Colorimetric and Electrochemical Methods for the Detection of SARS-CoV-2 Main Protease by Peptide-Triggered Assembly of Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
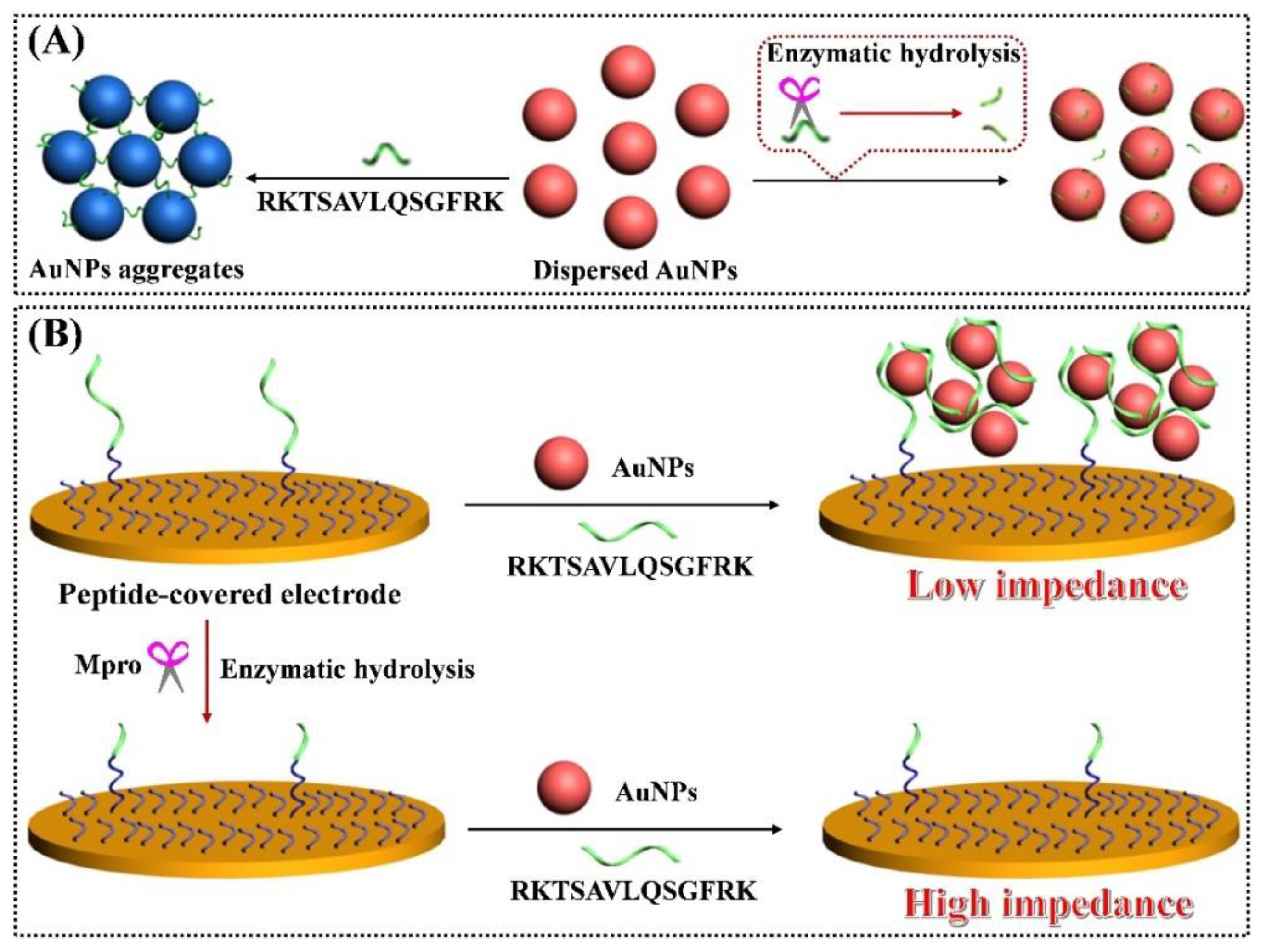
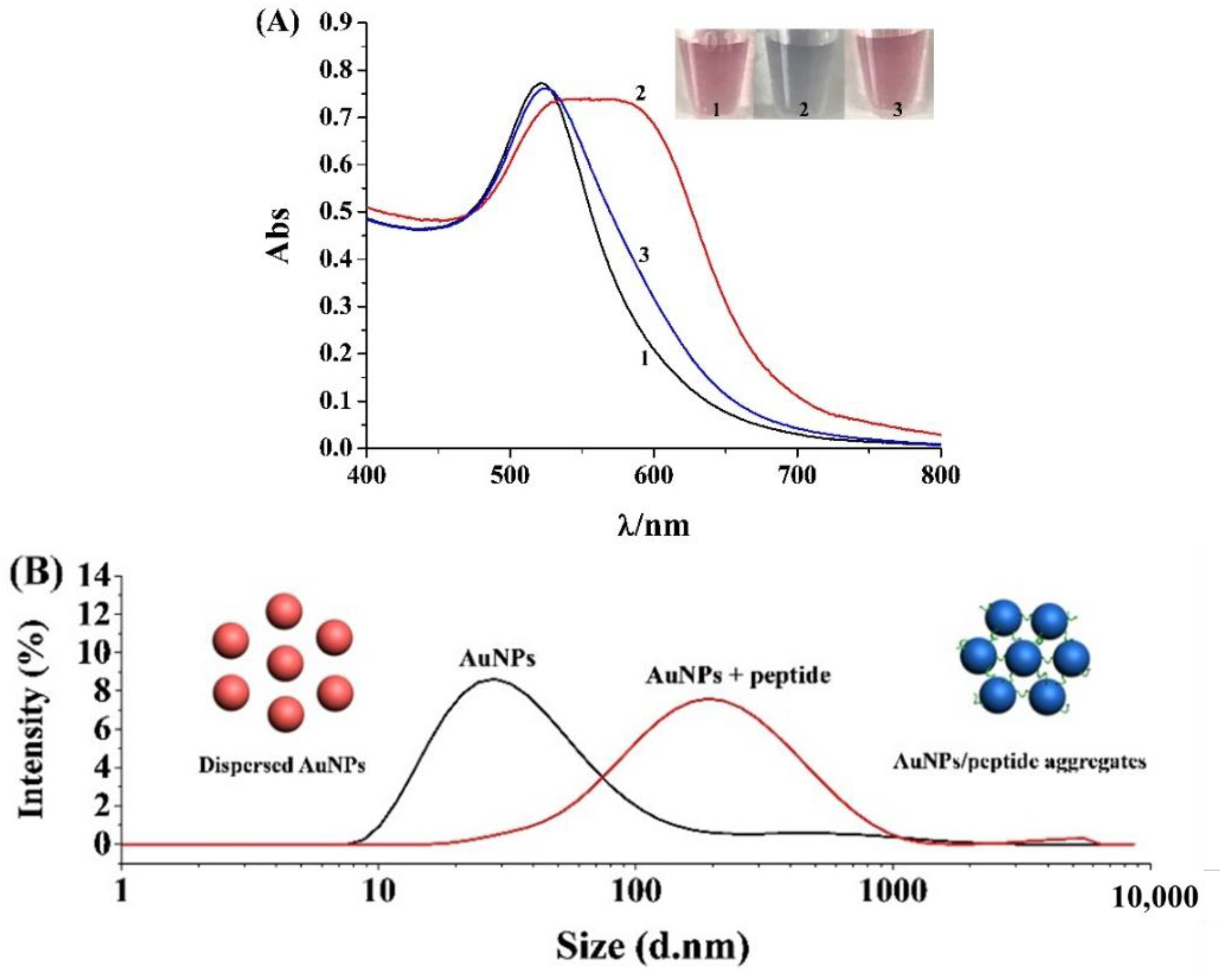
2.1. Feasibility for Colorimetric Analysis of Mpro
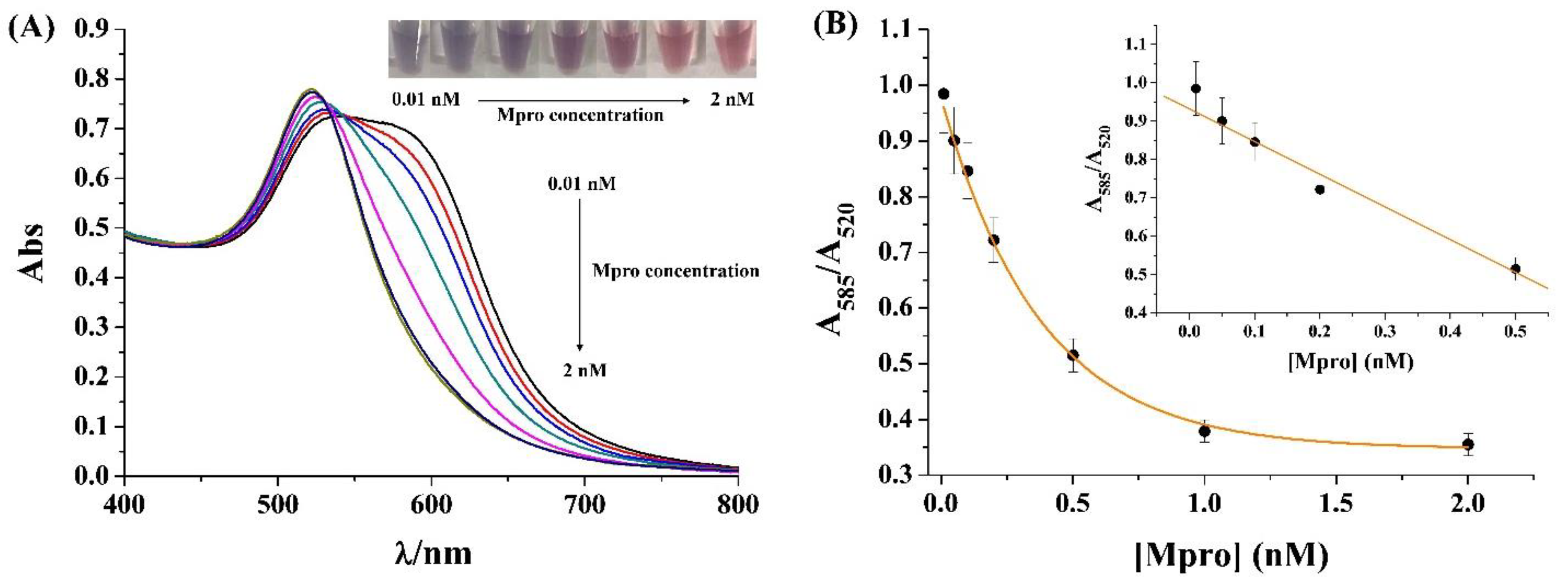
2.2. Sensitivity for Colorimetric Analysis of Mpro
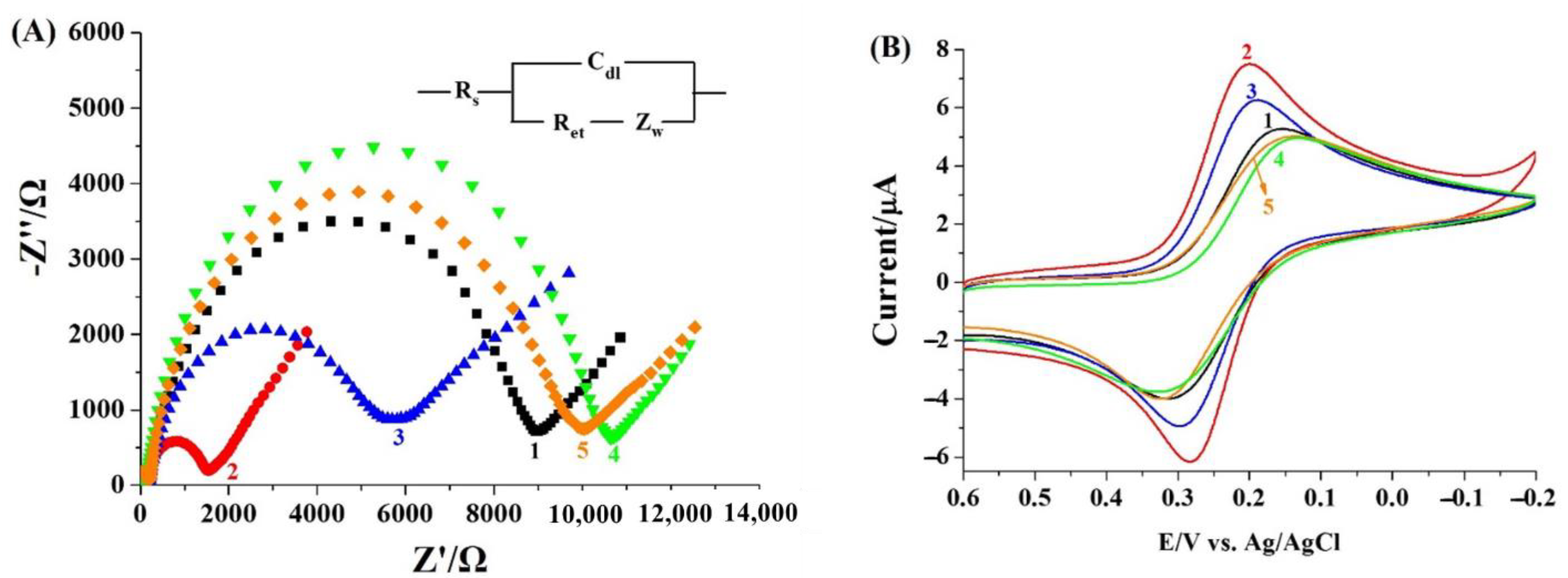
2.3. Feasibility for Electrochemical Detection of Mpro
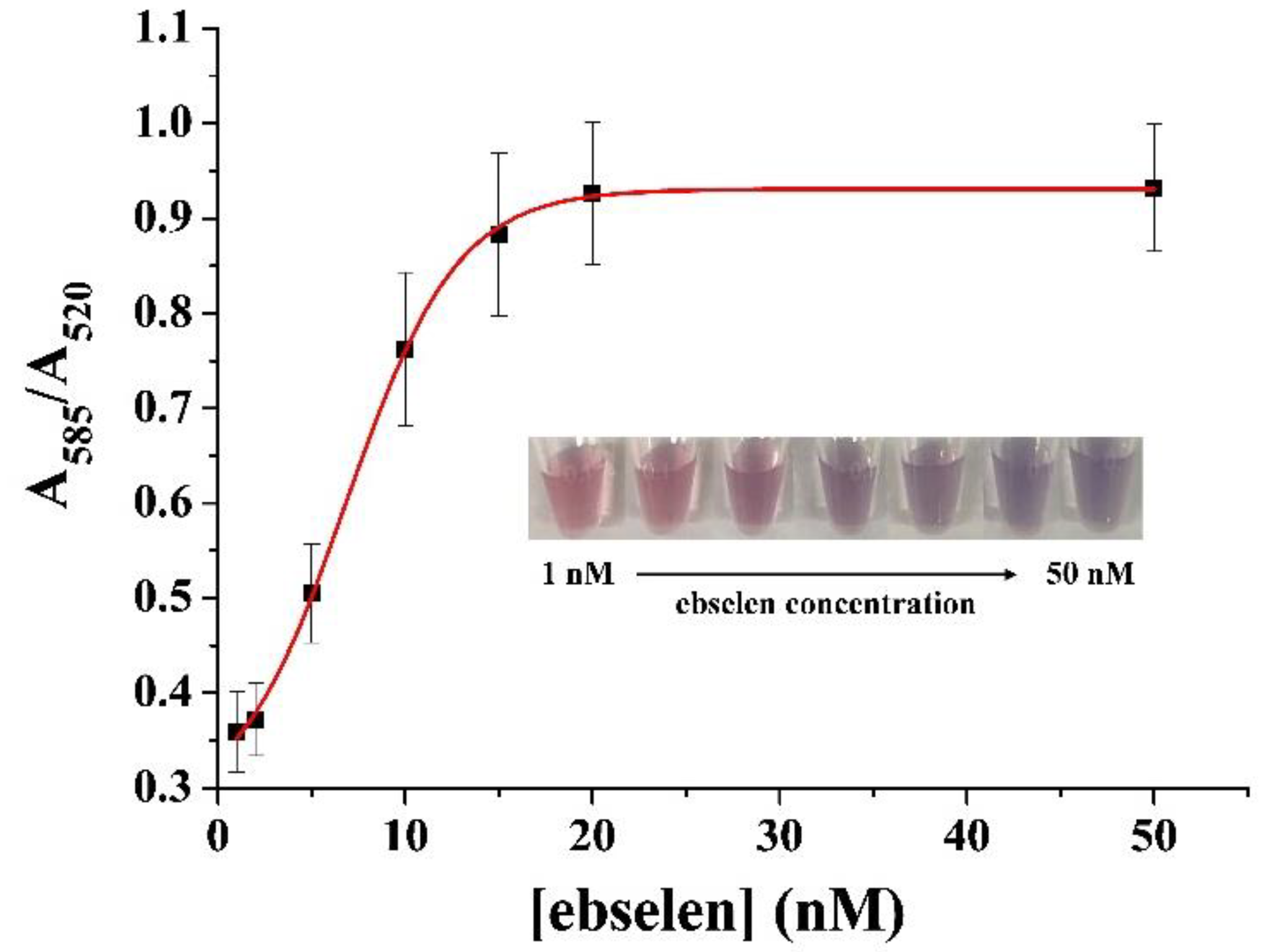
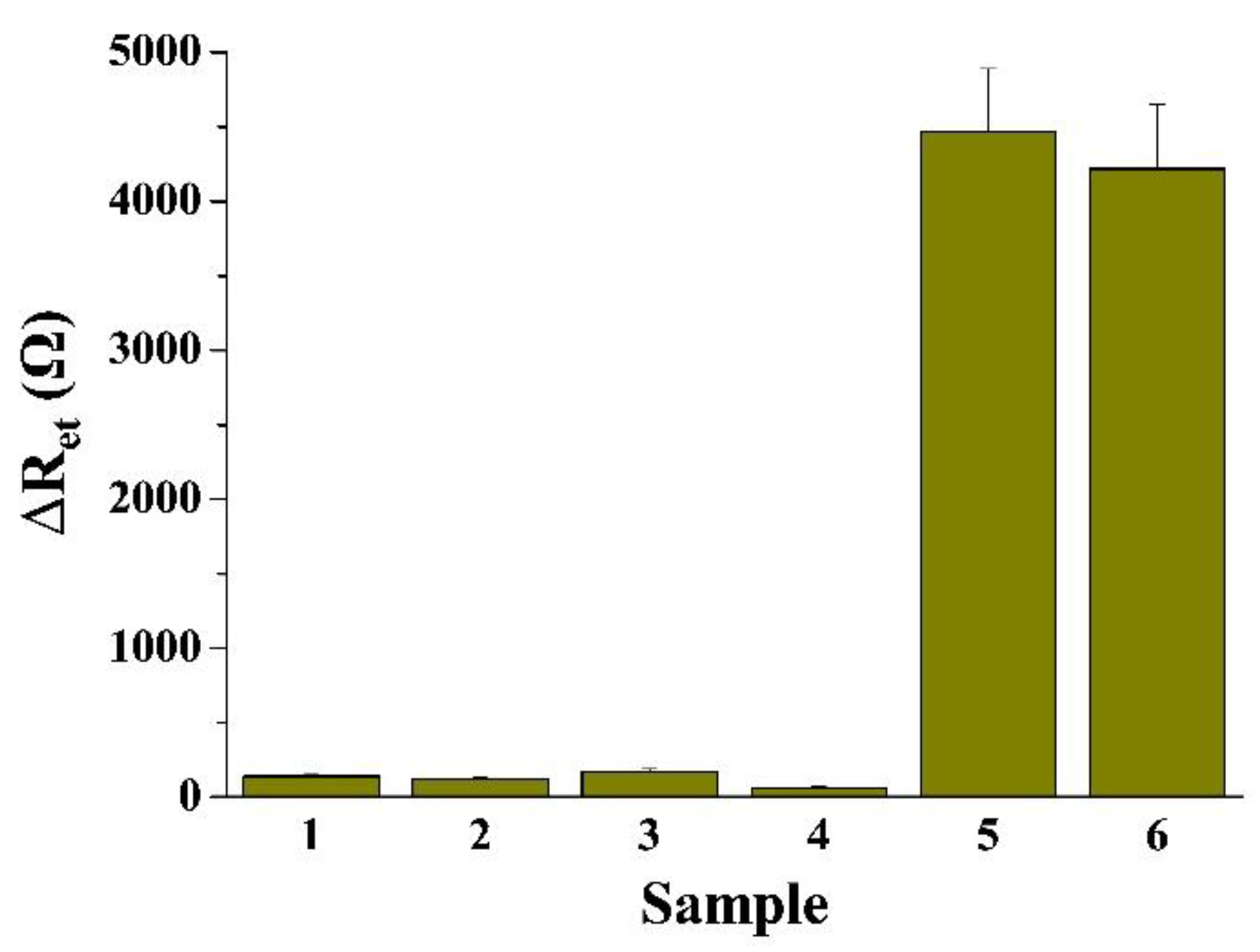
2.4. Sensitivity and Selectivity of the Electrochemical Method
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Peptide-Triggered Aggregation of AuNPs
3.3. Colorimetric Assays of Mpro Activity
3.4. Preparation of Sensing Electrode
3.5. Electrochemical Detection of Mpro
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Zhang, B.; Jiang, X.-M.; Su, H.; Li, J.; Zhao, Y.; Xie, X.; Jin, Z.; Peng, J.; Liu, F.; et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 2020, 368, 1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, A.; Mandal, K. Repurposing an antiviral drug against SARS-CoV-2 main protease. Angew. Chem. Int. Ed. 2021, 60, 23492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α α-ketoamide inhibitors. Science 2020, 368, 409. [Google Scholar] [CrossRef] [Green Version]
- Olubiyi, O.O.; Olagunju, M.; Keutmann, M.; Loschwitz, J.; Strodel, B. High throughput virtual screening to discover inhibitors of the main protease of the coronavirus SARS-CoV-2. Molecules 2020, 25, 3193. [Google Scholar] [CrossRef]
- Wang, J.; Lv, M.; Xia, H.; Du, J.; Zhao, Y.; Li, H.; Zhang, Z. Minimalist design for a hand-held SARS-Cov2 sensor: Peptide-induced covalent assembly of hydrogel enabling facile fiber-optic detection of a virus marker protein. ACS Sens. 2021, 6, 2465–2471. [Google Scholar] [CrossRef]
- Chauhan, N.; Soni, S.; Gupta, A.; Jain, U. New and developing diagnostic platforms for COVID-19: A systematic review. Expert. Rev. Mol. Diagn. 2020, 20, 971. [Google Scholar] [CrossRef]
- Soni, S.; Pudake, R.N.; Jain, U.; Chauhan, N. A systematic review on SARS-CoV-2-associated fungal coinfections. J. Med. Virol. 2022, 94, 99. [Google Scholar] [CrossRef]
- Moore, C.; Borum, R.M.; Mantri, Y.; Xu, M.; Fajtová, P.; O’Donoghue, A.J.; Jokerst, J.V. Activatable carbocyanine dimers for photoacoustic and fluorescent detection of protease activity. ACS Sens. 2021, 6, 2356–2365. [Google Scholar] [CrossRef]
- Brown, A.S.; Ackerley, D.F.; Calcott, M.J. High-throughput screening for inhibitors of the SARS-CoV-2 protease using a FRET-biosensor. Molecules 2020, 25, 4666. [Google Scholar] [CrossRef]
- Cihlova, B.; Huskova, A.; Böserle, J.; Nencka, R.; Boura, E.; Silhan, J. High-throughput fluorescent assay for inhibitor screening of proteases from RNA viruses. Molecules 2021, 26, 3792. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Chen, P.; Wang, Y.; Zhang, J.; Aili, D.; Liedberg, B. Biofunctionalized gold nanoparticles for colorimetric sensing of botulinum neurotoxin a light chain. Anal. Chem. 2014, 86, 2345. [Google Scholar] [CrossRef]
- Mauriz, E. Clinical applications of visual plasmonic colorimetric sensing. Sensors 2020, 20, 6214. [Google Scholar] [CrossRef]
- Siddiquee, S.; Saallah, S.; Bohari, N.A.; Ringgit, G.; Roslan, J.; Naher, L.; Nudin, N.F.H. Visual and optical absorbance detection of melamine in milk by melamine-induced aggregation of gold nanoparticles. Nanomaterials 2021, 11, 1142. [Google Scholar] [CrossRef]
- Guarise, C.; Pasquato, L.; Filippis, V.D.; Scrimin, P. Gold nanoparticles-based protease assay. Proc. Natl. Acad. Sci. USA 2006, 103, 3978. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Chen, C.-P.; Wu, T.-H.; Yang, C.-H.; Lin, C.-W.; Chen, C.-Y. Gold nanoparticle-based colorimetric strategies for chemical and biological sensing applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Aldewachi, H.S.; Woodroofe, N.; Turega, S.; Gardiner, P.H.E. Optimization of gold nanoparticle-based real-time colorimetric assay of dipeptidyl peptidase IV activity. Talanta 2017, 169, 13. [Google Scholar] [CrossRef]
- Chen, G.; Xie, Y.; Zhang, H.; Wang, P.; Cheung, H.-Y.; Yang, M.; Sun, H. A general colorimetric method for detecting protease activity based on peptide-induced gold nanoparticle aggregation. RSC Adv. 2014, 4, 6560. [Google Scholar] [CrossRef]
- Kim, C.-J.; Lee, D.-I.; Kim, C.; Lee, K.; Lee, C.-H.; Ahn, I.-S. Gold nanoparticles-based colorimetric assay for Cathepsin B activity and the efficiency of its inhibitors. Anal. Chem. 2014, 86, 3825. [Google Scholar] [CrossRef]
- Su, S.; Yu, T.; Hu, J.; Xianyu, Y. A bio-inspired plasmonic nanosensor for angiotensin-converting enzyme through peptide-mediated assembly of gold nanoparticles. Biosens. Bioelectron. 2022, 195, 113621. [Google Scholar] [CrossRef]
- Xia, N.; Zhou, B.; Huang, N.; Jiang, M.; Zhang, J.; Liu, L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens. Bioelectron. 2016, 85, 625. [Google Scholar] [CrossRef]
- Xia, N.; Wang, X.; Yu, J.; Wu, Y.; Cheng, S.; Xing, Y.; Liu, L. Design of electrochemical biosensors with peptide probes as the receptors of targets and the inducers of gold nanoparticles assembly on electrode surface. Sens. Actuat. B Chem. 2017, 239, 834. [Google Scholar] [CrossRef]
- Gu, Y.; Jiang, Z.; Ren, D.; Shang, Y.; Hu, Y.; Yi, L. Electrochemiluminescence sensor based on the target recognition-induced aggregation of sensing units for Hg2+ determination. Sens. Actuat. B Chem. 2021, 337, 129821. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, C.; Chang, Y.; Ma, H.; Hao, Y. Two sensitive electrochemical strategies for the detection of protein kinase activity based on the 4-mercaptophenylboronic acid-induced in situ assembly of silver nanoparticles. Sens. Actuat. B Chem. 2017, 248, 178. [Google Scholar] [CrossRef]
- Wei, T.; Dong, T.; Wang, Z.; Bao, J.; Tu, W.; Dai, Z. Aggregation of individual sensing units for signal accumulation: Conversion of liquid-phase colorimetric assay into enhanced surface-tethered electrochemical analysis. J. Am. Chem. Soc. 2015, 137, 8880–8883. [Google Scholar] [CrossRef]
- Xia, N.; Wang, X.; Zhou, B.; Wu, Y.; Mao, W.; Liu, L. Electrochemical detection of amyloid-beta oligomers based on the signal amplification of a network of silver nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 19303. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, L.; Ke, W.; Zheng, F.; Li, X. Electroactive Au@Ag nanoparticle assembly driven signal amplification for ultrasensitive chiral recognition of D/LTrp. ACS Sustain. Chem. Eng. 2019, 7, 5157–5166. [Google Scholar] [CrossRef]
- Zhou, M.; Han, L.; Deng, D.; Zhang, Z.; He, H.; Zhang, L.; Luo, L. 4-Mercaptobenzoic acid modified silver nanoparticles-enhanced electrochemical sensor for highly sensitive detection of Cu2+. Sens. Actuat B. Chem. 2019, 291, 164. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, Y.; Wu, S.; Jalalah, M.; Alsareii, S.A.; Yin, Y.; Harraz, F.A.; Li, G. Co-assembly of peptides and carbon nanodots: Sensitive analysis of transglutaminase 2. ACS Appl. Mater. Interfaces 2021, 13, 36919–36925. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yu, J.; Bo, B.; Shu, Y.; Li, G. A simple and general approach to assay protease activity with electrochemical technique. Biosens. Bioelectron. 2013, 45, 1. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chen, C.-P.; Lee, C.-H.; Chen, C.-Y.; Lin, C.-W. Colorimetric detection of human chorionic gonadotropin using catalytic gold nanoparticles and apeptide aptamer. Chem. Commun. 2014, 50, 14443. [Google Scholar] [CrossRef] [Green Version]
- Nowinski, A.K.; Sun, F.; White, A.D.; Keefe, A.J.; Jiang, S. Sequence, structure, and function of peptide self-assembled monolayers. J. Am. Chem. Soc. 2012, 134, 6000–6005. [Google Scholar] [CrossRef] [Green Version]
- Xia, N.; Huang, Y.; Cui, Z.; Liu, S.; Deng, D.; Liu, L.; Wang, J. Impedimetric biosensor for assay of caspase-3 activity and evaluation of cell apoptosis using self-assembled biotin-phenylalanine network as signal enhancer. Sens. Actuat. B Chem. 2020, 320, 128436. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Liu, G.; La, M.; Liu, L. Colorimetric and Electrochemical Methods for the Detection of SARS-CoV-2 Main Protease by Peptide-Triggered Assembly of Gold Nanoparticles. Molecules 2022, 27, 615. https://doi.org/10.3390/molecules27030615
Feng Y, Liu G, La M, Liu L. Colorimetric and Electrochemical Methods for the Detection of SARS-CoV-2 Main Protease by Peptide-Triggered Assembly of Gold Nanoparticles. Molecules. 2022; 27(3):615. https://doi.org/10.3390/molecules27030615
Chicago/Turabian StyleFeng, Yunxiao, Gang Liu, Ming La, and Lin Liu. 2022. "Colorimetric and Electrochemical Methods for the Detection of SARS-CoV-2 Main Protease by Peptide-Triggered Assembly of Gold Nanoparticles" Molecules 27, no. 3: 615. https://doi.org/10.3390/molecules27030615
APA StyleFeng, Y., Liu, G., La, M., & Liu, L. (2022). Colorimetric and Electrochemical Methods for the Detection of SARS-CoV-2 Main Protease by Peptide-Triggered Assembly of Gold Nanoparticles. Molecules, 27(3), 615. https://doi.org/10.3390/molecules27030615






