infection model) P. aeruginosa, A. baumannii; (Porcine central venous catheterization model) N/A | Precision-structured diblock copolymer brush (H(N)-b-S)
| [24] |
| NO Donors/Polymeric Materials | Advantages | Disadvantages |
|---|---|---|
| NO gas | FDA approved; Direct NO delivery to lung infection sites and surface of wound infections; Side effects easily reversed by stopping NO gas | React with oxygen to give potent pulmonary irritants like NO2 and with hemoglobin to give methemoglobin |
| Metal-nitrosyl complexes | Metal-nitrosyl complexes, such as sodium nitroprusside (SNP), is FDA approved and long history of use clinically | Possibility of cyanide toxicity when using SNP for prolonged treatment |
| Ru-nitrosyl complexes | Photo-responsive | Relatively new and less well studied for antimicrobial purposes |
| S-nitrosothiols (RSNO) | Present endogenously; Some, such as GSNO, have well studied metabolism and low toxicity; NO release can be modulated through various means, including light irradiation; Easily incorporated into polymeric scaffold | Spontaneous release of NO and formation of disulfide bonds in solution; Trans-nitrosylation reaction with other thiol groups present in the body; Multiple mechanisms of degradation by bacteria |
| N-diazeniumdiolates (NONOates) | Broad range of reproducible NO release kinetics; Easily incorporated into polymeric materials containing amine moieties by passing NO gas at high pressure; Stable in powder form and in alkaline solutions | Spontaneous NO release in solution under physiological conditions. Not used clinically |
| Furoxans | Well-studied NO release with applications in various NO mediated biological processes; Prolong duration of action compared to other NO donors; Thermally stable; May be conjugated to other groups for codelivery of antimicrobials and NO donor | Appears to have other non-NO dependent effects on evaluated bacteria (i.e., P. aeruginosa) that is not well studied or explained with NO release |
| Hybrid NO donor | Targeted NO release using antibiotics or antimicrobial peptides; Synergistic effect at eradicating bacteria/biofilm with both targeted NO release and QS inhibition or antimicrobial action | Earlier generations of some hybrid NO donors, such as C3D, require induction of β-lactamase production for activity |
| NO-releasing polymeric materials | ||
| Chitosan-based NO-releasing materials | Chitosan scaffold is biodegradable, biocompatible and has innate antimicrobial activity; Cationic chitosan promotes association with negatively charged bacterial membranes; Primary amine groups offer a straightforward means of incorporating NO-releasing moieties | In cases like NO-releasing chitosan oligosaccharide (COS/NO), cationic chitosan may improve cohesion of negatively charged biofilms |
| Alginate-based NO-releasing materials | Alginate is biodegradable and biocompatible; NO-releasing moieties easily introduced via abundant hydroxyl and carboxylic acid groups; NO release easily tunable by modifying high/low molecular weight alginate used | |
| NO-releasing cyclodextrins | Hydrophobic central cavity and hydrophilic exterior could enable delivery of hydrophobic antimicrobial compounds along with NO release | |
| NO-releasing silica nanoparticles | Innate antimicrobial activity of nanoparticles. Physiochemical properties, such as shape, sizes, and surface charge can be easily modified to improve NO delivery and bacteria eradication | Cytotoxicity reported in some designs |
| NO-releasing polymeric nanoparticles | Specificity and controlled release of NO can be achieved by incorporating photo-responsive groups and surface-charge switchable components; Able to co-deliver antibiotic with NO release to enhance bacterial or biofilm eradication; Other properties, e.g., magnetic field responsive NO-NP, may also be obtained | |
| NO-releasing dendrimers | High NO payloads within a single framework; Polymerization of antibiotics enable simultaneous delivery of NO with antibiotic and improve bacteria and biofilm eradication | Cytotoxicity may be associated with higher generation dendrimers and certain chemical modifications/ dendrimers |
| NO-releasing gel, polymer, and coatings | NO-releasing surfaces used in blood contacting medical devices may be designed to generate an NO flux representative of endothelial cells; Additional coating along with NO release can extent the anti-fouling lifespan of the material | Leaching of NO may occur depending on the design |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poh, W.H.; Rice, S.A. Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications. Molecules 2022, 27, 674. https://doi.org/10.3390/molecules27030674
Poh WH, Rice SA. Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications. Molecules. 2022; 27(3):674. https://doi.org/10.3390/molecules27030674
Chicago/Turabian StylePoh, Wee Han, and Scott A. Rice. 2022. "Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications" Molecules 27, no. 3: 674. https://doi.org/10.3390/molecules27030674
APA StylePoh, W. H., & Rice, S. A. (2022). Recent Developments in Nitric Oxide Donors and Delivery for Antimicrobial and Anti-Biofilm Applications. Molecules, 27(3), 674. https://doi.org/10.3390/molecules27030674





