Subsynaptic Distribution, Lipid Raft Targeting and G Protein-Dependent Signalling of the Type 1 Cannabinoid Receptor in Synaptosomes from the Mouse Hippocampus and Frontal Cortex
Abstract
1. Introduction
2. Results
2.1. Validation of the Enriched Synaptosomal Fraction from Mouse Frontal Cortex
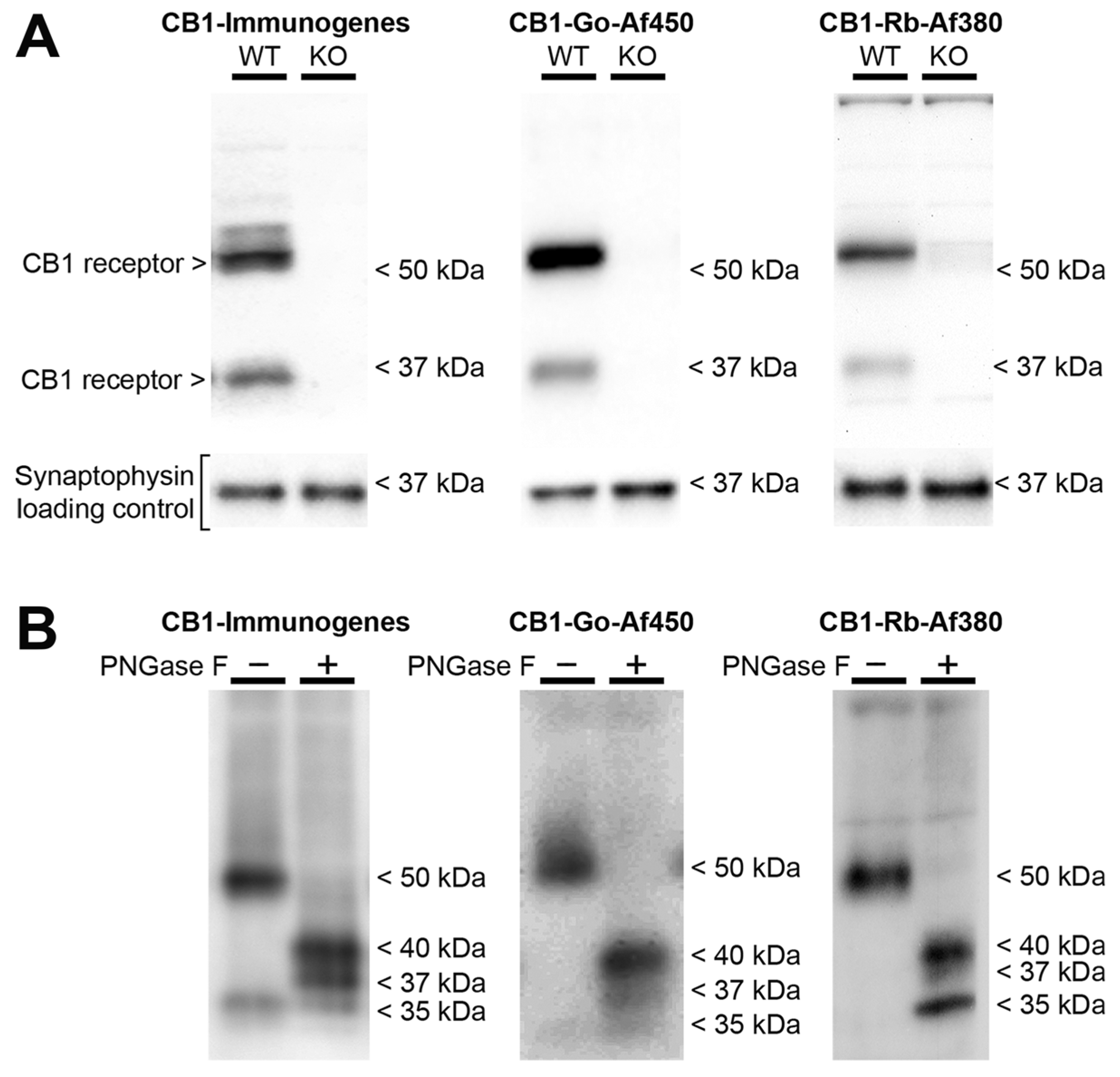
2.2. Characteristics of the Immunoreactive Signals Provided by Anti-CB1 Antibodies in Frontal Cortical Synaptosomes Derived from Wild Type Mice
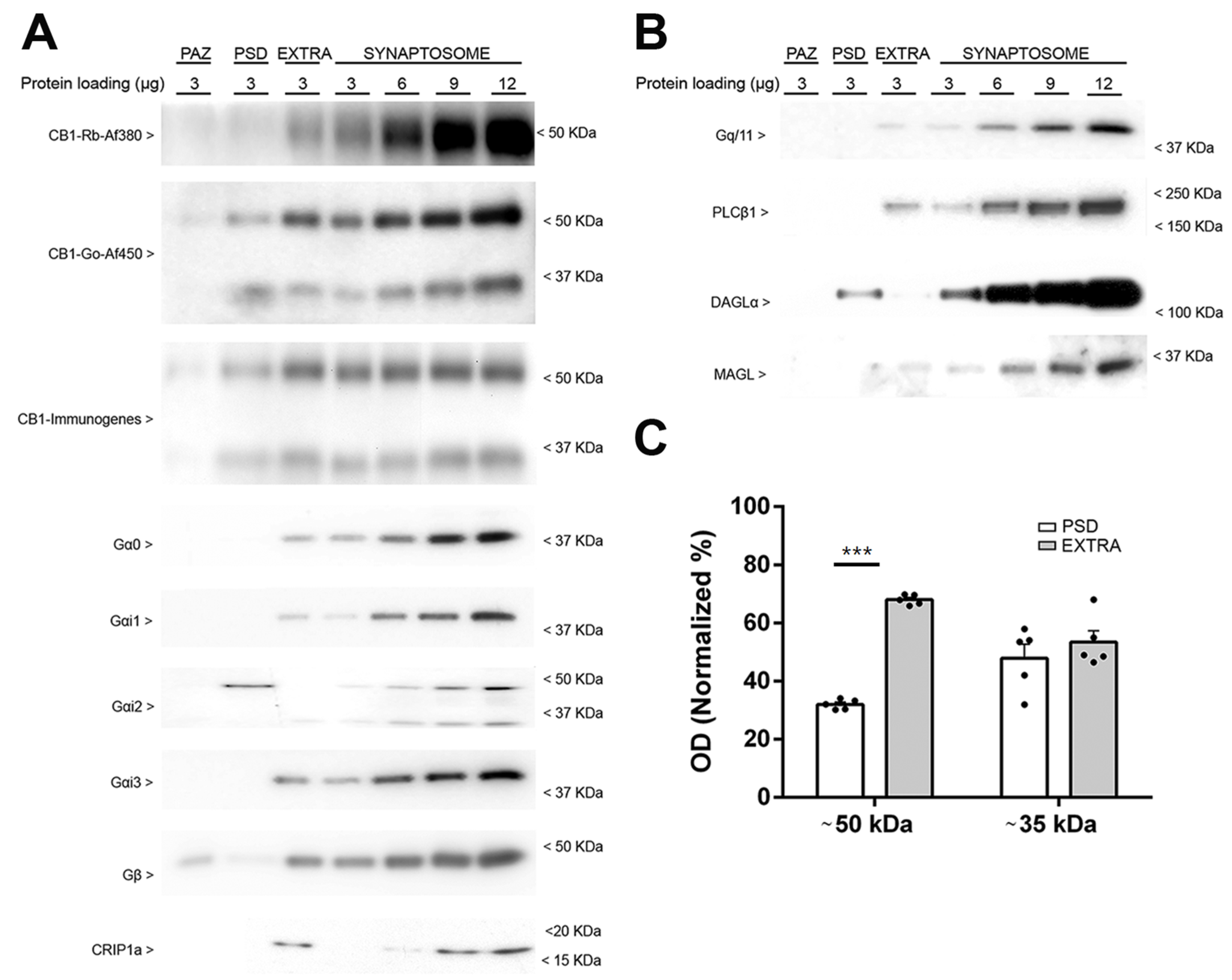
2.3. Subsynaptic Compartmentalization of the CB1 Receptor and Other Proteins of the Endocannabinoid System in Frontal Cortex Synaptosomes Derived from Wild-Type and CB1-RS Mice
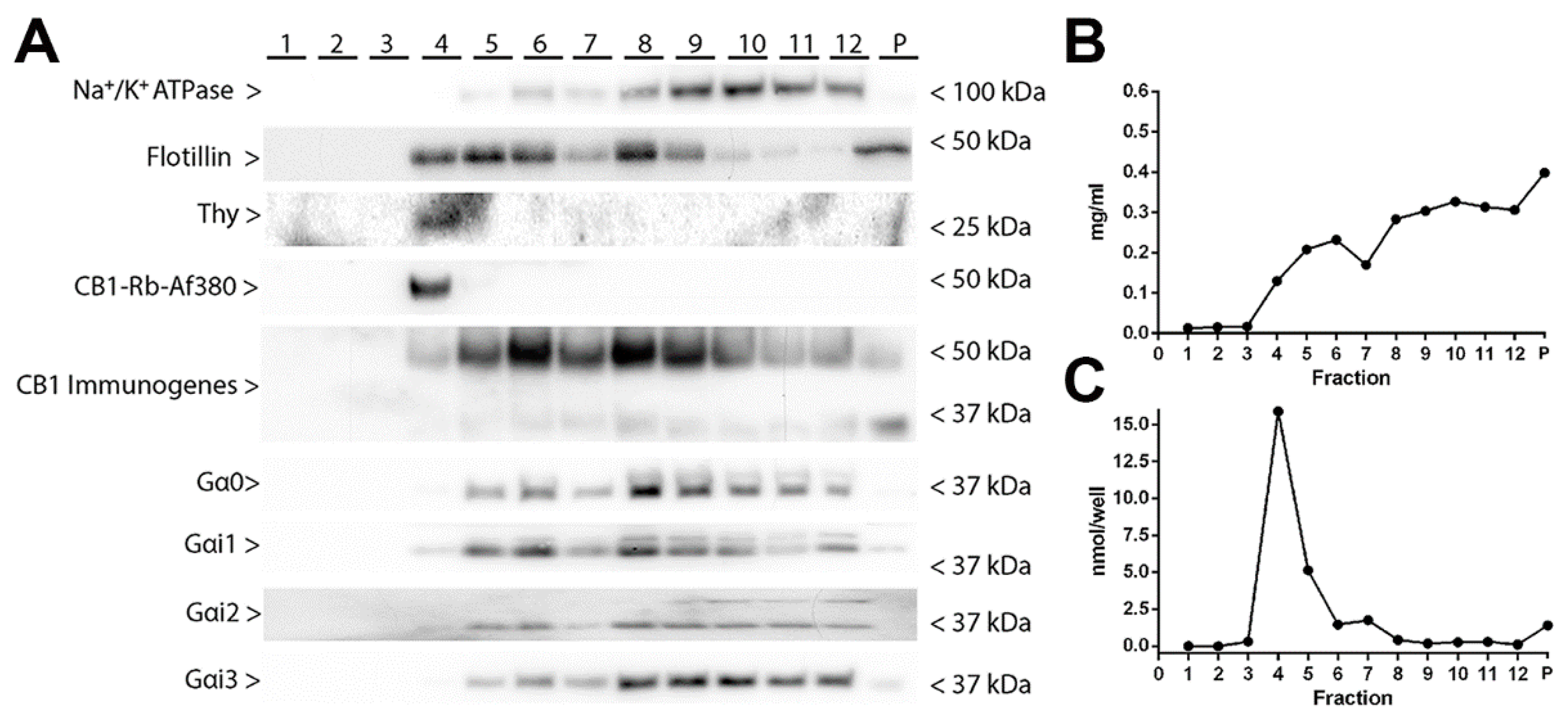
2.4. Localization of CB1 Receptors in Lipid Raft and Non-Lipid Raft Microdomains of Synaptosomal Plasma Membranes Obtained from Frontal Cortical Brain Tissue of Wild-Type and CB1-RS Mice
2.5. Analysis of the Coupling of the CB1 Receptor to Gαi/o Proteins in Frontal Cortical and Hippocampal Synaptosomes Obtained from CB1-RS and Wild-Type Brain Mice
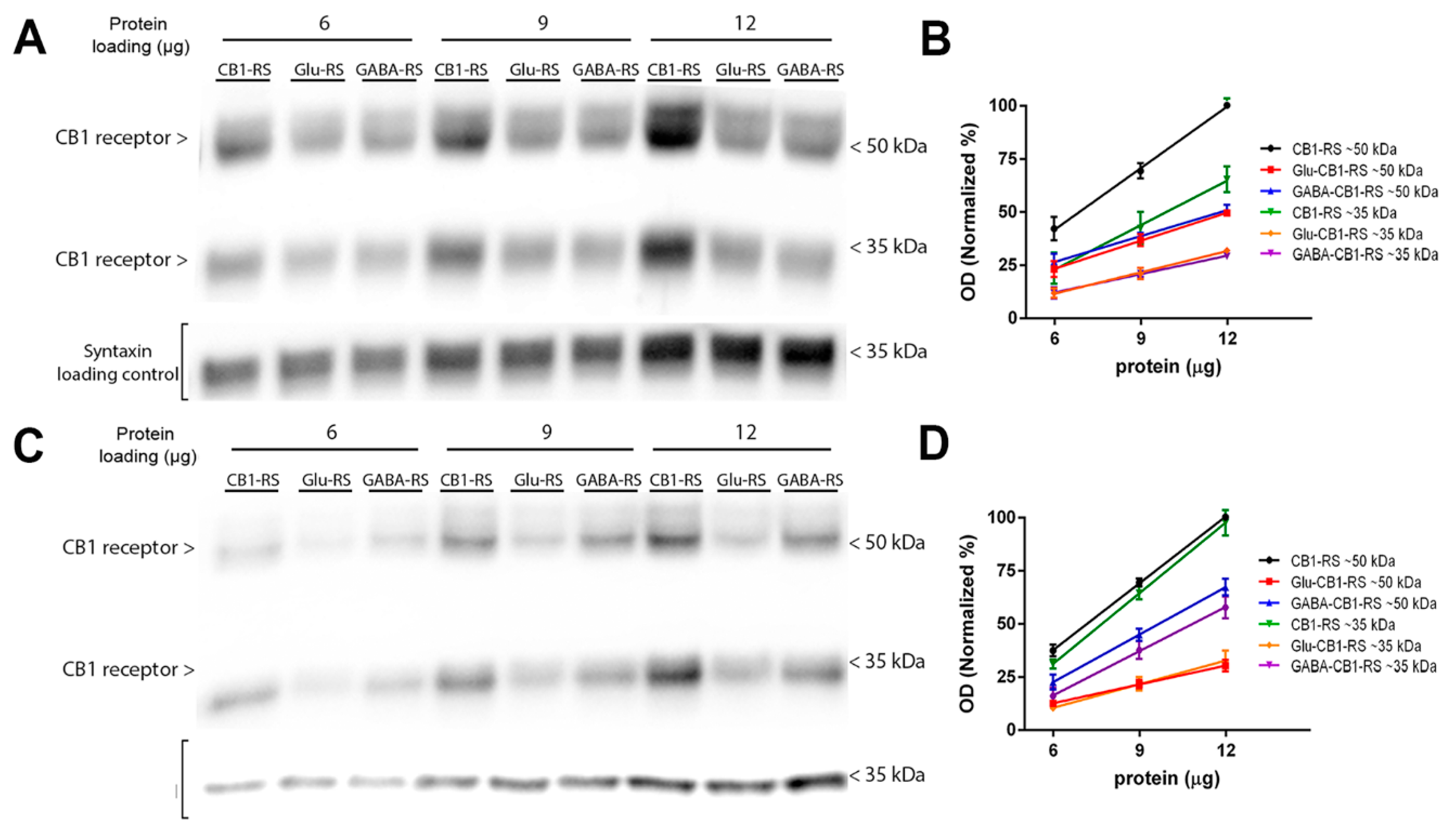
2.6. Analysis of the CB1 Receptor Protein Expression and Gαi/o Protein Coupling in Synaptosomes Obtained from Frontal Cortical and Hippocampal Tissue of Glu-CB1-RS, GABA-CB1-RS and CB1-RS Mice
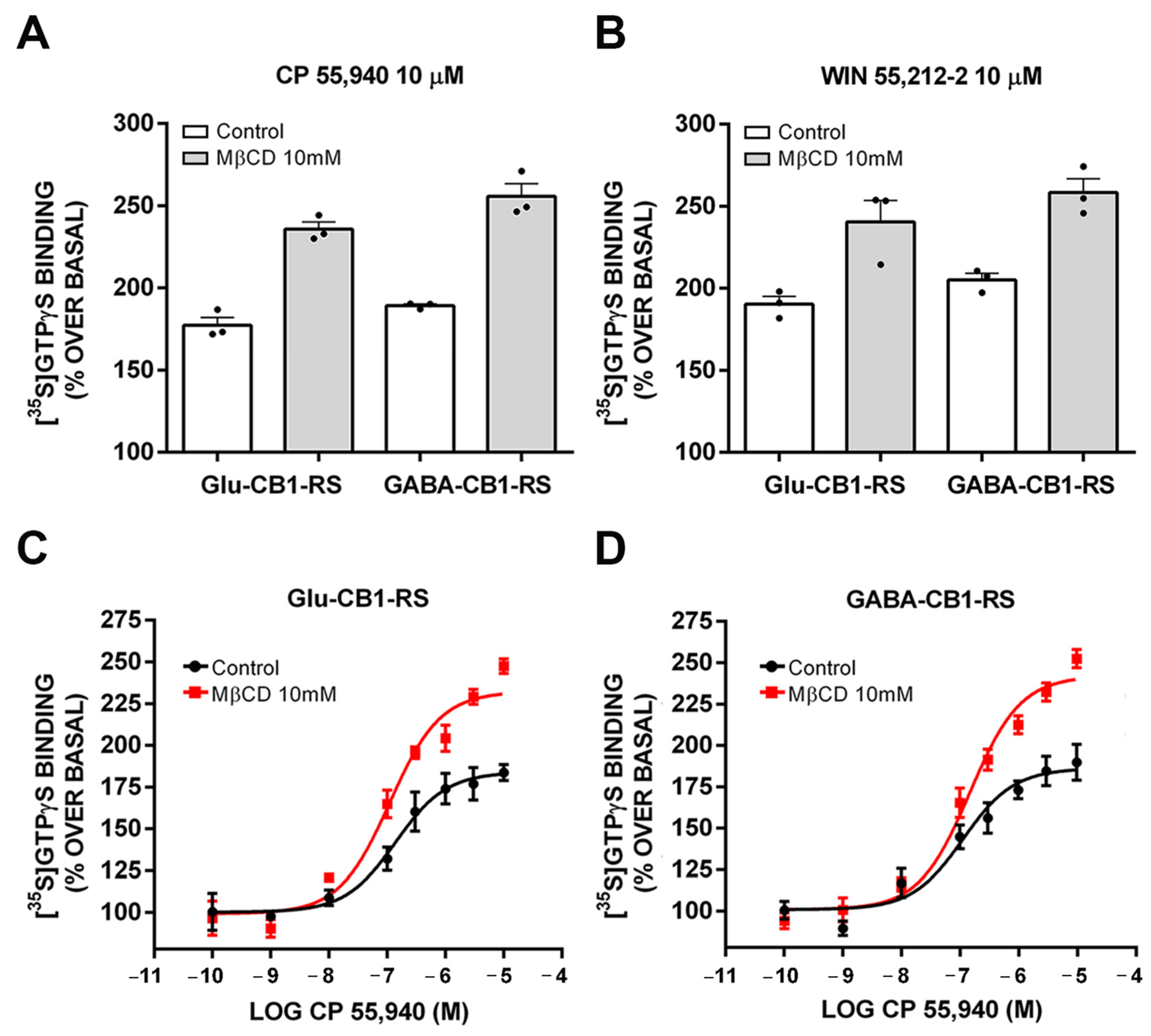
2.7. Analysis of the CB1 Receptor Coupling to Gαi/o Proteins in Control and MβCD Pretreated Synaptosomes Obtained from Frontal Cortical Tissue of Glu-CB1-RS, GABA-CB1-RS, and CB1-RS Mice
3. Discussion
4. Materials and Methods
4.1. Animal Procedures and Brain Tissue Preparation
4.2. Chemicals and Antibodies
4.3. Preparation of Mouse Synaptosomal Membranes and Purification of Subsynaptic Fractions
4.4. Isolation of “Lipid Rafts” from Cortical Synaptosomal Membranes
4.5. Immunofluorescence Assay for Frontal Cortical Synaptosomes
4.6. Treatment of Cortical Synaptosomal Fractions with Deglycosylating Enzymes
4.7. Treatment of Cortical Synaptosomes with Methyl-β-cyclodextrin
4.8. Western Blot Assay in Purified Fractions of Synaptosomal Membranes
4.9. Agonist Stimulated [35S]GTPγS Binding Assay in Synaptosomal Membranes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Monory, K.; Massa, F.; Egertová, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [CrossRef]
- Bellocchio, L.; Lafenêtre, P.; Cannich, A.; Cota, D.; Puente, N.; Grandes, P.; Chaouloff, F.; Piazza, P.V.; Marsicano, G. Bimodal control of stimulated food intake by the endocannabinoid system. Nat. Neurosci. 2010, 13, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Rey, A.A.; Purrio, M.; Viveros, M.P.; Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA B receptors in the balance of gabaergic and glutamatergic neurotransmission. Neuropsychopharmacology 2012, 37, 2624–2634. [Google Scholar] [CrossRef] [PubMed]
- Ruehle, S.; Remmers, F.; Romo-Parra, H.; Massa, F.; Wickert, M.; Wortge, S.; Haring, M.; Kaiser, N.; Marsicano, G.; Pape, H.-C.; et al. Cannabinoid CB1 Receptor in Dorsal Telencephalic Glutamatergic Neurons: Distinctive Sufficiency for Hippocampus-Dependent and Amygdala-Dependent Synaptic and Behavioral Functions. J. Neurosci. 2013, 33, 10264–10277. [Google Scholar] [CrossRef]
- Remmers, F.; Lange, M.D.; Hamann, M.; Ruehle, S.; Pape, H.-C.; Lutz, B. Addressing sufficiency of the CB1 receptor for endocannabinoid-mediated functions through conditional genetic rescue in forebrain GABAergic neurons. Brain Struct. Funct. 2017, 222, 3431–3452. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, A.; Puente, N.; Elezgarai, I.; Ruehle, S.; Lutz, B.; Reguero, L.; Gerrikagoitia, I.; Marsicano, G.; Grandes, P. Anatomical characterization of the cannabinoid CB 1 receptor in cell-type-specific mutant mouse rescue models. J. Comp. Neurol. 2017, 525, 302–318. [Google Scholar] [CrossRef]
- De Giacomo, V.; Ruehle, S.; Lutz, B.; Häring, M.; Remmers, F. Differential glutamatergic and GABAergic contributions to the tetrad effects of Δ9-tetrahydrocannabinol revealed by cell-type-specific reconstitution of the CB1 receptor. Neuropharmacology 2020, 179, 108287. [Google Scholar] [CrossRef]
- Monory, K.; Blaudzun, H.; Massa, F.; Kaiser, N.; Lemberger, T.; Schütz, G.; Wotjak, C.T.; Lutz, B.; Marsicano, G. Genetic Dissection of Behavioural and Autonomic Effects of Δ9-Tetrahydrocannabinol in Mice. PLoS Biol. 2007, 5, e269. [Google Scholar] [CrossRef]
- Steindel, F.; Lerner, R.; Häring, M.; Ruehle, S.; Marsicano, G.; Lutz, B.; Monory, K. Neuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampus. J. Neurochem. 2013, 124, 795–807. [Google Scholar] [CrossRef]
- Phillips, G.R.; Huang, J.K.; Wang, Y.; Tanaka, H.; Shapiro, L.; Zhang, W.; Shan, W.S.; Arndt, K.; Frank, M.; Gordon, R.E.; et al. The presynaptic particle web: Ultrastructure, composition, dissolution, and reconstitution. Neuron 2001, 32, 63–77. [Google Scholar] [CrossRef]
- Kofalvi, A.; Rodrigues, R.J.; Ledent, C.; Mackie, K.; Vizi, E.S.; Cunha, R.A.; Sperlágh, B. Involvement of Cannabinoid Receptors in the Regulation of Neurotransmitter Release in the Rodent Striatum: A Combined Immunochemical and Pharmacological Analysis. J. Neurosci. 2005, 25, 2874–2884. [Google Scholar] [CrossRef]
- Ostrom, R.S.; Insel, P.A. Methods for the Study of Signaling Molecules in Membrane Lipid Rafts and Caveolae. Methods Mol. Biol. 2006, 332, 181–192. [Google Scholar] [CrossRef]
- Yoneda, T.; Kameyama, K.; Esumi, K.; Daimyo, Y.; Watanabe, M.; Hata, Y. Developmental and Visual Input-Dependent Regulation of the CB1 Cannabinoid Receptor in the Mouse Visual Cortex. PLoS ONE 2013, 8, e53082. [Google Scholar] [CrossRef]
- Echeazarra, L.; García del Caño, G.; Barrondo, S.; González-Burguera, I.; Saumell-Esnaola, M.; Aretxabala, X.; López de Jesús, M.; Borrega-Román, L.; Mato, S.; Ledent, C.; et al. Fit-for-purpose based testing and validation of antibodies to amino- and carboxy-terminal domains of cannabinoid receptor 1. Histochem. Cell Biol. 2021, 1–24. [Google Scholar] [CrossRef]
- Fukudome, Y.; Ohno-Shosaku, Ã.T.; Matsui, Ã.M.; Omori, Y.; Fukaya, M.; Tsubokawa, H.; Taketo, M.M.; Watanabe, M.; Manabe, T.; Kano, M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses. Eur. J. Neurosci. 2004, 19, 2682–2692. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cueto, C.; Hernández-Gálvez, M.; Hillard, C.J.; Maciel, P.; García-García, L.; Valdeolivas, S.; Pozo, M.A.; Ramos, J.A.; Gómez-Ruiz, M.; Fernández-Ruiz, J. Dysregulation of the endocannabinoid signaling system in the cerebellum and brainstem in a transgenic mouse model of spinocerebellar ataxia type-3. Neuroscience 2016, 339, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Fontaine, C.J.; Ramos, A.; Reguero, L.; Gerrikagoitia, I.; de Fonseca, F.R.; Suarez, J.; Barrondo, S.; et al. Intermittent ethanol exposure during adolescence impairs cannabinoid type 1 receptor-dependent long-term depression and recognition memory in adult mice. Neuropsychopharmacology 2020, 45, 309–318. [Google Scholar] [CrossRef]
- Egaña-Huguet, J.; Bonilla-Del Río, I.; Gómez-Urquijo, S.M.; Mimenza, A.; Saumell-Esnaola, M.; Borrega-Roman, L.; García del Caño, G.; Sallés, J.; Puente, N.; Gerrikagoitia, I.; et al. The Absence of the Transient Receptor Potential Vanilloid 1 Directly Impacts on the Expression and Localization of the Endocannabinoid System in the Mouse Hippocampus. Front. Neuroanat. 2021, 15, 1–17. [Google Scholar] [CrossRef]
- De Jesús, M.L.; Sallés, J.; Meana, J.J.; Callado, L.F. Characterization of CB1 cannabinoid receptor immunoreactivity in postmortem human brain homogenates. Neuroscience 2006, 140, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Howlett, A.C. Rat brain cannabinoid receptors are N-linked glycosylated proteins. Life Sci. 1995, 56, 1983–1989. [Google Scholar] [CrossRef]
- Esteban, P.F.; Garcia-Ovejero, D.; Paniagua-Torija, B.; Moreno-Luna, R.; Arredondo, L.F.; Zimmer, A.; Arevalo-Martin, A.; Molina-Holgado, E. Revisiting CB1 cannabinoid receptor detection and the exploration of its interacting partners. J. Neurosci. Methods 2020, 337, 108680. [Google Scholar] [CrossRef]
- Nordström, R.; Andersson, H. Amino-Terminal Processing of the Human Cannabinoid Receptor 1. J. Recept. Signal Transduct. 2006, 26, 259–267. [Google Scholar] [CrossRef]
- Ruehle, S.; Wager-Miller, J.; Straiker, A.; Farnsworth, J.; Murphy, M.N.; Loch, S.; Monory, K.; Mackie, K.; Lutz, B. Discovery and characterization of two novel CB1 receptor splice variants with modified N-termini in mouse. J. Neurochem. 2017, 142, 521–533. [Google Scholar] [CrossRef]
- Egertová, M.; Elphick, M.R. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1. J. Comp. Neurol. 2000, 422, 159–171. [Google Scholar] [CrossRef]
- Wager-Miller, J.; Westenbroek, R.; Mackie, K. Dimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an example. Chem. Phys. Lipids 2002, 121, 83–89. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Howlett, A.C. CB 1 receptor-G protein association. Eur. J. Biochem. 2001, 268, 499–505. [Google Scholar] [CrossRef]
- Diniz, C.R.A.F.; Biojone, C.; Joca, S.R.L.; Rantamäki, T.; Castrén, E.; Guimarães, F.S.; Casarotto, P.C. Dual mechanism of TRKB activation by anandamide through CB1 and TRPV1 receptors. PeerJ 2019, 7, e6493. [Google Scholar] [CrossRef]
- Plummer, T.H.; Tarentino, A.L. Purification of the oligosaccharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology 1991, 1, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Tarentino, A.L.; Plummer, T.H. [4] Enzymatic deglycosylation of asparagine-linked glycans: Purification, properties, and specificity of oligosaccharide-cleaving enzymes from Flavobacterium meningosepticum. Methods Enzymol. 1994, 230, 44–57. [Google Scholar] [PubMed]
- Lee, C.R.; Park, Y.H.; Min, H.; Kim, Y.R.; Seok, Y.J. Determination of protein phosphorylation by polyacrylamide gel electrophoresis. J. Microbiol. 2019, 57, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Nyíri, G.; Cserép, C.; Szabadits, E.; Mackie, K.; Freund, T.F. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience 2005, 136, 811–822. [Google Scholar] [CrossRef]
- Niehaus, J.L.; Liu, Y.; Wallis, K.T.; Egertová, M.; Bhartur, S.G.; Mukhopadhyay, S.; Shi, S.; He, H.; Selley, D.E.; Howlett, A.C.; et al. CB 1 Cannabinoid Receptor Activity Is Modulated by the Cannabinoid Receptor Interacting Protein CRIP 1a. Mol. Pharmacol. 2007, 72, 1557–1566. [Google Scholar] [CrossRef]
- Guggenhuber, S.; Alpar, A.; Chen, R.; Schmitz, N.; Wickert, M.; Mattheus, T.; Harasta, A.E.; Purrio, M.; Kaiser, N.; Elphick, M.R.; et al. Cannabinoid receptor-interacting protein Crip1a modulates CB1 receptor signaling in mouse hippocampus. Brain Struct. Funct. 2016, 221, 2061–2074. [Google Scholar] [CrossRef]
- Rodrı́guez, J.J.; Mackie, K.; Pickel, V.M. Ultrastructural Localization of the CB1 Cannabinoid Receptor in μ-Opioid Receptor Patches of the Rat Caudate Putamen Nucleus. J. Neurosci. 2001, 21, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Katona, I.; Sperlágh, B.; Maglóczky, Z.; Sántha, E.; Köfalvi, A.; Czirják, S.; Mackie, K.; Vizi, E.; Freund, T. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience 2000, 100, 797–804. [Google Scholar] [CrossRef]
- Katona, I.; Sperlágh, B.; Sı́k, A.; Käfalvi, A.; Vizi, E.S.; Mackie, K.; Freund, T.F. Presynaptically Located CB1 Cannabinoid Receptors Regulate GABA Release from Axon Terminals of Specific Hippocampal Interneurons. J. Neurosci. 1999, 19, 4544–4558. [Google Scholar] [CrossRef]
- Katona, I.; Urban, G.M.; Wallace, M.; Ledent, C.; Jung, K.-M.; Piomelli, D.; Mackie, K.; Freund, T.F. Molecular Composition of the Endocannabinoid System at Glutamatergic Synapses. J. Neurosci. 2006, 26, 5628–5637. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Fukaya, M.; Uchigashima, M.; Miura, E.; Kamiya, H.; Kano, M.; Watanabe, M. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 2006, 26, 4740–4751. [Google Scholar] [CrossRef]
- Fukaya, M.; Uchigashima, M.; Nomura, S.; Hasegawa, Y.; Kikuchi, H.; Watanabe, M. Predominant expression of phospholipase Cβ1 in telencephalic principal neurons and cerebellar interneurons, and its close association with related signaling molecules in somatodendritic neuronal elements. Eur. J. Neurosci. 2008, 28, 1744–1759. [Google Scholar] [CrossRef]
- Montaña, M.; García del Caño, G.; López de Jesús, M.; González-Burguera, I.; Echeazarra, L.; Barrondo, S.; Sallés, J. Cellular neurochemical characterization and subcellular localization of phospholipase C β1 in rat brain. Neuroscience 2012, 222, 239–268. [Google Scholar] [CrossRef]
- García del Caño, G.; Aretxabala, X.; González-Burguera, I.; Montaña, M.; López de Jesús, M.; Barrondo, S.; Barrio, R.J.; Sampedro, C.; Goicolea, M.A.; Sallés, J. Nuclear diacylglycerol lipase-α in rat brain cortical neurons: Evidence of 2-arachidonoylglycerol production in concert with phospholipase C-β activity. J. Neurochem. 2015, 132, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-M.; Astarita, G.; Zhu, C.; Wallace, M.; Mackie, K.; Piomelli, D. A Key Role for Diacylglycerol Lipase-α in Metabotropic Glutamate Receptor-Dependent Endocannabinoid Mobilization. Mol. Pharmacol. 2007, 72, 612–621. [Google Scholar] [CrossRef]
- Howlett, A.C.; Qualy, J.M.; Khachatrian, L.L. Involvement of G(i) in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol. Pharmacol. 1986, 29, 307–313. [Google Scholar]
- Mukhopadhyay, S.; McIntosh, H.H.; Houston, D.B.; Howlett, A.C. The CB1 cannabinoid receptor juxtamembrane C-terminal peptide confers activation to specific G proteins in brain. Mol. Pharmacol. 2000, 57, 162–170. [Google Scholar]
- Straiker, A.J.; Borden, C.R.; Sullivan, J.M. G-Protein α Subunit Isoforms Couple Differentially to Receptors that Mediate Presynaptic Inhibition at Rat Hippocampal Synapses. J. Neurosci. 2002, 22, 2460–2468. [Google Scholar] [CrossRef]
- Costas-Insua, C.; Moreno, E.; Maroto, I.B.; Ruiz-Calvo, A.; Bajo-Grañeras, R.; Martín-Gutiérrez, D.; Diez-Alarcia, R.; Vilaró, M.T.; Cortés, R.; García-Font, N.; et al. Identification of BiP as a CB 1 Receptor-Interacting Protein That Fine-Tunes Cannabinoid Signaling in the Mouse Brain. J. Neurosci. 2021, 41, 7924–7941. [Google Scholar] [CrossRef] [PubMed]
- Techlovská, Š.; Chambers, J.N.; Dvořáková, M.; Petralia, R.S.; Wang, Y.-X.; Hájková, A.; Nová, A.; Franková, D.; Prezeau, L.; Blahos, J. Metabotropic glutamate receptor 1 splice variants mGluR1a and mGluR1b combine in mGluR1a/b dimers in vivo. Neuropharmacology 2014, 86, 329–336. [Google Scholar] [CrossRef][Green Version]
- Akama, K.T.; Thompson, L.I.; Milner, T.A.; McEwen, B.S. Post-synaptic Density-95 (PSD-95) Binding Capacity of G-protein-coupled Receptor 30 (GPR30), an Estrogen Receptor That Can Be Identified in Hippocampal Dendritic Spines. J. Biol. Chem. 2013, 288, 6438–6450. [Google Scholar] [CrossRef] [PubMed]
- Daigle, T.L.; Kwok, M.L.; Mackie, K. Regulation of CB 1 cannabinoid receptor internalization by a promiscuous phosphorylation-dependent mechanism. J. Neurochem. 2008, 106, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Wager-Miller, J.; Mackie, K. The CB1 cannabinoid receptor C-terminus regulates receptor desensitization in autaptic hippocampal neurones. Br. J. Pharmacol. 2012, 165, 2652–2659. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Battista, N.; Fezza, F.; Finazzi-Agrò, A.; Maccarrone, M. Lipid Rafts Control Signaling of Type-1 Cannabinoid Receptors in Neuronal Cells. J. Biol. Chem. 2005, 280, 12212–12220. [Google Scholar] [CrossRef] [PubMed]
- Sarnataro, D.; Grimaldi, C.; Pisanti, S.; Gazzerro, P.; Laezza, C.; Zurzolo, C.; Bifulco, M. Plasma membrane and lysosomal localization of CB1 cannabinoid receptor are dependent on lipid rafts and regulated by anandamide in human breast cancer cells. FEBS Lett. 2005, 579, 6343–6349. [Google Scholar] [CrossRef]
- Oddi, S.; Dainese, E.; Fezza, F.; Lanuti, M.; Barcaroli, D.; De Laurenzi, V.; Centonze, D.; Maccarrone, M. Functional characterization of putative cholesterol binding sequence (CRAC) in human type-1 cannabinoid receptor. J. Neurochem. 2011, 116, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Sýkora, J.; Bouřová, L.; Hof, M.; Svoboda, P. The effect of detergents on trimeric G-protein activity in isolated plasma membranes from rat brain cortex: Correlation with studies of DPH and Laurdan fluorescence. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 324–332. [Google Scholar] [CrossRef]
- Marsicano, G.; Lutz, B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 1999, 11, 4213–4225. [Google Scholar] [CrossRef]
- Kawamura, Y. The CB1 Cannabinoid Receptor Is the Major Cannabinoid Receptor at Excitatory Presynaptic Sites in the Hippocampus and Cerebellum. J. Neurosci. 2006, 26, 2991–3001. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Kubota, Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 1997, 7, 476–486. [Google Scholar] [CrossRef]
- Lakso, M.; Pichel, J.G.; Gorman, J.R.; Sauer, B.; Okamoto, Y.; Lee, E.; Alt, F.W.; Westphal, H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA 1996, 93, 5860–5865. [Google Scholar] [CrossRef]
- Marsicano, G.; Wotjak, C.T.; Azad, S.C.; Bisogno, T.; Rammes, G.; Cascio, M.G.; Hermann, H.; Tang, J.; Hofmann, C.; Zieglgänsberger, W.; et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 2002, 418, 530–534. [Google Scholar] [CrossRef]
- Dodd, P.R.; Hardy, J.A.; Oakley, A.E.; Edwardson, J.A.; Perry, E.K.; Delaunoy, J.-P. A rapid method for preparing synaptosomes: Comparison, with alternative procedures. Brain Res. 1981, 226, 107–118. [Google Scholar] [CrossRef]
- Garro, M.A.; López de Jesús, M.; Ruı́z de Azúa, I.; Callado, L.F.; Javier Meana, J.; Sallés, J. Regulation of phospholipase Cβ activity by muscarinic acetylcholine and 5-HT2 receptors in crude and synaptosomal membranes from human cerebral cortex. Neuropharmacology 2001, 40, 686–695. [Google Scholar] [CrossRef]
- Stigliani, S.; Zappettini, S.; Raiteri, L.; Passalacqua, M.; Melloni, E.; Venturi, C.; Tacchetti, C.; Diaspro, A.; Usai, C.; Bonanno, G. Glia re-sealed particles freshly prepared from adult rat brain are competent for exocytotic release of glutamate. J. Neurochem. 2006, 96, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Barrondo, S.; Sallés, J. Allosteric modulation of 5-HT1A receptors by zinc: Binding studies. Neuropharmacology 2009, 56, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Fleming, W.W.; Westfall, D.P.; De la Lande, I.S.; Jellett, L.B. Log-normal distribution of equiefective doses of norepinephrine and acetylcholine in several tissues. J. Pharmacol. Exp. Ther. 1972, 181, 339–345. [Google Scholar]
- Christopoulos, A. Assessing the distribution of parameters in models of ligand–receptor interaction: To log or not to log. Trends Pharmacol. Sci. 1998, 19, 351–357. [Google Scholar] [CrossRef]
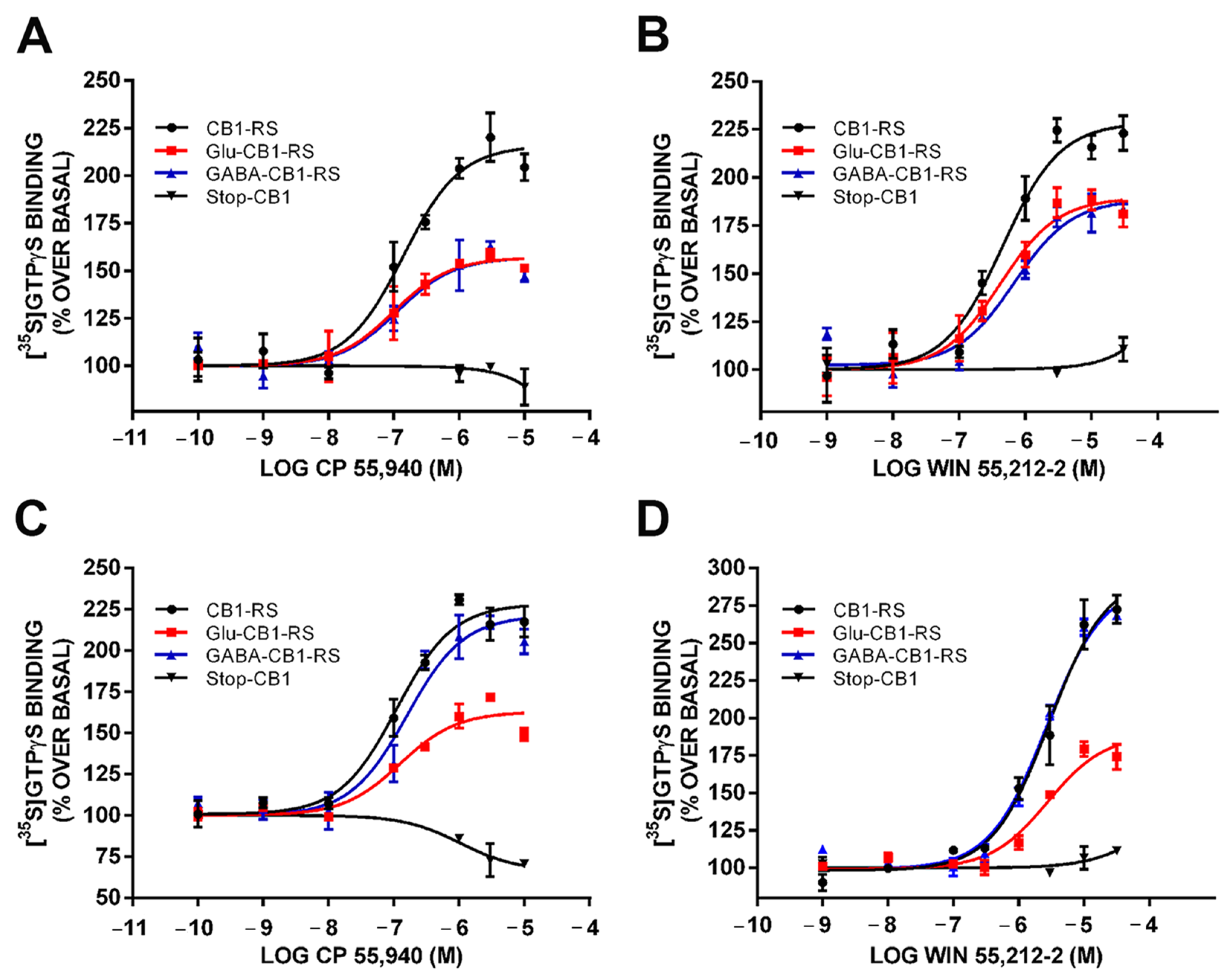
| CB1-RS | Glu-CB1-RS | GABA-CB1-RS | |
|---|---|---|---|
| Frontal Cortex | |||
| CP 55,940 | |||
| %Emax | 211.7 ± 2.37 | 157.11 ± 1.79 * | 159.00 ± 1.10 * |
| pEC50 | 6.85 ± 0.07 | 6.77 ± 0.12 | 6.72 ± 0.12 |
| WIN 55,221-2 | |||
| %Emax | 248.0 ± 18.74 | 189.55 ± 12.17 * | 184.8 ± 6.39 * |
| pEC50 | 6.75 ± 0.11 | 6.14 ± 0.12 | 6.13 ± 0.04 |
| Hippocampus | |||
| CP 55,940 | |||
| %Emax | 248.60 ± 16.36 | 183.43 ± 14.4 * | 240.60 ± 10.67 # |
| pEC50 | 6.61 ± 0.09 | 6.59 ± 0.16 | 6.61 ± 0.09 |
| WIN 55,221-2 | |||
| %Emax | 270.90 ± 9.68 | 195.85 ± 5.44 * | 258.1 ± 12.70 |
| pEC50 | 6.11 ± 0.12 | 5.93 ± 0.13 | 6.02 ± 0.12 |
| Basal (cpm) | 28,040 ± 2102 | 23,344 ± 1767 * | 23,559 ± 1725 * |
| Glu-CB1-RS | GABA-CB1-RS | |||
|---|---|---|---|---|
| Control | MβCD | Control | MβCD | |
| %Emax | 170.95 ± 3.23 | 225.87 ± 8.75 * | 167.43 ± 9.17 | 217 ± 14.20 * |
| pEC50 | 6.61 ± 0.12 | 6.78 ± 0.06 | 7.02 ± 0.08 | 6.96 ± 0.13 |
| Basal (cpm) | 11,175 ± 264 | 7443 ± 267 * | 10,324 ± 457 | 8696 ± 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saumell-Esnaola, M.; Barrondo, S.; García del Caño, G.; Goicolea, M.A.; Sallés, J.; Lutz, B.; Monory, K. Subsynaptic Distribution, Lipid Raft Targeting and G Protein-Dependent Signalling of the Type 1 Cannabinoid Receptor in Synaptosomes from the Mouse Hippocampus and Frontal Cortex. Molecules 2021, 26, 6897. https://doi.org/10.3390/molecules26226897
Saumell-Esnaola M, Barrondo S, García del Caño G, Goicolea MA, Sallés J, Lutz B, Monory K. Subsynaptic Distribution, Lipid Raft Targeting and G Protein-Dependent Signalling of the Type 1 Cannabinoid Receptor in Synaptosomes from the Mouse Hippocampus and Frontal Cortex. Molecules. 2021; 26(22):6897. https://doi.org/10.3390/molecules26226897
Chicago/Turabian StyleSaumell-Esnaola, Miquel, Sergio Barrondo, Gontzal García del Caño, María Aranzazu Goicolea, Joan Sallés, Beat Lutz, and Krisztina Monory. 2021. "Subsynaptic Distribution, Lipid Raft Targeting and G Protein-Dependent Signalling of the Type 1 Cannabinoid Receptor in Synaptosomes from the Mouse Hippocampus and Frontal Cortex" Molecules 26, no. 22: 6897. https://doi.org/10.3390/molecules26226897
APA StyleSaumell-Esnaola, M., Barrondo, S., García del Caño, G., Goicolea, M. A., Sallés, J., Lutz, B., & Monory, K. (2021). Subsynaptic Distribution, Lipid Raft Targeting and G Protein-Dependent Signalling of the Type 1 Cannabinoid Receptor in Synaptosomes from the Mouse Hippocampus and Frontal Cortex. Molecules, 26(22), 6897. https://doi.org/10.3390/molecules26226897







