New Carbonic Anhydrase-II Inhibitors from Marine Macro Brown Alga Dictyopteris hoytii Supported by In Silico Studies
Abstract
:1. Introduction
2. Results and Discussion
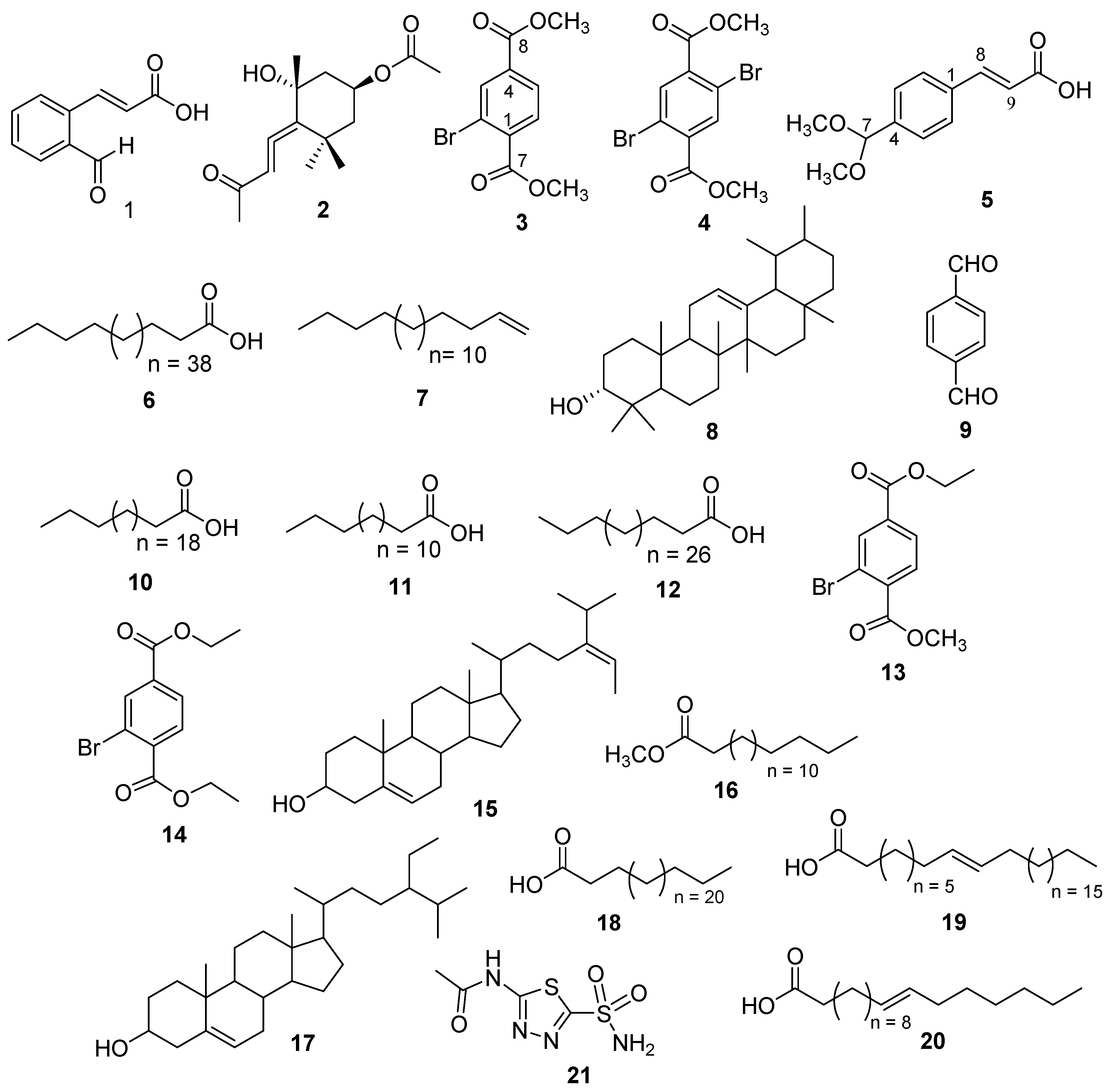
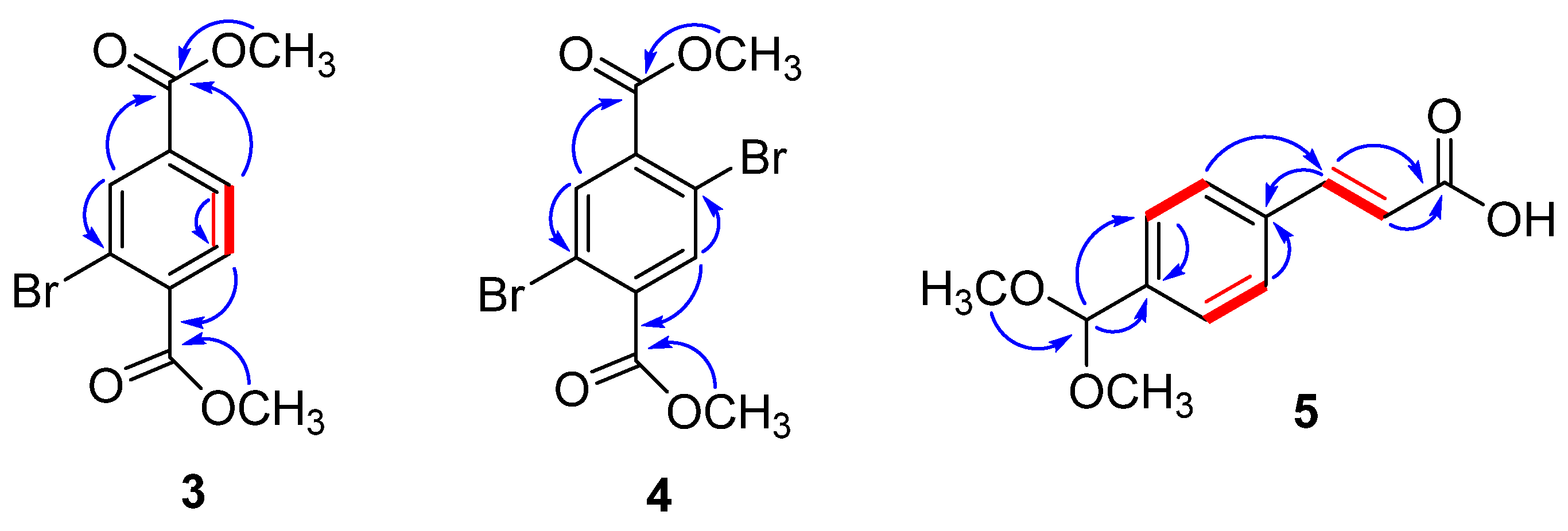
2.1. Structure Elucidation of Compounds 1–12
2.2. Carbonic Anhydrase-II Inhibition and Structural-Activity Relationship
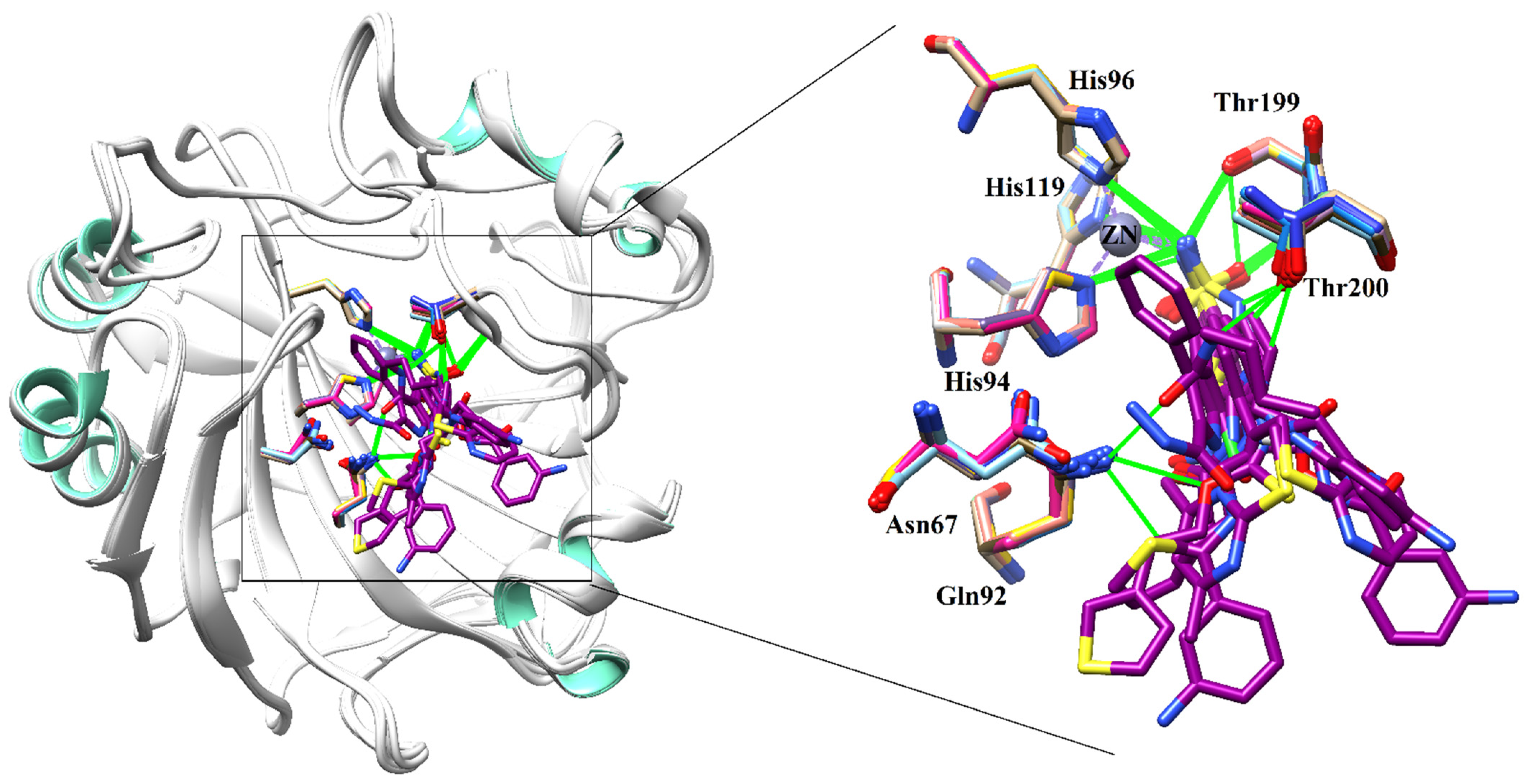
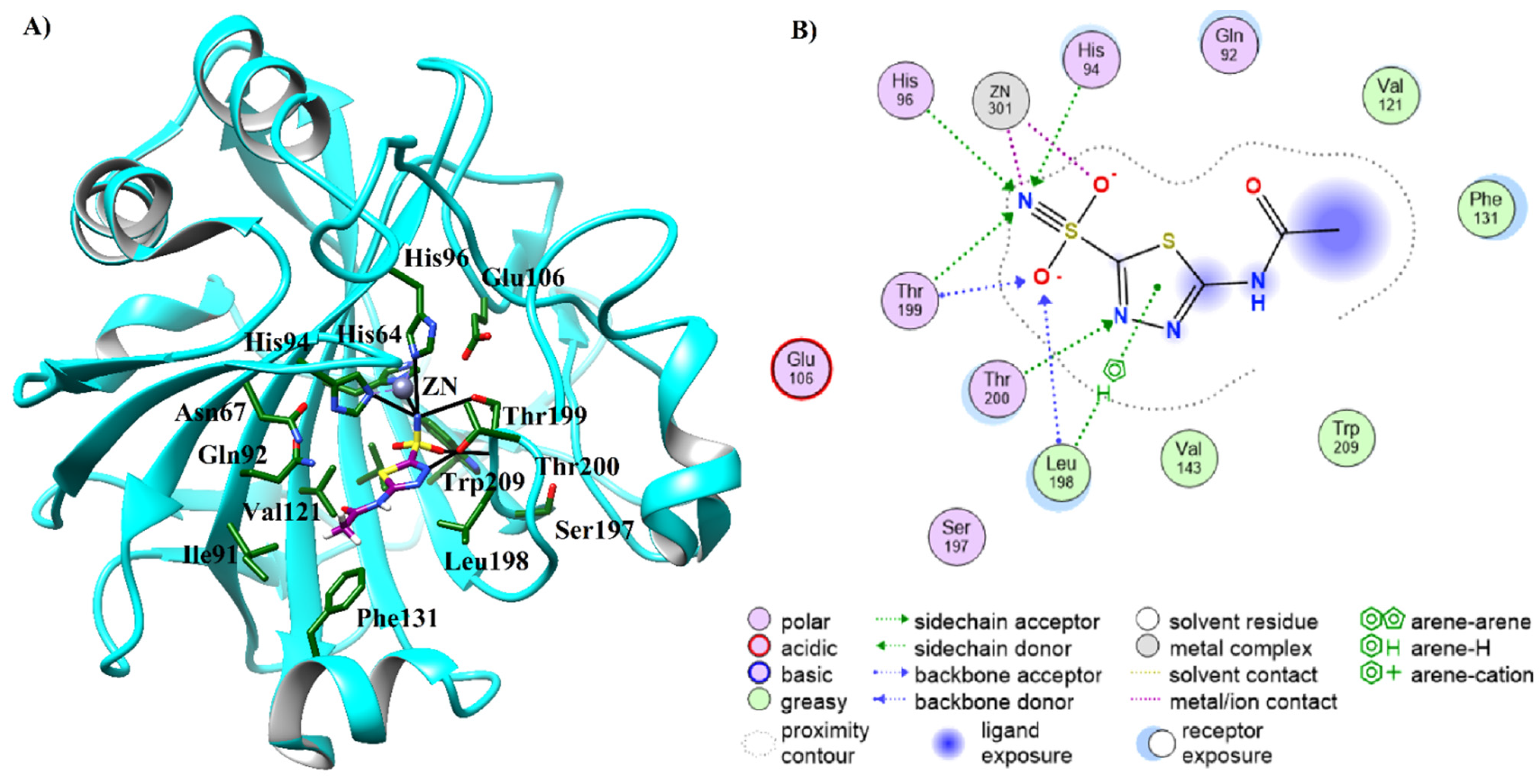
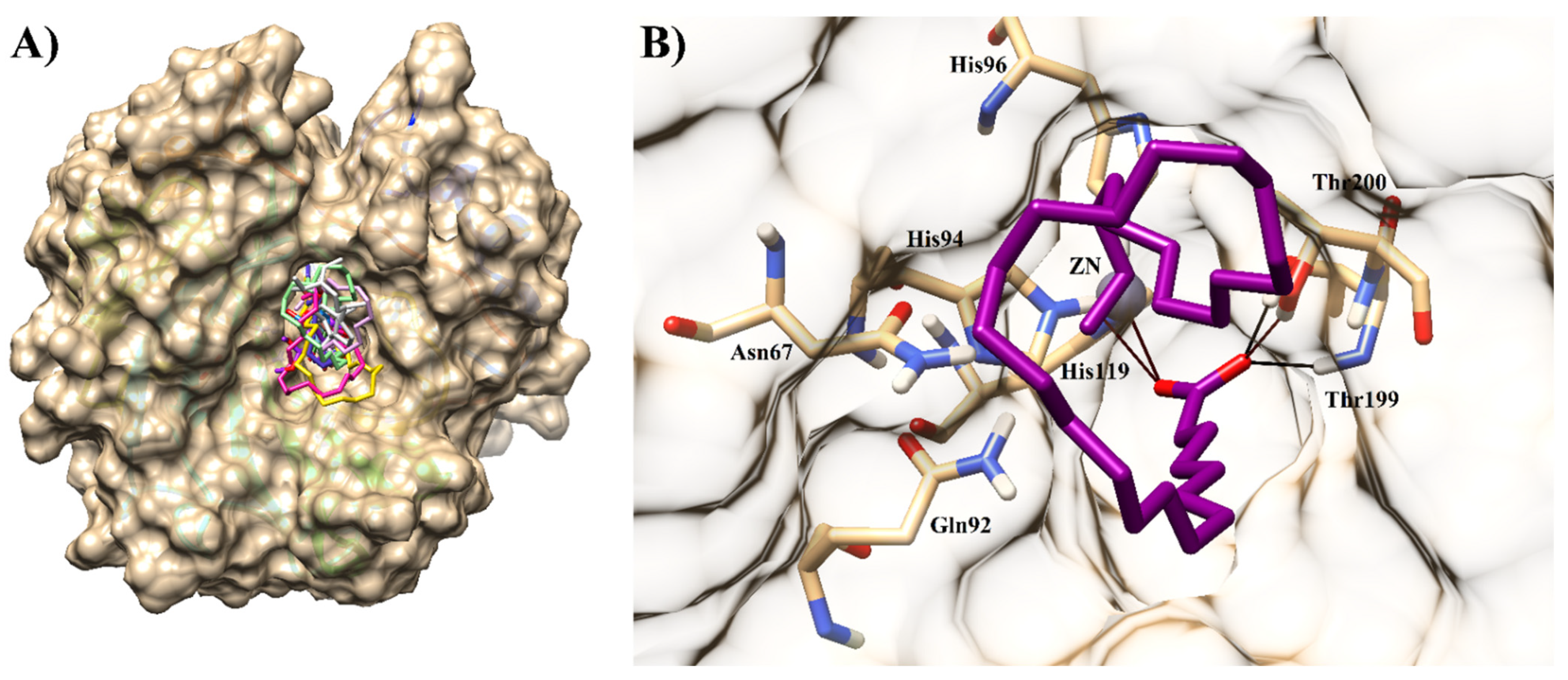
2.3. Molecular Docking of Carbonic Anhydrase-II Inhibitors
2.4. Pharmacokinetic Prediction of Compounds 1, 3, 5, 7, 9–10, 12–13, 15, 18, and 19
3. Material and Methods
3.1. General Instrumentation
3.2. Sample Collection and Identification
3.3. Extraction, Isolation, and Purification
3.4. Carbonic Anhydrase II Inhibition
3.5. Molecular Docking and ADMET Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J. Metabolites from Macroalgae and Its Applications in the Cosmetic Industry: A Circular Economy Approach. Resources 2020, 9, 101. [Google Scholar] [CrossRef]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Biris-Dorhoi, E.-S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A Sustainable Source of Chemical Compounds with Biological Activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a Source of Valuable Antimicrobial Compounds: Extraction and Applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Melendres, A.R.; Ilano, A. Bio-oil Product from Wild Brown Macro-algae Dunggandunggan (Padinasp) in Asturias and Carmen, Cebu, Philippines. Int. J. Med. Plants Nat. Prod. 2017, 3, 27–36. [Google Scholar]
- Rajasulochana, A.; Dhamotharan, R.; Krishnamoorthy, P.; Subbiah, M. Antibacterial Activity of the Extracts of Marine Red and Brown. J. Am. Sci. 2009, 5, 20–25. [Google Scholar]
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Pettus, J.A. Isolation and structure determination of dictyopterenes C’ and D’ from Dictyopteris. Stereospecificity in the cope rearrangement of dictyopterenes A and B. J. Am. Chem. Soc. 1971, 93, 3087–3088. [Google Scholar] [CrossRef]
- Schnitzler, I.; Pohnert, G.; Hay, M.; Boland, W. Chemical defense of brown algae (Dictyopteris spp.) against the herbivorous amphipod Ampithoe longimana. Oecologia 2001, 126, 515–521. [Google Scholar] [CrossRef]
- Zatelli, G.A.; Philippus, A.C.; Falkenberg, M. An overview of odoriferous marine seaweeds of the Dictyopteris genus: Insights into their chemical diversity, biological potential and ecological roles. Rev. Bras. Farmacogn. 2018, 28, 243–260. [Google Scholar] [CrossRef]
- Feng, M.-T.; Wang, T.; Liu, A.-H.; Li, J.; Yao, L.-G.; Wang, B.; Guo, Y.-W.; Mao, S.-C. PTP1B inhibitory and cytotoxic C-24 epimers of Δ28-24-hydroxy stigmastane-type steroids from the brown alga Dictyopteris undulata Holmes. Phytochemistry 2018, 146, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.-Y.; Wen, W.; Li, X.-M.; Xue, Q.-Z.; Xiao, H.-L.; Wang, B.-G. Brominated Selinane Sesquiterpenes from the Marine Brown Alga Dictyopteris divaricata. Mar. Drugs 2009, 7, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Xu, X.; Li, S.; Wang, S.; Zhao, J.; Yang, Y.; Fan, X.; Shi, J.; He, L. Minor sesquiterpenes with new carbon skeletons from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2006, 69, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Rafiq, K.; Khan, A.; Halim, S.A.; Ali, L.; Al-Saady, N.; Al-Balushi, A.H.; Al-Busaidi, H.K.; Al-Harrasi, A. α-Glucosidase inhibition and molecular docking studies of natural brominated metabolites from marine macro brown alga Dictyopteris hoytii. Mar. Drugs 2019, 17, 666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segawa, M.; Yamano, K.; Shirahama, H. A germacrane-type sesquiterpene from the brown alga Dictyopteris divaricata. Phytochemistry 1990, 29, 973–974. [Google Scholar] [CrossRef]
- Zbakh, H.; Zubía, E.; De Los Reyes, C.; Calderón-Montaño, J.M.; Motilva, V. Anticancer Activities of Meroterpenoids Isolated from the Brown Alga Cystoseira usneoides against the Human Colon Cancer Cells HT-29. Foods 2020, 9, 300. [Google Scholar] [CrossRef] [Green Version]
- Song, F.; Fan, X.; Xu, X.; Zhao, J.; Yang, Y.; Shi, J. Cadinane sesquiterpenes from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2004, 67, 1644–1649. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Suzuki, M. Cyclozonarone, a sesquiterpene-substituted benzoquinone derivative from the brown alga Dictyopteris undulata. Phytochemistry 1996, 41, 749–752. [Google Scholar] [CrossRef]
- Song, F.; Xu, X.; Li, S.; Wang, S.; Zhao, J.; Cao, P.; Yang, Y.; Fan, X.; Shi, J.; He, L.; et al. Norsesquiterpenes from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2005, 68, 1309–1313. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A.; Mistysyn, J. Odoriferous C11 hydrocarbons from Hawaiian Dictyopteris. J. Org. Chem. 1974, 39, 2201–2207. [Google Scholar] [CrossRef]
- Dimou, M.; Ioannou, E.; Daskalaki, M.G.; Tziveleka, L.A.; Kampranis, S.C.; Roussis, V. Disulfides with Anti-inflammatory Activity from the Brown Alga Dictyopteris membranacea. J. Nat. Prod. 2016, 79, 584–589. [Google Scholar] [CrossRef]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Belattmania, Z.; Reani, A.; Mustapha, B.; Zrid, R.; Samir, E.A.; Hassouani, M.; Eddaoui, A.; Bentiss, F.; Sabour, B. Antimicrobial, antioxidant and alginate potentials of Dictyopteris polypodioides (Dictyotales, Phaeophyceae) from the Moroccan Atlantic coast. Der Pharma Chem. 2016, 8, 216–226. [Google Scholar]
- Kim, K.N.; Ham, Y.M.; Moon, J.Y.; Kim, M.J.; Kim, D.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. In vitro cytotoxic activity of Sargassum thunbergii and Dictyopteris divaricata (Jeju seaweeds) on the HL-60 tumour cell line. Int. J. Pharmacol. 2009, 5, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Ghorai, S.; Pulya, S.; Ghosh, K.; Panda, P.; Ghosh, B.; Gayen, S. Structure-activity relationship of human carbonic anhydrase-II inhibitors: Detailed insight for future development as anti-glaucoma agents. Bioorg. Chem. 2020, 95, 103557. [Google Scholar] [CrossRef] [PubMed]
- Achal, V.; Pan, X. Characterization of Urease and Carbonic Anhydrase Producing Bacteria and Their Role in Calcite Precipitation. Curr. Microbiol. 2010, 62, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Bingol, Z.; Koca, M.; Dagoglu, S.; Pınar, N.M.; Demirci, B.; Gulcin, İ.; Brestic, M.; Sytar, O. Identification of non-alkaloid natural compounds of Angelica purpurascens (Avé-Lall.) Gilli. (Apiaceae) with cholinesterase and carbonic anhydrase inhibition potential. Saudi Pharm. J. 2020, 28, 1–14. [Google Scholar] [CrossRef]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef]
- Costa, G.; Gidaro, M.C.; Vullo, D.; Supuran, C.T.; Alcaro, S. Active Components of Essential Oils as Anti-Obesity Potential Drugs Investigated by in Silico Techniques. J. Agric. Food Chem. 2016, 64, 5295–5300. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Saeed, M.; Adhikari, A.; Khan, K.M.; Khan, H. Isolation of a new bioactive cinnamic acid derivative from the whole plant of Viola betonicifolia. J. Enzyme Inhib. Med. Chem. 2013, 28, 997–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauroozi, D.; Pejic, M.; Schwartz, P.-O.; Wachtler, M.; Bäuerle, P. Synthesis and solvent-free polymerisation of vinyl terephthalate for application as an anode material in organic batteries. RSC Adv. 2016, 6, 111350–111357. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66{,} UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]
- Song, C.L.; Liu, K.; Zhang, A.J.; Xu, Z.G.; Zhang, H.L. Dimethyl 2,5-bis-(5-hexyl-thio-phen-2-yl)benzene-1,4-dioate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o1059. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.J.; Bauer, N.; Dirkes, D.; Kaplan, J.; Peng, Z.; Zhang, H.; Ye, L.; Liu, S.; Gao, F.; Ade, H.; et al. The crucial role of end group planarity for fused-ring electron acceptors in organic solar cells. Mater. Chem. Front. 2019, 3, 1642–1652. [Google Scholar] [CrossRef]
- Daluge, S.M.; Wolberg, G.; Livingston, D.A.; SmithKline Beecham Corp. Substituted (1,3-bis(cyclohexylmethyl)-1,2,3,6-tetrahydro-2,6-dioxo-9h-purin-8-yl) Phenyl Derivatives, Their Preparation and Their Use in the Treatment of Inflammatory Conditions and Immune Disorders. US Patent (WO/1998/035966), 20 August 1998. [Google Scholar]
- Lehmann-Lintz, T.; Lustenberger, P.; Jürgen Roth, G.; Schindler, M.; Thomas, L.; Georg Mueller, S.; Stenkamp, D.; Lotz, R.R.H.; Rudolf, K. 3-(4-piperidine-1ylmethyl-phenyl)-propion Acid-phenylamide-derivatives and Related Compounds Used in the Form of mch Antagonists (Melanine Concentrating Hormone) for Treating Eating Disorders. US Patent (WO2005063239A1), 14 July 2005. [Google Scholar]
- Bernatek, E.; Frengen, C. Ozonolysis of Phenols. III. 1- and 2-Naphthol. Acta Chem. Scand. 1962, 16, 2421–2428. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, B.G.; Moza, P.N. Synthesis of Substituted β-Lactams. J. Med. Chem. 1966, 9, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Hattab, M.; Culioli, G.; Valls, R.; Richou, M.; Piovetti, L. Apo-fucoxanthinoids and loliolide from the brown alga Cladostephus spongiosus f. verticillatus (Heterokonta, Sphacelariales). Biochem. Syst. Ecol. 2008, 36, 447–451. [Google Scholar] [CrossRef]
- Doi, Y.; Ishibashi, M.; Yamaguchi, N.; Kobayashi, J. Isolation of Apo-9’-fucoxanthinone from the Cultured Marine Dinoflagellate Amphidinium sp. J. Nat. Prod. 1995, 58, 1097–1099. [Google Scholar] [CrossRef]
- Fengzhi, R.; Huihua, Q.; Xinhui, L.; Yimin, Z. Studies on the chemical constituents of Callicarpa bodinieri Levl. Nat. Prod. Res. Dev. 2001, 13, 33–34. [Google Scholar]
- Dailey, O.D.; Severson, R.F.; Arrendale, R.F. Nonpolar Lipids of Amaranthus palmeri S. Wats. 2. Unsaturated Esters and Free Fatty Acids, Sterols, and Triterpenols. J. Agric. Food Chem. 1997, 45, 3914–3920. [Google Scholar] [CrossRef]
- Sulaimon, L.; Anise, E.O.; Obuotor, E.M.; Samuel, T.; Moshood, A.; Olajide, M.; Fatoke, T. In vitro antidiabetic potentials, antioxidant activities and phytochemical profile of african black pepper (Piper guineense). Clin. Phytoscience 2020, 6, 90. [Google Scholar] [CrossRef]
- Dekebo, A.; Dagne, E.; Aasen, O.R.G.; Aasen, A.J. Triterpenes from the resin of Boswellia neglecta. Bull. Chem. Soc. Ethiop. 2002, 16, 87–90. [Google Scholar] [CrossRef]
- Ur Rehman, N.; Halim, S.A.; Al-Azri, M.; Khan, M.; Khan, A.; Rafiq, K.; Al-Rawahi, A.; Csuk, R.; Al-Harrasi, A. Triterpenic Acids as Non-Competitive α-Glucosidase Inhibitors from Boswellia elongata with Structure-Activity Relationship: In Vitro and In Silico Studies. Biomolecules 2020, 10, 751. [Google Scholar] [CrossRef]
- Sulzer, P.; Hartungen, E.; Hanel, G.; Feil, S.; Winkler, K.; Mutschlechner, P.; Haidacher, S.; Schottkowsky, R.; Gunsch, D.; Seehauser, H.; et al. A Proton Transfer Reaction-Quadrupole interface Time-Of-Flight Mass Spectrometer (PTR-QiTOF): High speed due to extreme sensitivity. Int. J. Mass Spectrom. 2014, 368, 1–5. [Google Scholar] [CrossRef]
- Dong, J.; Xu, F.; Dong, Z.; Zhao, Y.; Yan, Y.; Jin, H.; Li, Y. Fabrication of two dual-functionalized covalent organic polymers through heterostructural mixed linkers and their use as cationic dye adsorbents. RSC Adv. 2018, 8, 19075–19084. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhao, R.; Zou, Z. Chemical constituents from root bark of Tripterygium hypoglaucum. China J. Chin. Mater. Med. 2011, 36, 2503–2506. [Google Scholar]
- Krishnan, K.R.; James, F.; Mohan, A. Isolation and characterization of n-hexadecanoic acid from Canthium parviflorum leaves. J. Chem. Pharm. Res. 2016, 8, 614–617. [Google Scholar]
- Mir, M. Isolation, Characterization and Bioactivities of Sambucus wightiana derived Dotriacontanoic acid. Int. J. Trend Sci. Res. Dev. 2019, 1, 1323–1332. [Google Scholar] [CrossRef]
- KALIMUTHU, S.; Latha, S.; Selvamani, P.; Pandiyan, R.; BALAMURUGAN, B.; CHANDRASEKAR, T.M. Isolation, Characterization and Antibacterial Evaluation on Long Chain Fatty Acids from Limnophila polystachya Benth. Asian J. Chem. 2011, 23, 791–794. [Google Scholar]
- Innocenti, A.; Sarıkaya, S.B.O.; Gülçin, İ.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I–XIV with a series of natural product polyphenols and phenolic acids. Bioorg. Med. Chem. 2010, 18, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Chemical Computing Group Inc. Molecular Operating Environment (MOE); Chemical Computing Group Inc.: Montreal, QC, Canada, 2014. [Google Scholar]
- Biswas, S.; McKenna, R.; Supuran, C.T. Effect of incorporating a thiophene tail in the scaffold of acetazolamide on the inhibition of human carbonic anhydrase isoforms I., II, IX and XII. Bioorg. Med. Chem. Lett. 2013, 23, 5646–5649. [Google Scholar] [CrossRef] [PubMed]
| Compounds | % Inhibition | IC50 ± SEM |
|---|---|---|
| 1 | 79.4 | 44.9 ± 1.10 |
| 2 | 17.9 | NA |
| 3 | 16.6 | NA |
| 4 | 72.8 | 13.4 ± 1.35 |
| 5 | 93.9 | 17.7 ± 2.01 |
| 6 | ND | ND |
| 7 | 88.0 | 16.3 ± 0.31 |
| 8 | ND | ND |
| 9 | 93.9 | 53.1 ± 1.20 |
| 10 | 77.7 | 71.6 ± 1.05 |
| 11 | 20.7 | NA |
| 12 | 90.3 | 11.6 ± 0.56 |
| 13 | 87.3 | 39.5 ± 0.73 |
| 14 | ND | ND |
| 15 | 69.8 | 31.3 ± 1.34 |
| 16 | 37.4 | NA |
| 17 | ND | ND |
| 18 | 77.5 | 17.0 ± 1.32 |
| 19 | 87.3 | 24.3 ±1.38 |
| 20 | 29.5 | NA |
| Acetazolamide | 86.4 | 18.2 ± 1.23 |
| Compounds. | IC50 ± S.E.M | Docking Score (kcal/mol) | Binding Interactions | |||
|---|---|---|---|---|---|---|
| Ligand | Receptor | Bonds | Distance (Å) | |||
| 12 | 11.6 ± 0.56 | −9.53 | O93 | NE2-HIS94 | HBA | 3.15 |
| O94 | N-THR199 | HBA | 3.06 | |||
| O94 | OG1-THR199 | HBA | 3.05 | |||
| O94 | OG1-THR200 | HBA | 3.45 | |||
| O93 | ZN | Ionic | 2.37 | |||
| O93 | ZN | Ionic | 2.37 | |||
| O94 | ZN | Ionic | 3.15 | |||
| 4 | 13.4 ± 1.35 | −5.06 | O14 | NE2-HIS94 | HBA | 3.15 |
| O14 | ZN | Ionic | 2.38 | |||
| 7 | 16.3 ± 0.31 | −9.00 | C8 | 5-ring-HIS94 | H-π | 3.13 |
| 18 | 17.0 ± 1.32 | −9.35 | O78 | NE2-HIS94 | HBA | 2.83 |
| O79 | N-THR199 | HBA | 3.03 | |||
| O78 | ZN | Ionic | 2.30 | |||
| O78 | ZN | Ionic | 2.30 | |||
| 5 | 17.7 ± 2.01 | −5.39 | O18 | N-THR199 | HBA | 3.15 |
| O18 | OG1-THR199 | HBA | 3.44 | |||
| O19 | NE2-HIS94 | HBA | 2.89 | |||
| O19 | NE2-HIS96 | HBA | 3.36 | |||
| O19 | ZN | Ionic | 2.27 | |||
| O19 | ZN | Ionic | 2.27 | |||
| 19 | 24.3 ±1.38 | −9.66 | O83 | N-THR199 | HBA | 3.03 |
| O83 | OG1-THR199 | HBA | 2.90 | |||
| O82 | ZN | Ionic | 2.42 | |||
| O83 | ZN | Ionic | 2.67 | |||
| O82 | ZN | Ionic | 2.42 | |||
| O83 | ZN | Ionic | 2.67 | |||
| 15 | 31.3 ± 1.34 | −5.19 | O61 | NE2-HIS94 | HBD | 2.81 |
| O61 | ZN | Ionic | 2.52 | |||
| 13 | 39.5 ± 0.73 | −5.83 | O14 | NE2-HIS94 | HBA | 3.15 |
| O14 | ZN | Ionic | 2.55 | |||
| 1 | 44.9 ± 1.10 | −5.70 | O19 | N-THR199 | HBA | 3.38 |
| O19 | OG1-THR199 | HBA | 2.90 | |||
| O13 | ZN | Ionic | 2.88 | |||
| O19 | ZN | Ionic | 2.34 | |||
| O19 | ZN | Ionic | 2.34 | |||
| 9 | 53.1 ± 1.20 | −4.58 | O13 | OG1-THR199 | HBA | 3.02 |
| O13 | ZN | Ionic | 2.49 | |||
| 10 | 71.6 ± 1.05 | −9.35 | O66 | NE2-HIS94 | HBA | 2.93 |
| O67 | N-THR199 | HBA | 3.05 | |||
| O66 | ZN | Ionic | 2.26 | |||
| O66 | ZN | Ionic | 2.26 | |||
| Acetazolamide | 18.2 ± 1.23 | −9.47 | N1 | NE2-HIS94 | HBA | 2.89 |
| N1 | NE2-HIS96 | HBA | 3.32 | |||
| N1 | OG1-THR199 | HBA | 3.28 | |||
| O1 | N-THR199 | HBA | 2.90 | |||
| O1 | OG1-THR199 | HBA | 3.54 | |||
| N1 | ZN | Ionic | 1.82 | |||
| N1 | ZN | Ionic | 1.82 | |||
| O2 | ZN | Ionic | 3.33 | |||
| 5-ring | CD2-LEU198 | π-H | 3.75 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, K.; Khan, A.; Ur Rehman, N.; Halim, S.A.; Khan, M.; Ali, L.; Hilal Al-Balushi, A.; Al-Busaidi, H.K.; Al-Harrasi, A. New Carbonic Anhydrase-II Inhibitors from Marine Macro Brown Alga Dictyopteris hoytii Supported by In Silico Studies. Molecules 2021, 26, 7074. https://doi.org/10.3390/molecules26237074
Rafiq K, Khan A, Ur Rehman N, Halim SA, Khan M, Ali L, Hilal Al-Balushi A, Al-Busaidi HK, Al-Harrasi A. New Carbonic Anhydrase-II Inhibitors from Marine Macro Brown Alga Dictyopteris hoytii Supported by In Silico Studies. Molecules. 2021; 26(23):7074. https://doi.org/10.3390/molecules26237074
Chicago/Turabian StyleRafiq, Kashif, Ajmal Khan, Najeeb Ur Rehman, Sobia Ahsan Halim, Majid Khan, Liaqat Ali, Abdullah Hilal Al-Balushi, Haitham Khamis Al-Busaidi, and Ahmed Al-Harrasi. 2021. "New Carbonic Anhydrase-II Inhibitors from Marine Macro Brown Alga Dictyopteris hoytii Supported by In Silico Studies" Molecules 26, no. 23: 7074. https://doi.org/10.3390/molecules26237074
APA StyleRafiq, K., Khan, A., Ur Rehman, N., Halim, S. A., Khan, M., Ali, L., Hilal Al-Balushi, A., Al-Busaidi, H. K., & Al-Harrasi, A. (2021). New Carbonic Anhydrase-II Inhibitors from Marine Macro Brown Alga Dictyopteris hoytii Supported by In Silico Studies. Molecules, 26(23), 7074. https://doi.org/10.3390/molecules26237074











