Organic Nanoplatforms for Iodinated Contrast Media in CT Imaging
Abstract
1. Introduction
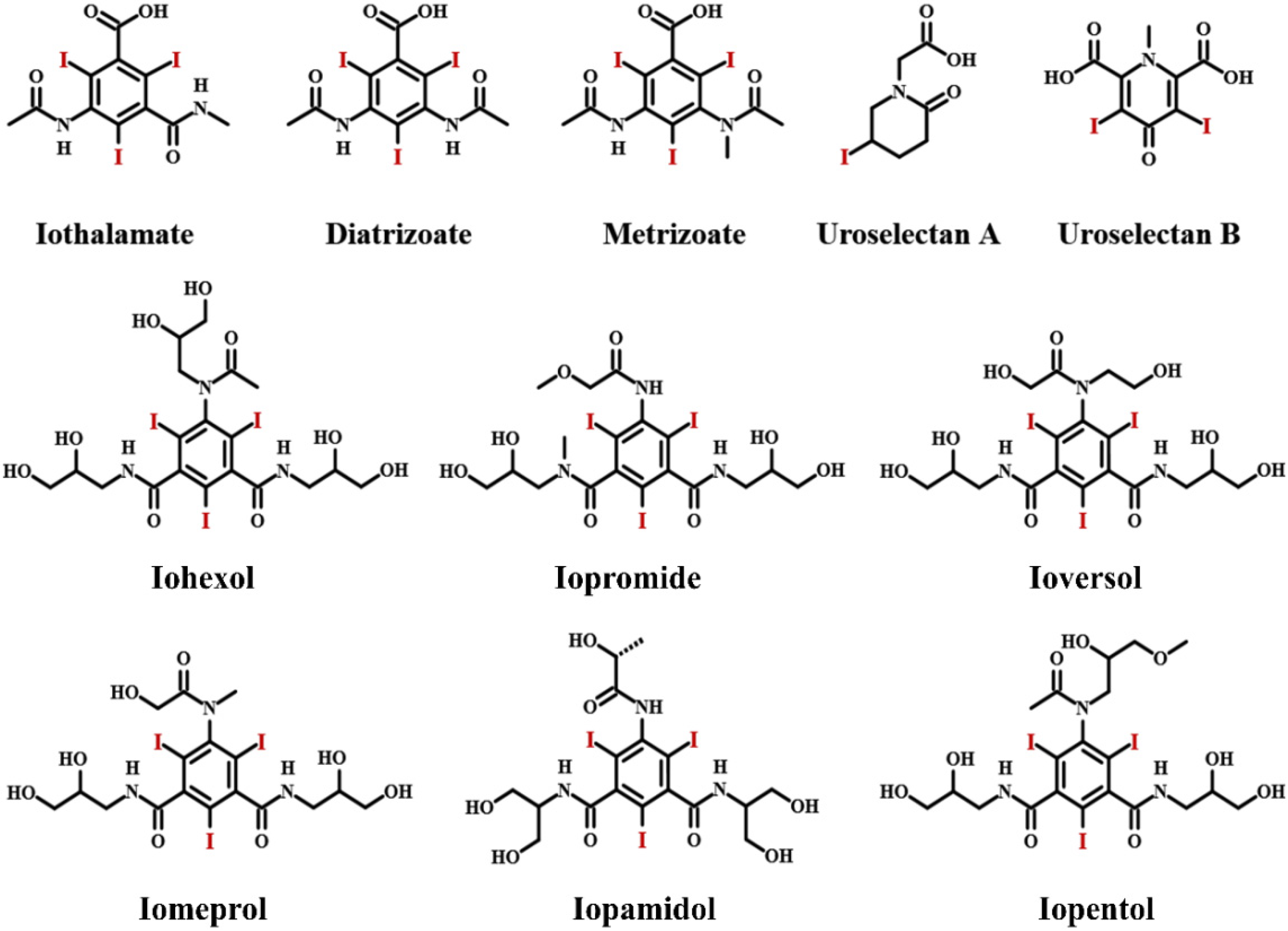
2. Iodinated Contrast Media
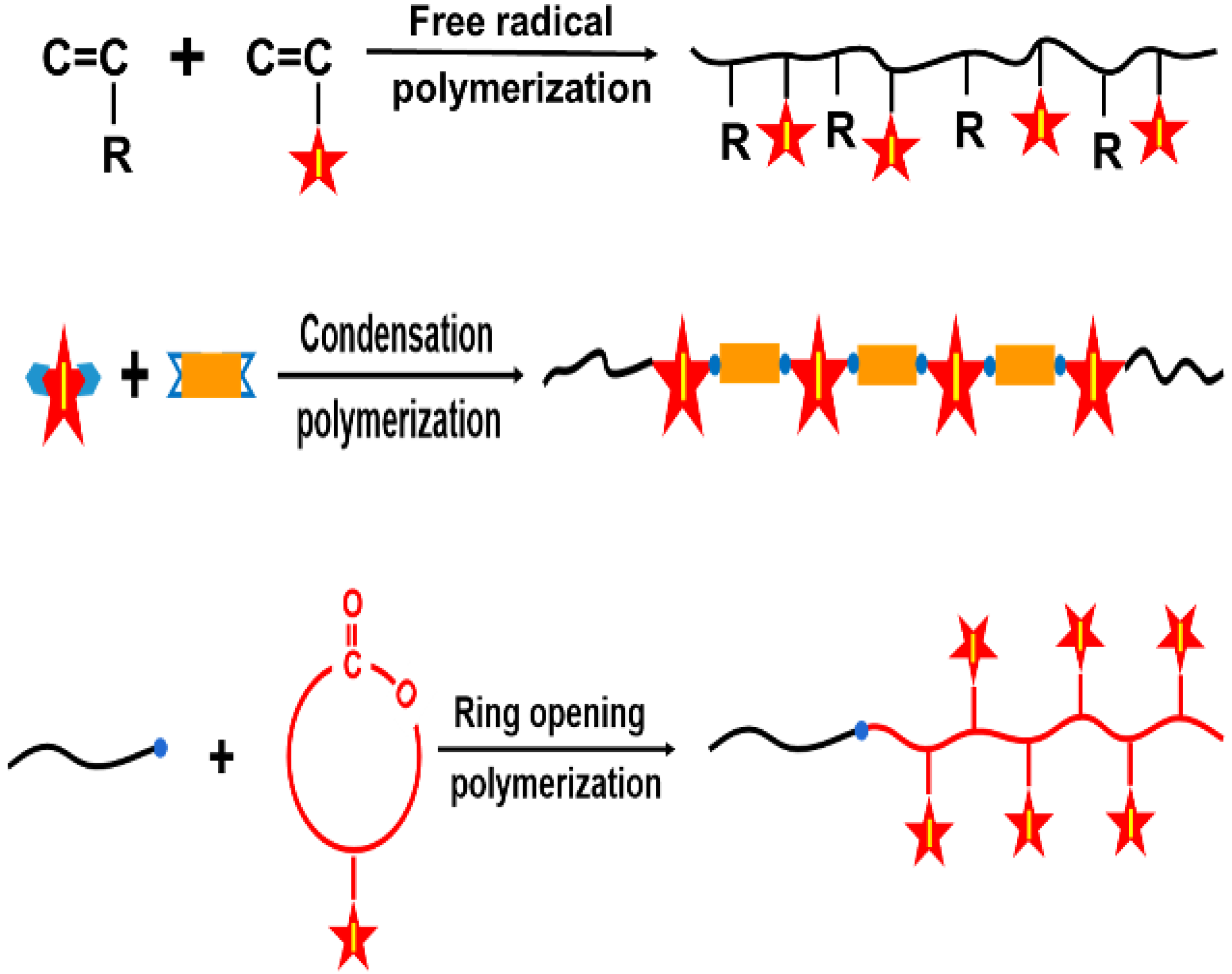
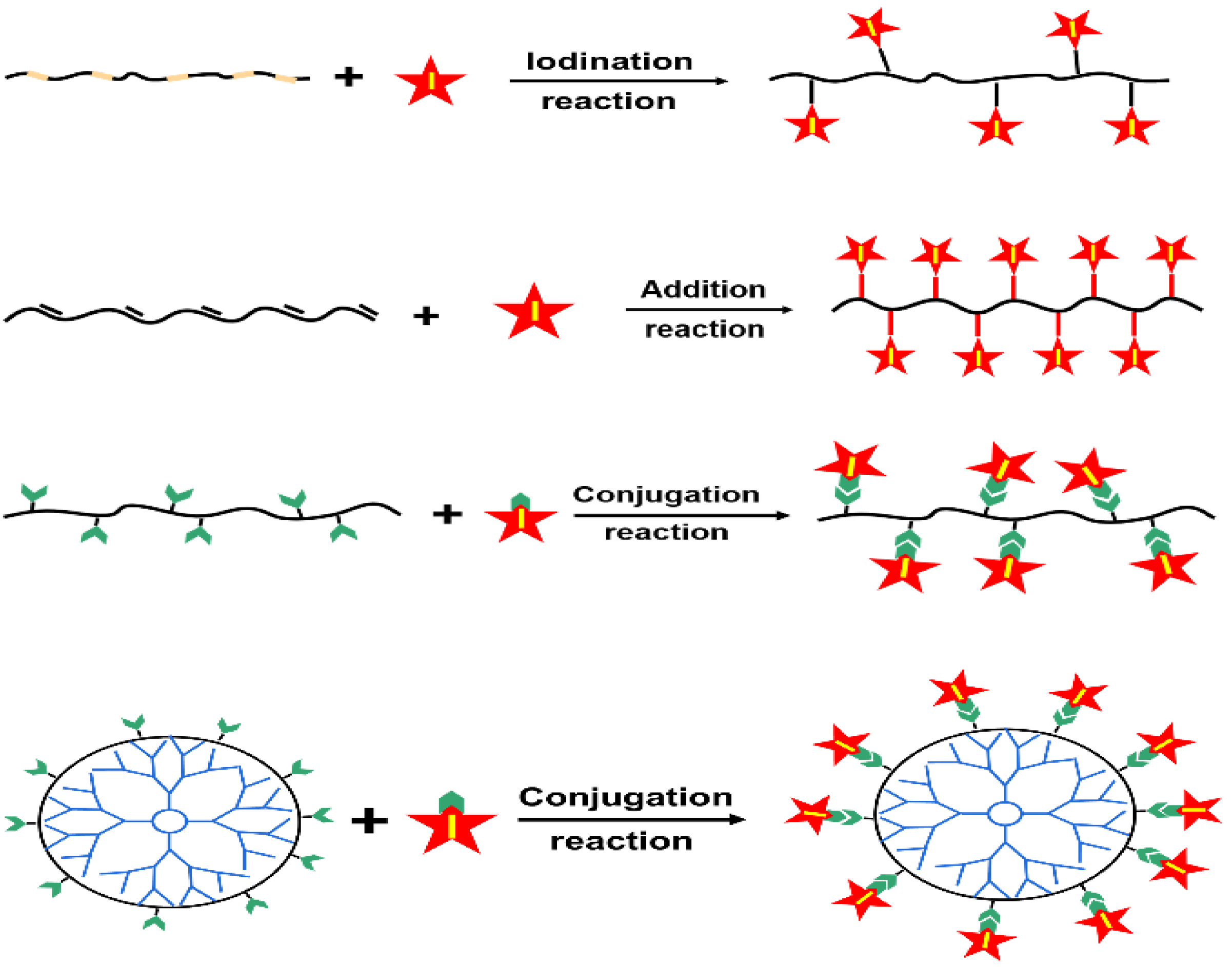
3. Iodinated Macromolecular Contrast Media
4. Organic Nanoparticles for ICMs Delivery
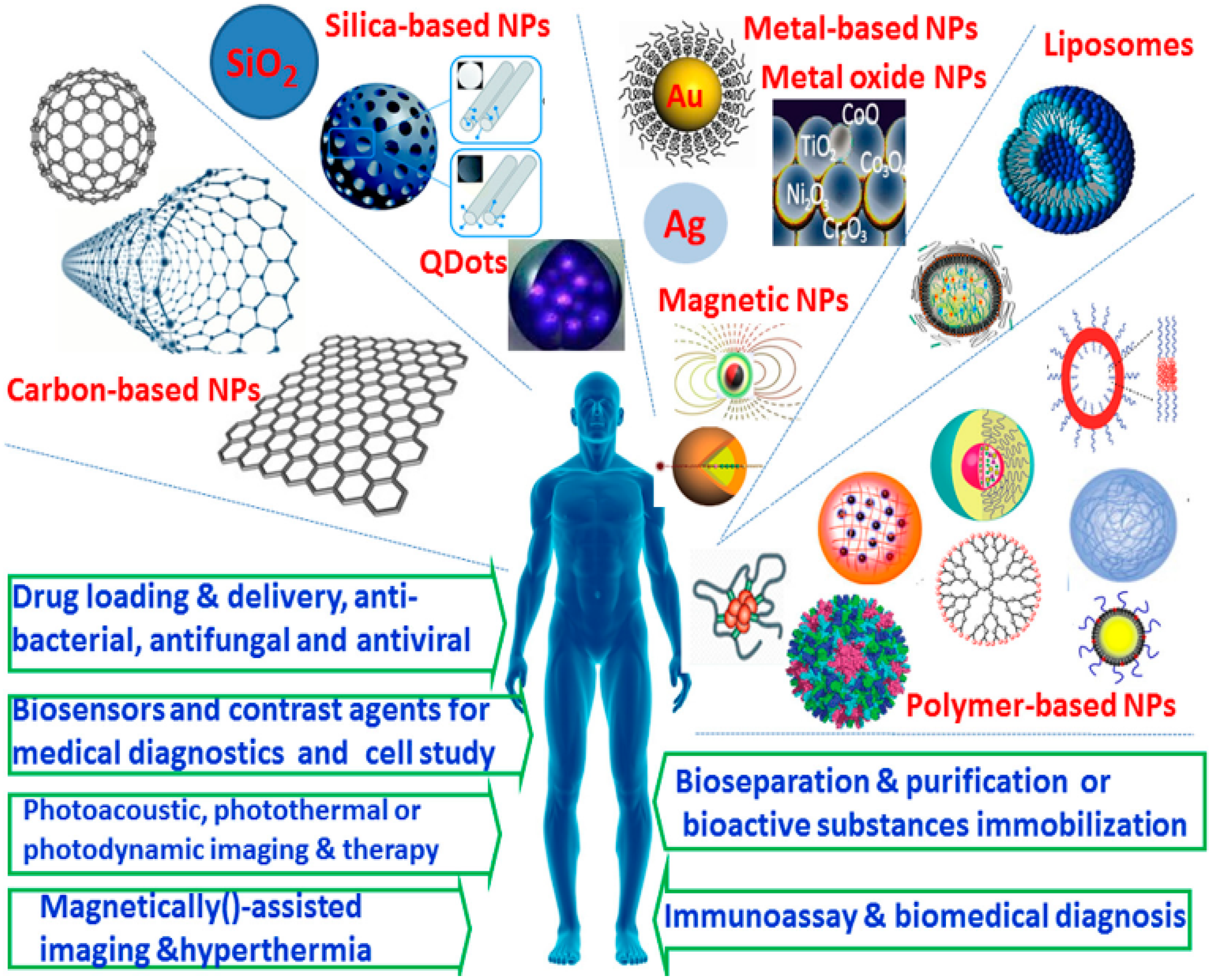
4.1. Nanoparticles for Biomedical Applications
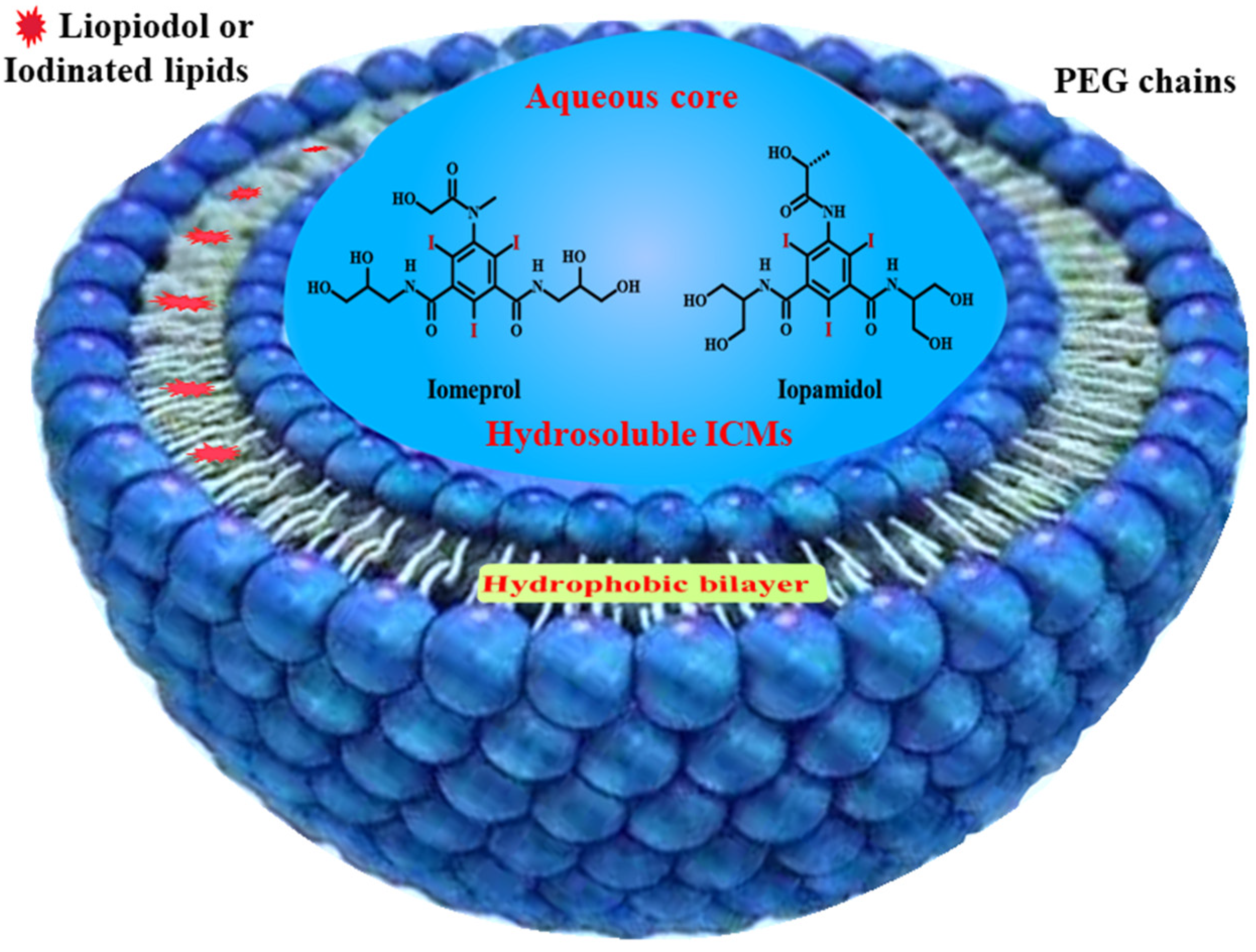
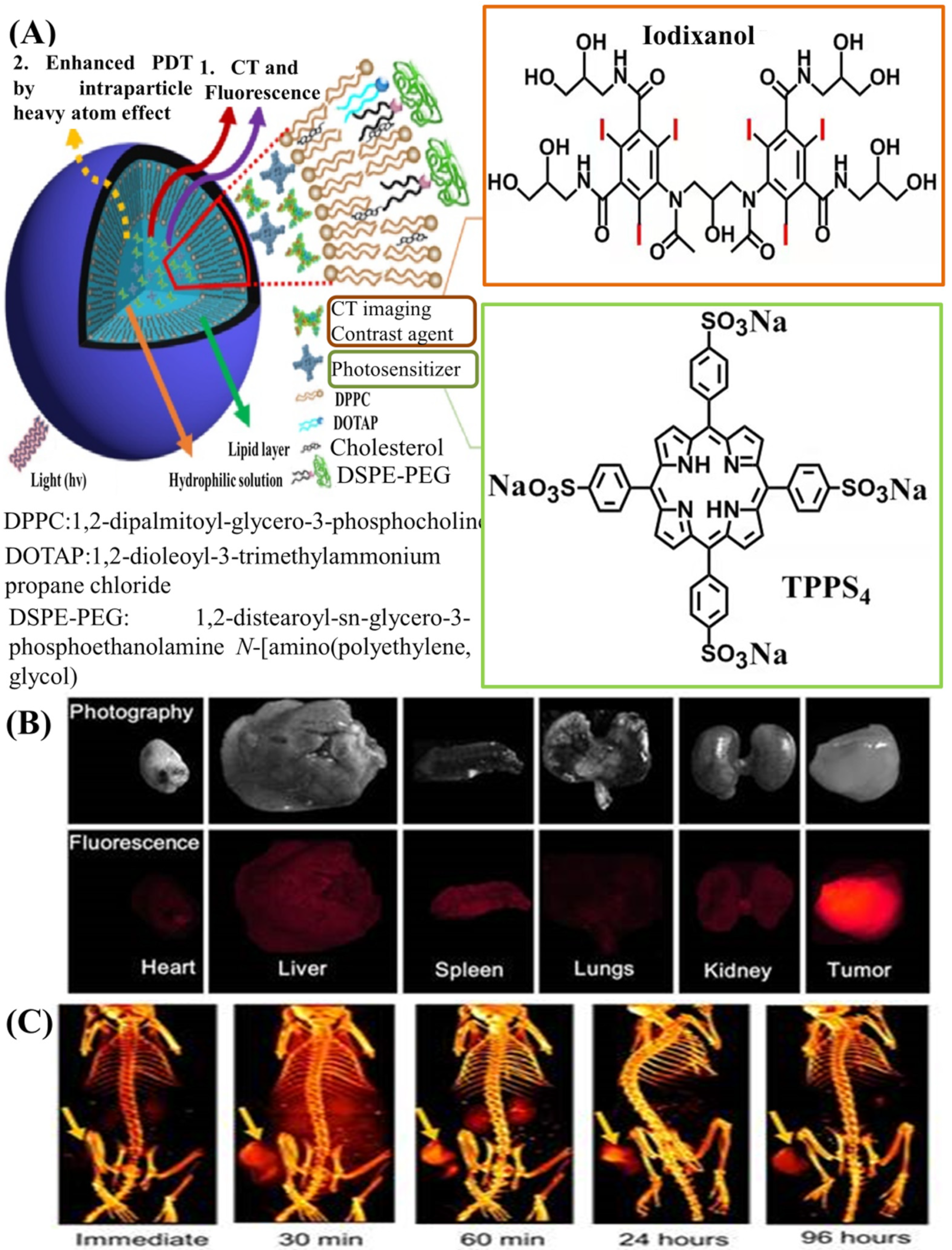
4.2. Liposomes for ICMs Delivery
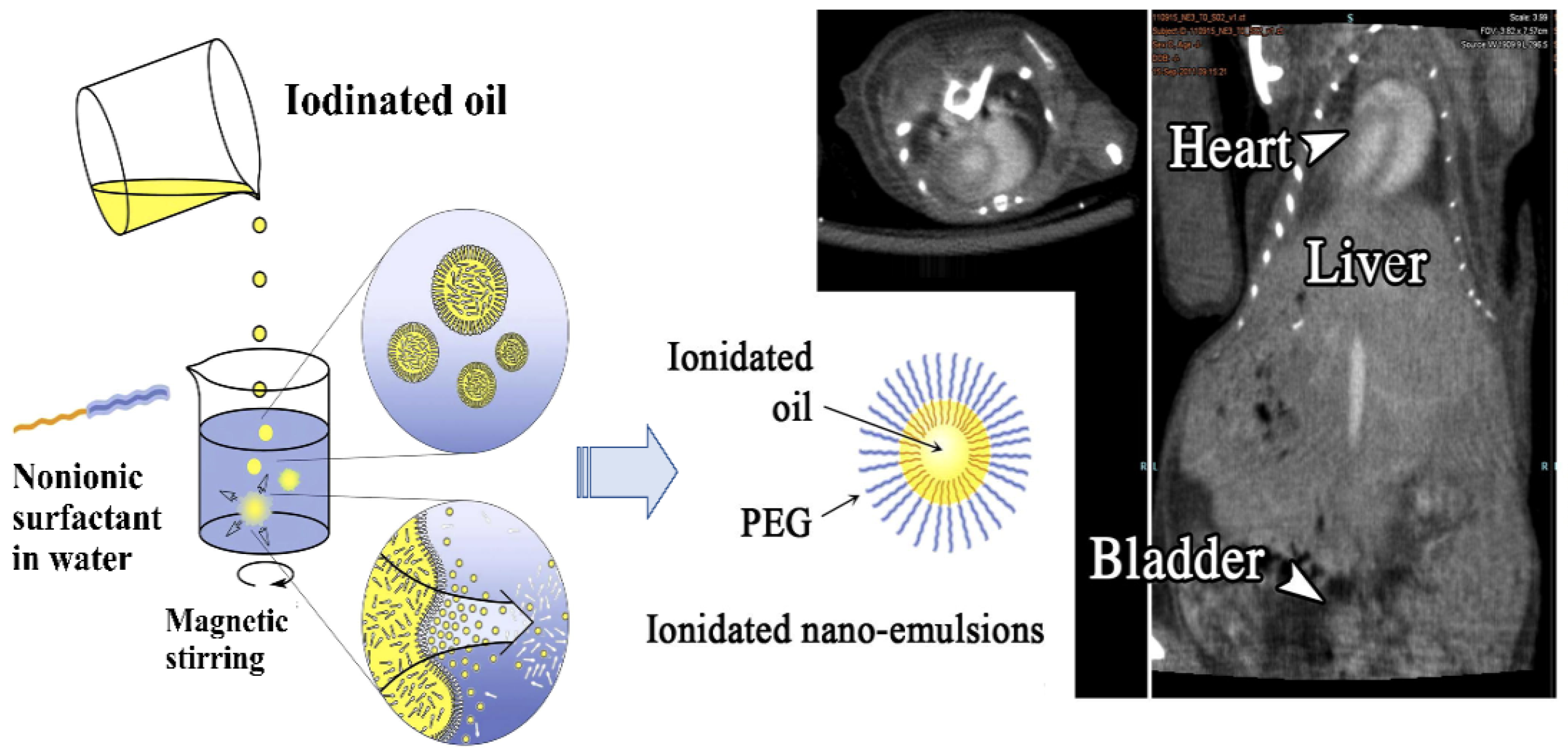
4.3. Nanoemulsions for ICMs Delivery
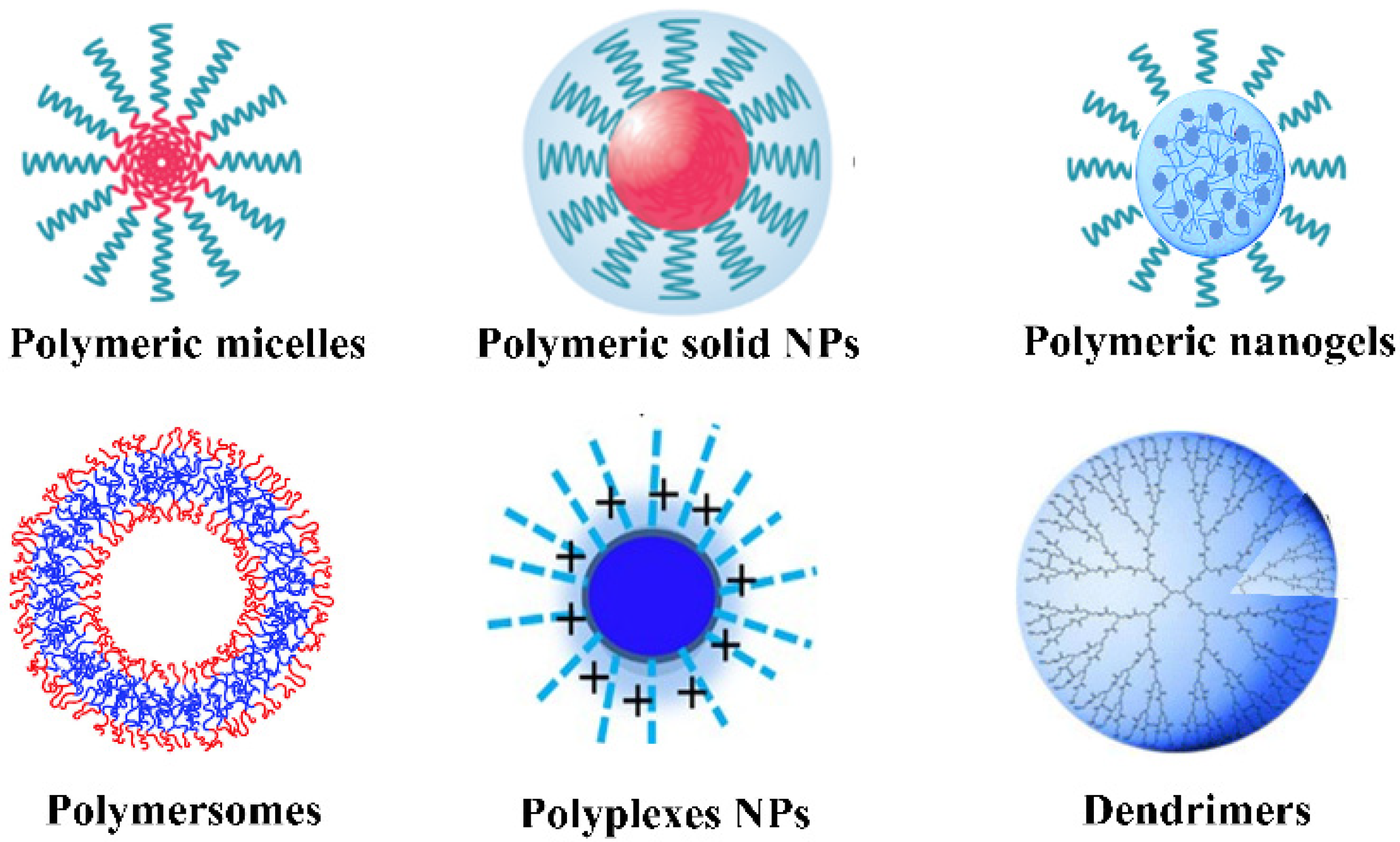
4.4. Polymeric Nanoparticles for ICMs Delivery
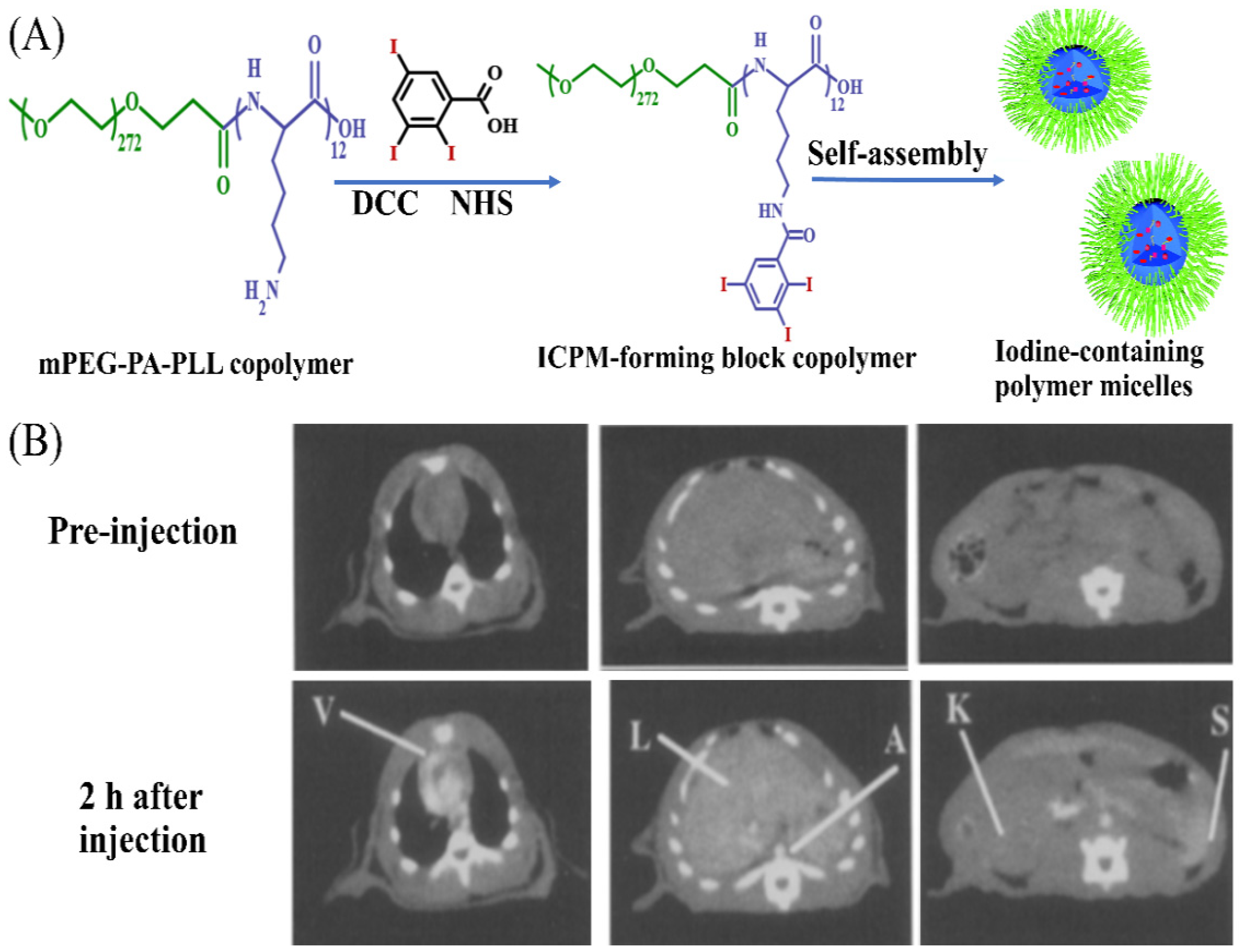
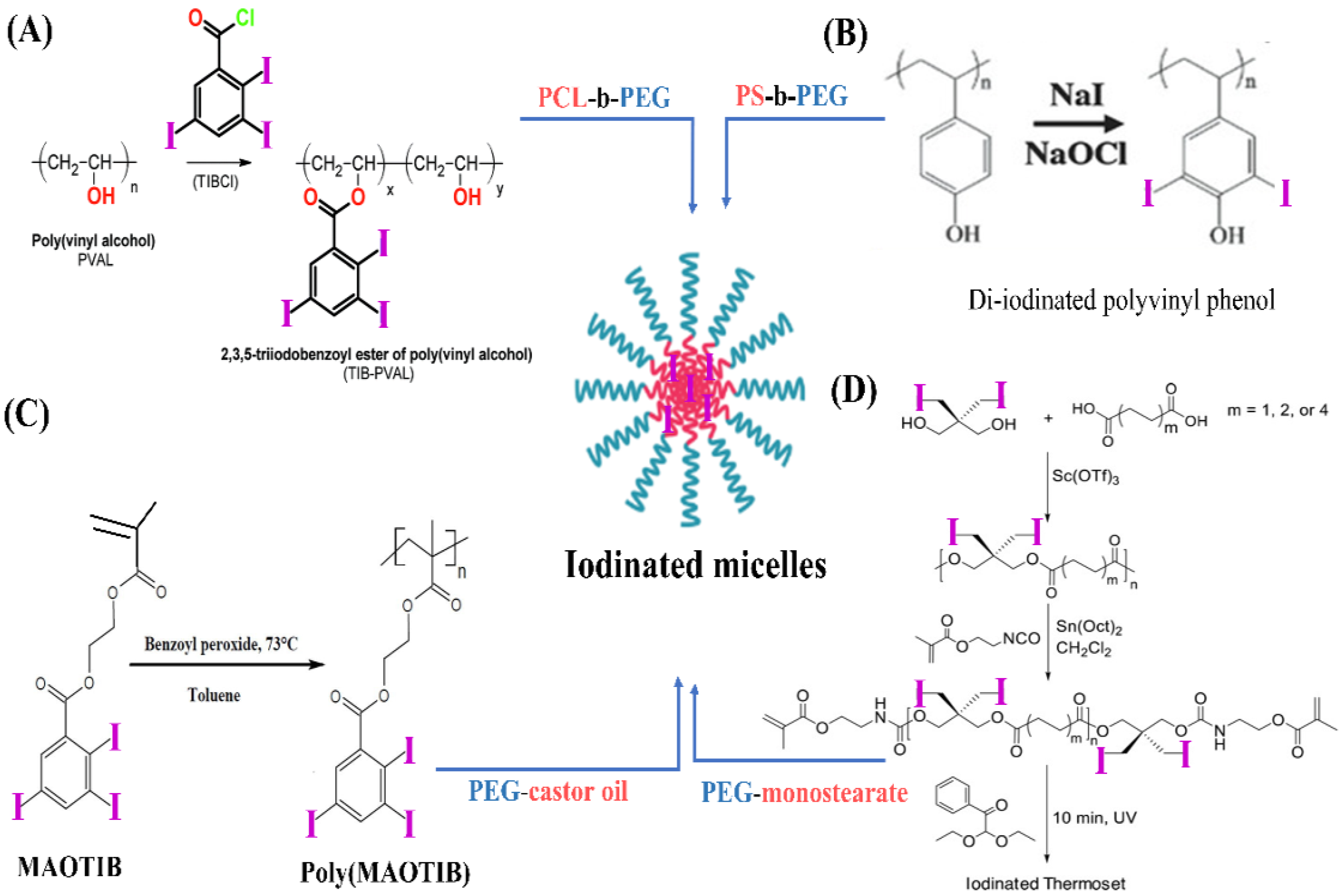
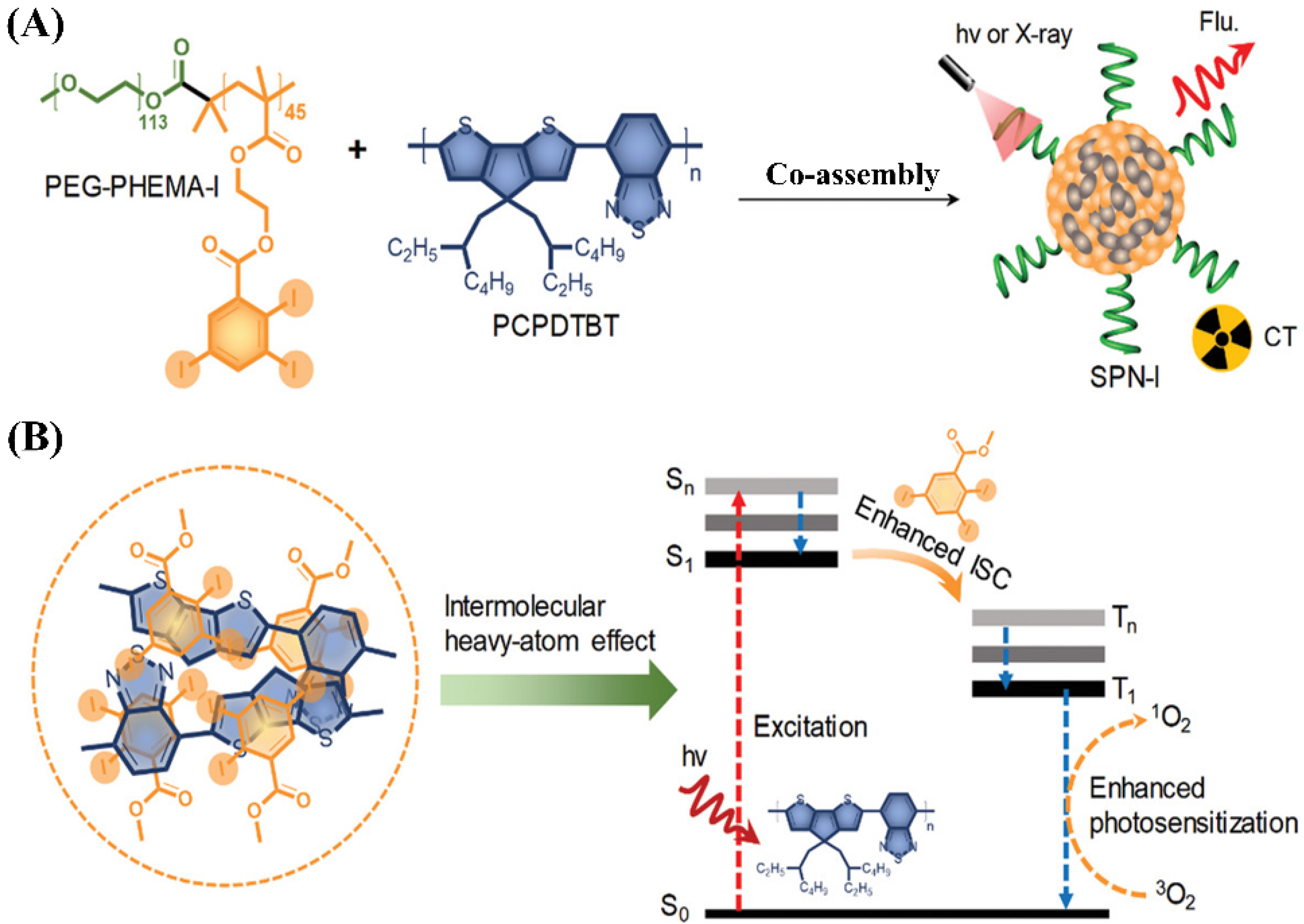
4.4.1. Polymeric Micelles
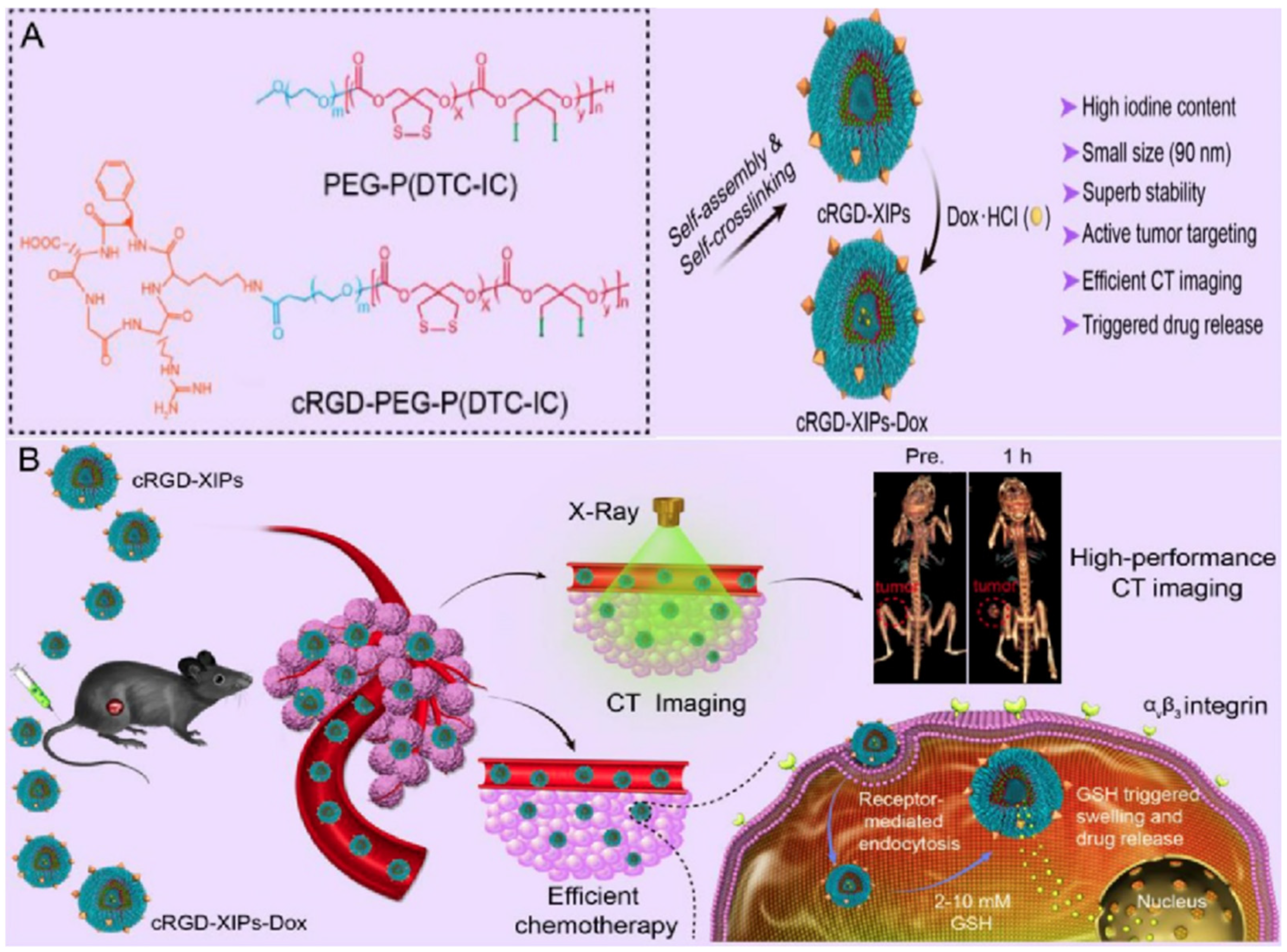
4.4.2. Polymersomes
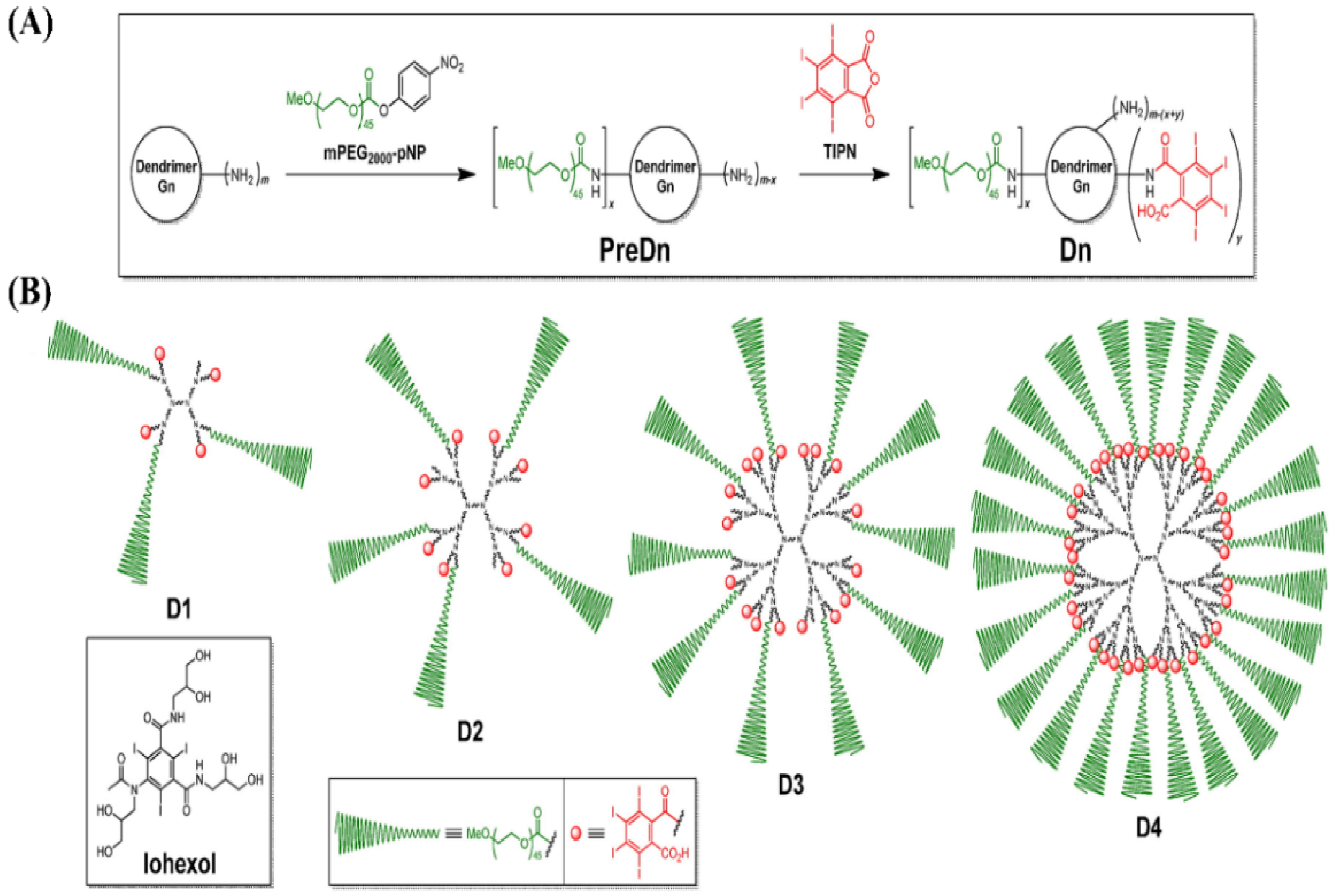
4.4.3. Dendrimers
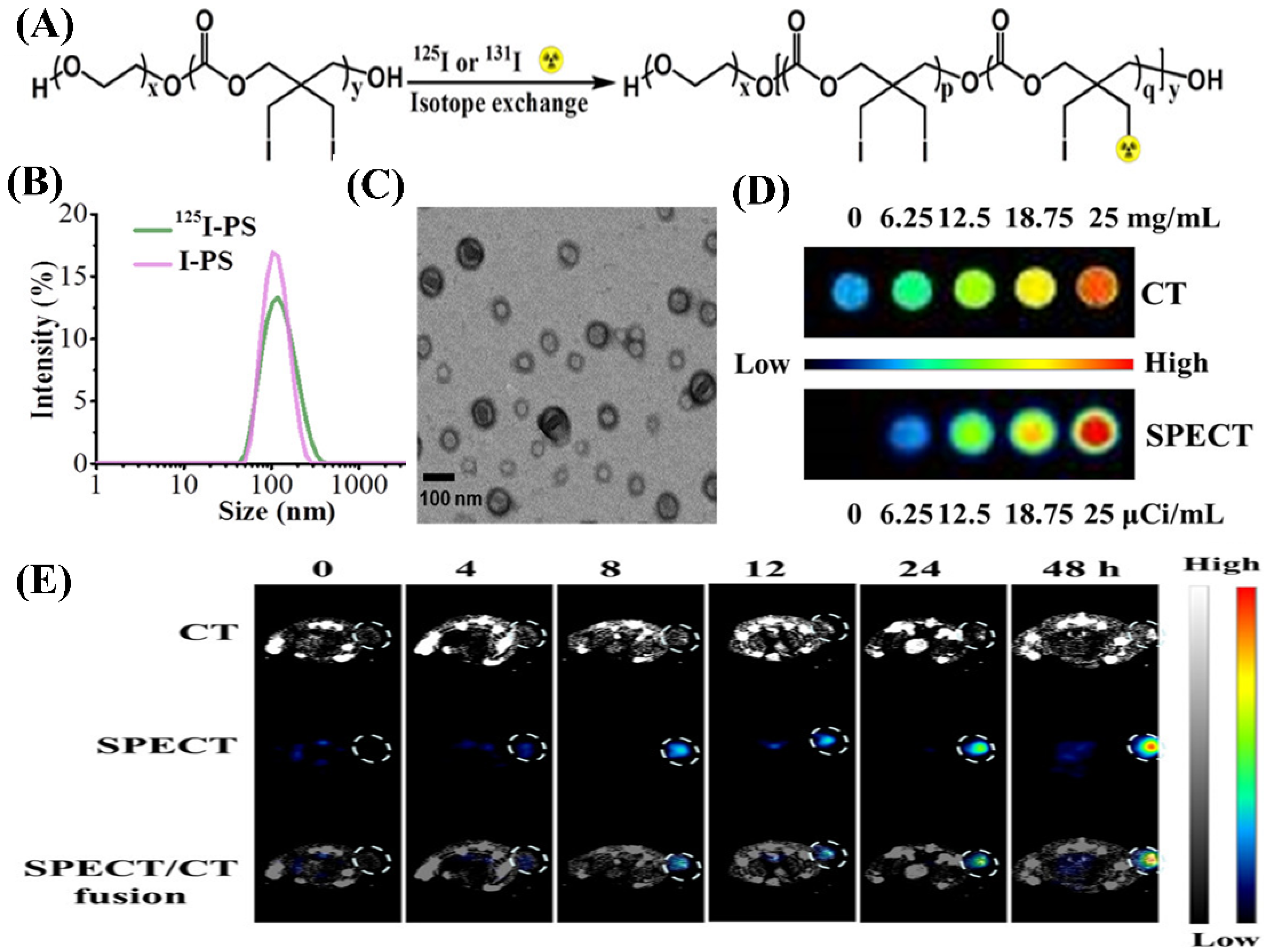
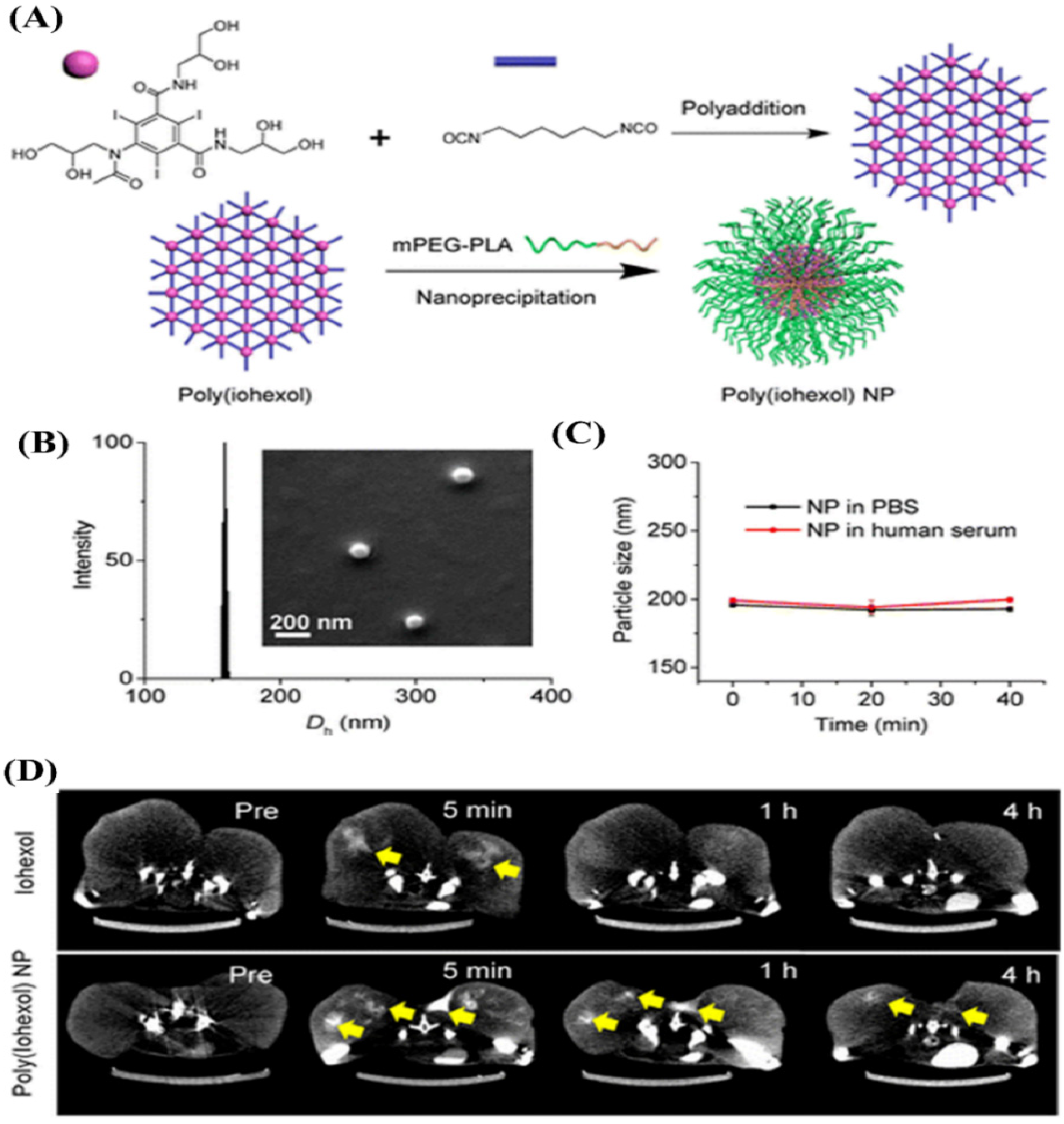
4.4.4. Polymeric Solid Nanoparticles
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Tempany, C.M.C.; McNeil, B.J. Advances in biomedical imaging. J. Am. Med. Assoc. 2001, 285, 562–567. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Akram, S.; Vandamme, T.F. Biomedical imaging: Principles, technologies, clinical aspects, contrast agents, limitations and future trends in nanomedicines. Pharm. Res. 2019, 36, 78. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.M.; Thomson, A.J.W. Preclinical ultrasound imaging—A review of techniques and imaging applications. Front. Phys. 2020, 8, 124. [Google Scholar] [CrossRef]
- Armana, R.; Habibb, Z. PET versus SPECT: Strengths, limitations and challenges. Nucl. Med. Commun. 2008, 29, 193–207. [Google Scholar] [CrossRef]
- Leeuwen, F.W.B.V.; Hardwick, J.C.H.; Erkel, A.R.V. Luminescence-based imaging approaches in the field of interventional molecular imaging. Radiology 2015, 276, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Gu, Y.; Basu, S.; Berglund, A.; Eschrich, S.A.; Schabath, M.B.; Forster, K.; Aerts, H.J.W.L.; Dekker, A.; Fenstermacher, D.; et al. Radiomics: The process and the challenges. Magn. Reson. Imaging. 2012, 30, 1234–1248. [Google Scholar] [CrossRef]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-sized CT contrast agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef] [PubMed]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef]
- LIU, Y.; AI, K.; LU, L. Nanoparticulate X-ray computed tomography contrast agents: From design validation to in vivo applications. Acc. Chem. Res. 2012, 45, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, J.C.; Hafeli, U.O. Utilization of nanoparticles as X-ray contrast agents for diagnostic imaging applications. Contrast. Media Mol. I 2015, 10, 81–95. [Google Scholar] [CrossRef]
- Gignac, P.M.; Kley, N.J.; Clarke, J.A.; Colbert, M.W.; Morhardt, A.C.; Cerio, D.; Cost, I.N.; Cox, P.G.; Daza, J.D.; Early, C.M.; et al. Diffusible iodine-based contrast-enhanced computed tomography (diceCT): An emerging tool for rapid, high-resolution, 3-D imaging of metazoan soft tissues. J. Anat. 2016, 228, 889–909. [Google Scholar] [CrossRef]
- Koc, M.M.; Aslan, N.; Kao, A.P.; Barber, A.H. Evaluation of X-ray tomography contrast agents: A review of production, protocols, and biological applications. Microsc. Res. Techniq. 2019, 82, 812–848. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Zuber, G.; Vandamme, T. Contrast agents for preclinical targeted X-ray imaging. Adv. Drug Deliv. Rev. 2014, 76, 116–133. [Google Scholar] [CrossRef]
- Thomsen, H.S. Gadolinium-or iodine-based contrast media: Which choice? Acta. Radiol. 2014, 55, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart. J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef]
- Ronco, C.; Stacul, F.; McCullough, P.A. Subclinical acute kidney injury (AKI) due to iodine-based contrast media. Eur. Radiol. 2013, 23, 319–323. [Google Scholar] [CrossRef]
- Meng, X.; Wu, Y.; Bu, W. Functional CT contrast nanoagents for the tumor microenvironment. Adv. Healthc. Mater. 2021, 10, 2000912. [Google Scholar] [CrossRef] [PubMed]
- Krause, W. Delivery of diagnostic agents in computed tomography. Adv. Drug Deliv. Rev. 1999, 37, 159–173. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Park, Y.I.; Lee, N.; Hyeon, T. Recent development of inorganic nanoparticles for biomedical imaging. ACS Cent. Sci. 2018, 4, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Ehlerding, E.B.; Grodzinski, P.; Cai, W.; Liu, C.H. Big potential from small agents: Nanoparticles for imaging-based companion diagnostics. ACS Nano. 2018, 12, 2106–2121. [Google Scholar] [CrossRef]
- Annapragada, A. Advances in nanoparticle imaging technology for vascular pathologies. Annu. Rev. Med. 2015, 66, 177–193. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, Z. Advances in multifunctional nano-sized CT contrast agents. Chinese Sci. Bull. 2015, 60, 3424–3437. [Google Scholar] [CrossRef][Green Version]
- Mulder, W.J.M.; van Leent, M.M.T.; Lameijer, M.; Fisher, E.A.; Fayad, Z.A.; Perez-Medina, C. High-density lipoprotein nanobiologics for precision medicine. Acc. Chem. Res. 2018, 51, 127–137. [Google Scholar] [CrossRef]
- Cao, J.; Wei, Y.; Zhang, Y.; Wang, G.; Ji, X.; Zhong, Z. Iodine-rich polymersomes enable versatile SPECT/CT imaging and potent radioisotope therapy for tumor in vivo. ACS Appl. Mater. Interfaces 2019, 11, 18953–18959. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, C.; Gu, J.; Jiang, Y.; Yang, X.; Li, S.; Gao, W.; An, T.; Duan, H.; Fu, J.; et al. Hyaluronic acid stabilized iodine-containing nanoparticles with Au nanoshell coating for X-ray CT imaging and photothermal therapy of tumors. ACS Appl. Mater. Interfaces 2016, 8, 27622–27631. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Serra, C.A.; Bouquey, M.; Collot, M.; Anton, H.; Weickert, J.L.; Messaddeq, N.; Vandamme, T.F. A new formulation of poly(MAOTIB) nanoparticles as an efficient contrast agent for in vivo X-ray imaging. Acta. Biomater. 2018, 66, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Thaxton, C.S.; Rink, J.S.; Naha, P.C.; Cormode, D.P. Lipoproteins and lipoprotein mimetics for imaging and drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 116–131. [Google Scholar] [CrossRef]
- Attia, M.F.; Brummel, B.R.; Lex, T.R.; Van Horn, B.A.; Whitehead, D.C.; Alexis, F. Recent advances in polyesters for biomedical imaging. Adv. Healthc. Mater. 2018, 7, 1800798. [Google Scholar] [CrossRef]
- Zou, Y.; Wei, Y.; Wang, G.; Meng, F.; Gao, M.; Storm, G.; Zhong, Z. Nanopolymersomes with an ultrahigh iodine content for high-performance X-ray computed tomography imaging In vivo. Adv. Mater. 2017, 29, 1603997. [Google Scholar] [CrossRef]
- Liu, D.; Cornel, E.J.; Du, J. Renoprotective angiographic polymersomes. Adv. Funct. Mater. 2020, 31, 2007330. [Google Scholar] [CrossRef]
- Torchilin, V.P. PEG-based micelles as carriers of contrast agents for different imaging modalities. Adv. Drug Deliv. Rev. 2002, 54, 235–252. [Google Scholar] [CrossRef]
- Trubetskoy, V.S. Polymeric micelles as carriers of diagnostic agents. Adv. Drug Deliv. Rev. 1999, 37, 81–88. [Google Scholar] [CrossRef]
- Torchilin, V.P. Polymeric contrast agents for medical imaging. Curr. Pharm. Biotechnol. 2000, 1, 183–215. [Google Scholar] [CrossRef]
- Tian, L.; Lu, L.; Feng, J.; Melancon, M.P. Radiopaque nano and polymeric materials for atherosclerosis imaging, embolization and other catheterization procedures. Acta. Pharm. Sin. B 2018, 8, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Galperin, A.; Margel, D.; Baniel, J.; Dank, G.; Biton, H.; Margel, S. Radiopaque iodinated polymeric nanoparticles for X-ray imaging applications. Biomaterials 2007, 28, 4461–4468. [Google Scholar] [CrossRef]
- Jin, E.; Lu, Z.R. Biodegradable iodinated polydisulfides as contrast agents for CT angiography. Biomaterials 2014, 35, 5822–5829. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.R.; Brosnan, S.M.; Burk, L.M.; Lee, Y.Z.; Luft, J.C.; Ashby, V.S. Iodinated polyesters as a versatile platform for radiopaque biomaterials. J. Polym. Sci. Pol. Chem. 2017, 55, 2171–2177. [Google Scholar] [CrossRef]
- El Habnouni, S.; Blanquer, S.; Darcos, V.; Coudane, J. Aminated PCL-based copolymers by chemical modification of poly(α-iodo-ε-caprolactone-co-ε-caprolactone). J. Polym. Sci. Pol. Chem. 2009, 47, 6104–6115. [Google Scholar] [CrossRef]
- Horák, D.; Metalová, M.; Rypáček, F. New radiopaque polyHEMA-based hydrogel particles. J Biomed. Mater. Res. 1997, 34, 183–188. [Google Scholar] [CrossRef]
- Davy, K.W.M.; Anseau, M.R.; Berry, C. Iodinated methacrylate copolymers as X-ray opaque denture base acrylics. J. Dent. 1997, 25, 499–505. [Google Scholar] [CrossRef]
- He, J.; Vallittu, P.K.; Lassila, L.V. Preparation and characterization of high radio-opaque e-glass fiber-reinforced composite with iodine containing methacrylate monomer. Dent. Mater. 2017, 33, 218–225. [Google Scholar] [CrossRef]
- Aviv, H.; Bartling, S.; Kieslling, F.; Margel, S. Radiopaque iodinated copolymeric nanoparticles for X-ray imaging applications. Biomaterials 2009, 30, 5610–5616. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, A.; Thanoo, B.C. Synthesis and polymerization of some iodine- containing monomers for biomedical applications. J. Appl. Polym. Sci. 1992, 44, 743–748. [Google Scholar] [CrossRef]
- Rode, C.; Schmidt, A.; Wyrwa, R.; Weisser, J.; Schmidt, K.; Moszner, N.; Gottlöber, R.-P.; Heinemann, K.; Schnabelrauch, M. Synthesis and processability into textile structures of radiopaque, biodegradable polyesters and poly(ester-urethanes). Polym. Int. 2014, 63, 1732–1740. [Google Scholar] [CrossRef]
- Tang, C.; York, A.W.; Mikitsh, J.L.; Wright, A.C.; Chacko, A.-M.; Elias, D.R.; Xu, Y.; Lim, H.-K.; Prud’homme, R.K. Preparation of PEGylated iodine- loaded nanoparticles via polymer-directed self-assembly. Macromol. Chem. Phys. 2018, 219, 1700592. [Google Scholar] [CrossRef]
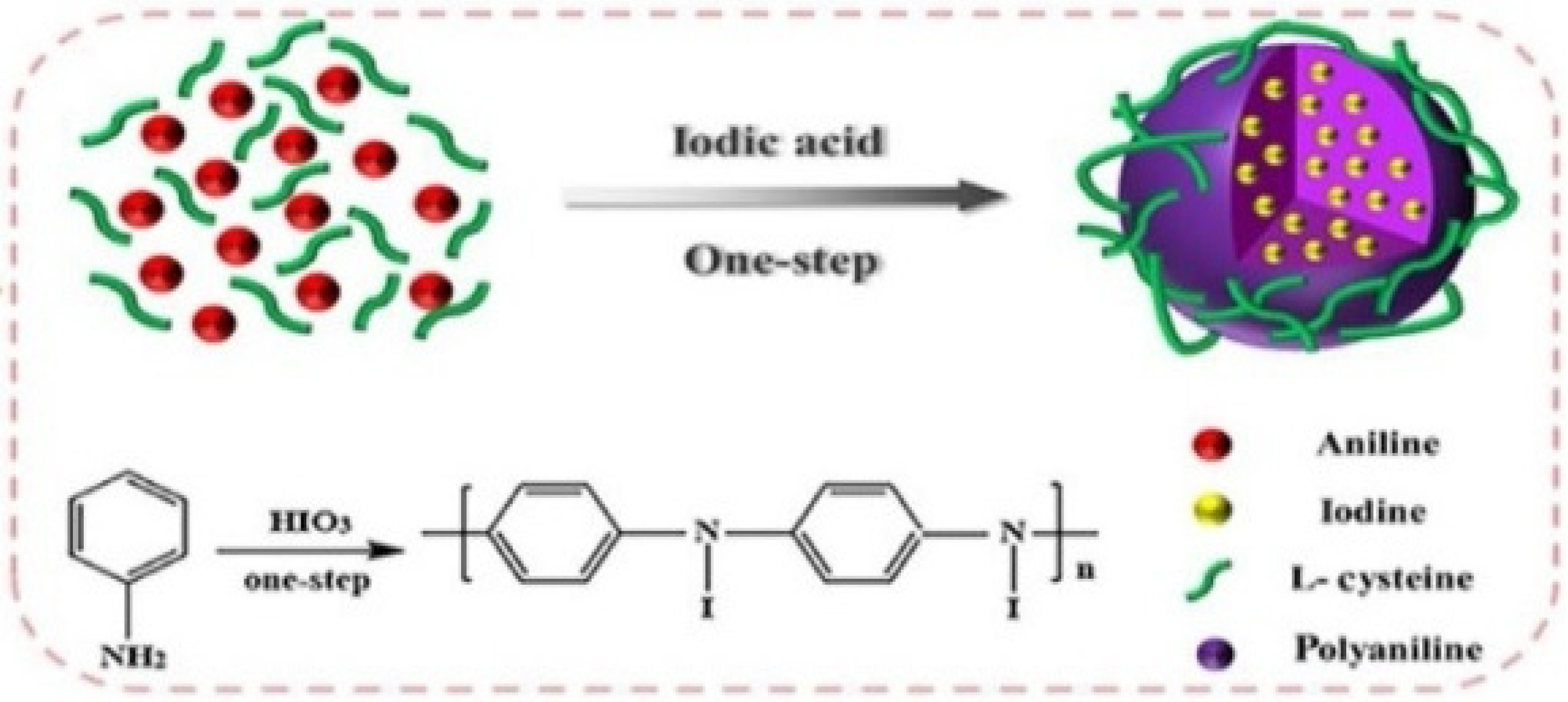
- Zou, Q.; Huang, J.; Zhang, X. One-step synthesis of iodinated polypyrrole nanoparticles for CT imaging guided photothermal therapy of tumors. Small 2018, 14, 1803101. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yang, S.; Jiang, S.; Zhou, X.; Sha, Z.; He, C. One-step synthesis of multifunctional nanoparticles for CT/PA imaging guided breast cancer photothermal therapy. Colloid. Surface B 2021, 201, 111630. [Google Scholar] [CrossRef]
- Ghosh, P.; Das, M.; Rameshbabu, A.P.; Das, D.; Datta, S.; Pal, S.; Panda, A.B.; Dhara, S. Chitosan derivatives cross-linked with iodinated 2,5-dimethoxy-2,5-dihydrofuran for non-invasive imaging. ACS Appl. Mater. Interfaces 2014, 6, 17926–17936. [Google Scholar] [CrossRef]
- Lim, C.K.; Shin, J.; Kwon, I.C.; Jeong, S.Y.; Kim, S. Iodinated photosensitizing chitosan: Self-assembly into tumor-homing nanoparticles with enhanced singlet oxygen generation. Bioconjugate Chem. 2012, 23, 1022–1028. [Google Scholar] [CrossRef]
- Revel, D.; Chambon, C.; Havard, P.; Dandis, G.; Canet, E.; Corot, C.; Amiel, M. Iodinated polymer as blood-pool contrast agent computed tommography evaluation in rabbits. Invest. Radiol. 1991, 26, 57–59. [Google Scholar] [CrossRef]
- Balegamire, J.; Vandamme, M.; Chereul, E.; Si-Mohamed, S.; Azzouz Maache, S.; Almouazen, E.; Ettouati, L.; Fessi, H.; Boussel, L.; Douek, P.; et al. Iodinated polymer nanoparticles as contrast agent for spectral photon counting computed tomography. Biomater. Sci. 2020, 8, 5715–5728. [Google Scholar] [CrossRef]
- True3etskoy, V.S.; Gazelle, G.S.; Wow, G.L.; Torchilin, V.I.P. Block-copolymer of polyethylene glycol and polylysine as a carrier of organic iodine: Design of long-circulating particulate contrast medium for X-ray computed tomography. J. Drug Target. 1997, 4, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, J.; Cheng, Y.; Xiong, Z.; Tang, Y.; Guo, L.; Shi, X.; Zhao, J. Chlorotoxin-conjugated multifunctional dendrimers jlabeled with radionuclide 131I for single photon emission computed tomography imaging and radiotherapy of gliomas. ACS Appl. Mater. Interfaces 2015, 7, 19798–19808. [Google Scholar] [CrossRef]
- Fu, Y.; Nitecki, D.E.; Maltby, D.; Simon, G.H.; Berejnoi, K.; Raatschen, H.-J.; Yeh, B.M.; Shames, D.M.; Brasch, R.C. Dendritic iodinated contrast agents with PEG- cores for CT imaging: synthesis and preliminary characterization. Bioconjugate Chem. 2006, 17, 1043–1056. [Google Scholar] [CrossRef]
- You, S.; Jung, H.Y.; Lee, C.; Choe, Y.H.; Heo, J.Y.; Gang, G.T.; Byun, S.K.; Kim, W.K.; Lee, C.H.; Kim, D.E.; et al. High-performance dendritic contrast agents for X-ray computed tomography imaging using potent tetraiodobenzene derivatives. J. Control Release 2016, 226, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, H.; Yu, B.; Xu, F.J. PGMA-based cationic nanoparticles with polyhydric iodine units for advanced gene vectors. Bioconjugate Chem. 2016, 27, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Mottu, F.; Ruufenacht, D.A.; Laurentc, E.D. Iodine-containing cellulose mixed esters as radiopaque polymers for direct embolization of cerebral aneurysms and arteriovenous malformations. Biomaterials 2002, 23, 121–131. [Google Scholar] [CrossRef]
- Choi, D.; Jeon, S.; You, D.G.; Um, W.; Kim, J.Y.; Yoon, H.Y.; Chang, H.; Kim, D.E.; Park, J.H.; Kim, H.; et al. Iodinated echogenic glycol chitosan nanoparticles for X-ray CT/US dual imaging of tumor. Nanotheranostics 2018, 2, 117–127. [Google Scholar] [CrossRef]
- Padmanabhan, P.; Kumar, A.; Kumar, S.; Chaudhary, R.K.; Gulyas, B. Nanoparticles in practice for molecular-imaging applications: An overview. Acta. Biomater. 2016, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Jarzyna, P.A.; Mulder, W.J.; Fayad, Z.A. Modified natural nanoparticles as contrast agents for medical imaging. Adv. Drug Deliv. Rev. 2010, 62, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Jakhmola, A.; Anton, N.; Vandamme, T.F. Inorganic nanoparticles based contrast agents for X-ray computed tomography. Adv. Healthc. Mater. 2012, 1, 413–431. [Google Scholar] [CrossRef]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as contrast agents for in-vivo bioimaging: Current status and future perspectives. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, W.; Sun, W.; Yu, B.; Zhang, Q.; Lu, L. Nanoparticles: Untying the gordian knot in conventional computed tomography imaging. CCS Chem. 2021, 3, 1242–1257. [Google Scholar] [CrossRef]
- Rosen, J.E.; Yoffe, S.; Meerasa, A.; Verma1, M.; Gu, F.X. Nanotechnology and diagnostic imaging: New advances in contrast agent technology. J. Nanomedic. Nanotechnol. 2011, 2, 1000115. [Google Scholar] [CrossRef]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Stiriba, S.-E.; Frey, H.; Haag, R. Dendritic polymers in biomedical applications: From potential to clinical use in diagnostics and therapy. Angew. Chem. Int. Ed. 2002, 41, 1329–1334. [Google Scholar] [CrossRef]
- Shim, M.S.; Kwon, Y.J. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv. Drug Deliv. Rev. 2012, 64, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.A.; Salinas, Y.; Resmini, M. Smart polymeric nanoparticles as emerging tools for imaging-the parallel evolution of materials. Chemistry 2016, 22, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003, 2, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Park, K.; Nam, H.Y.; Lee, S.; Kim, K.; Kwon, I.C. Polymers for bioimaging. Prog. Polym. Sci. 2007, 32, 1031–1053. [Google Scholar] [CrossRef]
- Nottelet, B.; Darcos, V.; Coudane, J. Aliphatic polyesters for medical imaging and theranostic applications. Eur. J. Pharm. Biopharm. 2015, 97, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Skajaa, T.; Cormode, D.P.; Falk, E.; Mulder, W.J.; Fisher, E.A.; Fayad, Z.A. High-density lipoprotein-based contrast agents for multimodal imaging of atherosclerosis. Arteriocl. Throm. Vas. 2010, 30, 169–176. [Google Scholar] [CrossRef]
- Shilo, M.; Reuveni, T.; Motiei, M.; Popovtzer, R. Nanoparticles as computed tomography contrast agents: Current status and future perspectives. Nanomedicine 2012, 7, 257–269. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-soluble conjugated polymers for imaging, diagnosis, and therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, T.; Ding, J.; Xie, Z. Robust organic nanoparticles for noninvasive long-term fluorescence imaging. J. Mater. Chem. B 2019, 7, 6879–6889. [Google Scholar] [CrossRef]
- Margulis-Goshen, K.; Magdassi, S. Organic nanoparticles from microemulsions: Formation and applications. Curr. Opin. Colloid Interface Sci. 2012, 17, 290–296. [Google Scholar] [CrossRef]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The clinical translation of organic nanomaterials for cancer therapy: A focus on polymeric nanoparticles, micelles, liposomes and exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef] [PubMed]
- Al-Jamal, W.T.; Kostarelos, K. Liposomes: From a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1094–1104. [Google Scholar] [CrossRef]
- Boase, N.R.B.; Blakey, I.; Thurecht, K.J. Molecular imaging with polymers. Polym. Chem. 2012, 3, 1384–1389. [Google Scholar] [CrossRef]
- Krause, W.; Leike, J.; Sachse, A.; Schuhmann-Giampieri, G. Characterization of iopromide liposomes. Invest. Radiol. 1993, 28, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Silindir, M.; Erdogan, S.; Ozer, A.Y.; Dogan, A.L.; Tuncel, M.; Ugur, O.; Torchilin, V.P. Nanosized multifunctional liposomes for tumor diagnosis and molecular imaging by SPECT/CT. J. Liposome Res. 2013, 23, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.; Babich, J.; Weissig, V. Liposomes and micelles to target the blood pool for imaging purposes. J. Liposome. Res. 2000, 10, 483–499. [Google Scholar] [CrossRef]
- Xu, H.; Ohulchanskyy, T.Y.; Qu, J.; Yakovliev, A.; Ziniuk, R.; Yuan, Z.; Qu, J. Co-encapsulating indocyanine green and CT contrast agent within nanoliposomes for trimodal imaging and near infrared phototherapy of cancer. Nanomedicine 2020, 29, 102269. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. Nanotechnology for computed tomography: A real potential recently disclosed. Pharm. Res. 2014, 31, 20–34. [Google Scholar] [CrossRef]
- Ryan, P.J.; Davis, M.A.; DeGaeta, L.R.; Woda, B.; Melchior, D.L. Liposomes loaded with contrast material for image enhancement in computed tomography. Work in progress. Radiology 1984, 152, 759–762. [Google Scholar] [CrossRef]
- Havron, A.; Seltzer, S.E.; Davis, M.A.; Shulkin, P. Radiopaque liposomes: A promising new contrast material for computed tomography of the spleen. Radiology 1981, 140, 507–511. [Google Scholar] [CrossRef]
- Benita, S.; Poly, P.A.; Puisieux, F.; Delattre, J. Radiopaque liposomes: Effect of formulation conditions on encapsulation efficiency. J. Pharm. Sci. 1984, 73, 1751–1755. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagasaki, Y.; Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 2012, 64, 246–255. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Samei, E.; Saunders, R.S.; Badea, C.T.; Ghaghada, K.B.; Hedlund, L.W.; Qi, Y.; Yuan, H.; Bentley, R.C.; Jr, S.M. Micro-CT imaging of breast tumors in rodents using a liposomal, nanoparticle contrast agent. Int. J. Nanomed. 2009, 4, 277–282. [Google Scholar] [CrossRef]
- Ghaghada, K.B.; Badea, C.T.; Karumbaiah, L.; Fettig, N.; Bellamkonda, R.V.; Johnson, G.A.; Annapragada, A. Evaluation of tumor microenvironment in an animal model using a nanoparticle contrast agent in computed tomography imaging. Acad. Radiol. 2011, 18, 20–30. [Google Scholar] [CrossRef]
- Mukundan, S., Jr.; Ghaghada, K.B.; Badea, C.T.; Kao, C.Y.; Hedlund, L.W.; Provenzale, J.M.; Johnson, G.A.; Chen, E.; Bellamkonda, R.V.; Annapragada, A. A liposomal nanoscale contrast agent for preclinical CT in mice. Am. J. Roentgenol. 2006, 186, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.J.; Annapragada, A.; Hoffman, E.A.; Chen, E.; Ghaghada, K.B.; Sieren, J.; Beek, E.J.R.v. Imaging of pulmonary embolism and t-PA therapy effects using MDCT and liposomal iohexol blood pool agent: Preliminary results in a rabbit model. Acad. Radiol. 2007, 14, 355–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, H.S.; Kim, H.K. Multispectral image-guided surgery in patients. Nat. Biomed. Eng. 2020, 4, 245–246. [Google Scholar] [CrossRef]
- Koudrina, A.; DeRosa, M.C. Advances in medical imaging: Aptamer-and peptide-targeted MRI and CT contrast agents. ACS Omega. 2020, 5, 22691–22701. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Lammers, T.; Kiessling, F.E.; Hennink, W.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Control Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Gazelle, G.S.; Wolf, G.L.; Mclntire, G.L.; Bacon, E.R.; Na, G.; Halpern, E.E.; Toner, J.L. Hepatic imaging with iodinated nanoparticles: A comparison with iohexol in rabbits. Acad. Radiol. 1995, 2, 700–704. [Google Scholar] [CrossRef]
- Desser, T.S.; Rubin, D.L.; Muller, H.; McIntire, G.L.; Bacon, E.R.; Toner, J.L. Blood pool and liver enhancement in CT with liposomal lodixanol: Comparison with lohexol. Acad. Radiol. 1999, 6, 176–183. [Google Scholar] [CrossRef]
- Kweon, S.; Lee, H.-J.; Hyung, W.J.; Suh, J.; Lim, J.S.; Lim, S.-J. Liposomes coloaded with iopamidol/lipiodol as a RES-targeted contrast agent for computed tomography imaging. Pharm. Res. 2010, 27, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Meel, R.v.d.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Zheng, J.; Jaffray, D.; Allen, C. Quantitative CT imaging of the spatial and temporal distribution of liposomes in a rabbit tumor model. Mol. Pharm. 2009, 6, 571–580. [Google Scholar] [CrossRef]
- Ghaghada, K.B.; Sato, A.F.; Starosolski, Z.A.; Berg, J.; Vail, D.M. Computed tomography imaging of solid tumors using a liposomal-iodine contrast agent in companion dogs with naturally occurring cancer. PLoS. ONE 2016, 11, e0152718. [Google Scholar] [CrossRef]
- Kim, J.; Piao, Y.; Hyeon, T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef]
- Tsang, M.-K.; Wong, Y.-T.; Hao, J. Cutting-edge nanomaterials for advanced multimodal bioimaging applications. Small Methods 2018, 2, 1700265. [Google Scholar] [CrossRef]
- Rieffel, J.; Chitgupi, U.; Lovell, J.F. Recent advances in higher- order, multimodal, biomedical imaging agents. Small 2015, 11, 4445–4461. [Google Scholar] [CrossRef]
- Xu, H.; Ohulchanskyy, T.Y.; Yakovliev, A.; Zinyuk, R.; Song, J.; Liu, L.; Qu, J.; Yuan, Z. Nanoliposomes co-encapsulating CT imaging contrast agent and photosensitizer for enhanced, imaging guided photodynamic therapy of cancer. Theranostics 2019, 9, 1323–1335. [Google Scholar] [CrossRef]
- Jenjob, R.; Phakkeeree, T.; Seidi, F.; Theerasilp, M.; Crespy, D. Emulsion techniques for the production of pharmacological nanoparticles. Macromol. Biosci. 2019, 19, 1900063. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Chiper, M.; Akasov, R.; Anton, H.; Messaddeq, N.; Fournel, S.; Klymchenko, A.S.; Mély, Y.M.; Vandamme, T.F. Biodistribution of X-ray iodinated contrast agent in nano-emulsions is controlled by the chemical nature of the oily core. ACS Nano 2014, 8, 10537–10550. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.; Custers, E.; Lub, J.; van den Bosch, S.; Nicolay, K.; Grull, H. Block-copolymer-stabilized iodinated emulsions for use as CT contrast agents. Biomaterials 2010, 31, 6537–6544. [Google Scholar] [CrossRef]
- Li, X.; Anton, N.; Zuber, G.; Zhao, M.; Messaddeq, N.; Hallouard, F.; Fessi, H.; Vandamme, T.F. Iodinated alpha-tocopherol nano-emulsions as non-toxic contrast agents for preclinical X-ray imaging. Biomaterials 2013, 34, 481–491. [Google Scholar] [CrossRef]
- Hallouard, F.; Briancon, S.; Anton, N.; Li, X.; Vandamme, T.; Fessi, H. Iodinated nano-emulsions as contrast agents for preclinical X-ray imaging: Impact of the free surfactants on the pharmacokinetics. Eur. J. Pharm. Biopharm. 2013, 83, 54–62. [Google Scholar] [CrossRef]
- Attia, M.F.; Anton, N.; Akasov, R.; Chiper, M.; Markvicheva, E.; Vandamme, T.F. Biodistribution and toxicity of X-ray iodinated contrast agent in nano-emulsions in function of their size. Pharm. Res. 2016, 33, 603–614. [Google Scholar] [CrossRef]
- Hallouard, F.; Anton, N.; Zuber, G.; Choquet, P.; Li, X.; Arntz, Y.; Aubertin, G.; Constantinesco, A.; Vandamme, T.F. Radiopaque iodinated nano-emulsions for preclinical X-ray imaging. RSC Adv. 2011, 1, 792–801. [Google Scholar] [CrossRef]
- Hallouard, F.; Anton, N.; Choquet, P.; Constantinesco, A.; Vandamme, T. Iodinated blood pool contrast media for preclinical X-ray imaging applications-a review. Biomaterials 2010, 31, 6249–6268. [Google Scholar] [CrossRef]
- Hallouard, F.; Briancon, S.; Anton, N.; Li, X.; Vandamme, T.; Fessi, H. Influence of diblock copolymer PCL-mPEG and of various iodinated oils on the formulation by the emulsion-solvent diffusion process of radiopaque polymeric nanoparticles. J. Pharm. Sci. 2013, 102, 4150–4158. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Naha, P.C.; Fayad, Z.A. Nanoparticle contrast agents for computed tomography: A focus on micelles. Contrast. Media Mol. I 2014, 9, 37–52. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, Y.; Zhang, Y.; Xin, X.; Li, R.; Xie, C.; Fan, Q. Iodine-rich semiconducting polymer nanoparticles for CT/fluorescence dual-modal imaging-guided enhanced photodynamic therapy. Small 2020, 16, 1905641. [Google Scholar] [CrossRef] [PubMed]
- Discher, B.M.; Won, Y.-Y.; Ege, D.S.; Lee, J.C.-M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef]
- Discher, D.E.; Ahmed, F. Polymersomes. Annu. Rev. Biomed. Eng. 2006, 8, 323–341. [Google Scholar] [CrossRef]
- Pawar, P.V.; Gohil, S.V.; Jain, J.P.; Kumar, N. Functionalized polymersomes for biomedical applications. Polym. Chem. 2013, 4, 3160–3176. [Google Scholar] [CrossRef]
- Thevenot, J.; Oliveira, H.; Lecommandoux, S. Polymersomes for theranostics. J. Drug Deliv. Sci. Technol. 2013, 23, 38–46. [Google Scholar] [CrossRef]
- Leong, J.; Teo, J.Y.; Aakalu, V.K.; Yang, Y.Y.; Kong, H. Engineering polymersomes for diagnostics and therapy. Adv. Healthc. Mater. 2018, 7, 1701276. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wei, Y.; Sun, Y.; Bao, J.; Yao, F.; Li, Z.; Meng, F.; Hu, C.; Storm, G.; Zhong, Z. Cyclic RGD-functionalized and disulfide-crosslinked iodine-rich polymersomes as a robust and smart theranostic agent for targeted CT imaging and chemotherapy of tumor. Theranostics 2019, 9, 8061–8072. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jie, X.; Xu, L.; Chen, C.; Shen, W.; Cao, Y.; Lian, G.; Qi, R. Recent advances in dendrimer research for cardiovascular diseases. Biomacromolecules 2015, 16, 2588–2598. [Google Scholar] [CrossRef]
- Mignani, S.; Rodrigues, J.; Tomas, H.; Caminade, A.-M.; Laurent, R.; Shi, X.; Majoral, J.-P. Recent therapeutic applications of the theranostic principle with dendrimers in oncology. Sci. China. Mater. 2018, 61, 1367–1386. [Google Scholar] [CrossRef]
- Qiao, Z.; Shi, X. Dendrimer-based molecular imaging contrast agents. Prog. Polym. Sci. 2015, 44, 1–27. [Google Scholar] [CrossRef]
- Kesharwani, P.; Jain, K.; Jain, N.K. Dendrimer as nanocarrier for drug delivery. Prog. Polym. Sci. 2014, 39, 268–307. [Google Scholar] [CrossRef]
- Yordanov, A.T.; Lodder, A.L.; Woller, E.K.; Cloninger, M.J.; Patronas, N.; Milenic, D.; Brechbiel, M.W. Novel iodinated dendritic nanoparticles for computed tomography (CT) imaging. Nano. Lett. 2002, 2, 595–599. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, X.; Xu, Y.; Cheng, T.; Ou, H.; Li, Z.; An, Y.; Shen, W.; Liu, Y.; Shi, L. Polymerization-induced self-assembly of large-scale iohexol nanoparticles as contrast agents for X-ray computed tomography imaging. Polym. Chem. 2018, 9, 2926–2935. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; Ridwan, S.M.; Stanishevskiy, Y.; Smilowitz, N.R.; Davis, J.; Smilowitz, H.M. Small, Long blood half-life iodine nanoparticle for vascular and tumor imaging. Sci. Rep. 2018, 8, 13803. [Google Scholar] [CrossRef]
- Yin, Q.; Yap, F.Y.; Yin, L.; Ma, L.; Zhou, Q.; Dobrucki, L.W.; Fan, T.M.; Gaba, R.C.; Cheng, J. Poly(iohexol) nanoparticles as contrast agents for in vivo X-ray computed tomography imaging. J. Am. Chem. Soc. 2013, 135, 13620–13623. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Skajaa, T.; Schooneveld, M.M.V.; Koole, R.; Jarzyna, P.; Lobatto, M.E.; Calcagno, C.; Barazza, A.; Gordon, R.E.; Zanzonico, P.; et al. Nanocrystal core high-density lipoproteins: A multimodality contrast agent platform. Nano. Lett. 2008, 8, 3715–3723. [Google Scholar] [CrossRef]
- Ng, K.K.; Lovell, J.F.; Zheng, G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc. Chem. Res. 2011, 44, 1105–1113. [Google Scholar] [CrossRef]
- Hill, M.L.; Corbin, I.R.; Levitin, R.B.; Cao, W.; Mainprize, J.G.; Yaffe, M.J.; Zheng, G. In vitro assessment of poly-iodinated triglyceride reconstituted low-density lipoprotein: Initial steps toward CT molecular imaging. Acad. Radiol. 2010, 17, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- deKrafft, K.E.; Xie, Z.; Cao, G.; Tran, S.; Ma, L.; Zhou, O.Z.; Lin, W. Iodinated nanoscale coordination polymers as potential contrast agents for computed tomography. Angew. Chem. Int. Ed. 2009, 48, 9901–9904. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, Y.; Zhang, Y.; Li, S.; Yang, X.; Chen, Y.; Fu, J.; Wang, Y.; Yang, X. cRGD-modified and disulfide bond-crosslinked polymer nanoparticles based on iopamidol as a tumor-targeted CT contrast agent. Polym. Chem. 2020, 11, 889–899. [Google Scholar] [CrossRef]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef]
- Pan, D.; Williams, T.A.; Senpan, A.; Allen, J.S.; Scott, M.J.; Gaffney, P.J.; Wickline, S.A.; Lanza, G.M. Detecting vascular biosignatures with a colloidal, radio-opaque polymeric nanoparticle. J. Am. Chem. Soc. 2009, 131, 15522–15527. [Google Scholar] [CrossRef]
- Wang, K.; Peng, H.; Thurecht, K.J.; Puttick, S.; Whittaker, A.K. Multifunctional hyperbranched polymers for CT/19F MRI bimodal molecular imaging. Polym. Chem. 2016, 7, 1059–1069. [Google Scholar] [CrossRef]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Y.; Sun, W.; Zhou, B.; Zhu, J.; Peng, C.; Shen, M.; Shi, X. Polyaniline-loaded gamma-polyglutamic acid nanogels as a platform for photoacoustic imaging-guided tumor photothermal therapy. Nanoscale 2017, 9, 12746–12754. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Ma, X.; Guo, R.; Ye, Z.; Fu, H.; Fu, N.; Guo, Z.; Zhang, J.; Zhang, J. Organic Nanoplatforms for Iodinated Contrast Media in CT Imaging. Molecules 2021, 26, 7063. https://doi.org/10.3390/molecules26237063
Zhang P, Ma X, Guo R, Ye Z, Fu H, Fu N, Guo Z, Zhang J, Zhang J. Organic Nanoplatforms for Iodinated Contrast Media in CT Imaging. Molecules. 2021; 26(23):7063. https://doi.org/10.3390/molecules26237063
Chicago/Turabian StyleZhang, Peng, Xinyu Ma, Ruiwei Guo, Zhanpeng Ye, Han Fu, Naikuan Fu, Zhigang Guo, Jianhua Zhang, and Jing Zhang. 2021. "Organic Nanoplatforms for Iodinated Contrast Media in CT Imaging" Molecules 26, no. 23: 7063. https://doi.org/10.3390/molecules26237063
APA StyleZhang, P., Ma, X., Guo, R., Ye, Z., Fu, H., Fu, N., Guo, Z., Zhang, J., & Zhang, J. (2021). Organic Nanoplatforms for Iodinated Contrast Media in CT Imaging. Molecules, 26(23), 7063. https://doi.org/10.3390/molecules26237063