The Reserve/Maximum Capacity of Melatonin’s Synthetic Function for the Potential Dimorphism of Melatonin Production and Its Biological Significance in Mammals
Abstract
1. Introduction
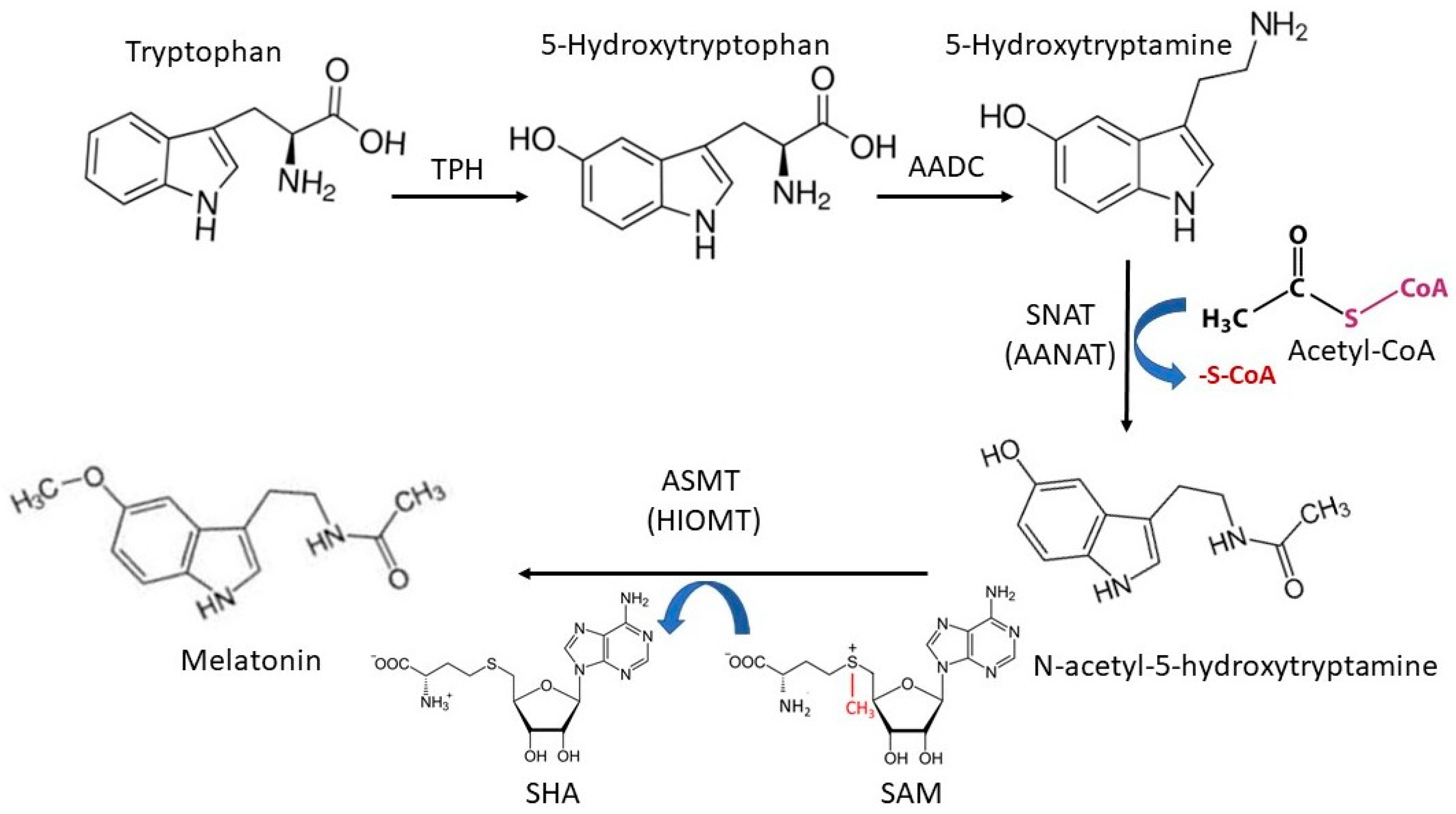
2. Melatonin’s Synthetic Pathway in Animals
3. Sites of Melatonin Synthesis
4. Potential Gender Bias in the Expression of ASMT in Mammals
5. Potential Gender Bias of Mitochondria-Related Melatonin Synthesis
6. Evidence to Support the Masked Dimorphism of Melatonin Production
7. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, D.-X.; Zheng, X.; Kong, J.; Manchester, L.; Hardeland, R.; Kim, S.; Xu, X.; Reiter, R. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: Relation to their biological functions. Int. J. Mol. Sci. 2014, 15, 15858–15890. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Poeggeler, B.; Alvares, F.L.; Ogden, G.B.; Reiter, R.J. Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cell. Mol. Biol. Res. 1995, 41, 391–395. [Google Scholar] [PubMed]
- Byeon, Y.; Lee, K.; Park, Y.-I.; Park, S.; Back, K. Molecular cloning and functional analysis of serotonin N-acetyltransferase from the cyanobacterium Synechocystis sp. PCC 6803. J. Pineal Res. 2013, 55, 371–376. [Google Scholar] [CrossRef]
- Hardeland, R.; Balzer, I.; Poeggeler, B.; Fuhrberg, B.; Una, H.; Behrmann, G.; Wolf, R.; Meyer, T.J.; Reiter, R.J. On the primary functions of melatonin in evolution: Mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free radicals. J. Pineal Res. 1995, 18, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.H.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.-X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Chattopadhyay, A.; Bandyopadhyay, D. Melatonin: An ancient note in a contemporary wrap. Melatonin Res. 2021, 4, 453–478. [Google Scholar] [CrossRef]
- Crespi, F.; Ratti, E.; TristF, D.G. Melatonin, a hormone monitorable in vivo by voltammetry? Analyst 1994, 119, 2193–2197. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.-S.; Hardeland, R.; Reiter, R.J. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef] [PubMed]
- Kuesel, J.T.; Hardeland, R.; Pfoertner, H.; Aeckerle, N. Reactions of the melatonin metabolite N(1)-acetyl-5-methoxykynuramine with carbamoyl phosphate and related compounds. J. Pineal Res. 2010, 48, 47–54. [Google Scholar] [CrossRef]
- Seever, K.; Hardeland, R. Novel pathway for N1-acetyl-5-methoxykynuramine: UVB-induced liberation of carbon monoxide from precursor N1-acetyl-N2-formyl-5-methoxykynuramine. J. Pineal Res. 2008, 44, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and 5-methoxytryptamine in non-metazoans. Reprod. Nutr. Dev. 1999, 39, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Brierley, A.S. Diel vertical migration. Curr. Biol. 2014, 24, R1074–R1076. [Google Scholar] [CrossRef]
- Sören Häfker, N.; Meyer, B.; Last, M.S.; Pond, D.W.; Hüppe, L.; Mathias Teschke, M. Circadian clock involvement in zooplankton diel vertical migration. Curr. Biol. 2017, 27, 2194–2201. [Google Scholar] [CrossRef] [PubMed]
- Antolín, I.; Obst, B.; Burkhardt, S.; Hardeland, R. Antioxidative protection in a high-melatonin organism: The dinoflagellate Gonyaulax polyedra is rescued from lethal oxidative stress by strongly elevated, but physiologically possible concentrations of melatonin. J. Pineal Res. 1997, 23, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Tosches, M.A.; Bucher, D.; Vopalensky, P.; Arendt, D. Melatonin signaling controls circadian swimming behavior in marine zooplankton. Cell 2014, 159, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991, 12, 151–180. [Google Scholar] [CrossRef] [PubMed]
- Robert, K.A.; Lesku, J.A.; Partecke, J.; Chamber, B. Artificial light at night desynchronizes strictly seasonal reproduction in a wild mammal. Proc. Biol. Sci. 2015, 282, 20151745. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Influence of pinealectomy on the breeding capability of hamsters maintained under natural photoperiodic and temperature conditions. Neuroendocrinology 1974, 13, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef]
- Majumder, R.; Datta, M.; Chattopadhyay, A.; Bandyopadhyay, D. Melatonin promotes gastric healing by modulating the components of matrix metalloproteinase signaling pathway: A novel scenario for gastric ulcer management. Melatonin Res. 2021, 4, 213–231. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Zmijewski, M.A.; Kim, T.K.; Slominski, R.M.; Reiter, R.J.; Fischer, T.W. Local melatoninergic system as the protector of skin integrity. Int. J. Mol. Sci. 2014, 15, 17705–17732. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Han, H.B.; Tian, X.Z.; Tan, D.-X.; Wang, L.; Zhou, G.B.; Shi-En Zhu, S.E.; Liu, G.S. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J. Pineal Res. 2012, 52, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Starcevic, A.; Akthar, S.; Dunlap, W.C.; Shick, J.M.; Hranueli, D.; Cullum, J.; Paul, F. Long, P.F. Enzymes of the shikimic acid pathway encoded in the genome of a basal metazoan, Nematostella vectensis, have microbial origins. Proc. Natl. Acad. Sci. USA 2008, 105, 2533–2537. [Google Scholar] [CrossRef]
- McKinney, J.; Knappskog, P.M.; Haavik, J. Different properties of the central and peripheral forms of human tryptophan hydroxylase. J. Neurochem. 2005, 92, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jiang, X.; Liu, G.; Wassie, T.; Girmay, S. An ancient mutation in the TPH1 gene is consistent with the changes in Mammalian reproductive rhythm. Int. J. Mol. Sci. 2019, 20, 6065. [Google Scholar] [CrossRef]
- Gaudet, S.J.; Slominski, A.; Etminan, M.; Pruski, D.; Paus, R.; Namboodiri, M.A.A. Identification and characterization of two isozymic forms of arylamine N-Acetyltransferase in Syrian hamster skin. J. Investig. Dermatol. 1993, 101, 660–665. [Google Scholar] [CrossRef]
- Slominski, A.; Pisarchik, A.; Semak, I.; Sweatman, T.; Wortsman, J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 2003, 270, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Corvera, A.; Cerrillo, I.; Naranjo, M.C.; Lardone, P.J.; Sanchez-Hidalgo, M.; Carrascosa-Salmoral, M.P.; Medrano-Campillo, P.; Guerrero, J.M.; Rubio, A.A. Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains. J. Pineal Res. 2009, 47, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ribelayga, C.; Pévet, P.; Simonneaux, V. HIOMT drives the photoperiodic changes in the amplitude of the melatonin peak of the Siberian hamster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1339–R1345. [Google Scholar] [CrossRef] [PubMed]
- Ceinos, R.M.; Chansard, M.; Revel, F.; Calgari, C.; Míguez, J.M.; Simonneaux, V. Analysis of adrenergic regulation of melatonin synthesis in Siberian hamster pineal emphasizes the role of HIOMT. Neurosignals 2004, 13, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, R.; Back, K.; Manchester, L.C.; Alatorre-Jimenez, M.A.; Reiter, R.J. On the significance of an alternate pathway of melatonin synthesis via 5-methoxytryptamine: Comparisons across species. J. Pineal Res. 2016, 61, 27–40. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Sagan, L. On the origin of mitosing cells. J. Theor. Biol. 1967, 14, 225-IN6. [Google Scholar] [CrossRef]
- Esser, C.; Ahmadinejad, N.; Wiegand, C.; Rotte, C.; Sebastiani, F.; Gelius-Dietrich, G.; Henze, K.; Kretschmann, E.; Richly, E.; Leister, D.; et al. A genome phylogeny for mitochondria among alpha-proteobacteria and a predominantly eubacterial ancestry of yeast nuclear genes. Mol. Biol. Evol. 2004, 21, 1643–1660. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice Oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef] [PubMed]
- Quintela, T.; Gonçalves, I.; Silva, M.; Duarte, A.C.; Guedes, P.; Andrade, K.; Freitas, F.; Talhada, D.; Albuquerque, T.; Tavares, S.; et al. Choroid plexus is an additional source of melatonin in the brain. J. Pineal Res. 2018, e12528. [Google Scholar] [CrossRef] [PubMed]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Qin, L.; Reiter, R.J. Melatonin: A mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int. J. Mol. Sci. 2016, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Agrimi, G.; Di Noia, M.A.; Marobbio, C.M.T.; Fiermonte, G.; Lasorsa, F.M.; Palmieri, F. Identification of the human mitochondrial S-adenosylmethionine transporter: Bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem. J. 2004, 379, 183–190. [Google Scholar] [CrossRef]
- Rosengarten, H.; Meller, E.; Friedhoff, A.J. In vitro enzymatic formation of melatonin by human erythrocytes. Res. Commun. Chem. Pathol. Pharmacol. 1972, 4, 457–465. [Google Scholar] [PubMed]
- Tan, D.-X.; Reiter, R.J. Mitochondria: The birth place, battle ground and the site of melatonin metabolism in cells. Melatonin Res. 2019, 2, 44–66. [Google Scholar] [CrossRef]
- Yi, H.; Donohue, S.J.; Klein, D.C.; McBride, O.W.H. Localization of the hydroxyindole-O-methyltransferase gene to the pseudoautosomal region: Implications for mapping of psychiatric disorders. Hum. Mol. Genet. 1993, 2, 127–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ried, K.; Rao, E.; Schiebel, K.; Rappold, G.A. Gene duplications as a recurrent theme in the evolution of the human pseudoautosomal region 1: Isolation of the gene ASMTL. Hum. Mol. Genet. 1998, 7, 1771–1778. [Google Scholar] [CrossRef]
- Mangs, A.H.; Morris, B.J. The Human Pseudoautosomal Region (PAR): Origin, function and future. Curr. Genom. 2007, 8, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, R.J.; Rappold, G. The pseudoautosomal regions, SHOX and disease. Curr. Opin. Genet. Dev. 2006, 16, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Soriano, P.; Keitges, E.A.; Schorderet, D.F.; Harbers, K.; Gartler, S.M.; Jaenisch, R. High rate of recombination and double crossovers in the mouse pseudoautosomal region during male meiosis. Proc. Natl. Acad. Sci. USA 1987, 84, 7218–7220. [Google Scholar] [CrossRef] [PubMed]
- Hinch, A.G.; Altemose, N.; Noor, N.; Donnelly, P.; Myers, S.M. Recombination in the human pseudoautosomal region PAR1. PLoS Genet. 2014, 10, e1004503. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Abe, K.; Mekada, K.; Yoshiki, A.; Kato, T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc. Natl. Acad. Sci. USA 2010, 107, 6412–6417. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Ruan, Y.; Lu, T.; Liu, C.; Jia, M.; Yue, W.; Liu, J.; Bourgeron, T.; Zhang, D. Sequencing ASMT identifies rare mutations in Chinese Han patients with autism. PLoS ONE 2013, 8, e53727. [Google Scholar] [CrossRef]
- Pagan, C.; Botros, H.G.; Poirier, K.; Dumaine, A.; Jamain, S.; Moreno, S.; de Brouwer, A.; Esch, H.V.; Delorme, R.; Launay, J.M.; et al. Mutation screening of ASMT, the last enzyme of the melatonin pathway, in a large sample of patients with intellectual disability. BMC Med. Genet. 2011, 12, 17. [Google Scholar] [CrossRef]
- Cruz-Machado, S.D.S.; Campos, L.M.G.; Fadini, C.C.; Anderson, G.; Regina, P.; Markus, R.P.; Luciana Pinato, L. Disrupted nocturnal melatonin in autism: Association with tumor necrosis factor and sleep disturbances. J. Pineal Res. 2021, 70, e12715. [Google Scholar] [CrossRef]
- Maruani, A.; Dumas, G.; Beggiato, A.; Traut, N.; Peyre, H.; Cohen-Freoua, A.; Amsellem, F.; Elmaleh, M.; Germanaud, D.; Launay, J.M.; et al. Morning plasma melatonin differences in autism: Beyond the impact of pineal gland volume. Front. Psychiatry 2019, 10, 11. [Google Scholar] [CrossRef]
- Veatch, O.J.; Pendergast, J.S.; Allen, M.J.; Leu, R.M.; Johnson, C.H.; Elsea, S.H.; Malow, B.A. Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay. J. Autism Dev. Disord. 2014, 45, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Melke, J.; Goubran Botros, H.; Chaste, P.; Betancur, C.; Nygren, G.; Anckarsäter, H.; Rastam, M.; Ståhlberg, O.; Gillberg, I.C.; Delorme, R.; et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 2008, 13, 90–98. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Unckless, R.L.; Herren, J.K. Population genetics of sexually antagonistic mitochondrial mutants under inbreeding. J. Theor. Biol. 2009, 260, 132–136. [Google Scholar] [CrossRef]
- Camus, M.F.; Dowling, D.K. Mitochondrial genetic effects on reproductive success: Signatures of positive intrasexual, but negative intersexual pleiotropy. Proc. Biol. Sci. 2018, 285, 20180187. [Google Scholar] [CrossRef] [PubMed]
- Rand, D.M.; Clark, A.G.; Kann, L.M. Sexually antagonistic cytonuclear fitness interactions in Drosophila melanogaster. Genetics 2001, 159, 173–187. [Google Scholar] [CrossRef]
- Frank, S.A.; Hurst, L.D. Mitochondria and male disease. Nature 1996, 383, 224. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, N.J.; Metcalf, V.J.; Allendorf, F.W. Mother’s curse: The effect of mtDNA on individual fitness and population viability. Trends Ecol. Evol. 2004, 19, 238–244. [Google Scholar] [CrossRef]
- Vaught, R.C.; Dowling, D.K. Maternal inheritance of mitochondria: Implications for male fertility? Reproduction 2018, 155, R159–R168. [Google Scholar] [CrossRef]
- Kuijper, B.; Lane, N.; Pomiankowski, A. Can paternal leakage maintain sexually antagonistic polymorphism in the cytoplasm? J. Evol. Biol. 2015, 28, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Polovina, E.S.; Parakatselaki, M.E.; Ladoukakis, E.D. Paternal leakage of mitochondrial DNA and maternal inheritance of heteroplasmy in Drosophila hybrids. Sci. Rep. 2020, 10, 2599. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, C.; Vaught, R.C.; Voigt, S.; Dobler, R.; Clancy, D.J.; Reinhardt, K.; Dowling, D.K. Interactions between cytoplasmic and nuclear genomes confer sex-specific effects on lifespan in Drosophila melanogaster. J. Evol. Biol. 2020, 33, 694–713. [Google Scholar] [CrossRef]
- Keaney, T.A.; Wong, H.W.S.; Dowling, D.K.; Jones, T.M.; Holman, L. Sibling rivalry versus mother’s curse: Can kin competition facilitate a response to selection on male mitochondria? Proc. Biol. Sci. 2020, 287, 20200575. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, P.W. Reversing mother’ s curse revisited. Evolution 2012, 66, 612–616. [Google Scholar] [CrossRef]
- Nagarajan-Radha, V.; Aitkenhead, I.; Clancy, D.J.; Chown, S.L.; Dowling, D.K. Sex-specific effects of mitochondrial haplotype on metabolic rate in Drosophila melanogaster support predictions of the Mother’s Curse hypothesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190178. [Google Scholar] [CrossRef] [PubMed]
- Iessi, E.; Cittadini, C.; Anticoli, S.; Fecchi, K.; Matarrese, P.; Ruggieri, A. Sex differences in antiviral immunity in SARS-CoV-2 infection: Mitochondria and mitomiR come into view. Acta Physiol. 2021, 231, e13571. [Google Scholar] [CrossRef]
- Claypool, L.E.; Wood, R.I.; Yellon, S.M.; Foster, D.L. The ontogeny of melatonin secretion in the lamb. Endocrinology 1989, 124, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Weiszenbacher, G.; Tatzer, E.; Gisinger, B.; Waldhauser, M.; Schemper, M.; Frisch, H. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J. Clin. Endocrinol. Metab. 1988, 66, 648–652. [Google Scholar] [CrossRef]
- Gunn, P.J.; Middleton, B.; Davies, S.K.; Revell, V.L.; Skene, D.J. Sex differences in the circadian profiles of melatonin and cortisol in plasma and urine matrices under constant routine conditions. Chronobiol. Int. 2016, 33, 39–50. [Google Scholar] [CrossRef]
- Delfs, T.M.; Baars, S.; Fock, C.; Schumacher, M.; Olcese, J.; Zimmermann, R.C. Sex steroids do not alter melatonin secretion in the human. Hum. Reprod. 1994, 9, 49–54. [Google Scholar] [CrossRef]
- Touitou, Y.; Fevre-Montange, M.; Proust, J.; Klinger, E.; Nakache, J.P. Age- and sex-associated modification of plasma melatonin concentrations in man. Relationship to pathology, malignant or not, and autopsy findings. Acta Endocrinol. 1985, 108, 135–144. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, melatonin, and the pro- and anti-inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin and brain inflammaging. Prog. Neurobiol. 2015, 127, 46–63. [Google Scholar] [CrossRef]
- Fuhrberg, B.; Hardeland, R.; Poeggeler, B.; Behrmann, C. Dramatic rises of melatonin and 5-Methoxytryptamine in Gonyaulax exposed to decreased temperature. Biol. Rhythm Res. 1997, 28, 144–150. [Google Scholar] [CrossRef]
- Jaworek, J.; Leja-Szpak, A.; Bonior, J.; Nawrot, K.; Tomaszewska, R.; Stachura, J.; Sendur, R.; Pawlik, W.; Brzozowski, T.; Konturek, S.J. Protective effect of melatonin and its precursor L-tryptophan on acute pancreatitis induced by caerulein overstimulation or ischemia/reperfusion. J. Pineal Res. 2003, 34, 40–52. [Google Scholar] [CrossRef]
- Ahmad, R.; Haldar, C. Immune responses to lipopolysaccharide challenge in a tropical rodent (Funambulus pennanti): Photoperiod entrainment and sex differences. Stress 2012, 15, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Vaghefi, S.S.E.; Mousavi, F.; Khaksari, M.; Asadikaram, G.; Soltani, Z. Sex-related changes in circadian rhythm of inflammatory and oxidative stress markers in CKD. Iran J. Kidney Dis. 2021, 15, 351–363. [Google Scholar]
- Tan, D.-X.; Manchester, L.C.; Sainz, R.M.; Mayo, J.C.; León, J.; Reiter, R.J. Physiological ischemia/reperfusion phenomena and their relation to endogenous melatonin production: A hypothesis. Endocrine 2005, 27, 149–158. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Korkmaz, A.; Ma, S. Obesity and metabolic syndrome: Association with chronodisruption, sleep deprivation, and melatonin suppression. Ann. Med. 2012, 44, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Reiter, R.J.; Pechanova, O.; Paulis, L. Experimental models of melatonin-deficient hypertension. Front. Biosci. 2013, 18, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Simko, F.; Reiter, R.J. Is melatonin deficiency a unifying pathomechanism of high risk patients with COVID-19? Life Sci. 2020, 256, 117902. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Esquifino, A.I.; Srinivasan, V.; Pandi-Perumal, S.R. Melatonin and the immune system in aging. Neuroimmunomodulation 2008, 15, 272–278. [Google Scholar] [CrossRef]
- Hardeland, R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef]
- Baltatu, O.C.; Amaral, F.G.; Campos, L.A.; Cipolla-Neto, J. Melatonin, mitochondria and hypertension. Cell. Mol. Life Sci. 2017, 74, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.-X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Bruss, Z.S.; Raja, A. Physiology, Stroke Volume; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise standards for testing and training: A scientific statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef] [PubMed]
- Cornélissen, G.; Halberg, F.; Schwartzkopff, O.; Delmore, P.; Katinas, G.; Hunter, D.; Tarquini, B.; Tarquini, R.; Perfetto, F.; Watanabe, Y.; et al. Chronomes, time structures, for chronobioengineering for “a full life”. Biomed. Instrum. Technol. 1999, 33, 152–187. [Google Scholar]
- Martín, M.; Macías, M.; León, J.; Escames, G.; Khaldy, H.; Acuña-Castroviejo, D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int. J. Biochem. Cell Biol. 2002, 34, 348–357. [Google Scholar] [CrossRef]
- Fang, Y.; Zhao, C.; Xiang, H.; Zhao, X.; Zhong, R. Melatonin inhibits formation of mitochondrial permeability transition pores and improves oxidative phosphorylation of frozen-thawed ram sperm. Front. Endocrinol. 2020, 10, 896. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Why men age faster but reproduce longer than women: mTOR and evolutionary perspectives. Aging 2010, 2, 265. [Google Scholar] [CrossRef]
- Tower, J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch. Biochem. Biophys. 2015, 576, 17–31. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin and healthy aging. Vitam. Horm. 2021, 115, 67–88. [Google Scholar] [CrossRef]
- Paulose, J.K.; Cassone, C.V.; Cassone, V.M. Aging, melatonin biosynthesis, and circadian clockworks in the gastrointestinal system of the laboratory mouse. Physiol. Genom. 2019, 51, 1–9. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Yang, M.; Tao, J.; Wu, H.; Zhang, L.; Yao, Y.; Liu, L.; Zhu, T.; Fan, H.; Cui, X.; Dou, H.; et al. Responses of transgenic melatonin-enriched goats on LPS stimulation and the proteogenomic profiles of their PBMCs. Int. J. Mol. Sci. 2018, 19, 2406. [Google Scholar] [CrossRef]
- Li, G.; Lv, D.; Yao, Y.; Wu, H.; Wang, J.; Deng, S.; Song, Y.; Guan, S.; Wang, L.; Ma, W.; et al. Overexpression of ASMT likely enhances the resistance of transgenic sheep to brucellosis by influencing immune-related signaling pathways and gut microbiota. FASEB J. 2021, 35, e21783. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.; Melenotte, C.; Lakbar, I.; Mezouar, S.; Devaux, C.; Raoult, D.; Bendiane, M.K.; Leone, M.; Mège, J.L. Sexual dimorphism and gender in infectious diseases. Front. Immunol. 2021, 12, 698121. [Google Scholar] [CrossRef]
- Ulhaq, Z.S.; Soraya, G.V.; Zambrano, L.E.A.; Garcia, C.P. Sexual dimorphism in SARS-CoV-2 infection. Acta Endocrinol. 2020, 16, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Pegiou, S.; Rentzeperi, E.; Koufakis, T.; Metallidis, S.; Kotsa, K. The role of sexual dimorphism in susceptibility to SARS-CoV-2 infection, disease severity, and mortality: Facts, controversies and future perspectives. Microbes Infect. 2021, 23, 104850. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Hardeland, H. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 2020, 25, 4410. [Google Scholar] [CrossRef] [PubMed]
- Kloc, M.; Ghobrial, R.M.; Kubiak, J.Z. The role of genetic sex and mitochondria in response to COVID-19 infection. Int. Arch. Allergy Immunol. 2020, 181, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Mehrzadi, S.; Karimi, M.Y.; Fatemi, A.; Reiter, R.J.; Hosseinzadeh, A. SARS-CoV-2 and other coronaviruses negatively influence mitochondrial quality control: Beneficial effects of melatonin. Pharmacol. Ther. 2021, 224, 107825. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Quizon, G.R.A.; Juco, M.J.M.; Roman, A.D.E.; Leon, D.G.; de Punzalan, F.E.R.; Guingon, R.B.L.; Morales, D.D.; Tan, D.-X.; Reiter, R.J. Melatonin as adjuvant treatment for Coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): A case series. Melatonin Res. 2020, 3, 297–310. [Google Scholar] [CrossRef]
- Hasan, Z.T.; Atrakji, M.Q.Y.M.A.A.; Mehuaiden, A.K. The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients. Int. J. Infect. Dis. 2021, 114, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; García-Mauriño, S.; Reiter, R.J.; Guerrero, J.M. Evidence of melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Muxel, S.M.; Laranjeira-Silva, M.F.; Carvalho-Sousa, C.E.; Floeter-Winter, L.M.; Markus, R.P. The RelA/cRel nuclear factor-κB (NF-κB) dimer, crucial for inflammation resolution, mediates the transcription of the key enzyme in melatonin synthesis in RAW 264.7 macrophages. J. Pineal Res. 2016, 60, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Mourier, A.; Tain, L.S.; Partridge, L.; Larsson, N.G.; Kühlbrandt, W. Changes of mitochondrial ultrastructure and function during ageing in mice and Drosophila. Elife 2017, 6, e24662. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, D.-X.; Hardeland, R. The Reserve/Maximum Capacity of Melatonin’s Synthetic Function for the Potential Dimorphism of Melatonin Production and Its Biological Significance in Mammals. Molecules 2021, 26, 7302. https://doi.org/10.3390/molecules26237302
Tan D-X, Hardeland R. The Reserve/Maximum Capacity of Melatonin’s Synthetic Function for the Potential Dimorphism of Melatonin Production and Its Biological Significance in Mammals. Molecules. 2021; 26(23):7302. https://doi.org/10.3390/molecules26237302
Chicago/Turabian StyleTan, Dun-Xian, and Rüdiger Hardeland. 2021. "The Reserve/Maximum Capacity of Melatonin’s Synthetic Function for the Potential Dimorphism of Melatonin Production and Its Biological Significance in Mammals" Molecules 26, no. 23: 7302. https://doi.org/10.3390/molecules26237302
APA StyleTan, D.-X., & Hardeland, R. (2021). The Reserve/Maximum Capacity of Melatonin’s Synthetic Function for the Potential Dimorphism of Melatonin Production and Its Biological Significance in Mammals. Molecules, 26(23), 7302. https://doi.org/10.3390/molecules26237302





