Physicochemical Characterization of the Loganic Acid–IR, Raman, UV-Vis and Luminescence Spectra Analyzed in Terms of Quantum Chemical DFT Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Molecular Geometry
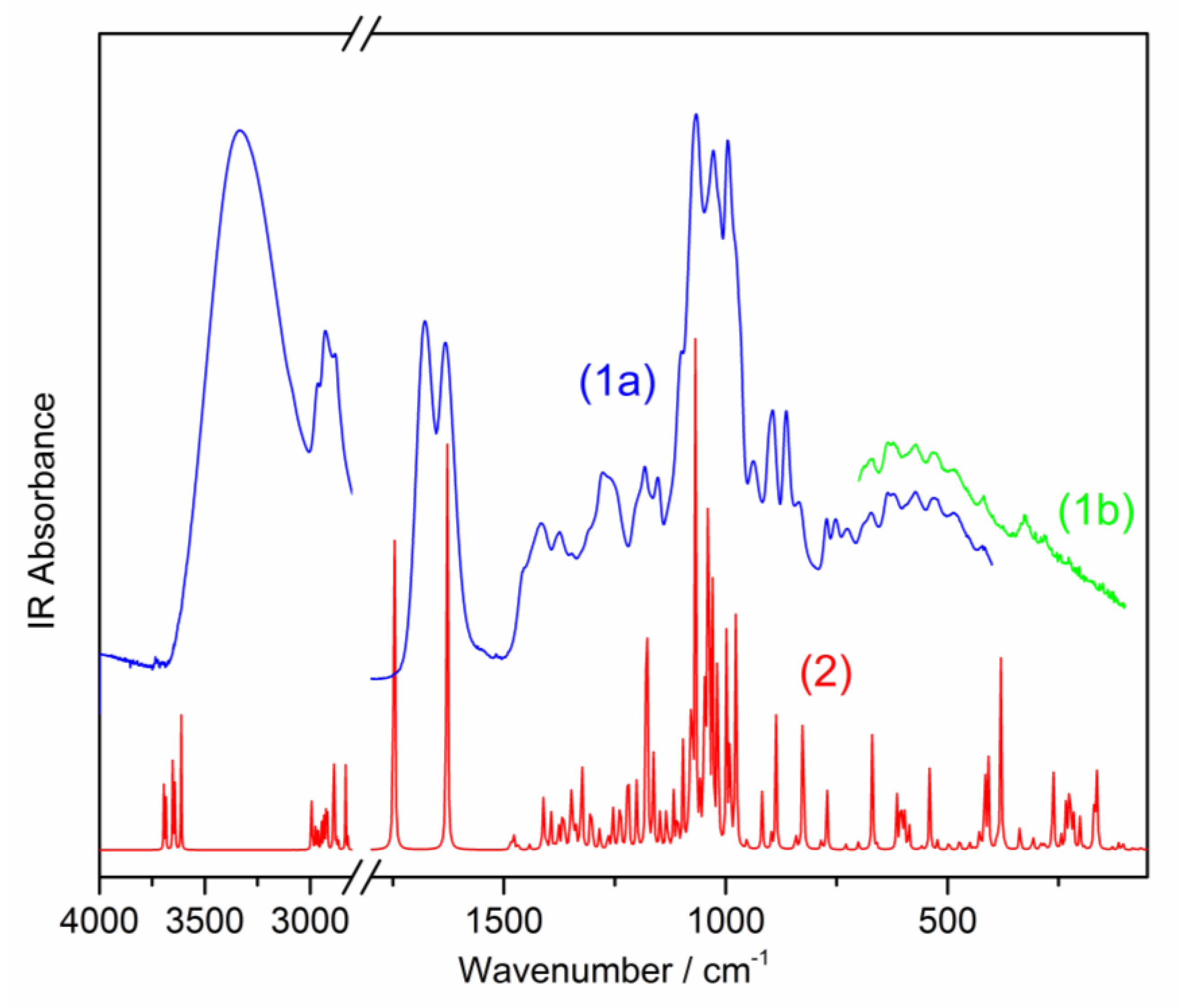
2.2. Vibrational Spectra
2.2.1. Vibrations of the Cyclopentane[c]pyran System (Φ, φ and Coupled Φφ)
2.2.2. Vibrations of the Pyran Ring
2.2.3. Carboxylic Group Vibrations
2.2.4. Vibrations of the C–O–C, i.e., θ-O-ΦBridged Bond
2.2.5. Vibrations of the CH, CH2, and CH3 Chromophores
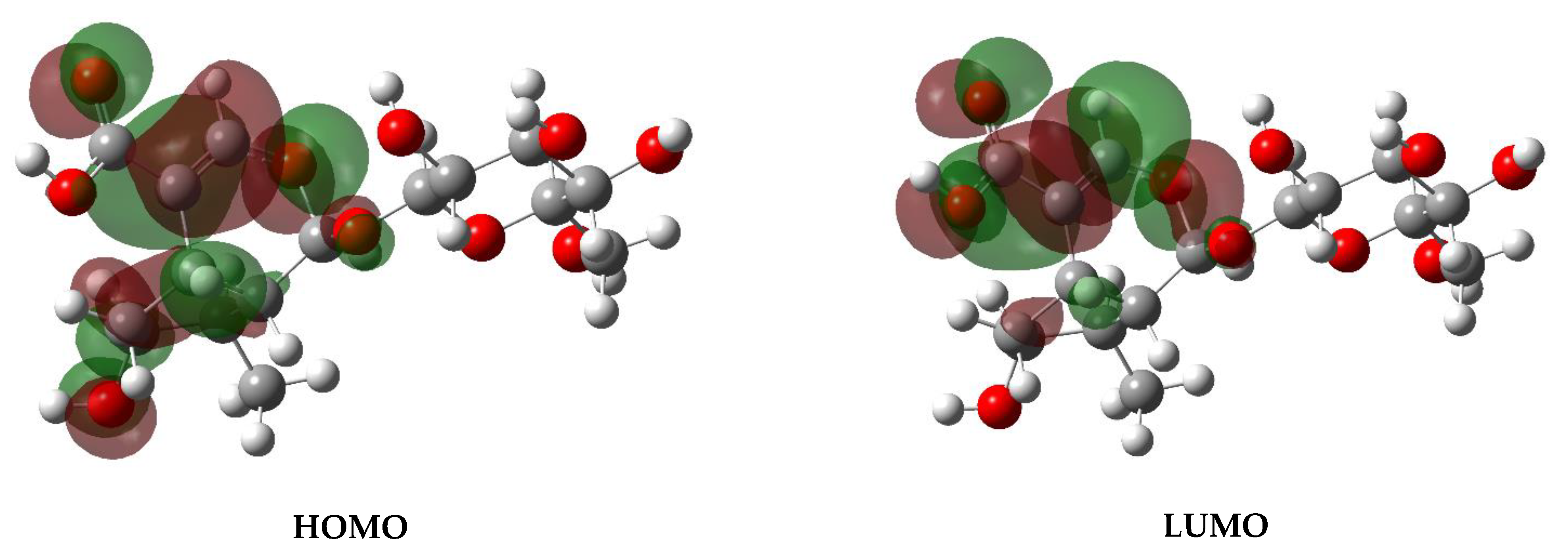
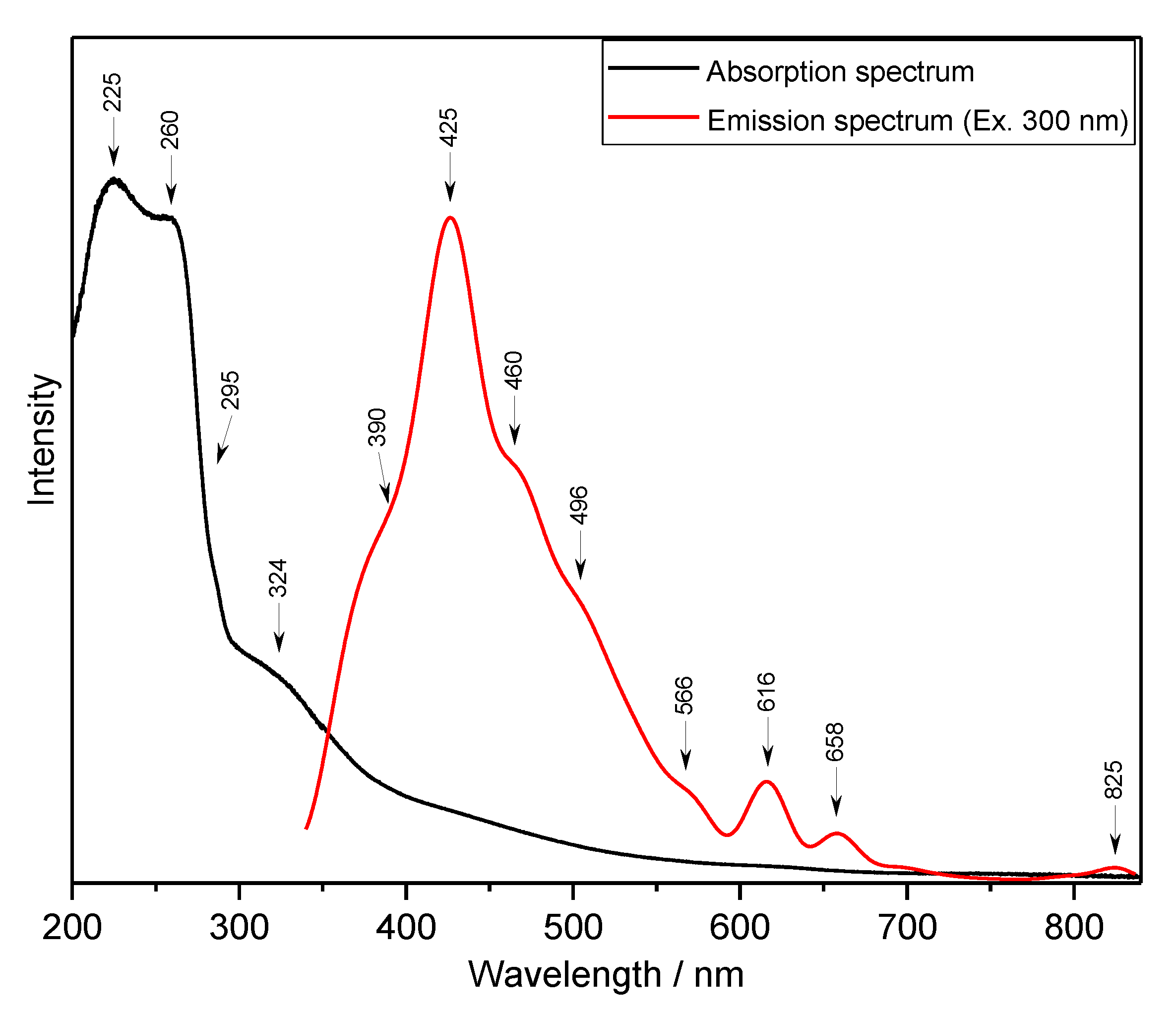
2.3. UV-Vis and Luminescence Spectra-Electron States and NBO Analysis
- The polar character of the C–O–C bridge follows from the positive 0.437 and 0.456 values of the charges on the carbon Cθand CΦatoms and the negative value of −0.591 on the oxygen atom.
- Oxygen atoms inside the θring and Φring exhibit the charges −0.61 and −0.59, respectively, i.e., they have similar character.
- All the hydroxyl groups appearing in the loganic molecule exhibit similar electron properties—the atomic charges of the oxygen atoms take the values from −0.735 to −0.755.
- The charges of the carbon atoms differ depending on the place inside the ring and substituent bonded to this atom. The carbon atoms of the pyran θring have positive charge (0.095, 0.107, 0.115, 0.105, and 0.437)—the greatest value corresponds to the atom bonded to the bridging oxygen. Another situation appears in the coupled cyclopentane-pyran Φφsystem. Its carbon atoms change their charge from positive values, 0.456, 0.259, and 0.202, to negative, −0.220, −0.224, −0.245, −0.255, and −0.403. The greatest value appears for the carbon joining the bridging oxygen atom and the smallest value belongs to the C15 atom in the φring.
- The peculiar situation is observed for the carboxyl group where the C25 atom has charge 0.822, the O9 oxygen atom of the C=O group has charge −0.633, the O8 oxygen atom of the OH group has charge ™0.711, and the H49 hydrogen atom has charge 0.483 (see atomic numbering in Figure 1).
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cipollini, D.; Stevenson, R.; Enright, S.; Eyles, A.; Bonello, P. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivore effects. J. Chem. Ecol. 2008, 34, 144–152. [Google Scholar] [CrossRef]
- Pengfei, Z.; Jianhua, Z.; Rongmin, Y.; Jiachen, Z. Enzyme inhibitors cause multiple effects on accumulation of monoterpene indole alkaloids in Catharanthus roseus cambial meristematic cell cultures. Pharmacogn. Mag. 2017, 13, 732–737. [Google Scholar]
- Ludwiczuk, A.; Skalicka-Woźniak, K.; Georgiev, M.I. Chapter 11-Terpenoids; Badal, S., Delgoda, R.B.T.-P., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 233–266. ISBN 978-0-12-802104-0. [Google Scholar]
- Kucharska, A.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.-Y.; Wang, R.; Shi, Y.-P. Chemical constituents from the fruits of Cornus officinalis. Biochem. Syst. Ecol. 2012, 45, 120–123. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Bowers, M.D. Iridoid and secoiridoid glycosides in a hybrid complex of bush honeysuckles (Lonicera spp., Caprifolicaceae): Implications for evolutionary ecology and invasion biology. Phytochemistry 2013, 86, 57–63. [Google Scholar] [CrossRef]
- Murata, J.; Roepke, J.; Gordon, H.; De Luca, V. The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant. Cell 2008, 20, 524–542. [Google Scholar] [CrossRef] [Green Version]
- Madyastha, K.M.; Guarnaccia, R.; Baxter, C.; Coscia, C.J. S-Adenosyl-L-methionine: Loganic acid methyltransferase. A carboxyl-alkylating enzyme from Vinca rosea. J. Biol. Chem. 1973, 248, 2497–2501. [Google Scholar] [CrossRef]
- Petronikolou, N.; Hollatz, A.J.; Schuler, M.A.; Nair, S.K. Loganic Acid Methyltransferase: Insights into the Specificity of Methylation on an Iridoid Glycoside. ChemBioChem 2018, 19, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Debnath, S. Monoterpenes: Iridoids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.M., Eds.; Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3009–3067. ISBN 9783642221439. [Google Scholar]
- Whitehead, S.R.; Tiramani, J.; Bowers, M.D. Iridoid glycosides from fruits reduce the growth of fungi associated with fruit rot. J. Plant. Ecol. 2016, 9, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Graikou, K.; Aligiannis, N.; Chinou, I.B.; Harvala, C. Cantleyoside-dimethyl-acetal and other iridoid glucosides from Pterocephalus perennis--antimicrobial activities. Z. Für Nat. C 2002, 57, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Szumny, A.; Magdalan, J.; Bielska, K.; Merwid-Ląd, A.; Woźniak, A.; Dzimira, S.; Piórecki, N.; Trocha, M. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine 2014, 21, 1774–1784. [Google Scholar] [CrossRef]
- Szumny, D.; Sozański, T.; Kucharska, A.Z.; Dziewiszek, W.; Piórecki, N.; Magdalan, J.; Chlebda-Sieragowska, E.; Kupczynski, R.; Szeląg, A.; Szumny, A. Application of Cornelian Cherry Iridoid-Polyphenolic Fraction and Loganic Acid to Reduce Intraocular Pressure. Evid.-Based Complementary Altern. Med. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Sozański, T.; Kucharska, A.; Dzimira, S.; Magdalan, J.; Szumny, D.; Matuszewska, A.; Nowak, B.; Piórecki, N.; Szeląg, A.; Trocha, M. Loganic acid and anthocyanins from cornelian cherry (Cornus mas L.) fruits modulate diet-induced atherosclerosis and redox status in rabbits. Adv. Clin. Exp. Med. 2018, 27, 1505–1513. [Google Scholar] [CrossRef]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef] [Green Version]
- Sozański, T.; Kucharska, A.Z.; Wiśniewski, J.; Fleszar, M.G.; Rapak, A.; Gomułkiewicz, A.; Dzięgiel, P.; Magdalan, J.; Nowak, B.; Szumny, D.; et al. The iridoid loganic acid and anthocyanins from the cornelian cherry (Cornus mas L.) fruit increase the plasma l-arginine/ADMA ratio and decrease levels of ADMA in rabbits fed a high-cholesterol diet. Phytomedicine 2019, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chaimum-aom, N.; Chomko, S.; Talubmook, C. Toxicology and Oral glucose Tolerance Test (OGTT) of Thai Medicinal Plant Used for Diabetes controls, Phyllanthus acidus L. (EUPHORBIACEAE). Pharmacogn. J. 2016, 9, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Jia, N.; Chu, W.; Li, Y.; Ding, L.; Duan, J.; Cui, J.; Cao, S.; Zhao, C.; Wu, Y.; Wen, A. Iridoid glycosides from the flowers of Gentiana macrophylla Pall. ameliorate collagen-induced arthritis in rats. J. Ethnopharmacol. 2016, 189, 1–9. [Google Scholar] [CrossRef]
- del Carmen Recio, M.; Giner, R.; Máñez, S.; Ríos, J. Structural Considerations on the Iridoids as Anti-Inflammatory Agents. Planta Med. 1994, 60, 232–234. [Google Scholar] [CrossRef]
- Ji, D.; Zhang, C.; Li, J.; Yang, H.; Shen, J.; Yang, Z. A New Iridoid Glycoside from the Roots of Dipsacus asper. Molecules 2012, 17, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Sozański, T.; Kucharska, A.Z.; Rapak, A.; Szumny, D.; Trocha, M.; Merwid-Ląd, A.; Dzimira, S.; Piasecki, T.; Piórecki, N.; Magdalan, J.; et al. Iridoid–loganic acid versus anthocyanins from the Cornus mas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis 2016, 254, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, J.; Yeo, S.; Kim, G.; Ko, E.-H.; Lee, S.; Li, W.; Choi, C.; Jeong, S.-Y. Antiadipogenic Effects of Loganic Acid in 3T3-L1 Preadipocytes and Ovariectomized Mice. Molecules 2018, 23, 1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesavan, R.; Chandel, S.; Upadhyay, S.; Bendre, R.; Ganugula, R.; Potunuru, U.R.; Giri, H.; Sahu, G.; Kumar, P.U.; Reddy, G.B.; et al. Gentiana lutea exerts anti-atherosclerotic effects by preventing endothelial inflammation and smooth muscle cell migration. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 293–301. [Google Scholar] [CrossRef]
- Ma, W.; Wang, K.-J.; Cheng, C.-S.; Yan, G.; Lu, W.-L.; Ge, J.-F.; Cheng, Y.-X.; Li, N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014, 153, 840–845. [Google Scholar] [CrossRef]
- Quideau, S. Flavonoids. Chemistry, Biochemistry and Applications; Andersen, Ø.M., Markham., K.R., Eds.; CRC Press: Weinheim, Germany, 2006; Volume 45, pp. 6786–6787. [Google Scholar]
- Wu, M.; Wu, P.; Liu, M.; Xie, H.; Jiang, Y.; Wei, X. Iridoids from Gentiana loureirii. Phytochemistry 2009, 70, 746–750. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Q.; Xiao, H.; Liang, X. Iridoid glucosides from Strychnos nux-vomica. Phytochemistry 2003, 64, 1341–1344. [Google Scholar] [CrossRef]
- Deng, S.; West, B.J.; Jensen, C.J. UPLC-TOF-MS Characterization and Identification of Bioactive Iridoids in Cornus mas Fruit. J. Anal. Methods Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergeron, C.; Marston, A.; Gauthier, R.; Hostettmann, K. Iridoids and secoiridoids from Gentiana linearis. Phytochemistry 1997, 44, 633–637. [Google Scholar] [CrossRef]
- Kim, J.S. Seeds of Cornus officinalis and Diabetic Cataracts. In Handbook of Nutrition, Diet and the Eye; Elsevier: Amsterdam, The Netherlands, 2014; pp. 451–458. [Google Scholar]
- Heckendorf, A.H.; Mattes, K.C.; Hutchinson, C.R.; Hagaman, E.W.; Wenkert, E. Stereochemistry and conformation of biogenetic precursors of indole alkaloids. J. Org. Chem. 1976, 41, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.-F.; Niemeyer, U.; Marx, P.; Glüsenkamp, K.-H. Iridoide—XII: Bestimmung der relativen konfiguration und konformation isomerer iridoidglycoside mit hilfe der 1H-, 13C-NMR- und massenspektroskopie. Tetrahedron 1980, 36, 1231–1236. [Google Scholar] [CrossRef]
- Tietze, L.-F.; Niemeyer, U.; Marx, P.; Glüsenkamp, K.-H.; Schwenen, L. Iridoide—XIII: Bestimmung der absoluten konfiguration und konformation isomerer iridoidglycoside mit hilfe chiroptischer methoden. Tetrahedron 1980, 36, 735–739. [Google Scholar] [CrossRef]
- Jones, P.G.; Sheldrick, G.M.; Glüsenkamp, K.-H.; Tietze, L.F. Loganin. Acta Crystallogr. Sect. B36 Struct. Crystallogr. Cryst. Chem. 1980, 36, 481–483. [Google Scholar] [CrossRef]
- Lentz, P.J.; Rossmann, M.G. The crystal structure of loganin penta-acetate monomethyl ether bromide. J. Chem. Soc. D Chem. Commun. 1969, 21, 1269a. [Google Scholar] [CrossRef]
- Coscia, C.J.; Guarnaccia, R.; Botta, L. Monoterpene biosynthesis. I. Occurrence and mevalonoid origin of gentiopicroside and loganic acid in Swertia caroliniensis. Biochemistry 1969, 8, 5036–5043. [Google Scholar] [CrossRef]
- Calis, I.; Lahloub, M.F.; Sticher, O. Loganin, loganic acid and periclymenoside, a new biosidic ester iridoid glucoside fromLonicera periclymenumL. (Caprifoliaceae). Helv. Chim. Acta 1984, 67, 160–165. [Google Scholar] [CrossRef]
- Skaltsounis, A.-L.; Tillequin, F.; Koch, M.; Pusset, J.; Chauvière, G. Iridoids from Scaevola racemigera 1. Planta Med. 1989, 55, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, L.; Caceres, A.; Morales, C.; De Simone, F.; Aquino, R. Iridoids from Lippia graveolens. Phytochemistry 1998, 49, 1829–1832. [Google Scholar] [CrossRef]
- Bianco, A.; Ramunno, A.; Melchioni, C. Iridoids from Seeds of Gentiana Lutea. Nat. Prod. Res. 2003, 17, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Song, W.; Zhu, C.; Lin, S.; Zhao, F.; Wu, X.; Yue, Z.; Liu, B.; Wang, S.; Yuan, S.; et al. Homosecoiridoids from the Flower Buds of Lonicera japonica. J. Nat. Prod. 2011, 74, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, A.Z.; Szumny, A.; Sokół-Łętowska, A.; Piórecki, N.; Klymenko, S.V. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J. Food Compos. Anal. 2015, 40, 95–102. [Google Scholar] [CrossRef]
- Cadet, F.; Bertrand, D.; Robert, P.; Maillot, J.; Dieudonné, J.; Rouch, C. Quantitative Determination of Sugar Cane Sucrose by Multidimensional Statistical Analysis of Their Mid-Infrared Attenuated Total Reflectance Spectra. Appl. Spectrosc. 1991, 45, 166–172. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; Wiley: New York, NY, USA, 2004; ISBN 9780470093078. [Google Scholar]
- Koczón, P.; Lewandowski, W.; Mazurek, A. Vibrational (FT-IR and FT-Raman) and NMR studies on selected metal (Ca, Mn, Zn) complexes with ortho-, meta-, and para-iodobenzoic acids. Vib. Spectrosc. 1999, 20, 143–149. [Google Scholar] [CrossRef]
- Saini, M.; Sangwan, R.; Khan, M.F.; Kumar, A.; Verma, R.; Jain, S. Specioside (SS) & verminoside (VS) (Iridoid glycosides): Isolation, characterization and comparable quantum chemical studies using density functional theory (DFT). Heliyon 2019, 5, e01118. [Google Scholar] [PubMed] [Green Version]
- EXTRASYNTHESE Loganic Acid. Available online: https://www.extrasynthese.com/loganic-acid.html (accessed on 4 October 2020).


| Bond Distances | Calc. | Exp. | Bond Angles | Calc. | Exp. |
|---|---|---|---|---|---|
| O1—C16 | 1.442 | 1.426 | C11—O1—C19 | 115.5 | 114.7 |
| O2—C16 | 1.475 | 1.432 | O1—C16—O2 | 108.7 | 109.6 |
| O2—C20 | 1.374 | 1.357 | C16—O2—C20 | 116.6 | 115.0 |
| C20—C17 | 1.354 | 1.333 | O2—C20—C17 | 125.1 | 124.4 |
| C17—C12 | 1.502 | 1.510 | C20—C17—C12 | 122.0 | 123.2 |
| C12—C11 | 1.561 | 1.549 | C17—C12—C11 | 110.7 | 110.7 |
| C11—C16 | 1.515 | 1.509 | C17—C12—C15 | 113.9 | 112.3 |
| C12—C15 | 1.551 | 1.551 | C12—C11—C16 | 113.2 | 113.0 |
| C15—C14 | 1.139 | 1.522 | C11—C16—O2 | 111.6 | 113.1 |
| C14—C13 | 1.562 | 1.522 | O1—C19—O4 | 107.7 | 107.5 |
| C13—C11 | 1.566 | 1.535 | C19—O4—C24 | 1132 | 111.7 |
| C17—C25 | 1.466 | 1.482 | O4—C24—C23 | 107.5 | 108.8 |
| C25—O9 | 1.243 | 1.197 | C24—C23—C22 | 110.0 | 110.7 |
| C25—O8 | 1.389 | 1.341 | C23—C22—C21 | 111.3 | 111.1 |
| C14—O3 | 1.457 | 1.439 | C22—C21—C19 | 109.1 | 108.4 |
| C13—C18 | 1.537 | 1.528 | C12—C15—C14 | 104.8 | 106.3 |
| O1—C19 | 1.432 | 1.396 | C15—C14—C13 | 105.8 | 103.9 |
| O4—C19 | 1.448 | 1.433 | C12—C11—C13 | 106.8 | 104.4 |
| O4—C24 | 1.473 | 1.422 | C16—C11—C13 | 112.6 | 114.2 |
| C23—C24 | 1.534 | 1.530 | C20—C17—C25 | 115.5 | 120.0 |
| C23—C22 | 1.526 | 1.529 | C17—C25—O9 | 126.7 | 123.5 |
| C22—C21 | 1.522 | 1.515 | C17—C25—O8 | 113.0 | 113.4 |
| C21—C19 | 1.523 | 1.511 | C15—C14—O3 | 113.1 | 111.9 |
| C24—C26 | 1.512 | 1.507 | C14—C13—C18 | 114.1 | 115.5 |
| C26—O10 | 1.453 | 1.418 | O4—C24—C26 | 107.8 | 107.0 |
| C23—O7 | 1.447 | 1.415 | C24—C23—O7 | 108.2 | 112.1 |
| C22—O6 | 1.450 | 1.414 | C23—C22—O6 | 106.6 | 111.1 |
| C21—O5 | 1.449 | 1.419 | C22—C21—O5 | 106.3 | 108.7 |
| Torsion angles | Calc. | Exp. | |||
| C20—C17—C12—C15 | −122.4 | −117.3 | |||
| O2—C16—C11—C13 | 68.0 | 67.5 | |||
| C16—O1—C19—O4 | −67.2 | −87.5 | |||
| C16—O1—C19—C21 | 174.9 | 153.2 | |||
| C14—C13—C11—C16 | −138.5 | −165.4 | |||
| No. | Calculated | Experimental | Assignment for Monomer | |||
|---|---|---|---|---|---|---|
| dimer | monomer | IR | RS | |||
| 1 | 3582 | 3582 | 3695(12,2) | ν(OH)-θ(99) | ||
| 2 | 3579 | 3579 | 3684(11,2) | ν(OH)-φ(98) | ||
| 3 | 3571 | 3571 | 3653(17,1) | ν(OH)-φ(99) | ||
| 4 | 3570 | 3570 | 3650(6,0) | ν(OH)-θ(98) | ||
| 5 | 3551 | 3551 | 3642(17,1) | 3400 sh | ν(OH⋅⋅⋅O)-θ(99) | |
| 6 | 3208 | 3208 | 3613(28,2) | 3334 vs,b | ν(OH⋅⋅⋅O)-Φ(98) (A group) | |
| 7 | 3098 | 3098 | 3081(0,2) | ν(CH)-Φ(99) | ||
| 8 | 3091 | 3091 | 2995(7,2) | 3086 sh | 3083 w | ν(CH)-Φ(99) |
| 9 | 3089 | 3089 | 2993(7,2) | νas(CH3)-Φ(99) | ||
| 10 | 3061 | 3061 | 2998(3,2) | νas(CH2)-φ(89) | ||
| 11 | 3026 | 3026 | 2974(10,1) | νas(CH3)-φ(97) | ||
| 12 | 3025 | 3025 | 2964(5,1) | ν(CH)-Φ(94) | ||
| 13 | 3014, 3008 | 3014, 3008 | 2946(10,3) | νas(CH2)-θ(93) | ||
| 14 | 2999 | 2999 | 2936(2,1) | ν(CH)-Φ(92) | ||
| 15 | 2997 | 2997 | 2934(10,2) | ν(CH)-θ(74) | ||
| 16 | 2992 | 2992 | 2925(6,2) | νas(CH2)-φ(82) | ||
| 17 | 2987 | 2987 | 2922(4,3) | ν(CH)-θ(85) | ||
| 18 | 2980 | 2980 | 2917(7,3) | ν(CH)-φ(75) | ||
| 19 | 2971 | 2971 | 2889(28,1) | νs(CH3)-φ(93) | ||
| 20 | 2967 | 2967 | 2885(8,1) | 2963 s | 2963 sh | ν(CH)-θ(98) |
| 21 | 2958 | 2958 | 2876(2,3) | ν(CH)-θ(90) | ||
| 22 | 2956 | 2956 | 2868(2,3) | 2928 s | 2936 vs | νs(CH2)-θ(91) |
| 23 | 2867 | 2833(16,3) | 2882 s | 2884 s | ν(CH)-θ(96) | |
| 24 | 2726 | 2822(2,0) | νs(CH2)-φ(89) | |||
| 25 | 1644 | 1638 | 1747(92,2) | 1677 s | 1682 s | ν(C=O)-Φ(A group) (69) |
| 26 | 1615 | 1605 | 1623(100,8) | 1632 s | 1634 vs | ν(Φ) (45) + ν(C=O) (A group) (17) |
| 27 | 1519 | 1519 | 1486(2,1) | 1517 vw | 1526 vw | δas(CH2)-θ(75) + δ(COH)-θ(11) |
| 28 | 1517 | 1517 | 1481(1,1) | δas(CH2)-φ(77) | ||
| 29 | 1511 | 1511 | 1477(2,1) | 1490 sh | δas(CH3)-φ(87) | |
| 30 | 1503 | 1503 | 1468(2,1) | 1481 sh | δas(CH3)-φ(90) | |
| 31 | 1460 | 1442(1,0) | 1458 w | 1459 m | δ(CH2OH)-θ(72) | |
| 32 | 1449 | 1449 | 1415(2,1) | 1454 sh | δ(COH)-θ(42) + δ(CCH)-θ(31) | |
| 33 | 1439 | 1404(9,0) | δs(CH3)-φ(56) + δ(CCH)-φ(24) | |||
| 34 | 1435 | 1434 | 1405(1,0) | δ(COH)-θ(53) + δ(CCH)-θ(34) | ||
| 35 | 1425 | 1425 | 1404(1,1) | ν(θ) (49) + ν(COC) (25) | ||
| 36 | 1416 | 1416 | 1396(1,1) | 1416 w | 1416 sh | ν(θ) (43) + ν(COC) (30) |
| 37 | 1410 | 1410 | 1394(7,0) | 1412 sh | 1408 sh | ν(φ) (59) |
| 38 | 1403 | 1403 | 1385(1,0) | ν(φ) (49) + δ(COH)-φ(34) | ||
| 39 | 1403 | 1402 | 1377(6,0) | 1405 sh | ν(θ) (58) + δ(CCH)-θ(24) | |
| 40 | 1397 | 1397 | 1375(0,1) | ν(Φ) (93) | ||
| 41 | 1382 | 1382 | 1368(5,1) | 1384 sh | ν(φ) (84) | |
| 42 | 1376 | 1376 | 1363(9,1) | 1378 sh | ν(φ) (78) | |
| 43 | 1371 | 1371 | 1349(9,0) | 1374 w | 1372 m | ν(φ) (38) + δ(CCH)-φ(29) |
| 44 | 1369 | 1369 | 1347(13,0) | 1363 sh | ν(θ) (54) + δ(CCH)-θ(27) | |
| 45 | 1355 | 1355 | 1341(2,0) | ν(COC) (27) + δ(CCH)-Φ(25) | ||
| 46 | 1353 | 1353 | 1337(4,1) | ν(COC) (29) + ν(Φ) (25) | ||
| 47 | 1351 | 1351 | 1329(3,1) | ν(Φ) (72) | ||
| 48 | 1345 | 1345 | 1325(5,0) | 1347 w | ν(θ) (50) + δ(COH)-θ(25) | |
| 49 | 1337 | 1337 | 1324(16,0) | 1340 sh | ν(θ) (55) + ν(COC) (32) | |
| 50 | 1330 | 1330 | 1304(14,0) | 1331 w | ν(φ) (44) + δ(CCH)-φ(23) | |
| 51 | 1323 | 1323 | 1299(2,0) | ν(θ) (60) + δ(COH)-θ(21) | ||
| 52 | 1308 | 1308 | 1284(6,0) | 1310 sh | ν(φ) (41) + δ(CCH)-φ(35) | |
| 53 | 1301 | 1295 | 1267(1,1) | ν(Φ) (47) + δ(CCH)-Φ(22) | ||
| 54 | 1295 | 1294 | 1264(2,0) | 1295 sh | ν(φ) (35) + δ(CCH)-φ(24) | |
| 55 | 1293 | 1292 | 1254(11,0) | ν(C-CH3)-θ(54) + δ(COH)-θ((19) | ||
| 56 | 1283 | 1283 | 1241(5,0) | 1276 m | 1276 sh | δ(CH2)-θ(64) + δ(COH)-θ(34) |
| 57 | 1261 | 1261 | 1238(12,1) | 1260 sh | 1264 w | δ(COH)-θ(55) |
| 58 | 1239 | 1239 | 1223(6,0) | 1254 sh | ν(φ)-(49) + δ(CCH)-φ(29) | |
| 59 | 1238 | 1238 | 1221(8,0) | δ(COH)-φ(44) + δ(CCH)-φ(35) | ||
| 60 | 1230 | 1230 | 1219(12,1) | δ(CCH)-θ(71) + ν(C–C)-θ(24) | ||
| 61 | 1222 | 1222 | 1201(15,0) | ν(φ) (35) + δ(CCH)–Φ(34) + ν(Φ) (29) | ||
| 62 | 1220 | 1239 | 1180(31,0) | ν(θ) (53) + δ(CCH)-θ(27) + δ(COH)-θ(10) | ||
| 63 | 1196 | 1194 | 1177(33,1) | 1202 sh | 1200 w | ν(φ) (47) + δ(C-COOH)-Φ(38) |
| 64 | 1191 | 1191 | 1163(22,0) | 1182 m | 1185 sh | δ(C-COOH)-Φ(44) + ν(Φ) (41) |
| 65 | 1163 | 1162 | 1148(9,1) | ν(Φ) (41) + δ(CCH)-Φ(36) | ||
| 66 | 1149 | 1148 | 1134(7,2) | 1153 m | 1150 sh | νas(C–O–C) (53) + ν(C-COH)-θ(41) |
| 67 | 1138 | 1138 | 1130 (4,0) | ν(C-CH3)-φ(76) + ν(C-C)-θ(22) | ||
| 68 | 1136 | 1136 | 1117(11,0) | ν(θ) (96) | ||
| 69 | 1121 | 1121 | 1108(10,1) | 1121 sh | 1124 sh | ν(C-CH3)-φ(65) + ν(C-OH)-φ(30) |
| 70 | 1101 | 1101 | 1097(20,1) | 1117 m | ν(C-OH) θ(51) + ν(θ) (45) | |
| 71 | 1088 | 1088 | 1091(1,1) | 1099 sh | ν(θ) (55) + δ(COH)-θ(42) | |
| 72 | 1080 | 1078 | 1083(10,1) | 1083 m | ν(θ) (74) + δ(COH)-θ(22) | |
| 73 | 1077 | 1075 | 1077(51,3) | ν(θ) (52) + δ(COH)-θ(46) | ||
| 74 | 1072 | 1069 | 1068(92,0) | 1067 w | δ(COH)-θ(54) + ν(C–C)-θ(42) | |
| 75 | 1066 | 1064 | 1058(9,1) | 1066 vs | ν(φ) (71) + δ(CCH)-φ(26) | |
| 76 | 1059 | 1058 | 1049(37,1) | ν(θ) (53) + ν(COH)-θ(43) | ||
| 77 | 1051 | 1051 | 1046(20,1) | 1047 sh | ν(C-OH)-φ(48) + ν(C-CH3)-φ(41) | |
| 78 | 1043 | 1042 | 1040(56,0) | ν(φ) (54) + δ(CCH)-φ(44) | ||
| 79 | 1035 | 1034 | 1039(54,1) | ν(θ) (94) | ||
| 80 | 1026 | 1026 | 1029(50,0) | 1028 vs | 1029 sh | ν(φ) (46) + ν(Φ) (45) |
| 81 | 1021 | 1019 | 1018(49,0) | ν(C-OH)-φ(51) + δ(C-CH3)-φ(44) | ||
| 82 | 1015 | 999(39,0) | 1016 sh | δ(φ) (96) | ||
| 83 | 1006 | 1006 | 990(28,0) | δ(θ) (96) | ||
| 84 | 997 | 997 | 979(6,0) | 995 vs | 999 w | δ(Φ) (97) |
| 85 | 994 | 993 | 978(11,1) | δ(θ) (55) + νs(C–O–C) (43) | ||
| 86 | 978 | 977 | 977(36,1) | 978 sh | 979 vw | δ(θ) (66) + νs(C–O–C) (32) |
| 87 | 971 | 971 | 971(11.1) | 967 sh | 968 sh | δ(θ) (52) + ν(C-OH) (41) |
| 88 | 959 | 958 | 952(2,0) | 937 w | 937 sh | νs(C–O–C) (52) + δ(Φ + φ) (46) |
| 89 | 917 | 916 | 918(11,0) | 901 sh | γ(C-CH3)-φ(74) + δ(φ) (23) | |
| 90 | 895 | 894 | 897(3,1) | 894 m | 897 w | δ(θ) (55) + δ(C-CH3)-θ(41) |
| 91 | 876 | 876 | 886(25,1) | 864 m | 865 w | δ(φ) (94) |
| 92 | 846 | 846 | 843(4,2) | 835 w | 837 w | γ(Φ) (95) |
| 93 | 812 | 811 | 826(36,1) | 806 vw | γ(Φ-φ) (88) | |
| 94 | 789 | 789 | 784(2,3) | 772 w | 796 vw | γ(Φ-COOH) (68) + γ(Φ) (31) |
| 95 | 757 | 756 | 772(12,1) | 752 w | 759 m | γ(Φ-COOH) (48) + γ(Φ) (46) |
| 96 | 741 | 729 | 730(1,5) | 726 w | 725 vw | γ(Φ-COOH) (61) + γ(Φ) (33) |
| 97 | 704 | 701 | 700(2,1) | 704 vw | δ(C–O–C) (49) + τ(Φ-COOH) (43) | |
| 98 | 682 | 679 | 669(22,2) | 673 w | 678 vw | δ(C–O–C) (78) + ρ(Φ-COOH) (21) |
| 99 | 650 | 650 | 660(1,0) | 667 sh | τ(Φ-COOH) (86) + γ(φ) (13) | |
| 100 | 615 | 615 | 614(10,0) | 634 w | 631 vw | τ(θ-CH2OH) (48) + τ(φ-COOH) (43) |
| 101 | 609 | 609 | 606(11,1) | 623 w | 614 vw | τ(Φ-COOH) (61) + γ(φ) (33) |
| 102 | 603(5,0) | 601 vw | γ(C–O–C) (51) + τ(Φ-COOH) (38) | |||
| 103 | 590 | 590 | 598(8,2) | 593 vw | γ(θ) (47) + γ(θ-OH) (44) | |
| 104 | 583 | 583 | 587(6,1) | 587 sh | γ(φ) (58) + τ(φ-CH3) (43) | |
| 105 | 564 | 560 | 560 (1,1) | 573 w | γ(θ-O-Φ) (93) | |
| 106 | 551 | 538 | 540(16,1) | 538 sh | γ(Φ) (89) | |
| 107 | 532 | 526 | 523(2,2) | 532 w 530 w | 533 m | τ(Φ-COOH) (64) + γ(Φ-C–O–C) (32) |
| 108 | 517 | 505 | 497(2,1) | 522 sh 505 w | τ(θ-OH) (39) + τ(θ-CH2OH) (37) + γ(θ) (20) | |
| 109 | 476 | 471 | 472(3,1) | 487 w | 485 w | γ(φ) (56) + τ(θ-OH) (37) |
| 110 | 465 | 462 | 451(2,2) | 475 vw | τ(θ-OH) (61) + γ(θ) (37) | |
| 111 | 452 | 448 | 442(1,1) | 455 vw | τ(θ-OH) (54) + γ(θ) (36) | |
| 112 | 441 | 441 | 428(5,2) | τ(θ-OH) (62) + τ(θ) (31) | ||
| 113 | 433 | 433 | 417(20,3) | τ(θ) (47) + γ(θ-O-Φ) (43) | ||
| 114 | 425 | 424 | 409(17,1) | 421 w | τ(φ-OH) (34) + γ(Φ) (32) + τ(θ) (31) | |
| 115 | 417 | 416 | 389(4,1) | 419 vw | γ(θ) (51) + τ(θ-OH) (43) | |
| 116 | 400 | 399 | 380(35,4) | τ(φ-OH)(31) + τ(φ-CH3) (30) + τ(θ-CH2OH) (29) | ||
| 117 | 386 | 382 | 371(1,1) | τ(φ-CH3) (33) + τ(θ-OH) (32) + τ(θ-O-Φ) (32) | ||
| 118 | 370 | 368 | 337(6,5) | 362 sh | γ(θ) (64) + τ(θ-CH2OH) (32) | |
| 119 | 336 | 335 | 312(1,2) | 333 w | ω(θ-O-Φφ) (94) | |
| 120 | 329 | 319 | 307(2,1) | 328 vw | τ(HO-θ-OH) (48) + τ(HO-φ/Φ) (45) | |
| 121 | 310 | 302 | 291(1,3) | τ(HO-φ-CH3) (94) | ||
| 122 | 298 | 292 | 287(1,1) | τ(HO-φ-CH3) (68) + ρ(θ-OH) (37) | ||
| 123 | 287 | 287 | 281(2,1) | ρ(φ-CH3) (38) + ρ(θ-OH) ((29) + ρ(φ-OH) (29) | ||
| 124 | 284 | 283 | 263(21,0) | τ(φ-OH) (95) | ||
| 125 | 270 | 268 | 244(3,1) | 266 w | τ(HO-θ-OH) (53) + τ(θ) (41) | |
| 126 | 263 | 251 | 233(8,2) | τ(θ-CH2OH) (57) + τ(HO-θ-OH) (41) | ||
| 127 | 244 | 244 | 228(5,2) | τ(φ) (47) + τ(φ-COOH) (45) | ||
| 128 | 234 | 233 | 224(18,3) | τ(φ-CH3) (96) | ||
| 129 | 230 | 230 | 217(10,3) | 227 vw | τ(θ-CH2OH) (53) + τ(HO-θ-OH) (46) | |
| 130 | 221 | 218 | 202(7,3) | τ(φ-COOH) (66) + τ(φ) (31) | ||
| 131 | 215 | 214 | 191(0,2) | 204 sh | τ(θ-CH2OH) (93) | |
| 132 | 184 | 184 | 171(7,3) | τ(Φ + φ) (98) | ||
| 133 | 171 | 170 | 165(23,14) | 175 w | τ(θ-O-Φ) (96) | |
| 134 | 161 | 160 | 160(2,6) | 150 vw | 146 w | τ(θ) (56) + τ(θ-CH2OH) (29) + τ(θ-O-Φ) (11) |
| 135 | 132, 126 | 130 | 128(1,1) | 125 w | τ(θ-CH2OH) (49) + τ(HO-θ-OH) (43) | |
| 136 | 115 | 116 | 115(1,4) | 115 sh | τ(HO-θ-OH) (34) + τ(θ-CH2OH) (36)+ τ(θ-O-Φ) (23) | |
| 137 | 107 | 101 | 106(2,1) | τ(Φ-φ) (42) + τ(C-COOH)-Φ(33) | ||
| 138 | 102, 97 | 89 | 103(2.2) | τ(θ-COH) (49) + τ(θ-CH2) (45) | ||
| 139 | 78 | 80 | 82(0,3) | τ(Φ-COOH) (58) + τ(Φ) (44) | ||
| 140 | 73 | 72 | 74(0,5) | τ(Φ) (54) + ρ(C-COOH)-Φ(33) | ||
| 141 | 65 | 64 | 65(1,21) | τ(θ) (48) + ρ(COH)-θ(40) | ||
| 142 | 39 | 38 | 39(0,32) | τ(θ-Φ) (57) + ρ(COH)-φ(36) | ||
| 143 | 33 | 30 | 32(0,25) | τ(θ-Φφ(70) + τ(COH)-φ(19) | ||
| 144 | 24 | 22 | 21(0,100) | τ(θ-Φφ) (79) | ||
| Atomic Numbering | Mulliken Atomic Charges | NBO Atomic Charge | Atomic Numbering | Mulliken Atomic Charges | NBO Atomic Charge |
|---|---|---|---|---|---|
| O1 | −0.548 | −0.591 | C26 | 0.251 | −0.015 |
| O2 | −0.470 | −0.549 | H27 | 0.037 | 0.223 |
| O3 | −0.503 | −0.749 | H28 | 0.058 | 0.225 |
| O4 | −0.548 | −0.610 | H29 | 0.049 | 0.200 |
| O5 | −0.522 | −0.736 | H30 | 0.035 | 0.161 |
| O6 | −0.544 | −0.755 | H31 | 0.049 | 0.202 |
| O7 | −0.545 | −0.748 | H32 | 0.047 | 0.211 |
| O8 | −0.430 | −0.711 | H33 | 0.063 | 0.191 |
| O9 | −0.430 | −0.633 | H34 | 0.061 | 0.197 |
| O10 | −0.500 | −0.735 | H35 | 0.063 | 0.208 |
| C11 | −0.083 | −0.245 | H36 | 0.057 | 0.192 |
| C12 | −0.085 | −0224 | H37 | 0.002 | 0.144 |
| C13 | −0.127 | −0.220 | H38 | 0.073 | 0.201 |
| C14 | 0.278 | 0.166 | H39 | 0.057 | 0.196 |
| C15 | −0.067 | −0.403 | H40 | 0.235 | 0.455 |
| C16 | 0.591 | 0.456 | H41 | 0.022 | 0.157 |
| C 17 | −0.201 | −0.255 | H42 | 0.036 | 0.169 |
| C18 | −0.119 | −0.563 | H43 | 0.041 | 0.179 |
| C19 | 0.473 | 0.437 | H44 | 0.033 | 0.160 |
| C20 | 0.275 | 0.259 | H45 | 0.042 | 0.176 |
| C21 | 0.246 | 0.095 | H46 | 0.247 | 0.454 |
| C22 | 0.246 | 0.107 | H47 | 0.256 | 0.470 |
| C23 | 0.275 | 0.115 | H48 | 0.263 | 0.470 |
| C24 | 0.180 | 0.105 | H49 | 0.249 | 0.483 |
| C25 | 0.594 | 0.822 | H50 | 0.243 | 0.455 |
| No | Molecular Orbitals | Energy | Energy Gap | Ionization Potential I | Electron Affinity A | Global Hardness η | Chemical Potential μ | Electro-Negativity χ | Global Softness σ | Global Electrophilicity ω |
|---|---|---|---|---|---|---|---|---|---|---|
| [eV] | [eV] | [eV] | [eV] | [eV] | [eV] | [eV] | [eV] | [eV] | ||
| 1. | H | −6.62 | 5.68 | 6.62 | 0.94 | 2.84 | −3.78 | 3.78 | 0.18 | 2.52 |
| L | −0.94 | |||||||||
| 2. | H − 1 | −7.30 | 7.74 | 7.30 | 0.44 | 3.87 | −3.43 | 3.43 | 0.13 | 1.53 |
| L + 1 | 0.44 | |||||||||
| 3. | H − 2 | −7.42 | 8.15 | 7.42 | 0.73 | 4.08 | −3.35 | 3.35 | 0.12 | 1.38 |
| L + 2 | 0.73 |
| Electron Levels | eV | nm | cm−1 | Oscillator Strength |
|---|---|---|---|---|
| Singlets | ||||
| S1 | 5.1236 | 241.99 | 41,324 | 0.0073 |
| S2 | 5.3784 | 230.52 | 43,380 | 0.2038 |
| S3 | 5.8312 | 212.62 | 47,032 | 0.0182 |
| S4 | 5.8778 | 210.94 | 47,407 | 0.0516 |
| S5 | 6.2700 | 197.74 | 50,571 | 0.0001 |
| S6 | 6.3440 | 195.44 | 51,167 | 0.0008 |
| S7 | 6.4366 | 192.62 | 51,916 | 0.0041 |
| S8 | 6.5325 | 189.80 | 52,687 | 0.0020 |
| S9 | 6.5971 | 187.94 | 53,208 | 0.0020 |
| S10 | 6.6393 | 186.74 | 53,550 | 0.0004 |
| Triplets | ||||
| T1 | 3.4084 | 363.76 | 27,491 | 0.0000 |
| T2 | 4.7521 | 260.90 | 38,329 | 0.0000 |
| T3 | 5.5947 | 221.61 | 45,124 | 0.0000 |
| T4 | 5.7579 | 215.33 | 46,440 | 0.0000 |
| T5 | 5.8279 | 212.74 | 47,006 | 0.0000 |
| T6 | 6.2618 | 198.00 | 50,505 | 0.0000 |
| T7 | 6.3102 | 196.48 | 50,896 | 0.0000 |
| T8 | 6.3757 | 194.46 | 51,424 | 0.0000 |
| T9 | 6.4022 | 193.66 | 51,637 | 0.0000 |
| T10 | 6.4411 | 192.49 | 51,951 | 0.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zając, A.; Michalski, J.; Ptak, M.; Dymińska, L.; Kucharska, A.Z.; Zierkiewicz, W.; Hanuza, J. Physicochemical Characterization of the Loganic Acid–IR, Raman, UV-Vis and Luminescence Spectra Analyzed in Terms of Quantum Chemical DFT Approach. Molecules 2021, 26, 7027. https://doi.org/10.3390/molecules26227027
Zając A, Michalski J, Ptak M, Dymińska L, Kucharska AZ, Zierkiewicz W, Hanuza J. Physicochemical Characterization of the Loganic Acid–IR, Raman, UV-Vis and Luminescence Spectra Analyzed in Terms of Quantum Chemical DFT Approach. Molecules. 2021; 26(22):7027. https://doi.org/10.3390/molecules26227027
Chicago/Turabian StyleZając, Adam, Jacek Michalski, Maciej Ptak, Lucyna Dymińska, Alicja Z. Kucharska, Wiktor Zierkiewicz, and Jerzy Hanuza. 2021. "Physicochemical Characterization of the Loganic Acid–IR, Raman, UV-Vis and Luminescence Spectra Analyzed in Terms of Quantum Chemical DFT Approach" Molecules 26, no. 22: 7027. https://doi.org/10.3390/molecules26227027
APA StyleZając, A., Michalski, J., Ptak, M., Dymińska, L., Kucharska, A. Z., Zierkiewicz, W., & Hanuza, J. (2021). Physicochemical Characterization of the Loganic Acid–IR, Raman, UV-Vis and Luminescence Spectra Analyzed in Terms of Quantum Chemical DFT Approach. Molecules, 26(22), 7027. https://doi.org/10.3390/molecules26227027








