Synthesis of High-Performance Aqueous Fluorescent Nanodispersions for Textile Printing—A Study of Influence of Moles Ratio on Fastness Properties
Abstract
:1. Introduction
2. Results and Discussions
2.1. UV-Vis Absorption of Rhodamine Dyes
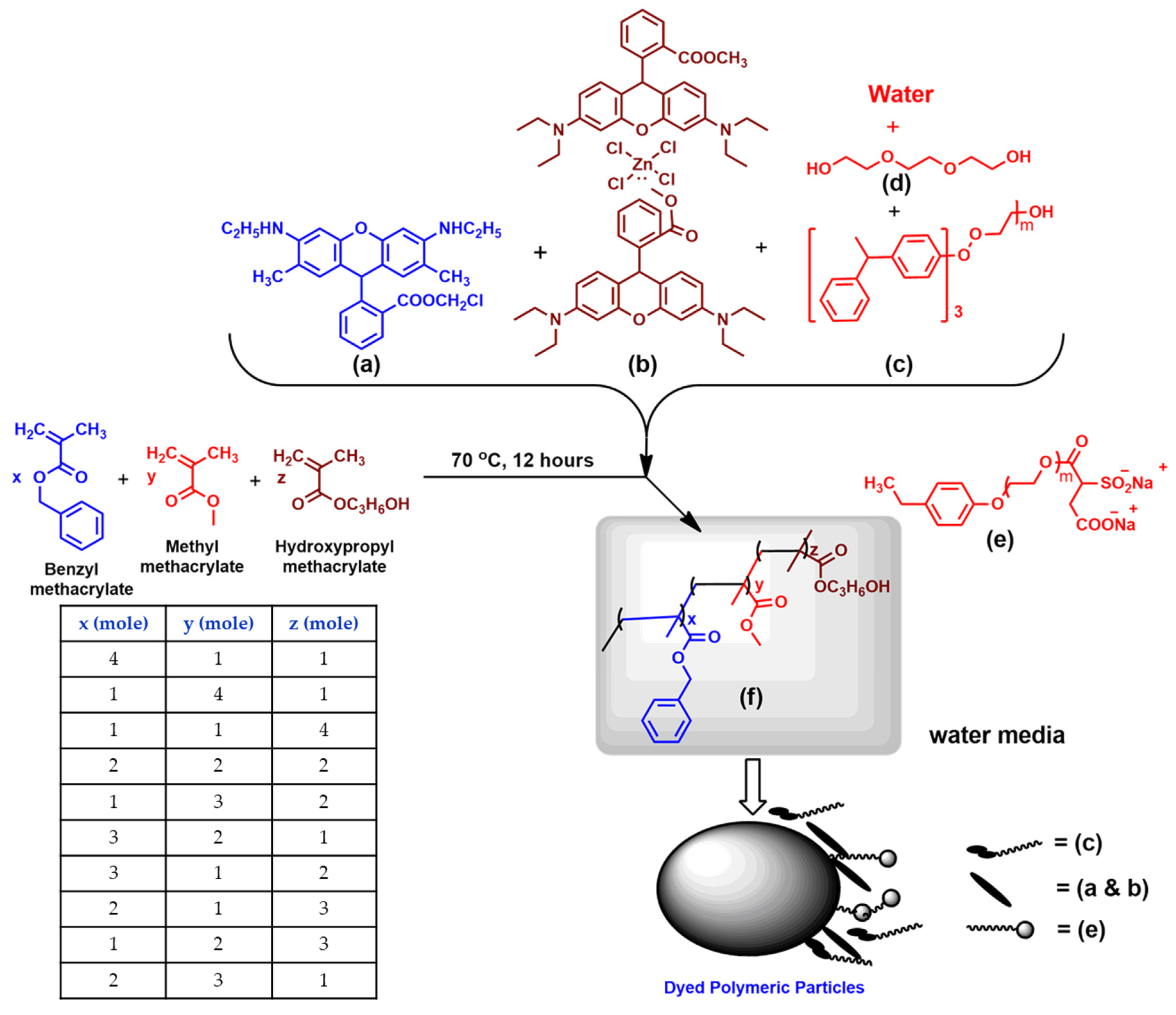
2.2. Synthesis of Fluorescent Dispersions
2.3. FT-IR Analysis of Fluorescent Dispersions
2.4. UV-Vis Absorption of Fluorescent Dispersions
2.5. SEM and TEM Analysis
2.6. Particle Size Distribution Curves
2.7. Wash Fastness and Color Migration Properties
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Fluorescent Dispersions
3.3. Characterization and Measurements
3.3.1. Measurement of Percent Solids
3.3.2. Measurement of Absorbance
3.3.3. Measurement of Particle Size
3.3.4. Observations by SEM and TEM Analysis
3.3.5. Printing Paste
3.3.6. Printing Technique
3.3.7. Measurement of Color Strength
3.3.8. Measurement of Colorfastness to Wash
3.3.9. Measurement of Color Migration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, H.; Sun, J. Wool Fabrics Colored with Fluorescent Pigment Emulsion: Their Color Performance and Fluorescent Properties. J. Nat. Fibers 2020, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P.; Fang, G.; Yi, P.; Yu, X.; Li, X.; Zeng, F.; Wu, S. Synthesis and characterization of novel reversible photoswitchable fluorescent polymeric nanoparticles via one-step miniemulsion polymerization. J. Phys. Chem. B 2011, 115, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, J.; Cui, N.; Yan, X.; Xie, Z.; Qi, D. Polymer/CI Pigment Red 170 hybrid latexes prepared by RAFT-mediated surfactant-free emulsion polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127409. [Google Scholar] [CrossRef]
- Jiang, J.; Shen, Y.; Yu, D.; Wu, M.; Yang, L.; Li, S. Sustainable washing-free printing of disperse dyes on polyester fabrics enabled by crosslinked fluorosilicone modified polyacrylate binders. Polym. Adv. Technol. 2021, 32, 641–650. [Google Scholar] [CrossRef]
- Chatterjee, K.; Ghosh, T.K. 3D printing of textiles: Potential roadmap to printing with fibers. Adv. Mater. 2020, 32, 1902086. [Google Scholar] [CrossRef] [PubMed]
- Christie, R. Chromic materials for technical textile applications. In Advances in the Dyeing and Finishing of Technical Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–36. [Google Scholar]
- Tincher, W.C.; Hu, Q.-A.; Li, X. Ink Jet Systems for Printing Fabric. Text. Chem. Color. 1998, 30, 24–27. [Google Scholar]
- Wang, L.; Cui, S.; Ni, H.; Wu, M. Preparation and application of polyacrylate binder for washing-free printing on polyester with disperse dyes. Text. Res. J. 2019, 89, 2721–2728. [Google Scholar] [CrossRef]
- Phillips, D.; Suesat, J.; Taylor, J.A.; Wilding, M.; Farrington, D.; Bone, J.; Dervan, S. Thermal migration of selected disperse dyes on poly (ethylene terephthalate) and poly (lactic acid)(Ingeo†) fibres. Color. Technol. 2004, 120, 260–264. [Google Scholar] [CrossRef]
- Wang, L.; Cui, S.; Ni, H.; Wu, M.; Wang, W. New washing-free printing binder based on organosilicon-modified polyacrylate for polyester fabric printing of disperse dyes. Prog. Org. Coat. 2018, 123, 75–81. [Google Scholar] [CrossRef]
- Guan, Y.; Mao, Y.-H.; Zheng, Q.-K.; Zheng, G.-H.; Tian, T. Transfer printing with disperse dyes on cotton fabric modified with an aqueous tolylene diisocyanate derivative. Fibers Polym. 2009, 10, 488–495. [Google Scholar] [CrossRef]
- Babendure, J.R.; Adams, S.R.; Tsien, R.Y. Aptamers switch on fluorescence of triphenylmethane dyes. J. Am. Chem. Soc. 2003, 125, 14716–14717. [Google Scholar] [CrossRef] [PubMed]
- Griffin, R.; Speers, S.; Elliott, L.; Todd, N.; Sogomo, W.; Kee, T. An improved high-performance liquid chromatography system for the analysis of basic dyes in forensic casework. J. Chromatogr. A 1994, 674, 271–280. [Google Scholar] [CrossRef]
- Shao, C.; Xiao, F.; Guo, H.; Yu, J.; Jin, D.; Wu, C.; Xi, L.; Tian, L. Utilizing polymer micelle to control dye J-aggregation and enhance its theranostic capability. Iscience 2019, 22, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, P.; Srivastava, R.; Koner, R.R.; Pramanik, A.; Mathew, J.; Sinha, S.; Rawat, M.; Anand, R.; Ghosh, S. Engineering fused coumarin dyes: A molecular level understanding of aggregation quenching and tuning electroluminescence via alkyl chain substitution. J. Mater. Chem. C 2014, 2, 6637–6647. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, V.J.; Ratcliffe, L.P.; Blanazs, A.; Warren, N.J.; Smith, A.J.; Mykhaylyk, O.O.; Armes, S.P. Tuning the critical gelation temperature of thermo-responsive diblock copolymer worm gels. Polym. Chem. 2014, 5, 6307–6317. [Google Scholar] [CrossRef]
- Podkocielna, B.; Bartnicki, A.; Gawdzik, B. New crosslinked hydrogels derivatives of 2-hydroxyethyl methacrylate: Synthesis, modifications and properties. Express Polym. Lett. 2012, 6, 759–771. [Google Scholar] [CrossRef]
- Canning, S.L.; Neal, T.J.; Armes, S.P. pH-responsive schizophrenic diblock copolymers prepared by polymerization-induced self-assembly. Macromolecules 2017, 50, 6108–6116. [Google Scholar] [CrossRef] [Green Version]
- Mable, C.; Thompson, K.; Derry, M.; Mykhaylyk, O.; Binks, B.; Armes, S. ABC triblock copolymer worms: Synthesis, characterization, and evaluation as pickering emulsifiers for millimeter-sized droplets. Macromolecules 2016, 49, 7897–7907. [Google Scholar] [CrossRef]
- Ho, K.M.; Li, W.Y.; Wong, C.H.; Li, P. Amphiphilic polymeric particles with core–shell nanostructures: Emulsion-based syntheses and potential applications. Colloid Polym. Sci. 2010, 288, 1503–1523. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Qureshi, U.F. Preparation and characterization of crosslinked acrylic acid/hydroxypropyl methyl cellulose hydrogels for drug delivery. Int. J. Pharm. Pharm. Sci. 2014, 6, 410. [Google Scholar]
- Jassal, M.; Bajaj, P. Developments in acrylic-based thickeners as substitute of emulsion thickeners for pigment printing. Indian J. Ffibre Text. Res. 2001, 26, 143–155. [Google Scholar]
- Abdel-Halim, E.; Emam, H.; El-Rafie, M. Utilization of hydroxypropyl cellulose and poly (acrylic acid)-hydroxypropyl cellulose composite as thickeners for textile printing. Carbohydr. Polym. 2008, 74, 938–941. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizao, C.; Farinha, J.P.S. Synthesis and Characterization of Perylenediimide Labeled Core− Shell Hybrid Silica− Polymer Nanoparticles. J. Phys. Chem. C 2009, 113, 18082–18090. [Google Scholar] [CrossRef]
- Kutanaee, H.N.; Aghaee, H.R. Synthesis and characterization of methyl methaacrylate and 2-methaacrylate and their application on pigment printing textile fabrics. Afr. J. Microbiol. Res. 2011, 5, 359–364. [Google Scholar]
- Kazemi, S.; Daryani, A.S.; Abdouss, M.; Shariatinia, Z. DFT computations on the hydrogen bonding interactions between methacrylic acid-trimethylolpropane trimethacrylate copolymers and letrozole as drug delivery systems. J. Theor. Comput. Chem. 2016, 15, 1650015. [Google Scholar] [CrossRef]
- Yu, X.; Yu, W.; Wang, X. DFT-based quantum theoretic QSPR studies of the glass transition temperatures of polyacrylates. J. Struct. Chem. 2009, 50, 821–826. [Google Scholar] [CrossRef]
- Ristić, N.; Šmelcerović, M.; Ristić, I. The effect of nonionic surfactant treatment on dyeing of cotton fabrics. Tekstil J. Text. Cloth. Technol. 2013, 62, 1–7. [Google Scholar]
- Sahil, M.; Khan, M.M.; Pervez, M.; Anshu, C.; Habib, M.; Quan, H. Synthesis of Polyacrylate Binder by Emulsion Polymerization and Application on Cotton. Asian J. Chem. 2017, 29. [Google Scholar] [CrossRef]
- Gupta, B.; Singh, H. Polymerization in microemulsion systems. Polym. Plast. Technol. Eng. 1992, 31, 635–658. [Google Scholar] [CrossRef]
- Najafi, H.; Yazdanshenas, M.E.; Rashidi, A.; Montazer, M. Synthesis and characterization of styrene-acrylic binders and their application on pigment printing of cotton and polyester textile fabrics. Asian J. Chem. 2009, 21, 4871. [Google Scholar]
- Shunmukham, S.; Hallenbeck, V.; Guile, R. Emulsion polymerization of styrene. II. Effect of agitation. J. Polym. Sci. 1951, 6, 691–698. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Krishnan, S.; Klein, A.; El-Aasser, M.; Sudol, E. Agitation effects in emulsion copolymerization of n-butyl methacrylate and N-methylol acrylamide. Polym. React. Eng. 2003, 11, 335–357. [Google Scholar] [CrossRef]
- Choudhary, M.S.; Varma, I. Copolymerizations of 2-hydroxyethyl methacrylate with alkyl methacrylates. Eur. Polym. J. 1979, 15, 957–959. [Google Scholar] [CrossRef]
- Varma, I.; Patnaik, S. Copolymers of 2-hydroxyethyl methacrylate and alkyl acrylates: Studies of molecular weight distribution and thermal behavior. J. Polym. Sci. Polym. Chem. 1979, 17, 3279–3289. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.M.; Liras, M.; Quijada-Garrido, I.; Gallardo, A.; Paris, R. Swelling control in thermo-responsive hydrogels based on 2-(2-methoxyethoxy) ethyl methacrylate by crosslinking and copolymerization with N-isopropylacrylamide. Polym. J. 2011, 43, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Kücük, I.; Kuyulu, A.; Okay, O. Effect of diluents on the porous structure of crosslinked poly (methyl methacrylate) beads. Polym. Bull. 1995, 35, 511–516. [Google Scholar] [CrossRef]
- Klein, S.M.; Manoharan, V.N.; Pine, D.J.; Lange, F.F. Preparation of monodisperse PMMA microspheres in nonpolar solvents by dispersion polymerization with a macromonomeric stabilizer. Colloid. Polym. Sci. 2003, 282, 7–13. [Google Scholar] [CrossRef]
- Tanrisever, T.; Okay, O.; Sönmezoğlu, I.Ç. Kinetics of emulsifier–free emulsion polymerization of methyl methacrylate. J. Appl. Polym. Sci. 1996, 61, 485–493. [Google Scholar] [CrossRef]
- Arunkumar, E.; Forbes, C.C.; Smith, B.D. Improving the properties of organic dyes by molecular encapsulation. Eur. J. Org. Chem. 2005, 2005, 4051–4059. [Google Scholar] [CrossRef]
- Cakal, E.; Cavus, S. Novel poly (N-vinylcaprolactam-co-2-(diethylamino) ethyl methacrylate) gels: Characterization and detailed investigation on their stimuli-sensitive behaviors and network structure. Ind. Eng. Chem. Res. 2010, 49, 11741–11751. [Google Scholar] [CrossRef]
- Gao, H.; Miasnikova, A.; Matyjaszewski, K. Effect of cross-linker reactivity on experimental gel points during ATRcP of monomer and cross-linker. Macromolecules 2008, 41, 7843–7849. [Google Scholar] [CrossRef]
- Lok, K.P.; Ober, C.K. Particle size control in dispersion polymerization of polystyrene. Can. J. Chem. 1985, 63, 209–216. [Google Scholar] [CrossRef]
- Shenava, S.M.; Amin, A.B.; Karant, R.M.; Venkata, S.J.; Ganugula, R. Synthesis of new rhodamine dyed copolymer nanodispersions for textiles-agglomeration and control with copolymer resins. Dyes Pigments 2016, 133, 424–434. [Google Scholar] [CrossRef]
- Smith, G.N.; Cunningham, V.J.; Canning, S.L.; Derry, M.J.; Cooper, J.; Washington, A.; Armes, S.P. Spin-echo small-angle neutron scattering (SESANS) studies of diblock copolymer nanoparticles. Soft Matter 2019, 15, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Raghavan, S.R.; Walls, H.J.; Khan, S.A. Rheology of silica dispersions in organic liquids: New evidence for solvation forces dictated by hydrogen bonding. Langmuir 2000, 16, 7920–7930. [Google Scholar] [CrossRef]
- Song, Y.; Fang, K.; Ren, Y.; Tang, Z.; Wang, R.; Chen, W.; Xie, R.; Shi, Z.; Hao, L. Inkjet printable and self-curable disperse dyes/P (St-BA-MAA) nanosphere inks for both hydrophilic and hydrophobic fabrics. Polymers 2018, 10, 1402. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, D.Y.; Lee, K.; Choe, S. Effect of crosslinking agents on the morphology of polymer particles produced by one-step seeded polymerization. Macromol. Res. 2009, 17, 250–258. [Google Scholar] [CrossRef]
- Musnickas, J.; Rupainytė, V.; Treigienė, R.; Ragelienė, L. Dye migration influences on colour: Characteristics of wool fabric dyed with acid dye. Fibres Text. East. Eur. 2005, 13, 65–69. [Google Scholar]














| BZMA (g) | MMA (g) | HPMA (g) | FD | BR | FD | BV | ||
|---|---|---|---|---|---|---|---|---|
| λmax (nm) | Abs. Max. | λmax (nm) | Abs. Max. | |||||
| 64.08 | 9.10 | 13.11 | BRD411 | 532.5 | 1.554 | BVD411 | 564.0 | 2.321 |
| 16.00 | 36.41 | 13.11 | BRD141 | 531.9 | 0.782 | BVD141 | 561.9 | 2.113 |
| 16.00 | 9.10 | 52.42 | BRD114 | 532.4 | 1.269 | BVD114 | 564.0 | 2.359 |
| 32.00 | 18.20 | 26.21 | BRD222 | 531.9 | 1.280 | BVD222 | 563.1 | 2.272 |
| 16.00 | 27.31 | 26.21 | BRD132 | 532.0 | 1.186 | BVD132 | 562.5 | 2.255 |
| 48.00 | 18.20 | 13.11 | BRD321 | 532.0 | 1.282 | BVD321 | 563.9 | 2.260 |
| 48.00 | 9.10 | 26.21 | BRD312 | 531.9 | 1.193 | BVD312 | 564.4 | 2.446 |
| 32.00 | 9.10 | 39.32 | BRD213 | 532.4 | 1.129 | BVD213 | 563.9 | 2.260 |
| 16.00 | 18.20 | 39.32 | BRD123 | 532.0 | 1.190 | BVD123 | 563.0 | 2.299 |
| 32.08 | 27.30 | 13.10 | BRD231 | 532.0 | 1.058 | BVD231 | 562.0 | 2.215 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shenava, S.M.; Kumar, J.V.S.; Ganugula, R.; Shaik, M.R.; Busquets, R.; Khan, M.R. Synthesis of High-Performance Aqueous Fluorescent Nanodispersions for Textile Printing—A Study of Influence of Moles Ratio on Fastness Properties. Molecules 2021, 26, 7075. https://doi.org/10.3390/molecules26237075
Shenava SM, Kumar JVS, Ganugula R, Shaik MR, Busquets R, Khan MR. Synthesis of High-Performance Aqueous Fluorescent Nanodispersions for Textile Printing—A Study of Influence of Moles Ratio on Fastness Properties. Molecules. 2021; 26(23):7075. https://doi.org/10.3390/molecules26237075
Chicago/Turabian StyleShenava, Shruthi Manjunath, J. V. Shanmukha Kumar, Rajkumar Ganugula, Mohammed Rafi Shaik, Rosa Busquets, and Mohammad Rizwan Khan. 2021. "Synthesis of High-Performance Aqueous Fluorescent Nanodispersions for Textile Printing—A Study of Influence of Moles Ratio on Fastness Properties" Molecules 26, no. 23: 7075. https://doi.org/10.3390/molecules26237075
APA StyleShenava, S. M., Kumar, J. V. S., Ganugula, R., Shaik, M. R., Busquets, R., & Khan, M. R. (2021). Synthesis of High-Performance Aqueous Fluorescent Nanodispersions for Textile Printing—A Study of Influence of Moles Ratio on Fastness Properties. Molecules, 26(23), 7075. https://doi.org/10.3390/molecules26237075








