Mutagenicity of Tectona grandis Wood Extracts and Their Ability to Improve Carbohydrate Yield for Kraft Cooking Eucalyptus Wood
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Eucalyptus Globulus Wood
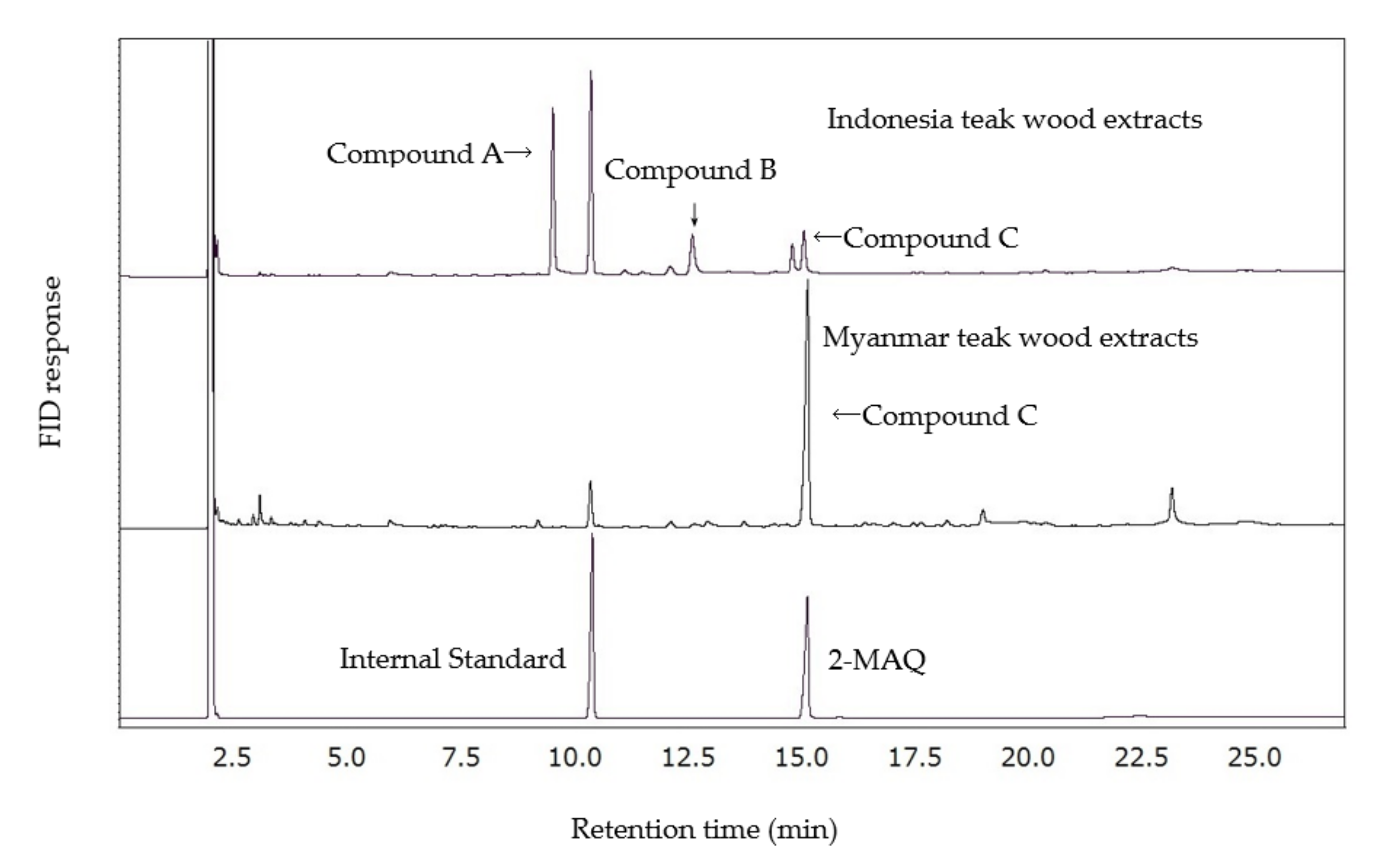
2.2. Contents of Natural 2-Methylanthraquinone in Myanmar and Indonesia Teak Wood
2.3. Degree of Residual Lignin and Carbohydrate Yield in Kraft Pulps
2.3.1. Effects of Teak Wood Extracts as Additives on Kraft Cooking
2.3.2. Effects of Additives on Carbohydrate Yield for Kraft Cooking
2.4. Residual Content of 2-Methylanthraquinone in Kraft Pulps
2.5. Mutagenicity of Teak Wood Extracts: S. typhimurium Ames Tests
3. Materials and Methods
3.1. Materials
3.2. Soluble 2-MAQ Preparation
3.3. Kraft Cooking
3.4. Chemical Analysis of Wood and Kraft Pulp
3.5. Extraction of Teak Wood and Determination of Residual 2-MAQ in Pulp
3.6. Mutagenicity of Teak Wood Extracts: Salmonella typhimurium Ames Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bach, B.; Fiehn, G. Neue Möglichkeiten zur Kohlehydratstabilisierung im alkalischen Holzaufschluß. Zellst. Pap. 1972, 21, 3–7. [Google Scholar]
- Hart, P.W.; Rudie, A.W. Anthraquinone a review of the rise and fall of a pulping catalyst. Tappi J. 2014, 13, 23–31. [Google Scholar] [CrossRef]
- Ohi, H. Function of anthraquinone as pulping additive and its possibilities. Jpn. Tappi J. 1994, 48, 531–544. [Google Scholar] [CrossRef][Green Version]
- Takahashi, S.; Homma, M.; Kajiyama, M.; Ohi, H.; Tanaka, J. Influence of soluble anthraquinone compound on bleaching load during kraft cooking of various woods. Jpn. Tappi J. 2011, 65, 792–798. [Google Scholar] [CrossRef][Green Version]
- Ohi, H. Rapid analysis of 2-methylanthraquinone in tropical hardwoods and its effects on polysulfide-AQ pulping. In Proceedings of the 11th International Symposium of Wood and Pulping Chemistry, Nice, France, 11–14 June 2001; Volume II, pp. 63–66. [Google Scholar]
- Leyva, A.; Dimmel, D.R.; Pullman, G.S. Teak extract as a catalyst for the pulping of loblolly pine. Tappi J. 1998, 81, 237. [Google Scholar]
- Butterworth, B.E.; Mathre, O.B.; Ballinger, K. The preparation of anthraquinone used in the National Toxicology Program cancer bioassay was contaminated with the mutagen 9-nitroanthracene. Mutagenesis 2001, 16, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Holton, H. Soda additive softwood pulping: A major new process. Pulp Pap. Can. 1977, 78, T218–T223. [Google Scholar]
- Hartshorn, G.S.; Peralta, R.L. Teak-the most important tropical hardwood. In Proceedings of the World Teak Conference, Bangkok, Thailand, 25–30 March 2013. [Google Scholar]
- Rizanti, D.W.; Darmawan, W.; George, B.; Merlin, A.; Dumarcay, S.; Chapuis, H.; Gérardin, C.; Gelhaye, E.; Raharivelomanana, P.; Sari, R.K.; et al. Comparison of teak wood properties according to forest management: Short versus long rotation. Ann. For. Sci. 2018, 75, 39. [Google Scholar] [CrossRef]
- Ladrach, W. Management of teak plantations for solid wood products. In ITSF News 2019: Special Report; Zobel Forestry Associates, Inc.: Bethesda, MD, USA, 2009. [Google Scholar]
- Vyas, P.; Yadav, D.K.; Khandelwal, P. Tectona grandis (teak)—A review on its phytochemical and therapeutic potential. Nat. Prod. Res. 2019, 33, 2338–2354. [Google Scholar] [CrossRef] [PubMed]
- Sumthong, P.; Romero-González, R.R.; Verpoorte, R. Identification of anti-wood rot compounds in Teak (Tectona grandis L.f.) sawdust extract. J. Wood Chem. Technol. 2008, 28, 247–260. [Google Scholar] [CrossRef]
- Yang, K.R.; Seo, H.S.; Lee, Y.S.; Choi, M.H.; Hong, J. A HT column GC/MS method for the determination of anthraquinone and its toxic impurities in paper products. Anal. Methods 2015, 7, 6060–6065. [Google Scholar] [CrossRef]
- National Toxicology Program (NTP) Technical Report. Toxicology and Carcinogenesis Studies of Anthraquinone (CAS No. 84-65-1) in F344/N Rats and B6C3F1 Mice (Feed Studies); NTP Technical Report Series No. 494; U.S. Department of Health and Human Services, Public Health Service, National Institute of Health, Research Triangle Park: Durham, NC, USA, 2005.
- Utami, S.P.; Tanifuji, K.; Putra, A.S.; Nakagawa-Izumi, A.; Ohi, H.; Evelyn, E. Effects of soluble anthraquinone application on prehydrolysis soda cooking of Acacia crassicarpa wood. Jpn. Tappi J. 2021, 75, 373–379. [Google Scholar] [CrossRef]
- Anita, Y.; Putra, A.S.; Tanifuji, K.; Nakagawa-Izumi, A.; Ohi, H.; Evelyn, E. Kraft cooking with teak wood extract and determining residual 2-methylanthraquinone in eucalyptus pulp. Jpn. Tappi J. 2021, 75, 153–163. [Google Scholar] [CrossRef]
- Lukmandaru, G.; Ashitani, T.; Takahashi, K. Color and chemical characterization of partially black-streaked heartwood in teak (Tectona grandis). J. For. Res. 2009, 20, 377–380. [Google Scholar] [CrossRef]
- Lukmandaru, G.; Takahashi, K. Radial distribution of quinones in plantation teak (Tectona grandis L.f.). Ann. For. Sci. 2009, 66, 605. [Google Scholar] [CrossRef]
- Lehto, J.; Alen, R. Chemical pretreatments of wood chips prior to alkaline pulping: A review of pretreatment alternatives, chemical aspects of the resulting liquors, and pulping outcomes. Bioresources 2015, 10, 8604–8656. [Google Scholar] [CrossRef]
- Wilson, M.J.; Sabbioni, G.; Rando, R.; Miller, C.A., III. Activation of aryl hydrocarbon receptor signaling by extracts of teak and other wood dusts. Environ. Toxicol. 2015, 30, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Salaghi, A.; Putra, A.S.; Rizaluddin, A.T.; Kajiyama, M.; Ohi, H. Totally chlorine-free bleaching of prehydrolysis soda pulp from plantation hardwoods consisting of various lignin structures. J. Wood Sci. 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Ames, B.N.; McCann, J.; Yamasaki, E. Methods for detecting carcinogens and mutagens with the salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975, 31, 347–363. [Google Scholar] [CrossRef]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef]

| Components (%) | |
|---|---|
| Glucan | 47.6 ± 1.8 |
| Xylan | 15.3 ± 0.5 |
| Other sugars | 1.08 ± 0.02 |
| Total lignin | 27.7 ± 0.2 |
| Ash | 0.47 ± 0.05 |
| Acetone extracts | 0.93 ± 0.03 |
| Unknown | 6.92 |
| Total (%) | 100 |
| Nitrobenzene oxidation | |
| Syringaldehyde (S)/vanillin (V) molar ratio | 4.60 ± 0.21 |
| S and V yields (mmol/g lignin) | 2.22 ± 0.05 |
| Acetone Extracts | 2-Methylanthraquinone | ||
|---|---|---|---|
| (%) | (%, in Acetone Extracts) | (%, in Wood) | |
| Myanmar teak wood | 7.95 ± 0.06 | 2.59 ± 0.20 | 0.21 ± 0.02 |
| Indonesia teak wood | 6.95 ± 0.39 | 2.58 ± 0.10 | 0.18 ± 0.01 |
| Active Alkali Dose (%) | Kappa Number | Pulp Yield (%) | |
|---|---|---|---|
| Myanmar teak wood extracts 1.16% dosage | 16 | 16.6 ± 0.1 | 58.8 ± 0.5 |
| 17 | 14.8 ± 0.7 | 58.3 ± 0.4 | |
| Indonesia teak wood extracts 1.22% dosage | 16 | 18.7 ± 0.6 | 55.6 ± 0.3 |
| 17 | 17.7 ± 0.6 | 55.4 ± 0.3 | |
| 2-Methylanthraquinone 0.03% dosage | 16 | 19.6 ± 1.2 | 57.3 ± 0.3 |
| 17 | 17.1 ± 1.3 | 56.8 ± 0.3 | |
| No additive | 16 | 22.6 ± 0.1 | 57.1 ± 0.1 |
| 17 | 19.9 ± 0.3 | 56.1 ± 0.1 |
| Active Alkali Dose (%) | Kappa Number | Residual 2-MAQ (mg/kg) | |
|---|---|---|---|
| Myanmar teak wood extracts | 16 | 16.6 | 2.42 ± 0.62 |
| 17 | 14.8 | 1.70 ± 1.54 | |
| Indonesia teak wood extracts | 16 | 18.7 | 2.86 ± 0.09 |
| 17 | 17.7 | 3.61 ± 0.07 | |
| 2-Methylanthraquinone | 16 | 19.6 | 6.00 ± 1.42 |
| 17 | 17.1 | 4.79 ± 1.46 |
| Strain | Dose of Extracts or 2-MAQ (μg/Plate) | Revertants/Plate + 10% Rat S9 | |||
|---|---|---|---|---|---|
| Indonesia Extracts | Myanmar Extracts [20] | 2-MAQ [20] | |||
| 1st Test | 2nd Test | ||||
| TA 100 | 0 | 109 ± 10 | 112 ± 9 | 98 ± 13.7 | 98 ± 13.7 |
| 156 | 145 ± 20 | 151 ± 14 | 117 ± 9.0 | - d | |
| 313 | 159 ± 22 | 165 ± 6 | 119 ± 10.1 | 108 ± 6.0 c | |
| 625 | 169 ± 22 | 208 ± 6 | 126 ± 5.9 | - d | |
| 1250 | 221 ± 29 | 226 ± 19 | 117 ± 6.9 | 101 ± 9.1 c | |
| 2500 | 139 ± 22 | 144 ± 9 | 150 ± 8.7 | 103 ± 9.2 c | |
| 5000 a | 79 ± 37 b | 0 ± 0 b | 199 ± 2.1 | 114 ± 3.2 c | |
| Trial summary | Positive | Positive | Positive | Negative | |
| Positive control e | 1.0 | 1611 ± 33 | 1464 ± 80 | 1566 ± 76.9 | 1566 ± 76.9 |
| TA98 | 0 | 21 ± 2 | 31 ± 5 | 23 ± 1.0 | 23 ± 1.0 |
| 156 | 25 ± 2 | 27 ± 5 | 24 ± 3.5 | - d | |
| 313 | 30 ± 8 | 32 ± 4 | 27 ± 6.0 | 30 ± 7.6 c | |
| 625 | 39 ± 13 | 45 ± 5 | 30 ± 6.1 | - d | |
| 1250 | 55 ± 17 | 65 ± 3 | 34 ± 4.0 | 26 ± 2.9 c | |
| 2500 | 74 ± 7 | 75 ± 12 | 31 ± 6.1 | 27 ± 6.6 c | |
| 5000 a | 0 ± 0 b | 0 ± 0 b | 28 ± 9.2 | 33 ± 3.2 c | |
| Trial summary | Positive | Positive | Negative | Negative | |
| Positive control e | 0.5 | 746 ± 71 | 785 ± 33 | 570 ± 20.2 | 570 ± 20.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anita, Y.; Utami, S.P.; Ohi, H.; Evelyn, E.; Nakagawa-Izumi, A. Mutagenicity of Tectona grandis Wood Extracts and Their Ability to Improve Carbohydrate Yield for Kraft Cooking Eucalyptus Wood. Molecules 2021, 26, 7171. https://doi.org/10.3390/molecules26237171
Anita Y, Utami SP, Ohi H, Evelyn E, Nakagawa-Izumi A. Mutagenicity of Tectona grandis Wood Extracts and Their Ability to Improve Carbohydrate Yield for Kraft Cooking Eucalyptus Wood. Molecules. 2021; 26(23):7171. https://doi.org/10.3390/molecules26237171
Chicago/Turabian StyleAnita, Yulia, Syelvia Putri Utami, Hiroshi Ohi, Evelyn Evelyn, and Akiko Nakagawa-Izumi. 2021. "Mutagenicity of Tectona grandis Wood Extracts and Their Ability to Improve Carbohydrate Yield for Kraft Cooking Eucalyptus Wood" Molecules 26, no. 23: 7171. https://doi.org/10.3390/molecules26237171
APA StyleAnita, Y., Utami, S. P., Ohi, H., Evelyn, E., & Nakagawa-Izumi, A. (2021). Mutagenicity of Tectona grandis Wood Extracts and Their Ability to Improve Carbohydrate Yield for Kraft Cooking Eucalyptus Wood. Molecules, 26(23), 7171. https://doi.org/10.3390/molecules26237171







