Self-Assembly of NaOL-DDA Mixtures in Aqueous Solution: A Molecular Dynamics Simulation Study
Abstract
:1. Introduction
2. Computational Details
2.1. Model Construction
2.2. Equilibrium Process
2.2.1. Energy Minimization
2.2.2. Equilibration
3. Results and Discussion
3.1. Aggregation Behavior
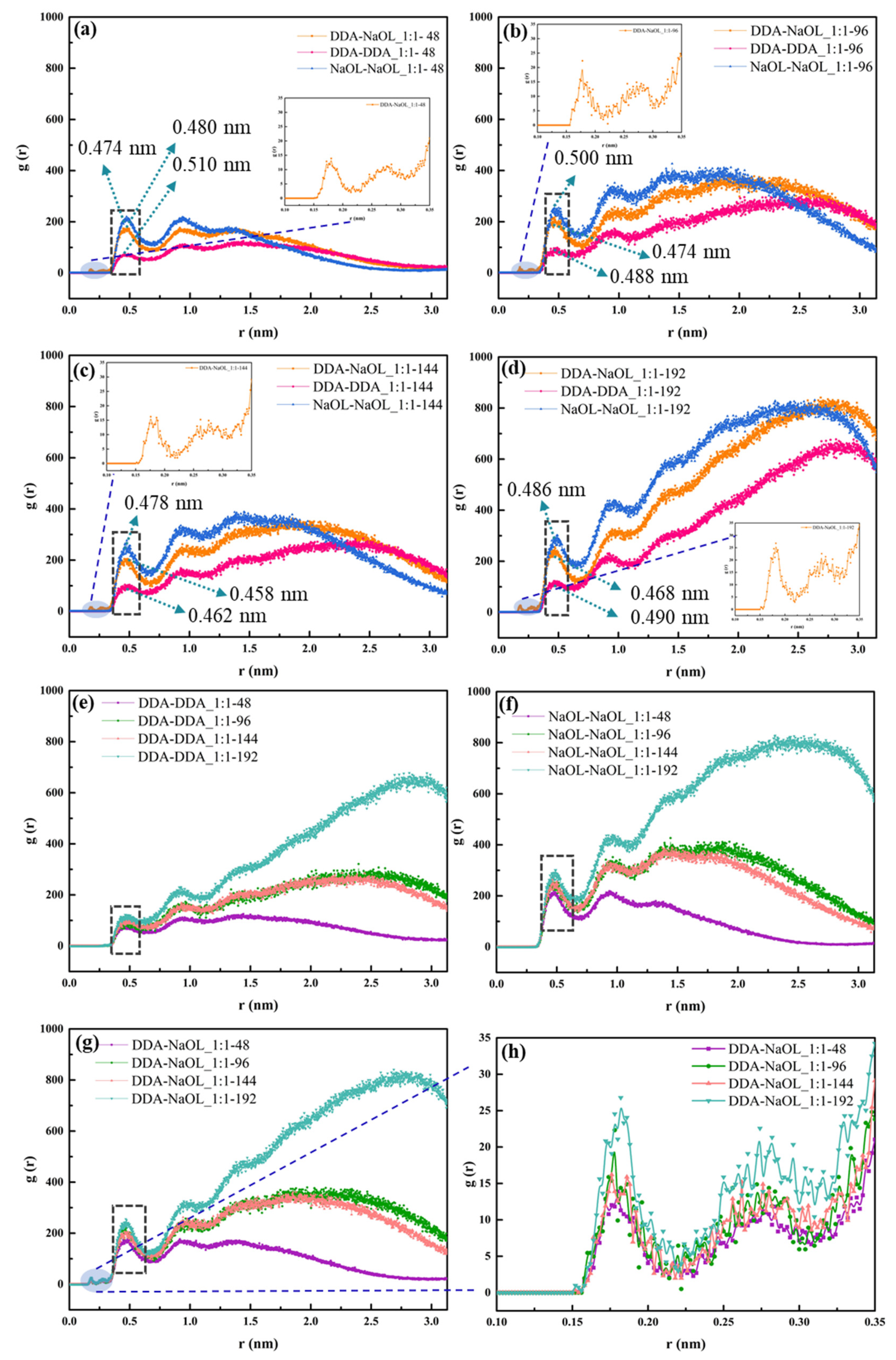
3.2. Radial Distribution Function
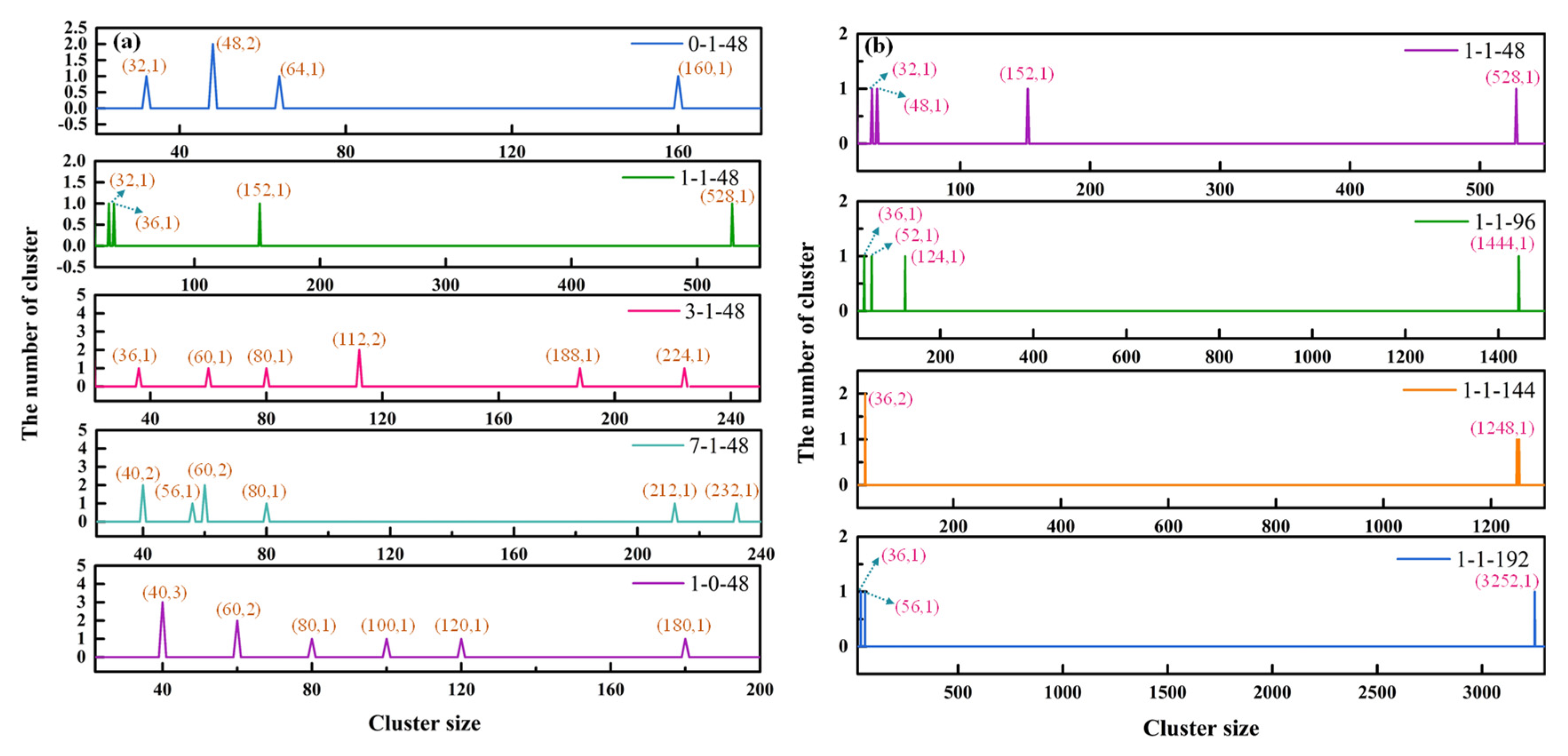
3.3. The Size Distribution of Cluster
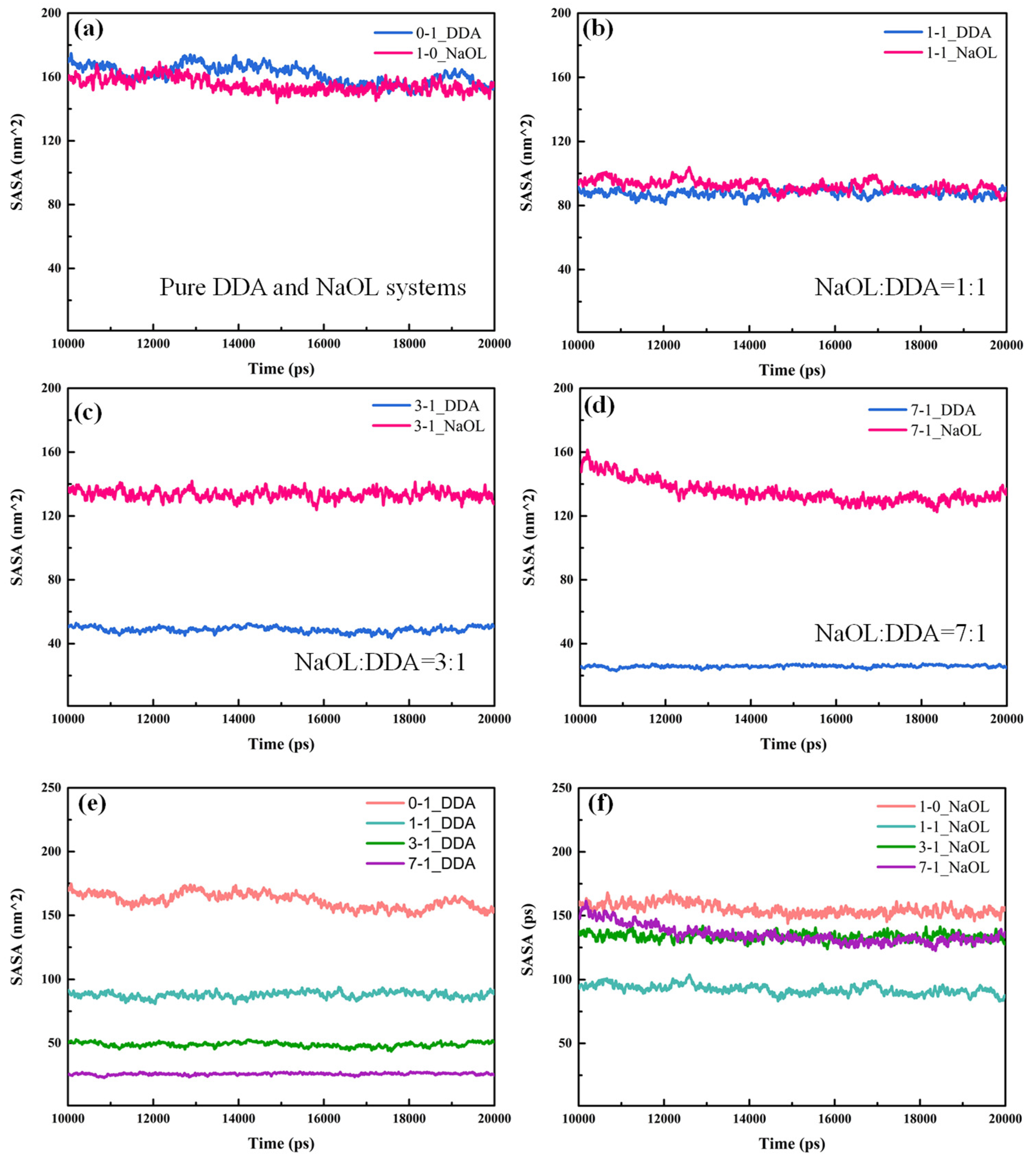
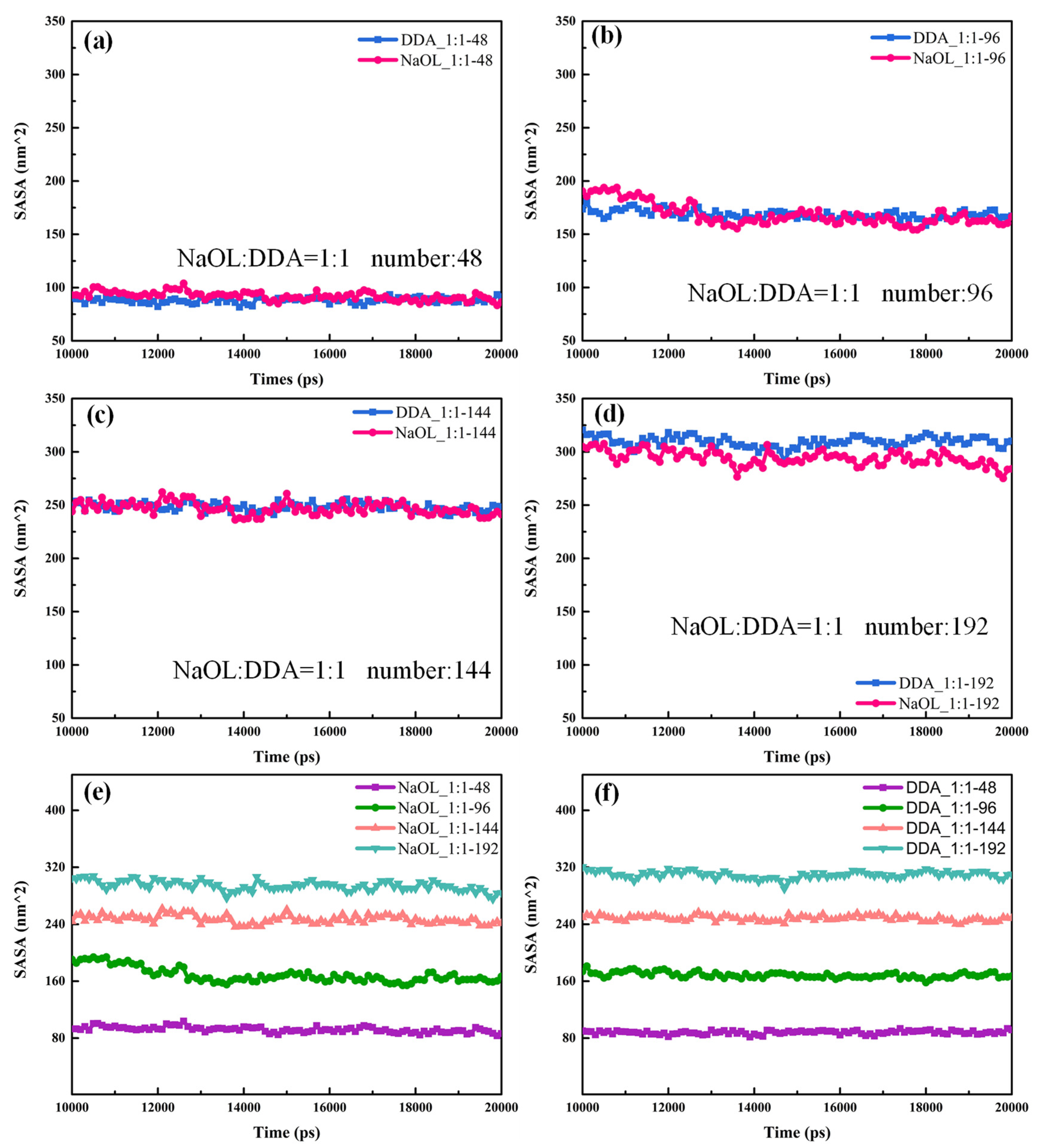
3.4. Solvent-Accessible Surface Area
3.5. Hydrogen Bond
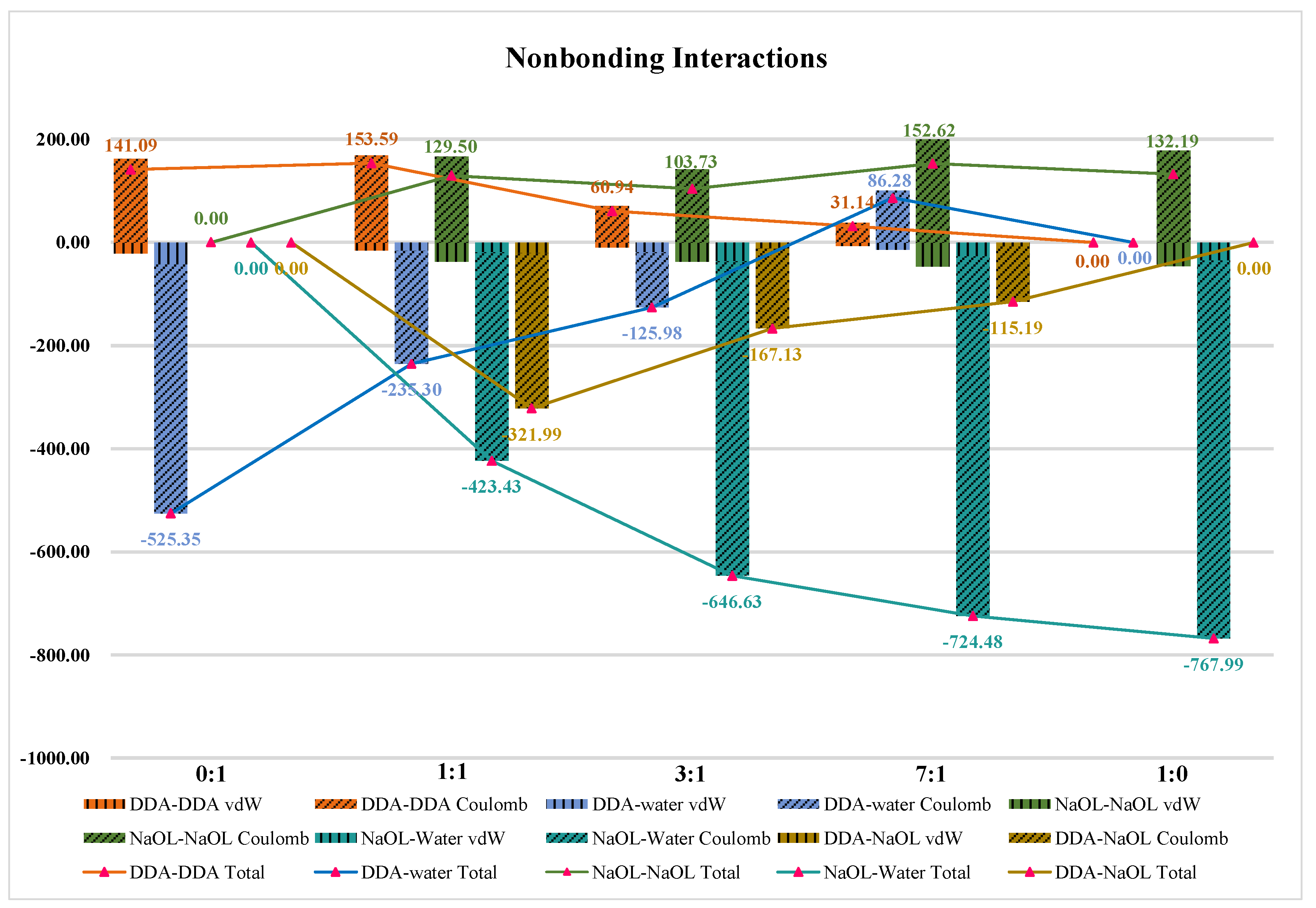
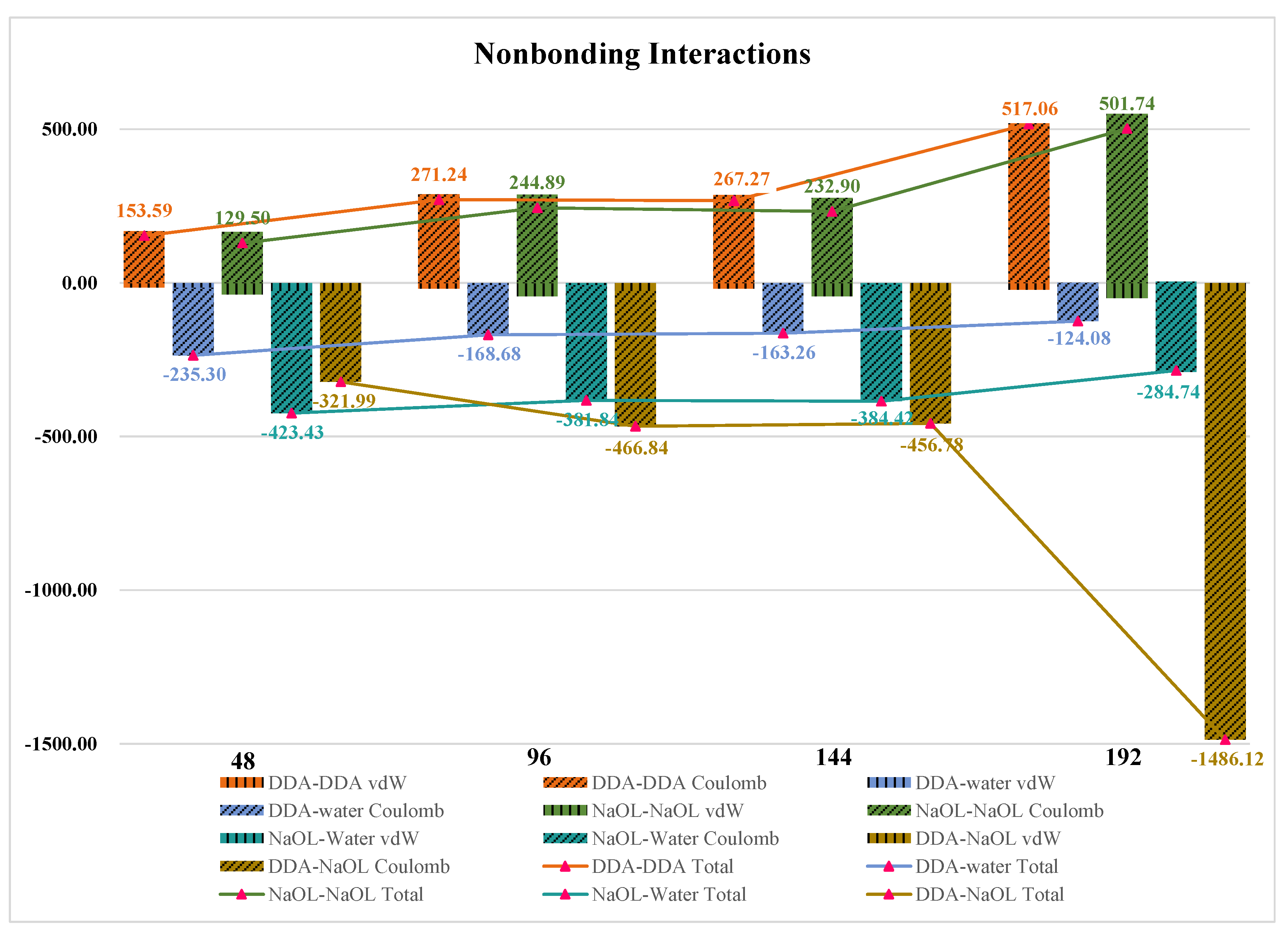
3.6. Nonbonding Interaction Energies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tucker, I.; Burley, A.; Petkova, R.; Hosking, S.; Thomas, R.; Penfold, J.; Li, P.; Ma, K.; Webster, J.; Welbourn, R. Surfactant/biosurfactant mixing: Adsorption of saponin/nonionic surfactant mixtures at the air-water interface. J. Colloid Interface Sci. 2020, 574, 385–392. [Google Scholar] [CrossRef]
- Ageev, A.A.; Volkov, B.A.; Kibalov, M.S.; Kukleva, K.K. Correlation between wetting and deterging abilities in mixed surfactant solutions. Fibre Chem. 2012, 44, 17–20. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, F.; Wang, Y.; Zhu, G. Studies on the formation of bifenthrin oil-in-water nano-emulsions prepared with mixed surfactants. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 389, 90–96. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, L. Enhanced desorption of phenanthrene from contaminated soil using anionic/nonionic mixed surfactant. Environ. Pollut. 2007, 147, 350–357. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; Gómez, M.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Influence of a mixed ionic/nonionic surfactant system and the emulsification process on the properties of paraffin emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 392, 38–44. [Google Scholar] [CrossRef]
- Jia, H.; Lian, P.; Leng, X.; Han, Y.; Wang, Q.; Jia, K.; Niu, X.; Guo, M.; Yan, H.; Lv, K. Mechanism studies on the application of the mixed cationic/anionic surfactant systems to enhance oil recovery. Fuel 2019, 258, 116156. [Google Scholar] [CrossRef]
- Liu, A.; Fan, P.-P.; Qiao, X.-X.; Li, Z.-H.; Wang, H.-F.; Fan, M.-Q. Synergistic effect of mixed DDA/surfactants collectors on flotation of quartz. Miner. Eng. 2020, 159, 106605. [Google Scholar] [CrossRef]
- Jiang, H.; Gao, Y.; Khoso, S.A.; Ji, W.; Hu, Y. A new approach for characterization of hydrophobization mechanisms of surfactants on muscovite surface. Sep. Purif. Technol. 2019, 209, 936–945. [Google Scholar] [CrossRef]
- Tyagi, G.; Seddon, D.; Khodaparast, S.; Sharratt, W.N.; Robles, E.S.; Cabral, J.T. Tensiometry and FTIR study of the synergy in mixed SDS:DDAO surfactant solutions at varying pH. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 618, 126414. [Google Scholar] [CrossRef]
- Georgieva, G.S.; Anachkov, S.E.; Lieberwirth, I.; Koynov, K.; Kralchevsky, P.A. Synergistic Growth of Giant Wormlike Micelles in Ternary Mixed Surfactant Solutions: Effect of Octanoic Acid. Langmuir 2016, 32, 12885–12893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ning, B.; Bai, Y.; Tai, X.; Wang, G. Solution properties of mixed system containing butynediol-ethoxylate polysiloxanes and polyether trisiloxane surfactant. Colloid Interface Sci. Commun. 2021, 41, 100367. [Google Scholar] [CrossRef]
- Vilasau, J.; Solans, C.; Gómez, M.J.; Dabrio, J.; Mújika-Garai, R.; Esquena, J. Phase behaviour of a mixed ionic/nonionic surfactant system used to prepare stable oil-in-water paraffin emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 384, 473–481. [Google Scholar] [CrossRef]
- Matsubara, H.; Kimura, T.; Miyao, R.; Shin, Y.; Ikeda, N. Relation between ionic surfactant concentration and thickness of foam film stabilized by ionic—nonionic surfactant mixed adsorbed films. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 625, 126915. [Google Scholar] [CrossRef]
- Yalcin, E.; Kelebek, S. Flotation kinetics of a pyritic gold ore. Int. J. Miner. Process. 2011, 98, 48–54. [Google Scholar] [CrossRef]
- Yehia, A.; El-Halim, S.A.; Sharada, H.; Fadel, M.; Ammar, M. Application of a fungal cellulase as a green depressant of hematite in the reverse anionic flotation of a high-phosphorus iron ore. Miner. Eng. 2021, 167, 106903. [Google Scholar] [CrossRef]
- Larsen, E.; Kleiv, R. Flotation of quartz from quartz-feldspar mixtures by the HF method. Miner. Eng. 2016, 98, 49–51. [Google Scholar] [CrossRef]
- Bhattarai, A.; Pathak, K.; Dev, B. Cationic and anionic surfactants interaction in water and methanol–water mixed solvent media. J. Mol. Liq. 2017, 229, 153–160. [Google Scholar] [CrossRef]
- Sachin, K.M.; Karpe, S.A.; Singh, M.; Bhattarai, A. Study on surface properties of sodiumdodecyl sulfate and dodecyltrimethylammonium bromide mixed surfactants and their interaction with dyes. Heliyon 2019, 5, e01510. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, S.; Chen, H.; Fu, D.; Liu, H. Compound System Containing Gemini Surfactant. Contemp. Chem. Ind. 2020, 49, 1781–1787. [Google Scholar]
- Wang, L.; Hu, Y.; Liu, J.; Sun, Y.; Sun, W. Flotation and adsorption of muscovite using mixed cationic–nonionic surfactants as collector. Powder Technol. 2015, 276, 26–33. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B.; Wójcik, W. The properties of mixtures of two cationic surfactants in water at water/air interface. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 264, 147–156. [Google Scholar] [CrossRef]
- Huang, L.; Maltesh, C.; Somasundaran, P. Adsorption Behavior of Cationic and Nonionic Surfactant Mixtures. J. Colloid Interface Sci. 1996, 177, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachin, K.M.; Karpe, S.A.; Kumar, D.; Singh, M.; Dominguez, H.; Ríos-López, M.; Bhattarai, A. A simulation study of self-assembly behaviors and micellization properties of mixed ionic surfactants. J. Mol. Liq. 2021, 336, 116003. [Google Scholar] [CrossRef]
- Sachin, K.M.; Karpe, S.A.; Singh, M.; Bhattarai, A. Self-assembly of sodium dodecylsulfate and dodecyltrimethylammonium bromide mixed surfactants with dyes in aqueous mixtures. R. Soc. Open Sci. 2019, 6, 181979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, Y.; Li, W.; Jin, Z. Role of Alcohol as a Cosurfactant at the Brine–Oil Interface under a Typical Reservoir Condition. Langmuir 2020, 36, 5198–5207. [Google Scholar] [CrossRef]
- Boek, E.; Padding, J.; Anderson, V.; Briels, W.; Crawshaw, J. Flow of entangled wormlike micellar fluids: Mesoscopic simulations, rheology and μ-PIV experiments. J. Non-Newton. Fluid Mech. 2007, 146, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z. Experimental and Molecular Simulation Research on Lepidolite Flotation Process. 2015. [Google Scholar]
- Wang, L.; Hu, Y.; Liu, R.; Liu, J.; Sun, W. Synergistic adsorption of DDA/alcohol mixtures at the air/water interface: A molecular dynamics simulation. J. Mol. Liquids 2017, 243, 1–8. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Liu, J.; Sun, W. Adsorption behavior of mixed cationic/anionic surfactants and their depression mechanism on the flotation of quartz. Powder Technol. 2016, 302, 15–20. [Google Scholar] [CrossRef]
- Jia, W.; Jiao, F.; Zhu, H.; Xu, L.; Qin, W. Mitigating the negative effects of feldspar slime on spodumene flotation using mixed anionic/cationic collector. Miner. Eng. 2021, 168, 106813. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.-H.; Xu, L.-H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite—Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Y.; Liu, G.; Liu, J.; Ma, L.; Niu, X.; Qu, X. A DFT prediction on the chemical reactivity of novel azolethione derivatives as chelating agents: Implications for copper minerals flotation and copper corrosion inhibition. J. Taiwan Inst. Chem. Eng. 2018, 93, 109–123. [Google Scholar] [CrossRef]
- Rissanou, A.N.; Georgilis, E.; Kasotakis, E.; Mitraki, A.; Harmandaris, V. Effect of Solvent on the Self-Assembly of Dialanine and Diphenylalanine Peptides. J. Phys. Chem. B 2013, 117, 3962–3975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, B.; Zhang, R.; Yang, N.; Zhang, L.; Sun, Y.; Jian, C.; Liu, L.; Xu, Z. Molecular Mechanisms of Suppressing Asphaltene Aggregation and Flocculation by Dodecylbenzenesulfonic Acid Probed by Molecular Dynamics Simulations. Energy Fuels 2019, 33, 5067–5080. [Google Scholar] [CrossRef]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef]
- Shi, L.; Sun, Q.; Wei, A. Molecular dynamics simulation of self—assembly behavior at gas—liquid interface of dodecyl carboxylic betaine. J. Northeast Pet. Univ. 2016, 40, 114–120. [Google Scholar]
- Sun, J.; Zhang, H.; Hu, M.; Meng, X.; Yuan, S. Molecular dynamics study on oil migration inside silica nanopore. Chem. Phys. Lett. 2017, 678, 186–191. [Google Scholar] [CrossRef]
- Shi, W.-X.; Guo, H.-X. Structure, Interfacial Properties, and Dynamics of the Sodium Alkyl Sulfate Type Surfactant Monolayer at the Water/Trichloroethylene Interface: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2010, 114, 6365–6376. [Google Scholar] [CrossRef]
- Chen, H.; Gizzatov, A.; Abdel-Fattah, A.I. Molecular Assembly of Surfactant Mixtures in Oil-Swollen Micelles: Implications for High Salinity Colloidal Stability. J. Phys. Chem. B 2020, 124, 568–576. [Google Scholar] [CrossRef]
- Mittal, K.L. Surfactants in Solution; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Guàrdia, E.; Marti, J.; Tarres, L.G.; Laria, D. A molecular dynamics simulation study of hydrogen bonding in aqueous ionic solutions. J. Mol. Liq. 2005, 117, 63–67. [Google Scholar] [CrossRef]
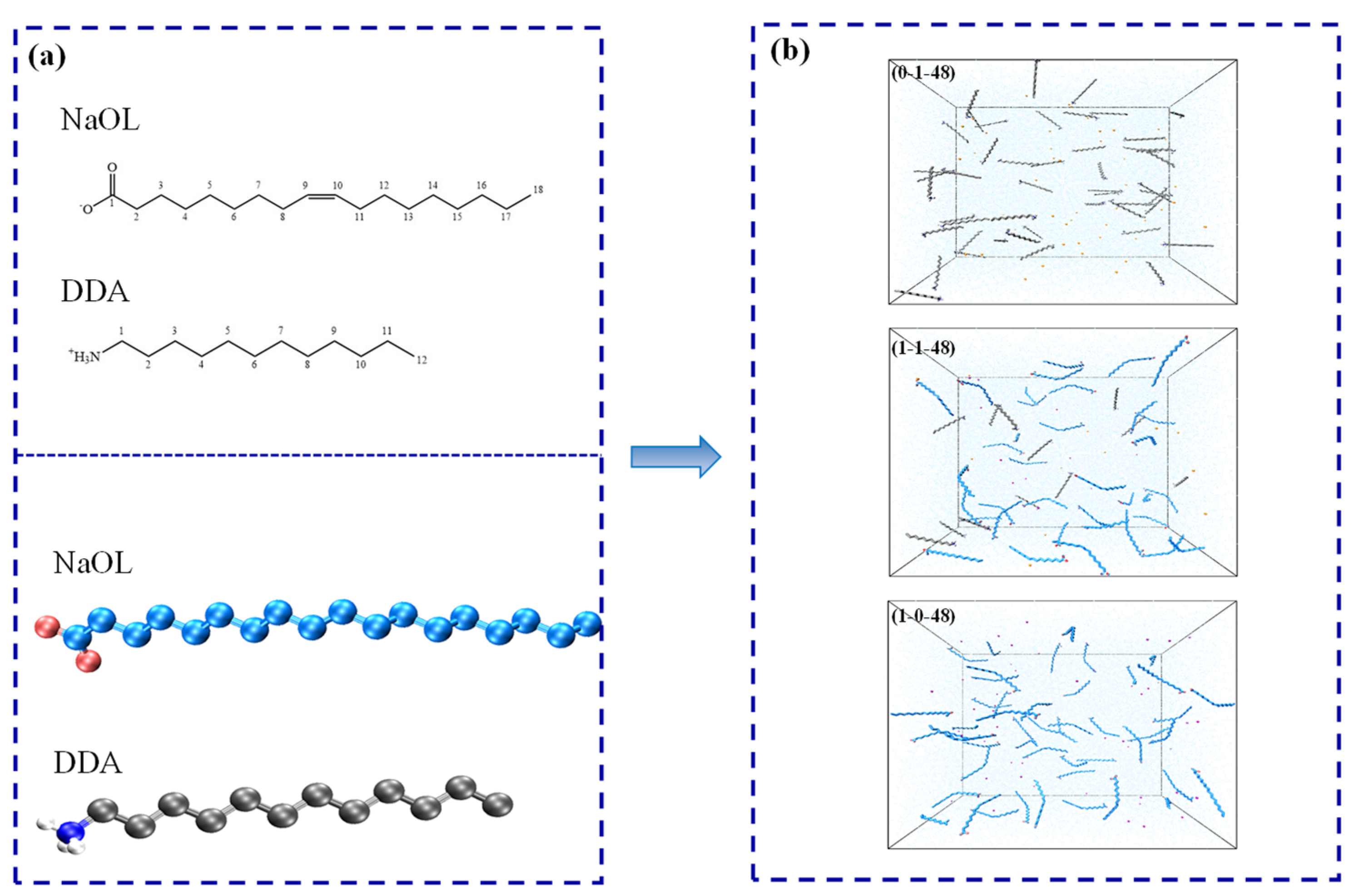

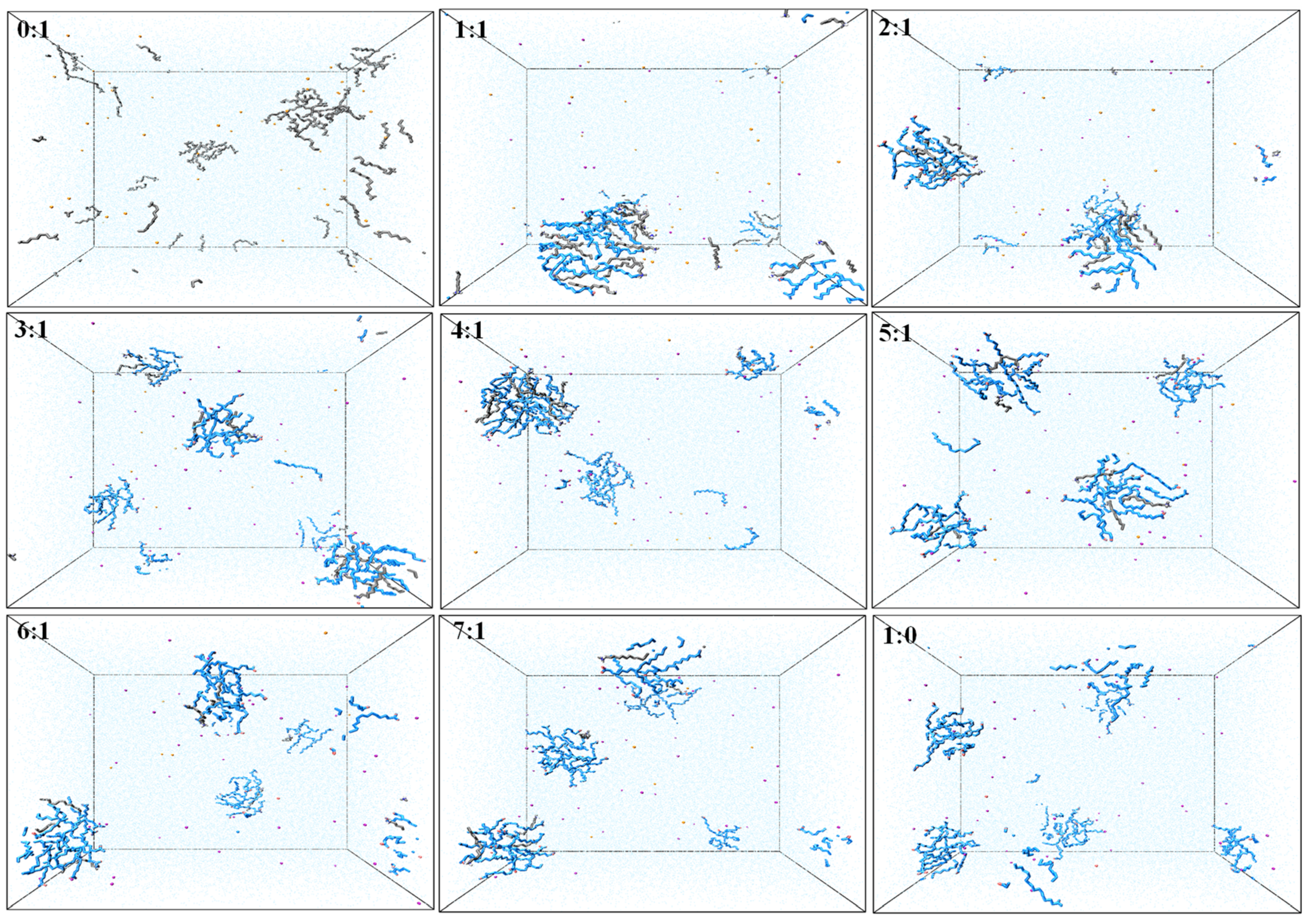
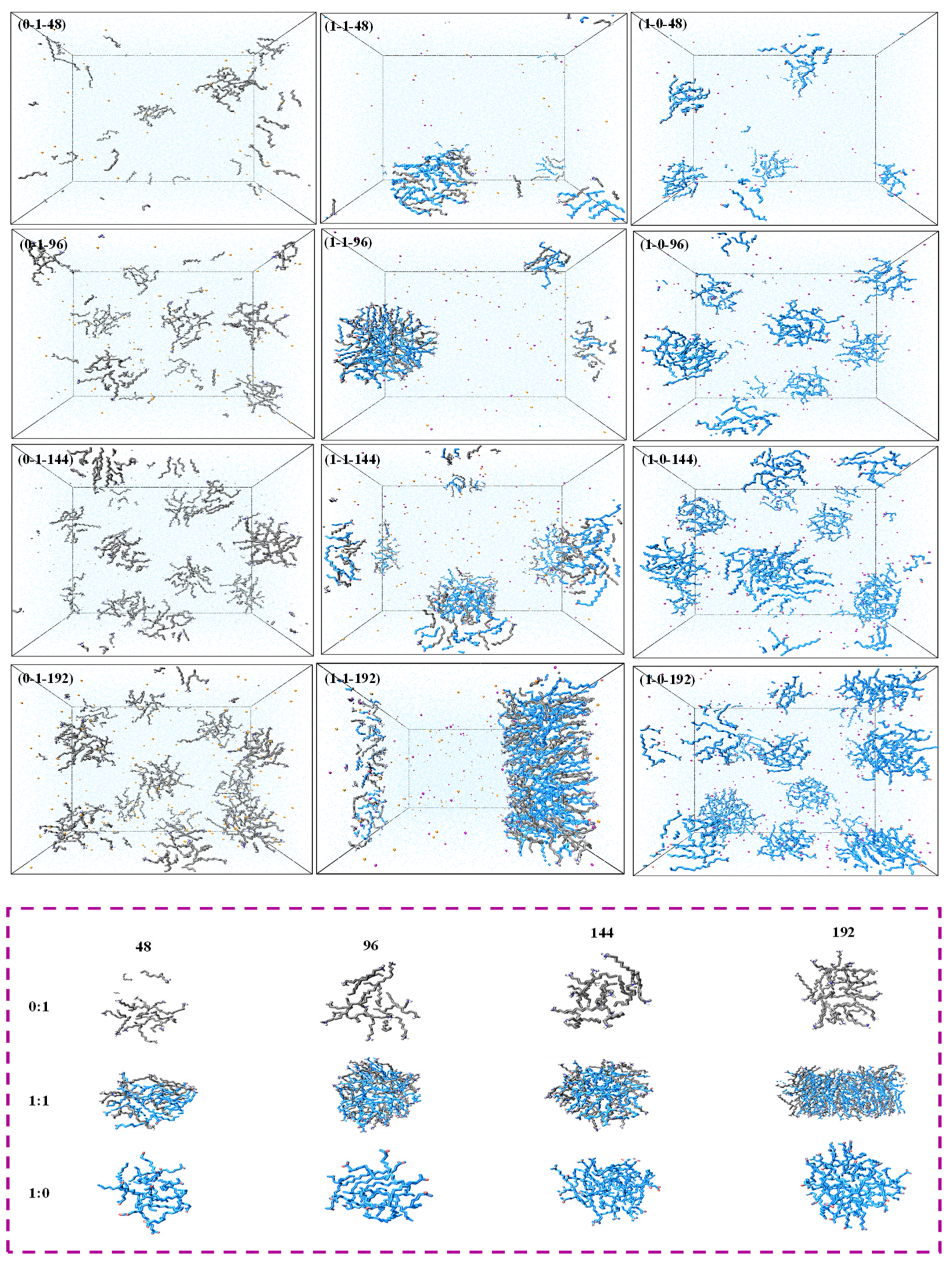
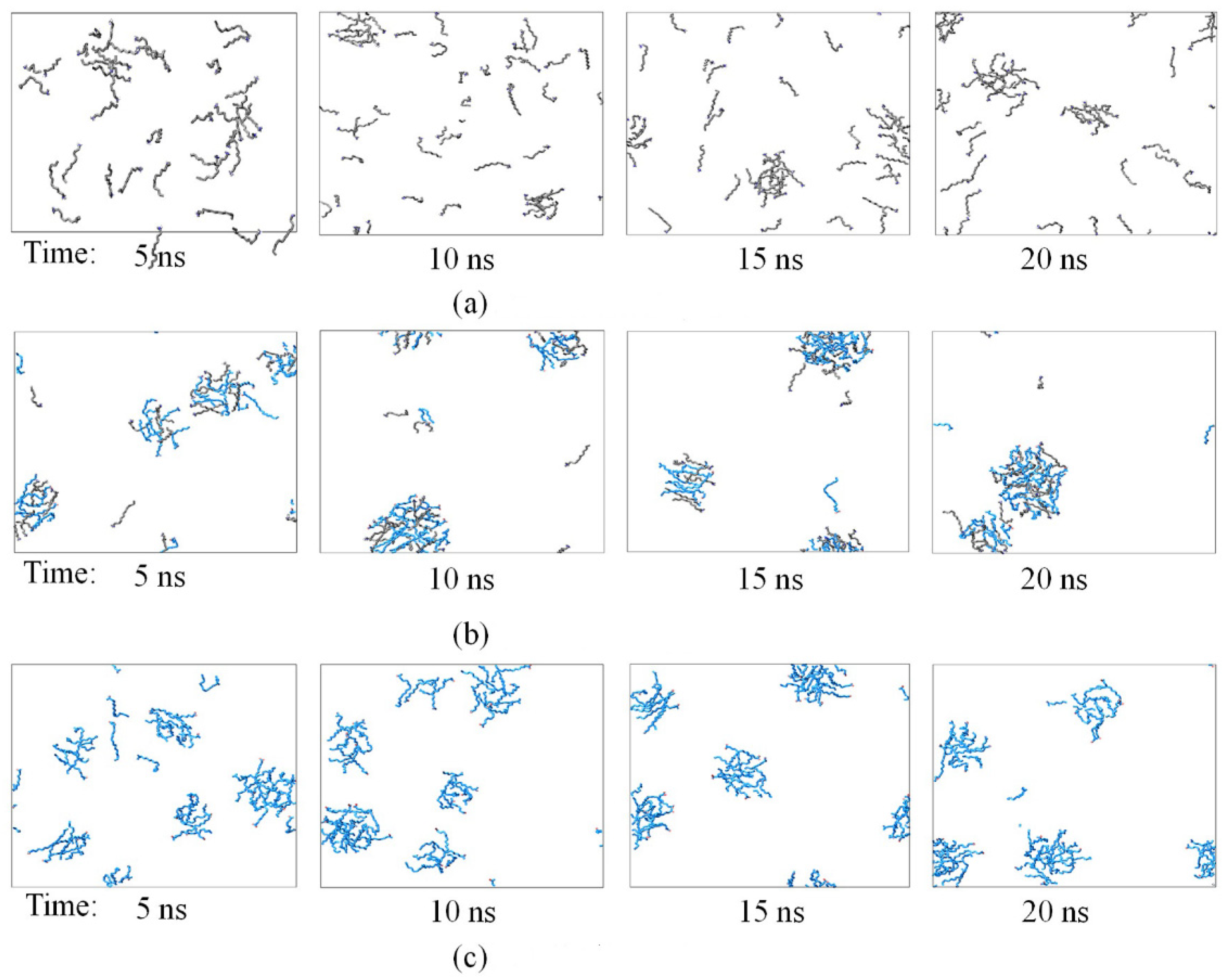

| NaOL/DDA | Total Number | OL− | DDAH | Na+ | CL− | H2O |
|---|---|---|---|---|---|---|
| 0:1 | 48 | 0 | 48 | 0 | 48 | 21824 |
| 96 | 0 | 96 | 0 | 96 | 21335 | |
| 144 | 0 | 144 | 0 | 144 | 20800 | |
| 192 | 0 | 192 | 0 | 192 | 20317 | |
| 1:1 | 48 | 24 | 24 | 24 | 24 | 21754 |
| 96 | 48 | 48 | 48 | 48 | 21166 | |
| 144 | 72 | 72 | 72 | 72 | 20574 | |
| 192 | 96 | 96 | 96 | 96 | 20007 | |
| 2:1 | 48 | 32 | 16 | 32 | 16 | 21713 |
| 3:1 | 48 | 36 | 12 | 36 | 12 | 21717 |
| 4:1 | 50 | 40 | 10 | 40 | 10 | 21677 |
| 5:1 | 48 | 40 | 8 | 40 | 8 | 21674 |
| 6:1 | 49 | 42 | 7 | 42 | 7 | 21688 |
| 7:1 | 48 | 42 | 6 | 42 | 6 | 21712 |
| 1:0 | 48 | 48 | 0 | 48 | 0 | 21660 |
| 96 | 96 | 0 | 96 | 0 | 21034 | |
| 144 | 144 | 0 | 144 | 0 | 20346 | |
| 192 | 192 | 0 | 192 | 0 | 19716 |
| System | DDA-Solvent | NaOL-Solvent | DDA-NaOL |
|---|---|---|---|
| NaOL/DDA | |||
| 0:1 | 128.52 | 0.00 | 0.00 |
| 1:1 | 55.93 | 129.02 | 11.06 |
| 3:1 | 28.12 | 214.10 | 5.44 |
| 7:1 | 12.53 | 250.06 | 4.32 |
| 1:0 | 0.00 | 294.39 | 0.00 |
| Number | DDA-Solvent | NaOL-Solvent | DDA-NaOL |
|---|---|---|---|
| 48 | 55.93 | 129.02 | 11.06 |
| 96 | 107.52 | 246.62 | 27.91 |
| 144 | 159.48 | 370.57 | 40.14 |
| 192 | 190.81 | 436.71 | 80.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Xu, R.; Liu, R.; Ge, P.; Sun, W.; Tian, M. Self-Assembly of NaOL-DDA Mixtures in Aqueous Solution: A Molecular Dynamics Simulation Study. Molecules 2021, 26, 7117. https://doi.org/10.3390/molecules26237117
Wang L, Xu R, Liu R, Ge P, Sun W, Tian M. Self-Assembly of NaOL-DDA Mixtures in Aqueous Solution: A Molecular Dynamics Simulation Study. Molecules. 2021; 26(23):7117. https://doi.org/10.3390/molecules26237117
Chicago/Turabian StyleWang, Li, Rui Xu, Ruohua Liu, Peng Ge, Wei Sun, and Mengjie Tian. 2021. "Self-Assembly of NaOL-DDA Mixtures in Aqueous Solution: A Molecular Dynamics Simulation Study" Molecules 26, no. 23: 7117. https://doi.org/10.3390/molecules26237117
APA StyleWang, L., Xu, R., Liu, R., Ge, P., Sun, W., & Tian, M. (2021). Self-Assembly of NaOL-DDA Mixtures in Aqueous Solution: A Molecular Dynamics Simulation Study. Molecules, 26(23), 7117. https://doi.org/10.3390/molecules26237117









