A Series of Novel Pentagonal-Bipyramidal Erbium(III) Complexes with Acyclic Chelating N3O2 Schiff-Base Ligands: Synthesis, Structure, and Magnetism
Abstract
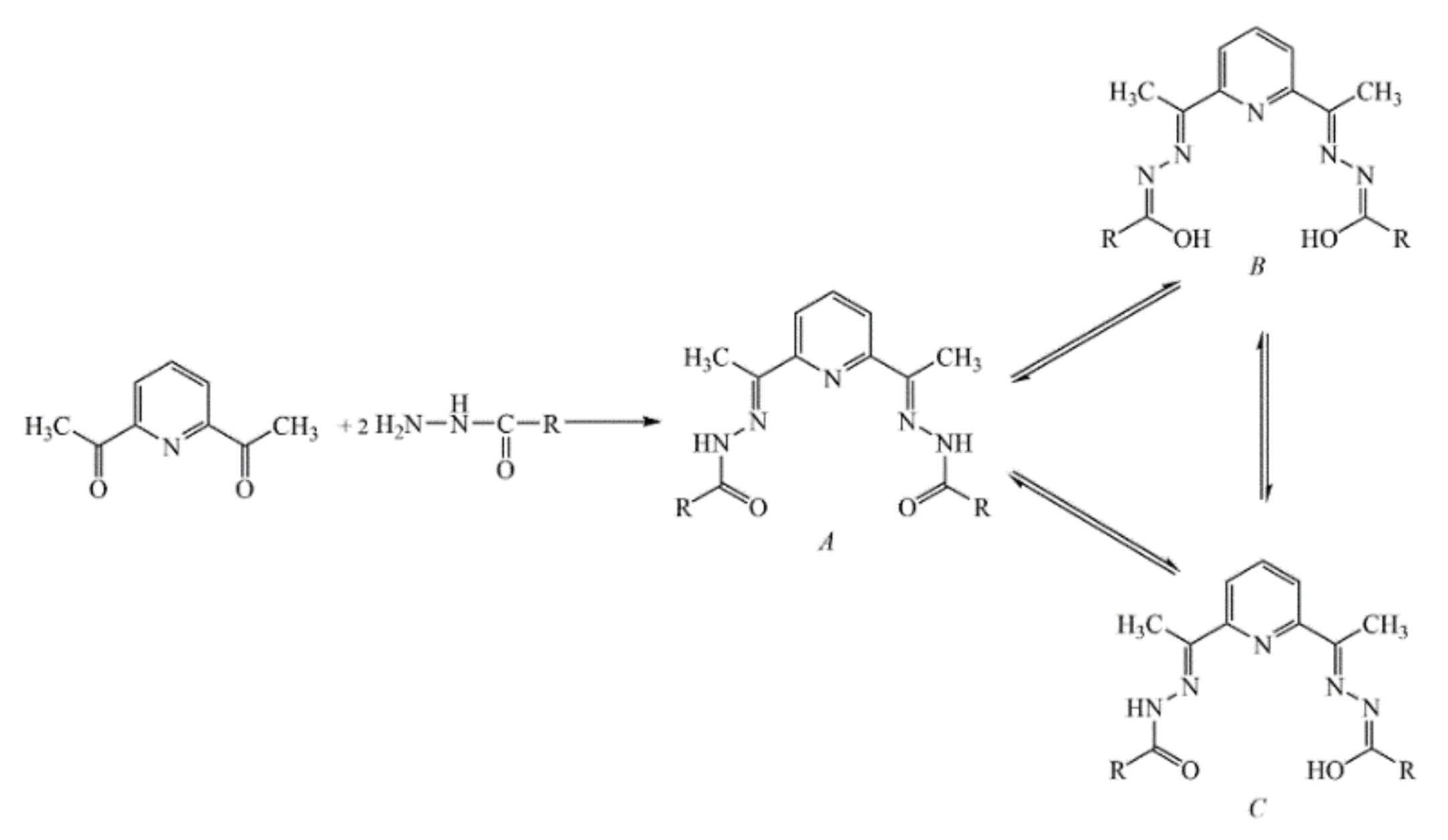
1. Introduction
2. Results and Discussion
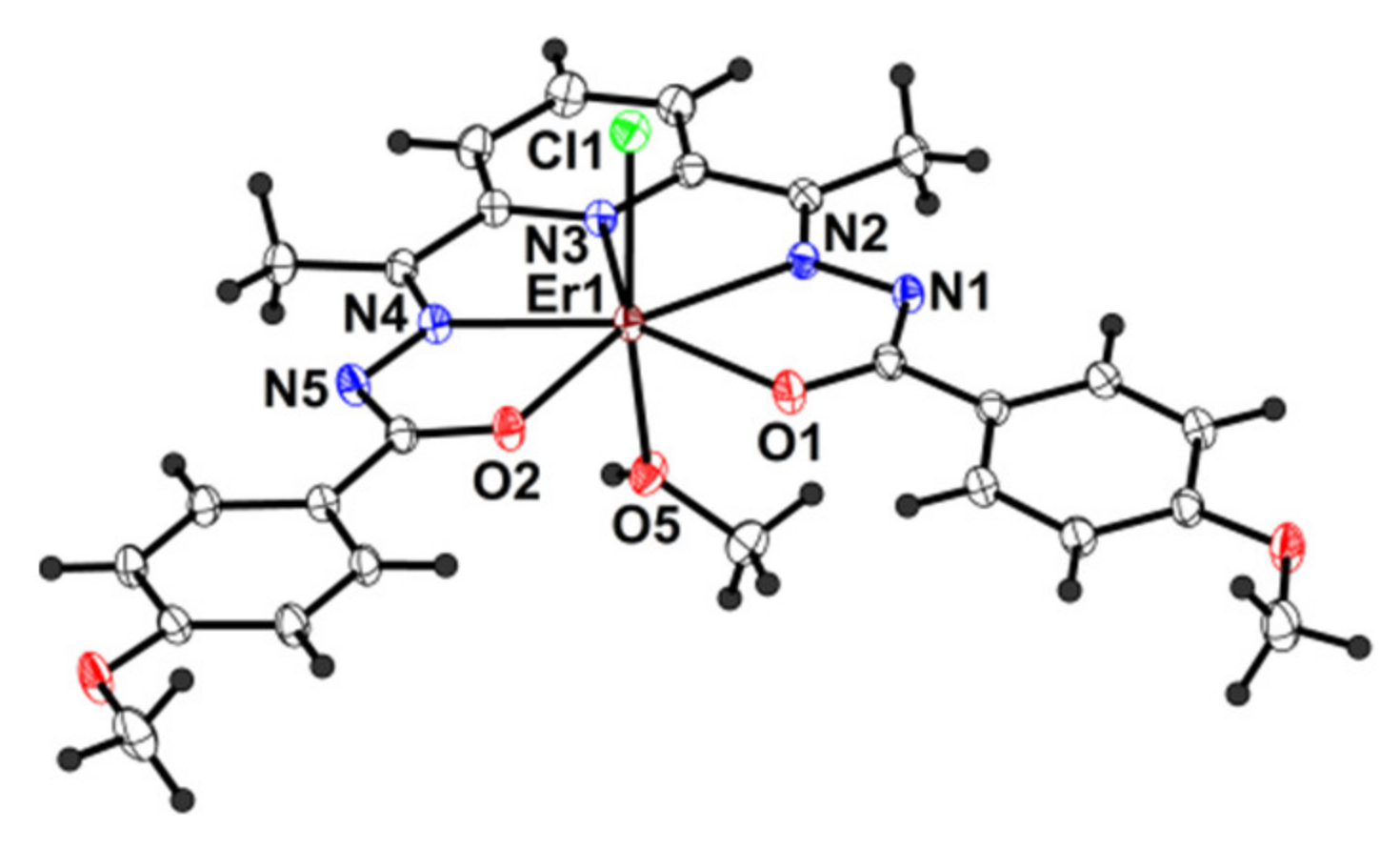
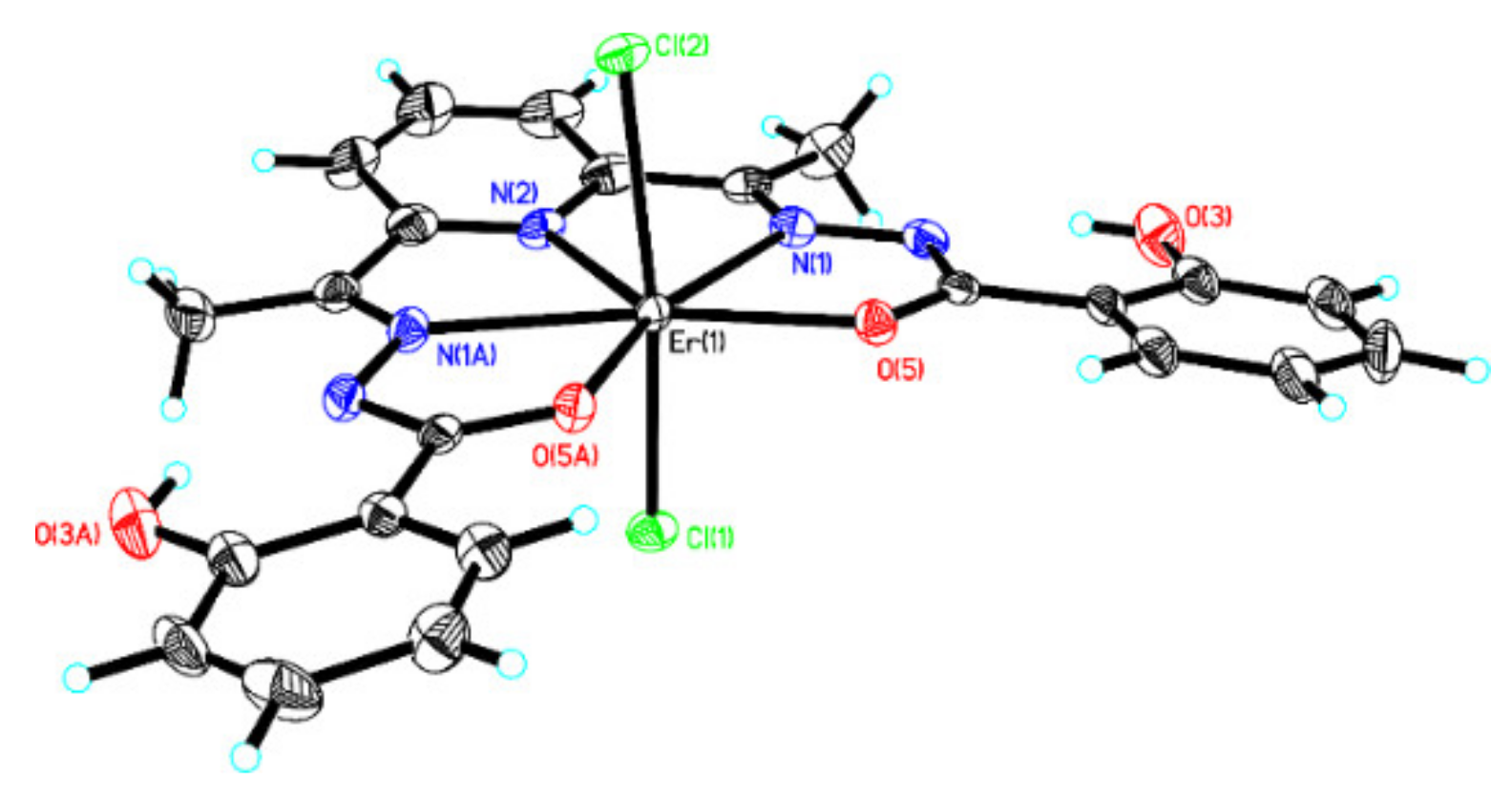
2.1. Synthesis and Molecular Structure
2.2. Description of the Structure
2.3. Magnetic Properties
2.3.1. Static (DC) Magnetic Properties
2.3.2. Crystal Field Analysis
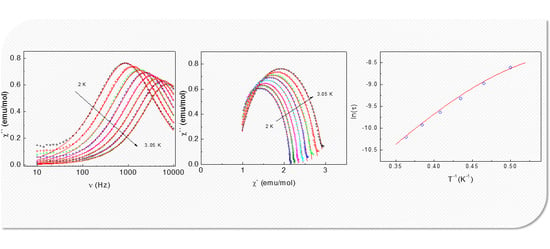
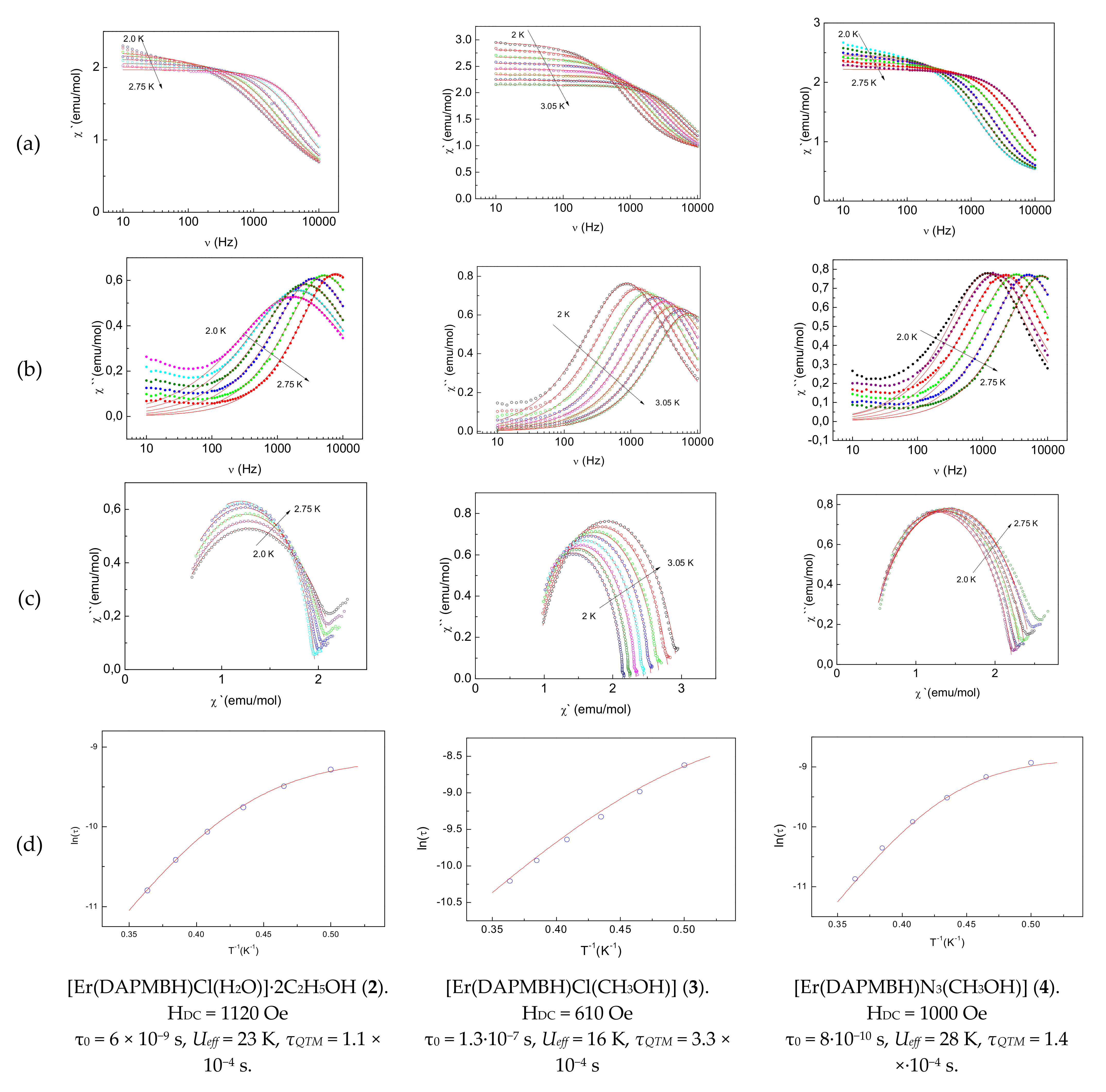
2.3.3. Dynamic (AC) Magnetic Properties
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. Crystal Structure Determination
3.3. Simulation of Static Magnetic Properties and CF Calculations
3.4. Computational Details
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
References
- Waldmann, O.A. Criterion for the Anisotropy Barrier in Single-Molecule Magnets. Inorg. Chem. 2007, 46, 10035–10037. [Google Scholar] [CrossRef]
- Neese, F.; Pantazis, D.A. What is not required to make a single molecule magnet. Faraday Discuss. 2011, 148, 229–238. [Google Scholar] [CrossRef]
- Feng, M.; Tong, M.-L. Single Ion Magnets from 3d to 5f; developments and strategies. Chem. Eur. J. 2018, 24, 7574–7594. [Google Scholar] [CrossRef]
- Mironov, V.S.; Chibotaru, L.F.; Ceulemans, A. Mechanism of a strongly anisotropic MoIII−CN−MnII spin−spin coupling in molecular magnets based on the [Mo(CN)7]4− heptacyanometalate: A new strategy for single-molecule magnets with high blocking temperatures. J. Am. Chem. Soc. 2003, 125, 9750–9760. [Google Scholar] [CrossRef]
- Mironov, V.S. New approaches to the problem of high-temperature single-molecule magnets. Dokl. Phys. Chem. 2006, 408, 130–136. [Google Scholar] [CrossRef]
- Craig, G.A.; Murrie, M. 3d single-ion magnets. Chem. Soc. Rev. 2015, 44, 2135–2147. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Avendaño, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar] [CrossRef]
- Layfield, R.A.; Murugesu, M. Lanthanides and Actinides in Molecular Magnetism; Wiley-VCH Verlag GmbH&Co, KGaA: Weinheim, Germany, 2015; pp. 1–368. [Google Scholar]
- Bar, A.K.; Pichon, C.; Sutter, J.-P. Magnetic anisotropy in two- to eight-coordinated transition-metal complexes: Recent developments in molecular magnetism. Coord. Chem. Rev. 2016, 308, 346–380. [Google Scholar] [CrossRef]
- Boca, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Rebilly, J.-N.; Charron, G.; Rivière, E.; Guillot, R.; Barra, A.-L.; Serrano, M.D.; van Slageren, J.; Mallah, T. Large Magnetic Anisotropy in Pentacoordinate NiII Complexes. Chem. Eur. J. 2008, 14, 1169–1177. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Wang, B.-W.; Sato, O.; Gao, S. First Fe(II)-based cyano-bridged single molecule magnet [CrIIIFeII2] with a large anisotropy. Chem. Commun. 2010, 46, 6959–6961. [Google Scholar] [CrossRef]
- Meng, Y.-S.; Jiang, S.D.; Wang, B.-W.; Gao, S. Understanding the magnetic anisotropy toward single-ion magnets. Acc. Chem. Res. 2016, 49, 2381–2389. [Google Scholar] [CrossRef]
- Gómes-Coca, S.; Cremades, E.; Aliaga-Alcaldeand, N.; Ruiz, E. Mononuclear single-molecule magnets: Tailoring the magnetic anisotropy of first-row transition-metal complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef]
- Saberand, M.R.; Dunbar, K.R. Ligands effects on the magnetic anisotropy of tetrahedral cobalt complexes. Chem. Commun. 2014, 50, 12266–12269. [Google Scholar]
- Huang, X.-C.; Zhou, C.; Shaoand, D.; Wang, X.-Y. Field-induced slow magnetic relaxation in cobalt(II) compounds with pentagonal bipyramid geometry. Inorg. Chem. 2014, 53, 12671–12673. [Google Scholar] [CrossRef]
- Bar, A.K.; Gogoi, N.; Pichon, C.; Goli, V.M.; Thlijeni, M.; Duhayon, C.; Suaud, N.; Guihery, N.; Barra, A.L.; Ramasesha, S.; et al. Pentagonal Bipyramid FeII Complexes: Robust Ising-Spin Units towards Heteropolynuclear Nanomagnets. Chem. -Eur. J. 2017, 23, 4380–4396. [Google Scholar] [CrossRef]
- Gogoi, N.; Thlijeni, M.; Duhayonand, C.; Sutter, J.-P. Heptacoordinated nickel(II) as an Ising-type anisotropic building unit: Illustration with a pentanuclear [(NiL)3{W(CN)8}2] complex. Inorg. Chem. 2013, 52, 2283–2285. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, J.-L.; Ungur, L.; Liu, J.; Li, Q.-W.; Wang, L.-F.; Ni, Z.-P.; Chibotaru, L.F.; Chen, X.-M.; Tong, M.-L. Symmetry-supported magnetic blocking at 20 K in pentagonal bipyramidal Dy(III) Single-Ion Magnets. J. Am. Chem. Soc. 2016, 138, 2829–2837. [Google Scholar] [CrossRef]
- Ruamps, R.; Batchelor, L.J.; Guillot, R.; Zakhia, G.; Barra, A.L.; Wernsdorfer, W.; Guihéryand, N.; Mallah, T. Ising-type magnetic anisotropy and single molecule magnet behaviour in mononuclear trigonal bipyramidal Co(ii) complexes. Chem. Sci. 2014, 5, 3418–3424. [Google Scholar] [CrossRef]
- Ruamps, R.; Batchelor, L.J.; Maurice, R.; Gogoi, N.; Jiménez-Lozano, P.; Guihéry, N.; de Graaf, C.; Barra, A.L.; Sutter, J.-P.; Mallah, T. Origin of the magnetic anisotropy in heptacoordinate NiII and CoII complexes. Chem. Eur. J. 2013, 19, 950–956. [Google Scholar] [CrossRef]
- Ruamps, R.; Maurice, R.; Batchelor, L.J.; Boggio-Pasqua, M.; Guillot, R.; Barra, A.L.; Liu, J.; Bendeif, E.-E.; Pillet, S.; Hill, S.; et al. Giant Ising-type magnetic anisotropy in trigonal bipyramidal Ni(II) complexes: Experiment and theory. J. Am. Chem. Soc. 2013, 135, 3017–3026. [Google Scholar] [CrossRef]
- Gavey, E.L.; Beldjoudi, Y.; Rawson, J.M.; Stamatatosand, T.C.; Pilkington, M. Slow relaxation in the first penta-aza Dy(iii) macrocyclic complex. Chem. Commun. 2014, 50, 3741–3743. [Google Scholar] [CrossRef]
- Unger, L.; Chibotaru, L.F. Strategies toward high-temperature lantanide-based single-molecule magnets. Inorg. Chem. 2016, 55, 10043–10056. [Google Scholar] [CrossRef]
- Pichon, C.; Suaud, N.; Duhayon, C.; Guihery, N.; Sutter, J.-P. Cyano-bridged Fe(II)–Cr(III) single-chain magnet based on pentagonal bipyramid units: On the added value of aligned axial anisotropy. J. Am. Chem. Soc. 2018, 140, 7698–7704. [Google Scholar] [CrossRef]
- Diaz-Ortega, I.F.; Herrera, J.M.; Dey, S.; Nojiri, H.; Rajaraman, G.; Colacio, E. The effect of the electronic structure and flexibility of the counteranions on magnetization relaxation in [Dy(L)2(H2O)5]3+ (L = phosphine oxide derivative) pentagonal bipyramidal SIMs. Inorg. Chem. Front. 2020, 7, 689–699. [Google Scholar] [CrossRef]
- Deng, Y.-F.; Yao, B.; Zhan, P.-Z.; Gan, D.; Zhang, Y.-Z.; Dunbar, K.R. Synthesis and magnetic studies of pentagonal bipyramidal metal complexes of Fe, Co and Ni. Dalton. Trans. 2019, 48, 3243–3248. [Google Scholar] [CrossRef]
- Manakin, Y.V.; Mironov, V.S.; Bazhenova, T.A.; Lyssenko, K.A.; Gilmutdinov, I.F.; Bikbaev, K.S.; Masitov, A.A.; Yagubskii, E.B. (Et4N)[MoIII(DAPBH)Cl2], the first pentagonal-bipyramidal Mo(iii) complex with a N3O2-type Schiff-base ligand: Manifestation of unquenched orbital momentum and Ising-type magnetic anisotropy. Chem. Commun. 2018, 54, 10084–10087. [Google Scholar] [CrossRef]
- Birk, F.J.; Pinkowicz, D.; Dunbar, K.R. The Heptacyanotungstate(IV) anion: A new 5 d transition-metal member of the rare heptacyanometallate family of anions. Angew. Chem. Int. Ed. 2016, 55, 11368–11371. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.-C.; Liu, J.-L.; Vieru, V.; Ungur, L.; Jia, J.-H.; Chibotaru, L.F.; Lan, Y.; Wernsdorfer, W.; Gao, S.; et al. A Stable Pentagonal Bipyramidal Dy(III) Single-Ion Magnet with a Record Magnetization Reversal Barrier over 1000 K. J. Am. Chem. Soc. 2016, 138, 5441–5450. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Chilton, N.F.; Winpenny, R.E.; Zheng, Y.-Z. On approaching the limit of molecular magnetic anisotropy: A near-perfect pentagonal bipyramidal dysprosium(III) single-molecule magnet. Angew. Chem. Int. Ed. 2016, 55, 16071–16074. [Google Scholar] [CrossRef]
- Gupta, S.K.; Rajeshkumar, T.; Rajaraman, G.; Murugavel, R. An air-stable Dy(iii) single-ion magnet with high anisotropy barrier and blocking temperature. Chem. Sci. 2016, 7, 5181–5191. [Google Scholar] [CrossRef]
- Ding, Y.-S.; Han, T.; Zhai, Y.-Q.; Reta, D.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.-Z. A Study of Magnetic Relaxation in Dysprosium(III) Single-Molecule Magnets. Chem. Eur. J. 2020, 26, 5893–5902. [Google Scholar] [CrossRef]
- Wu, H.; Li, M.; Yin, B.; Xia, Z.; Ke, H.; Wei, Q.; Xie, G.; Chen, S.; Gao, S. Fine-tuning the type of equatorial donor atom in pentagonal bipyramidal Dy(iii) complexes to enhance single-molecule magnet properties. Dalton Trans. 2019, 48, 16384–16394. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Jiang, S.-D.; Qin, S.-X. Prediction of the quantized axis of rare-earth ions: The electrostatic model with displaced point charges. Inorg. Chem. Front. 2015, 2, 613–619. [Google Scholar] [CrossRef]
- Gao, C.; Genoni, A.; Gao, S.; Jiang, S.; Soncini, A.; Overgaard, J. Observation of the asphericity of 4f-electron density and its relation to the magnetic anisotropy axis in single-molecule magnets. Nat. Chem. 2020, 12, 213–219. [Google Scholar] [CrossRef]
- Palenik, G.J.; Wester, D.W.; Rychlewska, U.; Palenik, R.C. Pentagonal-bipyramidal complexes. Synthesis and crystal structures of diaqua[2,6-diacetylpyridine bis(semicarbazone)]chromium(III) hydroxide dinitrate hydrate and dichloro[2,6-diacetylpyridine bis(semicarbazone)]iron(III) chloride dihydrate. Inorg. Chem. 1976, 15, 1814–1819. [Google Scholar] [CrossRef]
- Palenik, G.J.; Wester, D.W. Pentagonal-bipyramidal complexes. Crystal and molecular structures of chloroaqua(2,6-diacetylpyridine bis(semicarbazone))manganese(II), -iron(II), -cobalt(II), and -zinc(II) chloride dihydrates. Inorg. Chem. 1978, 17, 864–870. [Google Scholar] [CrossRef]
- Giordano, T.J.; Palenik, G.J.; Palenik, R.C.; Sullivan, D.A. Pentagonal-bipyramidal complexes. Synthesis and characterization of aqua(nitrato)[2,6-diacetylpyridine bis(benzoyl hydrazone)]cobalt(II) nitrate and diaqua[2,6-diacetylpyridine bis(benzoyl hydrazone)]nickel(II) nitrate dihydrate. Inorg. Chem. 1979, 18, 2445–2450. [Google Scholar] [CrossRef]
- Gerloch, M.; Morgenstern-Badarau, I.; Audiere, J.-P. Magnetic and spectral properties of the pentagonal-bipyramidal complex ions chloroaqua- and diaqua[2,6-diacetylpyridinebis(semicarbazone)]cobalt(II). Inorg. Chem. 1979, 18, 3220–3225. [Google Scholar] [CrossRef]
- Gerloch, M.; Morgenstern-Badarau, I. Magnetic and spectral properties of chloroaqua[2,6-diacetylpyridinebis(semicarbazone)]iron(II) and diaqua[2,6-diacetylpyridinebis(semicarbazone)]nickel(II): Ligand fields and bonding in pentagonal-bipyramidal complexes. Inorg. Chem. 1979, 18, 3225–3229. [Google Scholar] [CrossRef]
- Pelizzi, C.; Pelizzi, G. Investigation into aroylhydrazones as chelating agents. Synthesis and structural characterization of a tin(IV) complex with 2,6-diacetylpyridine bis(salicyloylhydrazone). J. Chem. Soc. Dalton Trans. 1980, 10, 1970–1973. [Google Scholar] [CrossRef]
- Lorenzini, C.; Pelizzi, C.; Pelizzi, G.; Predieri, G. Investigation into aroylhydrazones as chelating agents. Part 3. Synthesis and spectroscopic characterization of complexes of MnII, CoII, NiII, CuII, and ZnII with 2,6-diacetylpyridine bis(benzoylhydrazone) and X-ray structure of aquachloro[2,6-diacetylpyridine bis(benzoylhydrazone)]manganese(II) chloride. J. Chem. Soc. Dalton Trans. 1983, 4, 721–727. [Google Scholar]
- Bino, A.; Frim, R.; van Genderen, M.H.P. Three coordination modes of the pentadentate ligand 2,6-diacethylpyridinedisemicarbazone. Inorg. Chim. Acta 1987, 127, 95–101. [Google Scholar] [CrossRef]
- Ianelli, S.; Pelizzi, C.; Pelizzi, G.; Tarasconi, P. Heptacoordination in MnII, NiII, and CuII complexes of 2,6-diacetylpyridine bis (acetylhydrazone). J. Chem. Crystallogr. 1996, 26, 195–201. [Google Scholar] [CrossRef]
- Carcelli, M.; Ianelli, S.; Pelagatti, P.; Pelizzi, G. Structural characterization of a new ligand mode of 2,6-diacetylpyridine bis(semicarbazone), H2daps. Inorg. Chim. Acta 1999, 292, 121–126. [Google Scholar] [CrossRef]
- Ivanovic-Burmazovic, I.; Andjelkovic, K. Transition metal complexes with bis(hydrazone) ligands of 2,6-diacetylpyridine. Hepta-cordination of 3d metals. Adv. Inorg. Chem. 2004, 55, 315–360. [Google Scholar]
- Tamboura, F.B.; Haba, P.M.; Gaye, M.; Sall, A.S.; Barry, A.H.; Jouini, T. Structural studies of bis-(2,6-diacetylpyridine-bis-(phenylhydrazone)) and X-ray structure of its Y(III), Pr(III), Sm(III) and Er(III) complex. Polyhedron 2004, 23, 1191–1197. [Google Scholar] [CrossRef]
- Pichon, C.; Elrez, B.; Bereau, V.; Duhayon, C.; Sutter, J.-P. From heptacoordinated CrIII complexes with cyanide or isothiocyanate apical groups to 1D heterometallic assemblages with all-pentagonal-bipyramid coordination geometries. Eur. J. Inorg. Chem. 2018, 2018, 340–348. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Zorina, L.V.; Simonov, S.V.; Mironov, V.S.; Maximova, O.V.; Spillecke, L.; Koo, C.; Klingeler, R.; Manakin, Y.V.; Vasiliev, A.N.; et al. The first pentagonal-bipyramidal vanadium(iii) complexes with a Schiff-base N3O2 pentadentate ligand: Synthesis, structure and magnetic properties. Dalton Trans. 2020, 49, 15287–15298. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Zorina, L.V.; Simonov, S.V.; Manakin, Y.V.; Kornev, A.B.; Lyssenko, K.A.; Mironov, V.S.; Gilmutdinov, I.F.; Yagubskii, E.B. A novel family of hepta-coordinated Cr(III) complexes with a planar pentadentate N3O2 Schiff base ligand: Synthesis, structure and magnetism. Inorg. Chim. Acta 2021, 522, 120358. [Google Scholar] [CrossRef]
- Bar, A.K.; Kalita, P.; Sutter, J.-P.; Chandrasekhar, V. Pentagonal-bipyramid Ln(III) complexes exhibiting single-ion-magnet behavior: A rational synthetic approach for a rigid equatorial plane. Inorg. Chem. 2018, 57, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Corredoira-Vázguez, J.; Fondo, M.; Sanmartin-Matalobos, J.; García-Deibe, A.M. Attainment of pentagonal-bipyramidal LnIII.complexes from a planar pentadentate ligand. Proceedings 2020, 62, 2002. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, N.; Bar, A.K.; Dey, S.; Jana, A.; Rajaraman, G.; Sutter, J.-P.; Chandrasekhar, V. Pentagonal bipyramidal Ln(III) complexes containing an axial phosphine oxide ligand: Field-induced single-ion magnetism behavior of the Dy(III) analogues. Inorg. Chem. 2020, 59, 6603–6612. [Google Scholar] [CrossRef]
- Bazhenova, T.A.; Mironov, V.S.; Yakushev, I.A.; Svetogorov, R.D.; Maximova, O.V.; Manakin, Y.V.; Kornev, A.B.; Vasiliev, A.N.; Yagubskii, E.B. End-to-end azido-bridged lanthanide chain complexes (Dy, Er, Gd, and Y) with a pentadentate Schiff-base [N3O2] ligand: Synthesis, structure, and magnetism. Inorg. Chem. 2020, 59, 563–578. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2001; pp. 1–480. [Google Scholar]
- Hunter, C.A.; Sanders, J.K.M. The nature of pi-pi interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–970. [Google Scholar]
- Wyborne, B.G. Spectroscopic Properties of Rare Earths; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1965; pp. 1–241. [Google Scholar]
- Hüfner, S. Optical Spectra of Transparent Rare Earth Compounds, 1st ed.; Academic Press: New York, NY, USA, 1978; pp. 1–252. [Google Scholar]
- Eyring, L.; Gschneidner, K.A. Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1999; pp. 1–422. [Google Scholar]
- Gerloch, M.; McMeeking, R.F. Paramagnetic properties of unsymmetrical transition-metal complexes. J. Chem. Soc. Dalton Trans. 1975, 22, 2443–2451. [Google Scholar] [CrossRef]
- Newman, D.J. The orbit-lattice interaction for lanthanide ions. I. determination of empirical parameters. Aust. J. Phys. 1978, 31, 79–94. [Google Scholar] [CrossRef][Green Version]
- Newman, D.J.; Ng, B. The superposition model of crystal fields. Rep. Prog. Phys. 1989, 52, 699–762. [Google Scholar] [CrossRef]
- Bungenstock, C.; Tröster, T.; Holzapfel, W.B. Effect of pressure on free-ion and crystal-field parameters of Pr3+ in LOCl (L. = La, Pr, Gd). Phys. Rev. B 2000, 62, 7945–7955. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Spectral Intensities of the Trivalent Lanthanides and Actinides in Solution. II. Pm3+, Sm3+, Eu3+, Gd3+, Tb3+, Dy3+ and Ho3+. J. Chem. Phys. 1968, 49, 4412–4423. [Google Scholar] [CrossRef]
- Carnall, W.T.; Goodman, G.L.; Rajnak, K.; Rana, R.S. A systematic analysis of the spectra of the lanthanides doped into single crystal LaF3. J. Chem. Phys. 1989, 90, 3443–3457. [Google Scholar] [CrossRef]
- Mironov, V.S.; Li, L.E. Crystal field analysis of Pr3+ and Nd3+ ions in KR(WO4)2 (R = Y or Gd) potassium rare-earth tungstates. J. Alloy. Compd. 1998, 279, 83–92. [Google Scholar] [CrossRef]
- Mironov, V.S.; Galyametdinov, Y.G.; Ceulemans, A.; Binnemans, K. On the magnetic anisotropy of lanthanide-containing metallomesogens. J. Chem. Phys. 2000, 113, 10293–10303. [Google Scholar] [CrossRef]
- Mironov, V.S.; Galyametdinov, Y.G.; Ceulemans, A.; GörllerWalrand, C.; Binnemans, K. Room-temperature magnetic anisotropy of lanthanide complexes: A model study for various coordination polyhedra. J. Chem. Phys. 2002, 116, 4673–4685. [Google Scholar] [CrossRef]
- Chang, N.C.; Gruber, J.B.; Leavitt, R.P.; Morrison, C.A. Optical spectra, energy levels, and crystal-field analysis of tripositive rare earth ions in Y2O3. I. Kramers ions in C2 sites. J. Chem. Phys. 1982, 78, 3877–3889. [Google Scholar] [CrossRef]
- Jia, L.; Chen, Q.; Meng, Y.-S.; Sun, H.-L.; Gao, S. Elucidation of slow magnetic relaxation in a ferromagnetic 1D dysprosium chain through magnetic dilution. Chem. Commun. 2014, 50, 6052–6055. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J. Relaxation dynamics of dysprosium(iii) single molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef] [PubMed]
- Rigaku. CrysAlisPro Software System Version 1.171; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction Beamline for Studying Crystalline Samples at Kurchatov Synchrotron Radiation Source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Galván, I.F.; Vacher, M.; Alavi, A.; Angeli, C.; Aquilante, F.; Autschbach, J.; Bao, J.J.; Bokarev, S.I.; Bogdanov, N.A.; Carlson, R.K.; et al. OpenMolcas: From Source Code to Insight. J. Chem. Theory Comput. 2019, 15, 5925–5964. [Google Scholar] [CrossRef] [PubMed]
- Aquilante, F.; Autschbach, J.; Baiardi, A.; Battaglia, S.; Borin, V.A.; Chibotaru, L.F.; Conti, I.; de Vico, L.; Delcey, M.; Galván, I.F.; et al. Modern quantum chemistry with [Open]Molcas. J. Chem. Phys. 2020, 152, 214117. [Google Scholar] [CrossRef]
- Chibotaru, L.F.; Ungur, L. Ab initio calculation of anisotropic magnetic properties of complexes. I. Unique definition of pseudospin Hamiltonians and their derivation. J. Chem. Phys. 2012, 137, 64112. [Google Scholar] [CrossRef]
- Douglas, M.; Kroll, N.M. Quantum electrodynamical corrections to the fine structure of helium. Ann. Phys. 1974, 82, 89–155. [Google Scholar] [CrossRef]
- Hess, B.A. Applicability of the no-pair equation with free-particle projection operators to atomic and molecular structure calculations. Phys. Rev. A 1985, 32, 756–763. [Google Scholar] [CrossRef]
- Hess, B.A. Relativistic electronic-structure calculations employing a two-component no-pair formalism with external-field projection operators. Phys. Rev. A 1986, 33, 3742–3748. [Google Scholar] [CrossRef]
- Wolf, A.; Reiher, M.; Hess, B.A. The generalized Douglas-Kroll transformation. J. Chem. Phys. 2002, 117, 9215–9226. [Google Scholar] [CrossRef]





| 2 | 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|---|
| 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 |
| 29.2 | 33.17 | 32.1 | 35.87 | 21.9 | 26.29 | 9 | 26.24 |
| 49.7 | 54.6 | 62.4 | 60.3 | 50.5 | 44.58 | 22 | 60.15 |
| 99 | 84.29 | 95.9 | 96.98 | 69.9 | 92.06 | 74.1 | 159.71 |
| 197.8 | 174.94 | 182.9 | 190.25 | 146 | 233.03 | 130.3 | 199.31 |
| 272.8 | 307.39 | 293.2 | 286.1 | 185.5 | 288.5 | 203.7 | 281.43 |
| 301.2 | 452.97 | 310 | 441.15 | 211.9 | 409.12 | 207.8 | 399.21 |
| 321.6 | 488.93 | 336.2 | 472.05 | 278.7 | 429.09 | 245.5 | 423.48 |
| g-tensor components of the ground CF state | |||||||
| gx = 0.53 | 0.031 | gx = 1.74 | 0.353 | gx = 0.09 | 0.458 | gx = 2.07 | 0.439 |
| gy = 2.10 | 5.166 | gy = 1.98 | 3.347 | gy = 2.02 | 4.697 | gy = 4.88 | 0.547 |
| gz = 13.44 | 10.9 | gz = 13.95 | 12.954 | gz = 14.20 | 11.512 | gz = 12.37 | 15.04 |
| g-tensor components of the first excited CF state | |||||||
| gx = 1.96 | 0.348 | gx = 2.15 | 0.914 | gx = 2.75 | 0.925 | gx = 2.70 | 7.308 |
| gy = 5.09 | 2.642 | gy = 5.61 | 3.008 | gy = 5.24 | 1.799 | gy = 6.34 | 6.727 |
| gz = 9.68 | 6.157 | gz = 9.18 | 7.296 | gz = 8.92 | 5.048 | gz = 7.75 | 4.135 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhenova, T.A.; Kopotkov, V.A.; Korchagin, D.V.; Manakin, Y.V.; Zorina, L.V.; Simonov, S.V.; Yakushev, I.A.; Mironov, V.S.; Vasiliev, A.N.; Maximova, O.V.; et al. A Series of Novel Pentagonal-Bipyramidal Erbium(III) Complexes with Acyclic Chelating N3O2 Schiff-Base Ligands: Synthesis, Structure, and Magnetism. Molecules 2021, 26, 6908. https://doi.org/10.3390/molecules26226908
Bazhenova TA, Kopotkov VA, Korchagin DV, Manakin YV, Zorina LV, Simonov SV, Yakushev IA, Mironov VS, Vasiliev AN, Maximova OV, et al. A Series of Novel Pentagonal-Bipyramidal Erbium(III) Complexes with Acyclic Chelating N3O2 Schiff-Base Ligands: Synthesis, Structure, and Magnetism. Molecules. 2021; 26(22):6908. https://doi.org/10.3390/molecules26226908
Chicago/Turabian StyleBazhenova, Tamara A., Vyacheslav A. Kopotkov, Denis V. Korchagin, Yuriy V. Manakin, Leokadiya V. Zorina, Sergey V. Simonov, Ilya A. Yakushev, Vladimir S. Mironov, Alexander N. Vasiliev, Olga V. Maximova, and et al. 2021. "A Series of Novel Pentagonal-Bipyramidal Erbium(III) Complexes with Acyclic Chelating N3O2 Schiff-Base Ligands: Synthesis, Structure, and Magnetism" Molecules 26, no. 22: 6908. https://doi.org/10.3390/molecules26226908
APA StyleBazhenova, T. A., Kopotkov, V. A., Korchagin, D. V., Manakin, Y. V., Zorina, L. V., Simonov, S. V., Yakushev, I. A., Mironov, V. S., Vasiliev, A. N., Maximova, O. V., & Yagubskii, E. B. (2021). A Series of Novel Pentagonal-Bipyramidal Erbium(III) Complexes with Acyclic Chelating N3O2 Schiff-Base Ligands: Synthesis, Structure, and Magnetism. Molecules, 26(22), 6908. https://doi.org/10.3390/molecules26226908