Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress
Abstract
:1. Introduction
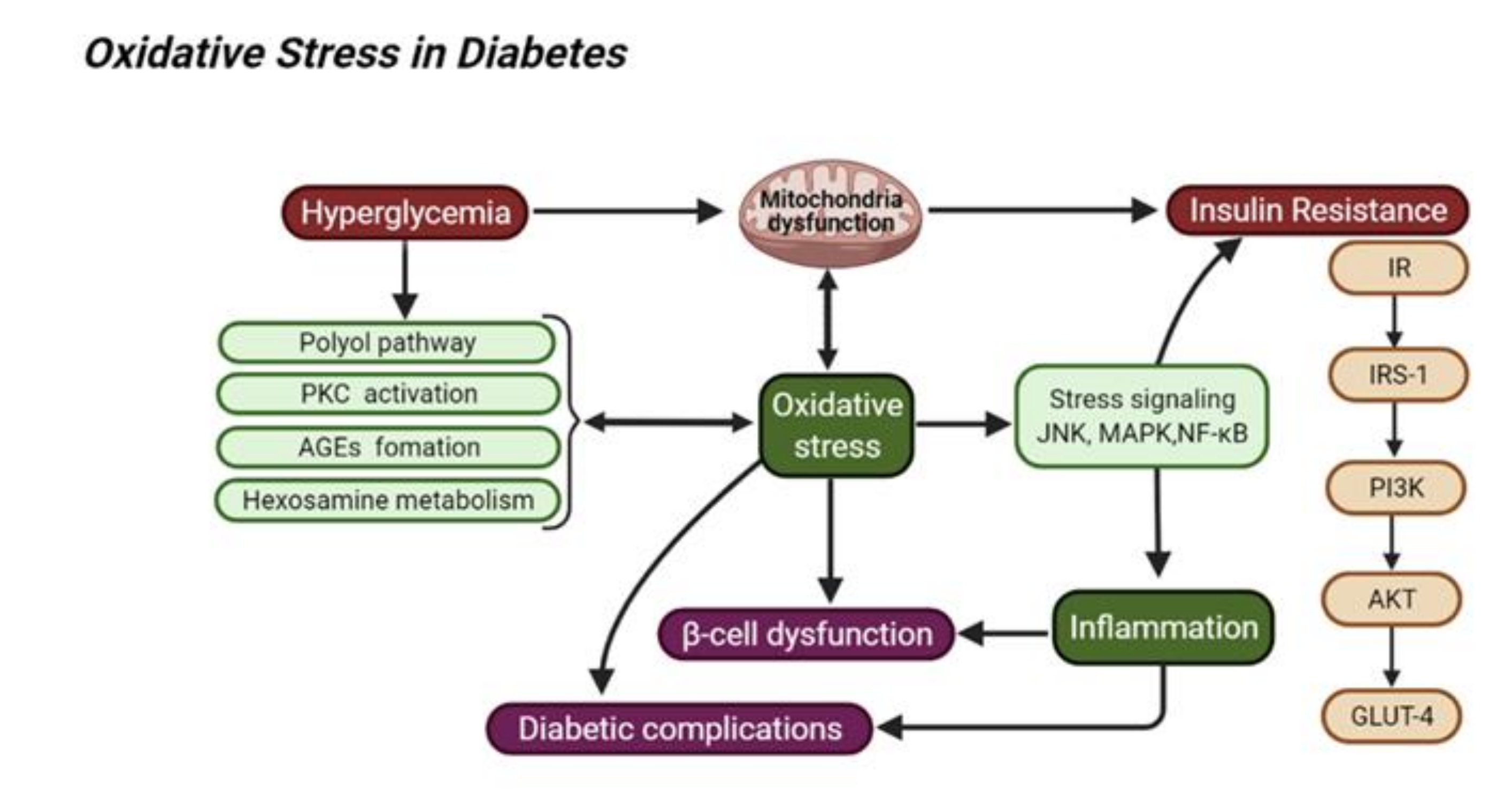
2. Oxidative Stress in Diabetes Mellitus and Its Related Complications
2.1. Hyperglycemia and Oxidative Stress
2.2. Insulin Resistance and Oxidative Stress
2.3. Inflammation and Oxidative Stress
3. Gallic Acid as a Natural Antioxidant
3.1. Gallic Acid in Edible Plants
3.2. Gallic Acid in Traditional Chinese Medicine
3.2.1. Sanguisorbae Radix (DiYu)
3.2.2. Chebulae Fructus (KeZi)
3.2.3. Pomegranate Rind (ShiLiuPi)
3.3. Gallic Acid as a Natural Bioactive Metabolite
3.4. Structure Modification of GA
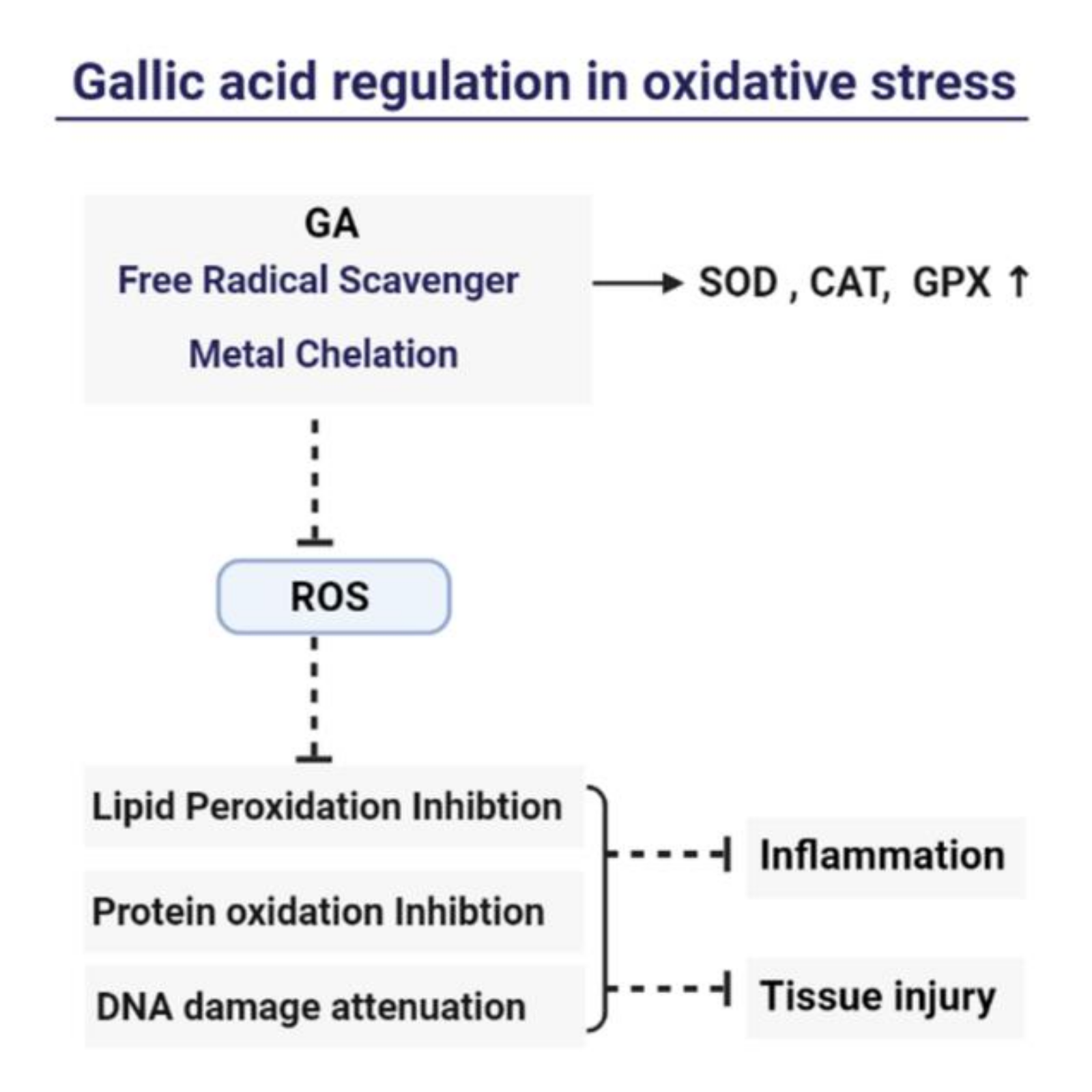
4. Mechanism of Gallic Acid’s Antioxidant and Anti-Inflammation
5. Gallic Acid for Diabetic Therapy
5.1. Diabetic Cardiovascular Diseases
5.2. Neurodegeneration
5.3. Diabetic Nephropathy
5.4. Liver Injury
5.5. Clinical Application
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Muriach, M.; Flores-Bellver, M.; Romero, F.J.; Barcia, J.M. Diabetes and the brain: Oxidative stress, inflammation, and autophagy. Oxidative Med. Cell. Longev. 2014, 2014, 102158. [Google Scholar] [CrossRef] [Green Version]
- Cepas, V.; Collino, M.; Mayo, J.C.; Sainz, R.M. Redox Signaling and Advanced Glycation Endproducts (AGEs) in Diet-Related Diseases. Antioxidants 2020, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Onyango, A.N. Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance. Oxidative Med. Cell. Longev. 2018, 2018, 4321714. [Google Scholar] [CrossRef] [Green Version]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Chhikara, N.; Kaur, R.; Jaglan, S.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive compounds and pharmacological and food applications of Syzygium cumini-A review. Food Funct. 2018, 9, 6096–6115. [Google Scholar] [CrossRef]
- Naveed, M.; BiBi, J.; Kamboh, A.A.; Suheryani, I.; Kakar, I.; Fazlani, S.A.; FangFang, X.; Kalhoro, S.A.; Yunjuan, L.; Kakar, M.U.; et al. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): A comprehensive overview. Biomed. Pharmacother. 2018, 100, 521–531. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Pan, M.H.; Lai, C.S.; Ho, C.T. Black tea: Chemical analysis and stability. Food Funct. 2013, 4, 10–18. [Google Scholar] [CrossRef]
- Pastoriza, S.; Mesias, M.; Cabrera, C.; Rufian-Henares, J.A. Healthy properties of green and white teas: An update. Food Funct. 2017, 8, 2650–2662. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.R.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef]
- Yang, J.H.; Yoo, J.M.; Cho, W.K.; Ma, J.Y. Anti-inflammatory effects of Sanguisorbae Radix water extract on the suppression of mast cell degranulation and STAT-1/Jak-2 activation in BMMCs and HaCaT keratinocytes. BMC Complement. Altern. Med. 2016, 16, 347. [Google Scholar] [CrossRef] [Green Version]
- Ban, J.Y.; Nguyen, H.T.; Lee, H.J.; Cho, S.O.; Ju, H.S.; Kim, J.Y.; Bae, K.; Song, K.S.; Seong, Y.H. Neuroprotective properties of gallic acid from Sanguisorbae radix on amyloid beta protein (25–35)-induced toxicity in cultured rat cortical neurons. Biol. Pharm. Bull. 2008, 31, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Seo, C.S.; Jeong, S.J.; Yoo, S.R.; Lee, N.R.; Shin, H.K. Quantitative Analysis and In vitro Anti-inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104–108. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.P.; Liao, M.; Dai, C.; Chen, J.F.; Yang, C.J.; Liu, M.; Chen, Z.G.; Yao, M.C. Sanguisorba officinalis L synergistically enhanced 5-fluorouracil cytotoxicity in colorectal cancer cells by promoting a reactive oxygen species-mediated, mitochondria-caspase-dependent apoptotic pathway. Sci. Rep. 2016, 6, 34245. [Google Scholar] [CrossRef] [Green Version]
- Suganthy, N.; Muniasamy, S.; Archunan, G. Safety assessment of methanolic extract of Terminalia chebula fruit, Terminalia arjuna bark and its bioactive constituent 7-methyl gallic acid: In vitro and in vivo studies. Regul. Toxicol. Pharmacol. 2018, 92, 347–357. [Google Scholar] [CrossRef]
- Gao, J.; Ajala, O.S.; Wang, C.Y.; Xu, H.Y.; Yao, J.H.; Zhang, H.P.; Jukov, A.; Ma, C.M. Comparison of pharmacokinetic profiles of Terminalia phenolics after intragastric administration of the aqueous extracts of the fruit of Terminalia chebula and a Mongolian compound medicine-Gurigumu-7. J. Ethnopharmacol. 2016, 185, 300–309. [Google Scholar] [CrossRef]
- Bag, A.; Kumar Bhattacharyya, S.; Kumar Pal, N.; Ranjan Chattopadhyay, R. Anti-inflammatory, anti-lipid peroxidative, antioxidant and membrane stabilizing activities of hydroalcoholic extract of Terminalia chebula fruits. Pharm. Biol. 2013, 51, 1515–1520. [Google Scholar] [CrossRef]
- Mukherjee, G.; Banerjee, R. Biosynthesis of tannase and gallic acid from tannin rich substrates by Rhizopus oryzae and Aspergillus foetidus. J. Basic Microbiol. 2004, 44, 42–48. [Google Scholar] [CrossRef]
- Sheng, Z.; Yan, X.; Zhang, R.; Ni, H.; Cui, Y.; Ge, J.; Shan, A. Assessment of the antidiarrhoeal properties of the aqueous extract and its soluble fractions of Chebulae Fructus (Terminalia chebula fruits). Pharm. Biol. 2016, 54, 1847–1856. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Georgiadis, I.; Karatzas, T.; Korou, L.M.; Katsilambros, N.; Perrea, D. Beneficial health effects of Chios Gum Mastic and peroxisome proliferator-activated receptors: Indications of common mechanisms. J. Med. Food 2015, 18, 1–10. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A.A.; Joseph, V.; Dan, V.M.; Jaleel, A.; Kumar, T.R.S.; Kartha, C.C. Untargeted metabolomics reveals alterations in metabolites of lipid metabolism and immune pathways in the serum of rats after long-term oral administration of Amalaki rasayana. Mol. Cell. Biochem. 2020, 463, 147–160. [Google Scholar] [CrossRef]
- Vazirian, M.; Khanavi, M.; Amanzadeh, Y.; Hajimehdipoor, H. Quantification of gallic acidin fruits of three medicinal plants. Iran. J. Pharm. Res. 2011, 10, 233–236. [Google Scholar]
- Andrade, P.M.L.; Baptista, L.; Britto, J.S.; Uetenabaro, A.P.T.; Costa, A.M.D. Co-production of tannase and gallic acid by a novel Penicillium rolfsii (CCMB 714). Prep. Biochem. Biotechnol. 2018, 48, 700–706. [Google Scholar] [CrossRef]
- Sharma, K.P.; John, P.J.; Goswami, P.; Soni, M. Enzymatic synthesis of gallic acid from tannic acid with an inducible hydrolase of Enterobacter spp. Biocatal. Biotransform. 2017, 35, 177–184. [Google Scholar] [CrossRef]
- Shakeri, A.; Zirak, M.R.; Sahebkar, A. Ellagic Acid: A Logical Lead for Drug Development? Curr. Pharm. Des. 2018, 24, 106–122. [Google Scholar] [CrossRef]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Barrio, R.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef]
- Barnes, R.C.; Krenek, K.A.; Meibohm, B.; Mertens-Talcott, S.U.; Talcott, S.T. Urinary metabolites from mango (Mangifera indica L. cv. Keitt) galloyl derivatives and in vitro hydrolysis of gallotannins in physiological conditions. Mol. Nutr. Food Res. 2016, 60, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Choi, B.S.Y.; Dyck, J.R.B.; Schertzer, J.D.; Marette, A. Host-Microbe Interplay in the Cardiometabolic Benefits of Dietary Polyphenols. Trends Endocrinol. Metab. 2019, 30, 384–395. [Google Scholar] [CrossRef]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Takagaki, A.; Nanjo, F. Metabolism of (−)-Epigallocatechin Gallate by Rat Intestinal Flora. J. Agric. Food Chem. 2010, 58, 1313–1321. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef]
- Shahrzad, S.; Aoyagi, K.; Winter, A.; Koyama, A.; Bitsch, I. Pharmacokinetics of Gallic Acid and Its Relative Bioavailability from Tea in Healthy Humans. J. Nutr. 2001, 131, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Inoue, M.; Nose, M.; Kojima, K.; Sakaguchi, N.; Isuzugawa, K.; Takeda, T.; Ogihara, Y. Metabolic Fate of Gallic Acid Orally Administered to Rats. Biol. Pharm. Bull. 1999, 22, 326–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdou, E.M.; Masoud, M.M. Gallic acid–PAMAM and gallic acid–phospholipid conjugates, physicochemical characterization and in vivo evaluation. Pharm. Dev. Technol. 2018, 23, 55–66. [Google Scholar] [CrossRef]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New Perspectives on the Efficacy of Gallic Acid in Cosmetics & Nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187. [Google Scholar]
- Bhattacharyya, S.; Ahammed, S.M.; Saha, B.P.; Mukherjee, P.K. The gallic acid-phospholipid complex improved the antioxidant potential of gallic acid by enhancing its bioavailability. AAPS PharmSciTech 2013, 14, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Wang, Y.; Jia, R.; Fang, F.; Liu, Y.; Cui, W. Computational and biological investigation of the soybean lecithin-gallic acid complex for ameliorating alcoholic liver disease in mice with iron overload. Food Funct. 2019, 10, 5203–5214. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-J.; Lee, Y.-C.; Huang, C.-H.; Chang, L.-S. Gallic acid-capped gold nanoparticles inhibit EGF-induced MMP-9 expression through suppression of p300 stabilization and NFκB/c-Jun activation in breast cancer MDA-MB-231 cells. Toxicol. Appl. Pharmacol. 2016, 310, 98–107. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Gontijo, A.V.L.; Borges, A.C.; Tanaka, M.H.; Lima, G.d.M.G.; Salvador, M.J.; Koga-Ito, C.Y. Gallic acid/hydroxypropyl-β-cyclodextrin complex: Improving solubility for application on in vitro/in vivo Candida albicans biofilms. PLoS ONE 2017, 12, e0181199. [Google Scholar] [CrossRef] [Green Version]
- Gim, S.Y.; Hong, S.; Kim, M.-J.; Lee, J. Gallic Acid Grafted Chitosan Has Enhanced Oxidative Stability in Bulk Oils. J. Food Sci. 2017, 82, 1608–1613. [Google Scholar] [CrossRef]
- Wang, P.; Liu, S. Development of a Gemini Interfacial Antioxidant for Oil in Water Emulsion with Gallic Acid and Dodecyl Gemini Chains. J. Agric. Food Chem. 2020, 68, 9953–9960. [Google Scholar] [CrossRef]
- Velderrain-Rodriguez, G.R.; Torres-Moreno, H.; Villegas-Ochoa, M.A.; Ayala-Zavala, J.F.; Robles-Zepeda, R.E.; Wall-Medrano, A.; Gonzalez-Aguilar, G.A. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhi, Q.-Q.; Li, J.-Y.; Keller, N.P.; He, Z.-M. The Antioxidant Gallic Acid Inhibits Aflatoxin Formation in Aspergillus flavus by Modulating Transcription Factors FarB and CreA. Toxins 2018, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Megson, I.L. Bioavailable Concentrations of Delphinidin and Its Metabolite, Gallic Acid, Induce Antioxidant Protection Associated with Increased Intracellular Glutathione in Cultured Endothelial Cells. Oxidative Med. Cell. Longev. 2017, 2017, 9260701. [Google Scholar] [CrossRef] [Green Version]
- Fischer, N.; Seo, E.-J.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef]
- Nair, G.G.; Nair, C.K.K. Radioprotective effects of gallic acid in mice. BioMed Res. Int. 2013, 2013, 953079. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Singh, A.; Mishra, A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013, 35, 473–485. [Google Scholar] [CrossRef]
- Yoshioka, K.; Kataoka, T.; Hayashi, T.; Hasegawa, M.; Ishi, Y.; Hibasami, H. Induction of apoptosis by gallic acid in human stomach cancer KATO III and colon adenocarcinoma COLO 205 cell lines. Oncol. Rep. 2000, 7, 1221–1224. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Chang, L.-S. Gallic acid downregulates matrix metalloproteinase-2 (MMP-2) and MMP-9 in human leukemia cells with expressed Bcr/Abl. Mol. Nutr. Food Res. 2012, 56, 1398–1412. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Lin, K.-N.; Jhang, L.-M.; Huang, C.-H.; Lee, Y.-C.; Chang, L.-S. Gallic acid abolishes the EGFR/Src/Akt/Erk-mediated expression of matrix metalloproteinase-9 in MCF-7 breast cancer cells. Chem. Biol. Interact. 2016, 252, 131–140. [Google Scholar] [CrossRef]
- Ho, H.-H.; Chang, C.-S.; Ho, W.-C.; Liao, S.-Y.; Wu, C.-H.; Wang, C.-J. Anti-metastasis effects of gallic acid on gastric cancer cells involves inhibition of NF-κB activity and downregulation of PI3K/AKT/small GTPase signals. Food Chem. Toxicol. 2010, 48, 2508–2516. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.-R.; Li, H.; Wang, S.-W. Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: Involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef]
- Radan, M.; Dianat, M.; Badavi, M.; Mard, S.A.; Bayati, V.; Goudarzi, G. In vivo and in vitro evidence for the involvement of Nrf2-antioxidant response element signaling pathway in the inflammation and oxidative stress induced by particulate matter (PM10): The effective role of gallic acid. Free Radic. Res. 2019, 53, 210–225. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, T.; Weng, A.; Huang, X.; Yao, Y.; Fu, Z.; Li, Y.; Liu, A.; Li, X.; Chen, D.; et al. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Through Enhancing Nrf2 Signaling. Front. Immunol. 2020, 11, 580593. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. Antioxidant mechanism of black garlic extract involving nuclear factor erythroid 2-like factor 2 pathway. Nutr. Res. Pract. 2017, 11, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Diaz, A.; Muñoz-Arenas, G.; Caporal-Hernandez, K.; Vázquez-Roque, R.; Lopez-Lopez, G.; Kozina, A.; Espinosa, B.; Flores, G.; Treviño, S.; Guevara, J. Gallic acid improves recognition memory and decreases oxidative-inflammatory damage in the rat hippocampus with metabolic syndrome. Synapse 2020, 75, 22186. [Google Scholar] [CrossRef]
- Tanaka, M.; Sugama, A.; Sumi, K.; Shimizu, K.; Kishimoto, Y.; Kondo, K.; Iida, K. Gallic acid regulates adipocyte hypertrophy and suppresses inflammatory gene expression induced by the paracrine interaction between adipocytes and macrophages in vitro and in vivo. Nutr. Res. 2020, 73, 58–66. [Google Scholar] [CrossRef]
- Paraiso, A.F.; Sousa, J.N.; Andrade, J.M.O.; Mangabeira, E.S.; Lelis, D.F.; de Paula, A.M.B.; Martins, A.; Lima, W.J.N.; Guimaraes, A.L.S.; Melo, G.A.; et al. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: A molecular and bioinformatic approach. Life Sci. 2019, 237, 116914. [Google Scholar] [CrossRef]
- D’Souza, J.J.; D’Souza, P.P.; Fazal, F.; Kumar, A.; Bhat, H.P.; Baliga, M.S. Anti-diabetic effects of the Indian indigenous fruit Emblica officinalis Gaertn: Active constituents and modes of action. Food Funct. 2014, 5, 635–644. [Google Scholar] [CrossRef]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic potential of gallic acid from Emblica officinalis: Improved glucose transporters and insulin sensitivity through PPAR-γ and Akt signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef]
- Bak, E.J.; Kim, J.; Jang, S.; Woo, G.H.; Yoon, H.G.; Yoo, Y.J.; Cha, J.H. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scand. J. Clin. Lab. Investig. 2013, 73, 607–614. [Google Scholar] [CrossRef]
- Lima Junior, J.P.; Franco, R.R.; Saraiva, A.L.; Moraes, I.B.; Espindola, F.S. Anacardium humile St. Hil as a novel source of antioxidant, antiglycation and alpha-amylase inhibitors molecules with potential for management of oxidative stress and diabetes. J. Ethnopharmacol. 2021, 268, 113667. [Google Scholar] [CrossRef]
- Hou, Y.; Ali, I.; Li, Z.; Sulaiman, A.; Aziz, S.; Chen, L.; Hussain, H.; Cui, L.; Wang, D.; Zheng, X. Separation of constituents from Bergenia stracheyi (Hook. F. & Thoms.) Engl. by high-speed countercurrent chromatography with elution mode and its antidiabetic and antioxidant in vitro evaluation. J. Sep. Sci. 2021, 44, 767–776. [Google Scholar] [CrossRef]
- Miranda Pedroso, T.F.; Bonamigo, T.R.; da Silva, J.; Vasconcelos, P.; Felix, J.M.; Cardoso, C.A.L.; Souza, R.I.C.; Dos Santos, A.C.; Volobuff, C.R.F.; Formagio, A.S.N.; et al. Chemical constituents of Cochlospermum regium (Schrank) Pilg. root and its antioxidant, antidiabetic, antiglycation, and anticholinesterase effects in Wistar rats. Biomed. Pharmacother. 2019, 111, 1383–1392. [Google Scholar] [CrossRef]
- Ghauri, S.; Raza, S.Q.; Imran, M.; Saeed, S.; Rashid, M.; Naseer, R. Assessment of alpha-amylase and alpha-glucosidase inhibitory potential of Citrus reticulata peel extracts in hyperglycemic/hypoglycemic rats. 3 Biotech 2021, 11, 167. [Google Scholar] [CrossRef]
- Khan, D.; Sharif, A.; Zafar, M.; Akhtar, B.; Akhtar, M.F.; Awan, S. Delonix regia a Folklore Remedy for Diabetes; Attenuates Oxidative Stress and Modulates Type II Diabetes Mellitus. Curr. Pharm. Biotechnol. 2020, 21, 1059–1069. [Google Scholar] [CrossRef]
- Roheem, F.O.; Mat Soad, S.Z.; Ahmed, Q.U.; Ali Shah, S.A.; Latip, J.; Zakaria, Z.A. Evaluation of the Enzyme Inhibitory and Antioxidant Activities of Entada spiralis Stem Bark and Isolation of the Active Constituents. Molecules 2019, 24, 1006. [Google Scholar] [CrossRef] [Green Version]
- Alegbe, E.O.; Terali, K.; Olofinsan, K.A.; Surgun, S.; Ogbaga, C.C.; Ajiboye, T.O. Antidiabetic activity-guided isolation of gallic and protocatechuic acids from Hibiscus sabdariffa calyxes. J. Food Biochem. 2019, 43, e12927. [Google Scholar] [CrossRef]
- Bouzghaya, S.; Amri, M.; Homble, F. Improvement of Diabetes Symptoms and Complications by an Aqueous Extract of Linum usitatissimum (L.) Seeds in Alloxan-Induced Diabetic Mice. J. Med. Food 2020, 23, 1077–1082. [Google Scholar] [CrossRef]
- Gondi, M.; Prasada Rao, U.J. Ethanol extract of mango (Mangifera indica L.) peel inhibits alpha-amylase and alpha-glucosidase activities, and ameliorates diabetes related biochemical parameters in streptozotocin (STZ)-induced diabetic rats. J. Food Sci. Technol. 2015, 52, 7883–7893. [Google Scholar] [CrossRef] [Green Version]
- Mahesh Kumar, P.; Venkataranganna, M.V.; Manjunath, K.; Viswanatha, G.L.; Ashok, G. Momordica cymbalaria fruit extract attenuates high-fat diet-induced obesity and diabetes in C57BL/6 mice. Iran. J. Basic Med. Sci. 2018, 21, 1083–1090. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, L.; Tang, M.; Zhang, S.; Liu, L.; Gao, L.; Ma, S.; Kong, M.; Yao, Q.; Feng, F.; et al. Analysis of mulberry leaf components in the treatment of diabetes using network pharmacology. Eur. J. Pharmacol. 2018, 833, 50–62. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Ojo, O.A.; Okesola, M.A.; Akinyemi, A.J.; Talabi, J.Y.; Idowu, O.T.; Fadaka, A.O.; Boligon, A.A.; Anraku de Campos, M.M. In vitro antioxidant activities and inhibitory effects of phenolic extract of Senecio biafrae (Oliv and Hiern) against key enzymes linked with type II diabetes mellitus and Alzheimer’s disease. Food Sci. Nutr. 2018, 6, 1803–1810. [Google Scholar] [CrossRef] [Green Version]
- Qamar, M.; Akhtar, S.; Ismail, T.; Yuan, Y.; Ahmad, N.; Tawab, A.; Ismail, A.; Barnard, R.T.; Cooper, M.A.; Blaskovich, M.A.T.; et al. Syzygium cumini(L.),Skeels fruit extracts: In vitro and in vivo anti-inflammatory properties. J. Ethnopharmacol. 2021, 271, 113805. [Google Scholar] [CrossRef]
- Ai, X.; Hou, Y.; Wang, X.; Wang, X.; Liang, Y.; Zhu, Z.; Wang, P.; Zeng, Y.; Li, X.; Lai, X.; et al. Amelioration of dry eye syndrome in db/db mice with diabetes mellitus by treatment with Tibetan Medicine Formula Jikan Mingmu Drops. J. Ethnopharmacol. 2019, 241, 111992. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Sajadimajd, S.; Iranpanah, A.; Khazaei, M.; Pourjabar, Z.; Hajimahmoodi, M.; Rahimi, R. In vitro and in vivo antidiabetic activity of Tamarix stricta Boiss.: Role of autophagy. J. Ethnopharmacol. 2021, 269, 113692. [Google Scholar] [CrossRef]
- Ramachandran, S.; Rajasekaran, A.; Adhirajan, N. In Vivo and In Vitro Antidiabetic Activity of Terminalia paniculata Bark: An Evaluation of Possible Phytoconstituents and Mechanisms for Blood Glucose Control in Diabetes. ISRN Pharmacol. 2013, 2013, 484675. [Google Scholar] [CrossRef]
- Katz, S.R.; Newman, R.A.; Lansky, E.P. Punica granatum: Heuristic treatment for diabetes mellitus. J. Med. Food 2007, 10, 213–217. [Google Scholar] [CrossRef]
- Raafat, K.; Samy, W. Amelioration of Diabetes and Painful Diabetic Neuropathy by Punica granatum L. Extract and Its Spray Dried Biopolymeric Dispersions. Evid Based Complement. Altern. Med. 2014, 2014, 180495. [Google Scholar] [CrossRef] [Green Version]
- Zulfqar, F.; Akhtar, M.F.; Saleem, A.; Akhtar, B.; Sharif, A.; Saleem, U. Chemical characterization, antioxidant evaluation, and antidiabetic potential of Pinus gerardiana (Pine nuts) extracts. J. Food Biochem. 2020, 44, e13199. [Google Scholar] [CrossRef]
- Youl, E.N.H.; Ouedraogo, C.A.P.; Gambo, M.; Ouedraogo, M.; Kiendrebeogo, M.; Traore, A.; Guissou, I.P. Antioxidant activity of crude ethanolic extract and fractions of Ziziphus mauritiana Lam. (Rhamnaceae) leaves from Burkina Faso. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 30. [Google Scholar] [CrossRef]
- LoRicco, J.G.; Xu, C.S.; Neidleman, J.; Bergkvist, M.; Greene, W.C.; Roan, N.R.; Makhatadze, G.I. Gallic Acid Is an Antagonist of Semen Amyloid Fibrils That Enhance HIV-1 Infection. J. Biol. Chem. 2016, 291, 14045–14055. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Carver, J.A.; Calabrese, A.N.; Pukala, T.L. Gallic acid interacts with α-synuclein to prevent the structural collapse necessary for its aggregation. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 1481–1485. [Google Scholar] [CrossRef]
- Owumi, S.; Najophe, E.S.; Farombi, E.O.; Oyelere, A.K. Gallic acid protects against Aflatoxin B1 -induced oxidative and inflammatory stress damage in rats kidneys and liver. J. Food Biochem. 2020, 44, e13316. [Google Scholar] [CrossRef]
- Esmaeilzadeh, M.; Heidarian, E.; Shaghaghi, M.; Roshanmehr, H.; Najafi, M.; Moradi, A.; Nouri, A. Gallic acid mitigates diclofenac-induced liver toxicity by modulating oxidative stress and suppressing IL-1beta gene expression in male rats. Pharm. Biol. 2020, 58, 590–596. [Google Scholar] [CrossRef]
- Sousa, J.N.; Paraiso, A.F.; Andrade, J.M.O.; Lelis, D.F.; Santos, E.M.; Lima, J.P.; Monteiro-Junior, R.S.; D’Angelo, M.; de Paula, A.M.B.; Guimaraes, A.L.S.; et al. Oral gallic acid improve liver steatosis and metabolism modulating hepatic lipogenic markers in obese mice. Exp. Gerontol. 2020, 134, 110881. [Google Scholar] [CrossRef]
- Hussein, R.M.; Anwar, M.M.; Farghaly, H.S.; Kandeil, M.A. Gallic acid and ferulic acid protect the liver from thioacetamide-induced fibrosis in rats via differential expression of miR-21, miR-30 and miR-200 and impact on TGF-beta1/Smad3 signaling. Chem. Biol. Interact. 2020, 324, 109098. [Google Scholar] [CrossRef]
| The Natural Plants | The Identified Phenolic Derivatives | Pharmacological Activities |
|---|---|---|
| Anacardium humile A.St-Hill. [63] | quercetin, catechin and GA | Antioxidant, antiglycation/α-mylase inhibitors |
| Bergenia stracheyi (Hook. f. & Thoms.) Engl. [64] | GA, 11-O-galloylbergenin, (-)-epicatechin 3-O-gallate | Antidiabetes, antioxidant |
| Tea leaves including black, green, and white tea [7] | caffeic acid (CA), GA | Colesterol-lowering, antioxidant |
| Chios Gum Mastic | oleanonic acid, oleanolic acid, GA | PPARs modulators, |
| Cochlospermum regium (Schrank) Pilg. Root [65] | GA, CA and ellagic acid | Antioxidant, antidiabetes, antiglycation, anticholinesterase |
| Citrus reticulata Blanco [66] | total phenolic chemicals | α-amylase and α-glucosidase inhibitory |
| Delonix regia (Bojer ex Hook.) Raf. [67] | quercetin, GA, CA, cinnamic acid, ferulic acid, and p-coumaric acid | Antioxidant, hypoglycemic, and hypolipidemic activities |
| Delonix regia (Bojer ex Hook.) Raf. | quercetin, gallic acid, caffeic acid, cinnamic acid, ferulic acid, and p-coumaric acid | Hypoglycemic, antioxidant, and hypolipidemic activities. |
| Entada spiralis Ridl. Stem Bark [68] | GA, (+)-catechin, (-)-epicatechin. | Antioxidant activity |
| Eugenia punicifolia (Kunth) DC. | myricetin-3-O-rhamnoside, quercetin-3-O-galactoside, quercetin-3-O-xyloside, quercetin-3-O-rhamnoside, kaempferol-3-O-rhamnoside, phytol, gallic acid, and trans-caryophyllene | Antidiabetic activity |
| Eugenia uniflora O. Berg | ellagic acid, GA and rutin | Antioxidant and anti-inflammatory activities |
| fermented legumes | gallic acid, catechin, caffeic acid, epicatechin, rutin, isoquercitrin, quercitrin, quercetin and kaempferol | Antidiabetic and anti-acetylcholinesterase activities |
| Gymnema montanum (Roxb.) Hook.f. | gallic acid, resveratrol, and quercetin | Protection against lipid peroxidation |
| Hibiscus sabdariffa L. [69] | GA and protocatechuic acids | Improvement of diabetes, hypertension, dyslipidemia, |
| Linum usitatissimum L. Seeds [70] | GA | Antidiabetic activity |
| Mango (Mangifera indica L.) peel [71] | ferulic acid, protocatechuic, chlorogenic, gallic, vanillic, and caffeic acids | Antioxidant, anti-inflammatory, antidiabetic activities, inhibition of α-amylase and α-glucosidase |
| Momordica cymbalaria Fenzl ex Naudin [72] | gallic acid and rutin | Antidiabetic and improvement of insulin resistance |
| Mulberry leaves [73] | GA | Modulation of insulin and inflammatory signaling |
| Senecio biafrae (Oliv. & Hiern) C.Jeffrey leaves [74] | GA, chlorogenic, caffeic acid, rutin, quercetin, and kaempferol. | Antidiabetic activity |
| Syzygium cumini (L.) Skeels kernels powder and fruits [75] | Myricetin, catechin, quinic acid, chlorogenic acid, ellagic acid, catechin, gallic acid, and caffeic acid | Antioxidant, anti-inflammatory, anticancer, antidiabetic, antibacterial, antifungal activities |
| Triphala Rasayana | GA, ellagic acid, chebulic acid, chebulinic acid, methyl gallate | Antidiabetes, anticonstipation, antiobesity |
| Jikan Mingmu Drop [76] | GA, ellagic acid, | Reduction of dry eye syndrome |
| Tamarix stricta Boiss [77] | GA | Antidiabetic activity via autophagy |
| Terminalia paniculata Roth Bark [78] | GA | Antidiabetic activity |
| Punica granatum L. [79,80] | punicalagin and ellagic, gallic, oleanolic, ursolic, and uallic acids | Antidiabetes, antipain in diabetic neuropathy |
| Pinus gerardiana Wall. ex D.Don [81] | GA | Inhibition of α-amylase, antioxidant, antihyperlipidemic antidiabetes |
| Ziziphus mauritiana Lam. leaves [82] | rich in polyphenols | Antioxidative stress in diabetes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules 2021, 26, 7115. https://doi.org/10.3390/molecules26237115
Xu Y, Tang G, Zhang C, Wang N, Feng Y. Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules. 2021; 26(23):7115. https://doi.org/10.3390/molecules26237115
Chicago/Turabian StyleXu, Yu, Guoyi Tang, Cheng Zhang, Ning Wang, and Yibin Feng. 2021. "Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress" Molecules 26, no. 23: 7115. https://doi.org/10.3390/molecules26237115
APA StyleXu, Y., Tang, G., Zhang, C., Wang, N., & Feng, Y. (2021). Gallic Acid and Diabetes Mellitus: Its Association with Oxidative Stress. Molecules, 26(23), 7115. https://doi.org/10.3390/molecules26237115








