The Future of Carica papaya Leaf Extract as an Herbal Medicine Product
Abstract
1. Introduction
2. Extraction
3. Nutraceuticals/Cosmeceuticals Product
4. Nutritional and Phytochemical Substances
5. Indications
5.1. Dengue
5.2. Malaria
5.3. Chikungunya
6. Good Manufacturing Practice
7. Herbal Medicine Product Registration
8. Safety
9. Perspective
10. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kondamudi, R.; Murthy, K.S.R.; Pullaiah, T. Euphorbiaceae-a critical review on plant tissue culture. Trop. Subtrop. Agroecosystems 2009, 10, 313–335. [Google Scholar]
- Romanelli, C.; Cooper, D.; Campbell-Lendrum, D.; Maiero, M.; Karesh, W.B.; Hunter, D.; Golden, C.D. Introduction to the state of knowledge review/Biodiversity and human health linkages: Concepts, determinants, drivers of change and approaches to integration. In Connecting Global Priorities: Biodiversity and Human Health: A State of Knowledge Review; World Health Organistion Convention on Biological Diversity: Quebec, QC, Canada, 2015; pp. 24–28. [Google Scholar]
- 10 Things You Can Do to Help Biodiversity. Available online: http://ecocitizen.tatasteel.com/eco-management/pdf/bio-diversity.pdf (accessed on 30 October 2021).
- Allan, P. November. Carica papaya responses under cool subtropical growth conditions. Int. Symp. Trop. Subtrop. Fruits 2000, 575, 757–763. [Google Scholar]
- Kinding, D.P.N. The financial eligibility of Indonesian calina/ california (Carica papaya L.) farm industry. PJSE 2021, 1, 24–33. [Google Scholar]
- Sekeli, R.; Hamid, M.H.; Razak, R.A.; Wee, C.Y.; Ong-Abdullah, J. Malaysian Carica papaya L. var. Eksotika: Current research strategies fronting challenges. Front. Plant Sci. 2018, 9, 1380. [Google Scholar] [CrossRef]
- Bunawan, H.; Baharum, S.N. Papaya Dieback in Malaysia: A StepTowards a New Insight of Disease Resistance. Iran. J. Biotechnol. 2015, 13, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mat Amin, N.; Bunawan, H.; Redzuan, R.A.; Jaganath, I.B.S. Erwinia mallotivora sp., a new pathogen of papaya (Carica papaya) in Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 39–45. [Google Scholar] [CrossRef]
- Sagadevan, P.; Selvakumar, S.; Raghunath, M.; Megala, R.; Janarthan, P.; Vinitha Ebziba, C. Medicinal properties of Carica papaya Linn: Review. Madr. J. Nov. Drug. Res. 2019, 3, 120–125. [Google Scholar] [CrossRef]
- Ugo, N.J.; Ade, A.R.; Joy, A.T. Nutrient Composition of Carica papaya Leaves Extracts. J. Food Nutr. Sci. Res. 2019, 2, 274–282. [Google Scholar]
- Rahman, A. Health Benefits, Chemistry and Mechanism of Carica papaya a Crowning Glory. Adv. Nat. Sci. 2013, 6, 26–27. [Google Scholar]
- Puji, R.P.N.; Hidayah, B.; Rahmawati, I.; Lestari, D.A.Y.; Fachrizal, A.; Novalinda, C. Increasing Multi-Business Awareness through “Prol Papaya” Innovation. IJHSSE 2018, 5, 2349-0381. [Google Scholar]
- Baskaran, C.; Velu, S.; Kumaran, K. The efficacy of Carica papaya leaf extract on some bacterial and a fungal strain by well diffusion method. Asian Pac. J. Trop. Dis. 2012, 2, S658–S662. [Google Scholar] [CrossRef]
- Nariya, A.; Jhala, D. Pharmacognostic study of Carica papaya leaf extract as inhibitors of reactive oxygen species. Int. Res. J. Pharm. 2017, 8, 13–17. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K.P.; Chanda, S.; Bhardwaj, V.; Tanwar, H.; Ganju, L.; Kumar, B.; Singh, S.B. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch. Virol. 2019, 164, 1095–1110. [Google Scholar] [CrossRef]
- Charan, J.; Saxena, D.; Goyal, J.P.; Yasobant, S. Efficacy and safety of Carica papaya leaf extract in the dengue: A systematic review and meta-analysis. Int. J. Appl. Basic Med. Res. 2016, 6, 249. [Google Scholar] [CrossRef]
- Sathyapalan, D.T.; Padmanabhan, A.; Moni, M.; P-Prabhu, B.; Prasanna, P.; Balachandran, S.; Trikkur, S.P.; Jose, S.; Edathadathil, F.; Anilkumar, J.O.; et al. Efficacy & safety of Carica papaya leaf extract (CPLE) in severe thrombocytopenia (≤30,000/μL) in adult dengue–Results of a pilot study. PLoS ONE 2020, 15, e0228699. [Google Scholar]
- Mardiyanto, N.; Dang, N.H.; Kumagai, E.; Kondo, A.; Iwata, S.; Morimoto, C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J. Ethnopharmacol. 2010, 127, 760–767. [Google Scholar]
- Wulansari, D.D.; Wulandari, D.D.; Risthanti, R.R.; Kirtishanti, A. Ameliorative effect of Carica papaya seed extract on diabetic rat model with muscle atrophy. MPI 2019, 2, 208–215. [Google Scholar] [CrossRef]
- Anwar, T.; Qureshi, H.; Parveen, N.; Bashir, R.; Qaisar, U.; Munazir, M.; Yasmin, S.; Basit, Z.; Mahmood, R.T.; Nayyar, B.G.; et al. Evaluation of bioherbicidal potential of Carica papaya leaves. Braz. J. Biol. Sci. 2019, 80, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Azizah, L.S.; Fasya, A.H. February. Effectiveness of pepaya leaf extract (Carica papaya L.) to control ectoparasite argulus on common carp (Cyprinus carpio). In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 236, p. 012106. [Google Scholar]
- Kurniawan, B.; Rapina, R.; Sukohar, A.; Nareswari, S. Effectiveness of the pepaya leaf (Carica papaya L.) ethanol extract as larvacide for Aedes aegypti Instar III. J. Major. 2015, 4, 76–84. [Google Scholar]
- Permadi, M.A.; Lubis, R.A.; Syawaludin, S.; Pasaribu, N.S. Utilization of papaya leaves (Carica papaya L.) to control onion pest Spodoptera exigua (lepidoptera: Noctuidae) lepidoptera (noctuidae). Biolink 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Ali, A.; Devarajan, S.; Waly, M.; Essa, M.M.; Rahman, M.S. Nutritional and medicinal value of papaya (Carica papaya L.). In Natural Products and Bioactive Compounds in Disease Prevention; Nova Science Publisher: New York, NY, USA, 2011; pp. 34–42. [Google Scholar]
- Megantara, S.; Mustarichie, R. Formulation of black hair dyes in the form of sticks from papaya seed extracts and powder. Int. Res. J. Pharm. 2018, 9, 69–74. [Google Scholar] [CrossRef]
- Pratiwi, I.; Rusita, Y.D. Face mask formulation of papaya leaf extract (Carica papaya L.) as anti-acne. JKKT 2018, 3, 84–89. [Google Scholar] [CrossRef]
- Haldar, S.; Mohapatra, S.; Singh, R.; Katiyar, C.K. Isolation and quantification of bioactive Carpaine from Carica papaya L. and its commercial formulation by HPTLC densitometry. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 388–393. [Google Scholar] [CrossRef]
- Mardiyanto, M. Formulation of ionic-gelation submicron particles loading extract papaya leaves (Carica papaya L.) with lactic acid isolates. JSTI 2019, 4, 77–81. [Google Scholar] [CrossRef][Green Version]
- Viqi, K.W. Formulation of Papaya Seeds Ethanolic Extract Transdermal Patch (Carica papaya L.) Using Hydroxypropil Metilcellulose (HPMC) Base. Doctoral Dissertation, Sekolah Tinggi Ilmu Kesehatan Nasional, Sukoharjo, Indonesia, 2020. [Google Scholar]
- Kumar, P.V. Dengue and drawbacks of marketed Carica papaya leaves supplements. IJGP 2016, 10, S72–S81. [Google Scholar]
- Patil, S.; Ayara, P. Carica papaya: Formulation and evaluation of new dosage form design. IJPSR 2019, 10, 1677–1685. [Google Scholar]
- Peristiowati, Y.; Siyoto, S.; Chusnatayaini, A. The use of sonication method for reducing size of liposomes in papaya leaf extract (Carica papaya L.) preparations as a candidate in treatment of cervical cancer. EJMCM 2020, 7, 4703–4709. [Google Scholar]
- Nugroho, B.H.; Citrariana, S.; Sari, I.N.; Oktari, R.N. Formulation and evaluation of SNEDDS (Self Nano-emulsifying Drug Delivery System) of papaya leaf extracts (Carica papaya L.) as an analgesic. Pharm. Sci. J. 2017, 13, 77–85. [Google Scholar]
- Zukhri, S.; Andasari, S.D.; Muchson, M. Formulation and physical quality evaluation of anti-acne cream from papaya leaf extract (Carica papaya L.). CERATA JIF 2018, 9, 63–67. [Google Scholar]
- Shenekar, P.N.; Ukirade, P.S.; Salunkhe, S.D.; Sutar, S.T.; Magdum, C.S.; Mohite, S.K.; Lokapure, S.G.; Metri, S.M. In vitro evaluation of sun protection factor of fruit extract of Carica papaya L. as a lotion formulation. Eur. J. Exp. Biol. 2014, 4, 44–47. [Google Scholar]
- Sari, C.M.A.; Andriani, D.; Wahyudi, D. Optimization of HPMC dan Carbopol combination in formulation of papaya seeds ethanolic extract gel (Carica papaya L.) and its antibacterial activity against Escherichia coli. JIFI 2020, 3, 241–252. [Google Scholar] [CrossRef]
- Prihandiwati, E.; Sari, A.K. Antibacterial activity evaluation in formulation of papaya leaf hydrocarbon ointment (Carica papaya L.) as one of wound healing agent alternatives. JIIS 2019, 4, 380–390. [Google Scholar] [CrossRef]
- Patel, N. Formulation and optimization of synthetic polymer based herbal emulgel for anti-microbial activity. JIAPS 2021, 6, 37–42. [Google Scholar]
- Hariyono, P.; Patramurti, C.; Candrasari, D.S.; Hariono, M. An integrated virtual screening of compounds from Carica papaya leaves against multiple protein targets of SARS-Coronavirus-2. RECHEM 2021, 3, 100113. [Google Scholar] [CrossRef]
- Olmoss, A. Papain, a plant enzyme of biological importance: A. Am. J. Biochem. Biotechnol. 2012, 8, 99–104. [Google Scholar]
- Vuorinen, E.; Valtonen, S.; Hassan, N.; Mahran, R.; Habib, H.; Malakoutikhah, M.; Kopra, K.; Härmä, H. Protease Substrate-Independent Universal Assay for Monitoring Digestion of Native Unmodified Proteins. Int. J. Mol. Sci. 2021, 22, 6362. [Google Scholar] [CrossRef] [PubMed]
- Sari Daun Pepaya. Available online: https://www.sidomuncul.co.id/en/product/sari_daun_pepaya.html (accessed on 30 October 2021).
- Mohammad Azmin, S.N.H.; Abdul Manan, Z.; Wan Alwi, S.R.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal processing and extraction technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Hariono, M.; Rollando, R.; Karamoy, J.; Hariyono, P.; Atmono, M.; Djohan, M.; Wiwy, W.; Nuwarda, R.; Kurniawan, C.; Salin, N.; et al. Bioguided fractionation of local plants against matrix metalloproteinase9 and its cytotoxicity against breast cancer cell models: In silico and in vitro study. Molecules 2020, 25, 4691. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Fauziah, L.; Wakidah, M. Extraction of papaya leaves (Carica papaya L.) using ultrasonic cleaner. EKSAKTA JSDA 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Utama, S.Y.A. The effect of papaya leaf extract (Carica papaya L.) to the bleeding time on mice with thrombocytopenia. IJND 2018, 6, 133–138. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, P.C.; Verma, A.K.; Sharma, A. Utilization of Carica papaya Herbal Leaf Extract for Preparation of a Nutraceutical Functional Beverage. Chem. Sci. Rev. Lett. 2020, 9, 39–49. [Google Scholar]
- Lonkala, S.; Reddy, A.R.N. Antibacterial activity of Carica papaya Leaves and Allium sativum cloves alone and in combination against multiple strains. Pharmacogn. J. 2019, 11, 600–602. [Google Scholar] [CrossRef]
- Nurjannah, N.; Hamidah, A.; Anggereini, E. Effect of Carica papaya Leaf Juice on Hematology of Mice (Mus musculus) with Anemia. Biosaintifika 2017, 9, 417–422. [Google Scholar]
- Hussain, S.M.; Sohrab, M.; Al-Mahmood, A.K.; Shuayb, M.; Al-Mansur, M.; Hasan, C. Clinical use of Carica papaya leaf extract in chemotherapy induced thrombocytopaenia. Int. J. Clin. Exp. Med. 2017, 10, 3752–3756. [Google Scholar]
- Pertiwi, D.; Hafiz, I.; Salma, R. Antibacterial Activity of Gel of Ethanol Extract of Papaya Leaves (Carica papaya L.) againts Propionobacterium acnes. Indones. J. Pharm. Clinical Res. 2019, 2, 01–06. [Google Scholar] [CrossRef]
- Sugito, S.; Suwandi, E. Effectivity of papaya leaf ethanolic extract (Carica papaya L.) toward the bacterial growth of Escherichia coli using diffusion method. J. Lab. Khatulistiwa. 2017, 1, 21–25. [Google Scholar] [CrossRef]
- Gredi, J. Analgesic effectivity of Chitosan-Papaya Leaf Ethanolic Extract Nanoparticle (Carica papaya L.) in male white mice (Mus Mucculus). Doctoral Dissertation, Tanjungpura University, Pontianak, Indonesia, 2015. [Google Scholar]
- Payangka, J.; Risma, R.; Wibowo, P. The influence of papaya leaf extract (Carica papaya) toward Aedes agypti INSTAR III mosquito larvae mortality. Med. Health Sci. J. 2019, 3, 7–16. [Google Scholar] [CrossRef]
- Yuliani, R.; Syahdeni, F. Ethanolic extract of papaya leaves (Carica papaya) and its fractions have no potential cytotoxicity on T47D Cells. Pharmacon JFI 2020, 17, 17–23. [Google Scholar] [CrossRef]
- Tewari, B.B.; Subramanian, G.; Gomathinayagm, R. Antimicrobial properties of Carica papaya (Papaya) different leaf extract against E. coli, S. aureus and C. albicans. Am. J. Pharmacol. Pharmacother. 2014, 1, 025–039. [Google Scholar]
- Kamilla, L.; Tumpuk, S.; Salim, M. Anti-Inflammatory of papaya leaf extract (Carica papaya L.) towards membrane stabilization of red blood cells. JKP 2021, 15, 1–7. [Google Scholar] [CrossRef]
- Hariono, M.; Hariyono, P.; Dwiastuti, R.; Setyani, W.; Yusuf, M.; Salin, N.; Wahab, H. Potential SARS-CoV-2 3CLpro inhibitors from chromene, flavonoid and hydroxamic acid compound based on FRET assay, docking and pharmacophore studies. RECHEM 2021, 3, 100195. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, S.; Muthuselvam, M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J. Agric. Res. 2009, 5, 572–576. [Google Scholar]
- Liana, Y. Comparative effectiveness of papaya leaf stew (Carica papaya L.) with turmeric acid (Curcuma domestica val-Tamarindus indica) against primary dysmenorrhea. SJM 2018, 1, 120–127. [Google Scholar] [CrossRef]
- Nisa, F.Z.; Astuti, M.; Haryana, S.M.; Murdiati, A. Antioxidant activity and total flavonoid of Carica papaya L. leaves with different varieties, maturity and solvent. Agritech 2019, 39, 54–59. [Google Scholar] [CrossRef]
- Singh, V.; Goyal, I.; Saini, A.; Chandra, R. Studying the effect of Carica papaya leaf extract on the shelf life of platelets. IJSR 2017, 6, 2319–7064. [Google Scholar]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Martin, K.I.; Glaser, D.A. Cosmeceuticals: The new medicine of beauty. Mo. Med. 2011, 108, 60. [Google Scholar]
- Medplusmart. Available online: https://www.medplusmart.com/compositionProducts/Carica-papaya-leaf-extract-1000-MG/29460 (accessed on 6 November 2021).
- HerbalGoodness. Available online: https://www.herbalgoodnessco.com/ (accessed on 30 October 2021).
- Sido Muncul. Available online: https://www.sidomuncul.co.id/ (accessed on 30 October 2021).
- Hawaian Herbal. Available online: https://hawaiian-Herbal (accessed on 30 October 2021).
- New Way Herbs. Available online: https://newwayherbs.com/ (accessed on 30 October 2021).
- INCI Decoder. Available online: https://incidecoder.com/ (accessed on 30 October 2021).
- Saeed, F.; Arshad, M.U.; Pasha, I.; Naz, R.; Batool, R.; Khan, A.A.; Nasir, M.A.; Shafique, B. Nutritional and phyto-therapeutic potential of papaya (Carica papaya L.): An overview. Int. J. Food Prop. 2014, 17, 1637–1653. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Singh, V.K. Carica papaya-a herbal medicine. IJRSB 2016, 4, 19–25. [Google Scholar]
- Vij, T.; Prashar, Y. A review on medicinal properties of Carica papaya Linn. Asian Pac. J. Trop. Dis. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Santana, L.F.; Inada, A.C.; Espirito Santo, B.L.S.D.; Filiú, W.F.; Pott, A.; Alves, F.M.; Guimarães, R.D.C.A.; Freitas, K.D.C.; Hiane, P.A. Nutraceutical potential of Carica papaya in metabolic syndrome. Nutrients 2019, 11, 1608. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.L.; Paridhavi, M.; Patel, J.A. Review on nutritional, medicinal and pharmacological properties of Papaya (Carica papaya L.). IJNPR 2008, 7, 364–373. [Google Scholar]
- Nwofia, G.E.; Ojimelukwe, P.; Eji, C. Chemical composition of leaves, fruit pulp and seeds in some Carica papaya (L.) morphotypes. Int. J. Med. Aromat. Plants 2012, 2, 200–206. [Google Scholar]
- Parle, M.; Gurditta, A. Basketful benefits of papaya. Int. Res. J. Pharm. 2011, 2, 6–12. [Google Scholar]
- Canini, A.; Alesiani, D.; D’Arcangelo, G.; Tagliatesta, P. Gas chromatography–mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J. Food Compost. Anal. 2007, 20, 584–590. [Google Scholar] [CrossRef]
- Akhila, S.; Vijayalakshmi, N.G. Phytochemical studies on Carica papaya leaf juice. Int. J. Pharm. Sci. Res. 2015, 6, 880. [Google Scholar]
- Nugroho, A.; Heryani, H.; Choi, J.S.; Park, H.J. Identification and quantification of flavonoids in Carica papaya leaf and peroxynitrite-scavenging activity. Asian Pac. J. Trop. Biomed. 2017, 7, 208–213. [Google Scholar] [CrossRef]
- Kaur, M.; Talniya, N.C.; Sahrawat, S.; Kumar, A.; Stashenko, E.E. Ethnomedicinal uses, phytochemistry and pharmacology of Carica papaya plant: A compendious review. Mini Rev. Org. Chem. 2019, 16, 463–480. [Google Scholar] [CrossRef]
- Julianti, T.; Oufir, M.; Hamburger, M. Quantification of the antiplasmodial alkaloid carpaine in papaya (Carica papaya) leaves. Planta Med. 2014, 80, 1138–1142. [Google Scholar] [CrossRef]
- Burdick, E.M. Carpaine: An alkaloid of Carica papaya: Its chemistry and pharmacology. Econ. Bot. 1971, 25, 363–365. [Google Scholar] [CrossRef]
- Azarkan, M.; El Moussaoui, A.; Van Wuytswinkel, D.; Dehon, G.; Looze, Y. Fractionation and purification of the enzymes stored in the latex of Carica papaya. J. Chromatogr. B. 2003, 790, 229–238. [Google Scholar] [CrossRef]
- Calvache, J.N.; Cueto, M.; Farroni, A.; de Escalada Pla, M.; Gerschenson, L.N. Antioxidant characterization of new dietary fiber concentrates from papaya pulp and peel (Carica papaya L.). J. Funct. Foods 2016, 27, 319–328. [Google Scholar] [CrossRef]
- Abo, K.A.; Fred-Jaiyesimi, A.A.; Jaiyesimi, A.E.A. Ethnobotanical studies of medicinal plants used in the management of diabetes mellitus in South Western Nigeria. J. Ethnopharmacol. 2008, 115, 67–71. [Google Scholar] [CrossRef]
- Isolation and Characterization of Secondary Metabolites from Carica Papaya Leaves, Report. Available online: https://www.researchgate.net/publication/329828494_Isolation_and_characterization_of_secondary_metabolites_from_carica_papaya_leaves (accessed on 9 November 2021).
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; AbuBakar, S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011, 8, 1–11. [Google Scholar] [CrossRef]
- Williams, D.J.; Pun, S.; Chaliha, M.; Scheelings, P.; O’Hare, T. An unusual combination in papaya (Carica papaya): The good (glucosinolates) and the bad (cyanogenic glycosides). J. Food Compos. Anal. 2013, 29, 82–86. [Google Scholar] [CrossRef]
- Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Aguilar-Domínguez, D.E.; Lobato-García, C.E.; Blé-Castillo, J.L.; López-Meraz, L.; Díaz-Zagoya, J.C.; Bermúdez-Ocaña, D.Y. Phytochemical screening and hypoglycemic activity of Carica papaya leaf in streptozotocin-induced diabetic rats. Rev. Bras. Farmacogn. 2014, 24, 341–347. [Google Scholar] [CrossRef]
- Zunjar, V.; Mammen, D.; Trivedi, B.M. Antioxidant activities and phenolics profiling of different parts of Carica papaya by LCMS-MS. Nat. Prod. Res. 2015, 29, 2097–2099. [Google Scholar] [CrossRef]
- Elsson, M.; Wijanarko, A.; Hermansyah, H.; Sahlan, M. Michaelis-menten parameters characterization of commercial papain enzyme “paya”. IOP Conf. Ser. Earth Environ. Sci. 2019, 217, 012037. [Google Scholar] [CrossRef]
- Hansch, C.; Smith, R.N.; Rockoff, A.; Calef, D.F.; Jow, P.Y.; Fukunaga, J.Y. Structure-activity relationships in papain and bromelain ligand interactions. Arch. Biochem. Biophys. 1977, 183, 383–392. [Google Scholar] [CrossRef]
- Cherrier, M.V.; Amara, P.; Talbi, B.; Salmain, M.; Fontecilla-Camps, J.C. Crystallographic evidence for unexpected selective tyrosine hydroxylations in an aerated achiral Ru–papain conjugate. Metallomics 2018, 10, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Vyas, S.; Agarwal, D.P.; Pradesh, M.; Pradesh, U. Therapeutic benefits of Carica papaya leaf extracts in dengue fever patients. Sch. J. Appl. Med. Sci. 2016, 4, 299–302. [Google Scholar]
- Ranasinghe, P.; Ranasinghe, P.; Abeysekera, W.K.M.; Premakumara, G.S.; Perera, Y.S.; Gurugama, P.; Gunatilake, S.B. In vitro erythrocyte membrane stabilization properties of Carica papaya L. leaf extracts. Pharmacogn. Res. 2012, 4, 196. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.M.R.; Khan, F.; Mohamed, I.N. Dengue Fever: Therapeutic Potential of Carica papaya L. Leaves. Front. Pharmacol. 2021, 12, 610912. [Google Scholar] [CrossRef]
- Zunjar, V.; Dash, R.P.; Jivrajani, M.; Trivedi, B.; Nivsarkar, M. Antithrombocytopenic activity of carpaine and alkaloidal extract of Carica papaya Linn. leaves in busulfan induced thrombocytopenic Wistar rats. J. Ethnopharmacol. 2016, 181, 20–25. [Google Scholar] [CrossRef]
- Lavanya, B.; Maheswaran, A.; Vimal, N.; Vignesh, K.; Yuvarani, K.; Varsha, R. Extraction and effects of papain obtained from leaves of Carica papaya: A remedy to dengue fever. Extraction 2018, 3, 44–46. [Google Scholar]
- Rajapakse, S.; de Silva, N.L.; Weeratunga, P.; Rodrigo, C.; Sigera, C.; Fernando, S.D. Carica papaya extract in dengue: A systematic review and meta-analysis. BMC Complement. Altern. Med. 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Sulaiman, S.N.; Hariono, M.; Salleh, H.M.; Chong, S.L.; Yee, L.S.; Zahari, A.; Wahab, H.A.; Derbré, S.; Awang, K. Chemical constituents from Endiandra kingiana (Lauraceae) as potential inhibitors for dengue type 2 NS2B/NS3 serine protease and its molecular docking. Nat. Prod. Commun. 2019, 14, 1934578X19861014. [Google Scholar] [CrossRef]
- Yap, B.K.; Lee, C.Y.; Choi, S.B.; Kamarulzaman, E.E.; Hariono, M.; Wahab, H.A. In Silico Identification of Novel Inhibitors. In Encyclopedia of Bioinformatics and Computational Biology; Ranganathan, S., Gribskov, M., Nakai, K., Schönbach, C., Eds.; Academic Press: Amsterdam, The Netherland, 2019; pp. 761–779. [Google Scholar]
- Sivasothy, Y.; Liew, S.Y.; Othman, M.A.; Abdul Wahab, S.M.; Hariono, M.; Mohd Nawi, M.S.; Abdul Wahab, H.; Awang, K. Natural DENV-2 NS2B/NS3 protease inhibitors from Myristica cinnamomea King. Trop. Biomed. 2021, 38, 79–84. [Google Scholar]
- Paranjape, S.M.; Harris, E. Control of dengue virus translation and replication. Dengue Virus 2010, 338, 15–34. [Google Scholar]
- Rodenhuis-Zybert, I.A.; Wilschut, J.; Smit, J.M. Dengue virus life cycle: Viral and host factors modulating infectivity. Cell. Mol. Life Sci. 2010, 67, 2773–2786. [Google Scholar] [CrossRef]
- Niyomrattanakit, P.; Winoyanuwattikun, P.; Chanprapaph, S.; Angsuthanasombat, C.; Panyim, S.; Katzenmeier, G. Identification of residues in the dengue virus type 2 NS2B cofactor that are critical for NS3 protease activation. J. Virol. 2004, 78, 13708–13716. [Google Scholar] [CrossRef]
- Idrees, S.; Ashfaq, U.A. A brief review on dengue molecular virology, diagnosis, treatment and prevalence in Pakistan. Genetic Vaccines Ther. 2012, 10, 1–10. [Google Scholar] [CrossRef]
- Erbel, P.; Schiering, N.; D’Arcy, A.; Renatus, M.; Kroemer, M.; Lim, S.P.; Yin, Z.; Keller, T.H.; Vasudevan, S.G.; Hommel, U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 2006, 13, 372–373. [Google Scholar] [CrossRef]
- Gutman, J.R.; Lucchi, N.W.; Cantey, P.T.; Steinhardt, L.C.; Samuels, A.M.; Kamb, M.L.; Kapella, B.K.; McElroy, P.D.; Udhayakumar, V.; Lindblade, K.A. Malaria and parasitic neglected tropical diseases: Potential syndemics with COVID-19? Am. J. Trop. Med. Hyg. 2020, 103, 572. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Mharakurwa, S.; Ndiaye, D.; Rathod, P.K.; Rosenthal, P.J. Antimalarial drug resistance: Literature review and activities and findings of the ICEMR network. Am. J. Trop. Med. Hyg. 2015, 93, 57. [Google Scholar] [CrossRef]
- Arrow, K.J.; Panosian, C.; Gelband, H. Antimalarial drugs and drug resistance. In Saving Lives, Buying Time: Economics of Malaria Drugs in an Age of Resistance; National Academies Press (US): Washington, DC, USA, 2004. [Google Scholar]
- Okpe, O.; Habila, N.; Ikwebe, J.; Upev, V.A.; Okoduwa, S.I.; Isaac, O.T. Antimalarial potential of Carica papaya and Vernonia amygdalina in mice infected with Plasmodium berghei. J. Trop. Med. 2016, 2016, 8738972. [Google Scholar] [CrossRef] [PubMed]
- Melariri, P.; Campbell, W.; Etusim, P.; Smith, P. In vitro antiplasmodial activities of extracts from five plants used singly and in combination against Plasmodium falciparum parasites. J. Med. Plant Res. 2012, 6, 5770–5779. [Google Scholar]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 1–18. [Google Scholar] [CrossRef]
- Julianti, T.; De Mieri, M.; Ebrahimi, S.; Neuburger, M.; Zimmermann, S.; Kaiser, M.; Hamburger, M. Potent antiplasmodial agents in Carica papaya L. Planta Med. 2013, 79, SL6. [Google Scholar] [CrossRef]
- Paixão, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health 2018, 3, e000530. [Google Scholar] [CrossRef]
- Tharanatha, V. Screening the Antiviral Activity of Carica papaya L. Leaves and Foeniculum Vulgare Fennel Grain Extracts against Chikungunya Virus. Doctoral Dissertation, Department of Virology, Sri Venkateswara University, Andhra Prades, India, 2017. [Google Scholar]
- Radhakrishnan, N.; Lam, K.W.; Norhaizan, M.E. Molecular docking analysis of Carica papaya Linn constituents as antiviral agent. Int. Food Res. J. 2017, 24, 1819–1825. [Google Scholar]
- Kaushik, S.; Sharma, V.; Chhikara, S.; Yadav, J.P.; Kaushik, S. Anti-chikungunya activity of green synthesized silver nanoparticles using Carica papaya leaves in animal cell culture model. Asian J. Pharm. Clin. Res. 2019, 12, 170–174. [Google Scholar]
- He, T.T.; Ung, C.O.L.; Hu, H.; Wang, Y.T. Good manufacturing practice (GMP) regulation of herbal medicine in comparative research: China GMP, cGMP, WHO-GMP, PIC/S and EU-GMP. Eur. J. Integr. Med. 2015, 7, 55–66. [Google Scholar] [CrossRef]
- Sosialisasi Peraturan Badan POM Nomor 14 Tahun 2021 Tentang Sertifikasi Cara Pembuatan Obat Tradisional Yang Baik (CPOTB). Available online: https://www.pom.go.id/new/view/more/berita/22602/Sosialisasi-Peraturan-Badan-POM-Nomor-14-tahun-2021-tentang-Sertifikasi-Cara-Pembuatan-Obat-Tradisional-yang-Baik--CPOTB--.html (accessed on 9 October 2021).
- Sido Muncul. Available online: https://www.sidomuncul.co.id/en/certification.html (accessed on 9 October 2021).
- Ramadoss, M.S.K.; Koumaravelou, K. Regulatory compliance of herbal medicines—A review. Int. J. Res. Pharm. Sci. 2019, 10, 3127–3135. [Google Scholar]
- Diniarti, I.; Iljanto, S. The strategy of increasing competitive ability in traditional medicine industry (IOT) in Central Java in 2017. JKKI 2017, 6, 184–192. [Google Scholar]
- Safety Assessment of Carica papaya (papaya)-Derived Ingredients as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/Papaya.pdf (accessed on 9 November 2021).
- Nkeiruka, U.E.; Chinaka, N.O. Anti-fertility effects of Carica papaya linn: Methanol leaf extracts in male wistar rats. J. Pharmacol. Toxicol. 2013, 8, 35–41. [Google Scholar] [CrossRef][Green Version]
- Halim, S.Z.; Abdullah, N.R.; Afzan, A.; Rashid, B.A.; Jantan, I.; Ismail, Z. Acute toxicity study of Carica papaya leaf extract in Sprague Dawley rats. J. Med. Plant Res. 2011, 5, 1867–1872. [Google Scholar]
- Peristiowati, Y.; Puspitasari, Y. Acute and subchronic toxicity tests of papaya leaf (Carica papaya Linn.) methanol extract on wistar strainwhite mice. J. Appl. Environ. Biol. Sci. 2017, 7, 9–14. [Google Scholar]
- Afzan, A.; Abdullah, N.R.; Halim, S.Z.; Rashid, B.A.; Semail, R.H.R.; Abdullah, N.; Jantan, I.; Muhammad, H.; Ismail, Z. Repeated dose 28-days oral toxicity study of Carica papaya L. leaf extract in Sprague Dawley rats. Molecules 2012, 17, 4326–4342. [Google Scholar] [CrossRef]
- Ekong, M.B.; Akpan, M.U.; Ekanem, T.B.; Akpaso, M.I. Morphometric malformations in fetal rats following treatment with aqueous leaf extract of Carica papaya. Asian J. Med. Sci. 2011, 2, 18–22. [Google Scholar] [CrossRef]
- Akinloye, O.O.; Morayo, O.M. Evaluation of andrological indices and testicular histology following chronic administration of aqueous extract of Carica papaya leaf in Wistar rat. Afr. J. Pharmacy Pharmacol. 2010, 4, 252–255. [Google Scholar]
- Lim, X.Y.; Chan, J.S.W.; Japri, N.; Lee, J.C.; Tan, T.Y.C. Carica papaya L. Leaf: A Systematic Scoping Review on Biological Safety and Herb-Drug Interactions. eCAM 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Hartini, Y.; Saputra, B.; Wahono, B.; Auw, Z.; Indayani, F.; Adelya, L.; Namba, G.; Hariono, M. Biflavonoid as potential 3-chymotrypsin-like protease (3CLpro) inhibitor of SARS-Coronavirus. RECHEM 2021, 3, 100087. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.S.; Maulana, S.; Widodo, A.; Pitopang, R.; Arba, M.; Hariono, M. GC-MS, LC-MS/MS, Docking and molecular dynamics approaches to identify potential SARS-CoV-2 3-chymotrypsin-like protease inhibitors from Zingiber officinale Roscoe. Molecules 2021, 26, 5230. [Google Scholar] [CrossRef] [PubMed]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Norahmad, N.A.; Abd Razak, M.R.M.; Misnan, N.M.; Jelas, N.H.M.; Sastu, U.R.; Muhammad, A.; Ho, T.C.D.; Jusoh, B.; Zolkifli, N.A.; Thayan, R.; et al. Effect of freeze-dried Carica papaya leaf juice on inflammatory cytokines production during dengue virus infection in AG129 mice. BMC Complement. Altern. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Ralapanawa, U.; Alawattegama, A.T.M.; Gunrathne, M.; Tennakoon, S.; Kularatne, S.A.M.; Jayalath, T. Value of peripheral blood count for dengue severity prediction. BMC Res. Notes 2018, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bilheiro, R.P.; Braga, A.D.; Limborço Filho, M.; Carvalho-Tavares, J.; Agero, U.; das Graças Carvalho, M.; Sanchez, E.F.; Salas, C.E.; Lopes, M.T. The thrombolytic action of a proteolytic fraction (P1G10) from Carica candamarcensis. Thromb. Res. 2013, 131, e175–e182. [Google Scholar] [CrossRef]
- Potential Herbal-Based Treatment for COVID-19, a Case for Papaya Leaves Extract. Available online: https://www.researchgate.net/publication/342053381_Papaya_leaves_extract_a_possible_weapon_against_COVID-19 (accessed on 9 October 2021).
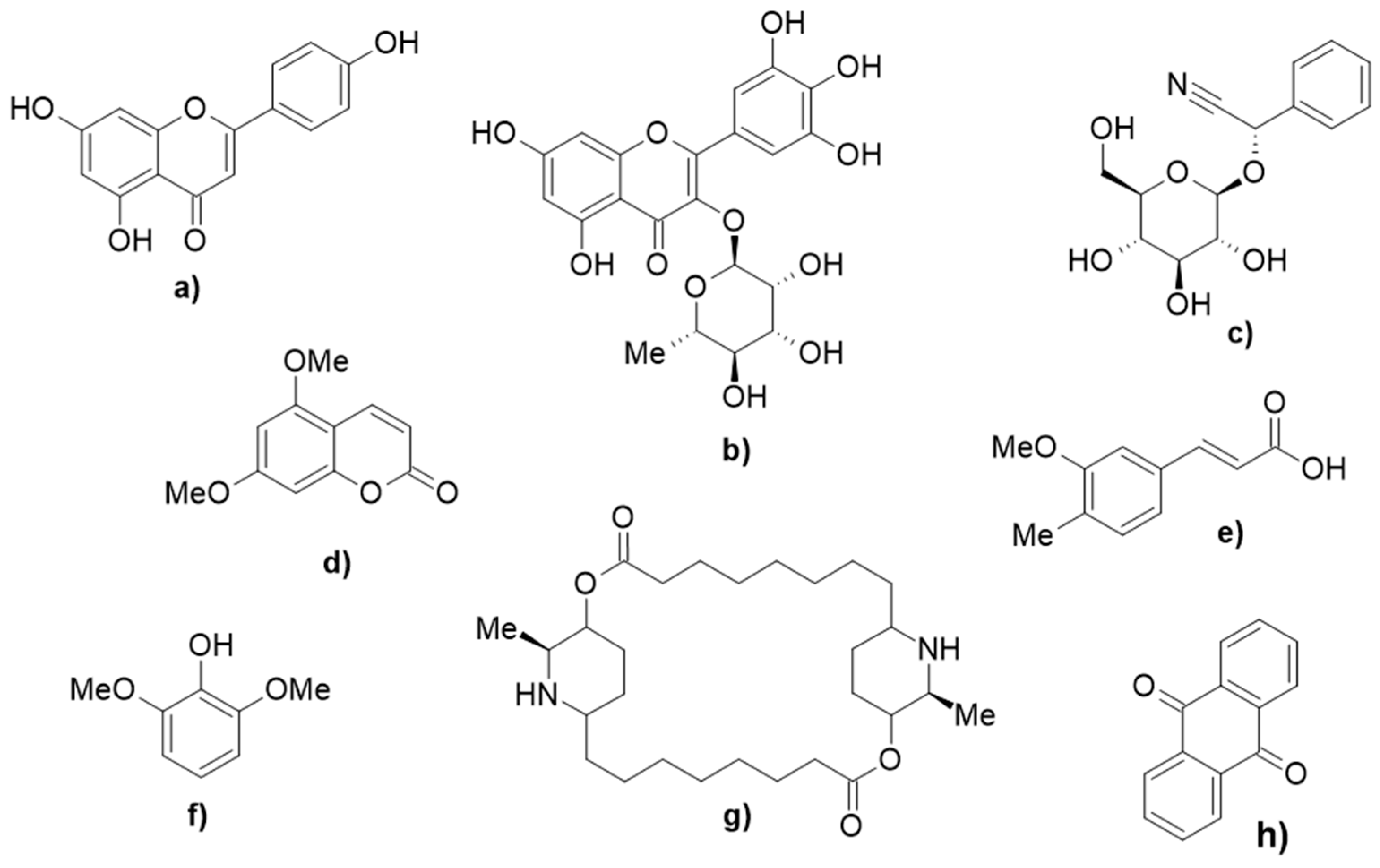
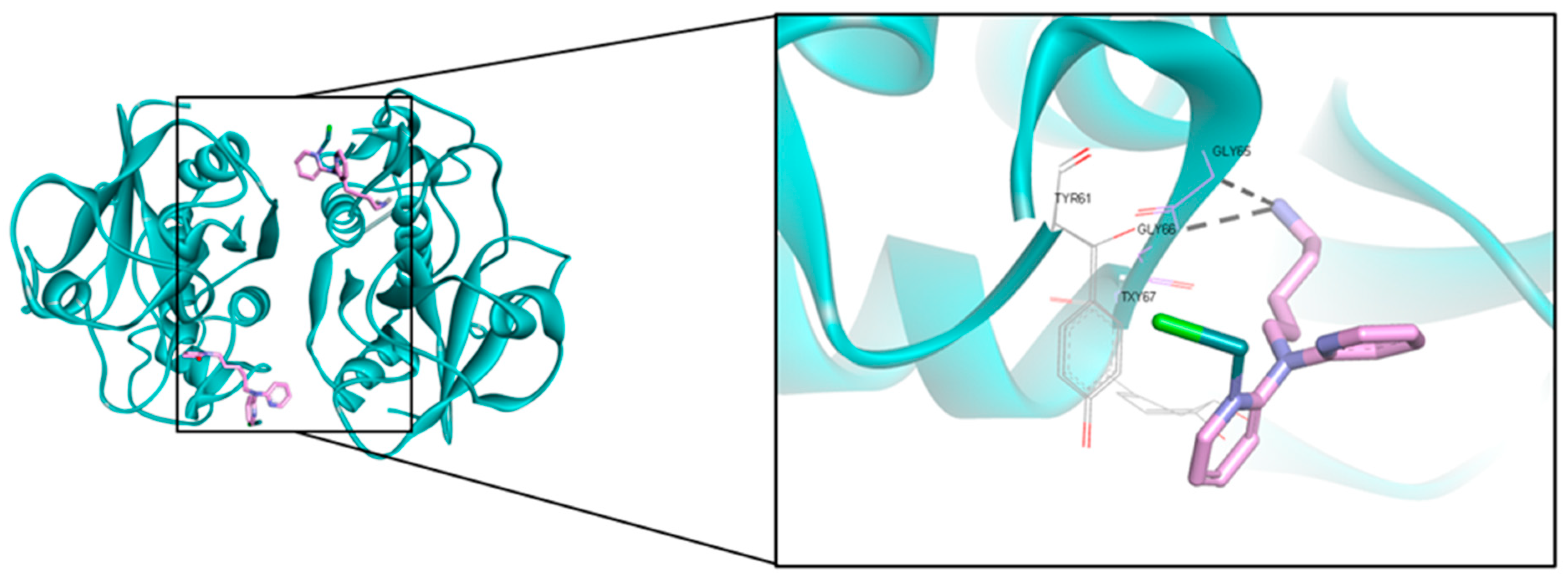

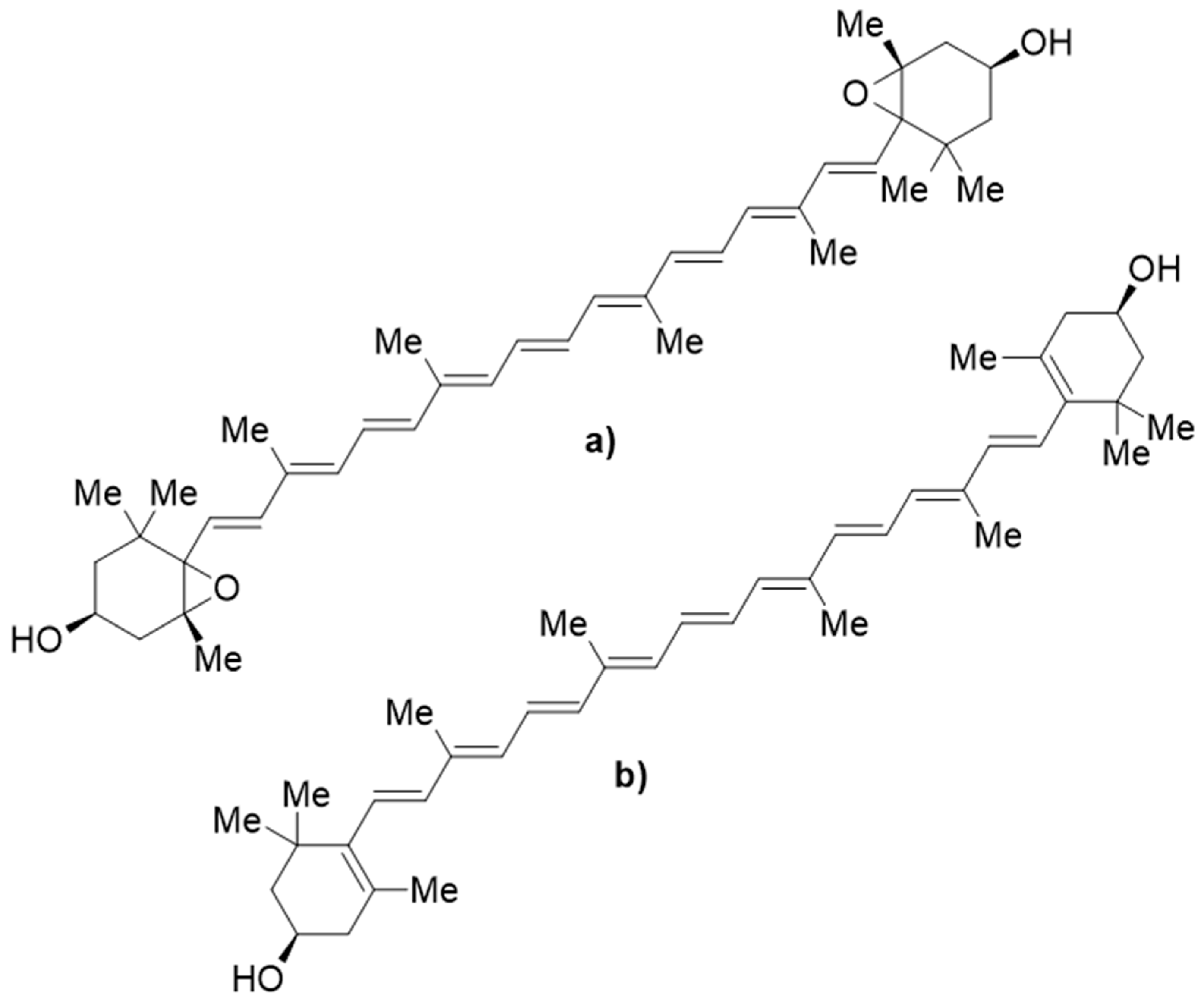
| Extraction Method | Solvents | References |
|---|---|---|
| Ultrasonic cleaner | Methanol 96% ethanol | [46] [47] |
| Hot presser | Water | [48] |
| Blender | Water | [23,49,50,51] |
| Maceration | 70% ethanol 96% ethanol 70% methanol 80% methanol Water | [25,52] [10,22,27,33,36,53,54,55,56] [57,58] [14,59] [20] |
| Mixer | cold water, hot water, cold ethanol, 70% | [60,61] |
| Microwave | methanol, 70% ethanol and water | [62] |
| Soxhlet | hexane, acetone, 60% ethanol, 40% ethanol and water | [63] |
| Nutrients | % | Nutrients | % | Nutrients | % |
|---|---|---|---|---|---|
| Proteins * | 5.8 | Phosphorous ** | 0.221 | Vitamin B3 ** | 0.0003 |
| Lipids * | 1.4 | Magnesium ** | 0.032 | Vitamin B2 ** | 0.0001 |
| Carbohydrates * | 78.2 | Iron ** | 0.006 | Vitamin B1 ** | 0.0004 |
| Fibre * | 13.1 | Calcium ** | 0.366 | Vitamin A ** | ND |
| Energy ** | 348.6 kcal | Vitamin C ** | 0.031 | Beta-carotene ** | 659.5 IU |
| Sodium ** | ND | Vitamin B9 ** | ND | ||
| Potassium ** | 0.534 | Vitamin B6 ** | ND |
| Class | Compounds | Pharmacological Effects |
|---|---|---|
| Flavonoids | apigenin, catechin, kaempferol, deoxykaempferol, deoxyquercetin, protocatechuic acid, galic acid | antioxidant, anti-bacterial, anti-dengue [89] |
| Flavonoid glycosides | quercetin 3-(2-rhamnosylrutinoside), kaempferol 3-(2-rhamnosylrutinoside), quercetin 3-rutinoside, myricetin 3-rhamnoside | antioxidant [81] |
| Cyanogenic glycosides | 2S-sambunigrin, R-prunasin | anticancer [90] |
| Coumarins | 5,7-dimethoxycoumarin, p-coumaric acid, o-coumaric acid, p-coumaric alcohol | antioxidant [79] |
| Quinones | anthraquinone | anti-diabetes [91] |
| Cinnamic acids | ferulic acid, chlorogenic acid, E-3-(4-hydroxy-3-(3,4,5-trimethoxybenzyl)phenyl)acrylic acid | |
| Phenols | 2,6-dimethoxyphenol | antioxidant [92] |
| Alkaloids | carpaine, pseudocarpaine, dehydrocarpaine I, dehydrocarpaine II, carposide, emetine | antimalarial [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hariono, M.; Julianus, J.; Djunarko, I.; Hidayat, I.; Adelya, L.; Indayani, F.; Auw, Z.; Namba, G.; Hariyono, P. The Future of Carica papaya Leaf Extract as an Herbal Medicine Product. Molecules 2021, 26, 6922. https://doi.org/10.3390/molecules26226922
Hariono M, Julianus J, Djunarko I, Hidayat I, Adelya L, Indayani F, Auw Z, Namba G, Hariyono P. The Future of Carica papaya Leaf Extract as an Herbal Medicine Product. Molecules. 2021; 26(22):6922. https://doi.org/10.3390/molecules26226922
Chicago/Turabian StyleHariono, Maywan, Jeffry Julianus, Ipang Djunarko, Irwan Hidayat, Lintang Adelya, Friska Indayani, Zerlinda Auw, Gabriel Namba, and Pandu Hariyono. 2021. "The Future of Carica papaya Leaf Extract as an Herbal Medicine Product" Molecules 26, no. 22: 6922. https://doi.org/10.3390/molecules26226922
APA StyleHariono, M., Julianus, J., Djunarko, I., Hidayat, I., Adelya, L., Indayani, F., Auw, Z., Namba, G., & Hariyono, P. (2021). The Future of Carica papaya Leaf Extract as an Herbal Medicine Product. Molecules, 26(22), 6922. https://doi.org/10.3390/molecules26226922







