HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics
Abstract
1. Introduction
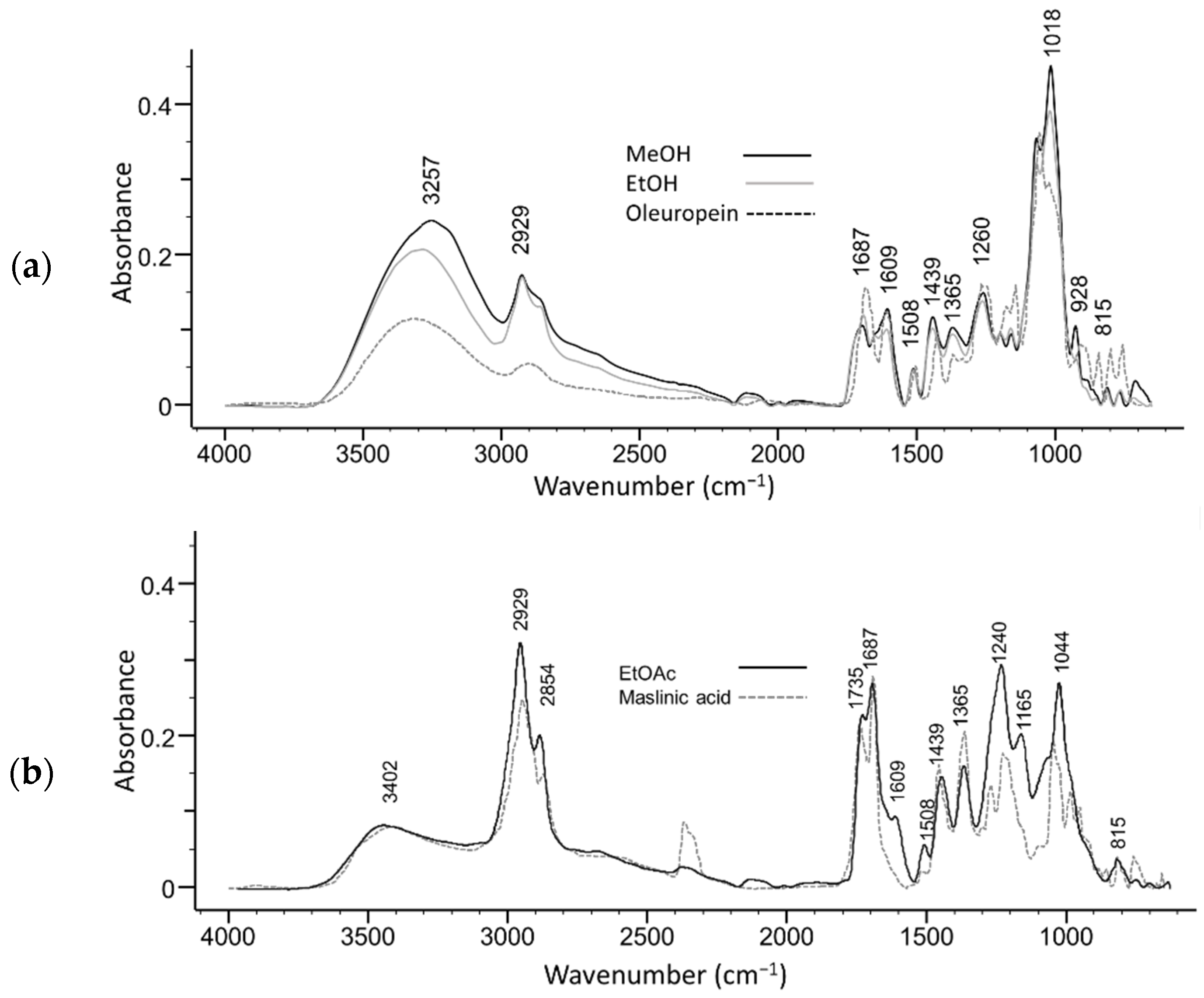
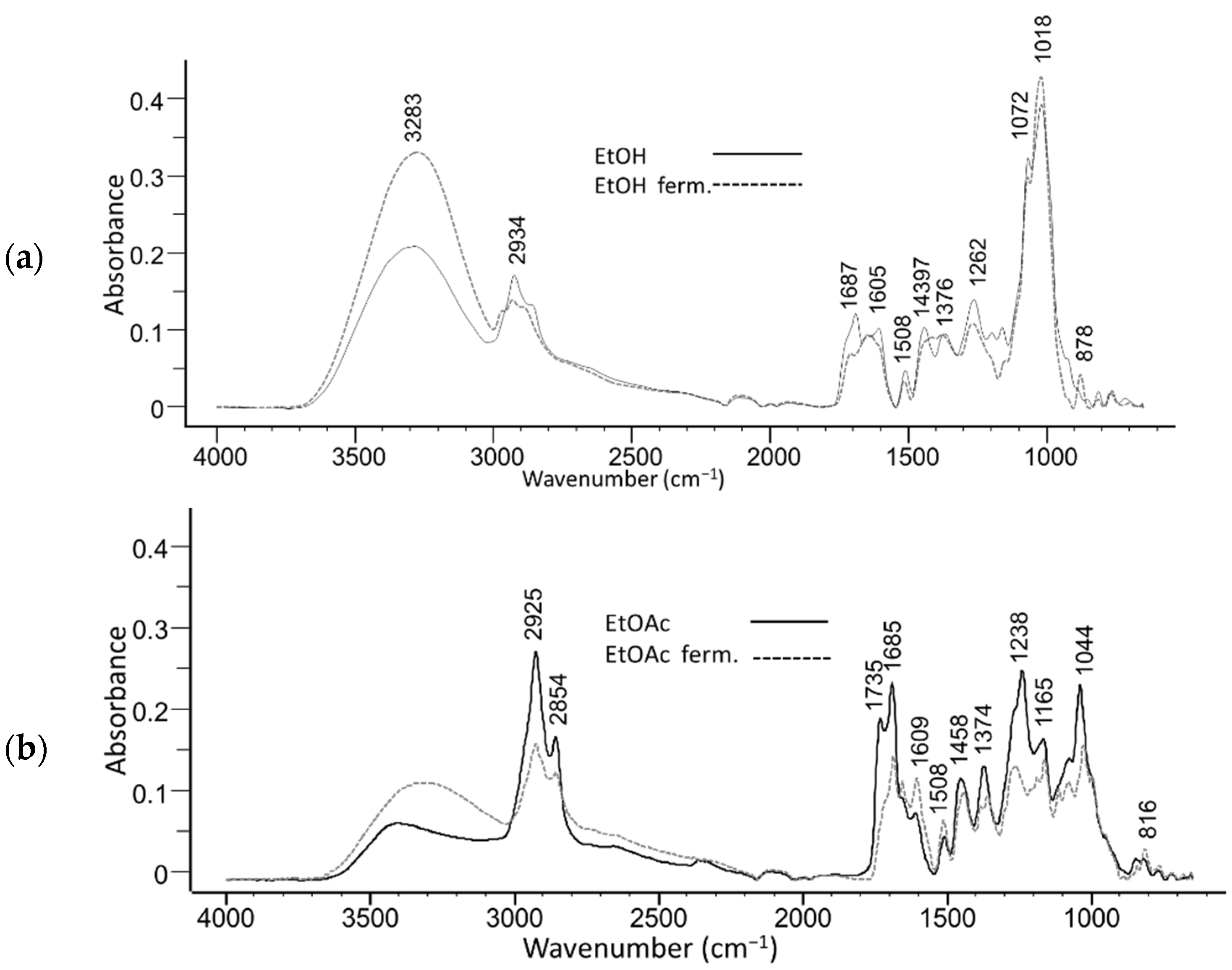
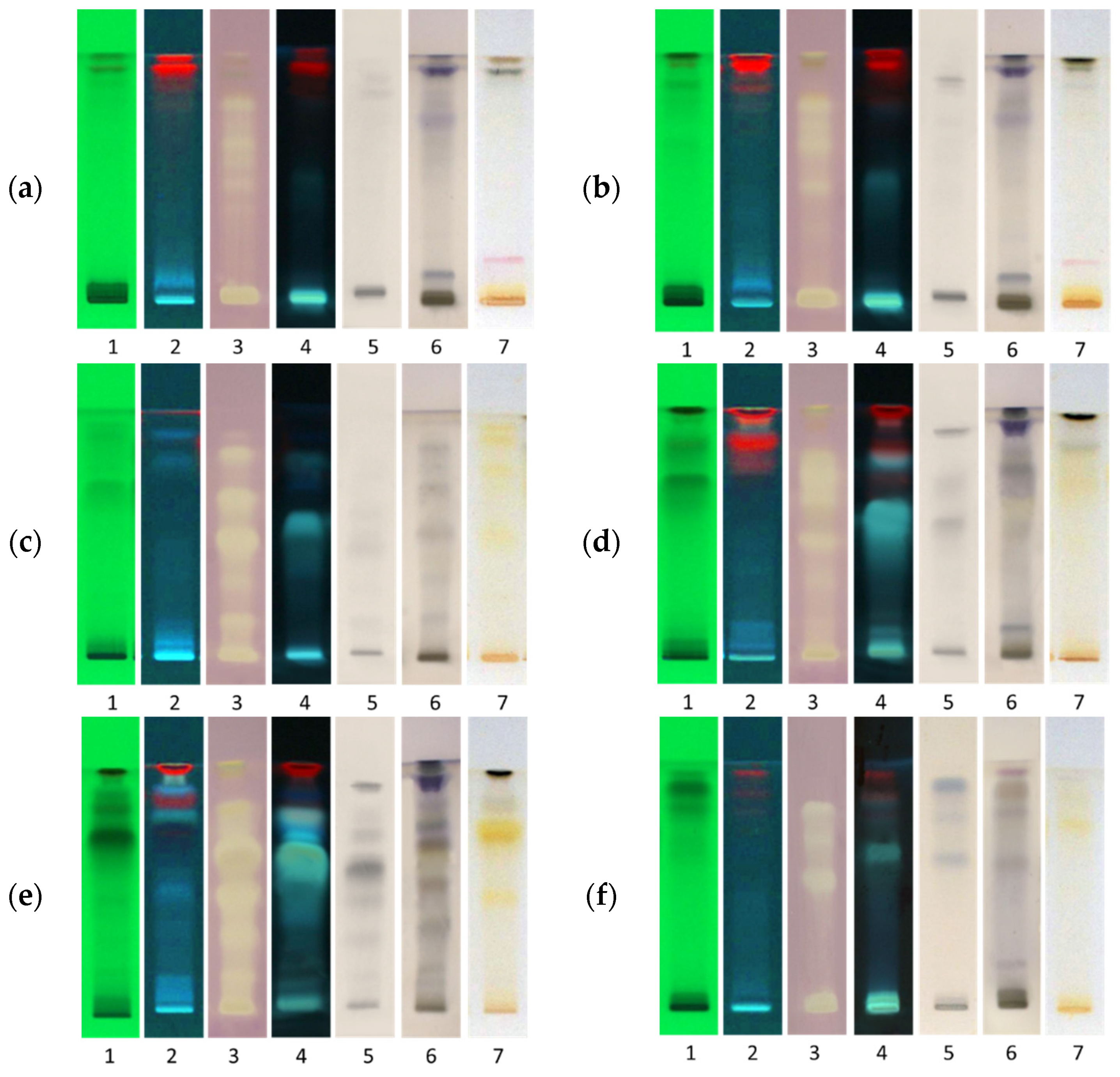
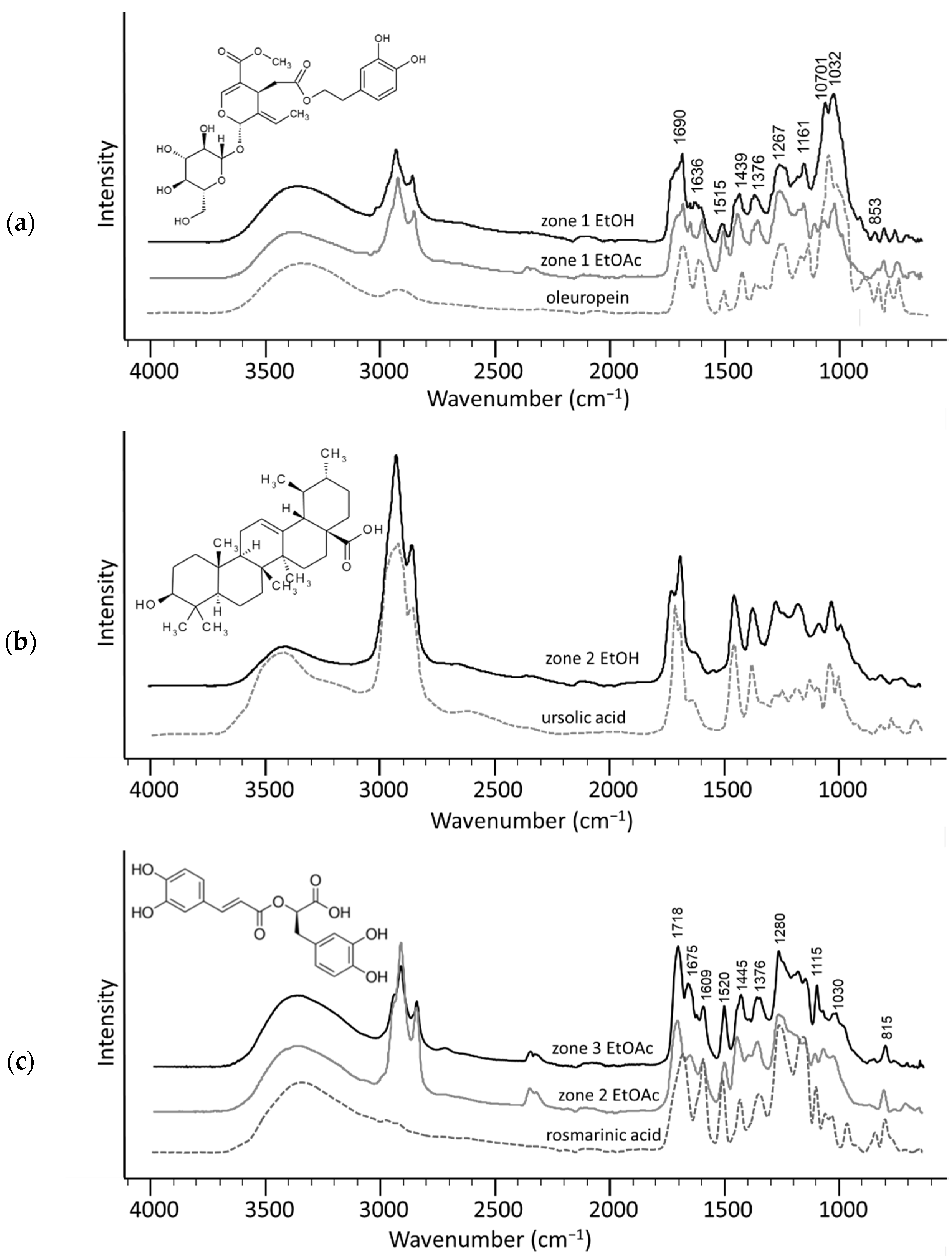
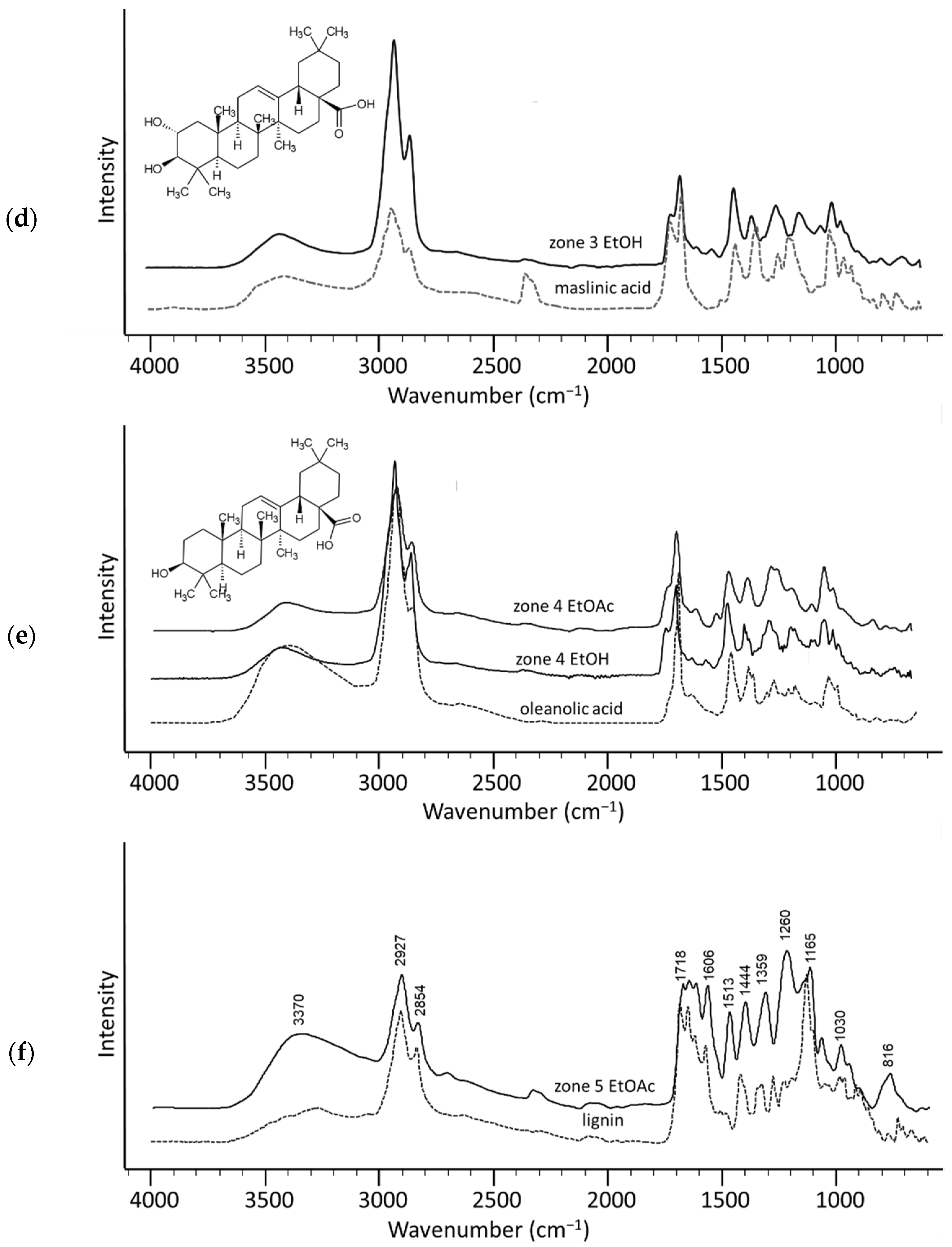
2. Results and Discussion
3. Materials and Methods
3.1. Solvents and Chemicals
3.2. Plant Extracts
3.3. Extractive Fermentation
3.4. Lignin Extraction
3.5. High-Performance Thin-Layer Chromatography
3.6. Post Chromatographic Derivatization
3.7. FTIR-ATR Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Langgut, D.; Cheddadi, R.; Carrión, J.S.; Cavanagh, M.; Colombaroli, D.; Eastwood, W.J.; Greenberg, R.; Litt, T.; Mercuri, A.M.; Miebach, A. The origin and spread of olive cultivation in the Mediterranean Basin: The fossil pollen evidence. Holocene 2019, 29, 902–922. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Bogani, P.; Grande, S.; Galli, C. Mediterranean food and health: Building human evidence. J. Physiol. Pharmacol. 2005, 56, 37–49. [Google Scholar] [PubMed]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G. Phenolic compounds of olive fruit: One- and two-dimensional nuclear magnetic resonance characterization of Nüzhenide and its distribution in the constitutive parts of fruit. J. Agric. Food Chem. 1999, 47, 12–18. [Google Scholar] [CrossRef] [PubMed]
- de la Torre-Carbot, K.; Jauregui, O.; Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; López-Sabater, M.C. Characterization and quantification of phenolic compounds in olive oils by solid-phase extraction, HPLC-DAD, and HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 4331–4340. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Sibbett, G.S.; Ferguson, L. Olive Production Manual, 2nd ed.; UCANR Publications: Oakland, CA, USA, 2005. [Google Scholar]
- Solarte-Toro, J.C.; Romero-Garcia, J.M.; Lopez-Linares, J.C.; Ramos, E.R.; Castro, E.; Alzate, C.A.C. Simulation approach through the biorefinery concept of the antioxidants, lignin and ethanol production using olive leaves as raw material. Chem. Eng. Trans. 2018, 70, 925–930. [Google Scholar]
- Keceli, T.M.; Harp, F. The effect of olive leaves and their harvest time on radical scavenging activity and oxidative stability of refined olive oil. Qual. Assur. Saf. Crops Foods 2014, 6, 141–149. [Google Scholar] [CrossRef]
- Briante, R.; Patumi, M.; Terenziani, S.; Bismuto, E.; Febbraio, F.; Nucci, R. Olea europaea L. leaf extract and derivatives: Antioxidant properties. J. Agric. Food Chem. 2002, 50, 4934–4940. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque de Castro, M.D. Dynamic ultrasound-assisted extraction of oleuropein and related biophenols from olive leaves. J. Chromatogr. A 2006, 1108, 76–82. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J.; Alcaraz, M. Radioprotective effects in vivo of phenolics extracted from Olea europaea L. leaves against X-ray-induced chromosomal damage: Comparative study versus several flavonoids and sulfur-containing compounds. J. Med. Food 2002, 5, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive leaf addition increases olive oil nutraceutical properties. Molecules 2019, 24, 545. [Google Scholar] [CrossRef]
- Wainstein, J.; Ganz, T.; Boaz, M.; Bar Dayan, Y.; Dolev, E.; Kerem, Z.; Madar, Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food 2012, 15, 605–610. [Google Scholar] [CrossRef]
- Gastaldo, P. Official compendium of the Italian flora. XVI. Fitoterapia 1974, 45, 199–217. [Google Scholar]
- Pieroni, A.; Heimler, D.; Pieters, L.; Van Poel, B.; Vlietinck, A.J. In vitro anti-complementary activity of flavonoids from oliva (Olea europaea L.) leaves. Pharmazie 1996, 51, 765–767. [Google Scholar]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Gegechkori, V.; Morton, D.W. The effect of extractive lacto-fermentation on the bioactivity and natural products content of Pittosporum angustifolium (gumbi gumbi) extracts. J. Chromatogr. A 2021, 1647, 462153. [Google Scholar] [CrossRef]
- Krüger, S.; Bergin, A.; Morlock, G.E. Effect-directed analysis of ginger (Zingiber officinale) and its food products, and quantification of bioactive compounds via high-performance thin-layer chromatography and mass spectrometry. Food Chem. 2018, 243, 258–268. [Google Scholar] [CrossRef]
- Morlock, G.E.; Ristivojevic, P.; Chernetsova, E. Combined multivariate data analysis of high-performance thin-layer chromatography fingerprints and direct analysis in real time mass spectra for profiling of natural products like propolis. J. Chromatogr. A 2014, 1328, 104–112. [Google Scholar] [CrossRef]
- Henion, J.; Maylin, G.A.; Thomson, B.A. Determination of drugs in biological samples by thin-layer chromatography tandem mass spectrometry. J. Chromatogr. A 1983, 271, 107–124. [Google Scholar] [CrossRef]
- Ovchinnikova, O.S.; Van Berkel, G.J. Thin-layer chromatography and mass spectrometry coupled using proximal probe thermal desorption with electrospray or atmospheric pressure chemical ionization. Rapid Commun. Mass Spectrom. 2010, 24, 1721–1729. [Google Scholar] [CrossRef]
- Van Berkel, G.J.; Ford, M.J.; Deibel, M.A. Thin-layer chromatography and mass spectrometry coupled using desorption electrospray ionization. Anal. Chem. 2005, 77, 1207–1215. [Google Scholar] [CrossRef]
- Paglia, G.; Ifa, D.R.; Wu, C.; Corso, G.; Cooks, R.G. Desorption electrospray ionization mass spectrometry analysis of lipids after two-dimensional high-performance thin-layer chromatography partial separation. Anal. Chem. 2010, 82, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Dytkiewitz, E.; Morlock, G.E. Analytical strategy for rapid identification and quantification of lubricant additives in mineral oil by high-performance thin-layer chromatography with UV absorption and fluorescence detection combined with mass spectrometry and infrared spectroscopy. J. AOAC Int. 2008, 91, 1237–1243. [Google Scholar] [PubMed]
- Sánchez-Quesada, C.; López-Biedma, A.; Warleta, F.; Campos, M.; Beltrán, G.; Gaforio, J.J. Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea. J. Agric. Food Chem. 2013, 61, 12173–12182. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Borriello, M.; Irace, G.; Cammarota, M.; Di Maro, A.; Sirangelo, I. Vanillin affects amyloid aggregation and non-enzymatic glycation in human insulin. Sci. Rep. 2017, 7, 15086. [Google Scholar] [CrossRef]
- Hue, N.V.; Craddock, G.R.; Adams, F. Effect of organic acids on aluminum toxicity in subsoils. Soil Sci. Soc. Am. J. 1986, 50, 28–34. [Google Scholar] [CrossRef]
- Pomar, F.; Merino, F.; Barceló, A.R. O-4-Linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol-HCl) reaction. Protoplasma 2002, 220, 17–28. [Google Scholar] [CrossRef]
- Sameni, J.; Krigstin, S.; Sain, M. Solubility of lignin and acetylated lignin in organic solvents. Bioresources 2017, 12, 1548–1565. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Olive pomace-derived biomasses fractionation through a two-step extraction based on the use of ultrasounds: Chemical characteristics. Foods 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Filannino, P.; Gobbetti, M.; De Angelis, M.; Di Cagno, R. Hydroxycinnamic acids used as external acceptors of electrons: An energetic advantage for strictly heterofermentative lactic acid bacteria. Appl. Environ. Microbiol. 2014, 80, 7574–7582. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Bellumori, M.; Pucci, L.; Gabriele, M.; Longo, V.; Paoli, P.; Melani, F.; Mulinacci, N.; Innocenti, M. Does Fermentation Really Increase the Phenolic Content in Cereals? A Study on Millet. Foods 2020, 9, 303. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Balyklova, K.S.; Gegechkori, V.; Morton, D.W. HPTLC and ATR/FTIR Characterization of Antioxidants in Different Rosemary Extracts. Molecules 2021, 26, 6064. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Abdelgaleel, M.A.; Salama, A.A.; Metwalli, S.M. Chemical and nutritional evaluation of olive leaves and selection the optimum conditions for extraction their phenolic compounds. J. Agric. Res. Kafr. El-Sheikh Univ. 2016, 42, 445–459. [Google Scholar]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Lin, Y.-T.; Labbe, R.G.; Shetty, K. Phenolic antioxidant mobilization in cranberry pomace by solid-state bioprocessing using food grade fungus Lentinus edodes and effect on antimicrobial activity against select food borne pathogens. Innov. Food Sci. Emerg. Technol. 2004, 5, 81–91. [Google Scholar] [CrossRef]
- Cho, K.M.; Hong, S.Y.; Math, R.K.; Lee, J.H.; Kambiranda, D.M.; Kim, J.M.; Islam, S.M.A.; Yun, M.G.; Cho, J.J.; Lim, W.J. Biotransformation of phenolics (isoflavones, flavanols and phenolic acids) during the fermentation of cheonggukjang by Bacillus pumilus HY1. J. Food Chem. 2009, 114, 413–419. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W.; Ristivojevic, P. Probing into the molecular requirements for antioxidant activity in plant phenolic compounds utilizing a combined strategy of PCA and ANN. Comb. Chem. High Throughput Screen. 2017, 20, 25–34. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Buijsman, M.N.C.P.; van Gameren, Y.; Cnossen, E.P.J.; de Vries, J.H.M.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Ferreira, O.; Pinho, S.P. Solubility of Flavonoids in Pure Solvents. Ind. Eng. Chem. Res. 2012, 51, 6586–6590. [Google Scholar] [CrossRef]
- Fabiani, R.; Rosignoli, P.; Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar] [CrossRef]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. Biofactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Genc, N.; Yildiz, I.; Chaoui, R.; Erenler, R.; Temiz, C.; Elmastas, M. Biosynthesis, characterization and antioxidant activity of oleuropein-mediated silver nanoparticles. Inorg. Nano-Met. Chem. 2020, 51, 411–419. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Alternat. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Perez-Jimenez, A.; Reyes-Zurita, F.J.; Garcia-Salguero, L.; Mokhtari, K.; Herrera-Merchan, A.; Medina, P.P.; Peragon, J.; Lupianez, J.A. Anti-cancer and anti-angiogenic properties of various natural pentacyclic tri-terpenoids and some of their chemical derivatives. Curr. Org. Chem. 2015, 19, 1. [Google Scholar] [CrossRef]
- Liu, J.; Rajendram, R.; Zhang, L. Effects of oleanolic acid and maslinic acid on glucose and lipid metabolism: Implications for the beneficial effects of olive oil on health. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Elsevier: London, UK, 2010; pp. 1423–1429. [Google Scholar]
- Martín, R.; Carvalho, J.; Ibeas, E.; Hernández, M.; Ruiz-Gutierrez, V.; Nieto, M.L. Acidic triterpenes compromise growth and survival of astrocytoma cell lines by regulating reactive oxygen species accumulation. Cancer Res. 2007, 67, 3741–3751. [Google Scholar] [CrossRef][Green Version]
- Montilla, M.P.; Agil, A.; Navarro, M.C.; Jiménez, M.I.; García-Granados, A.; Parra, A.; Cabo, M.M. Antioxidant activity of maslinic acid, a triterpene derivative obtained from Olea europaea. Planta Med. 2003, 69, 472–474. [Google Scholar] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Oriente, G. Isolamento e costituzione dell’acido rosmarinico (dal rosmarinus off.). Ric. Sci. 1958, 28, 2329–2333. [Google Scholar]
- Al-Sereiti, M.R.; Abu-Amer, K.M.; Sena, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 32, 124–130. [Google Scholar]
- Varsányi, G. Assignments for Vibrational Spectra of Seven Hundred Benzene Derivatives; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Smal, I.M.; Yu, Q.; Veneman, R.; Fränzel-Luiten, B.; Brilman, D.W.F. TG-FTIR Measurement of CO2-H2O co-adsorption for CO2 air capture sorbent screening. Energy Procedia 2014, 63, 6834–6841. [Google Scholar] [CrossRef]
- Tamaki, Y.; Mazza, G. Rapid determination of lignin content of straw using Fourier Transform Mid-Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kubovský, I.; Kačíková, D.; Kačík, F. Structural changes of oak wood main components caused by thermal modification. Polymers 2020, 12, 485. [Google Scholar] [CrossRef] [PubMed]
- Stark, N.M.; Yelle, D.J.; Agarwal, U.P. Techniques for characterizing lignin. In Lignin in Polymer Composites; Faruk, O., Sain, M., Eds.; Elsevier: Oxford, UK, 2016; pp. 49–66. [Google Scholar]
- Sethi, A. Systematic Lab. Experiments in Organic Chemistry; New Age International (P) Limited: New Delhi, India, 2006. [Google Scholar]
| Standard | Linear Regression Analysis | RSD | LOD (μg) | LOQ (μg) | Linear Range (µg/band) | |
|---|---|---|---|---|---|---|
| Gallic Acid | DPPH• | y = 109028x − 20474 (R2 = 0.98) | 3.98–8.48 | 0.33 | 1.12 | 0.4–5.0 |
| Gallic Acid | FeCl3 | y = 21093x + 15402 (R2 = 0.96) | 2.42–5.97 | 0.22 | 0.74 | 0.5–10.0 |
| β-Sitosterol | ASA | y = 5195.2x + 13732 (R2 = 0.95) | 2.6–6.87 | 0.43 | 1.48 | 0.5–8.0 |
| Rutin | FeCl3 | y = 8430.7x + 3798.4 (R2 = 0.97) | 0.77–2.35 | 0.29 | 0.96 | 1.0–7.0 |
| Polyphenolics | Antioxidants | Flavonoids | Terpenoids | |||||
|---|---|---|---|---|---|---|---|---|
| FeCl3 (pixels) | GAE (µg/20 µL) | DPPH• | GAE (µg/20 µL) | AlCl3 | RE (µg/20 µL) | ASA | SE (µg/20 µL) | |
| MeOH | 156,712 | 6.7 | 1,144,729 | 10.3 | 397,401 | 42.3 | 712,549 | 134.5 |
| EtOH | 165,300 | 7.1 | 1,213,229 | 10.9 | 590,339 | 65.0 | 974,103 | 184.9 |
| EtOH (F) | 118,785 | 4.9 | 2,249,023 | 20.4 | 765,880 | 85.7 | 590,527 | 111.0 |
| EtOAc | 235,488 | 10.4 | 1,944,948 | 17.6 | 1,567,426 | 179.9 | 1,145,825 | 217.9 |
| EtOAc (F) | 553,215 | 25.5 | 2,773,156 | 25.3 | 2,057,903 | 237.6 | 1,507,619 | 287.5 |
| EtOH (S) | 276,625 | 12.4 | 439,621 | 3.8 | 504,530 | 54.9 | 1,308,457 | 249.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agatonovic-Kustrin, S.; Gegechkori, V.; Petrovich, D.S.; Ilinichna, K.T.; Morton, D.W. HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules 2021, 26, 6892. https://doi.org/10.3390/molecules26226892
Agatonovic-Kustrin S, Gegechkori V, Petrovich DS, Ilinichna KT, Morton DW. HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules. 2021; 26(22):6892. https://doi.org/10.3390/molecules26226892
Chicago/Turabian StyleAgatonovic-Kustrin, Snezana, Vladimir Gegechkori, Dementyev Sergey Petrovich, Kobakhidze Tamara Ilinichna, and David William Morton. 2021. "HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics" Molecules 26, no. 22: 6892. https://doi.org/10.3390/molecules26226892
APA StyleAgatonovic-Kustrin, S., Gegechkori, V., Petrovich, D. S., Ilinichna, K. T., & Morton, D. W. (2021). HPTLC and FTIR Fingerprinting of Olive Leaves Extracts and ATR-FTIR Characterisation of Major Flavonoids and Polyphenolics. Molecules, 26(22), 6892. https://doi.org/10.3390/molecules26226892







